Effect of the Addition of Alkaline Earth and Lanthanide Metals for the Modification of the Alumina Support in Ni and Ru Catalysts in CO2 Methanation
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
- The real composition of the catalysts was determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) on a Perkin–Elmer Optima 3300DV. Moreover, after disaggregating the catalyst in acid solution (mixture of HCl 3:1 and HNO3), the composition and chemical element were identified by characteristic wavelength emission intensity.
- The crystalline species and an approximation of the average crystal size (estimated by XRD patterns and Scherrer equation [40]) of the catalysts were carried out on a PANalytical X’Pert Pro diffractometer using a CuKα radiation (λ = 0.15418 nm) at 40 kV and 30 mA and a scanning angle (2θ) of 20° to 90°.
- X-ray photoelectron spectroscopy (XPS) was obtained on a SPECS (Berlin, Germany) system equipped with a Phoibos 150 1D-DLD analyzer and an Al Kα (1486.6 eV) monochromatic radiation source with electrons output angle of 90°. XPS is a technique that allows the study of the species present on the surface of the catalyst and their chemical state.
- The temperature-programmed reduction of H2 (H2-TPR) and temperature programmed desorption of ammonia (NH3-TPD) or of CO2 (CO2-TPD) were carried out in a Micromeritics AutoChem II RS232, equipped with a thermal conductivity detector (TCD). The TPR profiles were recorded heating 50 mg of the sample from 323 K to 973 K, at a ramp rate of 10 K/min. Previously, the sample was heated from room temperature to 473 K for 1 h in Ar stream (30 Nml/min). Later, the sample was cooled down to 323 K and the Ar was replaced by 5 vol.