Nanosheet-Like Ho2O3 and Sr-Ho2O3 Catalysts for Oxidative Coupling of Methane
Abstract
:1. Introduction
2. Results and Discussion
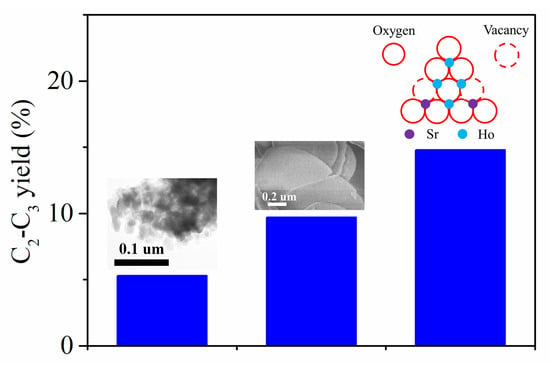
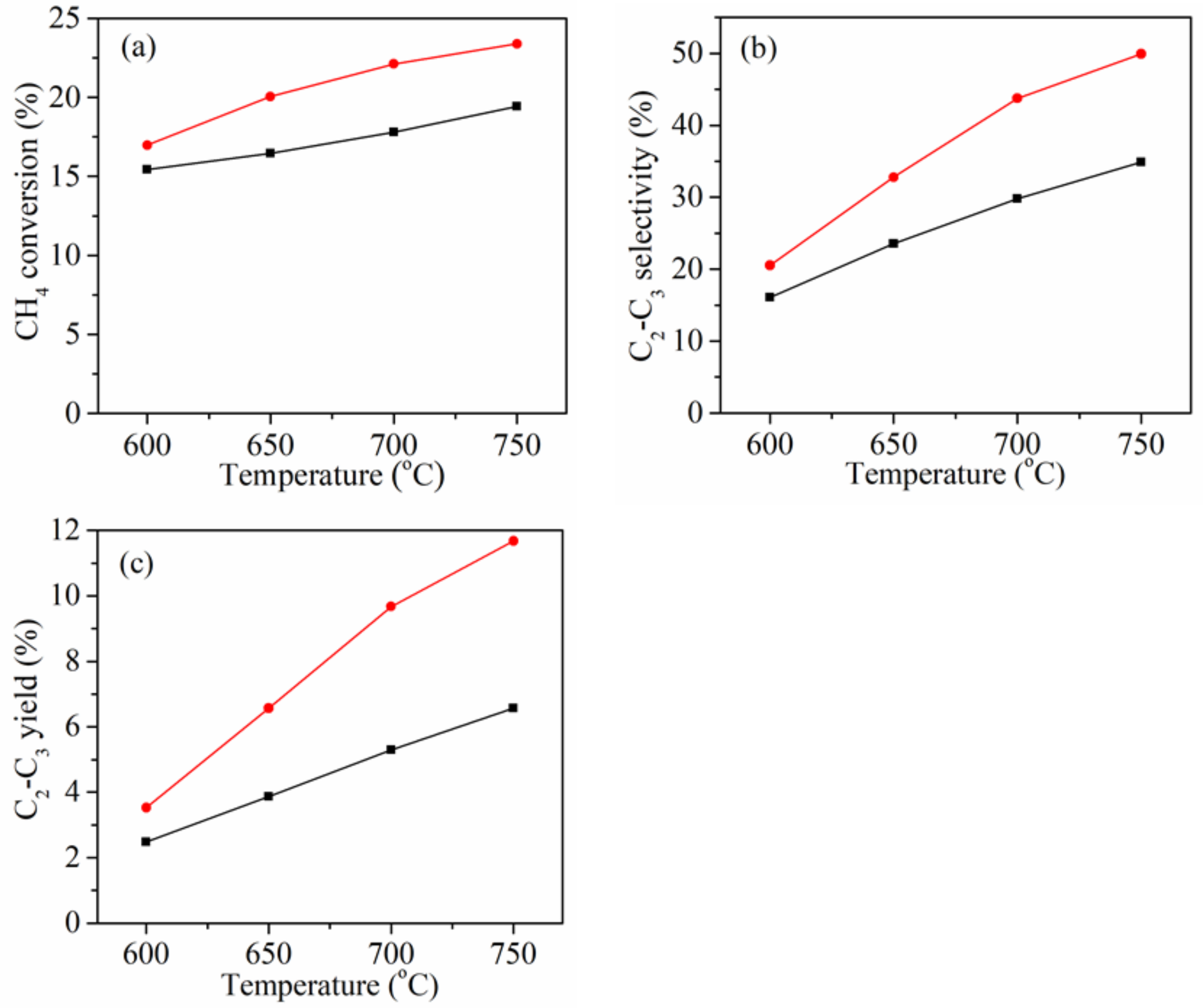
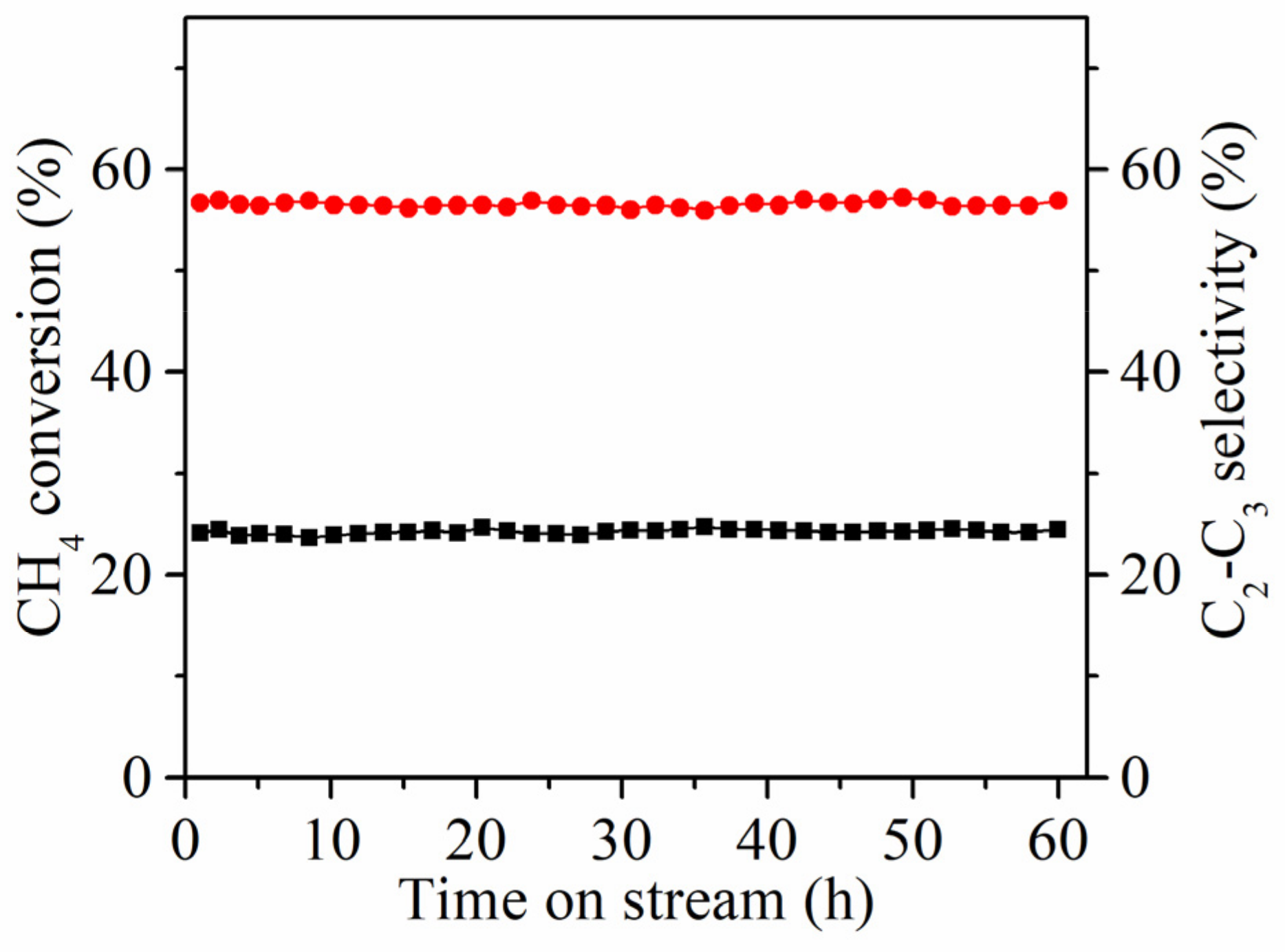
2.1. Catalytic Performances
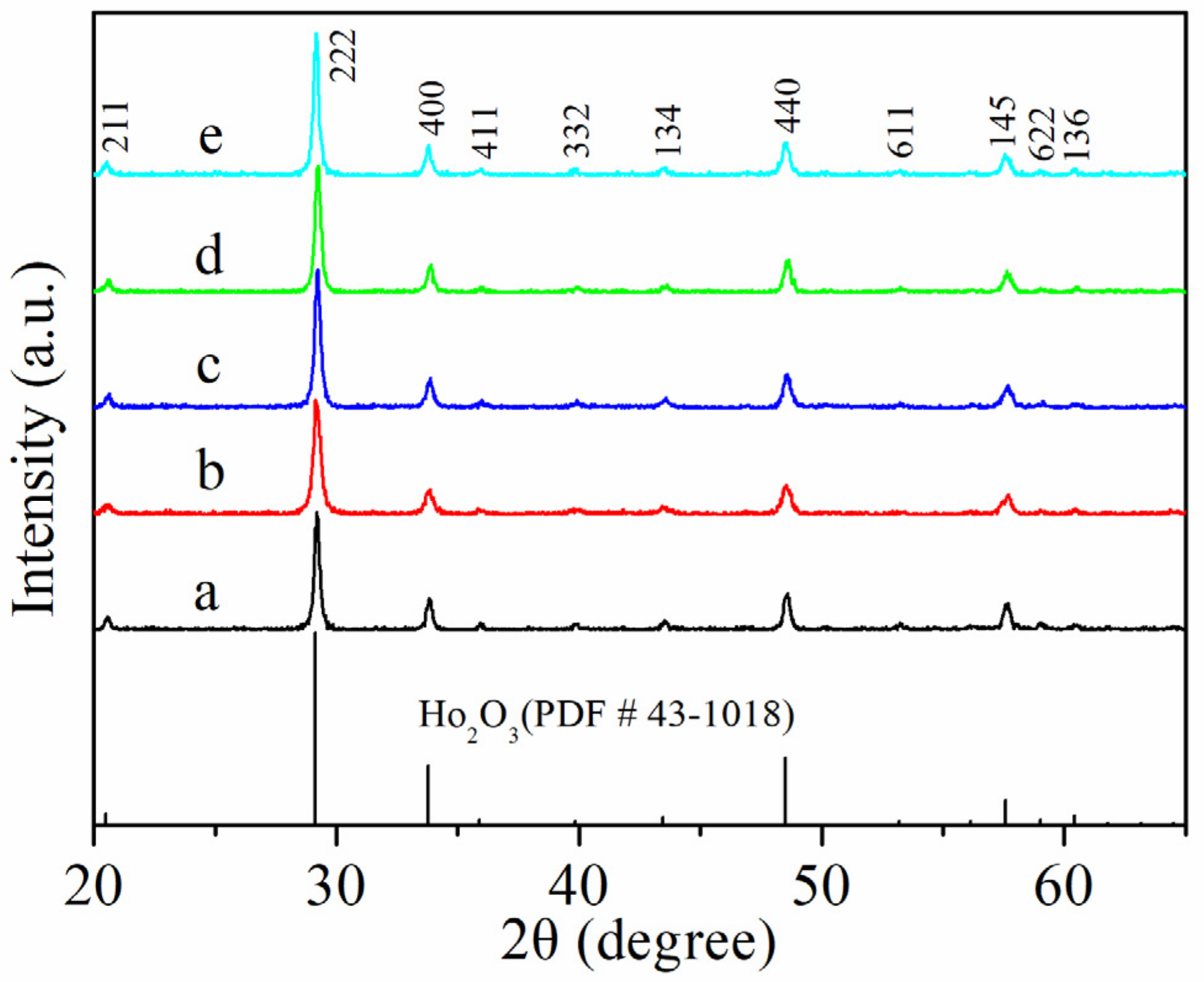
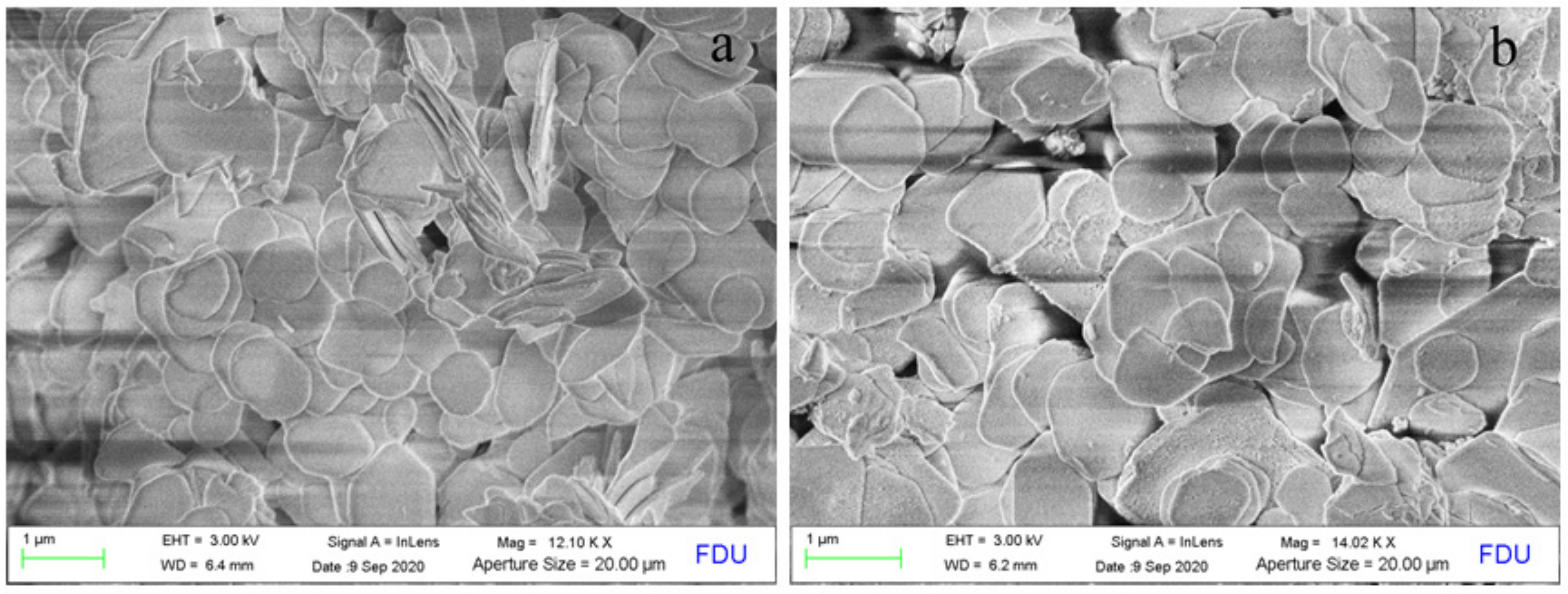
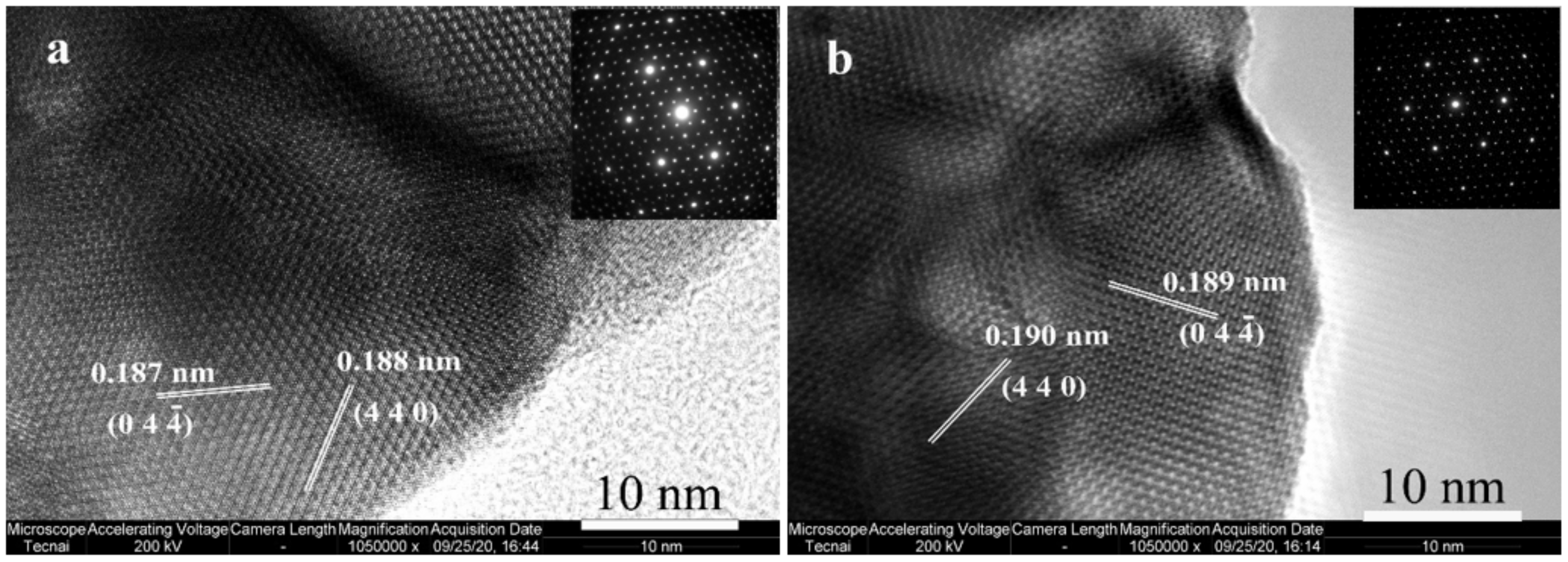
2.2. Structural and Textural Properties
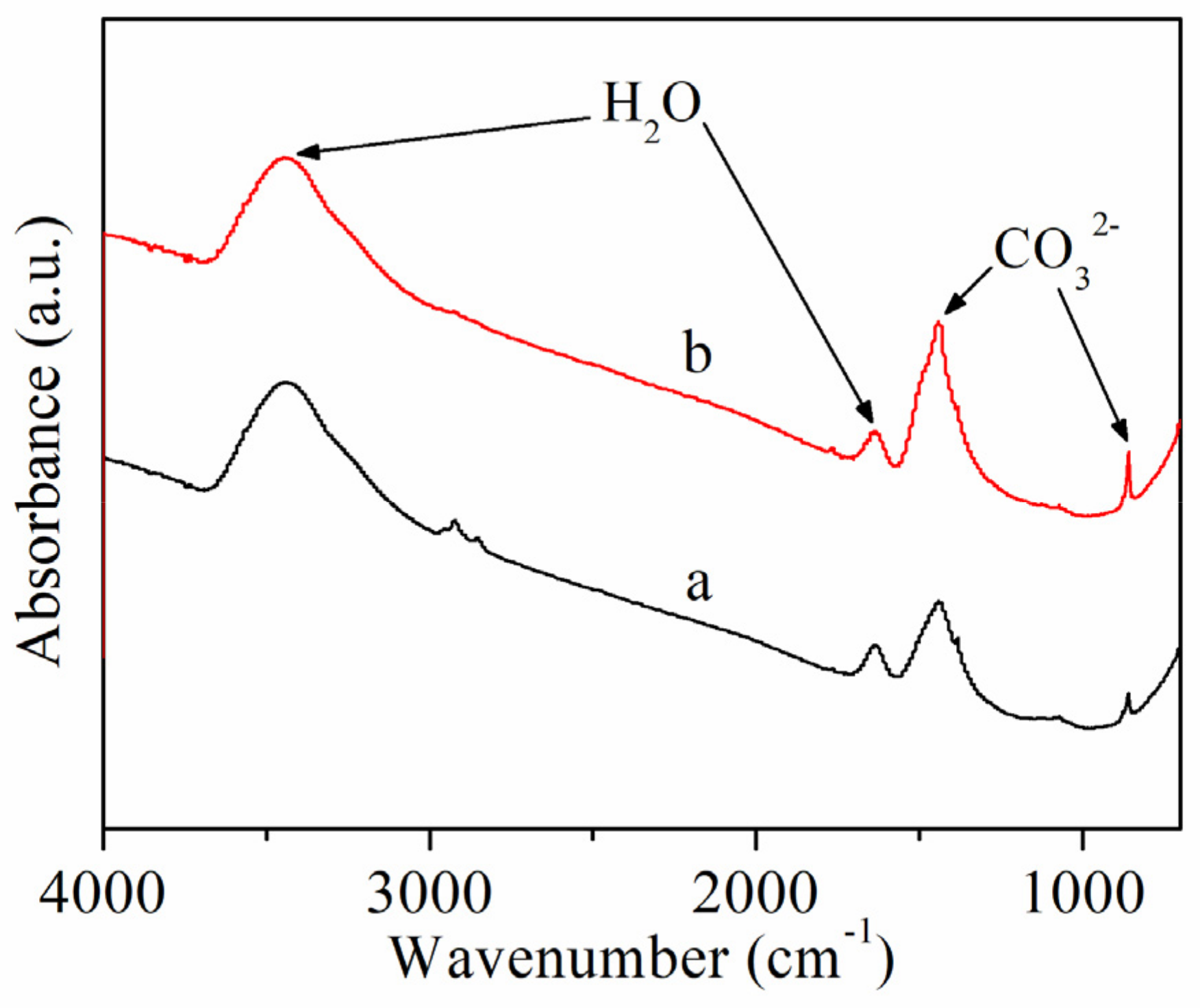
2.3. XPS and IR
2.4. Temperature-Programmed Desorption (TPD) of O2 and CO2
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization of the Catalyst
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.; Oyama, S. Oxidative coupling of methane to higher hydrocarbons. Catal. Rev. Sci. Eng. 1988, 30, 249–280. [Google Scholar] [CrossRef]
- Arndt, S.; Laugel, G.; Levchenko, S.; Horn, R.; Baerns, M.; Scheffler, M.; Schlögl, R.; Schomäcker, R. A critical assessment of Li/MgO-based catalysts for the oxidative coupling of methane. Catal. Rev. Sci. Eng. 2011, 53, 424–514. [Google Scholar] [CrossRef]
- Han, B.; Yang, Y.; Xu, Y.; Etim, U.J.; Qiao, K.; Xu, B.; Yan, Z. A review of the direct oxidation of methane to methanol. Chin. J. Catal. 2016, 37, 1206–1215. [Google Scholar] [CrossRef]
- Mesters, C. A selection of recent advances in C1 chemistry. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Galadima, A.; Muraza, O. Revisiting the oxidative coupling of methane to ethylene in the golden period of shale gas: A review. J. Ind. Eng. Chem. 2016, 37, 1–13. [Google Scholar] [CrossRef]
- Pierre, S.; Pan, X.L.; Bao, X.H. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: Challenges and prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar]
- Gambo, Y.; Jalil, A.A.; Triwahyono, S.; Abdulrasheed, A.A. Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene: A review. J. Ind. Eng. Chem. 2018, 59, 218–229. [Google Scholar] [CrossRef]
- Keller, G.E.; Bhasin, M.M. Synthesis of ethylene via oxidative coupling of methane: I. Determination of active catalysts. J. Catal. 1982, 73, 9–19. [Google Scholar] [CrossRef]
