Sample Environment for Operando Hard X-ray Tomography—An Enabling Technology for Multimodal Characterization in Heterogeneous Catalysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Design of the aRCTIC Setup
- A stable sample holder and support structure, allowing µm precise translation and maintaining the sample within the center of rotation;
- Free rotation in a complete 180° arc, avoiding missing wedge artifacts from incomplete tomography scans or incomplete sinograms;
- A support rod relatively transparent to hard X-rays and with minimal scattering, minimizing image artifacts at angles where the support rod may eclipse the X-ray beam;
- A closed gas-tight system allowing precise control of gas composition, flow rate and space velocity;
- An integrated online product analysis for operando measurement and quantification of catalyst performance, in terms of gas-phase reactant and product composition;
- A uniform source of heating to maintain stable catalytic performance during rotation and translation motions;
- A compact design for integration in beamline stages with a limited weight allowance. Small profile to allow positioning of optical components, detectors, other beamline apparatus, etc.
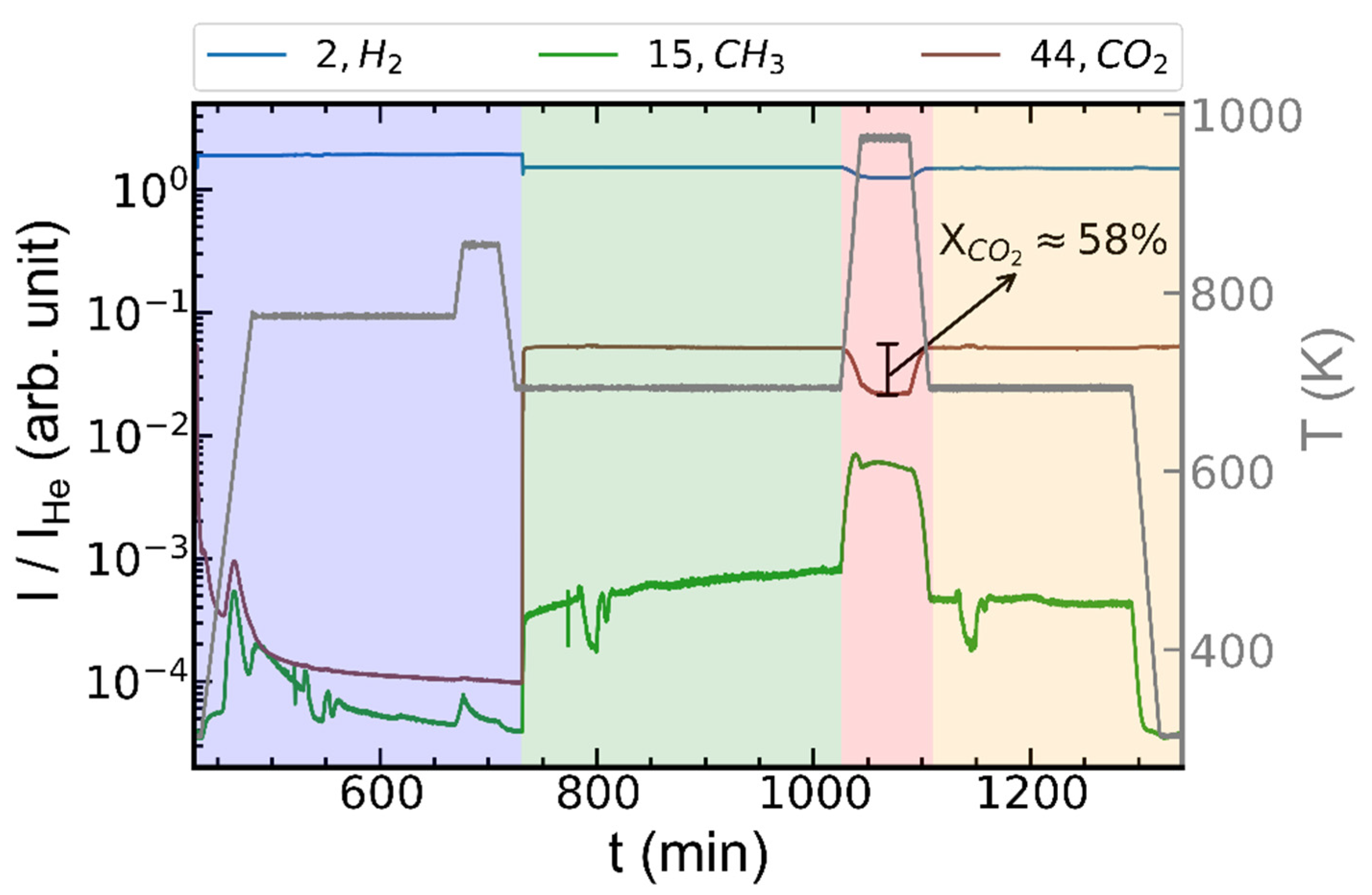
2.2. Operando XRD-CT Case Study—Catalytic Performance during CO2 Methanation
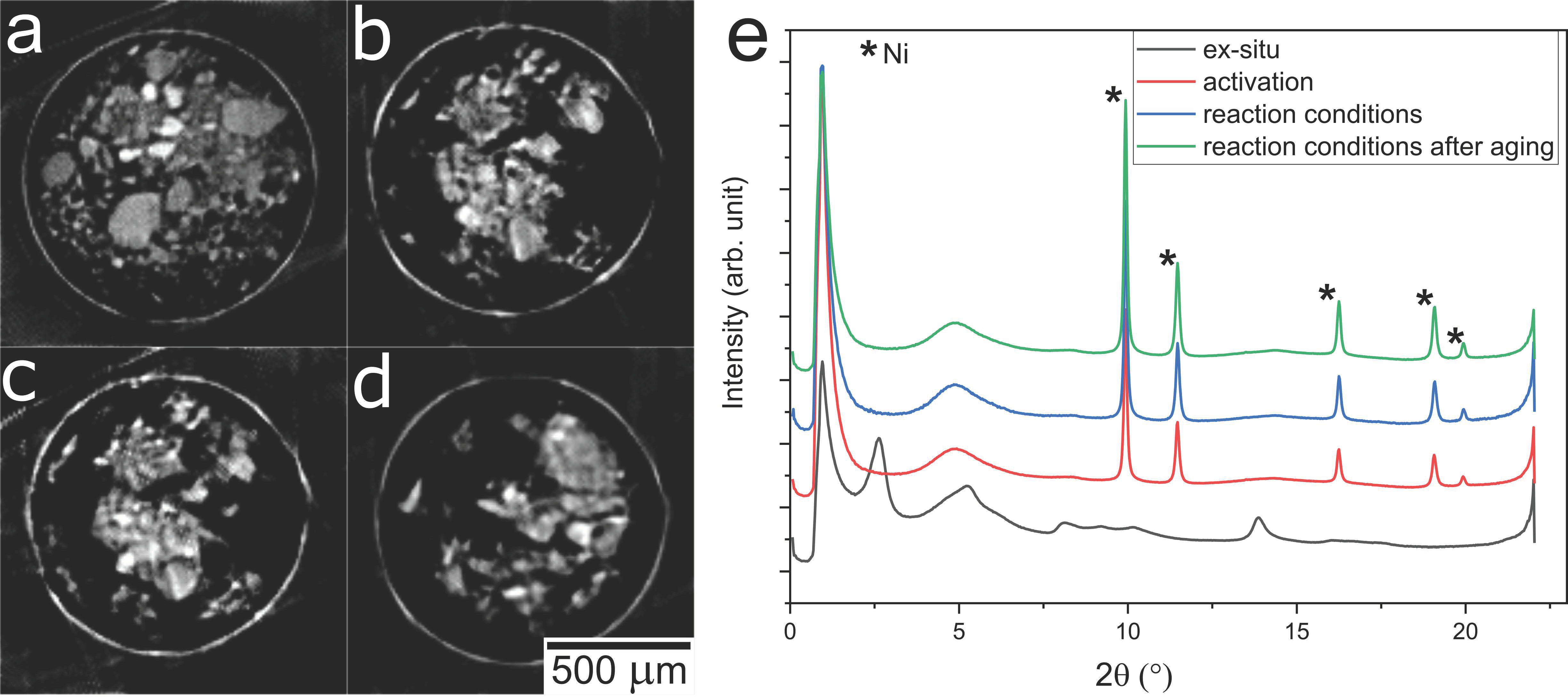
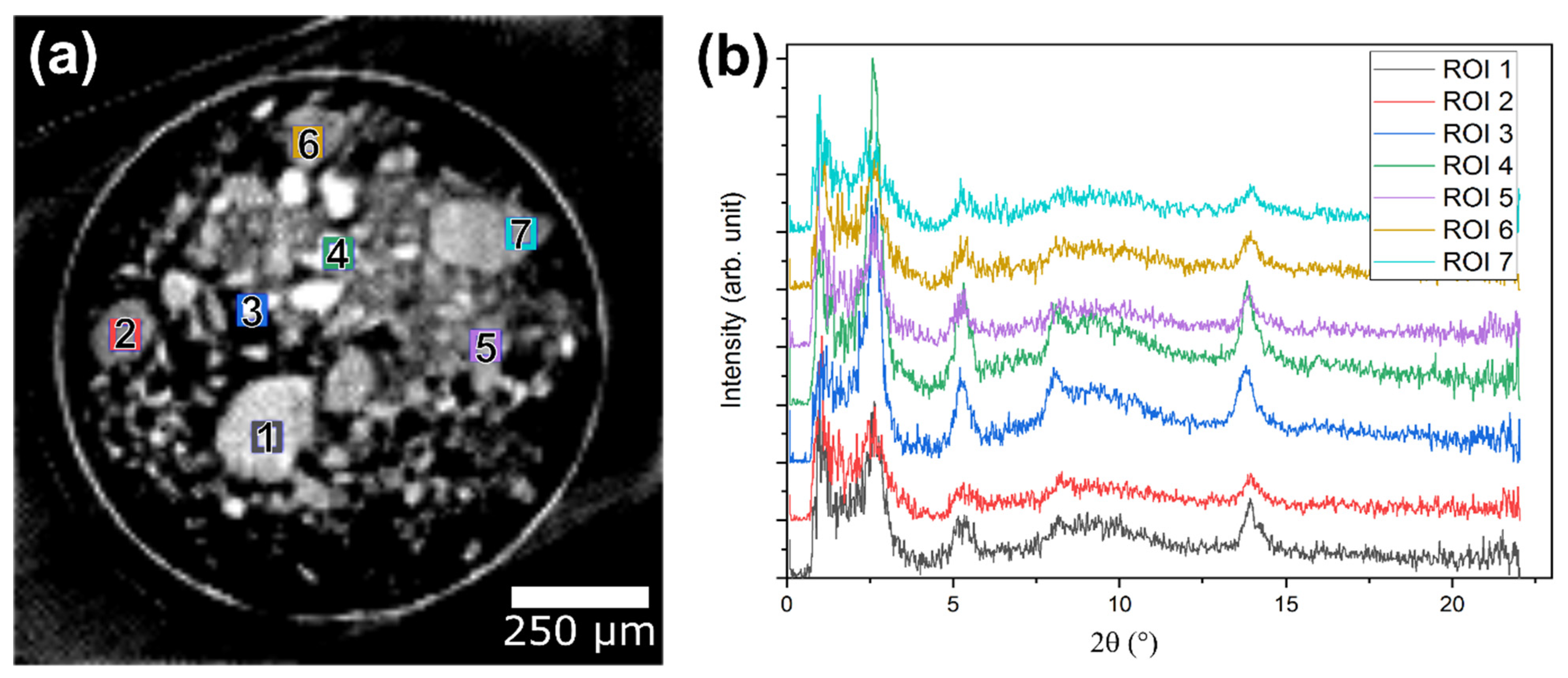
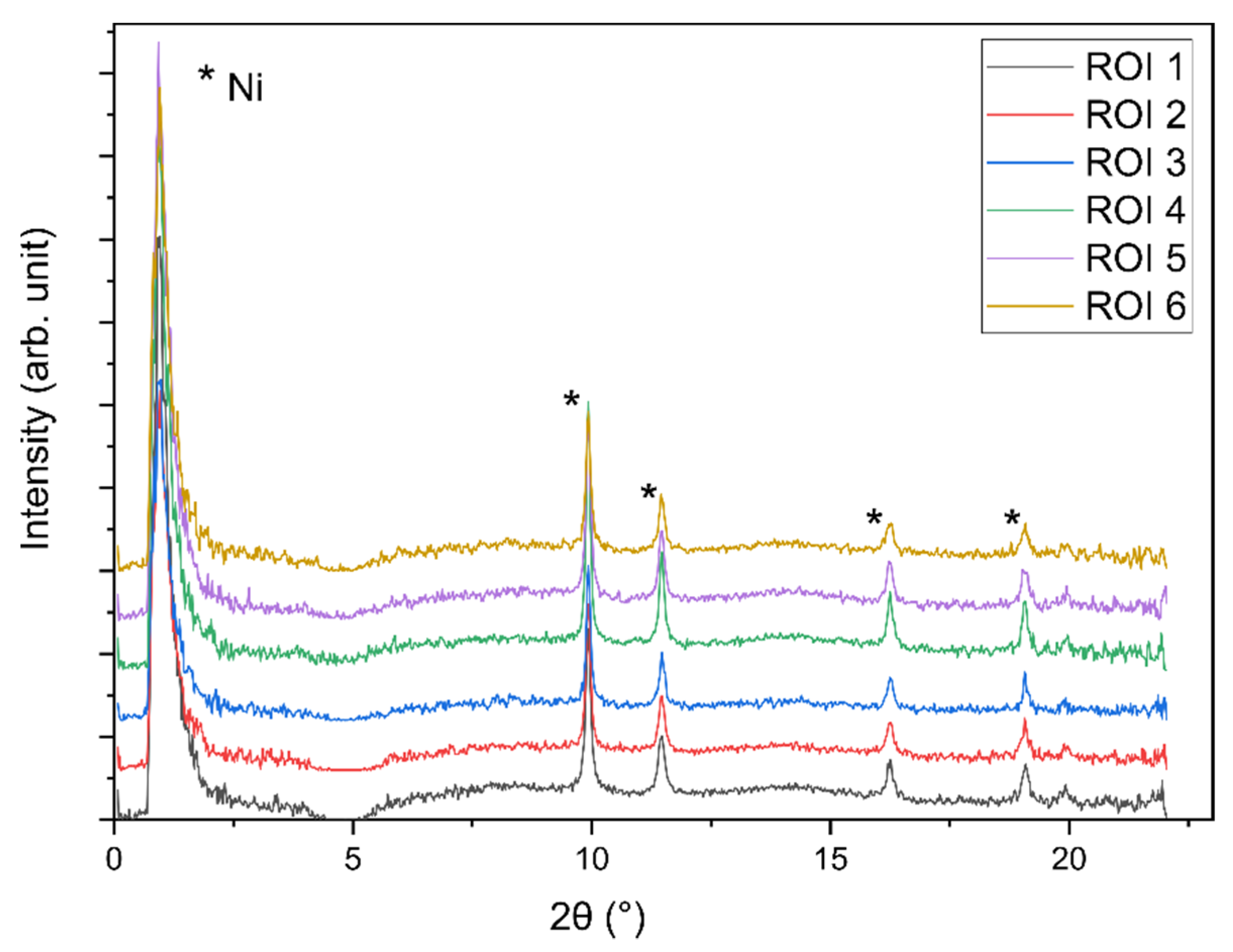
2.3. Operando XRD-CT Case Study—Tomography Measurements
2.4. ED-XAS-CT Feasibility Study—Catalytic Partial Oxidation of Methane
2.5. ED-XAS-CT Feasibility Study—Tomography Measurements
2.6. Evaluation of the aRCTIC Setup and Operando Tomography
3. Materials and Methods
3.1. Instrumentation of aRCTIC
3.2. Implementation of aRCTIC at P06 Microprobe Beamline
3.3. Catalytic Tests at P06 Microprobe Beamline
3.4. XRD-CT Data Acquisition
3.5. XRD-CT Data Treatment
3.6. Catalytic Tests at ESRF ID24 ED-XAS Beamline
3.7. ED-XAS-CT Data Acquisition
3.8. ED-XAS-CT Data Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bañares, M.A. Operando methodology: Combination of in situ spectroscopy and simultaneous activity measurements under catalytic reaction conditions. Catal. Today 2005, 100, 71–77. [Google Scholar] [CrossRef]