%, H2/Ar (45 Nml/min) stream and analyzed. The TPD profiles were recorded after reducing the sample with 5 vol.%, H2/Ar (45 Nml/min) stream, and heating to 723 K. Then, the sample was cooled with He, replacing this stream by 10 vol.% NH3/He or CO2 stream during 30 min. Recovering the He stream, NH3 desorbed per unit time and catalyst mass was determined heating the sample from 423 K to 1173 K, at a ramp rate of 10 K/min.
2.3. Activity Test
3. Results and Discussion
3.1. Catalyst Characterization
3.1.1. ICP Analysis
3.1.2. BET Measurements
3.1.3. H2-TPR Studies
3.1.4. NH3 and CO2-TPD Studies
3.1.5. XPS
3.1.6. XRD
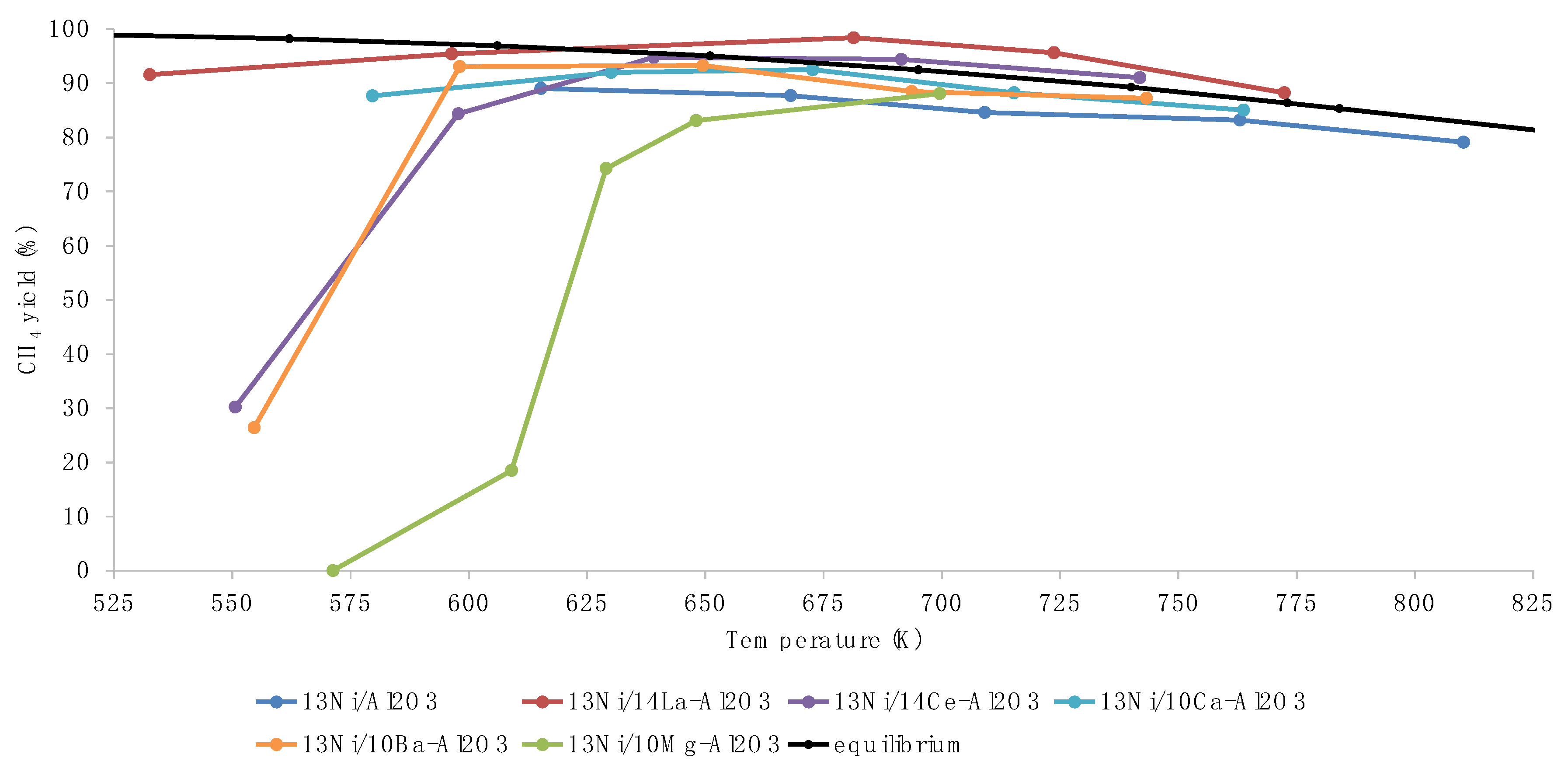
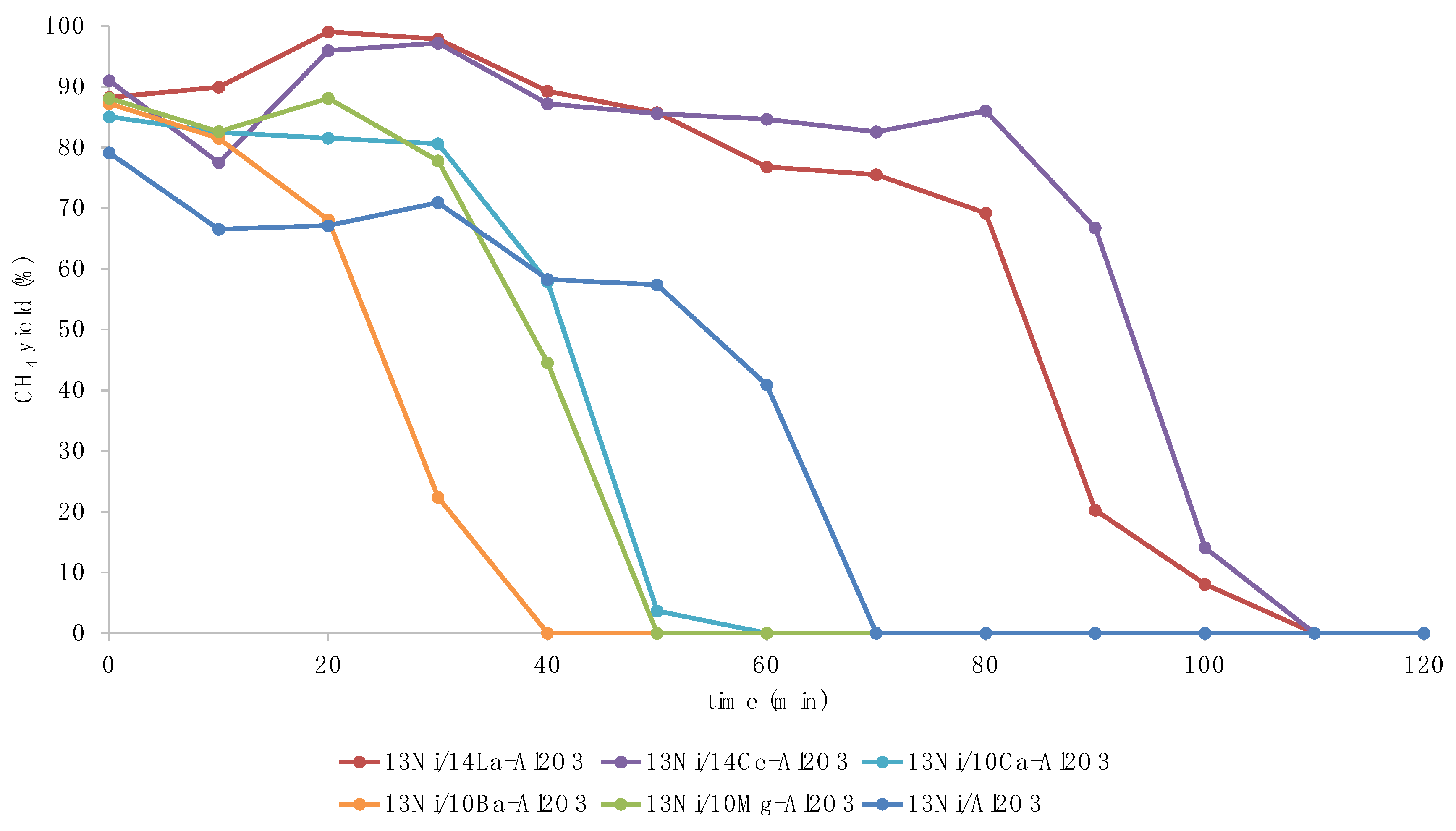
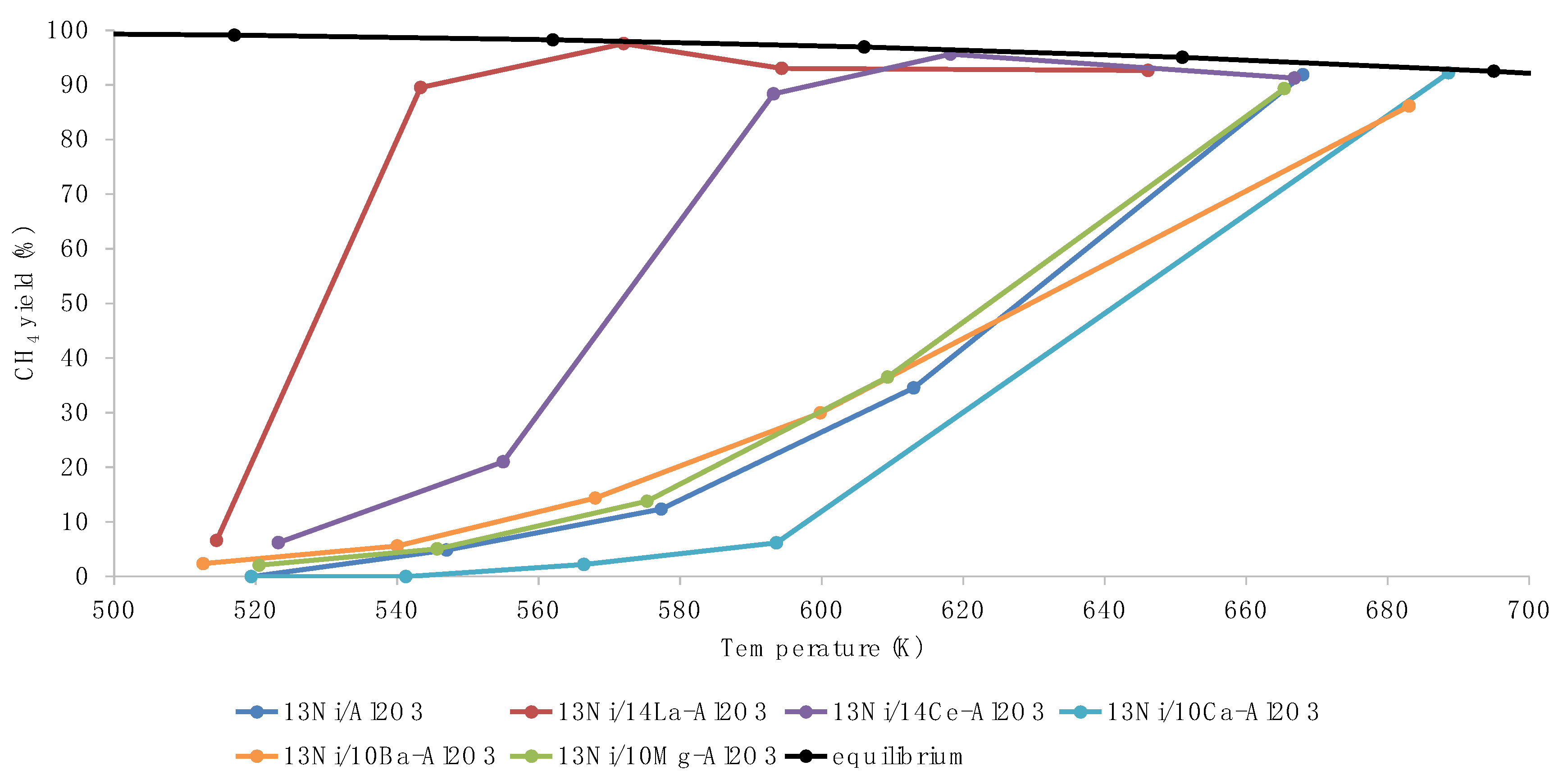
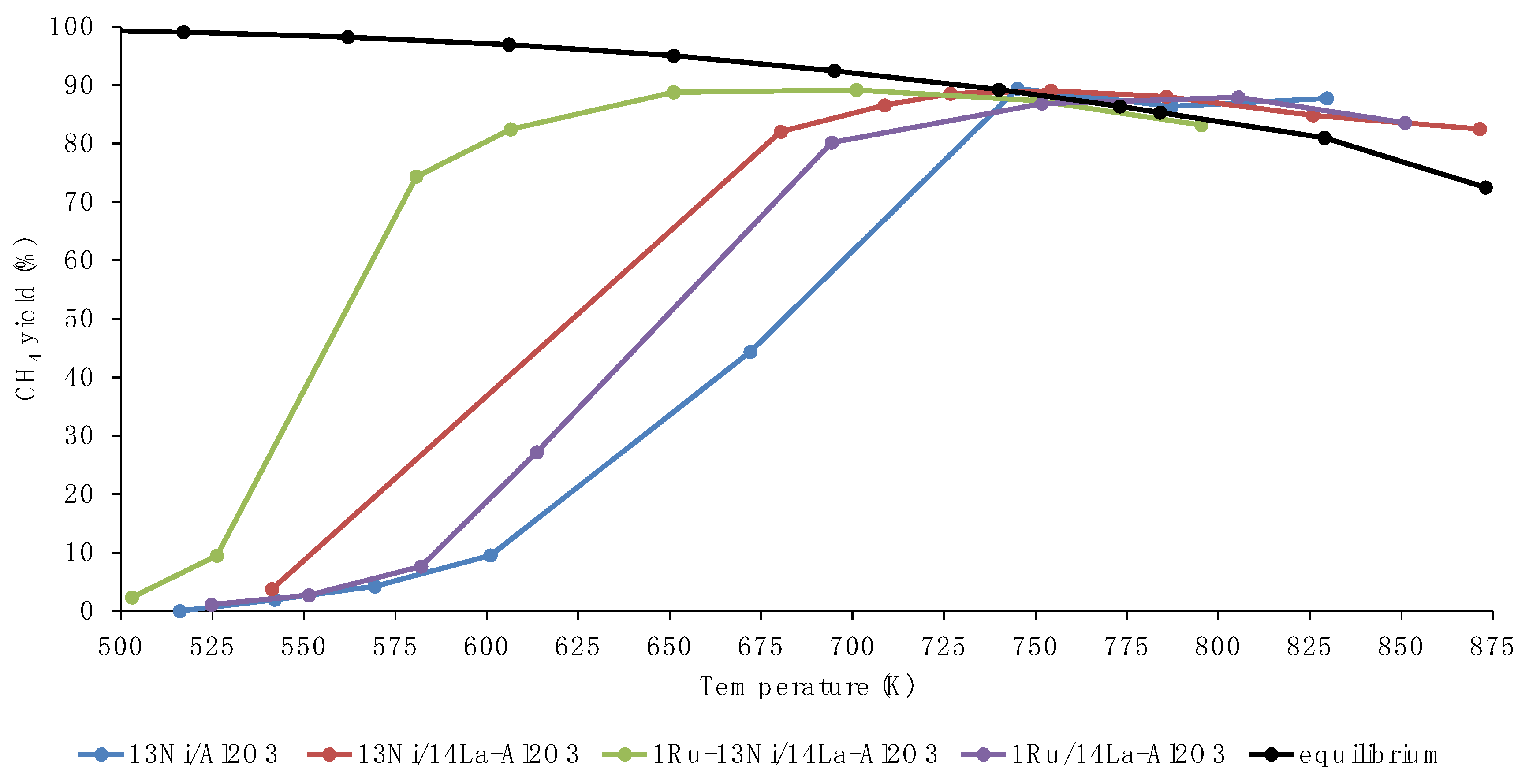
3.2. Activity Tests
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations. Climate Change. Available online: https://www.un.org/en/sections/issues-depth/climate-change/index.html (accessed on 24 September 2019).
- Tuckett, R. Greenhouse Gases. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; p. B9780124095472140000. [Google Scholar] [CrossRef]
- Khapre, A.; Jaiswal, A.; Kumar, S. Utilizing the greenhouse effect as a source to produce renewable energy. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 835–843. [Google Scholar]
- Allen, M.R.; Dube, O.P.; Solecki, W.; Aragón-Durand, F.; Cramer, S.; Humphreys, W.; Kainuma, M.; Kala, J.; Mahowald, N.; Mulugetta, Y.; et al. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- European Commission. Communication from the Commission. A Clean Planet for All, (2018). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52018DC0773&from=EN (accessed on 24 September 2019).
