Gold Nanoparticle-Decorated Bi2S3 Nanorods and Nanoflowers for Photocatalytic Wastewater Treatment
Abstract
1. Introduction
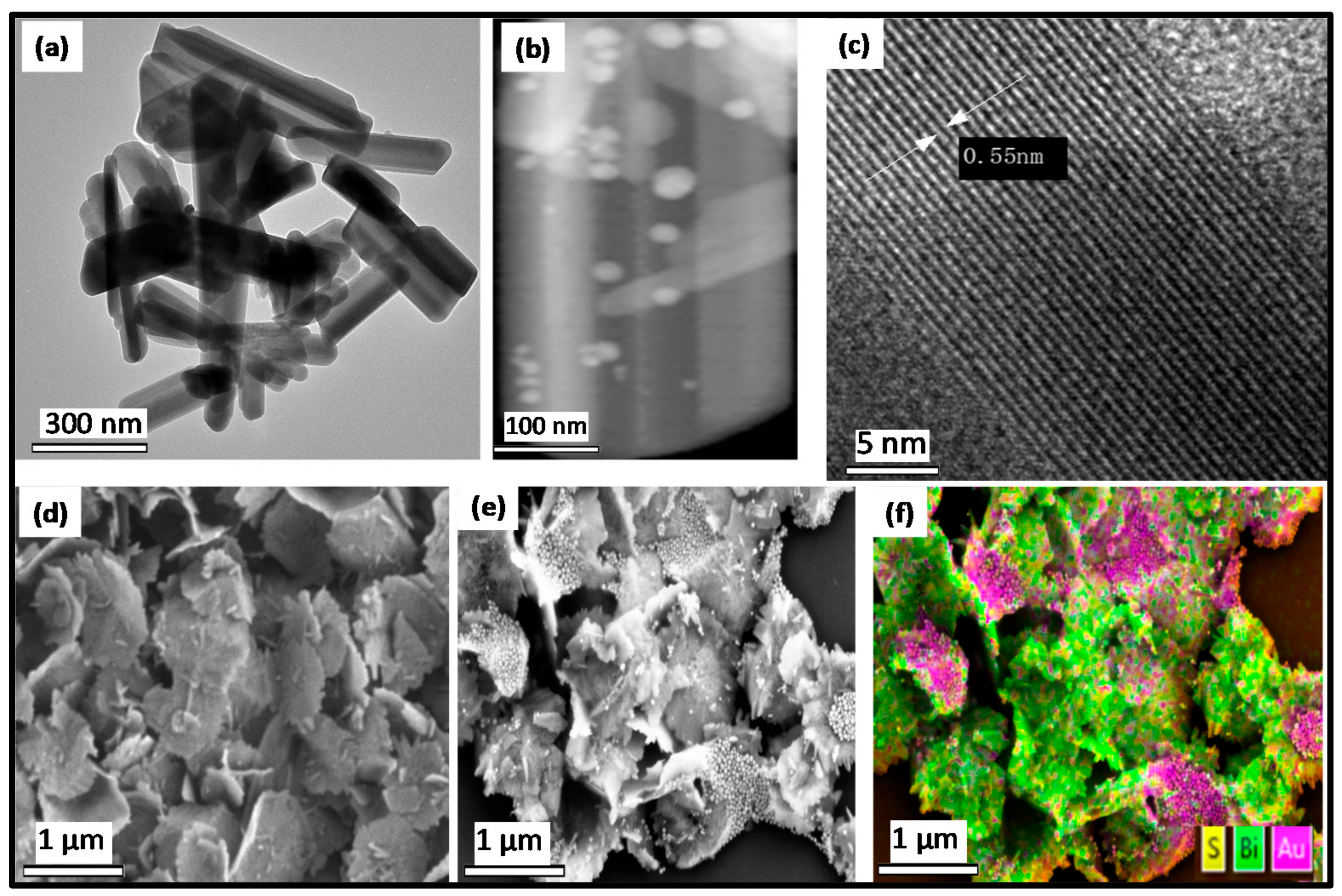
2. Results and Discussion
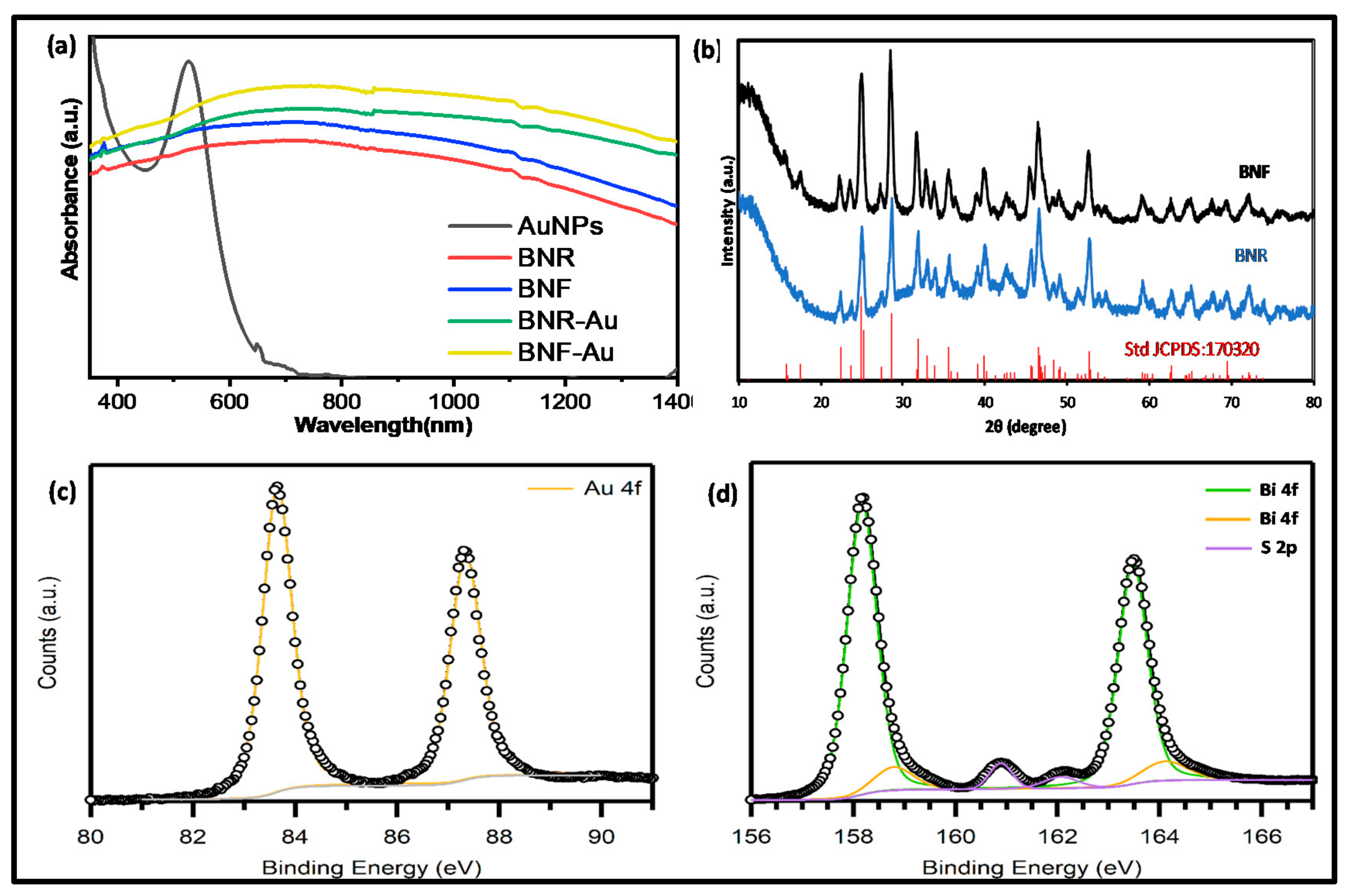
2.1. Optical Studies
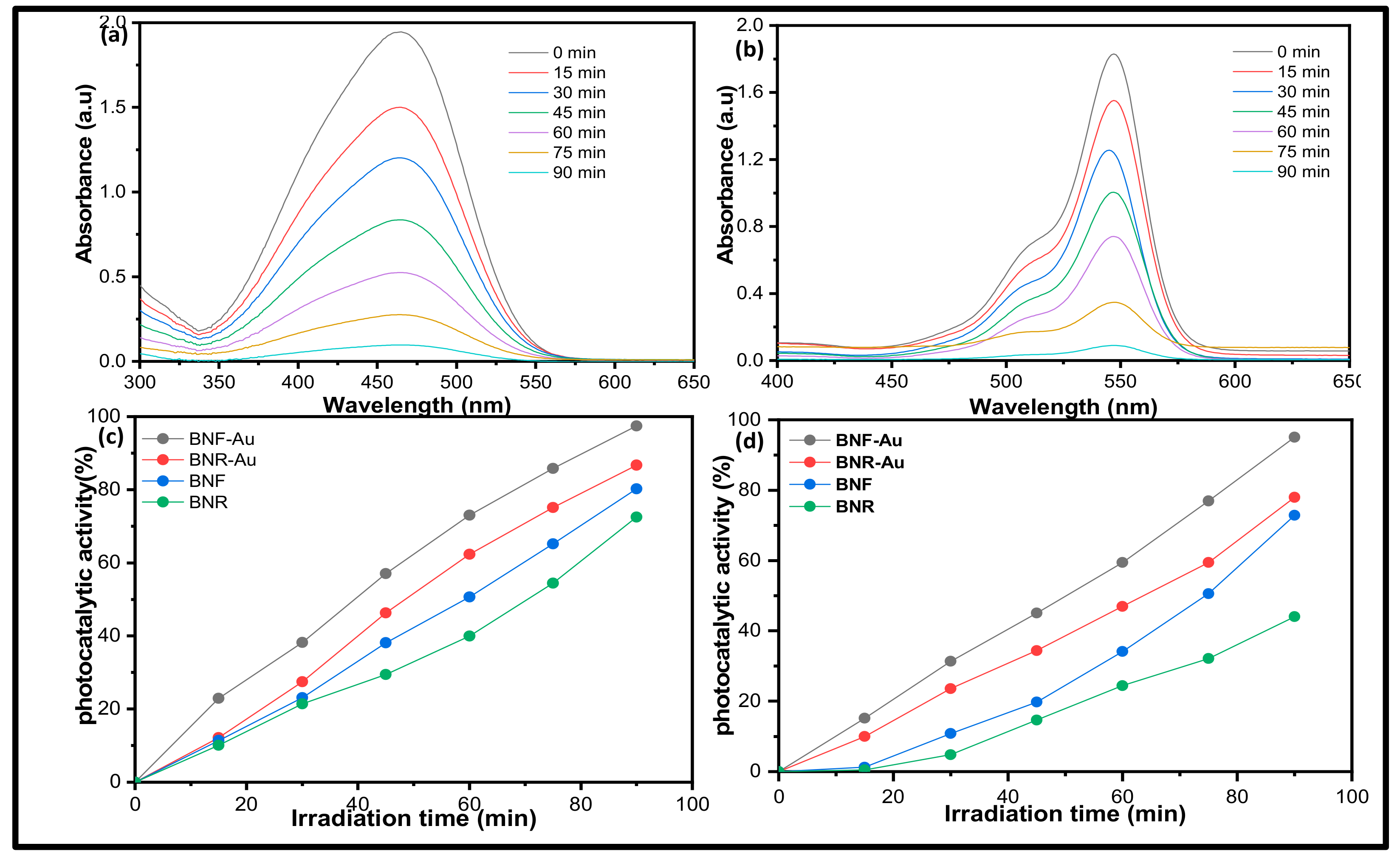
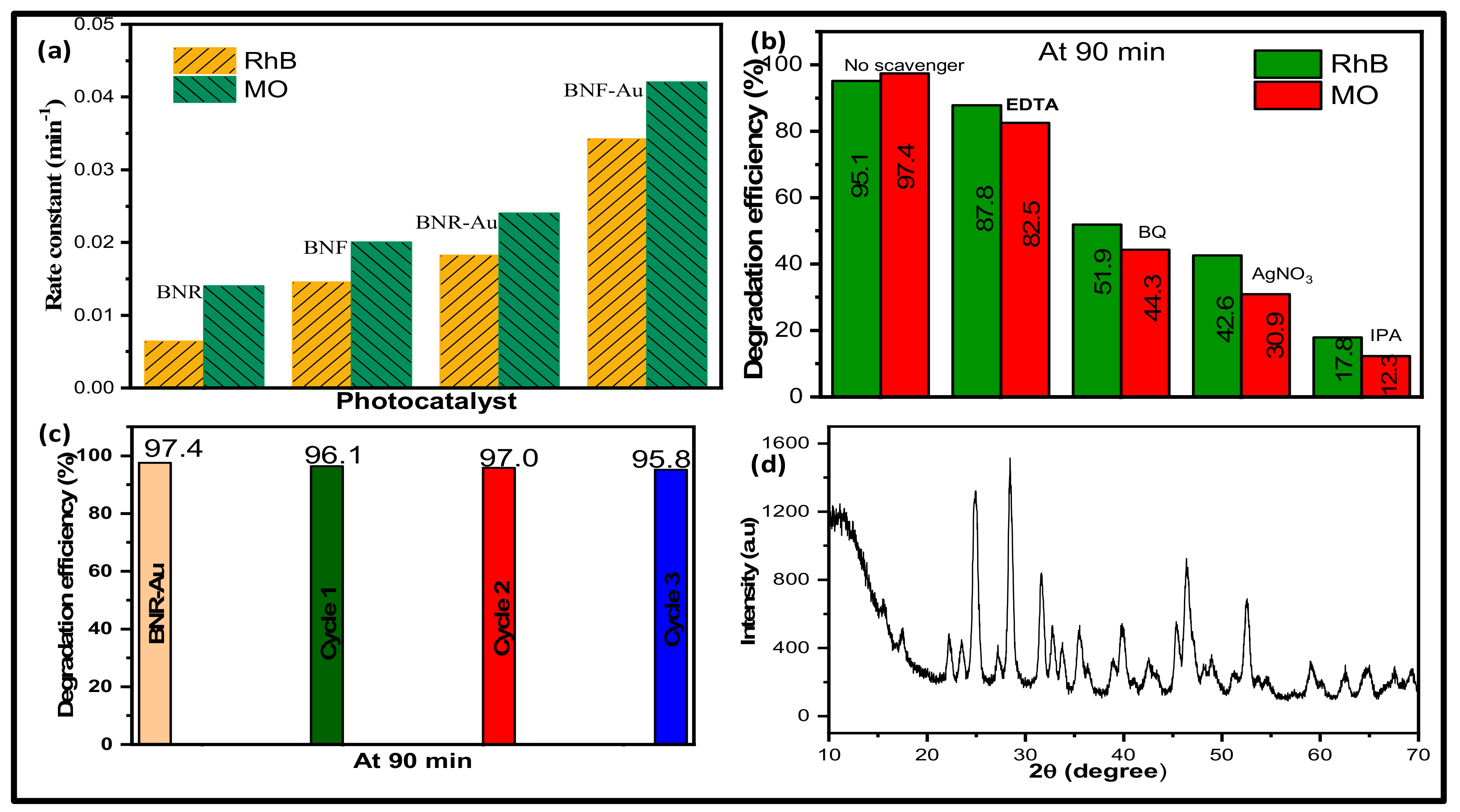
2.2. Photocatalytic Activity Study
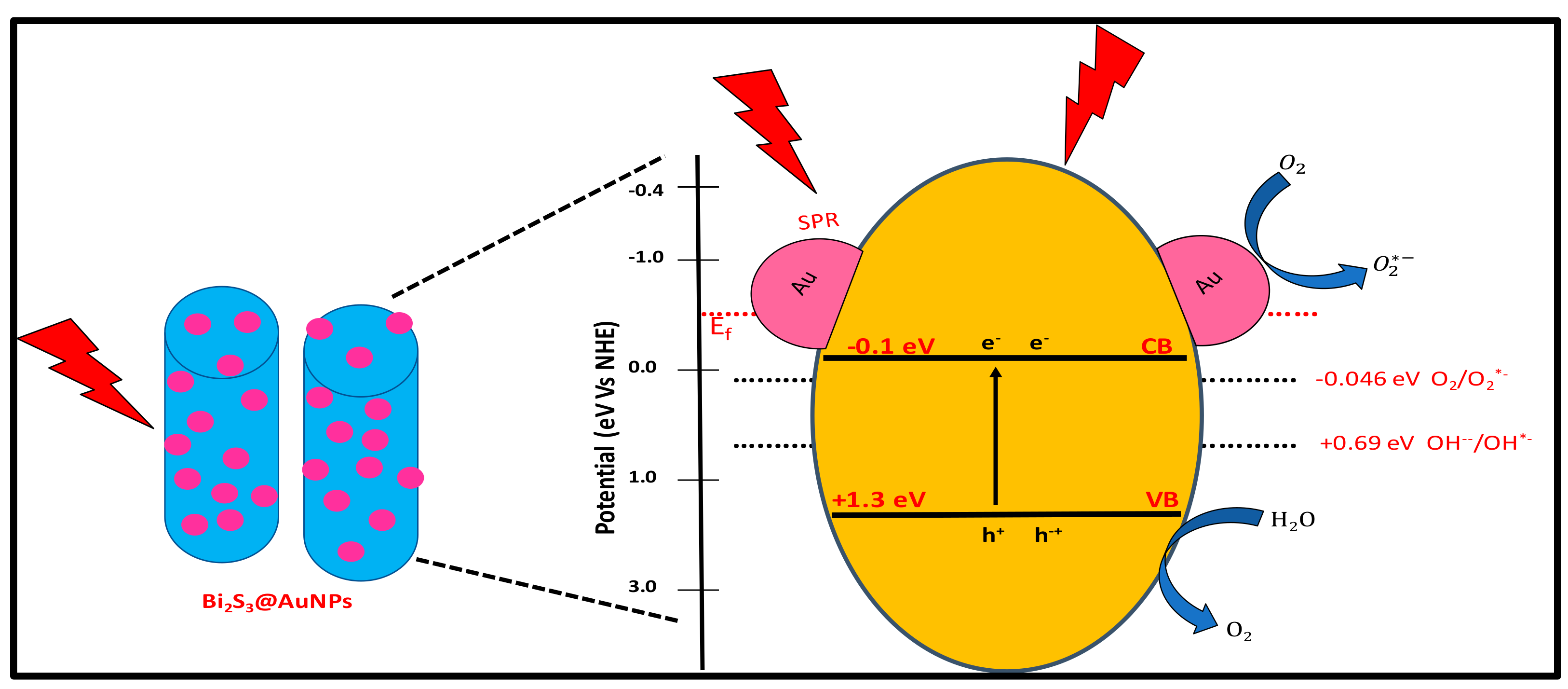
2.3. Photodegradation Mechanism
3. Materials and Methods
3.1. Methods
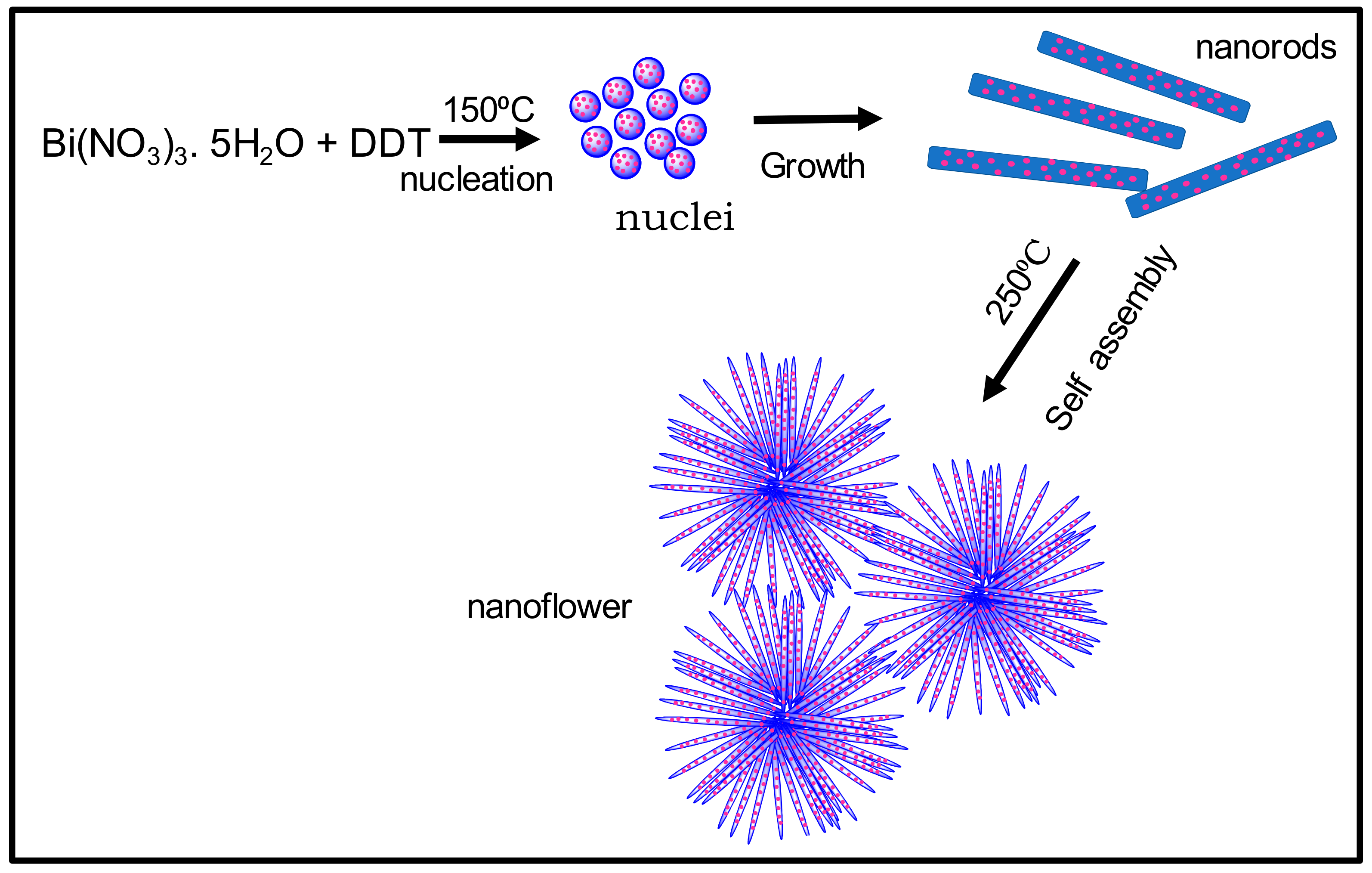
3.1.1. Synthesis of Bi2S3 Nanorods and Nanoflower Particles
3.1.2. Synthesis of BNR-Au and BNF-Au Particles
3.2. Photocatalysis Experiments
3.3. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vinodgopal, K.; Bedja, I.; Kamat, P.V. Nanostructured Semiconductor Films for Photocatalysis. Photoelectrochemical Behavior of SnO2/TiO2 Composite Systems and Its Role in Photocatalytic Degradation of a Textile Azo Dye. Chem. Mater. 1996, 8, 2180–2187. [Google Scholar] [CrossRef]
- Chatterjee, S.; Tyagi, A.K.; Ayyub, P. Efficient Photocatalytic Degradation of Rhodamine B Dye by Aligned Arrays of Self Assembled Hydrogen Titanate Nanotubes. J. Nanomater. 2014, 1–7. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of Synthetic Dyes from Wastewaters: A Review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumaar, S.; Porkodi, K.; Gomathi, R.; Geetha, M.A.; Manonmani, N. Sol∓gel Derived Silver Doped Nanocrystalline Titania Catalysed Photodegradation of Methylene Blue from Aqueous Solution. Dyes. Pigm. 2006, 69, 22–30. [Google Scholar] [CrossRef]
