Abstract
The ethanol dispersion method was employed to synthesize a series of MnOx/SAPO-34 catalysts using SAPO-34 with the hierarchical pore structure as the zeolite carrier, which were prepared by facile acid treatment with citric acid. Physicochemical properties of catalysts were characterized by XRD, XPS, BET, TEM, NH3-TPD, SEM, FT-IR, Py-IR, H2-TRP and TG/DTG. NH3-SCR performances of the hierarchical MnOx/SAPO-34 catalysts were evaluated at low temperatures. Results show that citric acid etching solution at a concentration of 0.1 mol/L yielded a hierarchical MnOx/SAPO-34-0.1 catalyst with ca.15 wt.% Mn loading, exhibiting optimal catalytic activity and SO2 tolerance at low temperatures. Almost 100% NO conversion and over 90% N2 selectivity at 120 °C under a gas hourly space velocity (GHSV) of 40,000 h−1 could be obtained over this sample. Furthermore, the NO conversion was still higher than 65% when 100 ppm SO2 was introduced to the reaction gas for 4 h. These could be primarily attributed to the large specific surface area, high surface acidity concentration and abundant chemisorbed oxygen species provided by the hierarchical pore structure, which could also increase the mass transfer of the reaction gas. This finding suggests that the NH3-SCR activity and SO2 poisoning tolerance of hierarchical MnOx/SAPO-34 catalysts at low temperatures can be improved by controlling the morphology of the catalysts, which might supply a rational strategy for the design and synthesis of Mn-based SCR catalysts.
1. Introduction
Nitrogen oxides (NOx), which are released from stationary and automobile exhausts, are major atmospheric pollutants that result in many environmental issues, such as haze, acid rain and photochemical smog [1]. To date, the best developed and most efficient flue gas cleaning technology for NOx abatement from stationary sources is the selective catalytic reduction (SCR) of NOx with ammonia [2,3,4]. Owing to their high efficiency in eliminating NOx, V2O5-WO3/TiO2 catalysts have been used commercially as an NH3-SCR catalyst. However, there are some drawbacks to these commercial catalysts, including biological toxicity and low SCR performance below 300 °C, which limit their application in NH3-SCR systems [1,4]. In recent years, the low-temperature NH3-SCR technology, working downstream after the electrostatic precipitator, where most of the flue gas is cooled down and the SO2/dusts are removed, has gained increasing attention [5,6].
Manganese-containing catalysts exhibit desirable catalytic activities at low temperatures, which received much attention in recent years [7,8,9,10,11]. However, Mn-based catalysts are easily deactivated when the treated flue gas contains SO2. For this reason, the sulfur-poisoning mechanism of catalysts caused by SO2 and SO3 has been widely explored. The active sites of catalysts were covered by ammonium bisulfate nitrate and metal sulfates deposited on the surface of catalysts, which potentially obstructed the SCR reaction at low temperatures [4,8,12]. Thus, fabricating Mn-based catalysts with both excellent catalytic activity and low SO2 sensitivity at low temperatures has attracted great interest in recent years.
Researchers have suggested that the structure and morphology of catalysts in the low-temperature SCR process could enhance their SO2 poisoning tolerance [13,14,15]. Recently, small-pore zeolites, such as SAPO-34, SSZ-13 and ZSM-5, have attracted more research owing to their exceptional catalytic activity and hydrothermal stability. Among them, SAPO-34 zeolite has a small eight-ring pore opening, a large CHA cavity and medium acidity, which has been extensively studied and used in NH3-SCR reactions because of its desirable catalytic performance [16,17,18,19]. However, these microporous zeolites still have many weaknesses, including diffusion limitation of the pore/channel and reaction gas inhibition due to ammonium bisulfate (ABS) deposition, leading to catalyst deactivation in practical NH3-SCR application. Hierarchical zeolites, which provide two or more levels of pore sizes, have attracted great attention. For example, Li et al. reported that 2.7 wt.% Cu/SAPO-34 with a hierarchical pore structure exhibited better SO2 resistance compared to 2.7 wt.% Cu/SAPO-34 [20]. Liu et al. found that a 1.0-Cu/SAPO-34 catalyst synthesized by the one-step hydrothermal method exhibited superior catalytic activity at a temperature range of 140–430 °C [21]. Additionally, Guo et al. concluded that the decomposition rate of ABS on the SBA-15 catalyst with a larger average pore size was faster than that on conventional SBA-15 [22]. To the best of our knowledge, few studies have focused on the preparation and performance of the hierarchical MnOx/SAPO-34 catalyst for low-temperature NH3-SCR.
In this study, the hierarchical SAPO-34 zeolite was prepared by facile acid treatment with citric acid. The SAPO-34 zeolites etched by citric acid solutions with different concentrations were termed H-SAPO-34-x (x = 0.01, 0.1 and 0.125), in which x represents the molar concentration of the citric acid etching solution. The ethanol dispersion method was used to synthesize a series of MnOx supported on hierarchical SAPO-34 catalysts. In addition, the low-temperature NH3-SCR activities and SO2 tolerance of the obtained catalysts were explored. Compared with the conventional MnOx/SAPO-34 catalyst, the effect of the hierarchical pore structure on SCR activity and SO2 resistance at low temperatures was investigated. The results indicate that hierarchical MnOx/SAPO-34 catalysts exhibited excellent low-temperature SCR activity and SO2 resistance. Among these hierarchical MnOx/SAPO-34 catalysts (denoted as MnOx/H-SAPO-34-y, y = 0.01, 0.1 and 0.125, in which y represents the molar concentration of the citric acid etching solution), MnOx/H-SAPO-34-0.1 exhibited optimal low-temperature NH3-SCR performance. Characterization analyses through XRD, SEM, TEM, FT-IR, Py-IR, NH3-TPD, H2-TPR, TG/DTG, XPS and BET demonstrated further exploration of the impact mechanisms of the hierarchical pore structure on SO2 resistance, SCR activity and stability at low temperatures.
2. Results and Discussion
2.1. Catalyst Characterization
2.1.1. XRD
The ordered meso-structures and crystalline nature of catalysts were confirmed by low- and wide-angle XRD, respectively. As shown in Figure 1a, all the samples exhibited typical SAPO-34 characteristic diffraction peaks at 9.6°, 12.8°, 15.9° and 20.6° [23], and there were no impurity peaks before or after citric acid treatment. No manganese oxide characteristic diffraction peaks were observed in the XRD results for both MnOx/H-SAPO-34-0.1 and MnOx/SAPO-34, indicating that the Mn species were highly dispersed or persisted in an amorphous state within the catalysts. As shown in Figure 1b, there was a diffraction peak within in the range of 0.5°–1.0° for both samples, which can be assigned to the (111) reflections of an fcc structure (Fm3m), suggesting that a mesoporous structure was formed in SAPO-34 zeolites [24].
