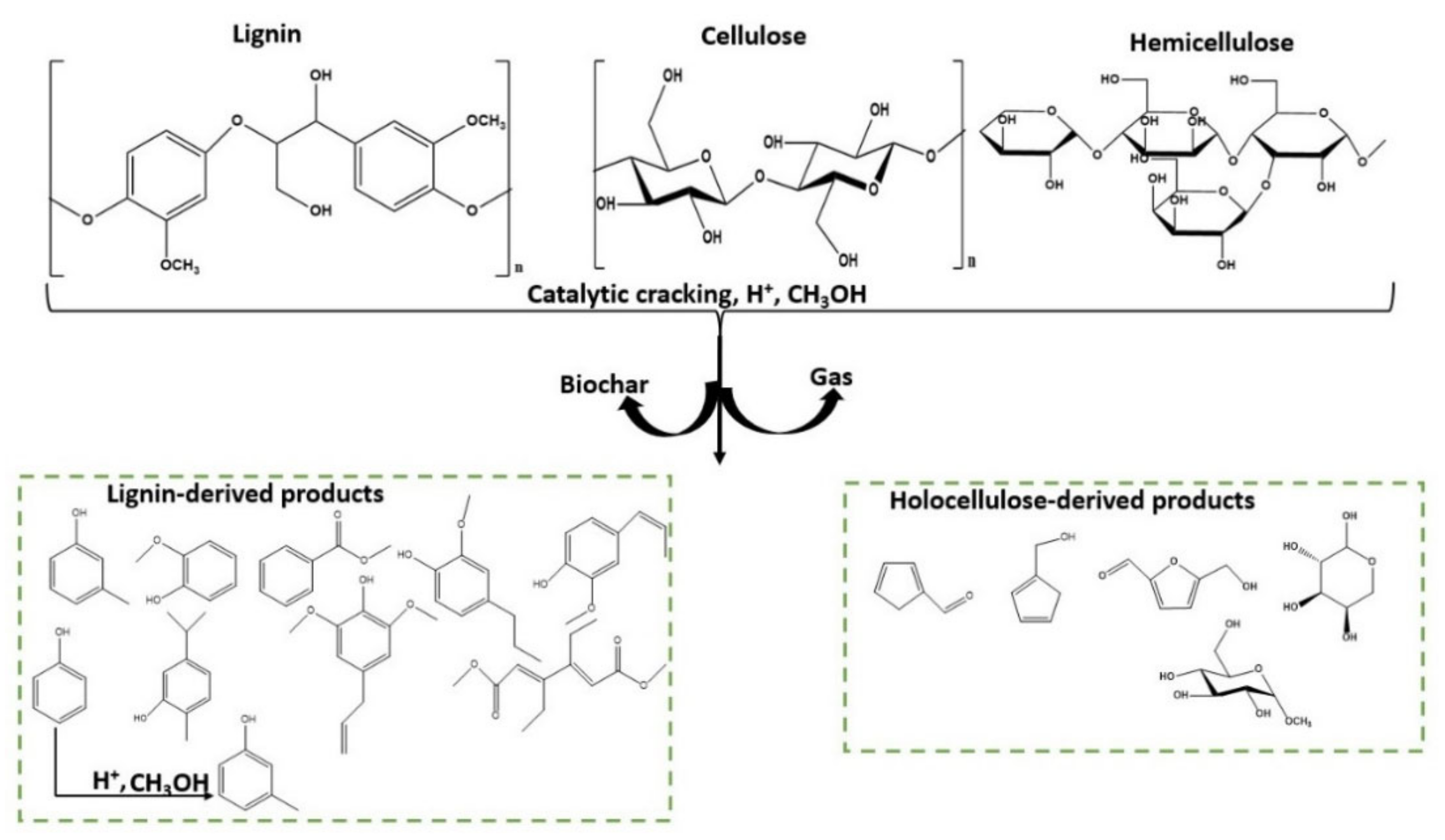
Catalytic Depolymerization of Date Palm Waste to Valuable C5–C12 Compounds
Abstract
1. Introduction
2. Results and Discussion
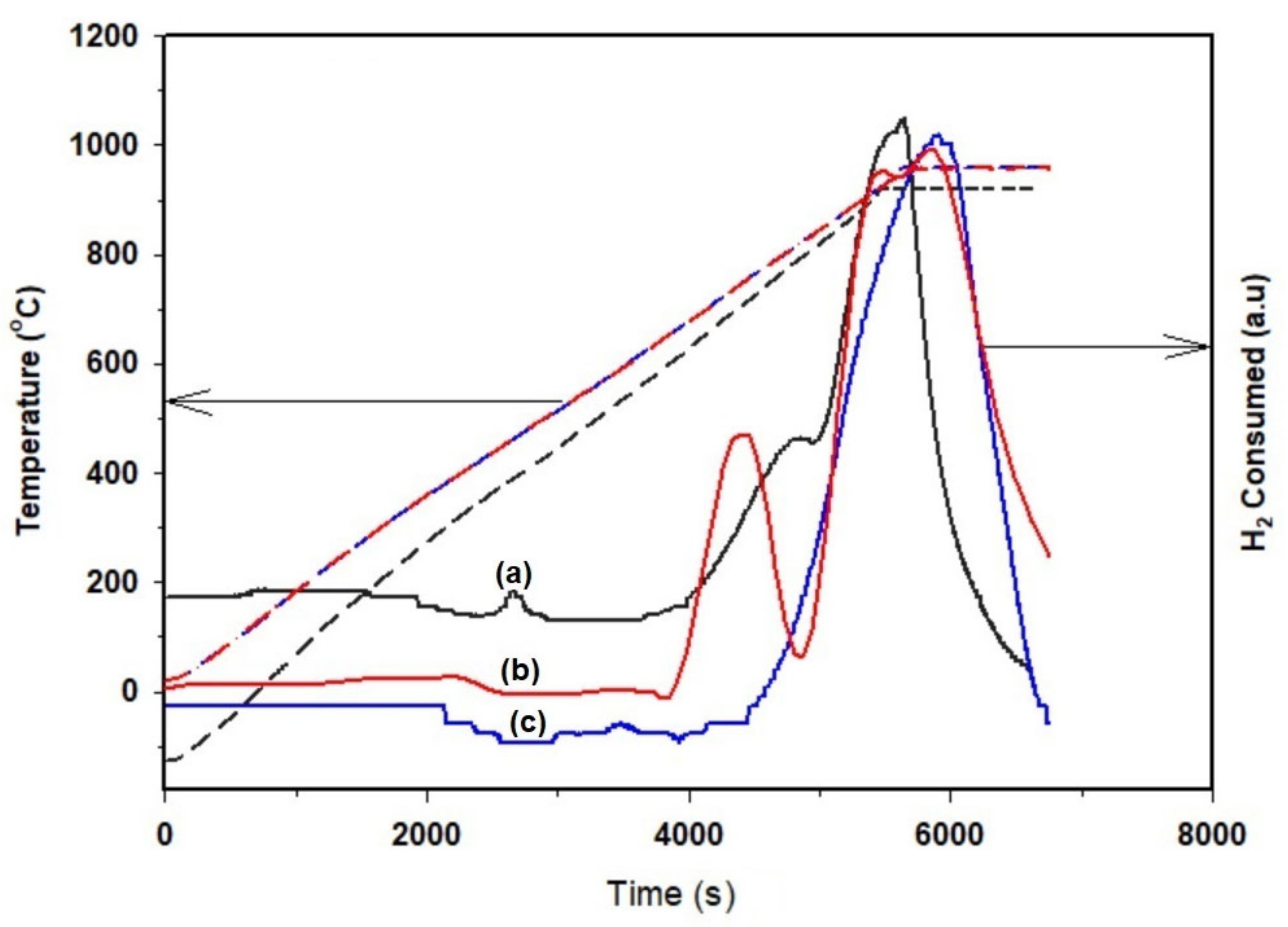
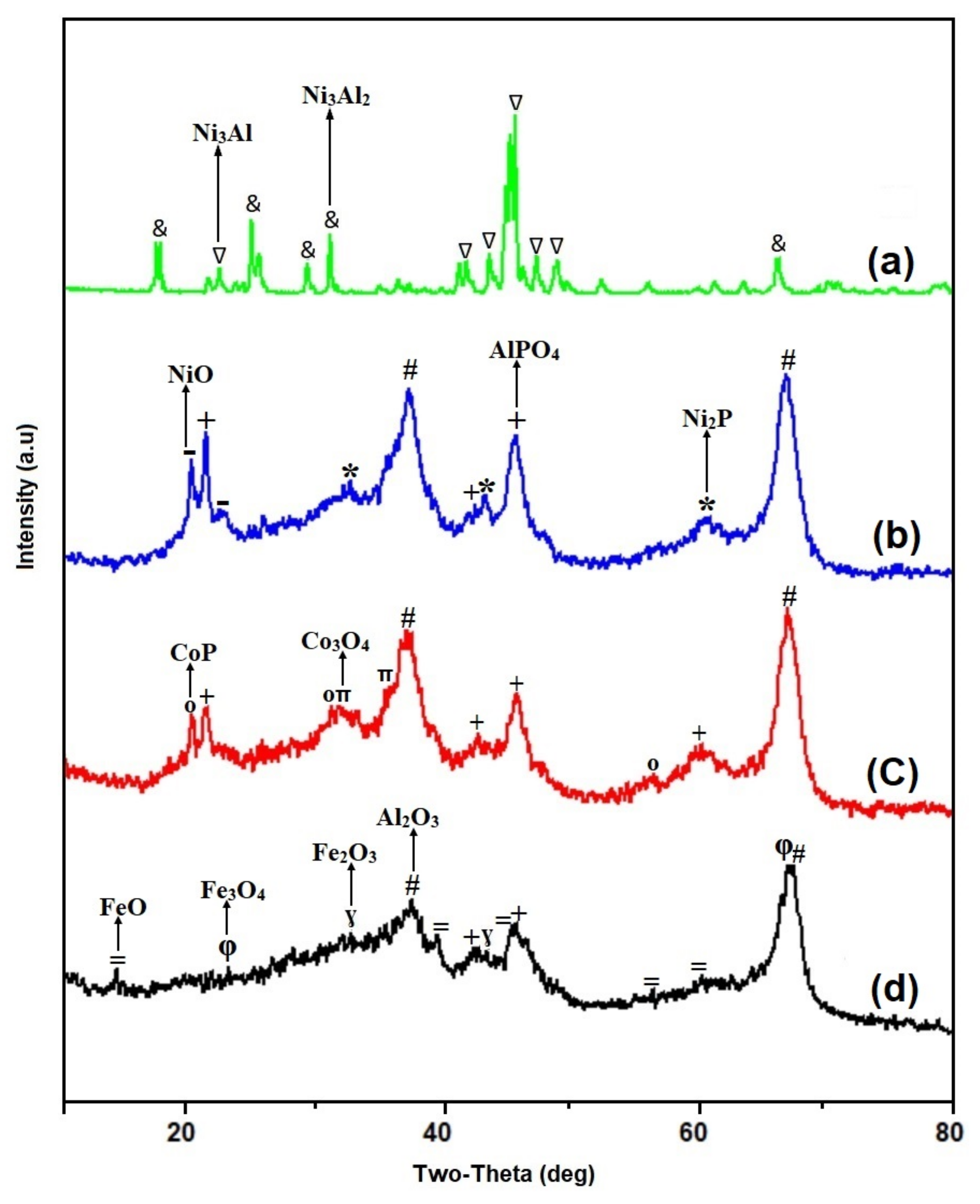
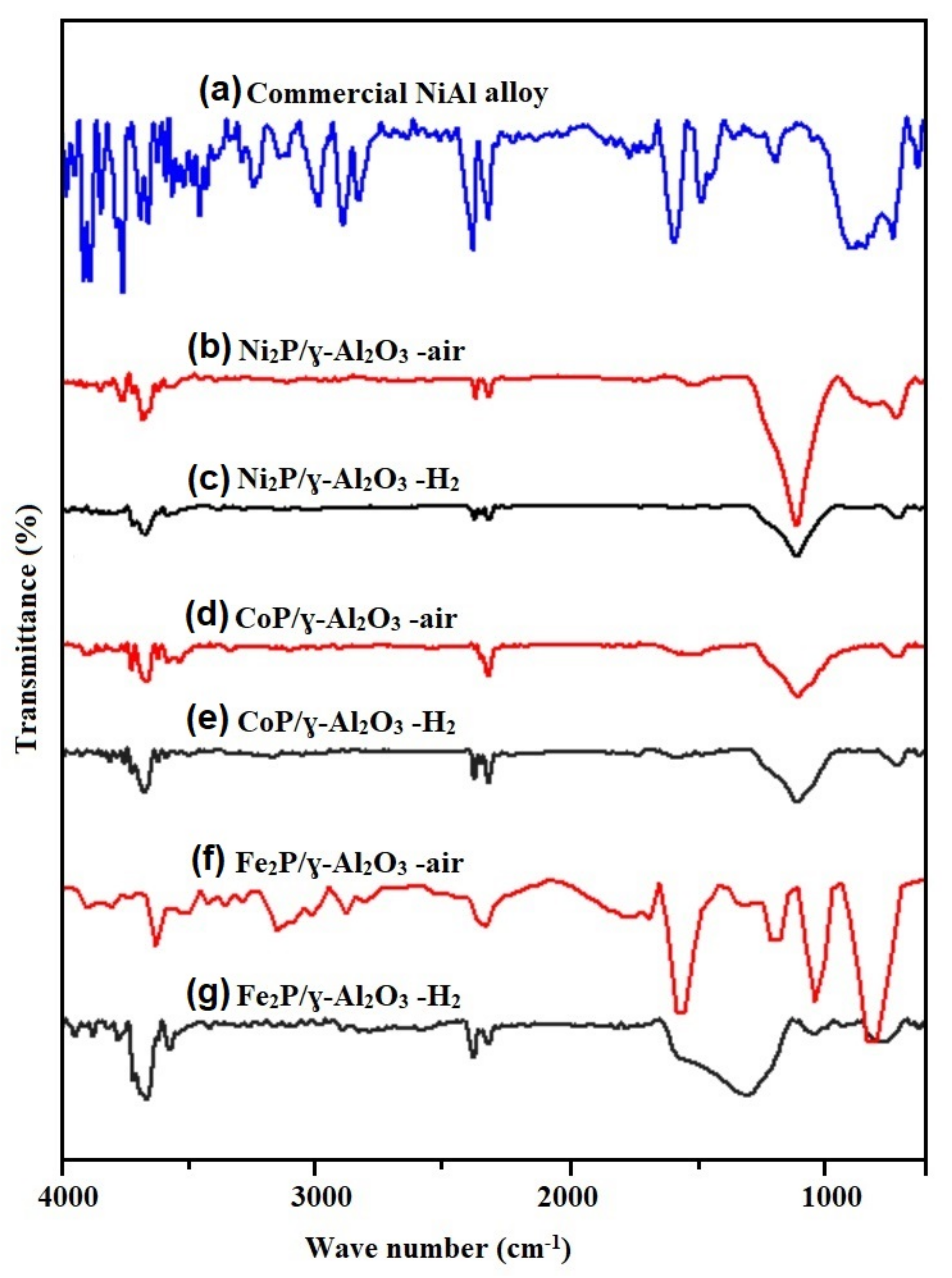
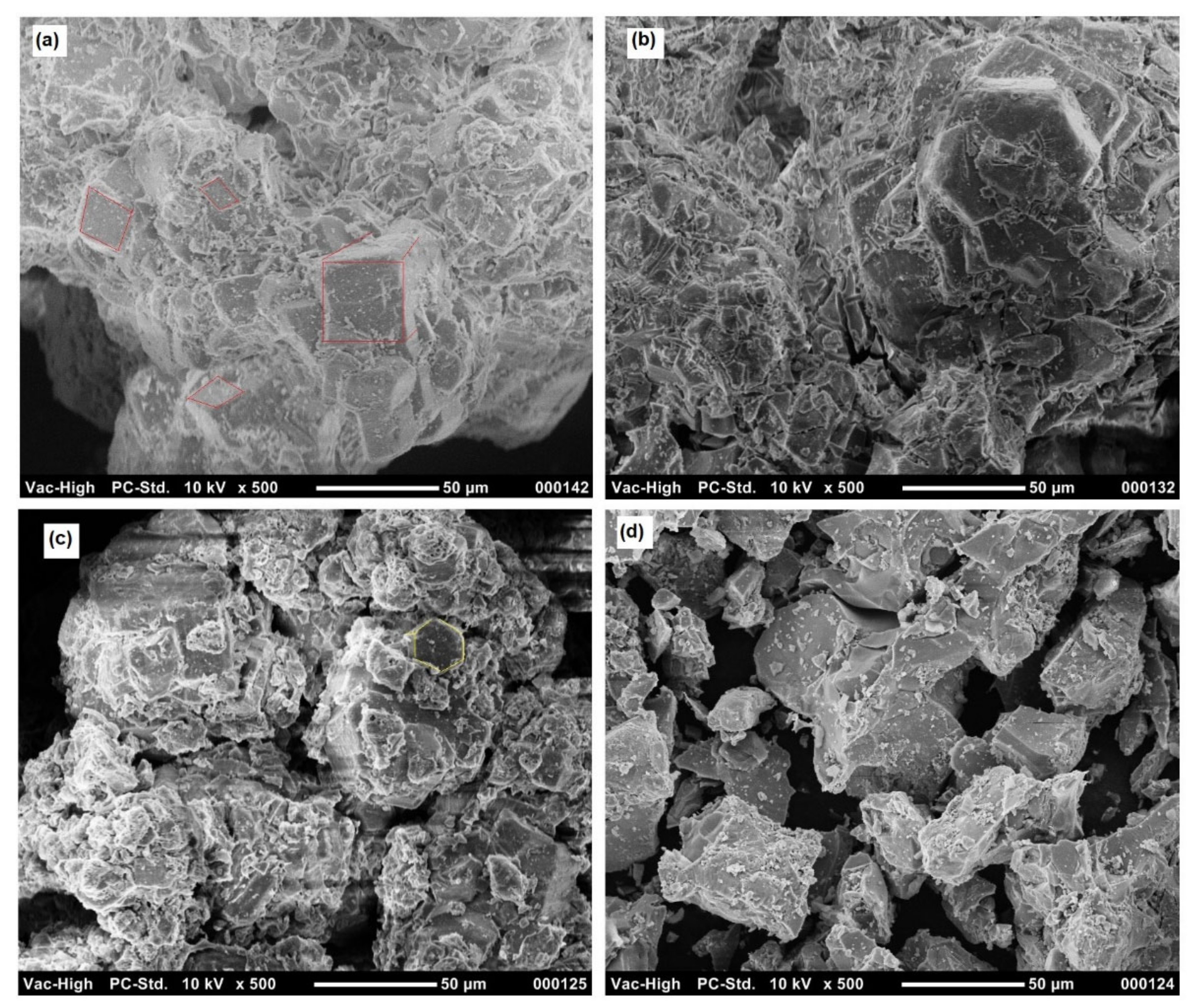
2.1. Catalyst and Biomass Characterization
| C | H | O | Lignin | Holocellulose | Extractives | HHV [MJ/kg] | Ash | Moisture |
|---|---|---|---|---|---|---|---|---|
| 40.6 | 5.1 | 45.6 | 26.4 | 45.0 | 16.0 ± 0.6 | 15.0 | 8.7 ± 1.2 | 6.72 ± 0.4 |
| Entry | Catalyst Name | SBET [m2 g−1] | Pore Volume [cm3 g−1] | Pore Size [nm] | |
|---|---|---|---|---|---|
| Calcination | TPR | ||||
| 1 | ɣ-Al2O3 | - | 258 | 0.39 a | 5.39 a |
| 2 | Commercial NiAl | - | 0.72 b | 0.000954 b | 53.3 b |
| 3 | Ni2P/ɣ-Al2O3 | 200.8 | 172.6 | 0.26 a | 4.31 a |
| 4 | CoP/ɣ-Al2O3 | 153.7 | 106.1 | 0.43 c | 7.67 c |
| 5 | Fe2P/ɣ-Al2O3 | 243.9 | 145.6 | 0.45 a | 5.74 a |
2.2. The Effects of Reaction Parameters on High-Value Product Distributions
3. Materials and Methods
3.1. Sample and Catalyst Preparation
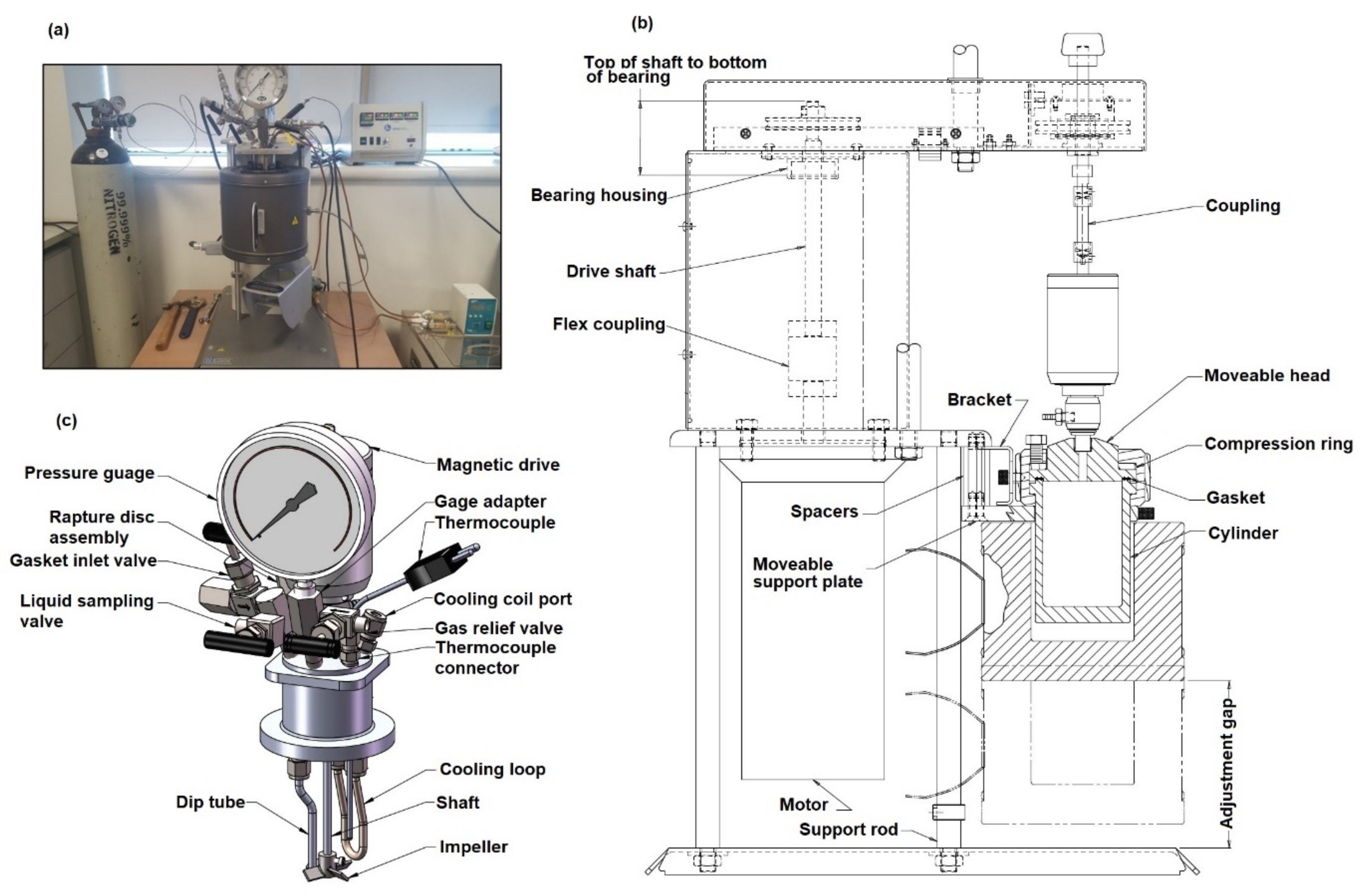
3.2. Experimental Apparatus and Reaction Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| BET | Brunauer-Emmett-Teller |
| TPR | Temperature-programmed reduction |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| TMPCs | Transition metal phosphide catalysts |
| HHV | Higher heating value |
| HDO | Hydrodeoxygenation |
| GC-MS | Gas chromatography-mass spectrometry |
| CA | Citric acid |
| ɣ-Al2O3 | Gamma alumina |
| HVCs | High value chemicals |
References
- Aguilera, R.F.; Eggert, R.G.C.C.G.L.; Tilton, J.E. Depletion and the Future Availability of Petroleum Resources. Energy J. 2009, 30, 1–6. [Google Scholar] [CrossRef]
- Marrs, R.H.; Freedman, B. Environmental Ecology: The Impacts of Pollution and Other Stresses on Ecosystem Structure and Function. J. Appl. Ecol. 1990, 27, 1093. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Nie, Y.; Lu, X.; Zhang, X.; He, H.; Pan, F.; Zhou, L.; Liu, X.; Ji, X.; Zhang, S. Cascade utilization of lignocellulosic biomass to high-value products. Green Chem. 2019, 21, 3499–3535. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Grassi, G. Biomass Pyrolysis Liquids Upgrading and Utilization, Commission of the European Communities; Elsevier: Amsterdam, The Netherlands, 1991; pp. 11–92. [Google Scholar]
- Wang, Y.T.; Fang, Z. Catalytic Biomass to Renewable Biofuels and Biomaterials. Catalysts 2020, 10, 480. [Google Scholar] [CrossRef]
- Morales, G.; Iglesias, J.; Melero, J.A. Sustainable Catalytic Conversion of Biomass for the Production of Biofuels and Bioproducts. Catalysts 2020, 10, 581. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid Hydrolysis of Lignocellulosic Biomass: Sugars and Furfurals Formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef]
- Yang, J.; Li, G.; Zhang, L.; Zhang, S. Efficient Production of N-Butyl Levulinate Fuel Additive from Levulinic Acid Using Amorphous Carbon Enriched with Oxygenated Groups. Catalysts 2018, 8, 14. [Google Scholar] [CrossRef]
