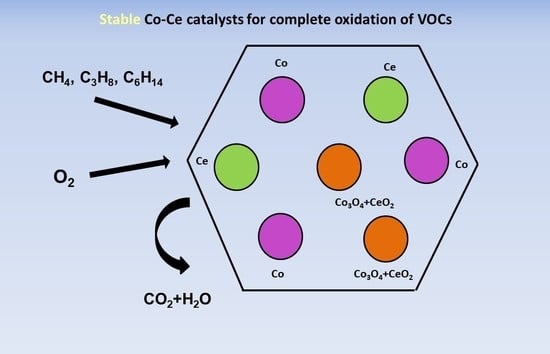
Co–Ce Oxides Supported on SBA-15 for VOCs Oxidation
Abstract
1. Introduction
2. Results and Discussion
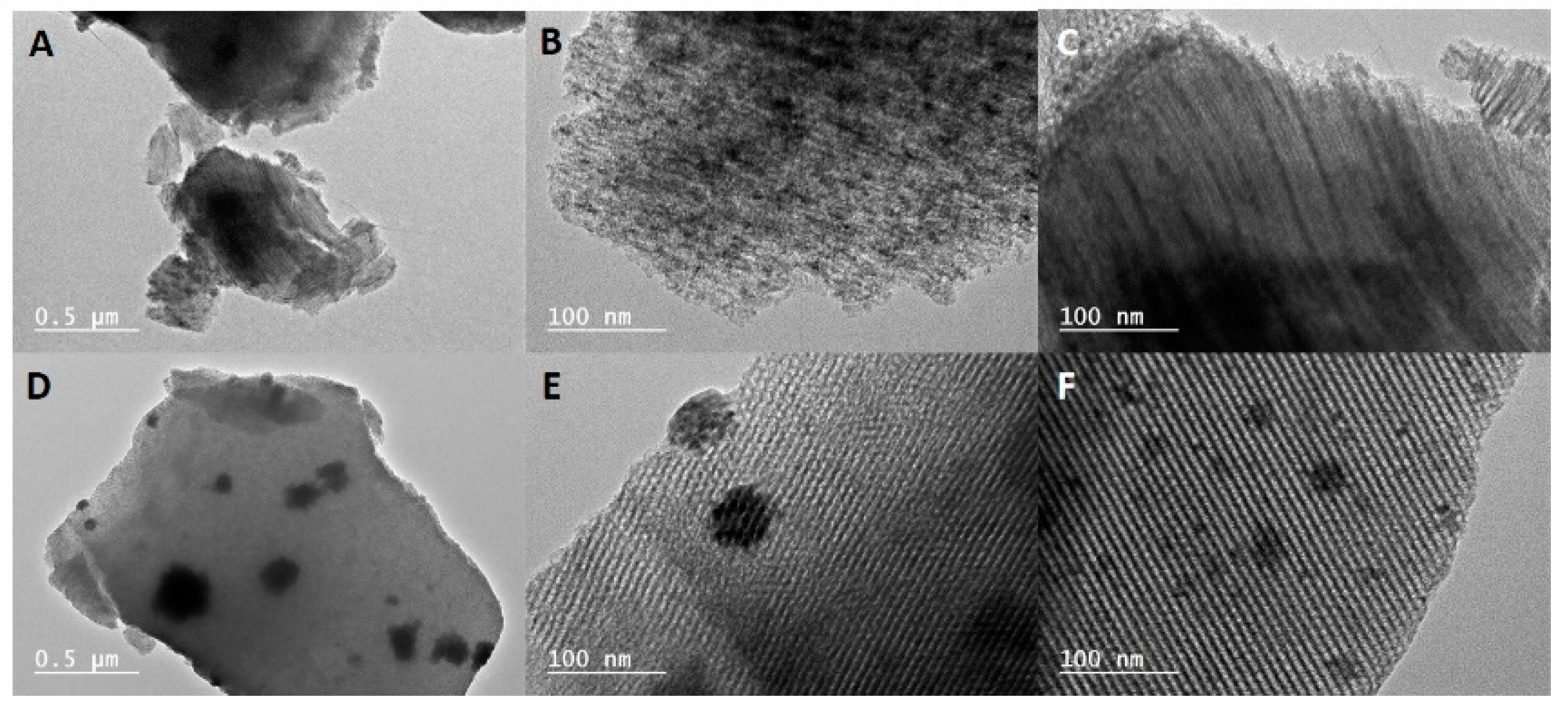
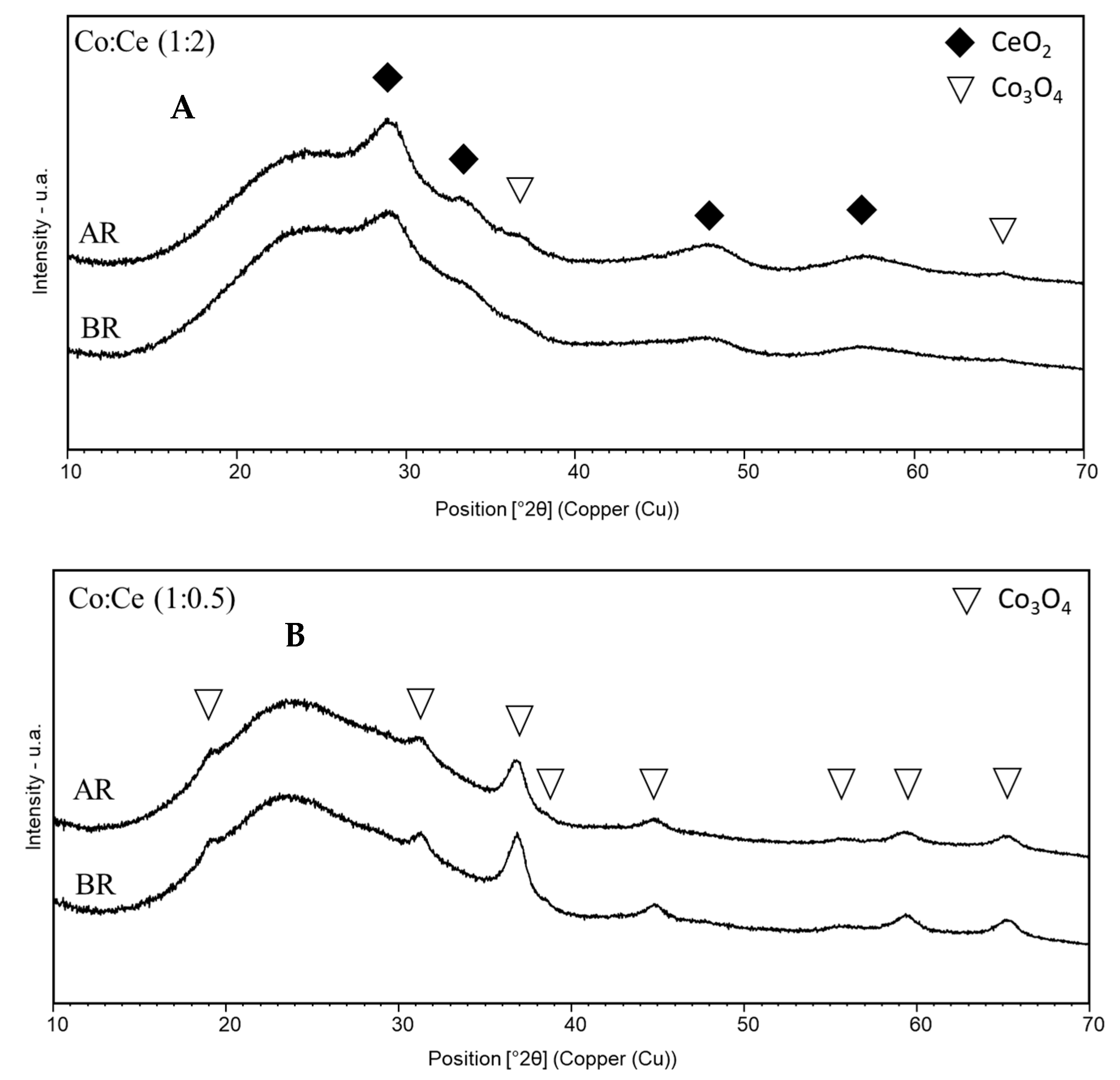
2.1. Characterization of the Mesoporous Catalysts before and after Reaction
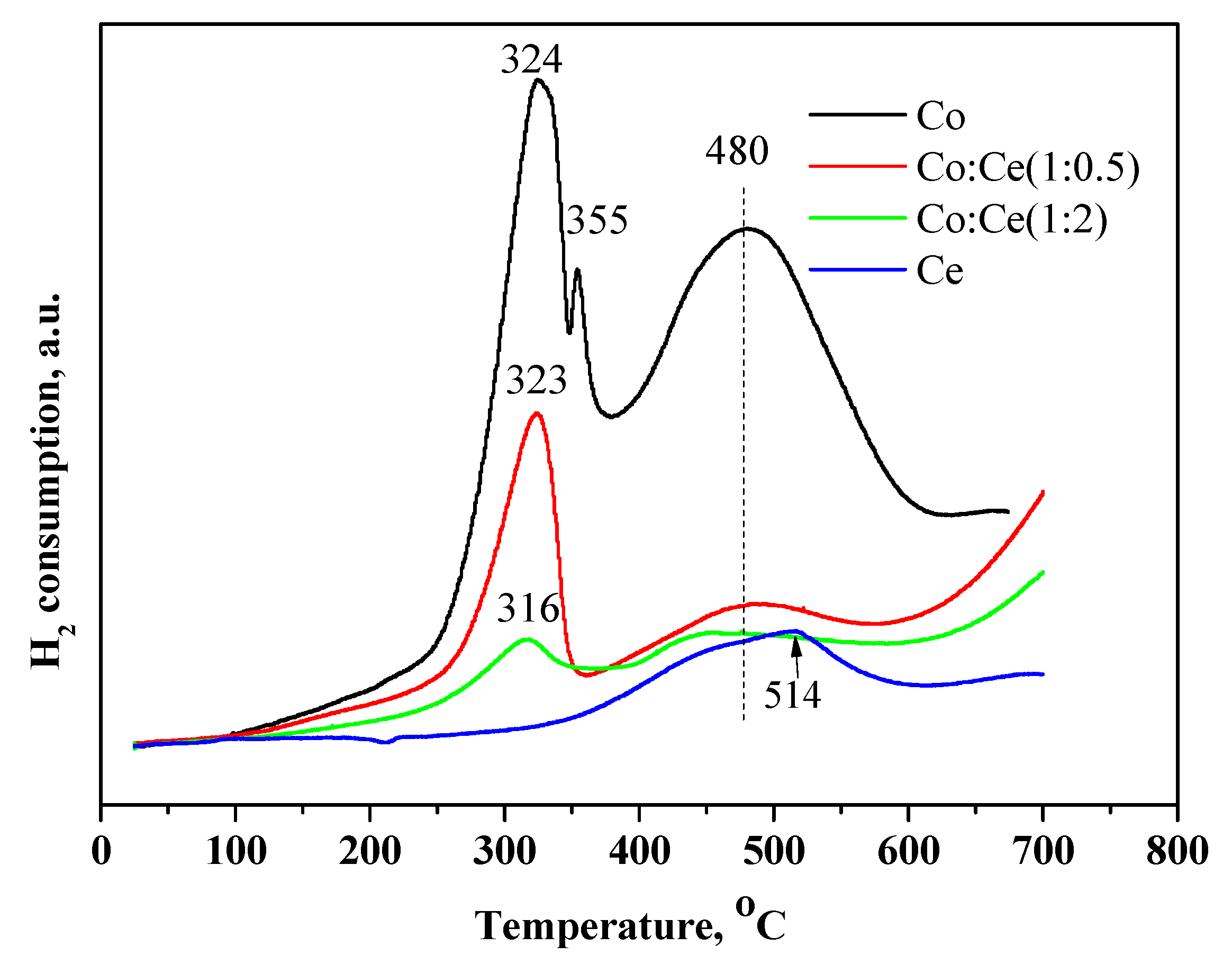
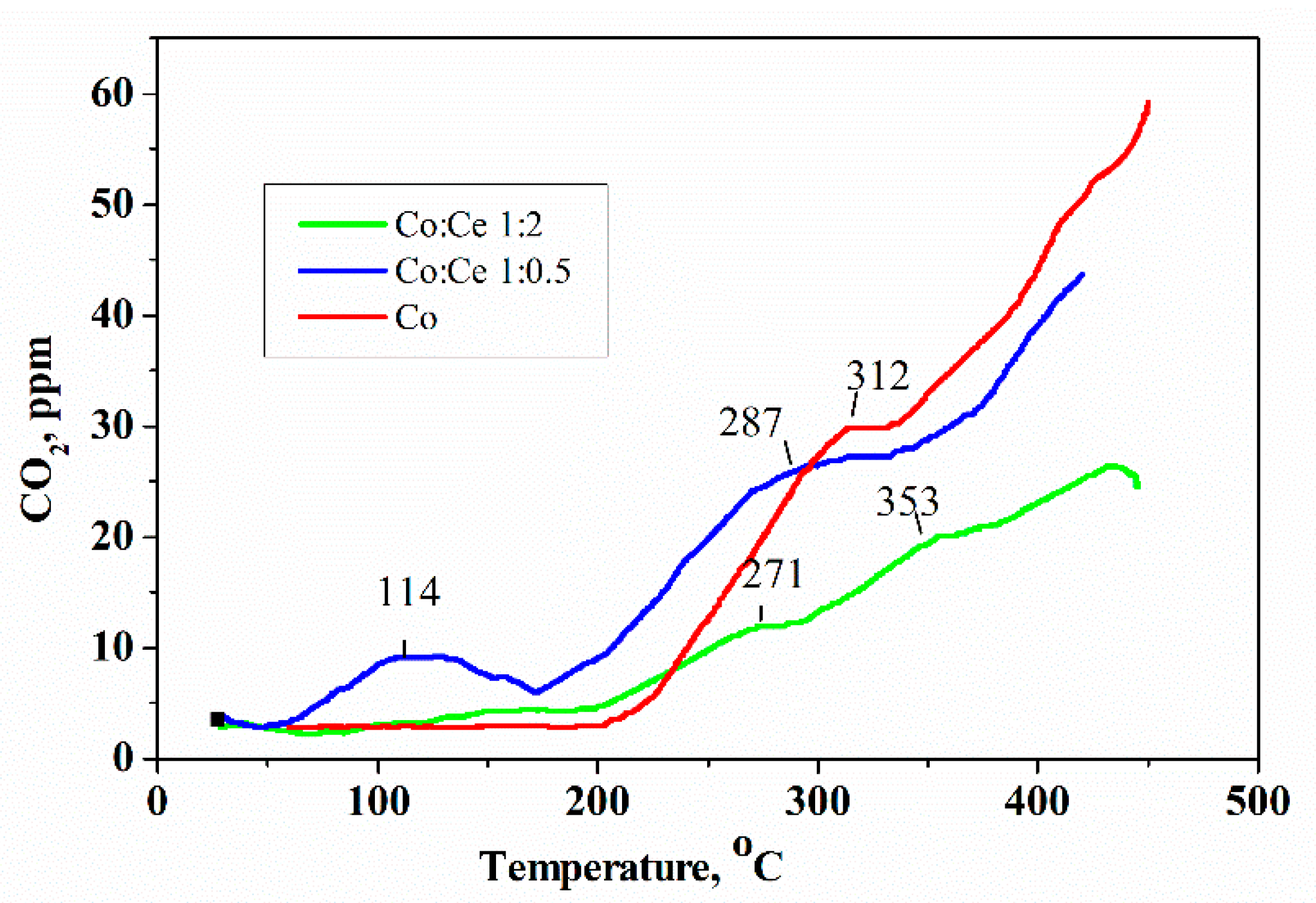
2.2. Characterization of the Supported Metal Species before and after Reaction
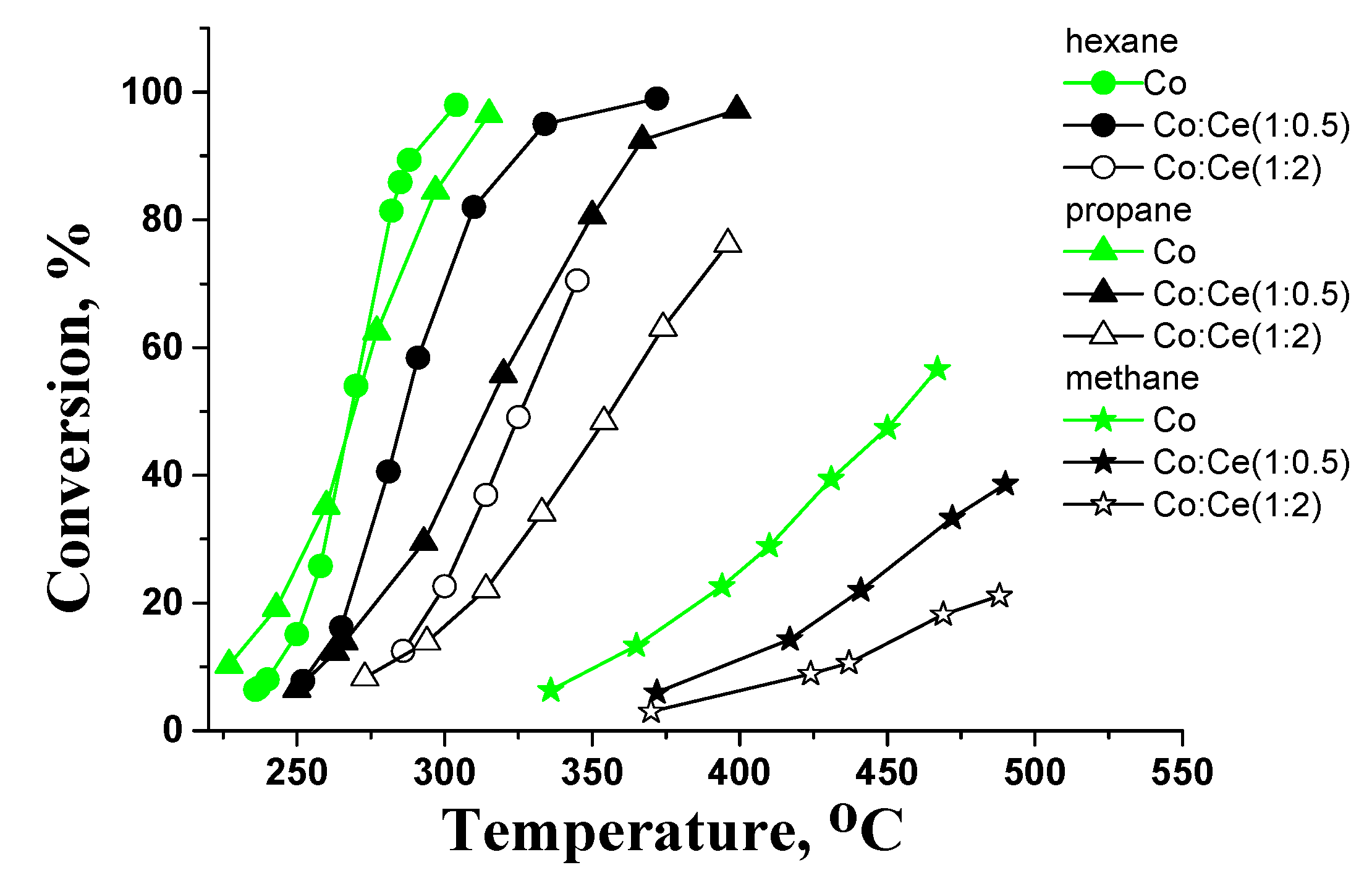
2.3. Catalytic Activity
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Spivey, J.J. Complete catalytic oxidation of volatile organics. Ind. Eng. Chem. Res. 1987, 26, 2165–2180. [Google Scholar] [CrossRef]
- Liotta, L.F.; Wu, H.; Pantaleo, G.; Venezia, A.M. Co3O4 nanocrystals and Co3O4 –MOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: A review. Catal. Sci. Technol. 2013, 3, 3085–3102. [Google Scholar] [CrossRef]
- Leyssensa, L.; Vincka, B.; Van Der Straeten, C.; Wuytse, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemichealth effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Wang, H.F.; Kavanagh, R.; Guo, Y.L.; Guo, Y.; Lu, G.Z.; Hu, P. Origin of extraordinarily high catalytic activity of Co3O4 and its morphological chemistry for CO oxidation at low temperature. J. Catal. 2012, 296, 110–119. [Google Scholar] [CrossRef]