- Ito, T.; Lunsford, J.H. Synthesis of ethylene and ethane by partial oxidation of methane over lithium-doped magnesium-oxide. Nature 1985, 314, 721–722. [Google Scholar] [CrossRef]
- Driscoll, D.J.; Martir, W.; Wang, J.X.; Lunsford, J.H. Formation of gas-phase methyl radicals over MgO. J. Am. Chem. Soc. 1985, 107, 58–63. [Google Scholar] [CrossRef]
- Peil, K.P.; Goodwin, J.G.; Marcelin, G. Surface phenomena during the oxidative coupling of methane over Li/MgO. J. Catal. 1991, 131, 143–155. [Google Scholar] [CrossRef]
- Nagaoka, K.; Karasuda, T.; Aika, K. The effect of SnO2 addition to Li/MgO catalysts for the oxidative coupling of methane. J. Catal. 1999, 181, 160–164. [Google Scholar] [CrossRef]
- Amin, N.A.S.; Pheng, S.E. Influence of process variables and optimization of ethylene yield in oxidative coupling of methane over Li/MgO catalyst. Chem. Eng. J. 2006, 116, 187–195. [Google Scholar] [CrossRef]
- Tang, L.; Yamaguchi, D.; Wong, L.; Burke, N.; Chiang, K. The promoting effect of ceria on Li/MgO catalysts for the oxidative coupling of methane. Catal. Today 2011, 178, 172–180. [Google Scholar] [CrossRef]
- Arndt, S.; Simon, U.; Kiefer, K.; Otremba, T.; Siemensmeyer, K.; Wollgarten, M.; Berthold, A.; Schmidt, F.; Görke, O.; Schomäcker, R.; et al. Li/MgO catalysts doped with alio-valent ions. Part I: Structure, composition, and catalytic properties. ChemCatChem 2017, 9, 3583–3596. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Li, S.; Lin, J.; Gu, J.; Yan, D. Preparation and characterization of catalyst for oxidative coupling of methane. J. Mol. Catal. 1992, 6, 254–262. [Google Scholar]
- Ji, S.; Xiao, T.; Li, S.; Chou, L.; Zhang, B.; Xu, C.; Hou, R.; York, A.P.E.; Green, M.L.H. Surface WO4 tetrahedron: The essence of the oxidative coupling of methane over M-W-Mn/SiO2 catalysts. J. Catal. 2003, 220, 47–56. [Google Scholar] [CrossRef]
- Wang, J.; Chou, L.; Zhang, B.; Song, H.; Zhao, J.; Yang, J.; Li, S. Comparative study on oxidation of methane to ethane and ethylene over Na2WO4-Mn/SiO2 catalysts prepared by different methods. J. Mol. Catal. A 2006, 245, 272–277. [Google Scholar] [CrossRef]
