1. Introduction
The layered material MCM-56 is a unique representative of two-dimensional (2D) zeolites as a delaminated form obtained by direct synthesis with high activity and ease of modification [
1,
2,
3,
4,
5]. It is the first layered zeolite exfoliated directly by soft-chemical treatment. MCM-56 can show activity higher than the conventional MWW forms MCM-22 and MCM-49 [
6] but is an intermediate during the preparation of MCM-49 and, upon prolonged synthesis, it converts to this 3D form. Recently, Xing et al. [
7] reported that addition of aniline to the MCM-56 synthesis delays the conversion to MCM-49 so it can be viewed as the final product (referred to as ‘temperature-controlled phase transformation’). Aniline is called a structure-promoting agent which facilitates crystallization but is not trapped within the framework; thus, it efficiently prevents layer condensation. Jiang et al. [
8] proposed an easy double templating method to control the crystallization of MCM-56, involving addition of tetraethyl ammonium hydroxide (TEAOH) to the HMI-containing synthesis mixture. This synthetic route yielded pure, delaminated MCM-56 material, with an extended phase stabilization period. The original MCM-56 prepared with HMI only has been extensively studied with regard to swelling and pillaring [
4]. To the best of our knowledge, there seems to be no reports concerning modification of the MCM-56 obtained with the additives mentioned above [
9].
The structure of MCM-56 is described as two-dimensional (2.5 nm thick MWW layers stacked randomly with only accidental interlayer bonds) [
1]. Acid centers located on the surface of the layers may potentially catalyze reactions of bulky molecules [
10]. As a common modification, a two-step process is often applied to open the structure and enhance accessibility of the acid centers by creating additional porosity between the zeolite layers through expansion of the interlayer space by swelling or intercalation followed by stabilization (e.g., pillaring with tetraethylorthosilicate/TEOS) or complete layer separation (delamination). The original pillared MWW zeolite was called MCM-36 [
11]. Ultrasound treatment has been reported to facilitate exfoliation and layer dispersion [
3,
12,
13,
14] but if layers are coated with surfactant (hydrophobic surface), the separation would entail exposure to hydrophilic environment. This should favor the status quo rather than enhanced separation. Of course, there may be mechanical enhancement due to fracturing of the layers.
Increased accessibility of acid centers in expanded layered zeolites makes them attractive as catalysts for the transformation of large organic molecules. The Friedel-Crafts reaction is most often carried out in the liquid phase with the use of halogens as alkylation agents and acid homogeneous catalysts, e.g., FeCl
3, AlCl
3, BF
3. Their application is associated with problems related to corrosion of the installation as well as recovery, separation, regeneration and disposal of the toxic catalyst waste [
15]. Due to stricter environmental protection requirements, attempts are being made to replace homogeneous catalysts with solid acids (e.g., zeolites) [
16] and perform alkylation reactions using alkyl alcohols instead of chlorides. MWW zeolites have shown particular benefits in this regard.
The aim of this work was to explore two pillaring procedures involving sonication treatment in an attempt to increase availability of the active centers. One procedure was adopted from the standard swelling and pillaring method often employed for modification of MWW zeolites, using excess amount of TEOS [
3,
11]. The other pillaring method was based on work by Letaïef et al. [
17], in which alcohols were used as a medium controlling hydrolysis of TEOS in pillaring of layered clays. These authors concluded that their system allows better controlled TEOS hydrolysis and may be an attractive alternative not only for clays but also other layered solids. Herein, this method is applied to MCM-56 as a model layered zeolite material. The obtained modified zeolites were tested in Friedel-Crafts alkylation of mesitylene with benzyl alcohol, a reaction involving bulky organic molecules, designed to test the activity of the external surfaces of the zeolites [
10,
18].