- Ertl, G.; Knözinger, H.; Schüth, F.; Weitkamp, J. Handbook of Heterogeneous Catalysis; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1997. [Google Scholar]
- Lanzafame, P.; Perathoner, S.; Centi, G.; Gross, S.; Hensen, E.J.M. Grand challenges for catalysis in the Science and Technology Roadmap on Catalysis for Europe: Moving ahead for a sustainable future. Catal. Sci. Technol. 2017, 7, 5182–5194. [Google Scholar] [CrossRef]
- Pelletier, J.D.A.; Basset, J.-M. Catalysis by Design: Well-Defined Single-Site Heterogeneous Catalysts. Acc. Chem. Res. 2016, 49, 664–677. [Google Scholar] [CrossRef]
- Weckhuysen, B.M. Determining the active site in a catalytic process: Operando spectroscopy is more than a buzzword. Phys. Chem. Chem. Phys. 2003, 5, 4351–4360. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Ford, M.E.; Gregory, D.; Hu, R.; Keturakis, C.J.; Lwin, S.; Tang, Y.; Yang, Z.; Zhu, M.; Bañares, M.A.; et al. A decade+ of operando spectroscopy studies. Catal. Today 2017, 283, 27–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, D.; Xu, X.; Sheng, Y.; Xu, J.; Han, Y.-F. Application of operando spectroscopy on catalytic reactions. Curr. Opin. Chem. Eng. 2016, 12, 1–7. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef]
- Védrine, J.C. Metal Oxides in Heterogeneous Oxidation Catalysis: State of the Art and Challenges for a More Sustainable World. ChemSusChem 2019, 12, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Copéret, C.; Allouche, F.; Chan, K.W.; Conley, M.P.; Delley, M.F.; Fedorov, A.; Moroz, I.B.; Mougel, V.; Pucino, M.; Searles, K.; et al. Bridging the Gap between Industrial and Well-Defined Supported Catalysts. Angew. Chem. Int. Ed. 2018, 57, 6398–6440. [Google Scholar] [CrossRef] [PubMed]
- Tsakoumis, N.E.; York, A.P.E.; Chen, D.; Rønning, M. Catalyst characterisation techniques and reaction cells operating at realistic conditions; towards acquisition of kinetically relevant information. Catal. Sci. Technol. 2015, 5, 4859–4883. [Google Scholar] [CrossRef]