- Esteban, J.; Yustos, P.; Ladero, M. Catalytic Processes from Biomass-Derived Hexoses and Pentoses: A Recent Literature Overview. Catalysts 2018, 8, 637. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, Z.; Zhang, F.; Li, X.; Tan, W.; Xi, B. Role of Humic Acid Chemical Structure Derived from Different Biomass Feedstocks on Fe (III) Bioreduction Activity: Implication for Sustainable Use of Bioresources. Catalysts 2019, 9, 450. [Google Scholar] [CrossRef]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin engineering. Curr. Opin. Plant Biol. 2008, 11, 278–285. [Google Scholar] [CrossRef]
- Gellerstedt, G.; Henriksson, G. Lignins: Major Sources, Structure and Properties, Monomers, Polymers and Composites from Renewable Resources; Belgacem, M., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 201–224. [Google Scholar]
- Jones, D.; Ormondroyd, G.; Curling, S.; Popescu, C.M.; Popescu, M.C. Chemical compositions of natural fibres. In Advanced High Strength Natural Fibre Composites in Construction; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–58. [Google Scholar]
- Baeyens, J.; Kang, Q.; Appels, L.; Dewil, R.; Lv, Y.; Tan, T. Challenges and opportunities in improving the production of bio-ethanol. Prog. Energy Combust. Sci. 2015, 47, 60–88. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Z.; Li, S.; Xue, S.; Sun, H. Novel Micro-Mesoporous Composite ZSM-5 Catalyst for Aromatics Production by Catalytic Fast Pyrolysis of Lignin Residues. Catalysts 2020, 10, 378. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; Lopes, A.M.D.C.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Zhou, C.H.; Xia, X.; Lin, C.X.; Tong, D.S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of re-newable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin: A Sustainable Alternative to Nonrenewable Materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Rong, H.; Gao, B.; Zhao, Y.; Sun, S.; Yang, Z.; Wang, Y.; Yue, Q.; Li, Q. Advanced lignin-acrylamide water treatment agent by pulp and paper industrial sludge: Synthesis, properties and application. J. Environ. Sci. 2013, 25, 2367–2377. [Google Scholar] [CrossRef]
- Araújo, J.D.P. Production of Vanillin from Lignin Present in the Kraft Black Liquor of the Pulp and Paper Industry. Ph.D. Thesis, Chemical Engineering-University of Porto, Porto, Portugal, 2008. [Google Scholar]
- Gosselink, R.J.A. Lignin as a Renewable Aromatic Resource for the Chemical Industry. Ph.D. Thesis, Chemistry—Wageningen University, Wageningen, Sweden, 2011. [Google Scholar]
- Wild, d.P.J.; Gosselink, R.J.A.; Huijgen, W.J.J. Lignin pyrolysis for profitable lignocellulosic biorefineries. Biofuels Bioprod. Biorefining 2014, 8, 645–657. [Google Scholar] [CrossRef]
- Li, C.; Chen, C.; Wu, X.; Tsang, C.W.; Mou, J.; Yan, J.; Liu, Y.; Lin, C.S.K. Recent advancement in lignin biorefinery: With special focus on enzymatic degradation and valorization. Bioresour. Technol. 2019, 291, 1898. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Renew. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Carpenter, D.; Westover, T.L.; Czernik, S.; Jablonski, W.S. Biomass feedstocks for renewable fuel production: A review of the impacts of feedstock and pretreatment on the yield and product distribution of fast pyrolysis bio-oils and vapors. Green Chem. 2014, 16, 384–406. [Google Scholar] [CrossRef]