- Yaashikaa, P.; Kumar, P.S.; Varjani, S.J.; Saravanan, A. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. J. CO2 Util. 2019, 33, 131–147. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Esa, Y.A.M.; Sapawe, N. A short review on carbon dioxide (CO2) methanation process. Mater. Today Proc. 2020, 31, 394–397. [Google Scholar] [CrossRef]
- Moioli, E.; Gallandat, N.; Züttel, A. Model based determination of the optimal reactor concept for Sabatier reaction in small-scale applications over Ru/Al2O3. Chem. Eng. J. 2019, 375, 121954. [Google Scholar] [CrossRef]
- Muroyama, H.; Tsuda, Y.; Asakoshi, T.; Masitah, H.; Okanishi, T.; Matsui, T.; Eguchi, K. Carbon dioxide methanation over Ni catalysts supported on various metal oxides. J. Catal. 2016, 343, 178–184. [Google Scholar] [CrossRef]
- Kuzmenko, D.; Nachtegaal, M.; Copéret, C.; Schildhauer, T. Molecular-level understanding of support effects on the regenerability of Ru-based catalysts in the sulfur-poisoned methanation reaction. J. Catal. 2019, 375, 74–80. [Google Scholar] [CrossRef]
- Dreyer, J.A.; Li, P.; Zhang, L.; Beh, G.K.; Zhang, R.; Sit, P.H.-L.; Teoh, W.Y. Influence of the oxide support reducibility on the CO2 methanation over Ru-based catalysts. Appl. Catal. B Environ. 2017, 219, 715–726. [Google Scholar] [CrossRef]
- Garbarino, G.; Bellotti, D.; Riani, P.; Magistri, L.; Busca, G. Methanation of carbon dioxide on Ru/Al 2 O 3 and Ni/Al 2 O 3 catalysts at atmospheric pressure: Catalysts activation, behaviour and stability. Int. J. Hydrog. Energy 2015, 40, 9171–9182. [Google Scholar] [CrossRef]
- Tada, S.; Ochieng, O.J.; Kikuchi, R.; Haneda, T.; Kameyama, H. Promotion of CO2 methanation activity and CH4 selectivity at low temperatures over Ru/CeO2/Al2O3 catalysts. Int. J. Hydrog. Energy 2014, 39, 10090–10100. [Google Scholar] [CrossRef]
- Beuls, A.; Swalus, C.; Jacquemin, M.; Heyen, G.; Karelovic, A.; Ruiz, P. Methanation of CO2: Further insight into the mechanism over Rh/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2012, 113–114, 2–10. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. Mechanistic study of low temperature CO2 methanation over Rh/TiO2 catalysts. J. Catal. 2013, 301, 141–153. [Google Scholar] [CrossRef]
- Hu, F.; Tong, S.; Lu, K.; Chen, C.-M.; Su, F.-Y.; Zhou, J.; Lu, Z.-H.; Wang, X.; Feng, G.; Zhang, R. Reduced graphene oxide supported Ni-Ce catalysts for CO2 methanation: The support and ceria promotion effects. J. CO2 Util. 2019, 34, 676–687. [Google Scholar] [CrossRef]
- Ye, R.-P.; Gong, W.; Sun, Z.; Sheng, Q.; Shi, X.; Wang, T.; Yao, Y.; Razink, J.J.; Lin, L.; Zhou, Z.; et al. Enhanced stability of Ni/SiO2 catalyst for CO2 methanation: Derived from nickel phyllosilicate with strong metal-support interactions. Energy 2019, 188, 116059. [Google Scholar] [CrossRef]
- García, I.G.; Izquierdo, U.; Barrio, V.; Arias, P.L.; Cambra, J.F. Power-to-Gas: Storing surplus electrical energy. Study of Al 2 O 3 support modification. Int. J. Hydrog. Energy 2016, 41, 19587–19594. [Google Scholar] [CrossRef]
- Zamani, A.; Ali, R.L.; Abu Bakar, W. Optimization of CO2 methanation reaction over M*/Mn/Cu–Al2O3 (M*: Pd, Rh and Ru) catalysts. J. Ind. Eng. Chem. 2015, 29, 238–248. [Google Scholar] [CrossRef]
- Garbarino, G.; Riani, P.; Magistri, L.; Busca, G. A study of the methanation of carbon dioxide on Ni/Al2O3 catalysts at atmospheric pressure. Int. J. Hydrog. Energy 2014, 39, 11557–11565. [Google Scholar] [CrossRef]