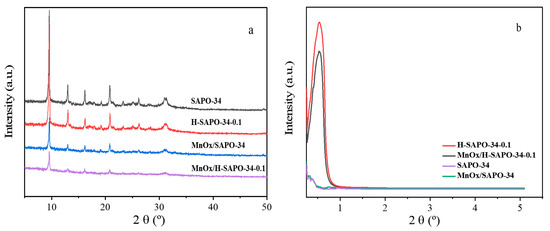
Figure 1.
XRD patterns of SAPO-34, H-SAPO-34-0.1, (a) MnOx/SAPO-34 and (b) MnOx/H-SAPO-34-0.1 catalysts.
2.1.2. SEM and TEM
SEM was used to observe the morphological features of both the synthesized and reference zeolites (Figure 2). Cubic morphology of the characteristic CHA structure was observed in all samples. As the molar concentration of citric acid increased, the surface roughness of the SAPO-34 molecular sieve gradually increased. When the molar concentration of citric acid etching solution was 0.125 mol/L, the structure of the catalyst collapsed, which caused its external specific areas to decrease. Compared with SAPO-34, which displayed well-developed crystal faces, hierarchical SAPO-34 crystals showed a rough surface because of the surface etching with citric acid solution. As expected, the MnOx/H-SAPO-34-0.1 zeolite retained its original structure well after loading manganese species, compared with the MnOx/SAPO-34 sample. The fact that the MnOx/H-SAPO-34-0.1 sample had much higher external specific surface areas than those of MnOx/SAPO-34 prepared by conventional methods might be due to the special structure of the former. Figure 3 shows the EDS elemental mapping of the MnOx/H-SAPO-34-0.1 catalyst. The elemental mapping results indicate that all elements, such as Al, Si, O, P and Mn species, were distributed homogeneously in the MnOx/H-SAPO-34-0.1 catalyst. Consistent with the results of low-angle XRD, the TEM image for the MnOx/H-SAPO-34-0.1 catalyst displayed in Figure 4 clearly shows that some mesopores were present within the sample. The HRTEM images (Figure 5) illustrate the lattice fringes of the MnOx/H-SAPO-34-0.1 catalyst. The lattice fringe spacings of 0.311 and 0.383 nm shown in Figure 5a corresponded to the (111) plane of MnO2 and the (211) plane of Mn3O4, respectively, while the lattice fringe spacings of 0.690 and 0.272 nm shown in Figure 5b corresponded to the (110) plane of MnO2 and the (222) plane of Mn2O3, respectively. The most active crystal plane in the NH3-SCR reaction was considered to be the (110) plane of MnO2, which was highly dispersed in MnOx/H-SAPO-34-0.1 [25]. This was one of the reasons for the high SCR activity of the MnOx/H-SAPO-34-0.1 catalyst at low temperatures.
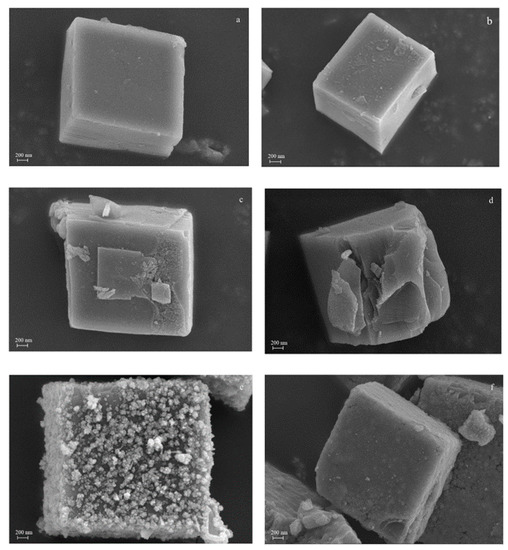
Figure 2.
SEM patterns of SAPO-34 (a), H-SAPO-34-0.01 (b), H-SAPO-34-0.1 (c), H-SAPO-34-0.125 (d), MnOx/SAPO-34 (e) and MnOx/H-SAPO-34-0.1 (f).
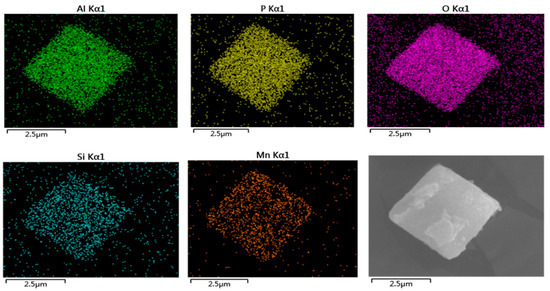
Figure 3.
EDS mapping of MnOx/H-SAPO-34-0.1 catalyst.
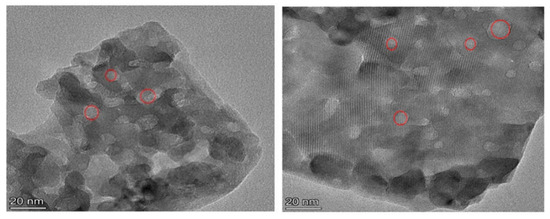
Figure 4.
TEM patterns of MnOx/H-SAPO-34-0.1 catalyst.
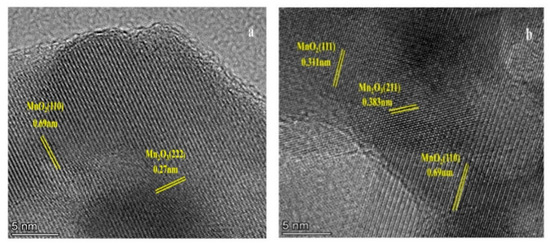
Figure 5.
HRTEM patterns of MnOx/H-SAPO-34-0.1 catalyst. (a) plane of MnO2 and Mn3O4 (b) plane of MnO2 and Mn2O3.
2.1.3. BET
The N2 adsorption–desorption isotherms and pore size distribution of all samples are shown in Figure 6. As seen in Figure 6a, MnOx/SAPO-34 presented typical type I adsorption–desorption isotherms in the low-pressure regions (P/P0 < 0.01). MnOx/H-SAPO-34-0.1 exhibited a representative characteristic type IV isotherm and a well-defined hysteresis loop at the pressure regions of 0.4 < P/P0 < 0.9 [26]. Hysteresis loops could be observed in the region of 0.4 < P/P0 < 0.9 for sample MnOx/H-SAPO-34-0.1, suggesting that there were secondary larger pores in the microporous structure of the SAPO-34 crystal [27]. The pore size distribution of samples in Figure 6b,c illustrates the existence of a mesopore structure with a pore size of approximately 5 nm. The results of the structural properties of the catalysts are listed in Table 1. Compared with conventional MnOx/SAPO-34, it was worth noting that the specific surface areas of MnOx/H-SAPO-34-0.1 increased from 248.9 to 428.26 m2/g. Meanwhile, the external specific areas of MnOx/H-SAPO-34-0.1 increased from 26.61 to 37.89 m2/g compared to the conventional MnOx/SAPO-34 catalyst. Significantly, with respect to SAPO-34 and MnOx/SAPO-34, both H-SAPO-34-0.1 and MnOx/H-SAPO-34-0.1 exhibited enhancements in mesopore volume and total pore volume, with a concomitant reduction in micropore volume. Combined with the SEM and TEM results, the BET results indicate that the hierarchical MnOx/SAPO-34 catalysts were successfully synthesized.