- Shen, D.; Jin, W.; Hu, J.; Xiao, R.; Luo, K. An overview on fast pyrolysis of the main constituents in lignocellulosic biomass to valued-added chemicals: Structures, pathways and interactions. Renew. Sustain. Energy Rev. 2015, 51, 761–774. [Google Scholar] [CrossRef]
- Luo, H.; Omar, A.M.M. Lignin extraction and catalytic upgrading from genetically modified poplar. Green Chem. 2017, 20, 745–753. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Ojo, O.S.; Tran, F.; Westwood, N.J. Isolation of Functionalized Phenolic Monomers through Selective Oxidation and C−O Bond Cleavage of the β-O-4 Linkages in Lignin. Angew. Chem. 2015, 127, 260–264. [Google Scholar] [CrossRef]
- Vardon, D.R.; Franden, M.A.; Johnson, C.W.; Karp, E.M.; Guarnieri, M.T.; Linger, J.G.; Salm, M.J.; Strathmann, T.J.; Beckham, G.T. Adipic acid production from lignin. Energy Environ. Sci. 2015, 689, 617–628. [Google Scholar] [CrossRef]
- Cheng, C.; Truong, J.; Barrett, J.A.; Shen, D.; Abu-Omar, M.M.; Ford, P.C. Hydrogenolysis of Organosolv Lignin in Ethanol/Isopropanol Media without Added Transition-Metal Catalyst. ACS Sustain. Chem. Eng. 2019, 8, 1023–1030. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Bosch, S.V.D.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fridrich, B.; De Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Kim, K.H.; Dutta, T.; Sun, J.; Simmons, B.; Singh, S. Biomass pretreatment using deep eutectic solvents from lignin derived phenols. Green Chem. 2018, 20, 809–815. [Google Scholar] [CrossRef]
- Si, X.; Lu, F.; Chen, J.; Lu, R.; Huang, Q.; Jiang, H.; Taarning, E.; Xu, J. A strategy for generating high-quality cellulose and lignin simultaneously from woody biomass. Green Chem. 2017, 19, 4849–4857. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.S.; Ghodake, G.S.; Kumar, G.; Oh, M.K.; Saratale, G.D. Combined effect of inorganic salts with calcium peroxide pretreatment for kenaf core biomass and their utilization for 2,3-butanediol production. Bioresour. Technol. 2018, 258, 26–32. [Google Scholar] [CrossRef] [PubMed]
- G.v. research, Cresol Market Size, Share & Trends Analysis Report by Product (Meta, Para, Ortho), By Region (North America, Asia Pacific, Europe, Central & South America, Middle East & Africa), and Segment Forecasts. Available online: https://www.grandviewresearch.com/industry-analysis/cresol-market (accessed on 10 November 2020).
- Wang, S.H. Pharmaceutical Composition Containing Guaiacol Derivatives and Syringol Derivatives Extracted from Natural Plant Vinegar. U.S. Patent Application No 10/578,583, 22 March 2007. [Google Scholar]
- Galiwango, E.; Marzuoqi, A.A.H.; Khaleel, A.A.; Omar, A.M.M. Investigation of Non-Isothermal Kinetics and Thermody-namic Parameters for the Pyrolysis of Different Date Palm Parts. Energies 2020, 13, 6553. [Google Scholar] [CrossRef]
- Azam, M.T.; Ahmad, A. Date Palm Waste: An Efficient Source for Production of Glucose and Lactic Acid. Sustain. Agric. Rev. 2019, 34, 155–178. [Google Scholar] [CrossRef]
