Insights into the Mechanism of the Bi/BiVO4 Composites for Improved Photocatalytic Activity
Abstract
1. Introduction
2. Results and Discussion
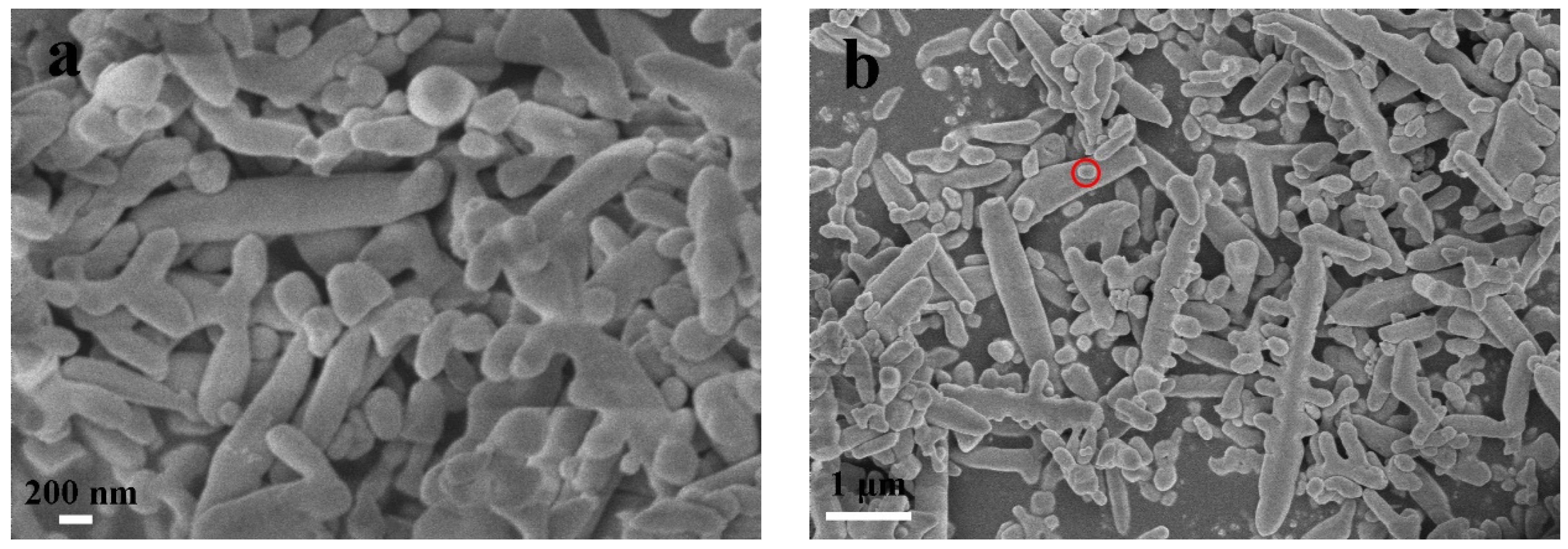
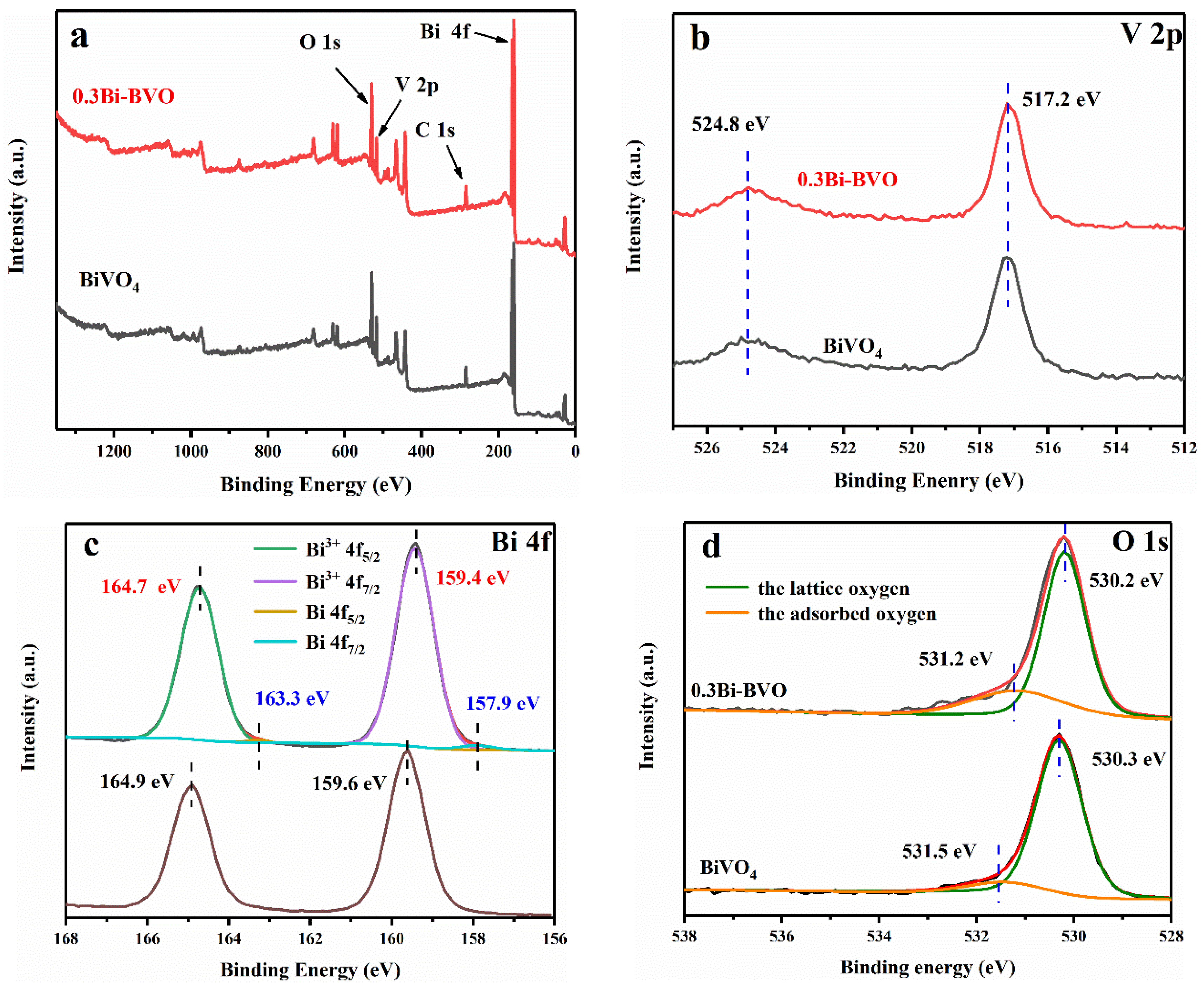
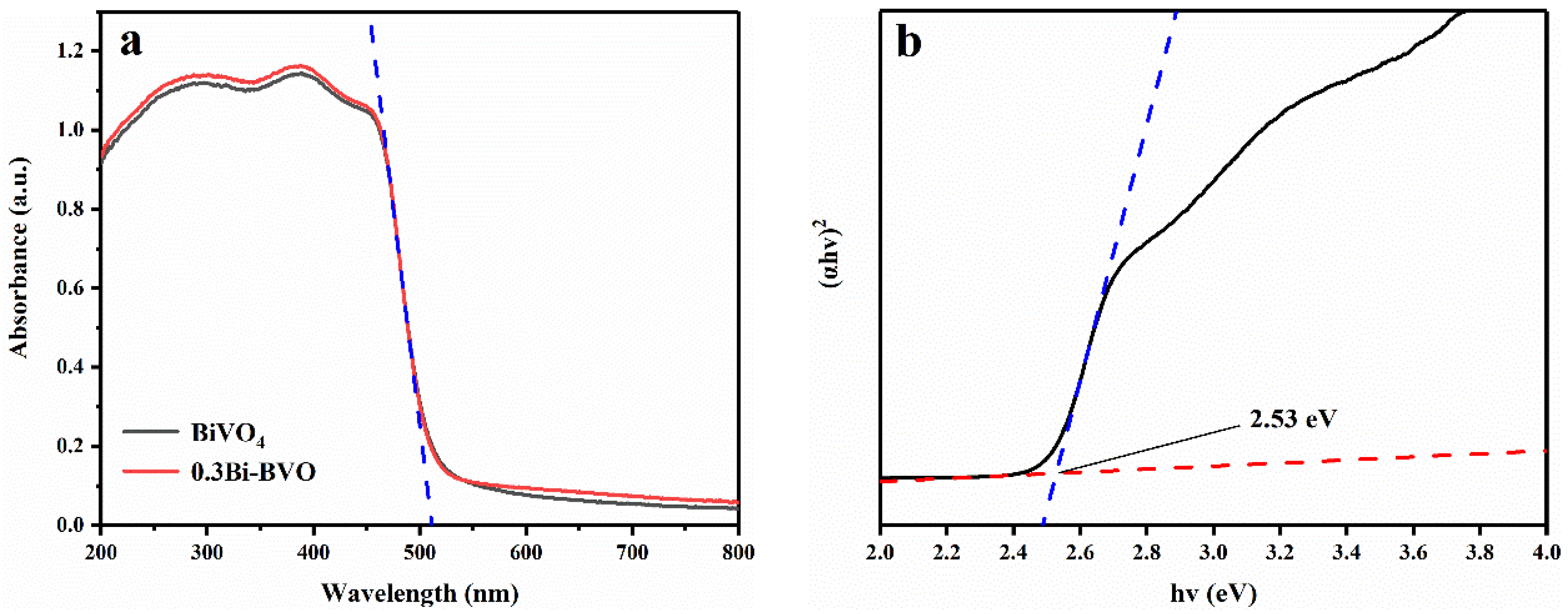
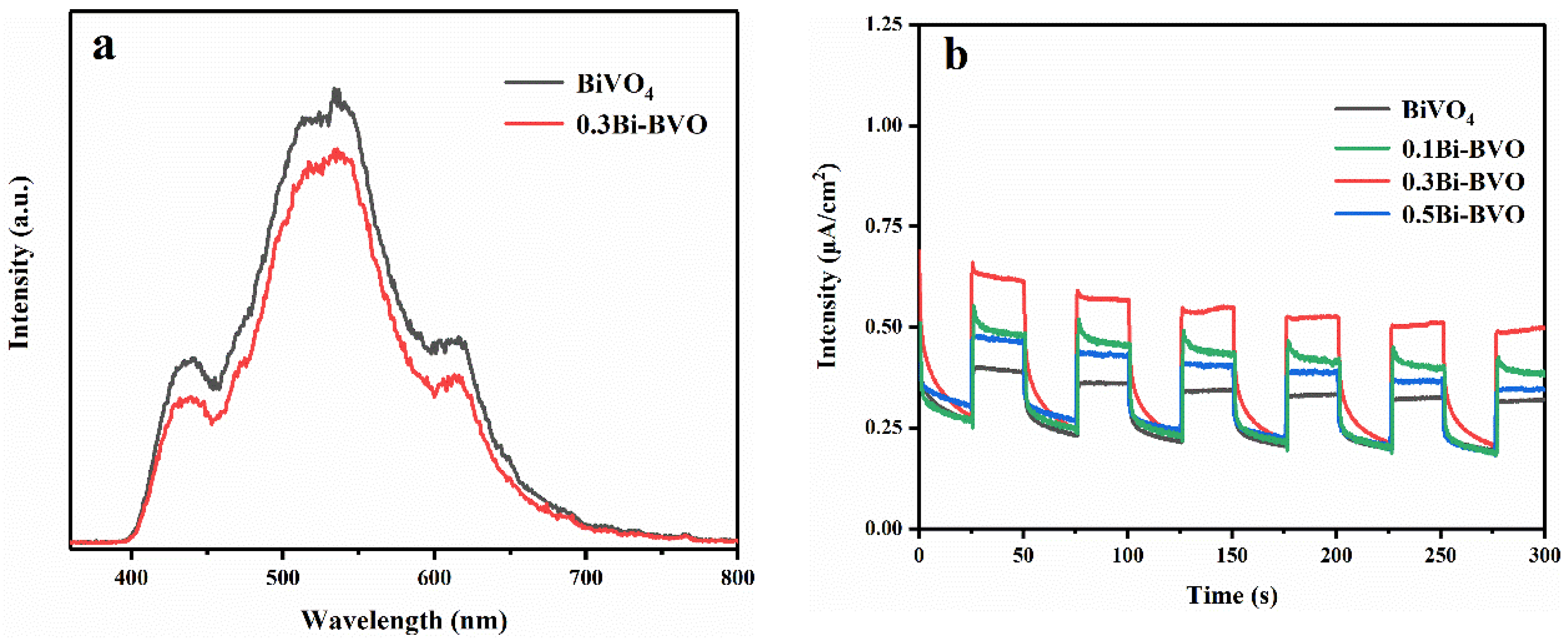
2.1. Characterization of the Prepared Samples
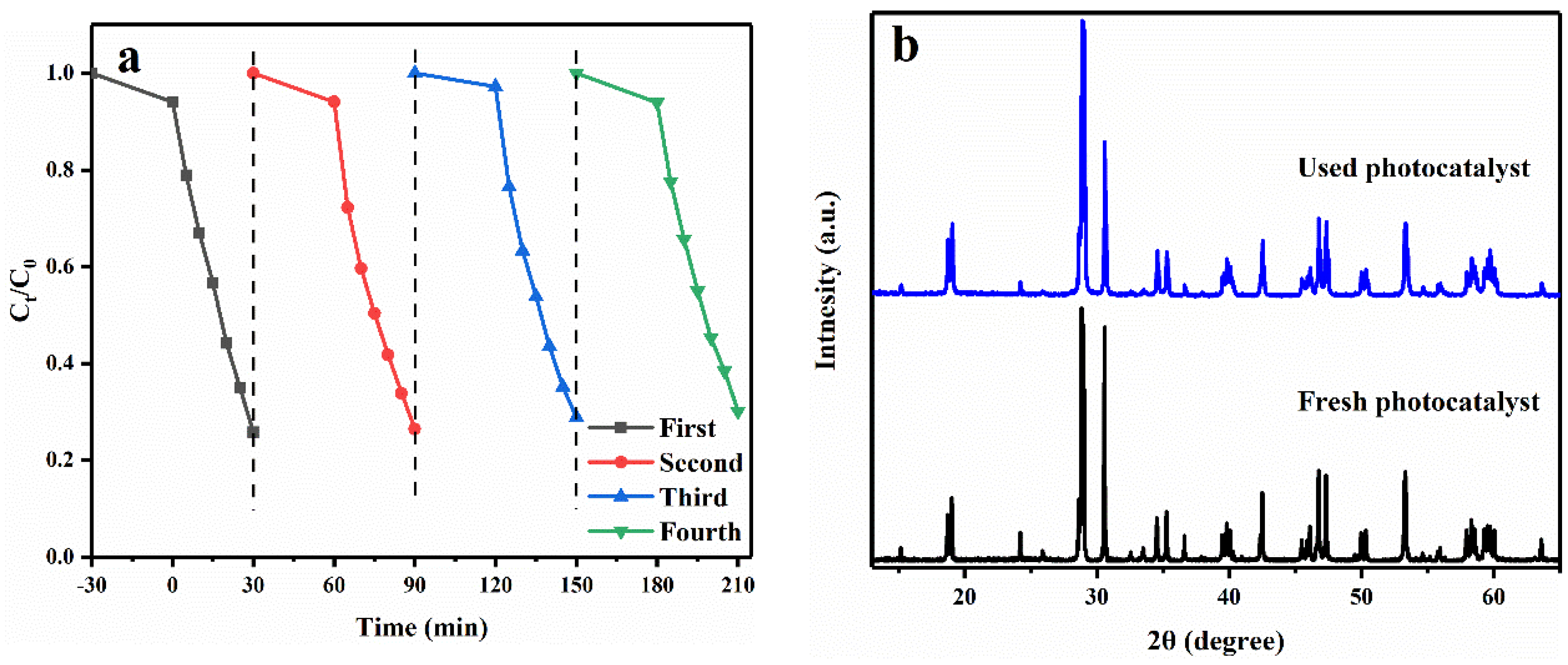
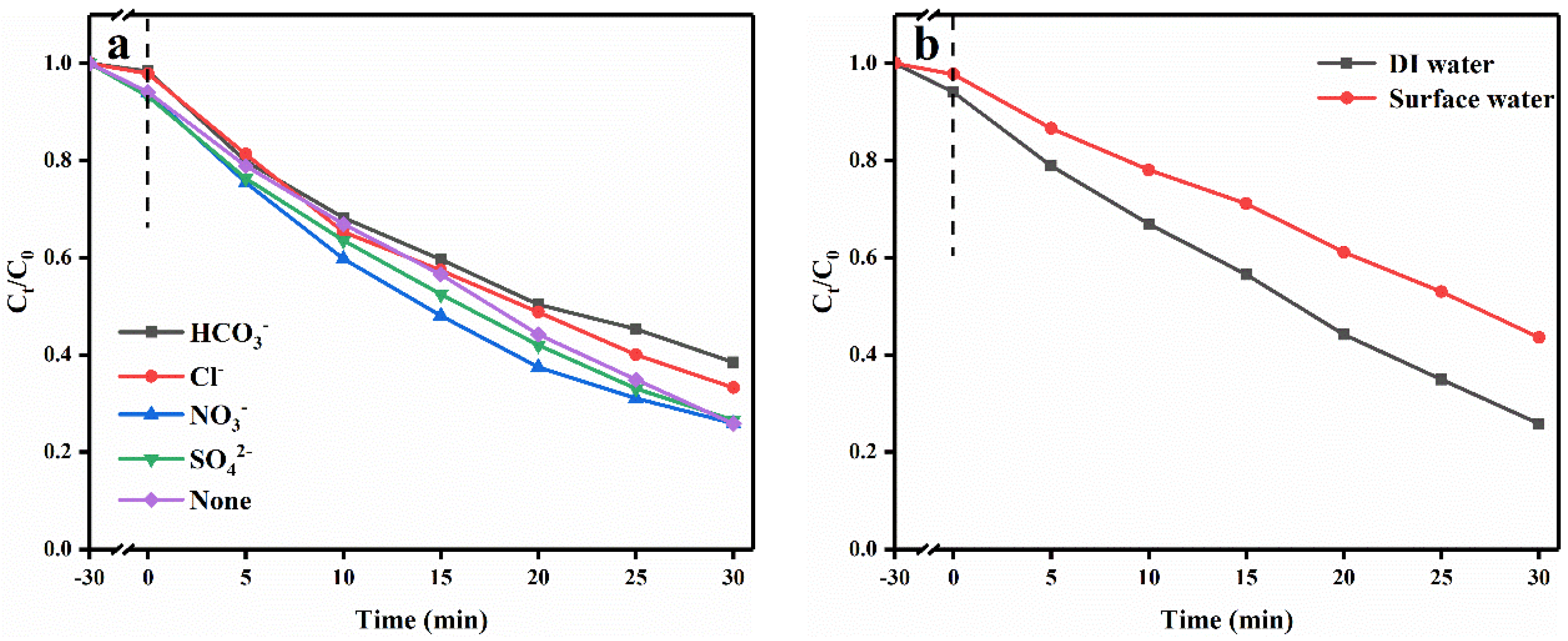
2.2. Photocatalytic Activity
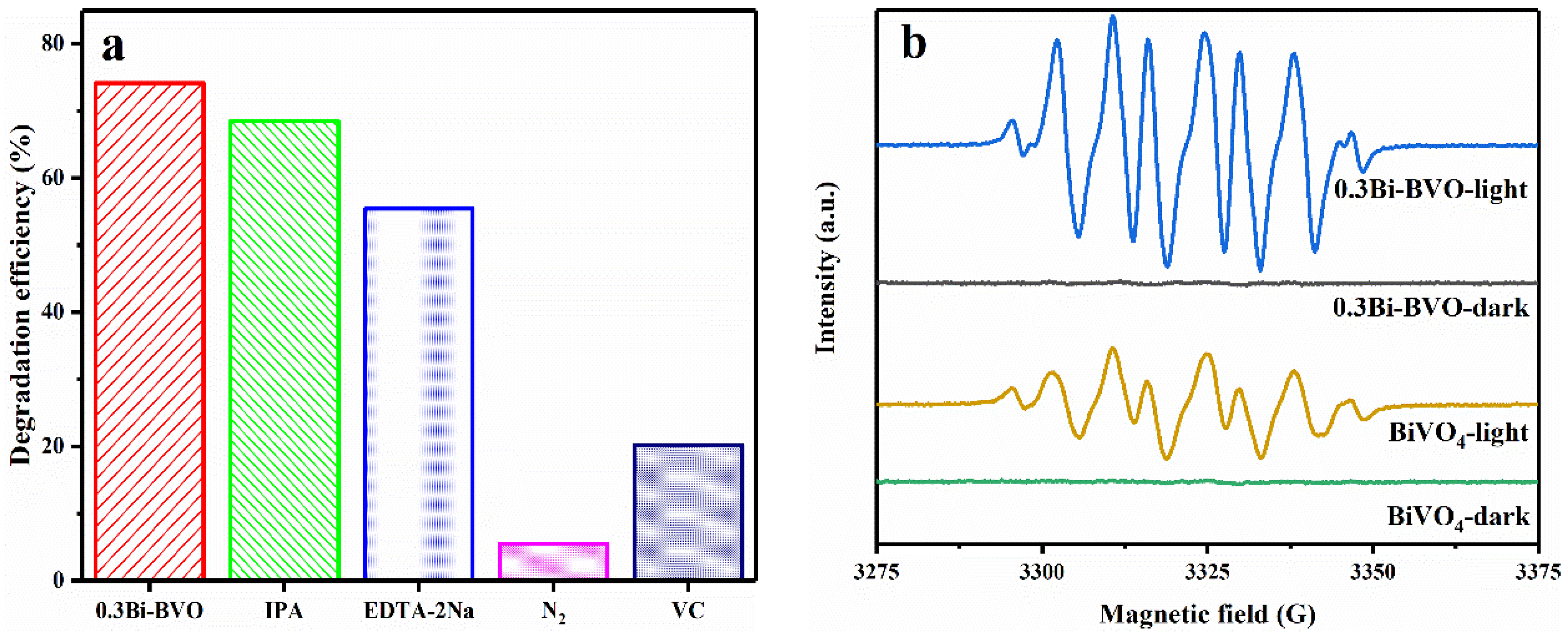
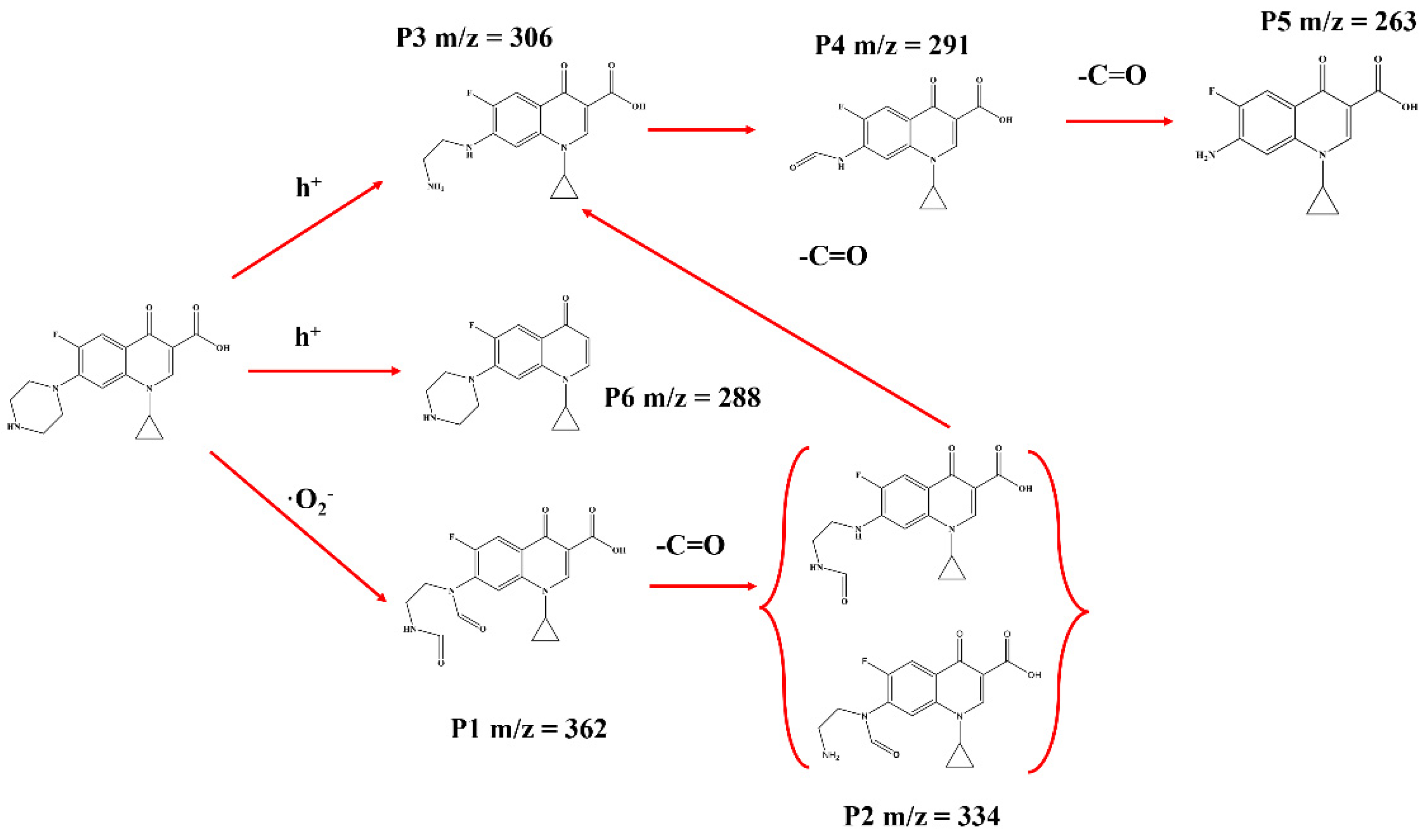
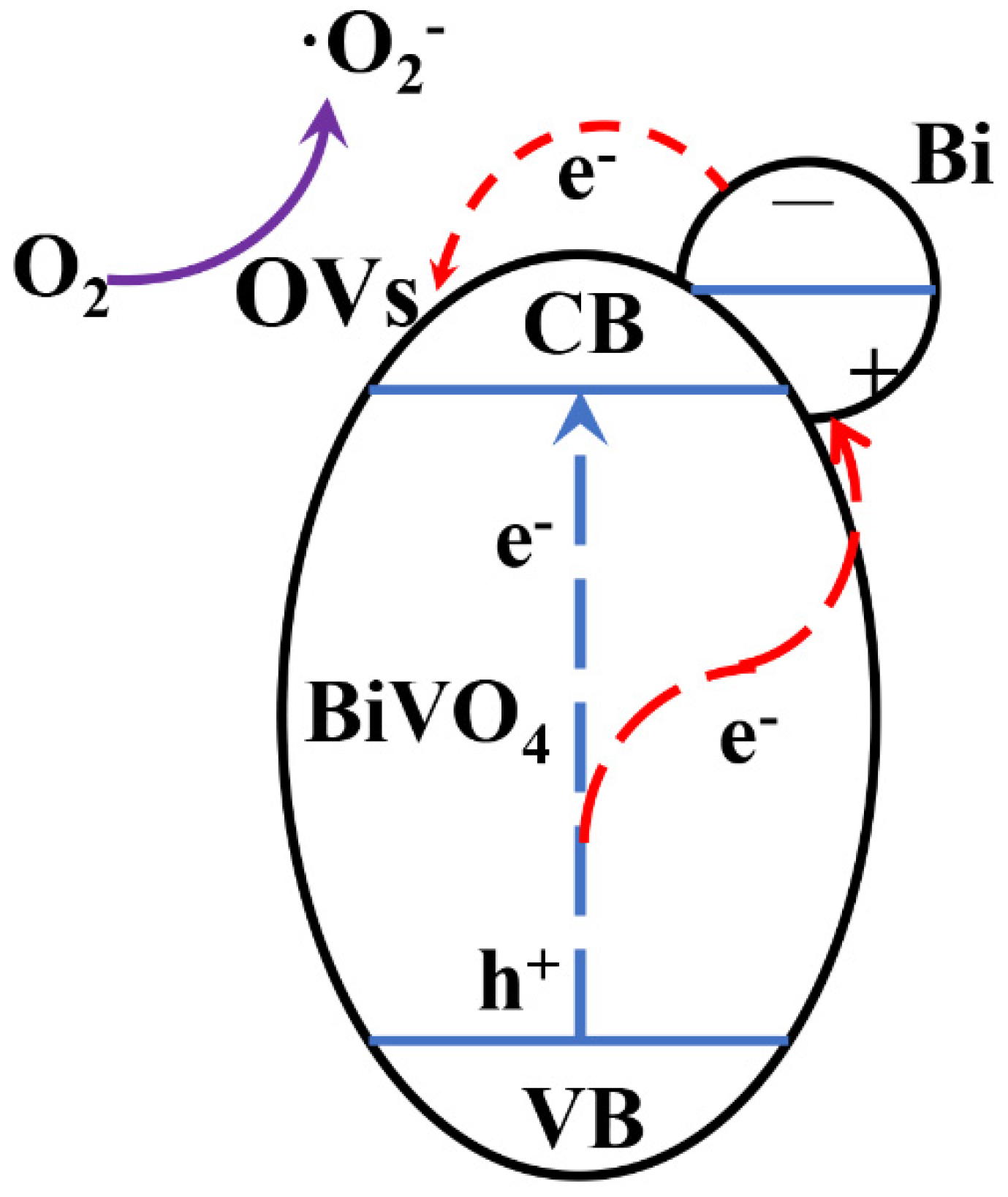
2.3. Photocatalytic Mechanism
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Photocatalyst
4.1.1. Synthesis of BiVO4
4.1.2. Synthesis of Bi/BiVO4
4.2. Characterization
4.3. Photocatalytic Degradation Experiments
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Salma, A.; Thoroe-Boveleth, S.; Schmidt, T.C.; Tuerk, J. Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J. Hazard. Mater. 2016, 313, 49–59. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Mai, D.T.; Stuckey, D.C.; Oh, S. Effect of ciprofloxacin on methane production and anaerobic microbial community. Bioresour. Technol. 2018, 261, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Sarkar, D.; Elzinga, E.J.; Punamiya, P.; Datta, R. Mechanisms of ciprofloxacin removal by nano-sized magnetite. J. Hazard. Mater. 2013, 246–247, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yao, J.; Huang, Y.; Gong, H.; Chu, W. Enhanced photocatalytic degradation of ciprofloxacin over Bi2O3/(BiO)2CO3 heterojunctions: Efficiency, kinetics, pathways, mechanisms and toxicity evaluation. Chem. Eng. J. 2018, 334, 453–461. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Eggen, T.; Asp, T.N.; Grave, K.; Hormazabal, V. Uptake and translocation of metformin, ciprofloxacin and narasin in forage- and crop plants. Chemosphere 2011, 85, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Polesel, F.; Lehnberg, K.; Dott, W.; Trapp, S.; Thomas, K.V.; Plósz, B.G. Factors influencing sorption of ciprofloxacin onto activated sludge: Experimental assessment and modelling implications. Chemosphere 2015, 119, 105–111. [Google Scholar] [CrossRef]