- Grunwaldt, J.-D.; Wagner, J.B.; Dunin-Borkowski, R.E. Imaging Catalysts at Work: A Hierarchical Approach from the Macro- to the Meso- and Nano-scale. ChemCatChem 2013, 5, 62–80. [Google Scholar] [CrossRef]
- Tao, F.; Crozier, P.A. Atomic-Scale Observations of Catalyst Structures under Reaction Conditions and during Catalysis. Chem. Rev. 2016, 116, 3487–3539. [Google Scholar] [CrossRef] [PubMed]
- Meirer, F.; Weckhuysen, B.M. Spatial and temporal exploration of heterogeneous catalysts with synchrotron radiation. Nat. Rev. Mater. 2018, 3, 324–340. [Google Scholar] [CrossRef]
- Korup, O.; Goldsmith, C.F.; Weinberg, G.; Geske, M.; Kandemir, T.; Schlögl, R.; Horn, R. Catalytic partial oxidation of methane on platinum investigated by spatial reactor profiles, spatially resolved spectroscopy, and microkinetic modeling. J. Catal. 2013, 297, 1–16. [Google Scholar] [CrossRef]
- Portela, R.; Perez-Ferreras, S.; Serrano-Lotina, A.; Bañares, M.A. Engineering operando methodology: Understanding catalysis in time and space. Front. Chem. Sci. Eng. 2018, 12, 509–536. [Google Scholar] [CrossRef]
- Weckhuysen, B.M. Chemical Imaging of Spatial Heterogeneities in Catalytic Solids at Different Length and Time Scales. Angew. Chem. Int. Ed. 2009, 48, 4910–4943. [Google Scholar] [CrossRef]
- Kalz, K.F.; Kraehnert, R.; Dvoyashkin, M.; Dittmeyer, R.; Gläser, R.; Krewer, U.; Reuter, K.; Grunwaldt, J.-D. Future Challenges in Heterogeneous Catalysis: Understanding Catalysts under Dynamic Reaction Conditions. ChemCatChem 2017, 9, 17–29. [Google Scholar] [CrossRef]
- Van Bokhoven, J.A.; Lamberti, C. X-ray Absorption and X-ray Emission Spectroscopy; John Wiley/Sons Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Bordiga, S.; Groppo, E.; Agostini, G.; van Bokhoven, J.A.; Lamberti, C. Reactivity of Surface Species in Heterogeneous Catalysts Probed by In Situ X-ray Absorption Techniques. Chem. Rev. 2013, 113, 1736–1850. [Google Scholar] [CrossRef]
- Frenkel, A.I.; Rodriguez, J.A.; Chen, J.G. Synchrotron Techniques for In Situ Catalytic Studies: Capabilities, Challenges, and Opportunities. ACS Catal. 2012, 2, 2269–2280. [Google Scholar] [CrossRef]