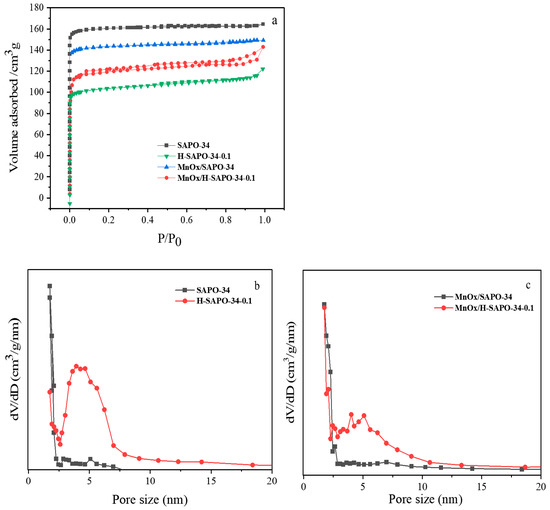
Figure 6.
N2 adsorption–desorption isotherms (a) and pore size distributions (b,c) of the synthesized catalysts.

Table 1.
Structural properties of the samples.
2.1.4. FT-IR
The chemical states of fresh and deactivated catalysts were studied by FT-IR spectroscopy and the results are illustrated in Figure 7. The sulfated MnOx/SAPO-34 and MnOx/H-SAPO-34-0.1 samples were marked as MnOx/SAPO-34-S and MnOx/H-SAPO-34-0.1-S, respectively. All of the catalysts displayed vibration absorptions centering at wave numbers of 1042 cm−1. This was mainly due to the asymmetric stretching of the Si-O-Si bond. The bands at around 1640 and 3314 cm−1 were caused by the stretching vibrations of H-OH and O-H bonds from H2O, respectively [28]. When the MnOx/SAPO-34 and MnOx/H-SAPO-34-0.1 were exposed to SO2 atmosphere, both of them showed a spectrum roughly similar to that of the fresh catalysts. However, one new peak appeared at 1420 cm−1, which could be assigned to NH4+ species chemisorbed on Brønsted acid sites. This implied that after the sulfur resistance test, ammonium species were formed on the surface of the catalyst. Concurrently, a new weak band appeared at 525 cm−1,which might be assigned to the characteristic frequencies of the SO42− [29]. These findings indicate that sulfate species may form during the SCR reaction in the presence of SO2 by binding to metal oxides or adsorbed NH3 species. From the above results, the bands at 1420 and 525 cm−1 were clearly visible for MnOx/SAPO-34 after the sulfur resistance test. However, these significant peaks were not obvious in the spectrum of MnOx/H-SAPO-34-0.1, indicating that sulfate species were only slightly deposited on this sample.
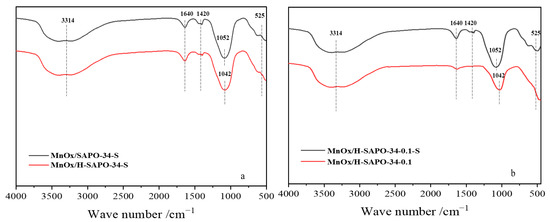
Figure 7.
FT-IR spectrum of the fresh and deactivated MnOx/SAPO-34 (a) and MnOx/H-SAPO-34-0.1 (b) catalysts.
2.1.5. NH3-TPD and Py-IR
NH3-TPD experiments were conducted to probe the surface acid properties of both hierarchical and conventional MnOx/SAPO-34 catalysts. As shown in Figure 8a and Table 2, the catalysts exhibited two desorption peaks in the whole temperature range. The peak from 170 to 220 °C was likely due to NH3 adsorbed on physical and weak acid sites. The desorption peak at 380–530 °C was assigned to NH3 adsorbed on the strong acid sites [30]. The strong acid amount of the hierarchical MnOx/H-SAPO-34-0.1 was the highest among the samples, which suggests that the larger pore structure and higher surface area enhanced the concentration and acidity of strong acid sites. Therefore, the hierarchical MnOx/SAPO-34 catalysts with larger specific surface areas, which could provide more strong acid sites on the surface of the catalysts for the adsorption and activation of NH3, exhibited the optimal NH3-SCR performance at low temperatures. Pyridine is larger than the eight-ring diameter of the CHA structure. The IR spectra of adsorbed pyridine was therefore related to the acid site in the mesopore channels [31]. The Py-IR measurement defined and established the types of acid sites in Figure 8b. Furthermore, the amounts of total and medium strong acid over the MnOx/SAPO-34 catalysts were acquired with the number of desorbed pyridine molecules at 200 °C. Brønsted acid (B) and Lewis acid (L) were found to be present on both MnOx/SAPO-34 and MnOx/H-SAPO-34-x, corresponding to the bands at around 1535 and 1445 cm−1, respectively. In addition, the peak at around 1490 cm−1 was assigned to both Brønsted and Lewis acid sites. As shown in Figure 8b, the Lewis acid sites were the main acid sites of the as-obtained catalysts, and there was also a small number of Brønsted acid sites. With the increase in the concentration of citric acid etching solution, the number of Brønsted acid sites increased gradually. In addition, the MnOx/H-SAPO-34-0.1 catalyst had the highest B/L ratio (0.65) among all of the catalysts, which was favorable for the oxidation of NO to NO2 for the reaction of NO +NH3 + O2.
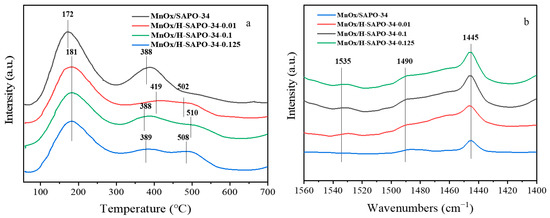
Figure 8.
NH3-TPD (a) and Py-IR (b) profiles on MnOx/SAPO-34 and hierarchical MnOx/SAPO-34 catalysts.

Table 2.
Acidity data of MnOx/SAPO-34 and hierarchical MnOx/ SAPO-34.
SO2 produced sulfur ammonium salt, which could cover and occupy some active sites and had a serious influence on the strong acidity of the catalysts. As seen in Figure 8, the MnOx/H-SAPO-34-0.1 sample had a higher amount of strong acid sites and Brønsted acid sites than the MnOx/SAPO-34 sample. This discovery indicates that the hierarchical pore structure could effectively inhibit the sulfation of active sites and provide more acid sites for the adsorption of NH3 on the surface of the catalyst, which was in accordance with the results of the SO2 resistance test.