- Eslami, A.; Amini, M.M.; Yazdanbakhsh, A.R.; Mohseni-Bandpei, A.; Safari, A.A.; Asadi, A. N,S co-doped TiO2 nanoparticles and nanosheets in simulated solar light for photocatalytic degradation of non-steroidal anti-inflammatory drugs in water: A comparative study. J. Chem. Technol. Biotechnol. 2016, 91, 2693–2704. [Google Scholar] [CrossRef]
- Kaur, A.; Kansal, S.K. Bi2WO6 nanocuboids: An efficient visible light active photocatalyst for the degradation of levofloxacin drug in aqueous phase. Chem. Eng. J. 2016, 302, 194–203. [Google Scholar] [CrossRef]
- Sood, S.; Mehta, S.K.; Sinha, A.S.K.; Kansal, S.K. Bi2O3/TiO2 heterostructures: Synthesis, characterization and their application in solar light mediated photocatalyzed degradation of an antibiotic, ofloxacin. Chem. Eng. J. 2016, 290, 45–52. [Google Scholar] [CrossRef]
- Gu, S.; Li, W.; Wang, F.; Wang, S.; Zhou, H.; Li, H. Synthesis of buckhorn-like BiVO4 with a shell of CeOx nanodots: Effect of heterojunction structure on the enhancement of photocatalytic activity. Appl. Catal. B-Environ. 2015, 170–171, 186–194. [Google Scholar] [CrossRef]
- Palaniselvam, T.; Shi, L.; Mettela, G.; Anjum, D.H.; Li, R.; Katuri, K.P.; Saikaly, P.E.; Wang, P. Vastly Enhanced BiVO4 Photocatalytic OER Performance by NiCoO2 as Cocatalyst. Adv. Mater. Interfaces 2017, 4, 1700540. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Feng, C.; Zeng, G.; Wang, J.; Zhou, Y.; Liu, Y.; Peng, B.; Feng, H. Construction of plasmonic Ag modified phosphorous-doped ultrathin g-C3N4 nanosheets/BiVO4 photocatalyst with enhanced visible-near-infrared response ability for ciprofloxacin degradation. J. Hazard. Mater. 2018, 344, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Uemura, J.; Kazoe, Y.; Kosar, S.; Makita, K.; Sugaya, T.; Matsui, T.; Fujita, D.; et al. Photocatalytic generation of hydrogen by core-shell WO3/BiVO4 nanorods with ultimate water splitting efficiency. Sci. Rep. 2015, 5, 11141. [Google Scholar] [CrossRef] [PubMed]