2. Results and Discussion
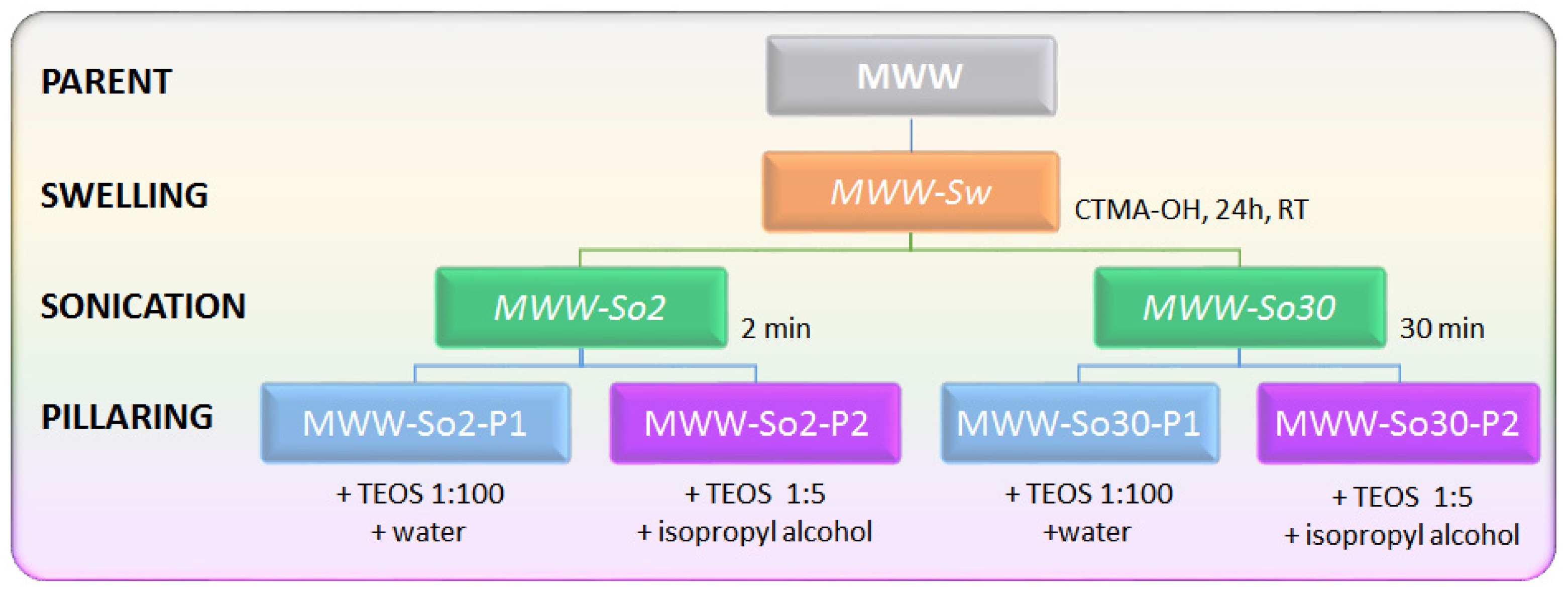
The general outline of post-synthetic modifications of MCM-56 zeolite employed in this study is presented in
Scheme 1. P1 denotes classical pillaring with the excess amount of TEOS, while P2, pillaring with TEOS in the presence of isopropanol. All of the final products were calcined in air at 540 °C. Detailed description of the synthesis and modifications is presented in
Section 3.1.
The analysis of the XRD patterns has shown that the basic MCM-56 (MWW) structure of all studied materials has been preserved during applied modifications. This identification is based on the position of the intralayer 100 reflection (2θ = 7.2°, Cu Kα radiation used throughout) and the presence of a wide “band” without the dip between 2θ = 8–10° indicating the lack of layer ordering in the c direction (
Figure 1b). The fact that position of the intralayer reflection (100) remains unchanged upon modification confirms that the internal structure of the MWW layers is preserved. The emergence of maxima at lower 2θ angles (
Figure 1a) indicates maintained layer separation after pillaring. The 001 reflection is observed with expanded materials and interlayer d-spacings ranges from 4.34 to 4.65 nm depending on the material (
Table 1).