- Arndt, S.; Otremba, T.; Simon, U.; Yildiz, M.; Schubert, H.; Schomäcker, R. Mn–Na2WO4/SiO2 as catalyst for the oxidative coupling of methane. What is really known? Appl. Catal. A 2012, 425–426, 53–61. [Google Scholar] [CrossRef]
- Ghose, R.; Hwang, H.T.; Varma, A. Oxidative coupling of methane using catalysts synthesized by solution combustion method: Catalyst optimization and kinetic studies. Appl. Catal. A 2014, 472, 39–46. [Google Scholar] [CrossRef]
- Elkins, T.W.; Hagelin-Weaver, H.E. Characterization of Mn-Na2WO4/SiO2 and Mn-Na2WO4/MgO catalysts for the oxidative coupling of methane. Appl. Catal. A 2015, 497, 96–106. [Google Scholar] [CrossRef]
- Fleischer, V.; Steuer, R.; Parishan, S.; Schomäcker, R. Investigation of the surface reaction network of the oxidative coupling of methane over Na2WO4/Mn/SiO2 catalyst by temperature programmed and dynamic experiments. J. Catal. 2016, 341, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Werny, M.J.; Wang, Y.; Girgsdies, F.; Schlögl, R.; Trunschke, A. Fluctuating storage of the active phase in a Mn-Na2WO4/SiO2 catalyst for the oxidative coupling of methane. Angew. Chem. Int. Ed. 2020, 59, 14921–14926. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, G.; Wang, Y.; Lu, Y. MnTiO3-driven low-temperature oxidative coupling of methane over TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst. Sci. Adv. 2017, 3, e1603180. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Zhao, Y.; Zhang, J.; Zhu, Y.; Sun, Y. Exploiting shape effects of La2O3 nanocatalysts for oxidative coupling of methane reaction. Nanoscale 2013, 5, 10844–10848. [Google Scholar] [CrossRef]
- Jiang, T.; Song, J.; Huo, M.; Yang, N.; Liu, J.; Zhang, J.; Sun, Y.; Zhu, Y. La2O3 catalysts with diverse spatial dimensionality for oxidative coupling of methane to produce ethylene and ethane. RSC Adv. 2016, 6, 34872–34876. [Google Scholar] [CrossRef]