- Sivula, K.; Formal, F.L.; Gratzel, M. Solar Water Splitting: Progress Using Hematite (α-Fe2O3) Photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Choi, K.S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, W.; Wang, L.; Sun, S. Enhancement of Visible-Light Photocatalysis by Coupling with Narrow-Band-Gap Semiconductor: A Case Study on Bi2S3/Bi2WO6. ACS Appl. Mater. Interfaces 2012, 4, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Su, R.R.; Yu, Y.X.; Xiao, Y.H.; Yang, X.; Zhang, W.D. Earth abundant ZnO/CdS/CuSbS2 core-shell nanowire arrays as highly efficient photoanode for hydrogen evolution. Int. J. Hydrogen Energy 2018, 43, 6040–6048. [Google Scholar] [CrossRef]
- Bai, Z.; Yan, X.; Li, Y.; Kang, Z.; Cao, S.; Zhang, Y. 3D-Branched ZnO/CdS Nanowire Arrays for Solar Water Splitting and the Service Safety Research. Adv. Energy Mater. 2016, 6, 1501459. [Google Scholar] [CrossRef]
- Xuelian, Y.; Alexey, S.; Xiaoqiang, A.; Zhishan, L.; Ibáñez, M.; Cabot, A. Cu2ZnSnS4Pt and Cu2ZnSnS4-Au Heterostructured Nanoparticles for Photocatalytic Water Splitting and Pollutant Degradation. J. Am. Chem. Soc. 2014, 136, 9236–9239. [Google Scholar] [CrossRef]