The nitrogen adsorption-desorption isotherms are shown in
Figure 2a. The isotherm exhibited by the parent material (MCM-56, denoted as MWW) is characteristic for a microporous solids with significant external surface area, reaching almost 40% of the total surface area (469 and 183 m
2/g, respectively) and some large, most probably interparticle mesopores, as suggested by a wide, H2b-type hysteresis loop [
19]. Isotherms for all modified samples show considerable increase of adsorption, corresponding to secondary mesoporosity introduced upon swelling and pillaring. The materials synthesized with the use of isopropanol (the P2 series: MWW-So2-P2 and MWW-So30-P2) exhibit increased adsorption at low relative pressure (p/p
0 up to 0.3), isotherms without hysteresis loops, revealing presence of wide micropores or narrow mesopores. It is evident that in the case of two other pillared samples (the P1 series: MWW-So2-P1 and MWW-So30-P1), adsorption isotherms are of type IV with H4 hysteresis loops. Closing of the hysteresis loop at p/p
0 equal to 0.5 is usually attributed to the presence of ink-bottle mesopores or interconnected, partially constricted mesopore systems. In this case, this would correspond to the overlapping effects of formation of narrow intraparticle mesopores, formed due to pillaring and much wider and less ordered interparticle mesopores.
The findings based on the N
2 adsorption data were corroborated by the results of Quasi-Equilibrated Temperature Programmed Desorption and Adsorption (QE-TPDA) of hexane and nonane. This characterization method, developed for characterization of micro- and mesoporous materials, has been proven useful in studies of modified zeolites [
20,
21,
22,
23,
24,
25]. The QE-TPDA profiles are recorded while the studied sample is cyclically heated and cooled in the flow of helium containing small admixture (<1 mol%) of the hydrocarbon vapor. The maxima that were recorded during heating correspond to desorption from the pores, while the minima that were observed during cooling correspond to adsorption. The higher the adsorption/desorption temperature, the smaller the corresponding pore size. By judicious choice of the adsorbate, its content in the carried gas and the heating/cooling rate, one may select a range of the pore sizes for detailed analysis.
The QE-TPDA profiles of hexane (
Figure 2b) are restricted to the adsorption in the micropores, since they were recorded at low relative partial pressure of the adsorptive, far from the saturation conditions (200 mbar at 25 °C). They exhibit narrow maxima at about 50 °C, observed just after starting heating of the sample in the desorption phase of the QE-TPDA experiment. These maxima represent larger micropores (e.g., distorted structural ones) or the strongest adsorption sites either on the external surface or on the surface of the mesopores. They are most intense for the –P2 (pillared with isopropanol present) zeolites, for which high contribution of wide micropores or narrow mesopores was found. Additional wide maxima that are observed in the high temperature range (100–200 °C) correspond to structural micropores formed by 10-rings channels, characteristic for the MWW layers. It is evident that to some extent that these micropores were retained after pillaring (
Table 1). However, as it is not possible to separate the low and high temperature desorption peaks in the QE-TPDA profiles of hexane, especially for the modified zeolites. In this technique, one may expect the presence of a broad continuous distribution of the pore sizes in the micropore region. For this reason, the micropore volumes calculated by integration of these profiles are larger than those obtained from the N
2 adsorption data, especially for the pillared materials.
The differences in porosity of the studied materials are better illustrated in
Figure 2c showing pore size distributions, calculated from the desorption branches of the N
2 sorption data, using the BJH scheme. For the –P2 materials modified with the use of isopropanol, the calculations confirm the presence of unique intermediate pores with the size of about 2 nm, while the P1 materials also contain larger mesopores with broad pore size distributions (4–10 nm in the case of MWW-So2-P1). As noted on previous occasions [
26], sharp peaks at 4 nm are common in modified layered materials and do not correspond to actual pore sizes, but result from the instability of liquid-like adsorbate at relative pressures below 0.45 p/p
0. For more detailed porosity analysis, the NLDFT method was applied. The corresponding plots are shown in
Figure S1.