- Swalus, C.; Jacquemin, M.; Poleunis, C.; Bertrand, P.; Ruiz, P. CO2 methanation on Rh/γ-Al2O3 catalyst at low temperature: “In situ” supply of hydrogen by Ni/activated carbon catalyst. Appl. Catal. B Environ. 2012, 125, 41–50. [Google Scholar] [CrossRef]
- Mihet, M.; Lazar, M.D. Methanation of CO2 on Ni/γ-Al2O3: Influence of Pt, Pd or Rh promotion. Catal. Today 2018, 306, 294–299. [Google Scholar] [CrossRef]
- Ashok, J.; Pati, S.; Hongmanorom, P.; Tianxi, Z.; Junmei, C.; Kawi, S. A review of recent catalyst advances in CO2 methanation processes. Catal. Today 2020, 356, 471–489. [Google Scholar] [CrossRef]
- Renda, S.; Ricca, A.; Palma, V. Study of the effect of noble metal promotion in Ni-based catalyst for the Sabatier reaction. Int. J. Hydrog. Energy 2020. [Google Scholar] [CrossRef]
- Bustinza, A.; Frías, M.; Liu, Y.; Garcia-Bordeje, E. Mono- and bimetallic metal catalysts based on Ni and Ru supported on alumina-coated monoliths for CO2 methanation. Catal. Sci. Technol. 2020, 10, 4061–4071. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]
- Liang, C.; Hu, X.; Wei, T.; Jia, P.; Zhang, Z.; Dong, D.; Zhang, S.; Liu, Q.; Hu, G. Methanation of CO2 over Ni/Al2O3 modified with alkaline earth metals: Impacts of oxygen vacancies on catalytic activity. Int. J. Hydrog. Energy 2019, 44, 8197–8213. [Google Scholar] [CrossRef]
- Garbarino, G.; Wang, C.; Cavattoni, T.; Finocchio, E.; Riani, P.; Flytzani-Stephanopoulos, M.; Busca, G. A study of Ni/La-Al2O3 catalysts: A competitive system for CO2 methanation. Appl. Catal. B Environ. 2019, 248, 286–297. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. The role of alkali and alkaline earth metals in the CO2 methanation reaction and the combined capture and methanation of CO2. Catalysts 2020, 10, 812. [Google Scholar] [CrossRef]
- Jomjaree, T.; Sintuya, P.; Srifa, A.; Koo-Amornpattana, W.; Kiatphuengporn, S.; Assabumrungrat, S.; Sudoh, M.; Watanabe, R.; Fukuhara, C.; Ratchahat, S. Catalytic performance of Ni catalysts supported on different CeO2 morphologies for low-temperature CO2 methanation. Catal. Today 2020. [Google Scholar] [CrossRef]
- Meeyoo, V.; Panchan, N.; Phongprueksathat, N.; Traitangwong, A.; Guo, X.; Li, C.; Rirksomboon, T. Low Temperature Methanation of CO2 on High Ni Content Ni-Ce-ZrOδ Catalysts Prepared via One-Pot Hydrothermal Synthesis. Catalysts 2019, 10, 32. [Google Scholar] [CrossRef]
- Charisiou, N.; Siakavelas, G.; Papageridis, K.; Baklavaridis, A.; Tzounis, L.; Avraam, D.; Goula, M. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO 2 and/or La 2 O 3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Maina, S.C.; Ballarini, A.D.; Vilella, J.I.; De Miguel, S.R. Study of the performance and stability in the dry reforming of methane of doped alumina supported iridium catalysts. Catal. Today 2020, 344, 129–142. [Google Scholar] [CrossRef]
- Cai, Z.; Li, J.; Liew, K.; Hu, J. Effect of La2O3-dopping on the Al2O3 supported cobalt catalyst for Fischer-Tropsch synthesis. J. Mol. Catal. A Chem. 2010, 330, 10–17. [Google Scholar] [CrossRef]
- Riani, P.; Valsamakis, I.; Cavattoni, T.; Escribano, V.S.; Busca, G.; Garbarino, G. Ni/SiO2-Al2O3 catalysts for CO2 methanation: Effect of La2O3 addition. Appl. Catal. B Environ. 2021, 284, 119697. [Google Scholar] [CrossRef]
- Méndez-Mateos, D.; Barrio, V.L.; Requies, J.M.; Cambra, J.F. A study of deactivation by H2S and regeneration of a Ni catalyst supported on Al2O3, during methanation of CO2. Effect of the promoters Co, Cr, Fe and Mo. RSC Adv. 2020, 10, 16551–16564. [Google Scholar] [CrossRef]