2.1.6. H2-TPR
H2-TPR characterization was performed to investigate the redox properties of the catalysts, and the corresponding H2-TPR profiles are shown in Figure 9. Both MnOx/H-SAPO-34-0.1 and MnOx/SAPO-34 catalysts presented two reduction peaks in the range of 200–800 °C. For the profile of MnOx/SAPO-34, the peaks at 416 and 513 °C could be assigned to MnO2/Mn2O3→Mn3O4 and Mn3O4→MnO, respectively [32]. Compared with the conventional MnOx/SAPO-34 catalyst, it is clear that the reduction peaks shifted to a lower temperature centering at 351 and 453 °C, suggesting that the reducibility of MnOx/H-SAPO-34-0.1 highly increased. Based on previous studies, the reduction peak area had a direct relationship with the consumed content of H2 [33]. It can be seen that the reduction peak area of MnOx/H-SAPO-34-0.1 that appeared at relatively low temperature was much larger than that of MnOx/SAPO-34, suggesting that the manganese species on the MnOx/H-SAPO-34-0.1 surface is highly dispersed, which is consistent with the results of the EDS mapping [34]. In addition, the Mn species with high valence states such as Mn4+ and Mn3+, which were conductive to the adsorption of NH3 and NO to form Mn4+-NH3 and Mn3+-NO3, could enhance the catalytic activity in the NH3-SCR reaction [35]. Therefore, the MnOx/H-SAPO-34-0.1 catalyst with higher reducibility exhibited optimal low-temperature performance.
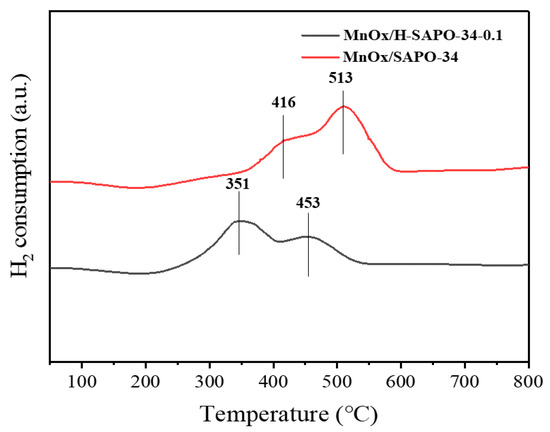
Figure 9.
H2-TPR profiles of MnOx/SAPO-34 and MnOx/H-SAPO-34-0.1 catalysts.
2.1.7. XPS
XPS characterization was performed to investigate the atomic distribution and composition of manganese, sulfur and oxygen ions on fresh and deactivated catalysts. The Mn 2p3/2, O 1s and S 2p photoelectron spectra are shown in Figure 10 and the assignments of the characteristic peaks in the XPS results of fresh and deactivated catalysts are listed in Table 3a. XPS-peak 4.1 software was used to perform the XPS peak fitting and background subtraction. By performing a peak fitting deconvolution, the Mn 2p3/2 spectra were divided into three peaks, Mn2+ (640.8 ± 0.3)eV, Mn3+ (641.8 ± 0.2)eV and Mn4+ (643.1 ± 0.5)eV [35,36]. O 1s spectra were separated into three characteristic peaks, which were ascribed to lattice oxygen species (O2− marked as Oα) at (529.8 ± 0.2)eV, chemisorbed oxygen species (O2− or O− marked as Oβ), including surface adsorbed oxygen and that of hydroxyl-like groups at (532.1 ± 0.2)eV and surface hydroxyl species/adsorbed water molecules (OH or H2O marked as Oγ) at (534.5 ± 0.2)eV [37,38]. In addition, the S 2p spectra can be detected in the deactivated samples (Figure 10c). The peaks at (169.7 ± 0.2)eV and (168.6 ± 0.2)eV are attributed to S6+/S4+ 2p3/2 and 2p1/2, respectively. Thus, it can be concluded that both SO42− and SO32− were generated on the surface of catalysts in the NH3-SCR reaction with the appearance of SO2 [39,40,41,42,43,44].
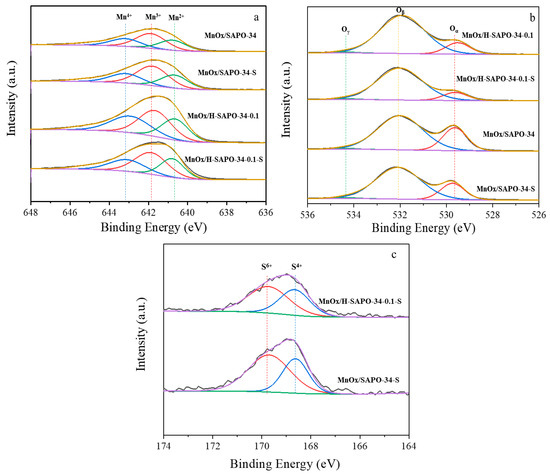
Figure 10.
High-resolution XPS spectra of all samples: (a) Mn 2p3/2, (b) O 1 s, (c) S 2p.
The results of surface compositions of all samples are shown in Table 3b. It was found that MnOx/H-SAPO-34-0.1 (72.45) had a higher (Mn3+ + Mn4+)/(Mn3+ + Mn2+ + Mn4+) ratio than that of MnOx/SAPO-34 (71.12), which is consistent with the results of H2-TPR. The ratios of (Mn3+ + Mn4+)/(Mn3+ + Mn2+ + Mn4+) for MnOx/H-SAPO-34-0.1 and MnOx/SAPO-34 decreased from 72.45% to 71.95% and from 71.12% to 69.13% after sulfation, respectively. However, this ratio over sulfated MnOx/H-SAPO-34-0.1 stayed at a high level, even higher than that over fresh MnOx/SAPO-34. It has been indicated that Mn4+ species and their redox processes promoted the catalytic cycle of NH3-SCR of NOx at low temperatures [37,45]. Therefore, MnOx/H-SAPO-34-0.1 exhibited excellent low-temperature activity and high tolerance to SO2.
Furthermore, compared with MnOx/SAPO-34 (72.69), MnOx/H-SAPO-34-0.1 (84.98) had a higher ratio of Oβ/(Oα + Oβ). It has been reported that higher percentages of Oβ promoted the catalytic cycle of NH3-SCR of NOx [46]. Therefore, the morphological features of MnOx/H-SAPO-34-0.1 indicate that the hierarchical pore structure could produce extra surface vacancies to activate oxygen [33]. The hierarchical pore structure appeared to facilitate the transformation of NO to NO2, which improved the SCR performance at low temperatures. It is worth noting that the atomic ratio of S in the deactivated MnOx/SAPO-34 catalyst was slightly higher than that in the deactivated MnOx/H-SAPO-34-0.1 sample, indicating that the sulfates did not accumulate in large quantities on the surface of the MnOx/H-SAPO-34-0.1-S sample, which is in keeping with the results of FT-IR. It has been reported that the deposition of ABS and the sulfurating of active component Mn were the main causes of catalyst deactivation [47]. Therefore, the hierarchical pore structure can attain a dynamic balance between the formation and decomposition of ammonium sulfate, which could provide an increased surface area for the reaction process and prolong the retention of reactants on the catalyst surface. Moreover, the special structure decreased the possibility of surface active sites being taken up by SO2 and prevented the formation of sulfates from blocking the active sites, leading to a high SO2 resistance [48].