- Abbasi, Z.; Haghighi, M.; Fatehifar, E.; Saedy, S. Synthesis and physicochemical characterizations of nanostructured Pt/Al2O3–CeO2 catalysts for total oxidation of VOCs. J. Hazard. Mater. 2011, 186, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Todorova, S.; Blin, J.L.; Naydenov, A.; Lebeau, B.; Kolev, H.; Gaudin, P.; Dotzeva, A.; Velinova, R.; Filkova, D.; Ivanova, I.; et al. Co3O4-MnOx oxides supported on SBA-15 for CO and VOCs oxidation. Catal. Today 2020, 357, 602–612. [Google Scholar] [CrossRef]
- Todorova, S.; Kadinov, G.; Tenchev, K.; Caballero, A.; Holgado, J.P.; Pereňiguez, R. Co3O4 + CeO2/SiO2 catalysts for n-hexane and CO oxidation. Catal. Lett. 2009, 155, 129–149. [Google Scholar] [CrossRef]
- Todorova, S.; Naydenov, A.; Kolev, H.; Tenchev, K.; Ivanov, G.; Kadinov, G. Effect of Co and Ce on silica supported manganese catalysts in the reactions of complete oxidation of n-hexane and ethyl acetate. J. Mater. Sci. 2011, 46, 7152–7159. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Galarneau, A.; Cambon, H.; Renzo, F.D.; Ryoo, R.; Choi, M.; Fajula, F. Microporosity and connections between pores in SBA-15 mesostructured silicas as a function of the temperature of synthesis. New J. Chem. 2003, 27, 73–79. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Adsorption Study of Surface and Structural Properties of MCM-41 Materials of Different Pore Sizes. J. Phys. Chem. B 1997, 101, 583–589. [Google Scholar] [CrossRef]