- Beale, A.M.; Jacques, S.D.M.; Weckhuysen, B.M. Chemical imaging of catalytic solids with synchrotron radiation. Chem. Soc. Rev. 2010, 39, 4656–4672. [Google Scholar] [CrossRef] [PubMed]
- Mino, L.; Borfecchia, E.; Segura-Ruiz, J.; Giannini, C.; Martinez-Criado, G.; Lamberti, C. Materials characterization by synchrotron x-ray microprobes and nanoprobes. Rev. Mod. Phys. 2018, 90, 025007. [Google Scholar] [CrossRef]
- Grunwaldt, J.-D.; Schroer, C.G. Hard and soft X-ray microscopy and tomography in catalysis: Bridging the different time and length scales. Chem. Soc. Rev. 2010, 39, 4741–4753. [Google Scholar] [CrossRef] [PubMed]
- Meirer, F.; Kalirai, S.; Morris, D.; Soparawalla, S.; Liu, Y.; Mesu, G.; Andrews, J.C.; Weckhuysen, B.M. Life and death of a single catalytic cracking particle. Sci. Adv. 2015, 1, e1400199. [Google Scholar] [CrossRef]
- Wise, A.M.; Weker, J.N.; Kalirai, S.; Farmand, M.; Shapiro, D.A.; Meirer, F.; Weckhuysen, B.M. Nanoscale Chemical Imaging of an Individual Catalyst Particle with Soft X-ray Ptychography. ACS Catal. 2016, 6, 2178–2181. [Google Scholar] [CrossRef] [PubMed]
- Basile, F.; Benito, P.; Bugani, S.; de Nolf, W.; Fornasari, G.; Janssens, K.; Morselli, L.; Scavetta, E.; Tonelli, D.; Vaccari, A. Combined Use of Synchrotron-Radiation-Based Imaging Techniques for the Characterization of Structured Catalysts. Adv. Funct. Mater. 2010, 20, 4117–4126. [Google Scholar] [CrossRef]
- Ihli, J.; Jacob, R.R.; Holler, M.; Guizar-Sicairos, M.; Diaz, A.; da Silva, J.C.; Sanchez, D.F.; Krumeich, F.; Grolimund, D.; Taddei, M.; et al. A three-dimensional view of structural changes caused by deactivation of fluid catalytic cracking catalysts. Nat. Commun. 2017, 8, 809. [Google Scholar] [CrossRef]
- Da Silva, J.C.; Mader, K.; Holler, M.; Haberthür, D.; Diaz, A.; Guizar-Sicairos, M.; Cheng, W.-C.; Shu, Y.; Raabe, J.; Menzel, A.; et al. Assessment of the 3 D Pore Structure and Individual Components of Preshaped Catalyst Bodies by X-ray Imaging. ChemCatChem 2015, 7, 413–416. [Google Scholar] [CrossRef]
- Price, S.W.T.; Ignatyev, K.; Geraki, K.; Basham, M.; Filik, J.; Vo, N.T.; Witte, P.T.; Beale, A.M.; Mosselmans, J.F.W. Chemical imaging of single catalyst particles with scanning μ-XANES-CT and μ-XRF-CT. Phys. Chem. Chem. Phys. 2015, 17, 521–529. [Google Scholar] [CrossRef]