- Fu, B.; Jiang, T.; Zhu, Y. Structural effect of one-dimensional samarium oxide catalysts on oxidative coupling of methane. J. Nanosci. Nanotechnol. 2018, 18, 3398–3404. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, M.; Miao, C.; Yue, Y.; Hua, W.; Gao, Z. Morphology effects of nanoscale Er2O3 and Sr-Er2O3 catalysts for oxidative coupling of methane. Catal. Lett. 2021, 1–10. [Google Scholar] [CrossRef]
- Song, J.; Sun, Y.; Ba, R.; Huang, S.; Zhao, Y.; Zhang, J.; Sun, Y.; Zhu, Y. Monodisperse Sr–La2O3 hybrid nanofibers for oxidative coupling of methane to synthesize C2 hydrocarbons. Nanoscale 2015, 7, 2260–2264. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, Y.; Song, J.; Ba, R.; Huang, S.; Zhao, Y.; Zhang, J.; Sun, Y.; Zhu, Y. Facet-controlled CeO2 nanocrystals for oxidative coupling of methane. J. Nanosci. Nanotechnol. 2016, 16, 4692–4700. [Google Scholar] [CrossRef]
- Wiktorczyk, T. Preparation and optical properties of holmium oxide thin films. Thin Solid Films 2002, 405, 238–242. [Google Scholar] [CrossRef]
- Mortazavi-Derazkola, S.; Zinatloo-Ajabshir, S.; Salavati-Niasari, M. New facile preparation of Ho2O3 nanostructured material with improved photocatalytic performance. J. Mater. Sci. 2017, 28, 1914–1924. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Mortazavi-Derazkola, S.; Salavati-Niasari, M. Simple sonochemical synthesis of Ho2O3-SiO2 nanocomposites as an effective photocatalyst for degradation and removal of organic contaminant. Ultrason. Sonochem. 2017, 39, 452–460. [Google Scholar] [CrossRef]
- Shiri, H.M.; Ehsani, A. A novel and facile route for the electrosynthesis of Ho2O3 nanoparticles and its nanocomposite with p-type conductive polymer: Characterization and electrochemical performance. Bull. Chem. Soc. Jpn. 2016, 89, 1201–1206. [Google Scholar] [CrossRef]
- Takenaka, S.; Kaburagi, T.; Yamanaka, I.; Otsuka, K. Oxidative coupling of methane over Li+-added Y2O3 catalyst prepared from Y(OH)3. Catal Today 2001, 71, 31–36. [Google Scholar] [CrossRef]
- Campbell, K.D.; Morales, E.; Lunsford, J.H. Gas-phase coupling of methyl radicals during the catalytic partial oxidation of methane. J. Am. Chem. Soc. 1987, 109, 7900–7901. [Google Scholar] [CrossRef]