- Bensidhom, G.; Trabelsi, B.H.A.; Alper, K.; Sghairoun, M.; Zaafouri, K.; Trabelsi, I. Pyrolysis of Date palm waste in a fixed-bed reactor: Characterization of pyrolytic products. Bioresour. Technol. 2018, 247, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Mallaki, M.; Fatehi, R. Design of a biomass power plant for burning date palm waste to cogenerate electricity and distilled water. Renew. Energy 2014, 63, 286–291. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Ajumobi, O.; Dzidzienyo, P. Pyrolysis of date palm waste to biochar using concentrated solar thermal energy: Economic and sustainability implications. Waste Manag. 2019, 93, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhong, P.; Li, Z.; Cui, X.; Zheng, H. Surface Structure and Catalytic Performance of Ni-Fe Catalyst for Low-Temperature CO Hydrogenation. J. Chem. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, Y.S.; Cha, J.Y.; Jo, Y.S.; Jeong, H.; Sohn, H.; Yoon, C.W.; Kim, Y.; Kim, K.B.; Nam, S.W. Development of porous nickel catalysts by low-temperature Ni–Al chemical alloying and post selective Al leaching, and their application for ammonia decomposition. Int. J. Hydrogen Energy 2020, 45, 19181–19191. [Google Scholar] [CrossRef]
- Ly, H.V.; Galiwango, E.; Kim, S.S.; Kim, J.; Choi, J.H.; Woo, H.C.; Othman, M.R. Hydrodeoxygenation of 2-furyl methyl ketone as a model compound of algal Saccharina Japonica bio-oil using iron phosphide catalyst. Chem. Eng. J. 2017, 317, 302–308. [Google Scholar] [CrossRef]
- Ly, H.V.; Kim, J.; Hwang, H.T.; Choi, J.H.; Woo, H.C.; Kim, S.S. Catalytic Hydrodeoxygenation of Fast Pyrolysis Bio-Oil from Saccharina japonica Alga for Bio-Oil Upgrading. Catalysts 2019, 9, 1043. [Google Scholar] [CrossRef]
- Oyama, S.; Lee, Y. The active site of nickel phosphide catalysts for the hydrodesulfurization of 4,6-DMDBT. J. Catal. 2008, 258, 393–400. [Google Scholar] [CrossRef]
- Winoto, H.P.; Ahn, B.S.; Jae, J. Production of γ-valerolactone from furfural by a single-step process using Sn-Al-Beta zeolites: Optimizing the catalyst acid properties and process conditions. J. Ind. Eng. Chem. 2016, 40, 62–71. [Google Scholar] [CrossRef]
- Wu, S.K.; Lai, P.C.; Lin, Y.C.; Wan, H.P.; Lee, H.T.; Chang, Y.H. Atmospheric Hydrodeoxygenation of Guaiacol over Alumina-, Zirconia-, and Silica-Supported Nickel Phosphide Catalysts. ACS Sustain. Chem. Eng. 2013, 1, 349–358. [Google Scholar] [CrossRef]
- Wang, X.; Clark, P.; Oyama, S. Synthesis, Characterization, and Hydrotreating Activity of Several Iron Group Transition Metal Phosphides. J. Catal. 2002, 208, 321–331. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Chen, G.; He, Z. Highly Loaded and Dispersed Ni2P/Al2O3 Catalyst with High Selectivity for Hydrogenation of Acetophenone. Catalysts 2018, 8, 309. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Z. Rationally Designed Functional Ni2P Nanoparticles as Co–Catalyst Modified CdS@ g-C3N4 Heterojunction for Efficient Photocatalytic Hydrogen Evolution. Chem. Sel. 2019, 4, 3602–3610. [Google Scholar]
- Choi, J.G.; Choi, H.K.; Jung, M.K.; Oh, H.G.; Choi, J.O. Characterization of supported cobalt catalysts by TPR and TPD. J. Ind. Eng. Chem. 1997, 3, 235–246. [Google Scholar]