2.1.8. TG/DTG
In order to explore the SO2 resistance of MnOx/H-SAPO-34-0.1 and MnOx/SAPO-34 catalysts, TG experiments were performed. The results are shown in Figure 11. As seen in Figure 11a, three weight losses are shown in the TG curves in the tested range. The weight loss below 200 °C was likely due to the evaporation of absorbed water, whereas the weight loss (A) between 200 and 450 °C was caused by the decomposition of ABS, and the latter weight loss (B) at 700–850 °C corresponded to the decomposition of MnSO4 [44]. To investigate the possible pore size effect, a decomposition experiment was also performed on the MnOx/H-SAPO-34-0.1 sample. The results are shown in Figure 11b. It was found that the TG signals of MnOx/H-SAPO-34-0.1 displayed similar trends to those of MnOx/SAPO-34. However, the weight loss (A) of MnOx/SAPO-34 and MnOx/H-SAPO-34-0.1 was measured at 4.05% and 3.39%, respectively. Smaller weight loss (A) was observed in MnOx/H-SAPO-34-0.1, indicating that less ABS formed on the sample, which was consistent with the XPS results. Meanwhile, the weight loss (B) of MnOx/SAPO-34 and MnOx/H-SAPO-34-0.1 was measured at 2.12% and 1.73%, respectively. The results of BET and NH3-TPD suggest that the MnOx/H-SAPO-34-0.1 catalyst with a larger specific surface area along with abundant acid sites, which could offer a high-efficiency place to trigger the SCR reaction, resulted in improved SO2 tolerance [49]. The TG/DTG analysis indicated that fewer sulfates were deposited on the surface of the MnOx/H-SAPO-34-0.1 catalyst, which provided evidence for the anti-SO2 capability of the hierarchical pore structure. Consistent with the results of NH3-TPD and Py-IR, the effect of the hierarchical pore structure was to make a balance between effective acid sites and the formation/decomposition of ABS, thus enhancing the deNOx properties and suppressing the blocking effect of SO2. Consequently, these phenomena suggest that the hierarchical pore structure promoted the diffusion of the reaction gas and minimized the sulfur poisoning of active Mn sites. This finding is consistent with the results obtained with regard to the sulfur poisoning resistance of the MnOx/H-SAPO-34-0.1 catalyst.
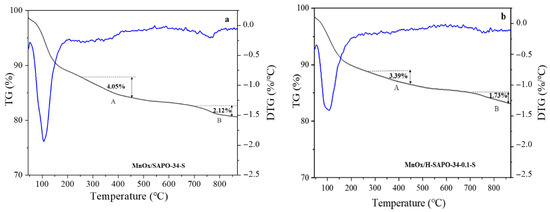
Figure 11.
TG-DTG patterns of sulfated MnOx/SAPO-34 (a) and MnOx/H-SAPO-34-0.1 (b) catalysts.
2.2. Catalytic Performance of the Low-Temperature NH3-SCR
2.2.1. Catalytic Activity Tests
As shown in Figure 12, performances of the hierarchical MnOx/SAPO-34 and MnOx/SAPO-34 catalysts during the NH3-SCR reaction were evaluated in the temperature range of 80–240 °C. To explore the influence of the hierarchical pore structure on the NH3-SCR reaction, the NO conversion over MnOx/SAPO-34 was measured for comparison. The MnOx/SAPO-34 showed little activity until the temperature reached 120 °C, and the NO conversion gradually increased to the maximum value (about 90%) at 180 °C. At low temperatures, hierarchical MnOx/SAPO-34 catalysts performed better than MnOx/SAPO-34. The NO conversion with different hierarchical MnOx/H-SAPO-34 catalysts as a function of the molar concentration of the citric acid etching solution in the NH3-SCR reaction is shown in Figure 12. Among the hierarchical MnOx/SAPO-34 catalysts, the MnOx/H-SAPO-34-0.1 catalyst exhibited the optimal NO conversion of 95% with an N2 selectivity over 90% in the temperature range of 80–240 °C. The superior NH3-SCR performance obtained with MnOx/H-SAPO-34-0.1 may due to the intercalation of mesoporous structures, which provided more channels and could substantially promote the mass transfer of reactants or products at low temperatures.
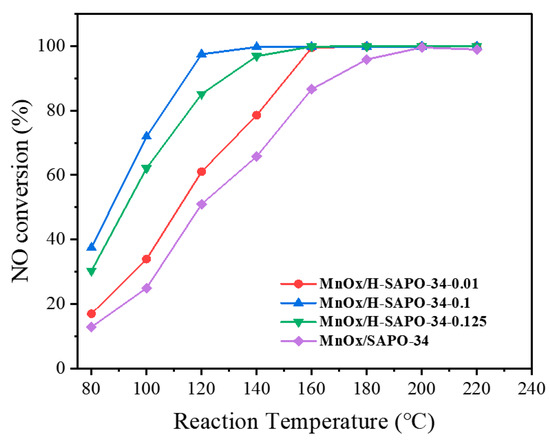
Figure 12.
SCR activity of MnOx/H-SAPO-34-0.01, MnOx/H-SAPO-34-0.1, MnOx/H-SAPO-34-0.125 and MnOx/SAPO-34 catalysts.
2.2.2. Impact of SO2 on Catalytic Activity
The effect of SO2 on the catalytic performance of MnOx/SAPO-34 and MnOx/H-SAPO-34-0.1 catalysts was investigated at 120 °C. As shown in Figure 13, when 100 ppm SO2 was added into the feed gas, a drop in NO conversion of approximately 80% occurred over MnOx/SAPO-34. After removing the SO2, the NO conversion did not fully recover. Nevertheless, the NO conversion of MnOx/H-SAPO-34-0.1 was still maintained at 100% after the 40-min test. The MnOx/SAPO-34 catalyst showed a relatively faster deactivation in a short period of time, indicating a notable difference in reaction efficiency. The SCR performance of MnOx/H-SAPO-34-0.1 decreased slowly and remained above 65% for the next 200 min. Pan et al. concluded that competitive adsorption between reactant molecules and toxicants on the surface of the catalyst may promote the deactivation of the catalyst [50]. Compared to traditional MnOx/SAPO-34 catalysts, the MnOx/H-SAPO-34-0.1 catalyst’s hierarchical pore structure was therefore found to be a key factor contributing to its high SO2 poisoning resistance. The hierarchical pore structure might inhibit ammonium bisulfate aggregation and facilitate dispersion of the active phase.
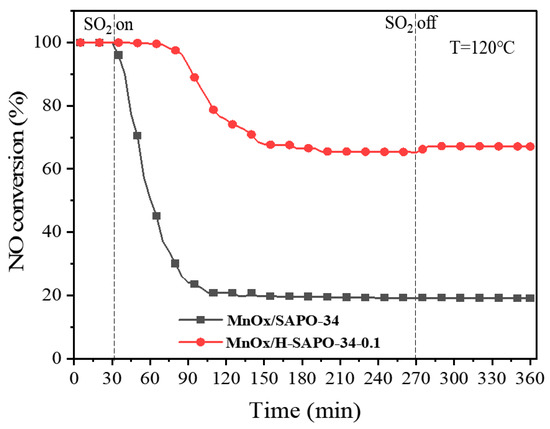
Figure 13.