Generally, the type of porosity shown by the modified materials for the classically pillared samples (the P1 series) seem to depend also on the sonication time: the longer the sonication, the higher the mesopore volume and the external surface area (
Table 1, 0.16 vs. 0.28 cm
3/g and 197 vs. 245 m
2/g, for the samples sonicated for 2 and 30 min, respectively). Interestingly, the micropore volume remains almost unchanged (the small increase from 0.05 to 0.06 cm
3/g is within the measurement accuracy). This would corroborate the conclusion that apart from the formation of intralayer mesopores, the mutual layer arrangement changed, hence the presence of the interlayer mesopores of wide size distribution. The porosity of the –P2 series is practically independent of the sonication time—for samples sonicated for 2 and 30 min, both the mesopore volumes and external surface area are similar (0.22 vs. 0.25 cm
3/g, and 148 vs. 143 m
2/g). These would correspond to the mechanism, proposed by Letaïef [
17], where hydrolysis of TEOS in presence of alcohol resulted in the pillared structure in which mainly the interlayer ordering was influenced.
The acid sites in aluminosilicates may be either of Brønsted or Lewis character. Acidic protons may come from Si-OH-Al groups (strong Brønsted acid sites)—the primary source of strong acid activity, surface silanols (non-acidic or weak Brønsted acid sites), or acidic OH groups associated with extraframework or partially framework Al species (usually weak Brønsted acid sites).
Figure 3 shows that for the studied materials the external, terminal silanols are characterized by the IR band at 3747 cm
−1 and the intensity of this band systematically increased and widened upon modification (better seen in
Figure S2). This is consistent with the introduction of amorphous silica as the pillaring material. Additionally, for pillared samples the main position of this band moves to 3743 cm
−1, which is characteristic of internal silanols located at the structural defects, including geminal silanols Si(OH)
2, due to the presence of OH groups from pillars made of amorphous SiO
2. Hydroxyls associated with extraframework or partial framework Al (3670 cm
−1) is visible in the spectrum of the parent material and the sample MWW-So2-P1. For all others, this band is practically invisible, and even if present, it may be hidden under the wide silanol tail that is characteristic of amorphous silica.
The Si-OH-Al hydroxyls, the strong Brønsted acid sites (BAS) for MWW structure are characterized by IR bands at 3624 cm
−1 with a very weak shoulder at ca. 3575 cm
−1 (marked by two arrows in
Figure 3). The former maximum corresponds to OH located in supercages, external cups and 10-member rings, while the latter to the OH groups in the double six-memberd rings connecting the supercages (corresponding to the OH group connected with the unusual, hidden T3 position of the Al atom) [
27,
28].
The intensity of the 3624 cm−1 maximum gradually decreases upon modification, which corresponds to a BAS concentration decrease from 900 to 140 μmol/g. It can be deduced that sonication time (2 vs. 30 min) has less effect on the acidity than pillaring procedure. The samples from the –P1 series were pillared with TEOS only, at the 1:100 w/w ratio, and therefore with substantial excess of silica. In contrast, the –P2 samples showed only small TEOS excess (1:5 w/w ratio) in the presence of the isopropyl alcohol. It is interesting to note that the concentration of acid centers in samples pillared with higher TEOS concentration is in fact much higher than for samples pillared with much lower TEOS concentration. At the same time, the amount of Al, determined by XRF, is not considerably decreased. The simplest explanation for this is that deposited silica covers the Al sites and blocks access to them. Other factors cannot be ruled out, e.g., migration of Al atoms into other (non-Brønsted) locations.
Judging solely on the concentration of the BAS in the final pillared samples, the alcohol-aided procedure proposed by the group of Ruiz-Hitzky [
17] would appear not to be optimal for layered zeolites, since its value is reduced by 70–80% compared to the classic pillaring (
Table 2,
Figure S4, BAS concentration 514 vs. 171, and 671 vs. 136 μmol/g, comparing MWW-So2-P1 with MWW-So2-P2 and MWW-So30-P1 with MWW-So30-P2, respectively) and decreases by more than 90% in comparison to the parent material (initial BAS concentration 899 μmol/g). This had only a minor effect on the overall conversion but at the same time enhanced turnover frequency, indicating there may be benefits to the isopropanol assisted pillaring.