- Hofmann, G.; Rochet, A.; Ogel, E.; Casapu, M.; Ritter, S.; Ogurreck, M.; Grunwaldt, J.-D. Aging of a Pt/Al2O3 exhaust gas catalyst monitored by quasi in situ X-ray micro computed tomography. RSC Adv. 2015, 5, 6893–6905. [Google Scholar] [CrossRef]
- Weker, J.N.; Huang, X.; Toney, M.F. In situ X-ray-based imaging of nano materials. Curr. Op. Chem. Eng. 2016, 12, 14–21. [Google Scholar] [CrossRef]
- Andrews, J.C.; Weckhuysen, B.M. Hard X-ray Spectroscopic Nano-Imaging of Hierarchical Functional Materials at Work. ChemPhysChem 2013, 14, 3655–3666. [Google Scholar] [CrossRef]
- Meunier, F.C. The design and testing of kinetically-appropriate operando spectroscopic cells for investigating heterogeneous catalytic reactions. Chem. Soc. Rev. 2010, 39, 4602–4614. [Google Scholar] [CrossRef]
- Doronkin, D.E.; Lichtenberg, H.; Grunwaldt, J.-D. XAFS Techniques for Catalysts, Nanomaterials, and Surfaces; Iwasawa, Y., Asakura, K., Tada, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 75–89. [Google Scholar]
- De Groot, F.M.F.; de Smit, E.; van Schooneveld, M.M.; Aramburo, L.R.; Weckhuysen, B.M. In-situ Scanning Transmission X-ray Microscopy of Catalytic Solids and Related Nanomaterials. ChemPhysChem 2010, 11, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Jimenez, I.D.; Cats, K.; Davidian, T.; Ruitenbeek, M.; Meirer, F.; Liu, Y.; Nelson, J.; Andrews, J.C.; Pianetta, P.; de Groot, F.M.F.; et al. Hard X-ray Nanotomography of Catalytic Solids at Work. Angew. Chem. Int. Ed. 2012, 51, 11986–11990. [Google Scholar] [CrossRef]
- Sheppard, T.L.; Price, S.W.T.; Benzi, F.; Baier, S.; Klumpp, M.; Dittmeyer, R.; Schwieger, W.; Grunwaldt, J.-D. In Situ Multimodal 3D Chemical Imaging of a Hierarchically Structured Core@Shell Catalyst. J. Am. Chem. Soc. 2017, 139, 7855–7863. [Google Scholar] [CrossRef] [PubMed]
- Price, S.W.T.; Martin, D.J.; Parsons, A.D.; Sławiński, W.A.; Vamvakeros, A.; Keylock, S.J.; Beale, A.M.; Mosselmans, J.F.W. Chemical imaging of Fischer-Tropsch catalysts under operating conditions. Sci. Adv. 2017, 3, e1602838. [Google Scholar] [CrossRef]