- Thalluri, S.M.; Hernández, S.; Bensaid, S.; Saracco, G.; Russo, N. Green-synthesized W- and Mo-doped BiVO4 oriented along the {040} facet with enhanced activity for the sun-driven water oxidation. Appl. Catal. B-Environ. 2016, 180, 630–636. [Google Scholar] [CrossRef]
- Zhong, X.; He, H.; Yang, M.; Ke, G.; Zhao, Z.; Dong, F.; Wang, B.; Chen, Y.; Shi, X.; Zhou, Y. In3+-doped BiVO4 photoanodes with passivated surface states for photoelectrochemical water oxidation. J. Mater. Chem. A 2018, 6, 10456–10465. [Google Scholar] [CrossRef]
- Jiang, D.; Xiao, P.; Shao, L.; Li, D.; Chen, M. RGO-Promoted All-Solid-State g-C3N4/BiVO4 Z-Scheme Heterostructure with Enhanced Photocatalytic Activity toward the Degradation of Antibiotics. Ind. Eng. Chem. Res. 2017, 56, 8823–8832. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; Chang, S.; Tian, B.; Zhang, J. Z-scheme CdS–Au–BiVO4 with enhanced photocatalytic activity for organic contaminant decomposition. Catal. Sci. Technol. 2017, 7, 124–132. [Google Scholar] [CrossRef]
- He, R.; Xu, D.; Cheng, B.; Yu, J.; Ho, W. Review on nanoscale Bi-based photocatalysts. Nanoscale Horiz. 2018, 3, 464–504. [Google Scholar] [CrossRef]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Van, C.N.; Chang, W.S.; Chen, J.-W.; Tsai, K.-A.; Tzeng, W.-Y.; Lin, Y.-C.; Kuo, H.-H.; Liu, H.-J.; Chang, K.-D.; Chou, W.-C.; et al. Heteroepitaxial approach to explore charge dynamics across Au/BiVO4 interface for photoactivity enhancement. Nano Energy 2015, 15, 625–633. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Liu, Z.; Yan, Y.; Zhu, Z. Preparation of noble metal Ag-modified BiVO4 nanosheets and a study on the degradation performance of tetracyclines. New J. Chem. 2020, 44, 13815–13823. [Google Scholar] [CrossRef]
- Dong, F.; Xiong, T.; Sun, Y.; Zhao, Z.; Zhou, Y.; Feng, X.; Wu, Z. A semimetal bismuth element as a direct plasmonic photocatalyst. Chem. Commun. 2014, 50, 10386–10389. [Google Scholar] [CrossRef]