- Dang-Bao, T.; Anh, N.P.; Phuong, P.H.; Van, N.T.T.; Nghia, T.N.D.; Tien, H.V.; Tri, N. Fabrication of cobalt-doped ceria nanorods for p-xylene deep oxidation: Effects of cobalt precursor and loading. Mater. Transact. 2020, 61, 1294–1300. [Google Scholar] [CrossRef]
- Larachi, F.; Pierre, J.; Adnot, A.; Bernis, A. 3d XPS study of composite CexMn1−xO2−y wet oxidation catalysts. Appl. Surf. Sci. 2002, 195, 236–250. [Google Scholar] [CrossRef]
- Matolín, V.; Cabala, M.; Cháb, V.; Matolínová, I.; Prince, K.C.; Škoda, M.; Šutara, F.; Skála, T.; Veltruská, K. A resonant photoelectron spectroscopy study of Sn(Ox) doped CeO2 catalysts. Surf. Interface Anal. 2008, 40, 225–230. [Google Scholar] [CrossRef]
- Bensalem, A.; Bozon-Verduraz, F.; Delamar, M.; Bugli, G. Preparation and characterization of highly dispersed silica-supported ceria. Appl. Catal. 1995, 121, 81–93. [Google Scholar] [CrossRef]
- Pu, Y.; Xuan, K.; Wang, F.; Li, A.; Zhao, N.; Xiao, F. Synthesis of dimethyl carbonate from CO2 and methanol over a hydrophobic Ce/SBA-15 catalyst. RSC Adv. 2018, 8, 27216. [Google Scholar] [CrossRef]