- Franks, G.V.; Meagher, L. The isoelectric points of sapphire crystals and alpha-alumina powder. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 99–110. [Google Scholar] [CrossRef]
- Schilhauer, T.J.; Biollaz, S.M.A. Synthetic natural gas: From coal, dry biomass, and power-to-gas applications. Focus Catal. 2016, 2016, 7. [Google Scholar] [CrossRef]
- Cheng, C.; Shen, D.; Xiao, R.; Wu, C. Methanation of syngas (H 2 /CO) over the different Ni-based catalysts. Fuel 2017, 189, 419–427. [Google Scholar] [CrossRef]
- Charisiou, N.; Siakavelas, G.; Papageridis, K.; Baklavaridis, A.; Tzounis, L.; Polychronopoulou, K.; Goula, M. Hydrogen production via the glycerol steam reforming reaction over nickel supported on alumina and lanthana-alumina catalysts. Int. J. Hydrog. Energy 2017, 42, 13039–13060. [Google Scholar] [CrossRef]
- Melchor-Hernández, C.; Gómez-Cortés, A.; Diaz, G. Hydrogen production by steam reforming of ethanol over nickel supported on La-modified alumina catalysts prepared by sol–gel. Fuel 2013, 107, 828–835. [Google Scholar] [CrossRef]
- Sing, K.S.; Williams, R.T. Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Bang, Y.; Han, S.J.; Gil Seo, J.; Youn, M.H.; Song, J.H.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over ordered mesoporous nickel–alumina catalyst. Int. J. Hydrog. Energy 2012, 37, 17967–17977. [Google Scholar] [CrossRef]
- Mo, W.; Ma, F.; Ma, Y.; Fan, X. The optimization of Ni–Al2O3 catalyst with the addition of La2O3 for CO2–CH4 reforming to produce syngas. Int. J. Hydrog. Energy 2019, 44, 24510–24524. [Google Scholar] [CrossRef]
- Bizkarra, K.; Barrio, V.; Yartu, A.; Requies, J.; Arias, P.L.; Cambra, J.F. Hydrogen production from n-butanol over alumina and modified alumina nickel catalysts. Int. J. Hydrog. Energy 2015, 40, 5272–5280. [Google Scholar] [CrossRef]
- García-Lario, A.L.; Aznar, M.; Martinez, I.; Grasa, G.S.; Murillo, R. Experimental study of the application of a NiO/NiAl 2 O 4 catalyst and a CaO-based synthetic sorbent on the Sorption Enhanced Reforming process. Int. J. Hydrog. Energy 2015, 40, 219–232. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, E.-G.; Joo, O.-S.; Jung, K.-D. Stabilization of Ni/Al2O3 catalyst by Cu addition for CO2 reforming of methane. Appl. Catal. A Gen. 2004, 269, 1–6. [Google Scholar] [CrossRef]
- Hossain, Z.; Chowdhury, M.B.; Charpentier, P.A. Effect of surface acidity of Al2O3 supported metal catalysts on catalytic activity and carbon deposition during SCWG of glucose. Biomass Bioenergy 2019, 124, 142–150. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; González-Marcos, J.A.; Bueno-López, A.; González-Velasco, J.R. Effect of metal loading on the CO2 methanation: A comparison between alumina supported Ni and Ru catalysts. Catal. Today 2020, 356, 419–432. [Google Scholar] [CrossRef]
- Song, K.H.; Yan, X.; Koh, D.J.; Kim, T.; Chung, J.-S. La effect on the long-term durability of Ni-Mg-Al 2 O 3 catalysts for syngas methanation. Appl. Catal. A Gen. 2017, 530, 184–192. [Google Scholar] [CrossRef]
- Charisiou, N.; Iordanidis, A.; Polychronopoulou, K.; Yentekakis, I.; Goula, M. Studying the stability of Ni supported on modified with CeO2 alumina catalysts for the biogas dry reforming reaction. Mater. Today Proc. 2018, 5, 27607–27616. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Sun, K.; Shao, Y.; Zhang, L.; Zhang, S.; Zhang, X.; Liu, Q.; Chen, Z.; Hu, X. Steam reforming of acetic acid over Ni–Ba/Al2O3 catalysts: Impacts of barium addition on coking behaviors and formation of reaction intermediates. J. Energy Chem. 2020, 43, 208–219. [Google Scholar] [CrossRef]