- Hu, J.; Xu, G.; Wang, J.; Lv, J.; Zhang, X.; Zheng, Z.; Xie, T.; Wu, Y. Photocatalytic properties of Bi/BiOCl heterojunctions synthesized using an in situ reduction method. New J. Chem. 2014, 38, 4913–4921. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Sun, Y.; Zhang, Y.; Yan, S.; Wu, Z. An Advanced Semimetal-Organic Bi Spheres-g-C3N4 Nanohybrid with SPR-Enhanced Visible-Light Photocatalytic Performance for NO Purification. Environ. Sci. Technol. 2015, 49, 12432–12440. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Zhang, G.; Li, J.; Wu, X. 0D Bi nanodots/2D Bi3NbO7 nanosheets heterojunctions for efficient visible light photocatalytic degradation of antibiotics: Enhanced molecular oxygen activation and mechanism insight. Appl. Catal. B-Environ. 2019, 240, 39–49. [Google Scholar] [CrossRef]
- Wang, B.; Feng, W.; Zhang, L.; Zhang, Y.; Huang, X.; Fang, Z.; Liu, P. In situ construction of a novel Bi/CdS nanocomposite with enhanced visible light photocatalytic performance. Appl. Catal. B-Environ. 2017, 206, 510–519. [Google Scholar] [CrossRef]
- Lai, M.; Zhao, J.; Chen, Q.; Feng, S.; Bai, Y.; Li, Y.; Wang, C. Photocatalytic toluene degradation over Bi-decorated TiO2: Promoted O2 supply to catalyst’s surface by metallic Bi. Catal. Today 2019, 335, 372–380. [Google Scholar] [CrossRef]
- Pan, Q.; Yang, K.; Wang, G.; Li, D.; Sun, J.; Yang, B.; Zou, Z.; Hu, W.; Wen, K.; Yang, H. BiVO4 nanocrystals with controllable oxygen vacancies induced by Zn-doping coupled with graphene quantum dots for enhanced photoelectrochemical water splitting. Chem. Eng. J. 2019, 372, 399–407. [Google Scholar] [CrossRef]
- Packiaraj, R.; Devendran, P.; Asath Bahadur, S.; Nallamuthu, N. Structural and electrochemical studies of Scheelite type BiVO4 nanoparticles: Synthesis by simple hydrothermal method. J. Mater. Sci. Mater. Electron. 2018, 29, 13265–13276. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, H.; Zong, X.; Xu, Q.; Ma, Y.; Li, G.; Li, C. Crystal facet dependence of water oxidation on BiVO4 sheets under visible light irradiation. Chem. Eur. J. 2011, 17, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, J.; Liu, J.; Li, Z.; Dai, K.; Liang, C. Bi SPR-Promoted Z-Scheme Bi2MoO6/CdS-Diethylenetriamine Composite with Effectively Enhanced Visible Light Photocatalytic Hydrogen Evolution Activity and Stability. ACS Sustain. Chem. Eng. 2017, 6, 696–706. [Google Scholar] [CrossRef]
- Tao, S.; Sun, S.; Zhao, T.; Cui, J.; Yang, M.; Yu, X.; Yang, Q.; Zhang, X.; Liang, S. One-pot construction of Ta-doped BiOCl/Bi heterostructures toward simultaneously promoting visible light harvesting and charge separation for highly enhanced photocatalytic activity. Appl. Surf. Sci. 2021, 543, 148798. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, S.; Cui, K.; Zhao, J.; Xie, S.; Li, J.; Xinghua, C. Cavity containing core-shell Bi@C nanowires toward high performance lithium ion batteries. J. Alloy. Compd. 2020, 842, 155796. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, H.; Wang, B.; Li, C.; Zhai, J.; Li, Q. Preparation and characterization of fly ash cenospheres supported CuO–BiVO4 heterojunction composite. Appl. Surf. Sci. 2014, 300, 51–57. [Google Scholar] [CrossRef]
- Regmi, C.; Kshetri, Y.K.; Kim, T.-H.; Pandey, R.P.; Ray, S.K.; Lee, S.W. Fabrication of Ni-doped BiVO4 semiconductors with enhanced visible-light photocatalytic performances for wastewater treatment. Appl. Surf. Sci. 2017, 413, 253–265. [Google Scholar] [CrossRef]
- Zhen, Y.; Yang, C.; Shen, H.; Xue, W.; Gu, C.; Feng, J.; Zhang, Y.; Fu, F.; Liang, Y. Photocatalytic performance and mechanism insights of a S-scheme g-C3N4/Bi2MoO6 heterostructure in phenol degradation and hydrogen evolution reactions under visible light. Phys. Chem. Chem. Phys. 2020, 22, 26278–26288. [Google Scholar] [CrossRef]
- Xiao, J.; Yang, W.; Li, Q. Bi quantum dots on rutile TiO2 as hole trapping centers for efficient photocatalytic bromate reduction under visible light illumination. Appl. Catal. B-Environ. 2017, 218, 111–118. [Google Scholar] [CrossRef]