- Yuan, Y.; Zhang, J.; Chen, H.; Hou, Q.; Shen, J. Preparation of Fe2P/Al2O3 and FeP/Al2O3 catalysts for the hydrotreating reac-tions. J. Energy Chem. 2019, 29, 116–121. [Google Scholar] [CrossRef]
- Usman, U.; Kubota, T.; Okamoto, Y. The Effects of Boron Addition and Presulfidation Temperature on the HDS Activity of a Co-Mos2/Al2O3 Catalyst. Indones. J. Chem. 2005, 5, 77–82. [Google Scholar] [CrossRef]
- Liu, C.; Shih, K.; Gao, Y.; Li, F.; Wei, L. Dechlorinating transformation of propachlor through nucleophilic substitution by dithionite on the surface of alumina. J. Soils Sediments 2012, 12, 724–733. [Google Scholar] [CrossRef]
- Djebaili, K.; Mekhalif, Z.; Boumaza, A.; Djelloul, A. XPS, FTIR, EDX, and XRD Analysis of Al2O3 Scales Grown on PM2000 Alloy. J. Spectrosc. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Peng, W.; Roy, P.; Favaro, L.; Amzallag, E.; Brubach, J.; Congeduti, A.; Cestelli, G.M.; Huntz, A.; Barros, J.; Tétot, R. Experimental and ab initio study of vibrational modes of stressed alumina films formed by oxidation of aluminium alloys under different atmospheres. Acta Mater. 2011, 59, 2723–2730. [Google Scholar] [CrossRef]
- Ramírez, P.J.; Mul, G.; Moulijn, J. In situ Fourier transform infrared and laser Raman spectroscopic study of the thermal decomposition of Co–Al and Ni–Al hydrotalcites. Vib. Spectrosc. 2001, 27, 75–88. [Google Scholar] [CrossRef]
- Kong, X.; Zheng, R.; Zhu, Y.; Ding, G.; Zhu, Y.; Li, Y.W. Rational design of Ni-based catalysts derived from hydrotalcite for se-lective hydrogenation of 5-hydroxymethylfurfural. Green Chem. 2015, 17, 2504–2514. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Accounts Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Su, S.; Liu, Y.; Ren, X.; Guo, W. Enhanced catalytic activity of MIL-101(Fe) with coordinatively unsaturated sites for activating persulfate to degrade organic pollutants. Environ. Sci. Pollut. Res. 2020, 27, 17194–17204. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Wu, J.; Chen, S. From lignocellulosic biomass to renewable cycloalkanes for jet fuels. Green Chem. 2015, 17, 4736–4747. [Google Scholar] [CrossRef]
- Ly, H.V.; Choi, J.H.; Woo, H.C.; Kim, S.S.; Kim, J. Upgrading bio-oil by catalytic fast pyrolysis of acid-washed Saccharina japonica alga in a fluidized-bed reactor. Renew. Energy 2019, 133, 11–22. [Google Scholar] [CrossRef]
- Shamanaev, I.V.; Deliy, I.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Bukhtiyarova, G.A. Enhancement of HDO Activity of MoP/SiO2 Catalyst in Physical Mixture with Alumina or Zeolites. Catalysts 2019, 10, 45. [Google Scholar] [CrossRef]
- Lv, X.; Hu, Z.; Zhao, H.; Liu, Y.; Yuan, Z. Self-supporting transition metal phosphides as electrocatalysts for hydrogen evolution reaction. Prog. Chem. 2018, 30, 947–957. [Google Scholar]
- Klein, I.; Saha, B.; Omar, A.M.M. Lignin depolymerization over Ni/C catalyst in methanol, a continuation: Effect of substrate and catalyst loading. Catal. Sci. Technol. 2015, 5, 3242–3245. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, Y.; Qiu, S.; Wang, M.; Ju, C.; Cao, H.; Fang, Y.; Tan, T. Lignin-first biorefinery: A reusable catalyst for lignin de-polymerization and application of lignin oil to jet fuel aromatics and polyurethane feedstock. Sustain. Energy Fuels 2018, 2, 637–647. [Google Scholar] [CrossRef]