- Carrero, A.; Vizcaíno, A.J.; Calles, J.A.; García-Moreno, L. Hydrogen production through glycerol steam reforming using Co catalysts supported on SBA-15 doped with Zr, Ce and La. J. Ener. Chem. 2017, 26, 42–48. [Google Scholar] [CrossRef]
- Huang, F.; Chen, C.; Wang, F.; Wang, B.; Zhang, L.; Lu, S.; Li, K. Effect of calcination temperature on the catalytic oxidation of formaldehyde over Co3O4–CeO2 catalysts. Catal. Surv. Asia 2017, 21, 143–149. [Google Scholar] [CrossRef]
- González, O.; Pérez, H.; Navarro, P.; Almeida, L.C.; Pacheco, J.G.; Montes, M. Use of different mesostructured materials based on silica as cobalt supports for the Fischer–Tropsch synthesis. Catal. Today 2009, 148, 140–147. [Google Scholar] [CrossRef]
- Vizcaino, A.J.; Carrero, A.; Calles, J.A. Comparison of ethanol steam reforming using Co and Ni catalysts supported on SBA-15 modified by Ca and Mg. Fuel Process. Technol. 2016, 146, 99–109. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, J.; Yuan, J.; Cai, T.; Xiao, B.; Liu, Z.; Zhao, K.; Yang, L.; He, D. Excellent low-temperature catalytic performance of nanosheet Co-Mn oxides for total benzene oxidation. Appl. Catal. A Gen. 2018, 566, 104–112. [Google Scholar] [CrossRef]
- Liu, C.-X.; Liu, Q.; Huang, X.-G.; Nie, X.-L.; Huang, Z. Identification of the active sites for low temperature CO oxidation over nanocrystalline Co3O4 catalysts. J. Chin. Chem. Soc. 2014, 61, 490–494. [Google Scholar] [CrossRef]