- Xie, Q.; He, W.; Liu, S.; Li, C.; Zhang, J.; Wong, P.K. Bifunctional S-scheme g-C3N4/Bi/BiVO4 hybrid photocatalysts toward artificial carbon cycling. Chin. J. Catal. 2020, 41, 140–153. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, Y.; Liu, J.; Liu, B.; Chen, X.; Ding, J.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Utilizing solar energy to improve the oxygen evolution reaction kinetics in zinc-air battery. Nat. Commun. 2019, 10, 4767. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, S.; Zhao, Z.; Shi, Z.; Yan, S.; Zou, Z. A Facet-Dependent Schottky-Junction Electron Shuttle in a BiVO4 {010}-Au-Cu2O Z-Scheme Photocatalyst for Efficient Charge Separation. Adv. Funct. Mater. 2018, 28, 1801214. [Google Scholar] [CrossRef]
- Gnayem, H.; Sasson, Y. Nanostructured 3D Sunflower-like Bismuth Doped BiOClxBr1–x Solid Solutions with Enhanced Visible Light Photocatalytic Activity as a Remarkably Efficient Technology for Water Purification. J. Phys. Chem. C 2015, 119, 19201–19209. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Kumar, A.; Shalini, G.S.; Naushad, M.; Kumar, A.; Kalia, S.; Guo, C.; Mola, G.T. Facile hetero-assembly of superparamagnetic Fe3O4/BiVO4 stacked on biochar for solar photo-degradation of methyl paraben and pesticide removal from soil. J. Photochem. Photobiol. A-Chem. 2017, 337, 118–131. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Xin, Y.; Chai, C.; Chen, Q. Construction of immobilized CuS/TiO2 nanobelts heterojunction photocatalyst for photocatalytic degradation of enrofloxacin: Synthesis, characterization, influencing factors and mechanism insight. J. Chem. Technol. Biotechnol. 2019, 94, 2219–2228. [Google Scholar] [CrossRef]
- Omrani, N.; Nezamzadeh-Ejhieh, A. A ternary Cu2O/BiVO4/WO3 nano-composite: Scavenging agents and the mechanism pathways in the photodegradation of sulfasalazine. J. Mol. Liq. 2020, 315, 113701. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef]