- Liu, W.S.; Guo, C.F.; Yao, M.L.; Lan, Y.C.; Zhang, H.; Zhang, Q.; Chen, S.; Opeil, C.P.; Ren, Z.F. Bi2S3 nanonetwork as precursor for improved thermoelectric performance. Nano Energy 2014, 4, 113. [Google Scholar] [CrossRef]
- Mukkabla, R.; Deepa, M.; Srivastava, A.K. Poly (3,4-ethylenedioxypyrrole)enurapped Bi2S3 nanoflowers for rigid and flexible supercapacitors. Electrochim. Acta 2015, 164–171. [Google Scholar] [CrossRef]
- Du, X.L.; Cai, F.S.; Wang, X.W. Enhanced thermoelectric performance of chloride doped bismuth sulfide prepared by mechanical alloying and spark plasma sintering. J. Alloys Compd. 2014, 587, 6–9. [Google Scholar] [CrossRef]
- Zahedi, E. Hydrostatic pressure effects on the electronic, optical, and photocatalytic properties of ribbon-like Bi2S3: A DFT study. Supperlattices Microstr. 2015, 81, 49–63. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, L.; Hu, C.G.; Wang, S.X.; Zhang, J.C.; Wu, W.D. Sensitive optical switch based on Bi2S3 single nanowire and nanowire film. J. Alloys Compd. 2014, 612, 301–305. [Google Scholar] [CrossRef]
- Sun, Z.; Hasan, T.; Ferrari, A.C. Ultrafast lasers mode-locked by nanotubes and graphene. Physica E 2012, 44, 1082–1091. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.P. Solution processing of graphene, topological insulators and other 2d crystals for ultrafast photonics. Opt. Mater. Express 2014, 4, 63–78. [Google Scholar] [CrossRef]
- Sun, Z.P.; Martinez, A.; Wang, F. Optical modulators with two-dimensional layered materials. Nat. Photonics 2016, 10, 227–238. [Google Scholar] [CrossRef]