- Kim, J.S.; Yu, J.K.; Lee, H.S.; Kim, J.Y.; Kim, Y.C.; Han, J.H.; Oh, I.H.; Rhee, Y.W. Effect of temperature, oxidant and catalyst loading on the performance of direct formic acid fuel cell. Korean J. Chem. Eng. 2005, 22, 661–665. [Google Scholar] [CrossRef]
- Zanuttini, M.; Lago, C.; Querini, C.; Peralta, M. Deoxygenation of m-cresol on Pt/γ-Al2O3 catalysts. Catal. Today 2013, 213, 9–17. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, Y.; Wang, Q.; Hui, K.S.; Wan, Z.; Song, R. Optimization of Catalyst Loading for Porous Copper Fiber Sintered Felts Used in Methanol Steam Reforming Microreactors. Chem. Eng. Technol. 2013, 36, 307–314. [Google Scholar] [CrossRef]
- Margellou, A.; Triantafyllidis, K.S. Catalytic Transfer Hydrogenolysis Reactions for Lignin Valorization to Fuels and Chemicals. Catalysts 2019, 9, 43. [Google Scholar] [CrossRef]
- Luo, H.; Klein, I.M.; Jiang, Y.; Zhu, H.; Liu, B.; Kenttämaa, H.I.; Abu-Omar, M.M. Total Utilization of Miscanthus Biomass, Lignin and Carbohydrates, Using Earth Abundant Nickel Catalyst. ACS Sustain. Chem. Eng. 2016, 4, 2316–2322. [Google Scholar] [CrossRef]
- Matson, T.D.; Barta, K.; Iretskii, A.V.; Ford, P.C. One-Pot Catalytic Conversion of Cellulose and of Woody Biomass Solids to Liquid Fuels. J. Am. Chem. Soc. 2011, 133, 14090–14097. [Google Scholar] [CrossRef]
- Teles, C.A.; de Souza, P.M.; Neto, R.R.C.; Griffin, M.B.; Mukarakate, C.; Orton, K.A.; Resasco, D.E.; Noronha, F.B. Catalytic upgrading of biomass pyrolysis vapors and model compounds using niobia supported Pd catalyst. Appl. Catal. B Environ. 2018, 238, 38–50. [Google Scholar] [CrossRef]
- Marquevich, M.; Czernik, S.; Chornet, E.; Montané, D. Hydrogen from Biomass: Steam Reforming of Model Compounds of Fast-Pyrolysis Oil. Energy Fuels 1999, 13, 1160–1166. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Qian, M.; Zhu, X.; Wu, J.; Chen, S. Enhancement of jet fuel range alkanes from co-feeding of lignocel-lulosic biomass with plastics via tandem catalytic conversions. Appl. Energy 2016, 173, 418–430. [Google Scholar] [CrossRef]
- Case, P.A.; Truong, C.; Wheeler, M.C.; De Sisto, W.J. Calcium-catalyzed pyrolysis of lignocellulosic biomass components. Bioresour. Technol. 2015, 192, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Erhard, A. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Toronto, BC, Canada, 2000; pp. 1–10. [Google Scholar]
- Barta, K.; Matson, T.D.; Fettig, M.L.; Scott, S.L.; Iretskii, A.V.; Ford, P.C. Catalytic disassembly of an organosolv lignin via hydrogen transfer from supercritical methanol. Green Chem. 2010, 12, 1640–1647. [Google Scholar] [CrossRef]
- Ouyang, X.; Huang, X.; Zhu, Y.; Qiu, X. Ethanol-Enhanced Liquefaction of Lignin with Formic Acid as an in Situ Hydrogen Donor. Energy Fuels 2015, 29, 5835–5840. [Google Scholar] [CrossRef]
- Azadi, P.; Farnood, R. Review of heterogeneous catalysts for sub- and supercritical water gasification of biomass and wastes. Int. J. Hydrogen Energy 2011, 36, 9529–9541. [Google Scholar] [CrossRef]