- Zhang, D.; Hao, J.; Wan, P.; Zhang, D.; Sun, Q.; Liu, Z.; Li, F.; Fang, H.; Wang, Y. Synergy of charge pre-separation and direct Z-scheme bridge in BiVO4{0 4 0}/Ag6Si2O7 photocatalyst boosting organic pollutant degradation. Appl. Surf. Sci. 2020, 513, 145832. [Google Scholar] [CrossRef]
- Xu, J.; Bian, Z.; Xin, X.; Chen, A.; Wang, H. Size dependence of nanosheet BiVO4 with oxygen vacancies and exposed {001} facets on the photodegradation of oxytetracycline. Chem. Eng. J. 2018, 337, 684–696. [Google Scholar] [CrossRef]
- Arunachalam, M.; Jun Seo, Y.; Jeon, S.; Ahn, K.-S.; Soo Kim, C.; Hyung Kang, S. Colloidal metal Ag nanowire as an efficient co-catalyst for enhancing the solar water oxidation of fluorinated BiVO4 photoelectrode. Chem. Eng. J. 2020, 394, 125016. [Google Scholar] [CrossRef]
- Yu, M.; Liang, H.; Zhan, R.; Xu, L.; Niu, J. Sm-doped g-C3N4/Ti3C2 MXene heterojunction for visible-light photocatalytic degradation of ciprofloxacin. Chin. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Hu, K.; Li, R.; Ye, C.; Wang, A.; Wei, W.; Hu, D.; Qiu, R.; Yan, K. Facile synthesis of Z-scheme composite of TiO2 nanorod/g-C3N4 nanosheet efficient for photocatalytic degradation of ciprofloxacin. J. Clean Prod. 2020, 253, 120055. [Google Scholar] [CrossRef]
- Yu, H.; Huang, B.; Wang, H.; Yuan, X.; Jiang, L.; Wu, Z.; Zhang, J.; Zeng, G. Facile construction of novel direct solid-state Z-scheme AgI/BiOBr photocatalysts for highly effective removal of ciprofloxacin under visible light exposure: Mineralization efficiency and mechanisms. J. Colloid Interface Sci. 2018, 522, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.; Niu, J.; Chen, M.; Yue, J. Synthesis of direct Z-Scheme Bi3TaO7/CdS composite photocatalysts with enhanced photocatalytic performance for ciprofloxacin degradation under visible light irradiation. J. Alloy. Compd. 2020, 834, 155061. [Google Scholar] [CrossRef]