- Ghosh, M.; Liu, J.; Chuang, S.S.C.; Jana, S.C. Fabrication of Hierarchical V2O5 Nanorods on TiO2 Nanofibers and Their Enhanced Photocatalytic Activity under Visible Light. ChemCatChem 2018, 13, 3305–3318. [Google Scholar] [CrossRef]
- Lu, F.; Wang, J.; Chang, Z.; Zeng, J. Uniform deposition of Ag nanoparticles on ZnO nanorod arrays grown on polyimide/Ag nanofibers by electrospinning, hydrothermal, and photoreduction processes. Mater. Des. 2019, 181, 108069. [Google Scholar] [CrossRef]
- Yuan, J.; Shi, E.; Choo, G.; Tang, X.; Sheng, Y.; Ding, J.; Xue, J. Synthesis of ZnO–Pt nanoflowers and their photocatalytic applications. Nanotechnology 2010, 21, 185606. [Google Scholar] [CrossRef]
- Arif, M.; Min, Z.; Yuting, L.; Yin, H.; Liu, X. Photocatalyst with enhanced photodecomposition and photocatalytic hydrogen evolution through Z-scheme process. J. Indust. Eng. Chem. 2019, 69, 345–357. [Google Scholar] [CrossRef]
- He, Q.; Huang, S.; Liu, M.; Li, P.; Sun, W.; Hou, L. Synthesis of CoS2/SnO2@MoS2 nanocube heterostructures for achieving enhanced electrocatalytic hydrogen evolution in acidic media. Inorg. Chem. Front. 2020, 7, 2660–2668. [Google Scholar] [CrossRef]