- Al-Zahrani, S.; Song, Q.; Lobban, L.L. Effects of CO2 during oxidative coupling of methane over Li/MgO: Mechanisms and models. Ind. Eng. Chem. Res. 1994, 33, 251–258. [Google Scholar] [CrossRef]
- Vislovskii, V.P.; Baidikova, I.V.; Mamedov, E.A.; Rizayev, R.G. Promoting effect of alkali oxides on Bi-Mn catalysts activity in oxidative coupling of methane. React. Kinet. Catal. Lett. 1992, 47, 193–197. [Google Scholar] [CrossRef]
- Baronetti, G.T.; Padrό, C.; Scelza, O.A.; Castro, A.A. Structure and reactivity of alkali-doped calcium oxide catalysts for oxidative coupling of methane. Appl. Catal. A 1993, 101, 167–183. [Google Scholar] [CrossRef]
- Zhao, M.; Ke, S.; Wu, H.; Xia, W.; Wan, H. Flower-like Sr-La2O3 microspheres with hierarchically porous structures for oxidative coupling of methane. Ind. Eng. Chem. Res. 2019, 58, 22847–22856. [Google Scholar] [CrossRef]
- Kharas, K.C.C.; Lunsford, J.H. Catalytic partial oxidation of methane over barium metaplumbate BaPbO3: Possible involvement of peroxide ion. J. Am. Chem. Soc. 1989, 111, 2336–2337. [Google Scholar] [CrossRef]
- Peng, X.D.; Richards, D.A.; Stair, P.C. Surface composition and reactivity of lithium-doped magnesium oxide catalysts for oxidative coupling of methane. J. Catal. 1990, 121, 99–109. [Google Scholar] [CrossRef]
- Ding, W.; Chen, Y.; Fu, X. Oxidative coupling of methane over Ce4+-doped Ba3WO6 catalysts: Investigation on oxygen species responsible for catalytic performance. Catal. Lett. 1994, 23, 69–78. [Google Scholar] [CrossRef]
- Hou, Y.H.; Han, W.C.; Xia, W.S.; Wan, H.L. Structure sensitivity of La2O2CO3 catalysts in the oxidative coupling of methane. ACS Catal. 2015, 5, 1663–1674. [Google Scholar] [CrossRef]
- Bai, Y.; Xia, W.; Weng, W.; Lian, M.; Zhao, M.; Wan, H. Influence of phosphate on La-based catalysts for oxidative coupling of methane. Chem. J. Chin. Univ. Chin. 2018, 39, 247–254. [Google Scholar]
- Sayle, T.X.T.; Parker, S.C.; Sayle, D.C. Oxidising CO to CO2 using ceria nanoparticles. Phys. Chem. Chem. Phys. 2005, 7, 2936–2941. [Google Scholar] [CrossRef]