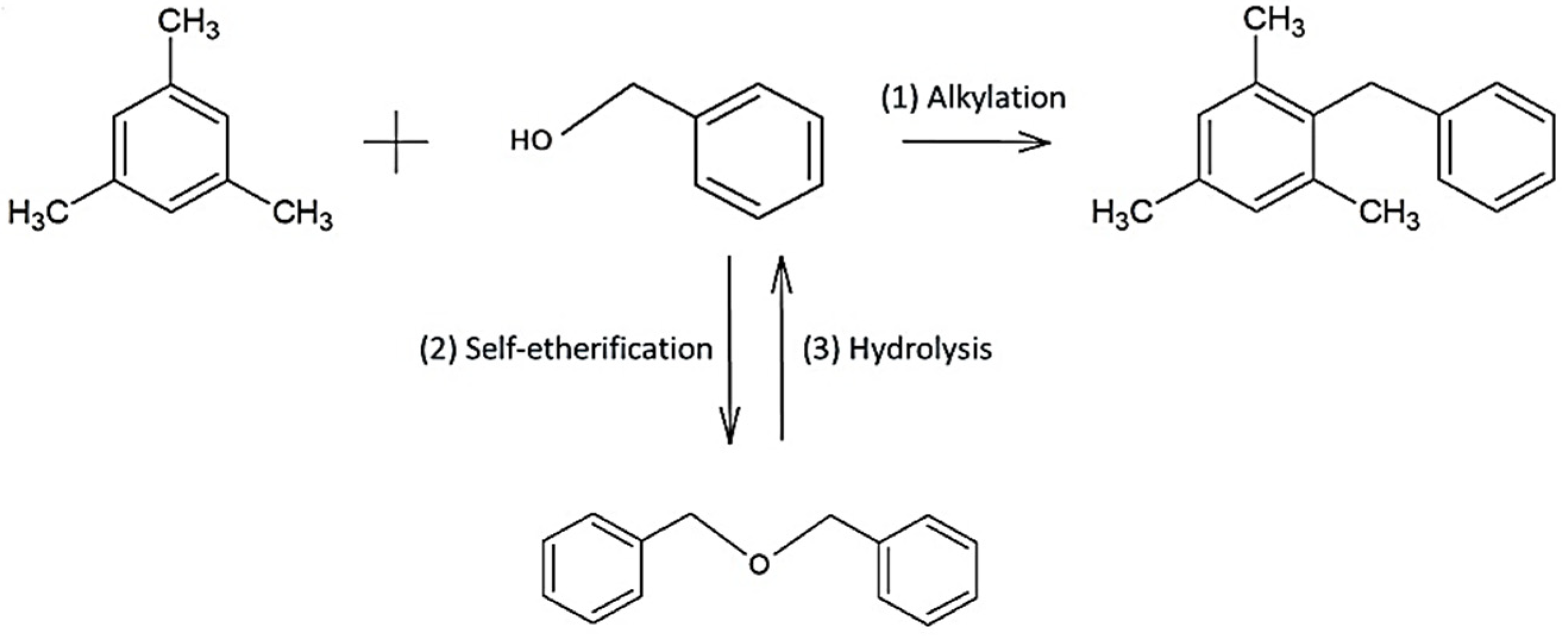
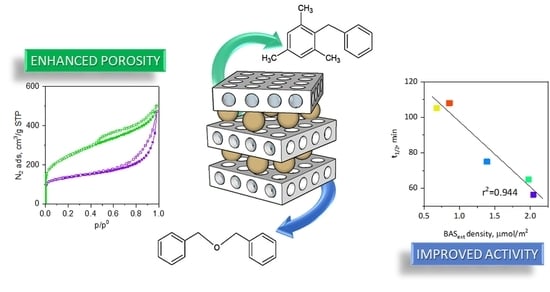
The goal of pillaring is an enhancement of mesoporosity to increase activity towards larger molecules. To evaluate this result, the Friedel-Crafts alkylation of mesitylene with benzyl alcohol was carried as the model test reactions. The main product in this reaction is 2-benzyl-1,3,5-trimethylbenzene (BTB), with dibenzyl ether (DBE) being the only side product (
Scheme 2).
To investigate the catalytic properties/activity of the studied MWW materials, we performed catalytic experiments in the liquid phase batch reactor.