- Sharma, S.; Khare, N. Sensitization of narrow band gap Bi2S3 hierarchical nanostructures with polyaniline for its enhanced visible-light photocatalytic performance. Colloid Polym. Sci. 2018, 296, 1479–1489. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, W.; Chen, L.; Cao, F.; Guo, J.; Li, L. Three-Dimensional WO3 Nanoplate/Bi2S3 Nanorod Heterojunction as a Highly Efficient Photoanode for Improved Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 40235–40243. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liang, S.; Liu, X.L.; Yang, D.J.; Zhou, L.; Wang, Q.Q. Synthesis of Dumbbell-like Gold-Metal Sulfide Core-Shell Nanorods with Largely Enhanced Transverse Plasmon Resonance in Visible Region and Efficiently Improved Photocatalytic Activity. Adv. Funct. Mater. 2015, 25, 898–904. [Google Scholar] [CrossRef]
- Manna, G.; Bose, R.; Pradhan, N. Photocatalytic Au–Bi2S3 Heteronanostructures. Angew. Chem. 2014, 53, 6743–6746. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Y.; Du, R.; Gan, L.; Yu, X. Synthesis of Bi2S3–Au Dumbbell Heteronanostructures with Enhanced Photocatalytic and Photoresponse Properties. Langmuir 2016, 32, 11639–11645. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.W.; Lai, C.S.; Du, A.Y.; Tiekink, R.E.T.; Loh, K.P. Growth of Bismuth Sulfide Nanowire Using Bismuth Trisxanthate Single Source Precursors. Chem. Mater. 2003, 15, 4544–4554. [Google Scholar] [CrossRef]
- Atar, F.B.; Battal, E.; Aygun, L.E.; Daglar, B.; Bayindir, M.; Okyay, A.K. Plasmonically enhanced hot electron based photovoltaic device: Erratum. Opt. Express 2013, 21, 7196–7201. [Google Scholar] [CrossRef]