- Vamvakeros, A.; Jacques, S.D.M.; di Michiel, M.; Matras, D.; Middelkoop, V.; Ismagilov, I.Z.; Matus, E.V.; Kuznetsov, V.V.; Drnec, J.; Senecal, P.; et al. 5D operando tomographic diffraction imaging of a catalyst bed. Nat. Commun. 2018, 9, 4751. [Google Scholar] [CrossRef] [PubMed]
- Matras, D.; Jacques, S.D.M.; Poulston, S.; Grosjean, N.; Bosch, C.E.; Rollins, B.; Wright, J.; di Michiel, M.; Vamvakeros, A.; Cernik, R.J.; et al. Operando and Postreaction Diffraction Imaging of the La–Sr/CaO Catalyst in the Oxidative Coupling of Methane Reaction. J. Phys. Chem. C 2019, 123, 1751–1760. [Google Scholar] [CrossRef]
- Vamvakeros, A.; Matras, D.; Jacques, S.D.M.; di Michiel, M.; Middelkoop, V.; Cong, P.; Price, S.W.T.; Bull, C.L.; Senecal, P.; Beale, A.M. Real-time tomographic diffraction imaging of catalytic membrane reactors for the oxidative coupling of methane. Catal. Today 2020, 364, 242–255. [Google Scholar] [CrossRef]
- Becher, J.; Sanchez, D.F.; Doronkin, D.E.; Zengel, D.; Meira, D.M.; Pascarelli, S.; Grunwaldt, J.-D.; Sheppard, T.L. Chemical gradients in automotive Cu-SSZ-13 catalysts for NOx removal revealed by operando X-ray spectrotomography. Nat. Catal. 2021, 4, 46–53. [Google Scholar] [CrossRef]
- Newton, M.A.; Checchia, S.; Knorpp, A.J.; Stoian, D.; van Beek, W.; Emerich, H.; Longo, A.; van Bokhoven, J.A. On isothermality in some commonly used plug flow reactors for X-ray based investigations of catalysts. Catal. Sci. Technol. 2019, 9, 3081–3089. [Google Scholar] [CrossRef]
- Weber, S.; Abel, K.L.; Zimmermann, R.T.; Huang, X.; Bremer, J.; Rihko-Struckmann, L.K.; Batey, D.; Cipiccia, S.; Titus, J.; Poppitz, D.; et al. Porosity and Structure of Hierarchically Porous Ni/Al2O3 Catalysts for CO2 Methanation. Catalysts 2020, 10, 1471. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Enger, B.C.; Lødeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Doronkin, D.E.; Kuriganova, A.B.; Leontyev, I.N.; Baier, S.; Lichtenberg, H.; Smirnova, N.V.; Grunwaldt, J.-D. Electrochemically Synthesized Pt/Al2O3 Oxidation Catalysts. Catal. Lett. 2016, 146, 452–463. [Google Scholar] [CrossRef]