- Sengupta, S.; Deo, G. Modifying alumina with CaO or MgO in supported Ni and Ni–Co catalysts and its effect on dry reforming of CH4. J. CO2 Util. 2015, 10, 67–77. [Google Scholar] [CrossRef]
- Tan, M.; Wang, X.; Shang, X.; Zou, X.; Lu, X.; Ding, W. Template-free synthesis of mesoporous γ-alumina-supported Ni–Mg oxides and their catalytic properties for prereforming liquefied petroleum gas. J. Catal. 2014, 314, 117–131. [Google Scholar] [CrossRef]
- Requies, J.; Cabrero, M.; Barrio, V.; Güemez, M.; Cambra, J.; Arias, P.; Pérez-Alonso, F.; Ojeda, M.; Peña, M.; Fierro, J. Partial oxidation of methane to syngas over Ni/MgO and Ni/La2O3 catalysts. Appl. Catal. A Gen. 2005, 289, 214–223. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Kuznetsov, V.V.; Ismagilov, Z.R. Effect of χ-alumina addition on H 2 S oxidation properties of pure and modified γ-alumina. Chin. J. Catal. 2018, 39, 258–274. [Google Scholar] [CrossRef]
- Zamani, M.; Delfani, A.M.; Jabbari, M. Scavenging performance and antioxidant activity of γ-alumina nanoparticles towards DPPH free radical: Spectroscopic and DFT-D studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Wulfsberg, G. Inorganic Chemistry; University Science Books: Mill Valley, CA, USA, 2000. [Google Scholar]
- Italiano, C.; Llorca, J.; Pino, L.; Ferraro, M.; Antonucci, V.; Vita, A. CO and CO2 methanation over Ni catalysts supported on CeO2, Al2O3 and Y2O3 oxides. Appl. Catal. B Environ. 2020, 264, 118494. [Google Scholar] [CrossRef]
- Liu, K.; Xu, X.; Xu, J.; Fang, X.; Liu, L.; Wang, X. The distributions of alkaline earth metal oxides and their promotional effects on Ni/CeO2 for CO2 methanation. J. CO2 Util. 2020, 38, 113–124. [Google Scholar] [CrossRef]
- NIST XPS Database. Selected Element Search Result. Available online: https://srdata.nist.gov/xps/EngElmSrchQuery.aspx?EType=PE&CSOpt=Retri_ex_dat&Elm=Ni (accessed on 12 July 2019).
- Paglia, G. Determination of the Structure of Y-Alumina Using Empirical and First Principle Calculations Combined with Supporting Experiments. Ph.D. Thesis, Curtin University of Technology, Perth, Australia, February 2004. [Google Scholar]
- Segal, F.M.; Corrêa, M.F.; Bacani, R.; Castanheira, B.; Politi, M.J.; Brochsztain, S.; Triboni, E.R. A Novel Synthesis Route of Mesoporous γ-Alumina from Polyoxohydroxide Aluminum. Mater. Res. 2018, 21. [Google Scholar] [CrossRef]
- Shen, X.; Garces, L.-J.; Ding, Y.; Laubernds, K.; Zerger, R.P.; Aindow, M.; Neth, E.J.; Suib, S.L. Behavior of H2 chemisorption on Ru/TiO2 surface and its application in evaluation of Ru particle sizes compared with TEM and XRD analyses. Appl. Catal. A Gen. 2008, 335, 187–195. [Google Scholar] [CrossRef]
- Chen, G.; Wang, R.; Zhao, W.; Kang, B.; Gao, D.; Li, C.; Lee, J.Y. Effect of Ru crystal phase on the catalytic activity of hydrolytic dehydrogenation of ammonia borane. J. Power Sources 2018, 396, 148–154. [Google Scholar] [CrossRef]
- Rah, I.J.; Kim, T.W.; Kim, J.; Lee, D.; Park, E.D. Selective CO oxidation in the hydrogen stream over Ru/Al@Al2O3 catalysts. Catal. Today 2020, 352, 148–156. [Google Scholar] [CrossRef]
- Lesbani, A.; Sitompul, S.O.C.; Mohadi, R.; Hidayati, N. Characterization and utilization of calcium oxide (CaO) thermally decomposed from fish bones as a catalyst in the production of biodiesel from waste cooking oil. Makara J. Technol. 2016, 20, 121. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Zhong, D.-L.; Duan, H.-L.; Song, C.-M.; Han, X.-T.; Ma, X.-R. Removal of naphthenic acids from crude oils by catalytic decomposition using Mg–Al hydrotalcite/γ-Al2O3 as a catalyst. Fuel 2014, 134, 499–504. [Google Scholar] [CrossRef]