- Chao, J.; Xing, S.; Zhao, J.; Qin, C.; Duan, D.; Zhao, Y.; He, Q. Bismuth sulfide nanoflowers as high performance near-infrared laser detectors and visible-light-driven photocatalysts. RSC Adv. 2016, 6, 55676–55681. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, J.; Zhang, J. Quantum dots and plasmonic Ag decorated WO3 nanorod photoanodes with enhanced photoelectrochemical performances. Int. J. Hydrogen Energy 2016, 41, 20529–20535. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: The role of Au0 and Auδ+ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Xiong, X.Y.; Ding, S.P.; Liu, X.F.; Jiang, Q.Q.; Hu, J.C. In-Situ topotactic synthesis and photocatalytic activity of plate-like BiOCl/2D networks Bi2S3 heterostructures. Appl. Catal. B Environ. 2018, 220, 570–580. [Google Scholar] [CrossRef]
- Grigas, J.; Talik, E.; Lazauskas, V. X-ray Photoelectron Spectra and Electronic Structure of Bi2S3 Crystals. Phys. Stat. Sol. 2002, 232, 220–230. [Google Scholar] [CrossRef]
- Hongxing, L.; Qinglin, Z.; Anlian, P.; Yanguo, W.; Bingsuo, Z.; Hong, J.F. Single-Crystalline Cu4Bi4S9 Nanoribbons: Facile Synthesis, Growth Mechanism, and Surface Photovoltaic Properties. Chem. Mater. 2011, 23, 1299–1305. [Google Scholar] [CrossRef]