- Pascarelli, S.; Mathon, O.; Munoz, M.; Mairs, T.; Susini, J. Energy-dispersive absorption spectroscopy for hard-X-ray micro-XAS applications. J. Synchrotron Radiat. 2006, 13, 351–358. [Google Scholar] [CrossRef]
- Sanchez, D.F.; Simionovici, A.S.; Lemelle, L.; Cuartero, V.; Mathon, O.; Pascarelli, S.; Bonnin, A.; Shapiro, R.; Konhauser, K.; Grolimund, D.; et al. 2D/3D Microanalysis by Energy Dispersive X-ray Absorption Spectroscopy Tomography. Sci. Rep. 2017, 7, 16453. [Google Scholar] [CrossRef] [PubMed]
- Ihli, J.; Ferreira Sanchez, D.; Jacob, R.R.; Cuartero, V.; Mathon, O.; Krumeich, F.; Borca, C.; Huthwelker, T.; Cheng, W.-C.; Shu, Y.; et al. Localization and Speciation of Iron Impurities within a Fluid Catalytic Cracking Catalyst. Angew. Chem. Int. Ed. 2017, 56, 14031–14035. [Google Scholar] [CrossRef] [PubMed]
- Vamvakeros, A.; Jacques, S.D.M.; di Michiel, M.; Senecal, P.; Middelkoop, V.; Cernik, R.J.; Beale, A.M. Interlaced X-ray diffraction computed tomography. J. Appl. Cryst. 2016, 49, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.D.M.; di Michiel, M.; Beale, A.M.; Sochi, T.; O’Brien, M.G.; Espinosa-Alonso, L.; Weckhuysen, B.M.; Barnes, P. Dynamic X-ray Diffraction Computed Tomography Reveals Real-Time Insight into Catalyst Active Phase Evolution. Angew. Chem. Int. Ed. 2011, 50, 10148–10152. [Google Scholar] [CrossRef]
- Vamvakeros, A.; Matras, D.; Jacques, S.D.M.; di Michiel, M.; Price, S.W.T.; Senecal, P.; Aran, M.A.; Middelkoop, V.; Stenning, G.B.G.; Mosselmans, J.F.W.; et al. Real-time multi-length scale chemical tomography of fixed bed reactors during the oxidative coupling of methane reaction. J. Catal. 2020, 386, 39–52. [Google Scholar] [CrossRef]
- Kieffer, J.; Valls, V.; Blanc, N.; Hennig, C. New tools for calibrating diffraction setups. J. Synchrotron Radiat. 2020, 27, 558–566. [Google Scholar] [CrossRef]
- Van Aarle, W.; Palenstijn, W.J.; Cant, J.; Janssens, E.; Bleichrodt, F.; Dabravolski, A.; de Beenhouwer, J.; Batenburg, K.J.; Sijbers, J. Fast and flexible X-ray tomography using the ASTRA toolbox. Opt. Express 2016, 24, 25129–25147. [Google Scholar] [CrossRef] [PubMed]