- Wang, F.; Feng, Y.; Chen, P.; Wang, Y.; Su, Y.; Zhang, Q.; Zeng, Y.; Xie, Z.; Liu, H.; Liu, Y.; et al. Photocatalytic degradation of fluoroquinolone antibiotics using ordered mesoporous g-C3N4 under simulated sunlight irradiation: Kinetics, mechanism, and antibacterial activity elimination. Appl. Catal. B-Environ. 2018, 227, 114–122. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Zhang, W.; Gao, C.; Zhang, Y.; Dong, F. Plasmonic Bi metal as cocatalyst and photocatalyst: The case of Bi/(BiO)2CO3 and Bi particles. J. Colloid Interface Sci. 2017, 485, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Feng, X.; Zhao, X.; Duan, Z.; Pan, J.; Chen, L.; Liu, Y. Bi/BiVO4 Chainlike Hollow Microstructures: Synthesis, Characterization, and Application as Visible-Light-Active Photocatalysts. ACS Appl. Nano Mater. 2018, 1, 2653–2661. [Google Scholar] [CrossRef]
- Xi, G.; Ye, J. Synthesis of bismuth vanadate nanoplates with exposed {001} facets and enhanced visible-light photocatalytic properties. Chem. Commun. 2010, 46, 1893–1895. [Google Scholar] [CrossRef]


| Samples | k (min−1) | R2 |
|---|---|---|
| None | 0.0010 | 0.9951 |
| BiVO4 | 0.0233 | 0.9985 |
| 0.1Bi-BVO | 0.0387 | 0.9799 |
| 0.2Bi-BVO | 0.0414 | 0.9794 |
| 0.3Bi-BVO | 0.0423 | 0.9869 |
| 0.4Bi-BVO | 0.0344 | 0.9855 |
| 0.5Bi-BVO | 0.0360 | 0.9975 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Sun, J.; Shen, T.; Deng, L.; Wang, X. Insights into the Mechanism of the Bi/BiVO4 Composites for Improved Photocatalytic Activity. Catalysts 2021, 11, 489. https://doi.org/10.3390/catal11040489
Song H, Sun J, Shen T, Deng L, Wang X. Insights into the Mechanism of the Bi/BiVO4 Composites for Improved Photocatalytic Activity. Catalysts. 2021; 11(4):489. https://doi.org/10.3390/catal11040489
Chicago/Turabian StyleSong, Hongchen, Jing Sun, Tingting Shen, Lang Deng, and Xikui Wang. 2021. "Insights into the Mechanism of the Bi/BiVO4 Composites for Improved Photocatalytic Activity" Catalysts 11, no. 4: 489. https://doi.org/10.3390/catal11040489
APA StyleSong, H., Sun, J., Shen, T., Deng, L., & Wang, X. (2021). Insights into the Mechanism of the Bi/BiVO4 Composites for Improved Photocatalytic Activity. Catalysts, 11(4), 489. https://doi.org/10.3390/catal11040489