- Kumar, S.; Khanchandani, S.; Thirumal, M.; Ganguli, A.K. Achieving enhanced visible-light-driven photocatalysis using type-II NaNbO3/CdS core/shell heterostructures. ACS Appl. Mater. Interfaces 2014, 6, 13221–13233. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Bi, G.; Cai, C.; Guo, G.; Wu, H.; Xu, Z. Enhanced photoluminescence properties of bismuth sulfide nanocrystals with core-shell Ag@SiO2. Opt. Lett. 2016, 1467–1469. [Google Scholar]
- Sharma, S.; Singh, S.; Khare, N. Synthesis of polyaniline/CdS (nanoflowers and nanorods) nanocomposites: A comparative study towards enhanced photocatalytic activity for degradation of organic dye. Colloid Polym. Sci. 2016, 294, 917–926. [Google Scholar] [CrossRef]
- Hȯller, R.P.M.; Jahn, I.J.; Cialla-May, D.; Chanana, M.; Popp, J.; Fery, A.; Kuttner, C. Biomacromolecular-Assembled Nanoclusters: Key Aspects for Robust Colloidal SERS Sensing. ACS Appl. Mater. Interfaces 2020, 12, 57302–57313. [Google Scholar] [CrossRef]
- Lee, W.P.C.; Tan, L.L.; Sumathi, S.; Chai, S.P. Copper-doped flower-like molybdenum disulfide/bismuth sulfide photocatalysts for enhanced solar water splitting. Int. J. Hydrogen Energy 2018, 43, 748–756. [Google Scholar] [CrossRef]
- Subramanyam, P.; Vinodkumar, T.; Deepa, M.; Subrahmanyam, C. Gold nanoparticle decorated bismuth sulfide nanorods for enhanced photoelectrochemical hydrogen production. J. Mater. Chem. C 2019, 7, 6398–6405. [Google Scholar] [CrossRef]