SO2 tolerance of MnOx/SAPO-34 and Mn/H-SAPO-34-0.1 in NH3-SCR reaction.
Reaction conditions: 800 ppm NH3, 800 ppm NO, 100 ppm SO2 (when needed), 5 vol.% O2, Ar to balance, T = 120 °C, gas hourly space velocity (GHSV) = 40,000 h−1.
3. Materials and Methods
3.1. Catalysts Preparation
3.1.1. Preparation of Hierarchical SAPO-34
The SAPO-34 zeolite with a hierarchical pore structure (H-SAPO-34) was obtained by efficient post-synthesis via citric acid etching based on previous studies [31]. The carrier catalyst in this study was a commercial SAPO-34 with a Si/Al mass ratio of 0.27 (XFNANO Company, Nanjing, China). Typically, a certain amount of citric acid (Aladdin, Shanghai, China) was dissolved in 100 mL of ethanol and stirred for 30 min at room temperature. An amount of 3 g SAPO-34 was then added to the solution with vigorous stirring for 30 min. Then, the solution was transferred into an oil bath at 90 °C for 6 h. The product was washed with deionized water, filtered and dried overnight at 110 °C. The as-synthesized products were calcined at 550 °C for 5 h to obtain H-SAPO-34. The SAPO-34 zeolites etched in citric acid solutions with different concentrations were termed H-SAPO-34-x (x = 0.01, 0.1 and 0.125), in which x represents the molar concentration of the citric acid etching solution.
3.1.2. Preparation of the Catalysts
The ethanol dispersion method was used to prepare MnOx/SAPO-34 catalysts with a hierarchical pore structure. Manganese nitrate (50% by weight in H2O, Aladdin, Shanghai, China) was used as a precursor. The mass fraction of manganese was 15% and 2.72 mL of 50 wt.% Mn (NO3)2 was dissolved in 50 mL ethanol and stirred at ambient temperature. An amount of 3g H-SAPO-34-x was then added while being stirred. The solution was treated with ultrasound for 30 min and stirred continuously at 80 °C until the solvent evaporated completely. The products were dried at 100 °C and calcined at 400 °C for 4 h. The synthesized catalysts were denoted as MnOx/H-SAPO-34-y (y = 0.01, 0.1 and 0.125, where y is the molar concentration of the citric acid etching solution). The ordinary MnOx/SAPO-34 catalyst was prepared following the same method for comparison.
3.2. Catalysts Characterization
X-ray powder diffraction (XRD) measurement in the 2θ range of 0°–50° was obtained with the SmartLab (3KW) Japan Rigaku (Tokyo, Japan) X-ray diffractometer D8 using Cu Ka radiation (λ = 1.5418 Å). The scanning step size was 0.02°·s−1. The distribution of Mn, Si, Al, P and O species was observed using a field emission SEM in JEOL JSM-6700F (Tokyo, Japan) with X-ray energy-dispersive spectrometry (EDS). The micro-structural characterization by transmission electron microscope (TEM) images was determined by JEM-2010 (JEOL, Tokyo, Japan) with a working voltage of 200 KV. BET specific surface area and pore characterization were tested using the N2 adsorption–desorption on an ASAP 2020 (Drive Norcross, GA, USA) analyzer at −195 °C. X-ray photoelectron spectroscopy (XPS) was performed through the spectrum (K-Alpha+ ULTRA DLD) equipped with an Al Kα (1487 eV) radiation source.
Thermogravimetry (TG) was conducted using a STA449C-QMS403 thermal analyzer (Netisch, Germany) at a temperature range of 200–900 °C, with a heating rate of 10 °C min−1 in 5% O2/Ar. A Thermo Nicolet iS50 Spectrometer with a resolution of 4 cm−1 was used to measure the FT-IR spectra of catalysts. NH3 temperature-programmed desorption (NH3-TPD) experiments were conducted on the Tp-5080 (Xianquan Industrial and Trading Co., Ltd., Tianjin, China). To explore pyridine adsorption, the sample was dehydrated at 450°C under a dynamic vacuum (1.5 × 10−3 Pa), followed by saturated adsorption of pyridine at room temperature. Py-IR spectra were then evacuated at 200 °C. H2-TPR was conducted using a Tp-5080 (Xianqua, China) at a temperature range of 0–800 °C.
3.3. Catalysts Evaluation
A fixed-bed quartz flow reactor at atmospheric pressure was used to carry out SCR activity tests for the catalysts. The reaction temperature was increased from 25 to 240 °C at a rate of 5 °C/min, with an isotherm step of 20 °C. An amount of 800 mg of 40–60 mesh catalysts was used in each test. The simulated gas was composed of 800 ppm NH3, 800 ppm NO, 5.0% O2 and 100 ppm SO2 (when needed) and balanced by Ar. All of the tests were performed with a total flow rate of 600 mL/min and a gas hourly space velocity (GHSV) of 40,000 h−1. A NO-NO2-NOx analyzer (Thermal Scientific, model 42i-HL, Waltham, MA, USA) was used to measure the concentrations of NO and NO2. The conversion of NO was calculated by
where [NO]inlet refers to the concentration of the NO inlet gas and [NO]outlet refers to the concentration of the NO outlet gas.
CNO = (1 − [NO]outlet/[NO]inlet) × 100%
4. Conclusions
In this work, citric acid treatment was used to synthesize a series of hierarchical MnOx/SAPO-34 catalysts. It was found that the hierarchical pore structure improved the low-temperature activity and SO2 resistance of the MnOx/SAPO-34 catalyst. Among them, MnOx/H-SAPO-34-0.1 presented the optimal SCR performance with more than 90% NO conversion at 110–240 °C. Moreover, the impact of introducing SO2 to MnOx/H-SAPO-34-0.1 on NO conversion was lesser than that observed in the MnOx/SAPO-34 catalyst. Numerous characterizations demonstrated that the hierarchical pore structure significantly increased the BET surface area and Mn4+ percentage as well as the acid site quantity of the MnOx/SAPO-34 zeolite, all of which were responsible for improving the SCR activity at low temperatures. The results of TG/DTG showed that fewer manganese sulfate species and ABS formed on the surface of the MnOx/H-SAPO-34-0.1-S catalyst. The XPS results indicated that the MnOx/H-SAPO-34-0.1 catalyst retained a high ratio of Mn oxides with a high valence state after sulfation. These phenomena could be ascribed to the fact that the hierarchical pore structure facilitated the decomposition of surface sulphates deposited on the catalyst during the SCR reaction, thus effectively reducing the SO2 poisoning of active Mn sites. Overall, the low-temperature SCR activity and SO2 tolerance of the MnOx/SAPO-34 zeolite were significantly improved by the hierarchical pore structure, which could supply a rational strategy for the further design of Mn-based SCR catalysts.