- Song, W.; Poyraz, A.S.; Meng, Y.; Ren, Z.; Chen, S.-Y.; Suib, S.L. Mesoporous Co3O4 with controlled porosity: Inverse micelle synthesis and high-performance catalytic CO oxidation at −60 °C. Chem. Mater. 2014, 26, 4629–4639. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.-C.; Chen, M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Dry citrate-precursor synthesized nanocrystalline cobalt oxide as highly active catalyst for total oxidation of propane. J. Catal. 2009, 263, 104–113. [Google Scholar] [CrossRef]
- Finocchio, E.; Busca, G.; Lorenzelli, V.; Escribano, V.S. FTIR studies on the selective oxidation and combustion of light hydrocarbons at metal oxide surfaces. Part 2—Propane and propene oxidation on Co3O4. J. Chem. Soc. Faraday Trans. 1996, 92, 1587–1593. [Google Scholar] [CrossRef]
- Solsona, B.; Vázquez, I.; Garcia, T.; Davies, T.E.; Taylor, S.H. Complete oxidation of short chain alkanes using a nanocrystalline cobalt oxide catalyst. Catal. Lett. 2007, 116, 116–121. [Google Scholar] [CrossRef]
- Kniep, B.L.; Ressler, T.; Rabis, A.; Girgsdies, F.; Baenitz, M.; Steglich, F.; Schlögl, R. Rational design of nanostructured copper–zinc oxide catalysts for the steam reforming of methanol. Angew. Chem. Int. Ed. 2004, 43, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Ling, F.; Li, J. Studies on MCM-48 supported cobalt catalyst for Fischer–Tropsch synthesis. J. Mol. Cat. A Chem. 2006, 244, 33–40. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Griboval-Constant, A.; Bechara, R.; Villain, F. Pore-size control of cobalt dispersion and reducibility in mesoporous silicas. J. Phys. Chem. B 2001, 105, 9805–9811. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Meng, M.; Zha, Y.-Q.; Guo, L.-H. Identification of the active sites for CO and C3H8 total oxidation over nanostructured CuO−CeO2 and Co3O4−CeO2 catalysts. J. Phys. Chem. C 2008, 112, 8694–8701. [Google Scholar] [CrossRef]
- Todorova, S.; Blin, J.L.; Naydenov, A.; Lebeau, B.; Karashanova, D.; Kolev, H.; Gaudin, P.; Dotzeva, A.; Velinova, R.; Filkova, D.; et al. Co-Mn oxides supported on hierarchical macro-mesoporous silica for CO and VOCs oxidation. Catal. Today 2021, 361, 94–101. [Google Scholar] [CrossRef]
- Todorova, S.; Yordanova, I.; Naydenov, A.; Kolev, H.; Cherkezova-Zheleva, Z.; Tenchev, K.; Kunev, B. Cobalt-manganese supported oxides as catalysts for complete n-hexane and methane oxidation: Relationship between structure and catalytic activity. Rev. Roum. Chim. 2014, 59, 259–265. [Google Scholar]