- Ye, L.; Su, Y.; Jin, X.; Xie, H.; Zhang, C. Recent advances in BiOX (X = Cl, Br and I) photocatalysts: Synthesis, modification, facet effects and mechanisms. Environ. Sci. Nano 2014, 1, 90–112. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Liu, X.; Li, H. A facile solvothermal approach of novel Bi2S3/TiO2/RGO composites with excellent visible light degradation activity for methylene blue. Appl. Surf. Sci. 2017, 396, 58–66. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.W.; Choi, W. Platinized WO3 as an environmental photocatalyst that generates OH radicals under visible light. Environ. Sci. Technol. 2010, 44, 6849–6854. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwaji, N.; Akinoglu, E.M.; Giersig, M. Gold Nanoparticle-Decorated Bi2S3 Nanorods and Nanoflowers for Photocatalytic Wastewater Treatment. Catalysts 2021, 11, 355. https://doi.org/10.3390/catal11030355
Nwaji N, Akinoglu EM, Giersig M. Gold Nanoparticle-Decorated Bi2S3 Nanorods and Nanoflowers for Photocatalytic Wastewater Treatment. Catalysts. 2021; 11(3):355. https://doi.org/10.3390/catal11030355
Chicago/Turabian StyleNwaji, Njemuwa, Eser Metin Akinoglu, and Michael Giersig. 2021. "Gold Nanoparticle-Decorated Bi2S3 Nanorods and Nanoflowers for Photocatalytic Wastewater Treatment" Catalysts 11, no. 3: 355. https://doi.org/10.3390/catal11030355
APA StyleNwaji, N., Akinoglu, E. M., & Giersig, M. (2021). Gold Nanoparticle-Decorated Bi2S3 Nanorods and Nanoflowers for Photocatalytic Wastewater Treatment. Catalysts, 11(3), 355. https://doi.org/10.3390/catal11030355