Figure 4a shows characteristic kinetic curves with linear dependence of conversion vs. time, implying constant reaction rate (or zero order kinetics). The 100% conversion of benzyl alcohol was obtained after ca. 180 min. Initially, formation of dibenzyl ether was observed (with maximum selectivity of ca. 10%), but later it was completely transformed to the main product (benzylated mesitylene) (
Figure 4b).
Comparison of the kinetic curves (
Figure 4a) shows high activity of the parent zeolite, with t
1/2 equal 56 min (
Table 2). Kinetic curves observed for all of the modified materials exhibited smaller slopes (i.e., lower overall reaction rate). However, comparison of the turnover frequency (TOF) data shows considerable increase of individual activity of the acid centers, especially in the materials pillared according to the P2 method (alcohol addition). Low values of acid sites concentration found for the P2 series may indicate high content of the amorphous silica introduced during modification, despite smaller TEOS excess. This seems justified by long reaction time of the pillared zeolite with TEOS.
The rate of the secondary reaction (transformation of DBE to BTB) was the slowest for the –P2 series, followed by the P1 one, while the transformation of DBE to BTB was the fastest for the parent material, but this is due to different overall reaction rate. If conversion vs. selectivity is drawn, then at the same conversion level there is no difference in selectivities (
Figure S3).
Lower concentration of the BAS in modified samples explains lower reaction rates and longer reaction half-time. On the other hand, it is important to note that even if BAS concentration dropped almost 7-fold (from 899 to 135 μmol/g), the 100% conversion is reached after 120 min for parent material and after ca. 240 min for MWW-So30-P2. It is evident that a single acid center becomes more active, which can be expressed quantitatively by calculation of the TOF values. The TOF values are the highest for materials pillared in the presence of alcohol (4.41 × 10
−3 and 5.23 × 10
−3 s
−1 for MWW-So2-P2 and MWW-So30-P2, respectively) comparing to 1.69 × 10
−3 and 2.09 × 10
−3 s
−1 for other samples. This may also be linked to the availability of the acid centers to bulky molecules. To check this availability, additional IR measurements were carried out and involved the adsorption of pivalonitrile (PN), a molecule which cannot diffuse through 10-membered rings in MWW zeolite and therefore can be used to quantify the BAS on the external surfaces (BAS
ext,
Table 2). It is evident that, even if the overall concentration of BAS decreased upon modifications, the accessibility increased (from 42% in the parent MWW, to 90% in MWW-So30-P2). Unfortunately, the decrease in BAS concentration was not compensated by the increased availability of these centers, and the actual concentration of BAS
ext increased only for MWW-So30-P1 (from 374 to 474 μmol/g) but decreased for any other sample. It should be noted that MWW-So30-P1, even though it possessed the highest concentration of the external acid sites, was not the most active. It seems that the important factor is BAS
ext density, corresponding to their actual accessibility for the reactants, calculated as the BAS
ext concentration divided by the externals surface (S
ext). If reactivity (in term of t
1/2) was plotted against BAS
ext density, the dependence was linear (
Figure 4c).
Another important factor influencing the catalytic activity of an individual acid center may be their acid strength. Acid strength may be determined either by the adsorption of strong bases, leading to proton transfer, or by the interaction with weaker bases, leading to the formation of a hydrogen bond. Both processes may be followed by IR spectroscopy. Acid strength, determined by thermodesorption of bases, may depend not only on the intrinsic strength of acid sites, but also on the presence or absence of diffusion limitations. In other words, diffusion may be slowed down in micropores falsely implying higher acid strength to OH groups located there. Adsorption of weaker bases, like CO, more directly depends on the intrinsic acid strength—the higher the red-shift of the IR maximum of given OH group upon interaction with the weak base, the stronger the acid center [
29]. This method has also drawbacks, since a hydrogen-bonded molecule may also interact with surrounding oxygens defining the zeolitic channel, and is subjected to channel size-dependent dispersive interactions [
30]. However, it has been proposed that the CO molecule are less influenced than other molecules (N
2 or acetonitrile) [
31].