Author Contributions
Writing, original draft preparation, L.Z.; writing, review and editing, L.Z., J.G. and C.Y.; funding acquisition, B.H.; supervision, B.H. All authors have read and agreed to the published version of the manuscript.
Funding
The project is financially supported by the National Natural Science Foundation of China (NSFC-51478191) and the Key Research and Development Plan of Guangdong Province (2019B110207001).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Zhang, T.; Chang, H.; Li, K.; Peng, Y.; Li, X.; Li, J. Different exposed facets VOx/CeO2 catalysts for the selective catalytic reduction of NO with NH3. Chem. Eng. J. 2018, 349, 184–191. [Google Scholar] [CrossRef]
- Xie, L.; Liu, F.; Ren, L.; Shi, X.; Xiao, F.-S.; He, H. Excellent Performance of One-Pot Synthesized Cu-SSZ-13 Catalyst for the Selective Catalytic Reduction of NOx with NH3. Environ. Sci. Technol. 2014, 48, 566–572. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Hammershoi, P.S.; Vennestrom, P.N.R.; Falsig, H.; Jensen, A.D.; Janssens, T.V.W. Importance of the Cu oxidation state for the SO2-poisoning of a Cu-SAPO-34 catalyst in the NH3-SCR reaction. Appl. Catal. B Environ. 2018, 236, 377–383. [Google Scholar] [CrossRef]
- Jangjou, Y.; Do, Q.; Gu, Y.; Lim, L.-G.; Sun, H.; Wang, D.; Kumar, A.; Li, J.; Grabow, L.C.; Epling, W.S. Nature of Cu Active Centers in Cu-SSZ-13 and Their Responses to SO2 Exposure. ACS Catal. 2018, 8, 1325–1337. [Google Scholar] [CrossRef]
- Wang, L.; Huang, B.; Su, Y.; Zhou, G.; Wang, K.; Luo, H.; Ye, D. Manganese oxides supported on multi-walled carbon nanotubes for selective catalytic reduction of NO with NH3: Catalytic activity and characterization. Chem. Eng. J. 2012, 192, 232–241. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.-W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Wang, C.; Sun, L.; Cao, Q.; Hu, B.; Huang, Z.; Tang, X. Surface structure sensitivity of manganese oxides for low-temperature selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 2011, 101, 598–605. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, Y.; Wu, Z. Low-temperature selective catalytic reduction of NO on MnOx/TiO2 prepared by different methods. J. Hazard. Mater. 2009, 162, 1249–1254. [Google Scholar] [CrossRef]
- Chang, H.; Chen, X.; Li, J.; Ma, L.; Wang, C.; Liu, C.; Schwank, J.W.; Hao, J. Improvement of Activity and SO2 Tolerance of Sn-Modified MnOx-CeO2 Catalysts for NH3-SCR at Low Temperatures. Environ. Sci. Technol. 2013, 47, 5294–5301. [Google Scholar] [CrossRef]
- Marban, G.; Fuertes, A.B. Kinetics of the low-temperature selective catalytic reduction of NO with NH3 over activated carbon fiber composite-supported iron oxides. Catal. Lett. 2002, 84, 13–19. [Google Scholar] [CrossRef]
- Li, L.; Sun, B.; Sun, J.; Yu, S.; Ge, C.; Tang, C.; Dong, L. Novel MnOx-CeO2 nanosphere catalyst for low-temperature NH3-SCR. Catal. Commun. 2017, 100, 98–102. [Google Scholar] [CrossRef]
- Yu, C.; Hou, D.; Huang, B.; Lu, M.; Peng, R.; Zhong, Z. A MnOx@Eu-CeOx nanorod catalyst with multiple protective effects: Strong SO2-tolerance for low temperature DeNOx processes. J. Hazard. Mater. 2020, 399, 123011. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Lee, J.; Kwak, S.-Y. Manganese oxides with hierarchical structures derived from coordination polymers and their enhanced catalytic activity at low temperature for selective catalytic reduction of NOx. Dalton Trans. 2019, 48, 16395–16401. [Google Scholar] [CrossRef] [PubMed]
- Fickel, D.W.; D’Addio, E.; Lauterbach, J.A.; Lobo, R.F. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B Environ. 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Korhonen, S.T.; Fickel, D.W.; Lobo, R.F.; Weckhuysen, B.M.; Beale, A.M. Isolated Cu2+ ions: Active sites for selective catalytic reduction of NO. Chem. Commun. 2011, 47, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, A.; Wang, W.; Xu, M.; Arnold, A.; Hunger, M. Thermal stability and dehydroxylation of Bronsted acid sites in silicoaluminophosphates H-SAPO-11, H-SAPO-81 H-SAPO-31, and H-SAPO-34 investigated by multi-nuclear solid-state NMR spectroscopy. Microporous Mesoporous Mater. 2002, 56, 267–278. [Google Scholar] [CrossRef]
- Li, R.; Wang, P.; Ma, S.; Yuan, F.; Li, Z.; Zhu, Y. Excellent selective catalytic reduction of NOx by NH3 over Cu/SAPO-34 with hierarchical pore structure. Chem. Eng. J. 2020, 379, 122376. [Google Scholar] [CrossRef]
- Liu, J.; Yu, F.; Liu, J.; Cui, L.; Zhao, Z.; Wei, Y.; Sun, Q. Synthesis and kinetics investigation of meso-microporous Cu-SAPO-34 catalysts for the selective catalytic reduction of NO with ammonia. J. Environ. Sci. 2016, 48, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Fan, G.; Gu, D.; Yu, S.; Ma, K.; Liu, A.; Tan, W.; Wang, J.; Du, X.; Zou, W.; et al. Pore Size Expansion Accelerates Ammonium Bisulfate Decomposition for Improved Sulfur Resistance in Low-Temperature NH3-SCR. ACS Appl. Mater. Interfaces 2019, 11, 4900–4907. [Google Scholar] [CrossRef]
- Arstad, B.; Lind, A.; Cavka, J.H.; Thorshaug, K.; Akporiaye, D.; Wragg, D.; Fjellvag, H.; Gronvold, A.; Fuglerud, T. Structural changes in SAPO-34 due to hydrothermal treatment. A NMR, XRD, and DRIFTS study. Microporous Mesoporous Mater. 2016, 225, 421–431. [Google Scholar] [CrossRef]
- Jin, H.X.; Gu, X.J.; Hong, B.; Lin, L.S.; Wang, C.Y.; Jin, D.F.; Peng, X.L.; Wang, X.Q.; Ge, H.L. Fabrication of Mesoporous Co3O4 from LP-FDU-12 via Nanocasting Route and Effect of Wall/Pore Size on Their Magnetic Properties. J. Phys. Chem. C 2012, 116, 13374–13381. [Google Scholar] [CrossRef]
- Shao, J.; Cheng, S.; Li, Z.; Huang, B. Enhanced Catalytic Performance of Hierarchical MnOx/ZSM-5 Catalyst for the Low-Temperature NH3-SCR. Catalysts 2020, 10, 311. [Google Scholar] [CrossRef]