- Bernal, S.; Botana, F.J.; Garcia, R.; Rodiguez-Izquierdo, J.M. Behaviour of rare earth sesquioxides exposed to atmospheric carbon dioxide and water. React. Soliak. 1987, 4, 23–40. [Google Scholar] [CrossRef]
- Djerdj, I.; Garnweitner, G.; Su, D.S.; Niederberger, M. Morphology-controlled nonaqueous synthesis of anisotropic lanthanum hydroxide nanoparticles. J. Solid State Chem. 2007, 180, 2154–2165. [Google Scholar] [CrossRef] [Green Version]
- Farrukh, M.A.; Imran, F.; Ali, S.; Khaleeq-ur-Rahman, M.; Naqvi, I.I. Micelle assisted synthesis of La2O3 nanoparticles and their applications in photodegradation of bromophenol blue. Russ. J. Appl. Chem. 2015, 88, 1523–1527. [Google Scholar] [CrossRef]
- Spinicci, R.; Tofanari, A. Characterization of catalysts for methane-coupling by means of temperature programmed desorption. Catal. Today 1990, 6, 473–479. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Xu, X.; Fang, X.; Xi, R.; Liu, Y.; Zheng, R.; Wang, X. Constructing La2B2O7 (B = Ti, Zr, Ce) compounds with three typical crystalline phases for the oxidative coupling of methane: The effect of phase structures, superoxide anions, and alkalinity on the reactivity. ACS Catal. 2019, 9, 4030–4045. [Google Scholar] [CrossRef]
- McFarland, E.W.; Metiu, H. Catalysis by Doped Oxides. Chem. Rev. 2013, 113, 4391–4427. [Google Scholar] [CrossRef]
- Liang, Q.; Wu, X.; Weng, D.; Xu, H. Oxygen activation on Cu/Mn−Ce mixed oxides and the role in diesel soot oxidation. Catal. Today 2008, 139, 113–118. [Google Scholar] [CrossRef]
- Papa, F.; Luminita, P.; Osiceanu, P.; Birjega, R.; Akane, M.; Balint, I. Acid–base properties of the active sites responsible for C2+ and CO2 formation over MO–Sm2O3 (M = Zn, Mg, Ca and Sr) mixed oxides in OCM reaction. J. Mol. Catal. A 2011, 346, 46–54. [Google Scholar] [CrossRef]
- Elkins, T.W.; Roberts, S.J.; Hagelin-Weaver, H.E. Effects of alkali and alkaline-earth metal dopants on magnesium oxide supported rare-earth oxide catalysts in the oxidative coupling methane. Appl. Catal. A 2016, 528, 175–190. [Google Scholar] [CrossRef]