In the present investigation, both methods were applied for three selected samples: MWW, MWW-So30-P1, and MWW-So30-P2. CO was adsorbed, the value of the red-shift of the Si-OH-Al groups was determined and compared with the results of pyridine thermodesorption (
Figure 5 and
Figure S5,
Table S1). The value of the bathochromic shift was comparable for all samples (312 to 325 cm
−1). In the study of pyridine thermodesorption, the fraction of the pyridinium ions still present after each desorption step was also comparable for all samples. Therefore, it may be concluded that the results of both experiments indicate no significant difference in the acid strength among the studied samples, and therefore the concentration of accessible BAS centers have the biggest influence on the catalytic activity.
3. Materials and Methods
3.1. Catalyst Synthesis
MCM-56 zeolite with aniline (AN) was synthesized according to the procedure described in the literature [
32]. The synthesis mixture comprised deionized water, 50% NaOH solution (Sigma Aldrich Poland, Poznań, Polska), sodium aluminate (Riedel-de-Haen, Seelze, Germany, 40–45% Na
2O, 50–56% Al
2O
3), hexamethyleneimine (HMI, 99%, Sigma Aldrich Poland, Poznań, Polska), aniline (AN, > 99%, Sigma Aldrich Poland, Poznań, Polska) and Aerosil (A200, Evonic, Piscataway, NJ, USA) in the following molar ratios: Si/Al = 12.5, OH/Si = 0.18, HMI/Si = 0.1, AN/Si = 0.2 and H
2O/Si = 45. The crystallization was carried out in a Teflon-lined autoclave (200 mL) at 143 °C for 352 h, static approximately half of the time and then rotated till completion. The products were isolated by centrifugation, washed three times with distilled water and dried in air. The parent MCM-56 sample is denoted as MWW.
Swelling process was carried out using cetyltrimethylammonium hydroxide solution (CTMA-OH, made in-house) [
33]. The mixtures, containing 0.5 g of as-made MCM-56 and 20 g CTMA-OH, were stirred for 24 h at room temperature. The products were isolated by centrifugation, washed three times with distilled water and dried in air.
The swollen samples were sonicated using Ultrasonic Processor VCX 130 (Sonics & Materials, Newtown, CT, USA) with stepped microtips (70% amplitude), either for 2 min (denoted as MWW-So2) or 30 min. (denoted as MWW-So30), acidified with HCl to pH below 2, separated, washed with water (three times) and dried in air [
3].
In the first series (–P1), pillaring of the swollen and sonicated zeolite was carried out using tetraethylorthosilicate (TEOS, Sigma Aldrich Poland, Poznań, Polska) with 1:100
w/
w ratio [
34]. The reaction mixtures were stirred at RT for 24 h. The solids were filtered on a Büchner funnel (without washing) and dried at room temperature in air.
In the second series (–P2), after swelling with CTMA-OH, the zeolites were pillared with an addition of isopropyl alcohol. The wet solids obtained after centrifugation from swelling agent were washed three times with distilled water. Immediately afterwards, 13 mL of 2-propanol was added, and the mixtures were stirred for 2 h. After that, 2.6 g tetraethylorthosilicate (TEOS) was added (1:5 w/w ratio in relation to the initial zeolite mass), and then the mixtures were stirred for 7 days at RT. The solids were filtered on a Büchner funnel (without washing) and dried at room temperature in air.
The final products were obtained after calcination at 540 °C for 6 h in air (ramp at 2 °C/min) and exchange with 1M NH4NO3 solution (three times, 0.5 g of the zeolite in 20 mL of solution, 1 h at room temperature), washed with deionized water, dried, and activated at 500 °C.
3.2. Catalyst Characterization
X-ray powder diffraction (XRD) was carried out using a Bruker AXS D8 Advance diffractometer (Bruker, Billerica, MA, USA) equipped with a graphite monochromator (Bruker, Billerica, MA, USA), position sensitive detector (Våntec-1, Bruker, Billerica, MA, USA) in Bragg-Brentano geometry in the range 1–10° 2θ and Rigaku MiniFlex diffractometer (Rigaku, TheWoodlands, TX, USA) in refection mode. CuKα radiation (ʎ = 0.154 nm) was used in the ranges 3–30° 2θ. The XRD patterns were collected with steps of 0.02°.