- Kozuch, S.; Martin, J.M.L. “Turning over” definitions in catalytic cycles. ACS Publ. 2012, 12, 2787–2794. [Google Scholar] [CrossRef]
- Hussaini, A.L.; Launay, F.; Galvez, E. Vanadium-Substituted Phosphomolybdic Acids for the Aerobic Cleavage of Lignin Models—Mechanistic Aspect and Extension to Lignin. Materials 2020, 13, 812. [Google Scholar] [CrossRef]
- Dence, C.W. The Determination of Lignin. In Methods in Lignin Chemistry; Springer: Berlin/Heidelberg, Germany, 1992; pp. 33–61. [Google Scholar]
- Galiwango, E.; Marzouqi, A.A.H. Investigation of Nonisothermal Combustion Kinetics of Isolated Lignocellulosic Biomass: A Case Study of Cellulose from Date Palm Biomass Waste; IntechOpen: London, UK, 2020. [Google Scholar]
- Galiwango, E.; Rahman, N.S.A.; Marzouqi, A.A.H.; Omar, A.M.M.; Khaleel, A.A. Isolation and characterization of cellulose and α-cellulose from date palm biomass waste. Heliyon 2019, 5, 2937. [Google Scholar] [CrossRef]
- Prins, R.; Pirngruber, G.; Weber, T. Metal phosphides and zeolite-like mesoporous materials as catalysts. Chimia 2001, 55, 791–795. [Google Scholar]
- Whiffen, V.M.L.; Smith, K.J. The Effect of Calcination Temperature on the Properties and Hydrodeoxygenation Activity of Ni2P Catalysts Prepared Using Citric Acid. In ACS Symposium Series; American Chemical Society (ACS): Washington, DC, USA, 2013; pp. 287–300. [Google Scholar]

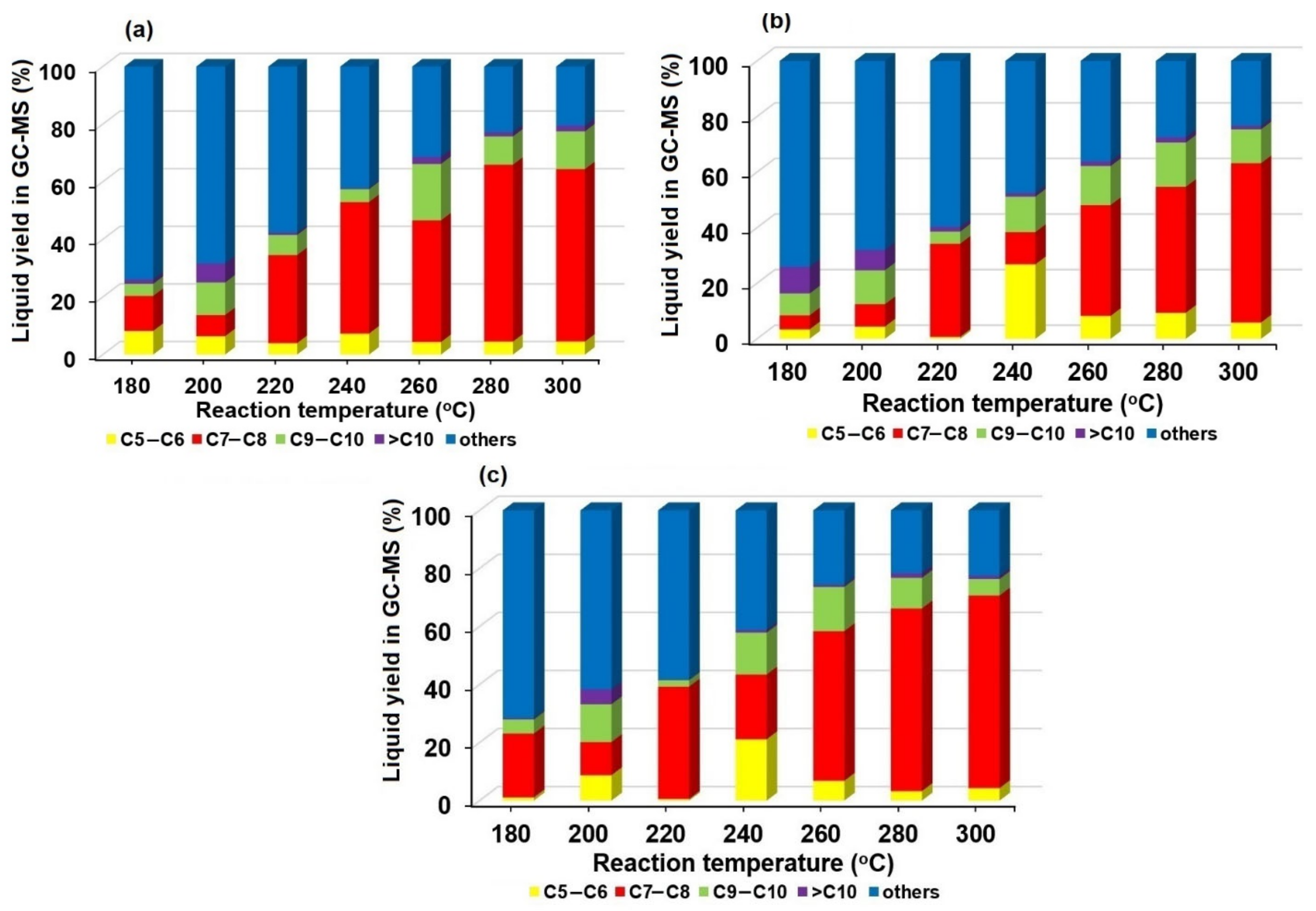

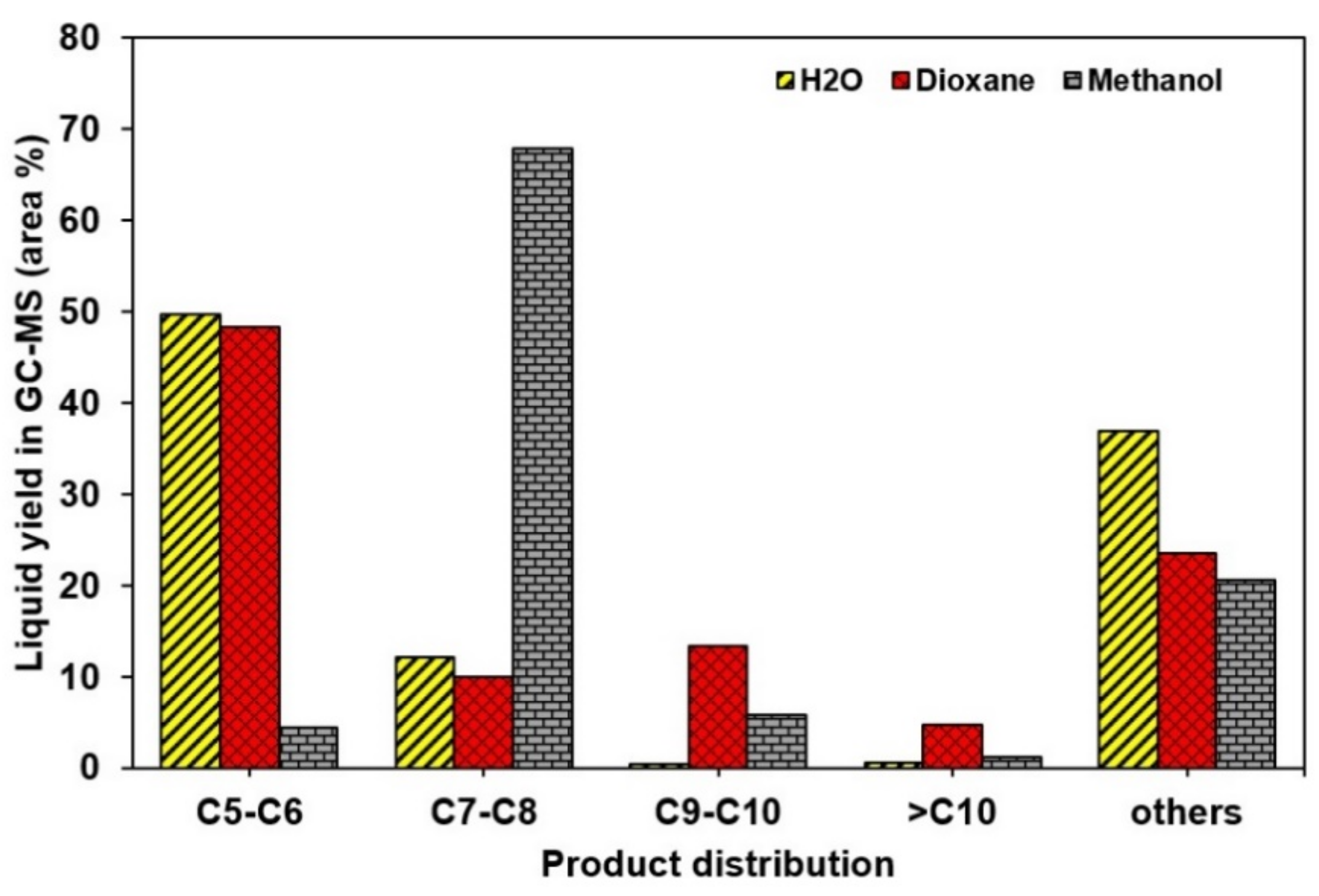
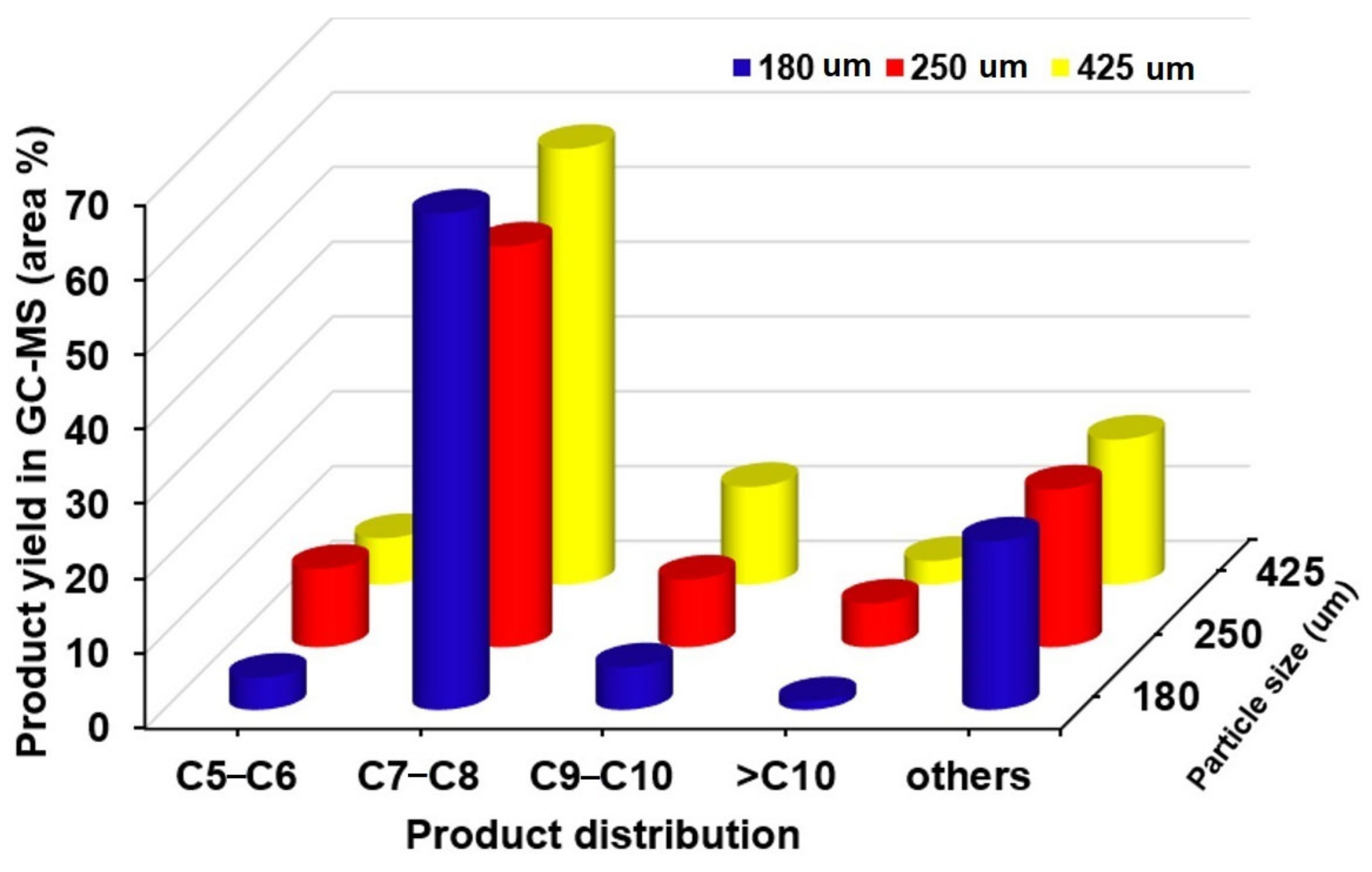
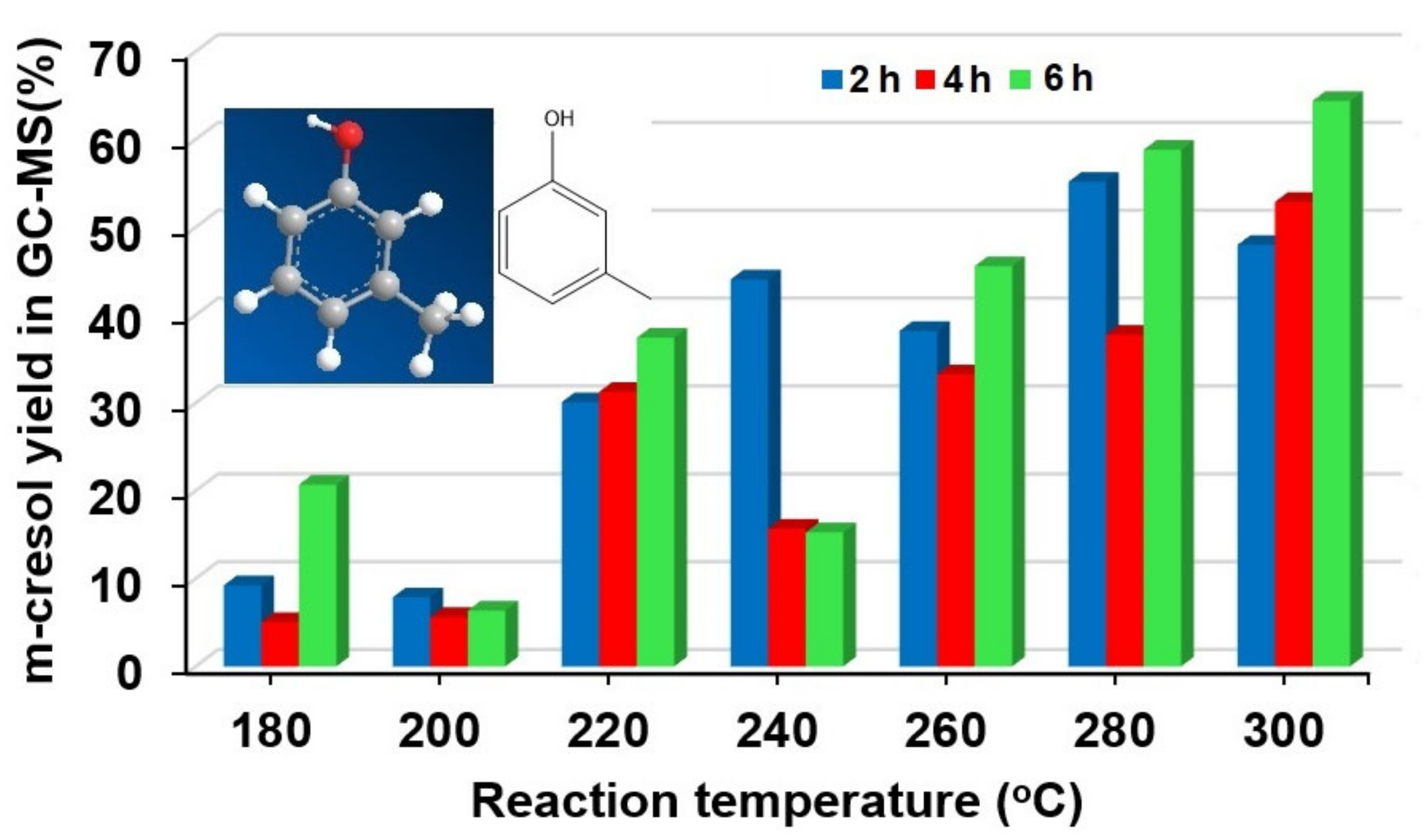
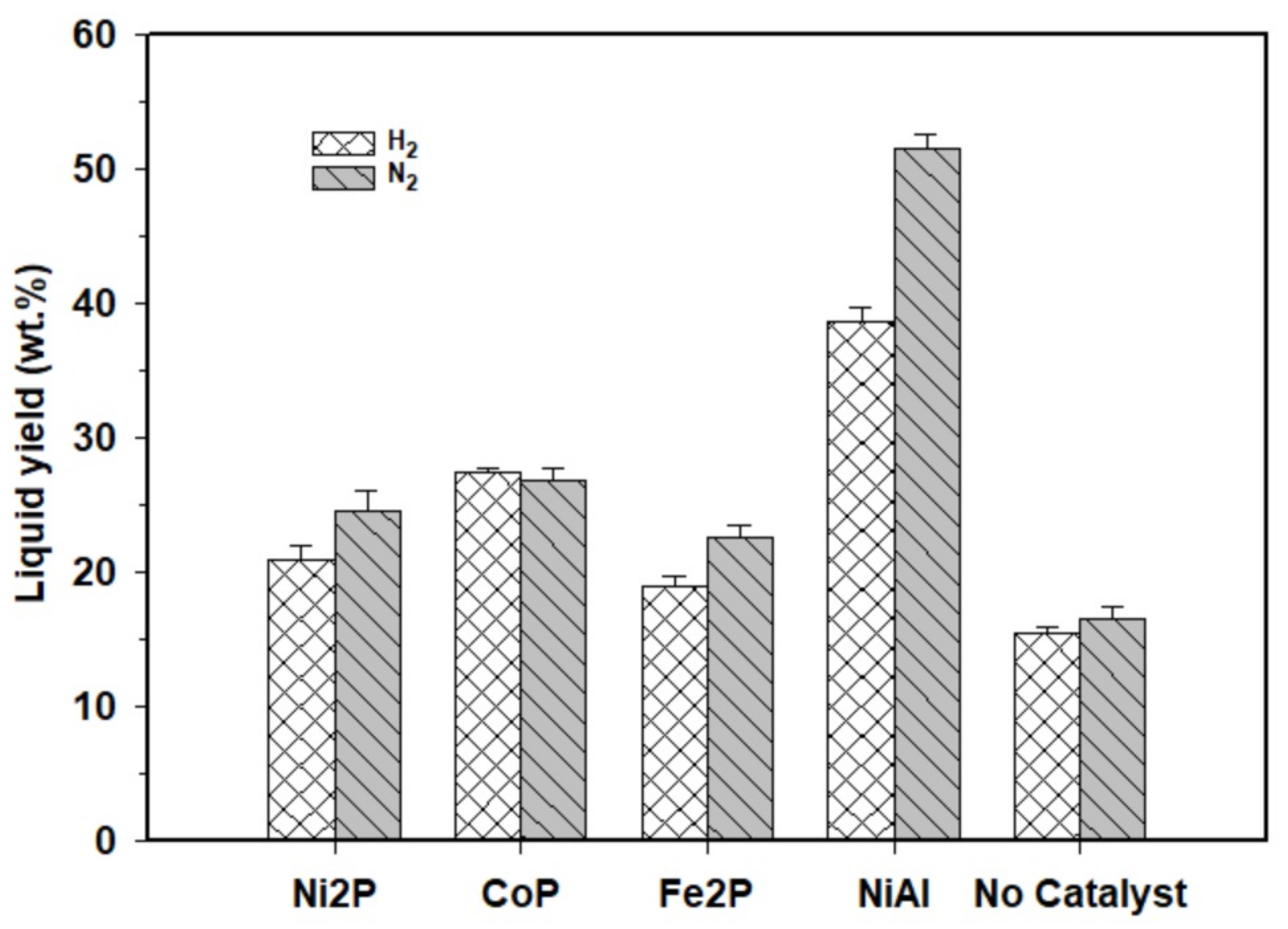
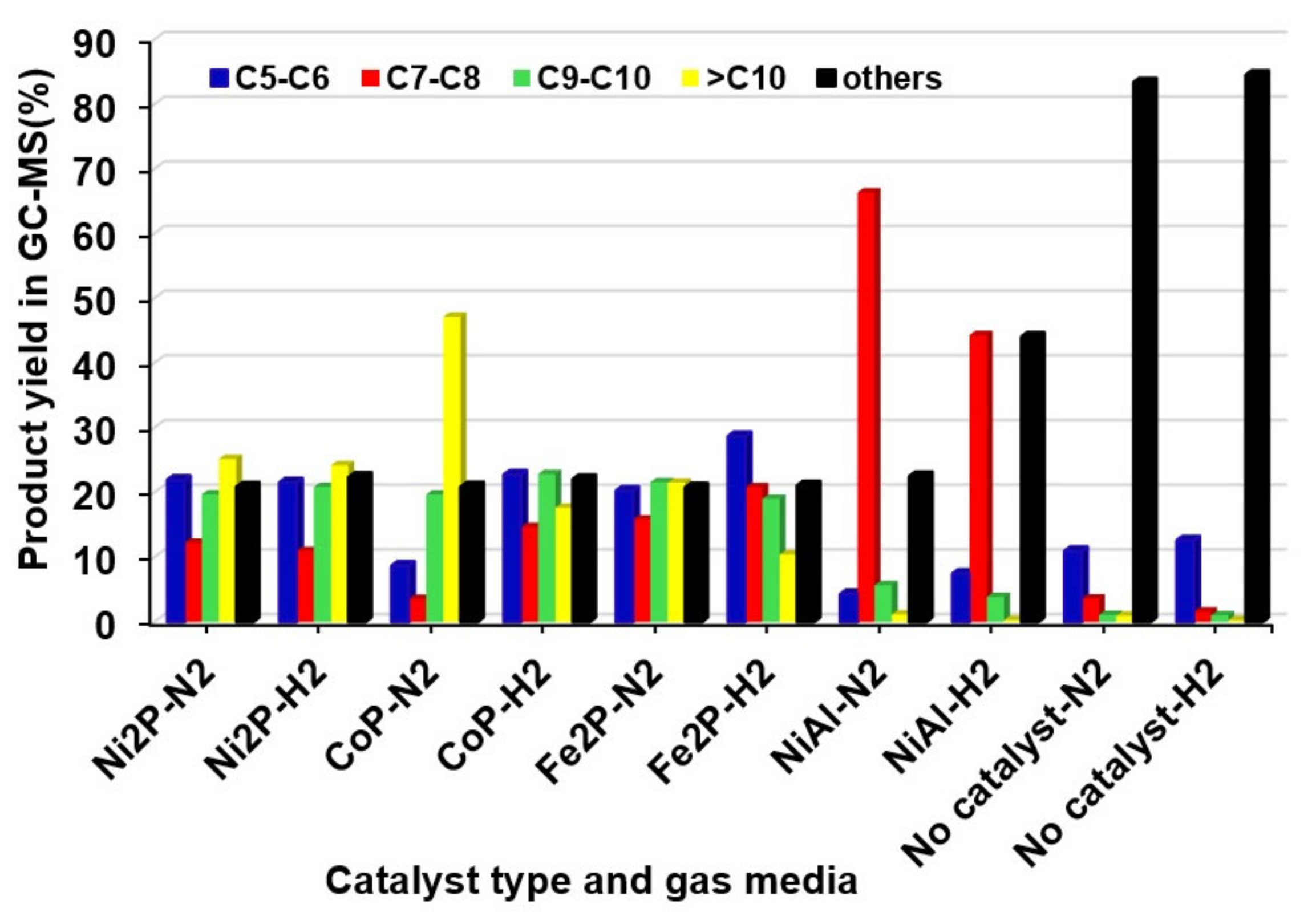
| Catalysts | NiAl Alloy | Ni2P/ɣ-Al2O3 | CoP/ɣ-Al2O3 | Fe2P/ɣ-Al2O3 | Without Catalyst | |
|---|---|---|---|---|---|---|
| Product yield (wt. %) | Solid | 14.1 ± 0.6 | 22.4 ± 0.7 | 23.5 ± 0.4 | 18.8 ± 0.7 | 32.0 ± 0.2 |
| Liquid | 51.5 ± 1.0 | 24.6 ± 1.5 | 26.9 ± 0.9 | 22.6 ± 1.0 | 16.6 ± 0.4 | |
| Gas | 34.4 ± 0.4 | 53.0 ± 0.2 | 49.6 ± 0.2 | 58.6 ± 0.2 | 51.4 ± 0.2 | |
| Catalyst | Solvent | T [°C] | PN2 after Reaction [bar] | Time [h] | Conversion * [%] | pH | Compound Name and Formula | Liquid selectivity GC-MS [%] | Liquid Yield [%] | Rate ** [h−1] |
|---|---|---|---|---|---|---|---|---|---|---|
| NiAl alloy | methanol | 180 | 16.3 | 2 | 26.0 ± 0.5 | 5.42 | m-cresol (C7H8O) | 35.5 | 9.2 ± 0.2 | 2.48 |
| NiAl alloy | methanol | 200 | 25.3 | 2 | 31.6 ± 0.2 | 5.05 | Isoeugenol(C10H12O2) | 24.9 | 7.9 ± 0.1 | 2.82 |
| NiAl alloy | methanol | 220 | 28.4 | 2 | 42.3 ± 1.1 | 5.39 | m-cresol (C7H8O) | 71.1 | 30.1 ± 0.8 | 3.53 |
| NiAl alloy | methanol | 240 | 30.4 | 2 | 57.8 ± 3.6 | 5.69 | m-cresol (C7H8O) | 76.3 | 44.1 ± 2.7 | 5.61 |
| NiAl alloy | methanol | 260 | 33.1 | 2 | 68.6 ± 1.1 | 5.42 | m-cresol (C7H8O) | 55.7 | 38.1 ± 0.6 | 6.56 |
| NiAl alloy | methanol | 280 | 38.3 | 2 | 77.2 ± 0.3 | 5.40 | m-cresol (C7H8O) | 71.5 | 55.2 ± 0.2 | 6.76 |
| NiAl alloy | methanol | 300 | 42.5 | 2 | 79.5 ± 0.4 | 5.47 | m-cresol (C7H8O) | 60.4 | 48.0 ± 0.2 | 7.34 |
| NiAl alloy | methanol | 180 | 16.8 | 4 | 25.9 ± 0.7 | 5.48 | 2,6-dimethoxy-4,2-propenyl phenol, DMPP (C11H14O3) | 19.5 | 5.1 ± 0.1 | 1.26 |