- Imperor-Clerc, M.; Bazin, D.; Appay, M.-D.; Beaunier, P.; Davidson, A. Crystallization of β-MnO2 nanowires in the pores of SBA-15 silicas: In situ investigation using synchrotron radiation. Chem. Mater. 2004, 16, 1813–1821. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–331. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G. Co3O4/CeO2 composite oxides for methane emissions abatement: Relationship between Co3O4–CeO2 interaction and catalytic activity. Appl. Catal. B 2006, 66, 217–227. [Google Scholar] [CrossRef]
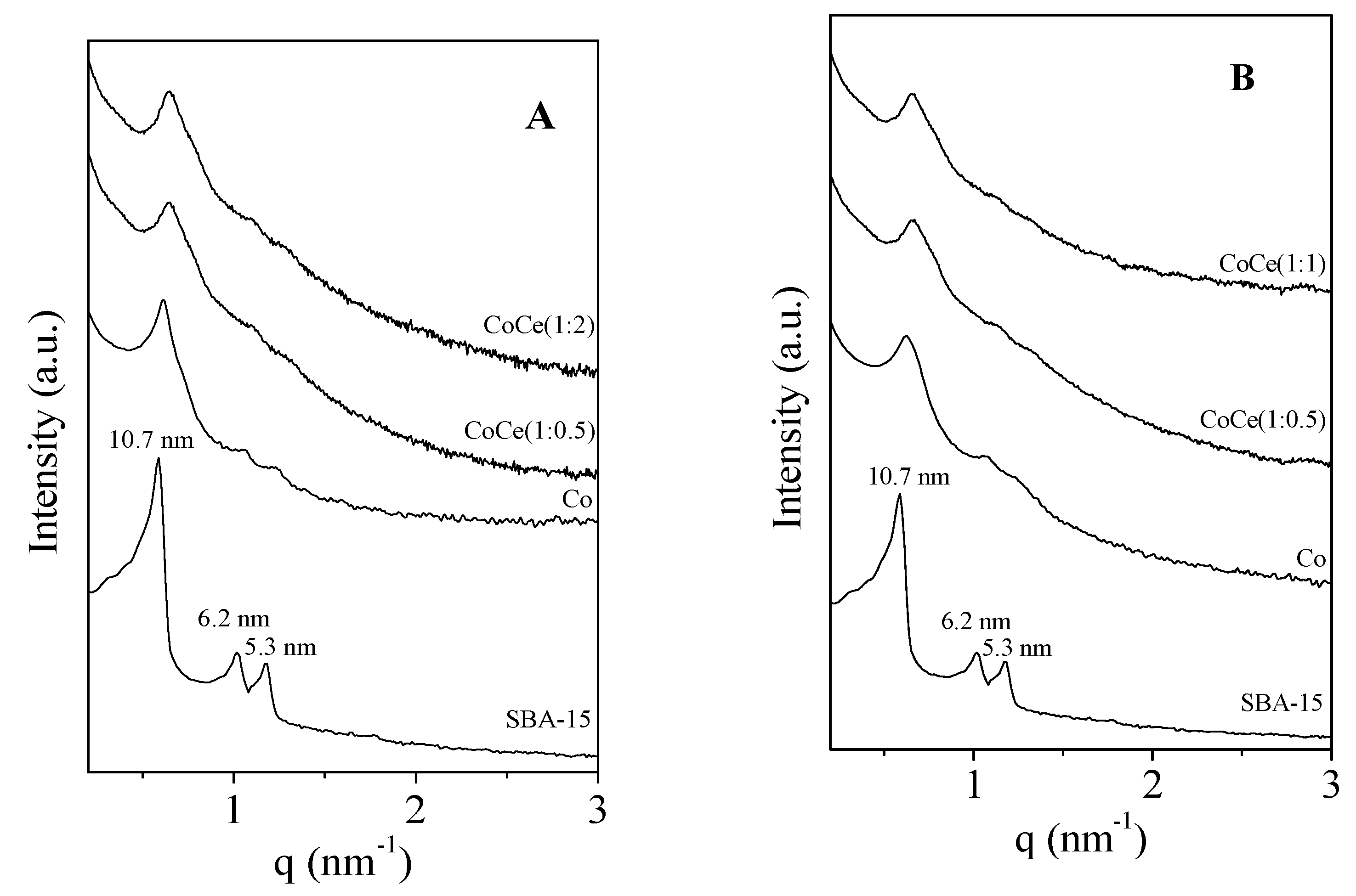
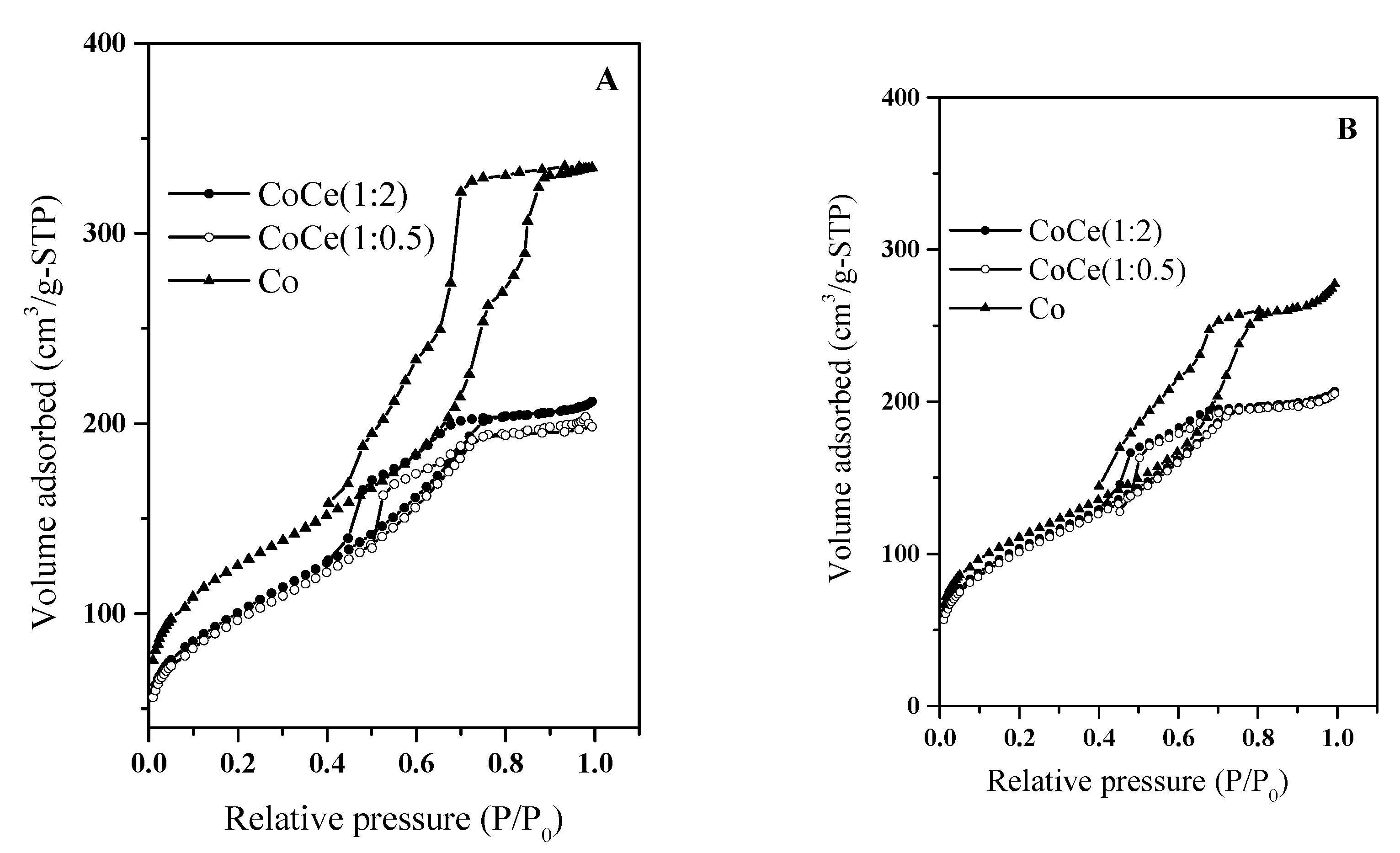


| Sample | D-Spacing | SBET | Sµ a | Vp b | Vµ a | Vmes c | ∅ d | |
|---|---|---|---|---|---|---|---|---|
| (nm) | (m2/g) | (m2/g) | (cm3/g) | (cm3/g) | (cm3/g) | (nm) | ||
| SBA-15 | 10.7 | 1020 | 251 | 1.19 | 0.09 | 1.10 | 10 (8) [6] | |
| Co-SBA-15 | BR | 10.1 | 454 | 114 | 0.52 | 0.04 | 0.48 | 8.5−10.2 (8.7) [6] |
| AR | 10.0 | 401 | 93 | 0.41 | 0.04 | 0.37 | 8.1 (7.3) | |
| Co:Ce(1:0.5) | BR | 10.0 | 347 | 28 | 0.31 | 0.01 | 0.30 | 5.3−7.4 (6.4) |
| AR | 10.0 | 367 | 37 | 0.31 | 0.01 | 0.30 | 5.5−7.4 (6.1) | |
| Co:Ce (1:2) | BR | 9.9 | 362 | 29 | 0.32 | 0.01 | 0.31 | 5.1−7.9 (5.6) |
| AR | 10.0 | 376 | 44 | 0.31 | 0.01 | 0.30 | 5.1−7.4 (6.1) |
| Sample\Element | O 1s | Si 2s | Co 2p1/2 | Ce 3d | Ce3+/Ce4+ |
|---|---|---|---|---|---|