- Bernal, S.; Blanco, G.; El Amarti, A.; Cifredo, G.; Fitian, L.; Galtayries, A.; Martín, J.; Pintado, J.M. Surface basicity of ceria-supported lanthana. Influence of the calcination temperature. Surf. Interface Anal. 2006, 38, 229–233. [Google Scholar] [CrossRef]
- Peng, L.; Xu, J.; Fang, X.; Liu, W.; Xu, X.; Liu, L.; Li, Z.; Peng, H.; Zheng, R.; Wang, X. SnO2 based catalysts with low-temperature performance for oxidative coupling of methane: Insight into the promotional effects of alkali-metal oxides. Eur. J. Inorg. Chem. 2018, 2018, 1787–1799. [Google Scholar] [CrossRef]
- Xu, J.; Peng, L.; Fang, X.; Fu, Z.; Liu, W.; Xu, X.; Peng, H.; Zheng, R.; Wang, X. Developing reactive catalysts for low temperature oxidative coupling of methane: On the factors deciding the reaction performance of Ln2Ce2O7 with different rare earth A sites. Appl. Catal. A 2018, 552, 117–128. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Liu, Y.; Fang, X.; Xu, X.; Liu, W.; Zheng, R.; Wang, X. Optimizing the reaction performance of La2Ce2O7-based catalysts for oxidative coupling of methane (OCM) at lower temperature by lattice doping with Ca cations. Eur. J. Inorg. Chem. 2019, 183–194. [Google Scholar] [CrossRef]
- Wang, Z.; Zou, G.; Luo, X.; Liu, H.; Gao, R.; Chou, L.; Wang, X. Oxidative coupling of methane over BaCl2-TiO2-SnO2 catalyst. J. Nat. Gas Chem. 2012, 21, 49–55. [Google Scholar] [CrossRef]
- Lee, H.I.; Lee, S.W.; Rhee, C.K.; Sohn, Y. Paramagnetic Ho2O3 nanowires, nano-square sheets, and nanoplates. Ceram. Int. 2018, 44, 17919–17924. [Google Scholar] [CrossRef]
- Ito, T.; Wang, J.X.; Lin, C.H.; Lunsford, J.H. Oxidative dimerization of methane over a lithium-promoted magnesium oxide catalyst. J. Am. Chem. Soc. 1985, 107, 5062–5068. [Google Scholar] [CrossRef]




| Catalyst | Conversion | Selectivity (%) | Selectivity | Yield of | |||||
|---|---|---|---|---|---|---|---|---|---|
| of CH4 (%) | C2H4 | C2H6 | C3H6 | C3H8 | CO2 | CO | of C2-C3 (%) | C2-C3 (%) | |
| Ho2O3-NS | 20.0 | 14.9 | 17.1 | 0.3 | 0.5 | 40.4 | 26.8 | 32.8 | 6.6 |
| 0.02Sr-Ho2O3-NS | 22.3 | 25.5 | 23.5 | 1.5 | 1.5 | 36.1 | 11.9 | 52.0 | 11.6 |
| 0.04Sr-Ho2O3-NS | 24.0 | 27.4 | 26.0 | 1.6 | 1.7 | 35.5 | 7.8 | 56.7 | 13.6 |