- Pascarelli, S.; Mathon, O.; Mairs, T.; Kantor, I.; Agostini, G.; Strohm, C.; Pasternak, S.; Perrin, F.; Berruyer, G.; Chappelet, P.; et al. The Time-resolved and Extreme-conditions XAS (TEXAS) facility at the European Synchrotron Radiation Facility: The energy-dispersive X-ray absorption spectroscopy beamline ID24. J. Synchrotron Radiat. 2016, 23, 353–368. [Google Scholar] [CrossRef] [PubMed]

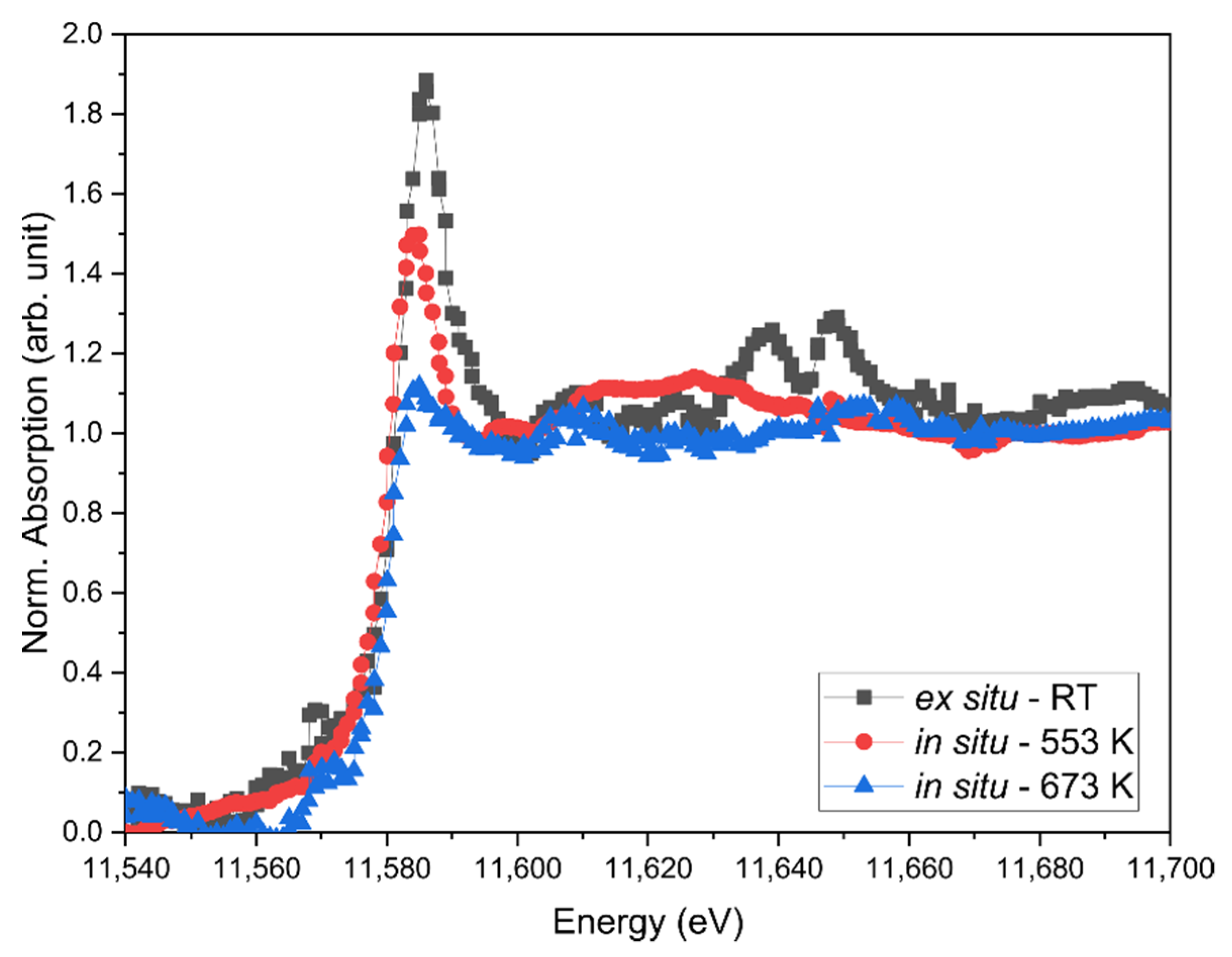
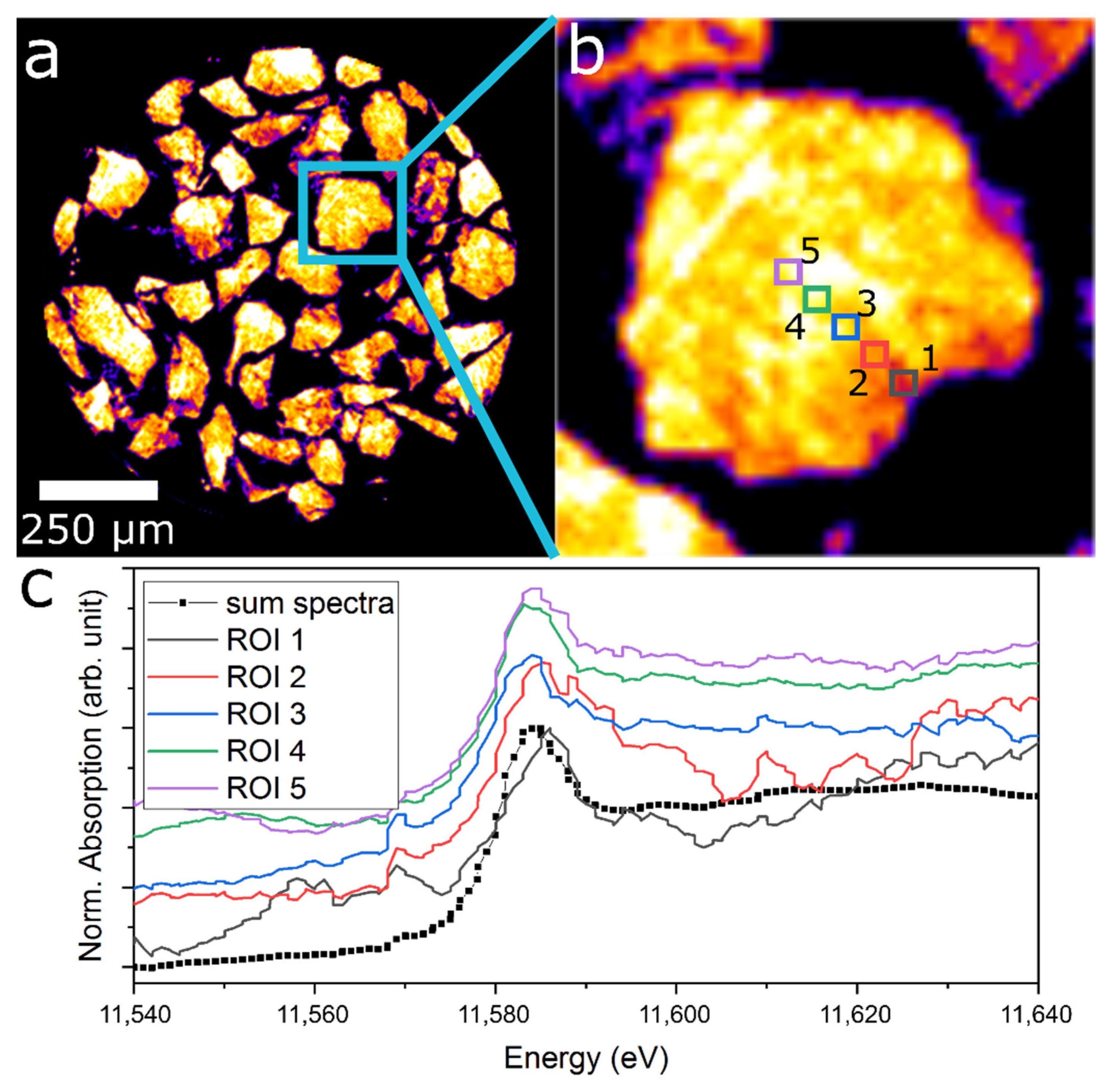
| Condition | FWHM of Reflection: 1 | |
|---|---|---|
| 2θ = 9.93° | 2θ = 11.47° | |
| Activation | 0.115 | 0.139 |
| Reaction conditions | 0.114 | 0.141 |
| Reaction conditions after thermal aging | 0.117 | 0.140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becher, J.; Weber, S.; Ferreira Sanchez, D.; Doronkin, D.E.; Garrevoet, J.; Falkenberg, G.; Motta Meira, D.; Pascarelli, S.; Grunwaldt, J.-D.; Sheppard, T.L. Sample Environment for Operando Hard X-ray Tomography—An Enabling Technology for Multimodal Characterization in Heterogeneous Catalysis. Catalysts 2021, 11, 459. https://doi.org/10.3390/catal11040459
Becher J, Weber S, Ferreira Sanchez D, Doronkin DE, Garrevoet J, Falkenberg G, Motta Meira D, Pascarelli S, Grunwaldt J-D, Sheppard TL. Sample Environment for Operando Hard X-ray Tomography—An Enabling Technology for Multimodal Characterization in Heterogeneous Catalysis. Catalysts. 2021; 11(4):459. https://doi.org/10.3390/catal11040459
Chicago/Turabian StyleBecher, Johannes, Sebastian Weber, Dario Ferreira Sanchez, Dmitry E. Doronkin, Jan Garrevoet, Gerald Falkenberg, Debora Motta Meira, Sakura Pascarelli, Jan-Dierk Grunwaldt, and Thomas L. Sheppard. 2021. "Sample Environment for Operando Hard X-ray Tomography—An Enabling Technology for Multimodal Characterization in Heterogeneous Catalysis" Catalysts 11, no. 4: 459. https://doi.org/10.3390/catal11040459
APA StyleBecher, J., Weber, S., Ferreira Sanchez, D., Doronkin, D. E., Garrevoet, J., Falkenberg, G., Motta Meira, D., Pascarelli, S., Grunwaldt, J.-D., & Sheppard, T. L. (2021). Sample Environment for Operando Hard X-ray Tomography—An Enabling Technology for Multimodal Characterization in Heterogeneous Catalysis. Catalysts, 11(4), 459. https://doi.org/10.3390/catal11040459