- Malinowski, J.; Dercz, G.; Prusik, K.; Pajak, L.; Pielaszek, R.; Pudlo, W. Structure studies on nanocrystalline powder of MgO xerogel prepared by sol-gel method. Mater. Sci. Pol. 2009, 27, 201–207. [Google Scholar]
- Selvarajan, K.E.P.; Balasubramanian, K. Preparation and studies of cerium dioxide (CeO2) nanoparticles by microwave-assisted solution method. Recent Res. Sci. Technol. 2010, 2, 37–41. [Google Scholar]
- Seki, H.; Saito, H.; Toko, K.; Hosono, Y.; Higo, T.; Gil Seo, J.; Maeda, S.; Hashimoto, K.; Ogo, S.; Sekine, Y. Effect of Ba addition to Ga-α-Al2O3 catalyst on structure and catalytic selectivity for dehydrogenation of ethane. Appl. Catal. A Gen. 2019, 581, 23–30. [Google Scholar] [CrossRef]
- Saravani, H.; Khajehali, M. Synthesis and characterization of lanthanum oxide and lanthanumoxid carbonate nanoparticles from thermalizes of [La(acacen)(NO3)(H2O) complex. Orient. J. Chem. 2016, 32, 491–498. [Google Scholar] [CrossRef]
- Claude, V.; Mahy, J.G.; Douven, S.; Lohay, T.; Micheli, F.; Lambert, S.D. Sol-gel Ni-based/γ-Al2O3 as efficient catalysts for toluene reforming: Catalytic activity during long-term experiments and in presence of H2S. J. Environ. Chem. Eng. 2020, 8, 104528. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zhao, G.; Yang, H.; Yuan, M.; An, X.; Zhou, H.; Qiao, Y.; Tian, Y. CO2 methanation over ordered mesoporous NiRu-doped CaO-Al2O3 nanocomposites with enhanced catalytic performance. Int. J. Hydrog. Energy 2018, 43, 239–250. [Google Scholar] [CrossRef]
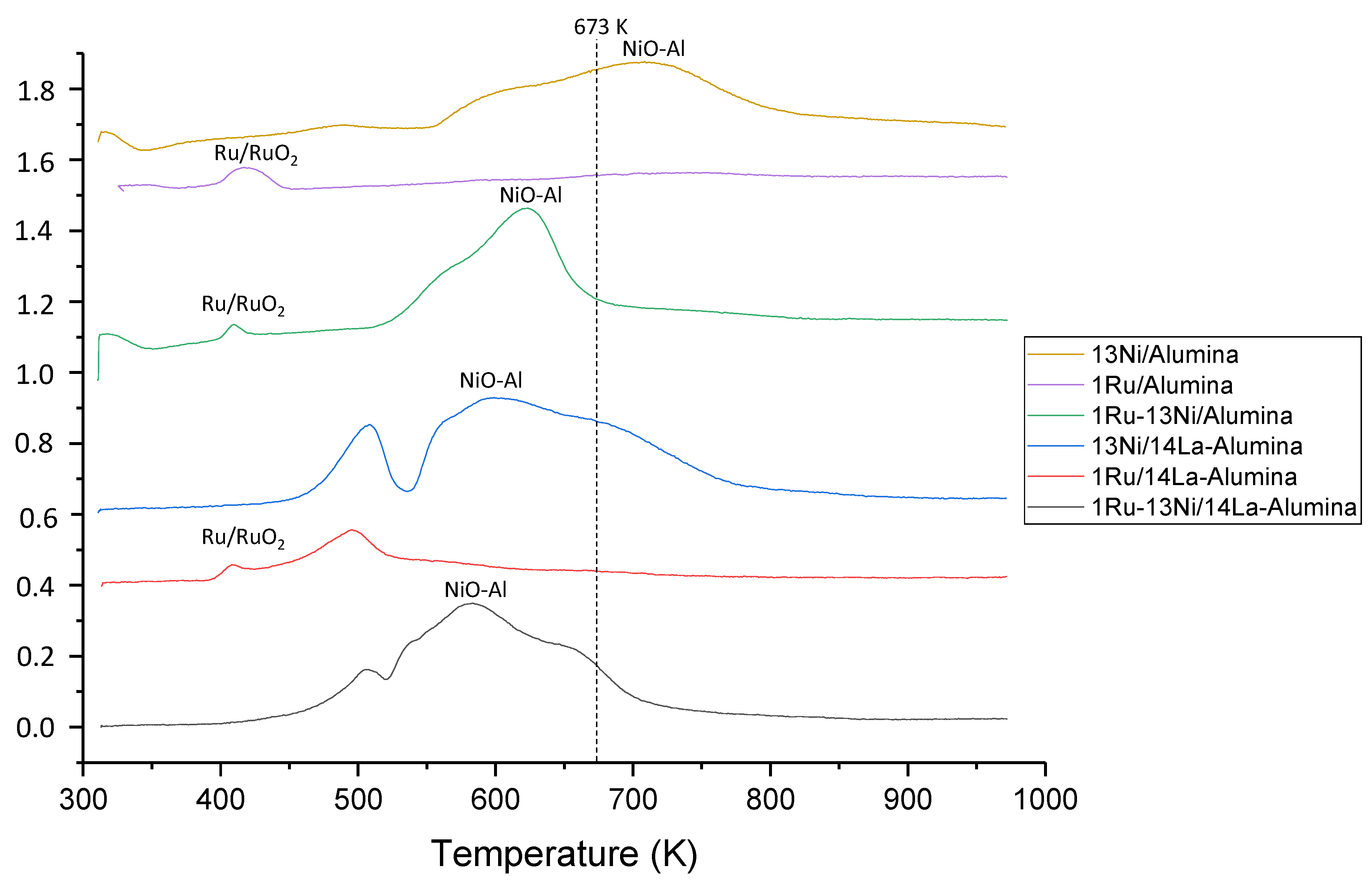

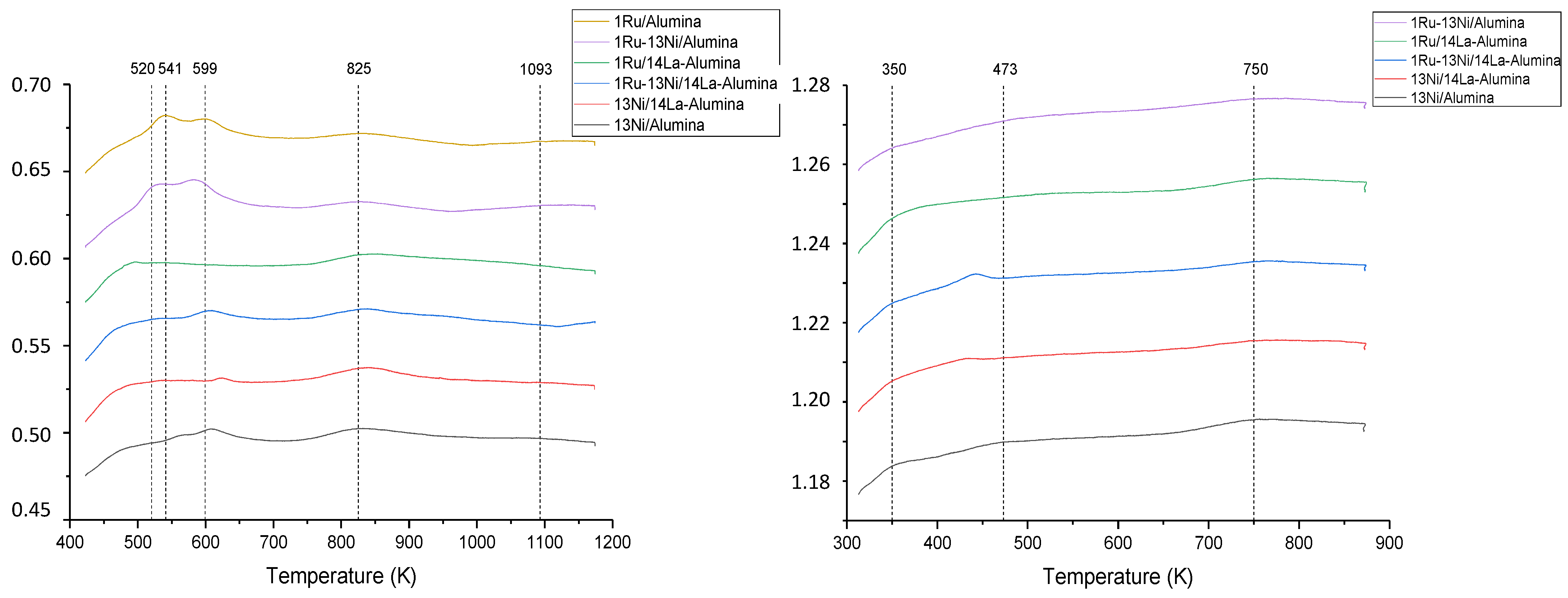
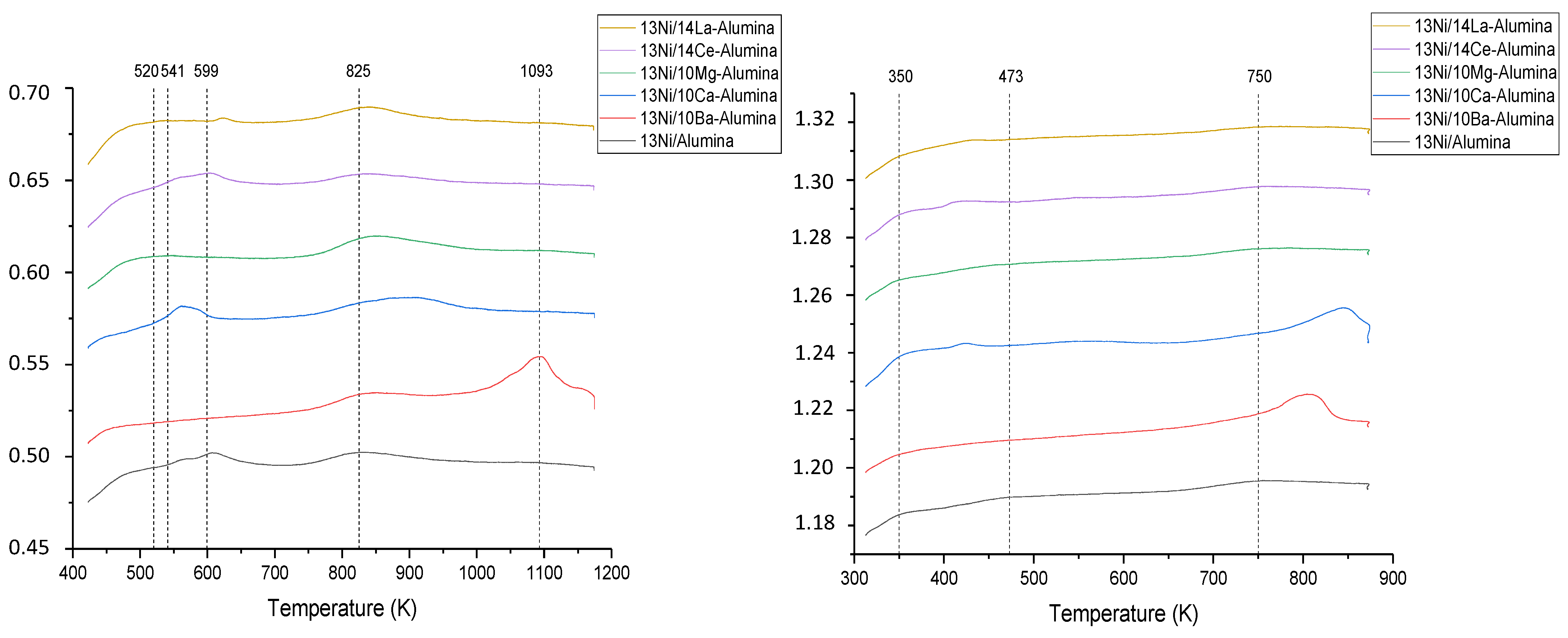

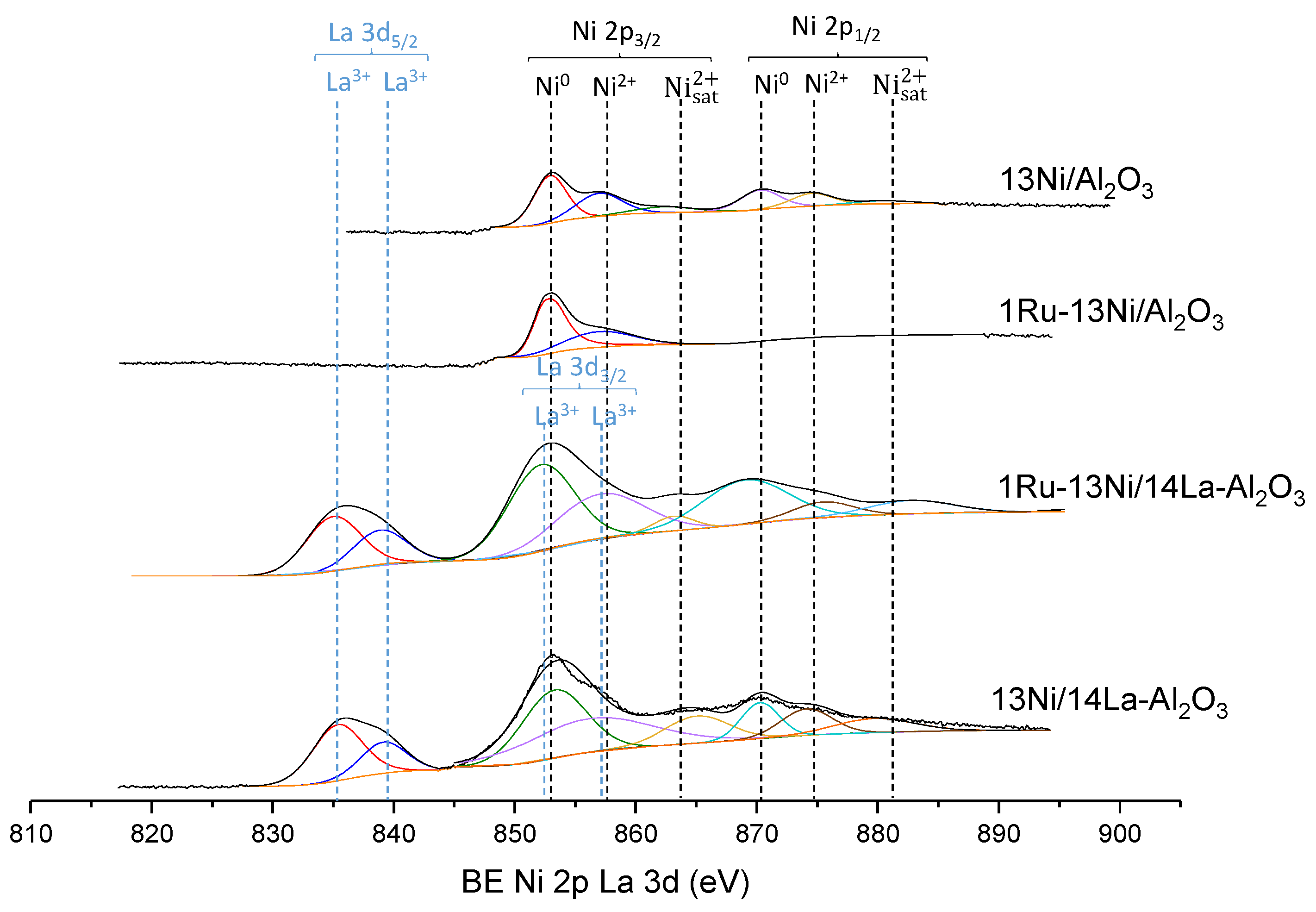
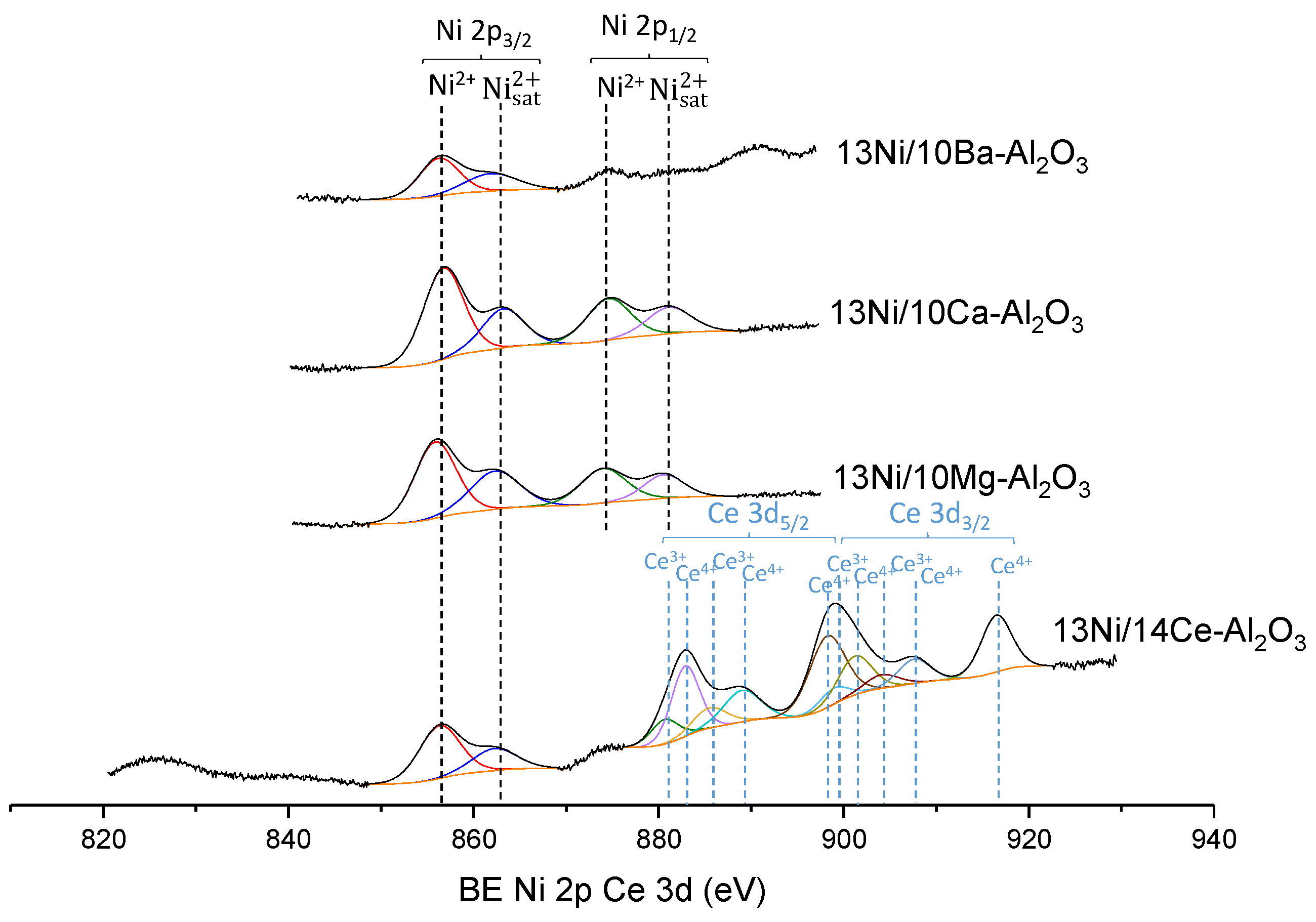
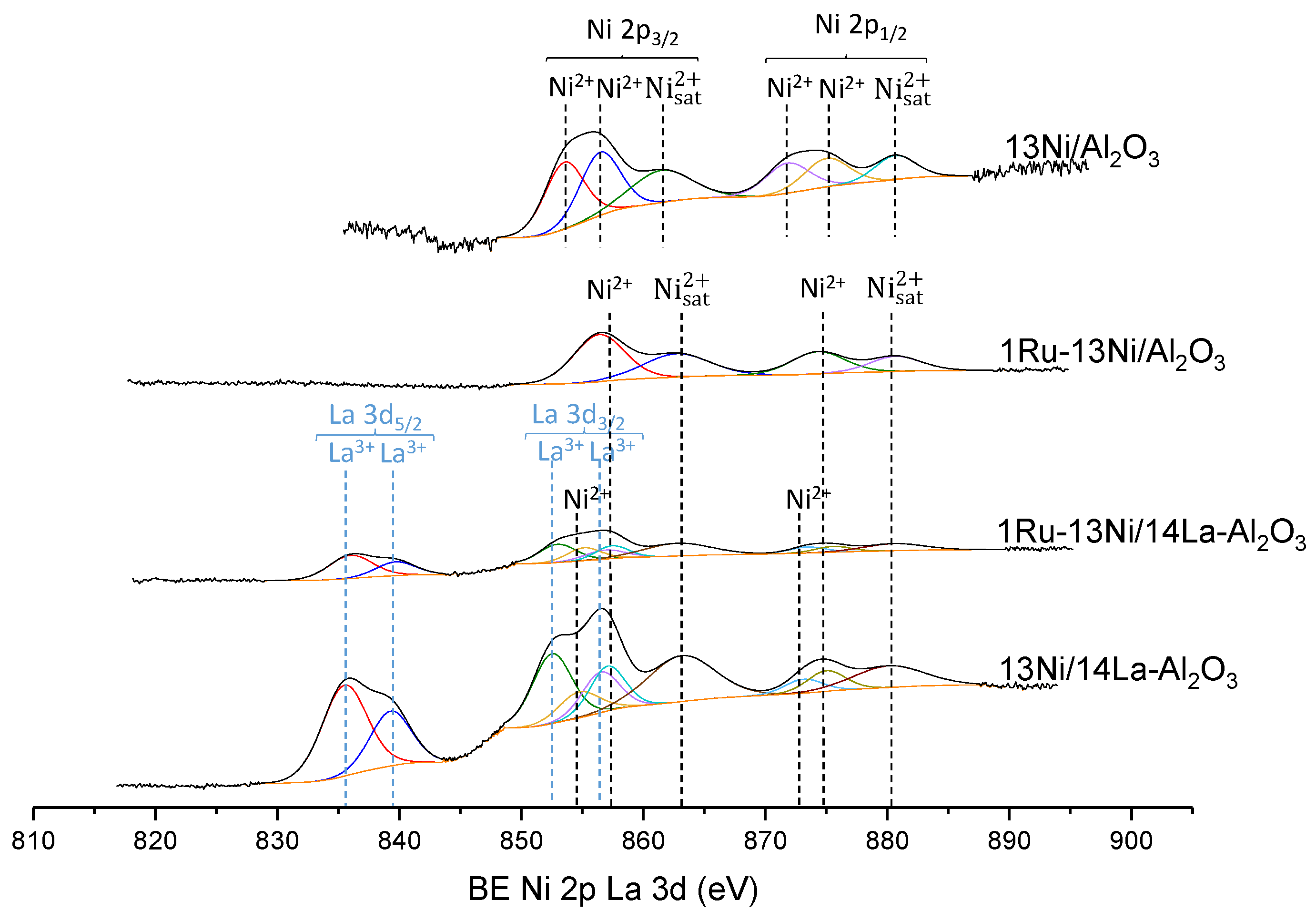
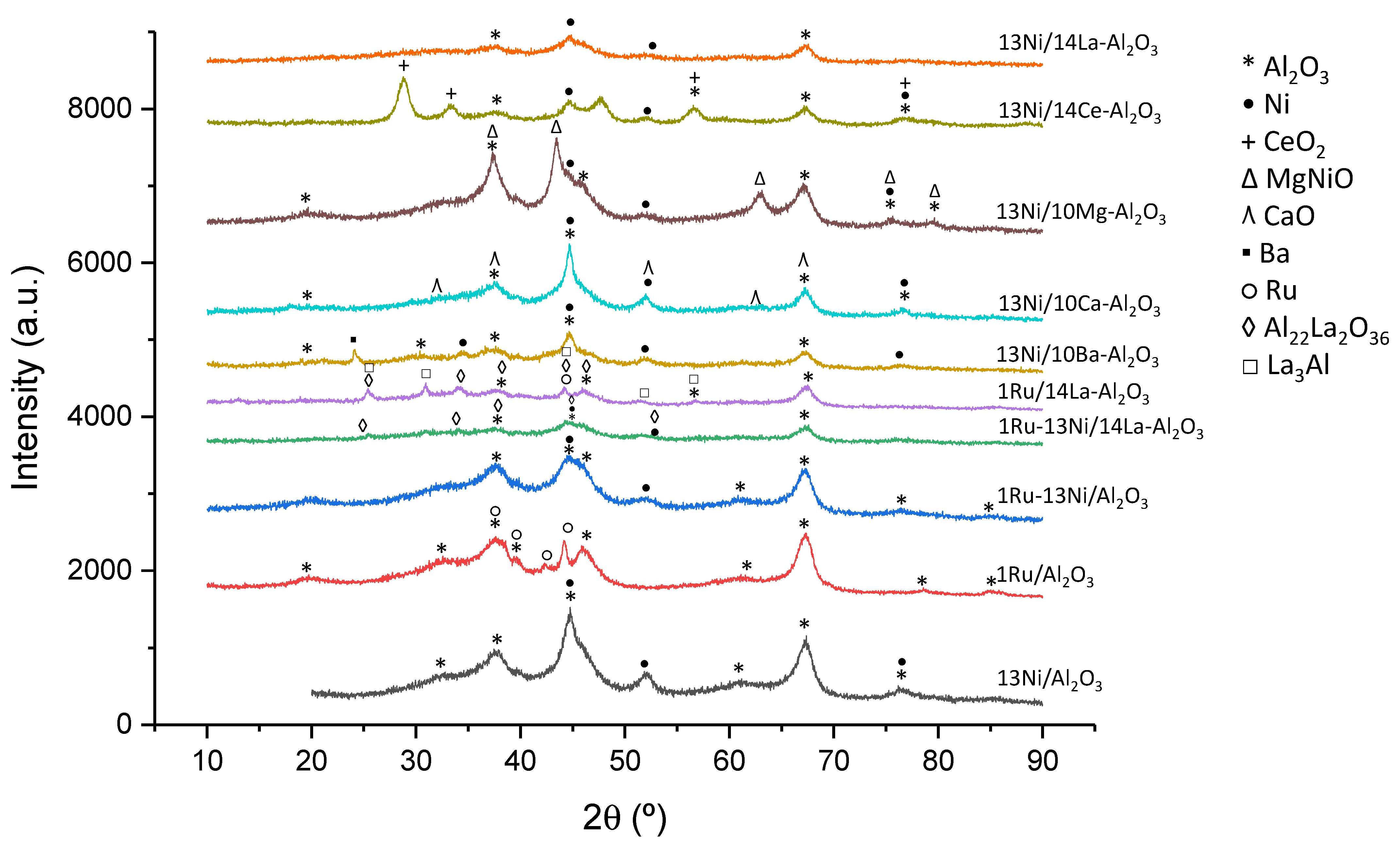
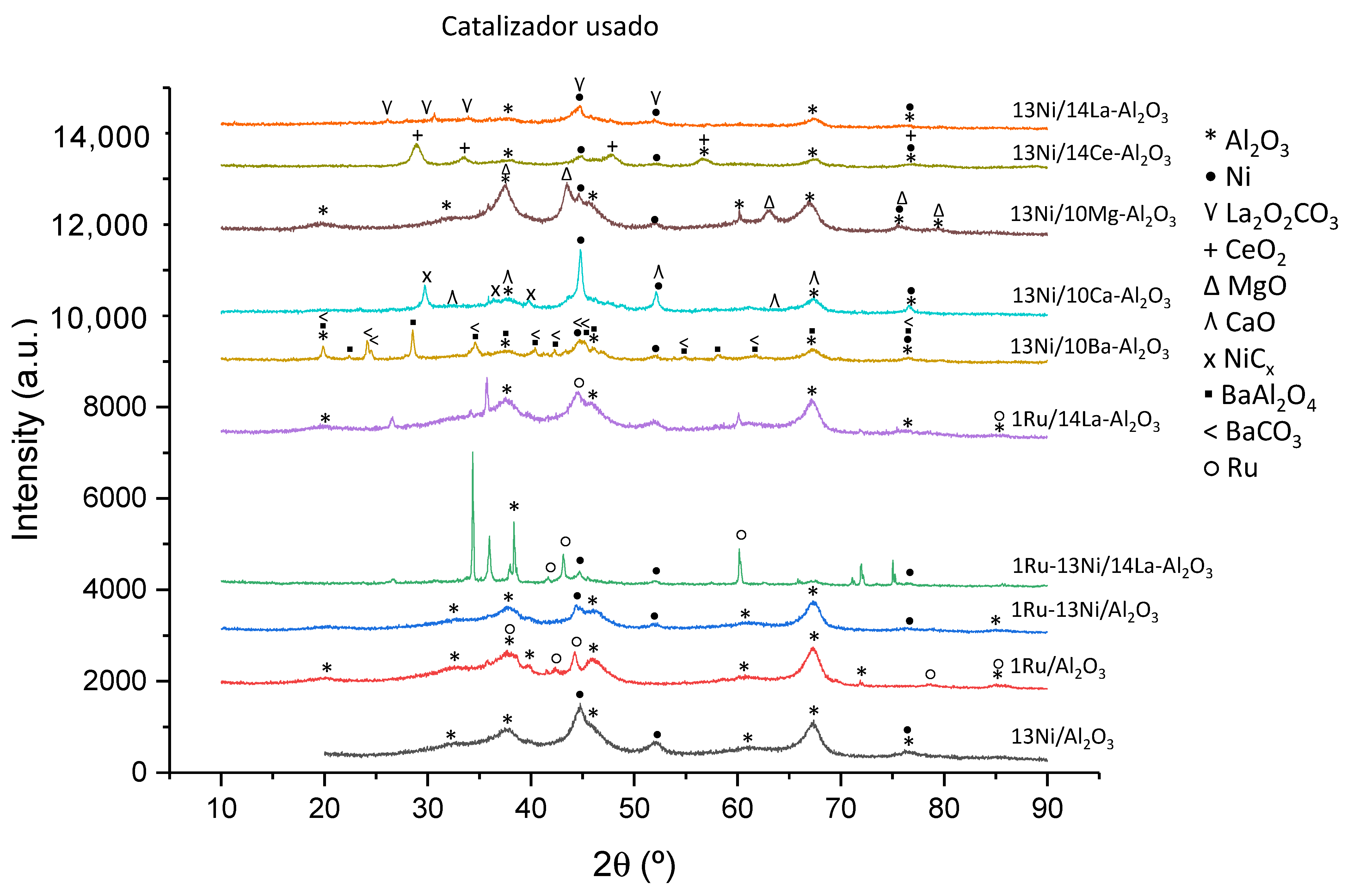


| Catalyst | Nominal Content | Measured Content by ICP | SBET a (m2/g) | VP b (cm3/g) | DP c (nm) | SXRD d1 (nm) | SXRD d2 (nm) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Ru | M | Ni | Ru | M | ||||||
| γ-Al2O3 | − | − | − | − | − | − | 202 | 0.81 | 7.7 | − | − |
| 13Ni/Al2O3 | 13 | − | − | 13.9 | − | − | 180 | 0.55 | 7.2 | 5 | 10 |
| 13Ni/10Ba-Al2O3 | 13 | − | 10 | 8.83 | − | 14.17 | 93 | 0.12 | 6.9 | 5 | 15 |
| 13Ni/10Ca-Al2O3 | 13 | − | 10 | 15.73 | − | 7.27 | 74 | 0.08 | 6.1 | 10 | 25 |
| 13Ni/10Mg-Al2O3 | 13 | − | 10 | 17.52 | − | 5.48 | 109 | 0.15 | 7.3 | 5 | 15 |
| 13Ni/14Ce-Al2O3 | 13 | − | 14 | 12.16 | − | 14.84 | 86 | 0.12 | 7.2 | 5 | 15 |
| 13Ni/14La-Al2O3 | 13 | − | 14 | 15.41 | − | 11.59 | 79 | 0.11 | 6.5 | 5 | 15 |
| 1Ru/14La-Al2O3 | − | 1 | 14 | − | 1.19 | 13.81 | 129 | 0.17 | 7.2 | − | − |
| 1Ru-13Ni/14La-Al2O3 | 13 | 1 | 14 | 14.79 | 0.52 | 12.69 | 93 | 0.12 | 7.1 | 5 | 15 |
| 1Ru-13Ni/Al2O3 | 13 | 1 | − | 13.73 | 0.27 | − | 163 | 0.22 | 7.5 | 5 | 15 |
| Catalyst | Acidity Amount μmol NH3/gcat | Basicity Amount μmol CO2/gcat | ||||
|---|---|---|---|---|---|---|
| Weak | Media | Strong | Weak | Media | Strong | |
| Temperature | <523 | 523 < T < 673 | >673 | <423 | 423 < T < 723 | >723 |
| 13Ni/Al2O3 | 127.3 | 337.9 | 1146.2 | 11.0 | 56.7 | 100.8 |
| 13Ni/10Ba-Al2O3 | 85.7 | 197.3 | 1407.3 | 12.7 | 64.6 | 127.4 |
| 13Ni/10Ca-Al2O3 | 83.7 | 260.2 | 1091.1 | 18.1 | 70.6 | 124.5 |
| 13Ni/10Mg-Al2O3 | 119.6 | 251.2 | 1116.5 | 15.6 | 79.3 | 143.0 |
| 13Ni/14Ce-Al2O3 | 143.1 | 383.2 | 1274.8 | 14.2 | 62.0 | 105.2 |
| 13Ni/14La-Al2O3 | 166.8 | 345.3 | 1268.7 | 15.2 | 70.8 | 118.5 |