| 0.06Sr-Ho2O3-NS | 20.6 | 21.9 | 24.9 | 1.2 | 1.5 | 38.9 | 11.6 | 49.5 | 10.2 |
| Catalyst | SBET | Average | a = b = c | O 1s BE e, FWHM f (eV) | (O− + O2−)/ O2− | |||
|---|---|---|---|---|---|---|---|---|
| (m2/g) | size (nm) | (nm) d | O2− | O− | CO32− | O2− | ||
| Ho2O3-NP | 7.9 | 17.5 ± 3.3 | 1.0560 | 529.3/1.7 | 530.8/1.7 | 531.8/1.2 | 532.7/1.2 | 1.4 |
| Ho2O3-NS | 6.1 | 771 ± 232 a 81.9 ± 21.0 b | 1.0561 | 529.3/1.6 | 530.6/1.5 | 531.6/1.1 | 532.5/1.4 | 1.7 |
| 0.02Sr-Ho2O3-NS | 7.5 | − c | 1.0571 | 529.3/1.5 | 530.8/1.7 | 531.8/1.2 | 532.6/1.4 | 1.9 |
| 0.04Sr-Ho2O3-NS | 7.7 | 761 ± 184 a 82.5 ± 27.9 b | 1.0580 | 529.6/1.5 | 530.9/1.6 | 532.0/1.4 | 532.8/1.3 | 2.2 |
| 0.06Sr-Ho2O3-NS | 7.2 | − c | 1.0589 | 529.2/1.8 | 530.7/1.5 | 531.6/1.2 | 532.5/1.3 | 2.0 |
| Catalyst | Peak Temperature (°C) | Amount of Desorbed O2 (μmol/g) | Amount of Basic Sites (μmol/g) | ||||
|---|---|---|---|---|---|---|---|
| I | II | I | II | Weak | Moderate | Total | |
| Ho2O3-NP | 99 | 426 | 2.3 | 12.8 | 3.2 | 16.3 | 19.5 |
| Ho2O3-NS | 85 | 344 | 2.2 | 15.9 | 7.0 | 30.1 | 37.1 |
| 0.02Sr-Ho2O3-NS | 109 | 263 | 9.2 | 19.8 | 16.7 | 61.8 | 78.5 |
| 0.04Sr-Ho2O3-NS | 137 | 275 | 11.3 | 24.2 | 16.2 | 69.9 | 86.1 |
| 0.06Sr-Ho2O3-NS | 118 | 309 | 9.9 | 20.7 | 14.6 | 63.0 | 77.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Miao, C.; Yue, Y.; Hua, W.; Gao, Z. Nanosheet-Like Ho2O3 and Sr-Ho2O3 Catalysts for Oxidative Coupling of Methane. Catalysts 2021, 11, 388. https://doi.org/10.3390/catal11030388
Fan Y, Miao C, Yue Y, Hua W, Gao Z. Nanosheet-Like Ho2O3 and Sr-Ho2O3 Catalysts for Oxidative Coupling of Methane. Catalysts. 2021; 11(3):388. https://doi.org/10.3390/catal11030388
Chicago/Turabian StyleFan, Yuqiao, Changxi Miao, Yinghong Yue, Weiming Hua, and Zi Gao. 2021. "Nanosheet-Like Ho2O3 and Sr-Ho2O3 Catalysts for Oxidative Coupling of Methane" Catalysts 11, no. 3: 388. https://doi.org/10.3390/catal11030388
APA StyleFan, Y., Miao, C., Yue, Y., Hua, W., & Gao, Z. (2021). Nanosheet-Like Ho2O3 and Sr-Ho2O3 Catalysts for Oxidative Coupling of Methane. Catalysts, 11(3), 388. https://doi.org/10.3390/catal11030388