- Tian, J.; Peng, H.; Xu, X.; Liu, W.; Ma, Y.; Wang, X.; Yang, X. High surface area La2Sn2O7 pyrochlore as a novel, active and stable support for Pd for CO oxidation. Catal. Sci. Technol. 2015, 5, 2270–2281. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Li, R.M.; Wang, M.J.; Li, Y.S.; Tong, Y.M.; Yang, P.P.; Zhu, Y.J. Two steps synthesis of CeTiOx oxides nanotube catalyst: Enhanced activity, resistance of SO2 and H2O for low temperature NH3-SCR of NOx. Appl. Catal. B Environ. 2021, 282, 119542. [Google Scholar] [CrossRef]
- Gao, J.; Han, Y.; Mu, J.; Wu, S.; Tan, F.; Shi, Y.; Li, X. 2D, 3D mesostructured silicas templated mesoporous manganese dioxide for selective catalytic reduction of NOx with NH3. J. Colloid Interface Sci. 2018, 516, 254–262. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.; Zhu, J.; Liu, Y.; Su, F.; Gao, S.; Xu, G. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with NH3. Appl. Catal. B Environ. 2010, 95, 160–168. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Shi, L.; Fang, C.; Li, H.; Gao, R.; Huang, L.; Zhang, J. In situ supported MnOx-CeOx on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2013, 5, 1127–1136. [Google Scholar] [CrossRef]
- Jin, W.; Wang, B.; Tuo, P.; Li, C.; Li, L.; Zhao, H.; Gao, X.; Shen, B. Selective Desilication, Mesopores Formation, and MTO Reaction Enhancement via Citric Acid Treatment of Zeolite SAPO-34. Ind. Eng. Chem. Res. 2018, 57, 4231–4236. [Google Scholar] [CrossRef]
- Li, J.; Guo, J.; Shi, X.; Wen, X.; Chu, Y.; Yuan, S. Effect of aluminum on the catalytic performance and reaction mechanism of Mn/MCM-41 for NH3-SCR reaction. Appl. Surf. Sci. 2020, 534, 147592. [Google Scholar] [CrossRef]
- Qiu, M.; Zhan, S.; Yu, H.; Zhu, D.; Wang, S. Facile preparation of ordered mesoporous MnCo2O4 for low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2015, 7, 2568–2577. [Google Scholar] [CrossRef]
- Sun, X.; Guo, R.-T.; Liu, J.; Fu, Z.-G.; Liu, S.-W.; Pan, W.-G.; Shi, X.; Qin, H.; Wang, Z.-Y.; Liu, X.-Y. The enhanced SCR performance of Mn/TiO2 catalyst by Mo modification: Identification of the promotion mechanism. Int. J. Hydrog. Energy 2018, 43, 16038–16048. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B Environ. 2013, 134, 251–257. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, J.; Yan, N.; Ma, L.; Chang, H. Low temperature selective catalytic reduction of NO with NH3 over Mn-Fe spinel: Performance, mechanism and kinetic study. Appl. Catal. B Environ. 2011, 110, 71–80. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, L.; Ning, P.; Gu, J.; Guan, Q. Surface characterization studies of CuO-CeO2-ZrO2 catalysts for selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2014, 317, 955–961. [Google Scholar] [CrossRef]
- Ren, Q.; Mo, S.; Peng, R.; Feng, Z.; Zhang, M.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Controllable synthesis of 3D hierarchical Co3O4 nanocatalysts with various morphologies for the catalytic oxidation of toluene. J. Mater. Chem. A 2018, 6, 498–509. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Wu, Z.B.; Liu, Y.; Lee, S.C.; Ho, W.K. DRIFT Study of the SO2 Effect on Low-Temperature SCR Reaction over Fe-Mn/TiO2. J. Phys. Chem. C 2010, 114, 4961–4965. [Google Scholar] [CrossRef]
- Wu, S.; Yao, X.; Zhang, L.; Cao, Y.; Zou, W.; Li, L.; Ma, K.; Tang, C.; Gao, F.; Dong, L. Improved low temperature NH3-SCR performance of FeMnTiOx mixed oxide with CTAB-assisted synthesis. Chem. Commun. 2015, 51, 3470–3473. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y.; Yan, N.; Wu, D.; He, H.; Qu, Z.; Yang, C.; Zhou, Q.; Jia, J. Nanosized Cation-Deficient Fe-Ti Spinel: A Novel Magnetic Sorbent for Elemental Mercury Capture from Flue Gas. ACS Appl. Mater. Interfaces 2011, 3, 209–217. [Google Scholar] [CrossRef]
- Liu, H.; Fan, Z.X.; Sun, C.Z.; Yu, S.H.; Feng, S.; Chen, W.; Chen, D.Z.; Tang, C.J.; Gao, F.; Dong, L. Improved activity and significant SO2 tolerance of samarium modified CeO2-TiO2 catalyst for NO selective catalytic reduction with NH3. Appl. Catal. B Environ. 2019, 244, 671–683. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, F.; Shan, W.; He, H. Improvement of Nb Doping on SO2 Resistance of VOx/CeO2 Catalyst for the Selective Catalytic Reduction of NOx with NH3. J. Phys. Chem. C 2017, 121, 7803–7809. [Google Scholar] [CrossRef]
- Li, B.; Ren, Z.; Ma, Z.; Huang, X.; Liu, F.; Zhang, X.; Yang, H. Selective catalytic reduction of NO by NH3 over CuO-CeO2 in the presence of SO2. Catal. Sci. Technol. 2016, 6, 1719–1725. [Google Scholar] [CrossRef]
- Bai, B.; Arandiyan, H.; Li, J. Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts. Appl. Catal. B Environ. 2013, 142, 677–683. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R.; Liu, Y.; Wang, H. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal. Commun. 2008, 9, 2217–2220. [Google Scholar] [CrossRef]
- Shao, J.; Lin, F.; Huang, Y.; Wang, Z.; Li, Y.; Chen, G.; Cen, K. MnOx fabrication with rational design of morphology for enhanced activity in NO oxidation and SO2 resistance. Appl. Surf. Sci. 2020, 503, 144064. [Google Scholar] [CrossRef]
- Li, C.; Tang, X.; Yi, H.; Wang, L.; Cui, X.; Chu, C.; Li, J.; Zhang, R.; Yu, Q. Rational design of template-free MnOx-CeO2 hollow nanotube as de-NOx catalyst at low temperature. Appl. Surf. Sci. 2018, 428, 924–932. [Google Scholar] [CrossRef]
- Ma, Z.; Sheng, L.; Wang, X.; Yuan, W.; Chen, S.; Xue, W.; Han, G.; Zhang, Z.; Yang, H.; Lu, Y.; et al. Oxide Catalysts with Ultrastrong Resistance to SO2 Deactivation for Removing Nitric Oxide at Low Temperature. Adv. Mater. 2019, 31, 1903719. [Google Scholar] [CrossRef]
- Pan, S.; Luo, H.; Li, L.; Wei, Z.; Huang, B. H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3. J. Mol. Catal. A Chem. 2013, 377, 154–161. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).