| Ce/SBA-15 | 64.07% | 35.04% | - | 0.88% | 45/55 |
| Co/SBA-15 | 1.22 (Co3+) | ||||
| Co:Ce (1:0.5) | 63.32% | 34.95% | 1.37% | 0.37% | Ce3+-100% |
| Co:Ce (1:2) | 64.04% | 34.03% | 1.49% | 0.45% | 55/45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blin, J.-L.; Michelin, L.; Lebeau, B.; Naydenov, A.; Velinova, R.; Kolev, H.; Gaudin, P.; Vidal, L.; Dotzeva, A.; Tenchev, K.; et al. Co–Ce Oxides Supported on SBA-15 for VOCs Oxidation. Catalysts 2021, 11, 366. https://doi.org/10.3390/catal11030366
Blin J-L, Michelin L, Lebeau B, Naydenov A, Velinova R, Kolev H, Gaudin P, Vidal L, Dotzeva A, Tenchev K, et al. Co–Ce Oxides Supported on SBA-15 for VOCs Oxidation. Catalysts. 2021; 11(3):366. https://doi.org/10.3390/catal11030366
Chicago/Turabian StyleBlin, Jean-Luc, Laure Michelin, Bénédicte Lebeau, Anton Naydenov, Ralitsa Velinova, Hristo Kolev, Pierrick Gaudin, Loïc Vidal, Anna Dotzeva, Krasimir Tenchev, and et al. 2021. "Co–Ce Oxides Supported on SBA-15 for VOCs Oxidation" Catalysts 11, no. 3: 366. https://doi.org/10.3390/catal11030366
APA StyleBlin, J.-L., Michelin, L., Lebeau, B., Naydenov, A., Velinova, R., Kolev, H., Gaudin, P., Vidal, L., Dotzeva, A., Tenchev, K., & Todorova, S. (2021). Co–Ce Oxides Supported on SBA-15 for VOCs Oxidation. Catalysts, 11(3), 366. https://doi.org/10.3390/catal11030366