| NiAl alloy | methanol | 200 | 25.7 | 4 | 32.0 ± 0.7 | 5.10 | 2-methoxy-4-propylphenol DHE (C10H14O2) | 17.6 | 5.6 ± 0.1 | 1.52 |
| NiAl alloy | methanol | 220 | 35.7 | 4 | 40.3 ± 1.5 | 5.37 | m-cresol (C7H8O) | 77.6 | 31.3 ± 1.2 | 1.85 |
| NiAl alloy | methanol | 240 | 46.3 | 4 | 52.4 ± 0.4 | 5.60 | Phenol (C6H6O) | 29.9 | 15.7 ± 0.1 | 2.50 |
| NiAl alloy | methanol | 260 | 46.5 | 4 | 63.9 ± 0.6 | 5.28 | Phenol (C6H6O) | 26.8 | 17.1 ± 0.2 | 3.06 |
| NiAl alloy | methanol | 280 | 44.1 | 4 | 72.5 ± 0.7 | 5.33 | m-cresol (C7H8O) | 52.1 | 37.8 ± 0.4 | 3.43 |
| NiAl alloy | methanol | 300 | 40.6 | 4 | 76.7 ± 0.5 | 5.31 | m-cresol (C7H8O) | 69.0 | 52.9 ± 0.4 | 3.50 |
| NiAl alloy | methanol | 180 | 16.8 | 6 | 28.4 ± 1.6 | 5.45 | m-cresol (C7H8O) | 72.8 | 20.7 ± 1.1 | 0.83 |
| NiAl alloy | methanol | 200 | 24.65 | 6 | 38.3 ± 2.2 | 5.09 | Isoeugenol(C10H12O2) | 16.6 | 6.4 ± 0.4 | 1.21 |
| NiAl alloy | methanol | 220 | 34.3 | 6 | 41.8 ± 2.8 | 5.41 | m-cresol (C7H8O) | 89.6 | 37.4 ± 2.5 | 1.33 |
| NiAl alloy | methanol | 240 | 37.7 | 6 | 58.8 ± 1.9 | 5.65 | m-cresol (C7H8O) | 26.0 | 15.3 ± 0.5 | 1.68 |
| NiAl alloy | methanol | 260 | 49.5 | 6 | 74.4 ± 2.2 | 5.38 | m-cresol (C7H8O) | 61.2 | 45.6 ± 1.4 | 2.33 |
| NiAl alloy | methanol | 280 | 44.6 | 6 | 78.4 ± 1.2 | 5.39 | m-cresol (C7H8O) | 75.1 | 58.8 ± 0.9 | 2.55 |
| NiAl alloy | methanol | 300 | 43.6 | 6 | 77.5 ± 0.5 | 5.68 | m-cresol (C7H8O) | 83.1 | 64.4 ± 0.4 | 2.58 |
| No catalyst | methanol | 300 | 7.5 | 6 | 16.6 ± 1.2 | 3.94 | Phenol (C6H6O) | 50.5 | 8.4 ± 0.6 | - |
| NiAl alloy | 1,4-dioxane | 300 | 55.6 | 6 | 76.4 ± 0.9 | 3.63 | Phenol (C6H6O) | 41.6 | 31.8 ± 0.4 | 2.21 |
| NiAl alloy | Water | 300 | 77.73 | 6 | 63.0 ± 0.6 | 3.51 | Phenol (C6H6O) | 54.0 | 34.00 ± 0.3 | 1.89 |
| Ni2P/ɣ-Al2O3 | methanol | 300 | 140.6 | 6 | 79.1 ± 1.4 | 4.77 | 3,4-diethyl-2,4-hexadienedioic acid, dimethyl ester (HDD-ester) | 20.5 | 16.2 ± 0.3 | 2.56 |
| CoP/ɣ-Al2O3 | methanol | 300 | 136.1 | 6 | 79.0 ± 2.5 | 4.86 | 3,4-diethyl-2,4-hexadienedioic acid, dimethyl ester (HDD-ester) | 22.6 | 17.8 ± 0.6 | 2.57 |
| Fe2P/ɣ-Al2O3 | methanol | 300 | 146.15 | 6 | 79.2 ± 2.1 | 4.82 | Phenol (C6H6O) | 35.9 | 28.4 ± 0.8 | 2.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galiwango, E.; Al-Marzuoqi, A.H.; Khaleel, A.A.; Abu-Omar, M.M. Catalytic Depolymerization of Date Palm Waste to Valuable C5–C12 Compounds. Catalysts 2021, 11, 371. https://doi.org/10.3390/catal11030371
Galiwango E, Al-Marzuoqi AH, Khaleel AA, Abu-Omar MM. Catalytic Depolymerization of Date Palm Waste to Valuable C5–C12 Compounds. Catalysts. 2021; 11(3):371. https://doi.org/10.3390/catal11030371
Chicago/Turabian StyleGaliwango, Emmanuel, Ali H. Al-Marzuoqi, Abbas A. Khaleel, and Mahdi M. Abu-Omar. 2021. "Catalytic Depolymerization of Date Palm Waste to Valuable C5–C12 Compounds" Catalysts 11, no. 3: 371. https://doi.org/10.3390/catal11030371
APA StyleGaliwango, E., Al-Marzuoqi, A. H., Khaleel, A. A., & Abu-Omar, M. M. (2021). Catalytic Depolymerization of Date Palm Waste to Valuable C5–C12 Compounds. Catalysts, 11(3), 371. https://doi.org/10.3390/catal11030371