Relative content of Al and Si was determined in the samples formulated into pellets, 13 mm in diameter, with the use of Energy-Dispersive XRF spectrometer (Thermo Scientific, ARL QUANT’X, Waltham, MA, USA). The X-rays of 4–50 kV (1 kV step) with the beam size of 1 mm were generated with the Rh anode. The detector used was a 3.5 mm Si(Li) drifted crystal with a Peltier cooling (ca. −90 °C). For quantitative analysis, calibration with a series of metallic standards and UniQuant software (Version 3, Thermo Fisher, West Palm Beach, FL, USA) were used.
Characterization of Lewis (LAS) and Brønsted (BAS) acid sites was carried out using adsorption of pyridine (absorption coefficients: ε
LAS = 0.165 cm
2/μmol, and ε
BAS = 0.044 cm
2/μmol), pivalonitrile and CO [
35,
36] followed by IR spectroscopy (Tensor 27 from Bruker, Billerica, Ettlingen, Germany, MTC detector, spectral resolution 2 cm
−1). Zeolites were pressed into self-supporting wafers and activated in situ at 500 °C for 1 h at high vacuum (10
−5 mbar) in a home-made quartz cell, equipped with CaF
2 windows. The cell construction allowed in situ activation, measurement of the spectra at chosen temperature and adsorption of gases and vapors inside the infrared spectrometer. Before adsorption of a probe molecule the system was cooled to the proper adsorption temperature: 170 °C for pyridine (Py), ambient temperature for pivalonitrile (PN), and ca. −100 °C for CO. After adsorption of the vapors (ca. 20 mbar equilibrium pressure for Py and PN, and ca. 5 mbar for CO) the gas phase together with weakly adsorbed species were evacuated at the adsorption temperature for 20 min (Py and PN) and 1 min for CO. In pyridine thermodesorption experiments, additional desorption steps at 350 and 450 °C were performed (20 min each), after each desorption sample was cooled down to 170 °C and the spectrum collected. All spectra were recalculated to the same pellet mass, equal 10 mg.
Nitrogen isotherms were determined by the standard method at −196 °C (liquid nitrogen temperature) using an ASAP2025 (Micromeritics, Norcross, GA, USA) static volumetric apparatus and autosorb iQ Gas Sorption System (Quantachrome, Boynton Beach, FL, USA). Before experiments, the samples were outgassed at 350 °C using a turbomolecular pump to remove pre-adsorbed water.
The porosity was also studied by quasi-equilibrated temperature programmed desorption and adsorption (QE-TPDA,home-made equipment) of hexane. The instrumentation and experimental procedures were described in detail previously [
20,
21,
22]. Prior to the QE-TPDA measurements a sample (6–7 mg) placed in a quartz tube was activated by heating up to 400 °C (10 °C/min) in flow of helium (7 cm
3/min), then cooled to room temperature. After activation, the hexane vapor was added to the helium stream resulting in isothermal sorption at room temperature. The signal of the thermal conductivity detector, consisting of desorption maxima and adsorption minima, recorded during cyclic heating and cooling of the sample at constant rate, represented a QE-TPDA profile.
The catalytic test reaction, liquid phase alkylation of mesitylene with benzyl alcohol, was carried out in a three-necked round-bottom equipped with a reflux condenser with heating in a multi-experiment workstation StarFish (Radleys Discovery Technologies, Saffron Walden, UK) under atmospheric pressure. The reaction temperature was 80 °C. First, reaction samples were activated at 500 °C for 5 h in air. Then 22 mL of mesitylene, 50 mg of the studied catalyst and 0.1 g of dodecane, as an internal standard, were mixed. The reaction mixture was maintained for 30 min at 80 °C and then 0.2 g of benzyl alcohol was added. This was considered as the beginning of the reaction. Liquid samples (0.4 mL) were withdrawn at regular intervals (0.5, 3, 5, 10, 20, 30, 60, 120, 180, 240, 300, and 360 min) and analyzed by the gas chromatograph PerkinElmer Clarus 600 GC (PerkinElmer, Shelton, CT, USA) with an FID detector (PerkinElmer, Shelton, CT, USA) using a 30 m packed column Elite-1MS (PerkinElmer, Shelton, CT, USA).