| 1Ru-13Ni/14La-Al2O3 | 167.4 | 378.8 | 1253.1 | 13.4 | 65.8 | 108.5 |
| 1Ru/14La-Al2O3 | 165.5 | 316.2 | 1180.7 | 10.8 | 64.9 | 112.3 |
| 1Ru-13Ni/Al2O3 | 169.1 | 470.7 | 1167.0 | 12.7 | 55.8 | 91.9 |
| 1Ru/Al2O3 | 153.6 | 411.3 | 974.6 | |||
| Fresh Catalyst | Used Catalyst | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Al (%) | O (%) | Ni (%) | Ru (%) | M (%) | Al (%) | O (%) | Ni (%) | Ru (%) | M (%) | |
| 13Ni/Al2O3 | 40.4 | 56.5 | 2.7 | − | − | 37.7 | 59.1 | 2.6 | − | − |
| 1Ru-13Ni/Al2O3 | 36.9 | 58.3 | 3.2 | − | − | 35.9 | 60.6 | 2.7 | 0.3 | − |
| 1Ru-13Ni/14La-Al2O3 | 38.6 | 57.0 | * | − | 4.4 | 24.5 | 69.8 | 3.9 | − | 1.8 |
| 13Ni/14La-Al2O3 | 35.7 | 60.3 | * | − | 3.4 | 26.7 | 64.9 | 5.3 | − | 3.1 |
| 13Ni/10Ba-Al2O3 | 32.7 | 59.2 | 2.1 | − | 6.0 | 28.9 | 62.9 | 2.9 | − | 5.3 |
| 13Ni/10Ca-Al2O3 | 27.7 | 55.2 | 3.8 | − | 13.4 | 21.2 | 65.5 | 6.5 | − | 6.8 |
| 13Ni/10Mg-Al2O3 | 33.3 | 55.6 | 4.8 | − | 6.3 | 30.6 | 58.1 | 5.2 | − | 6.1 |
| 13Ni/14Ce-Al2O3 | 32.3 | 60.8 | 1.9 | − | 5.1 | 23.7 | 67.5 | 4.2 | − | 4.5 |
| 1Ru/14La-Al2O3 | 38.1 | 57.3 | − | 0.6 | 3.5 | 28.5 | 67.0 | − | 0.7 | 3.1 |
| 1Ru/Al2O3 | 42.1 | 55.9 | − | 0.5 | − | 40.2 | 58.2 | − | 1.1 | − |
| Metallic Species | JCPDS Code | Value (2θ) | Bibliography |
|---|---|---|---|
| Al2O3 | 077-0396 | 19.7, 34, 37.5, 44.5, 57, 61, 67.4, 85 | [63,64] |
| Ni | 087-0712 | 44.5, 51.6, 76.7 | [17] |
| Ru | 088-1734 | 38.5, 42.3, 44.2, 78.6, 84.9 | [65,66] |
| Ba | 006-0235 | 24.1, 34.5 | [28,53] |
| CaO | 086-0402 | 32.2, 37.3, 53.8, 64.1, 67.3 | [34,68] |
| Mg0.4Ni0.6O | 034-0410 | 37.4, 43.5, 63, 75.5, 79.4 | [69,70] |
| MgO | 087-0653 | 37.5, 43.5, 63, 75.7, 79.2 | [69,70] |
| CeO2 | 075-0076 | 28.9, 33.4, 47.7, 56.6, 76.8 | [65,71] |
| Al22La2O36 | 028-0502 | 25.8, 34.1, 37.5, 44.5, 45.9, 51.6 | [29] |
| LaAlO3 | 031-0022 | 25.3, 30.8, 44.1, 51.3, 56.7 | [29] |
| BaAl2O4 | 017-0306 | 19.7, 22.2, 28.4, 34.5, 37.5, 40.3, 42.2, 45.1, 45.9, 54.7, 58, 61.5, 67.2, 76.4 | [34,53,72] |
| BaCO3 | 005-0378 | 19.7, 24, 24.4, 34.5, 40.3, 42.2, 44.6, 45.1, 54.7, 61.5, 76.4 | [34,72] |
| La2O2CO3 | 025-0424 | 26, 30.5, 33.74, 44.6, 51.82 | [73] |
| Species | Peak Position (°) | Crystal Size Approximation of Reduced Catalyst (nm) | Crystal Size Approximation of Used Catalyst (nm) |
|---|---|---|---|
| Ru | 44.2 | 20 | 5 (20 for the 1Ru/Al2O3) |
| Ni | 51.9 | <5 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méndez-Mateos, D.; Barrio, V.L.; Requies, J.M.; Cambra, J.F. Effect of the Addition of Alkaline Earth and Lanthanide Metals for the Modification of the Alumina Support in Ni and Ru Catalysts in CO2 Methanation. Catalysts 2021, 11, 353. https://doi.org/10.3390/catal11030353
Méndez-Mateos D, Barrio VL, Requies JM, Cambra JF. Effect of the Addition of Alkaline Earth and Lanthanide Metals for the Modification of the Alumina Support in Ni and Ru Catalysts in CO2 Methanation. Catalysts. 2021; 11(3):353. https://doi.org/10.3390/catal11030353
Chicago/Turabian StyleMéndez-Mateos, David, V. Laura Barrio, Jesús M. Requies, and José F. Cambra. 2021. "Effect of the Addition of Alkaline Earth and Lanthanide Metals for the Modification of the Alumina Support in Ni and Ru Catalysts in CO2 Methanation" Catalysts 11, no. 3: 353. https://doi.org/10.3390/catal11030353
APA StyleMéndez-Mateos, D., Barrio, V. L., Requies, J. M., & Cambra, J. F. (2021). Effect of the Addition of Alkaline Earth and Lanthanide Metals for the Modification of the Alumina Support in Ni and Ru Catalysts in CO2 Methanation. Catalysts, 11(3), 353. https://doi.org/10.3390/catal11030353







