Photoactive Heterostructures: How They Are Made and Explored
Abstract
Simple Summary
Abstract
1. Introduction
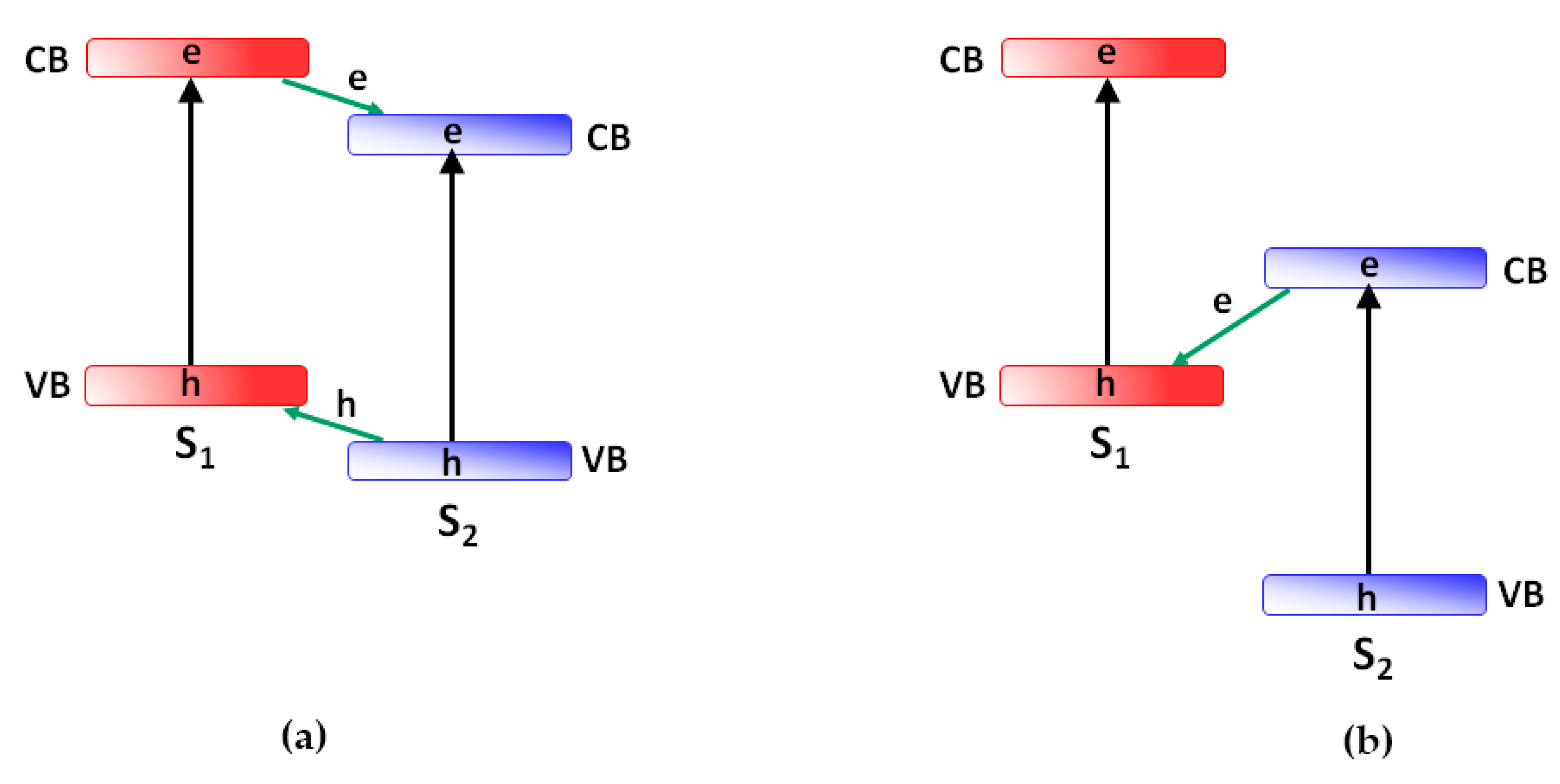
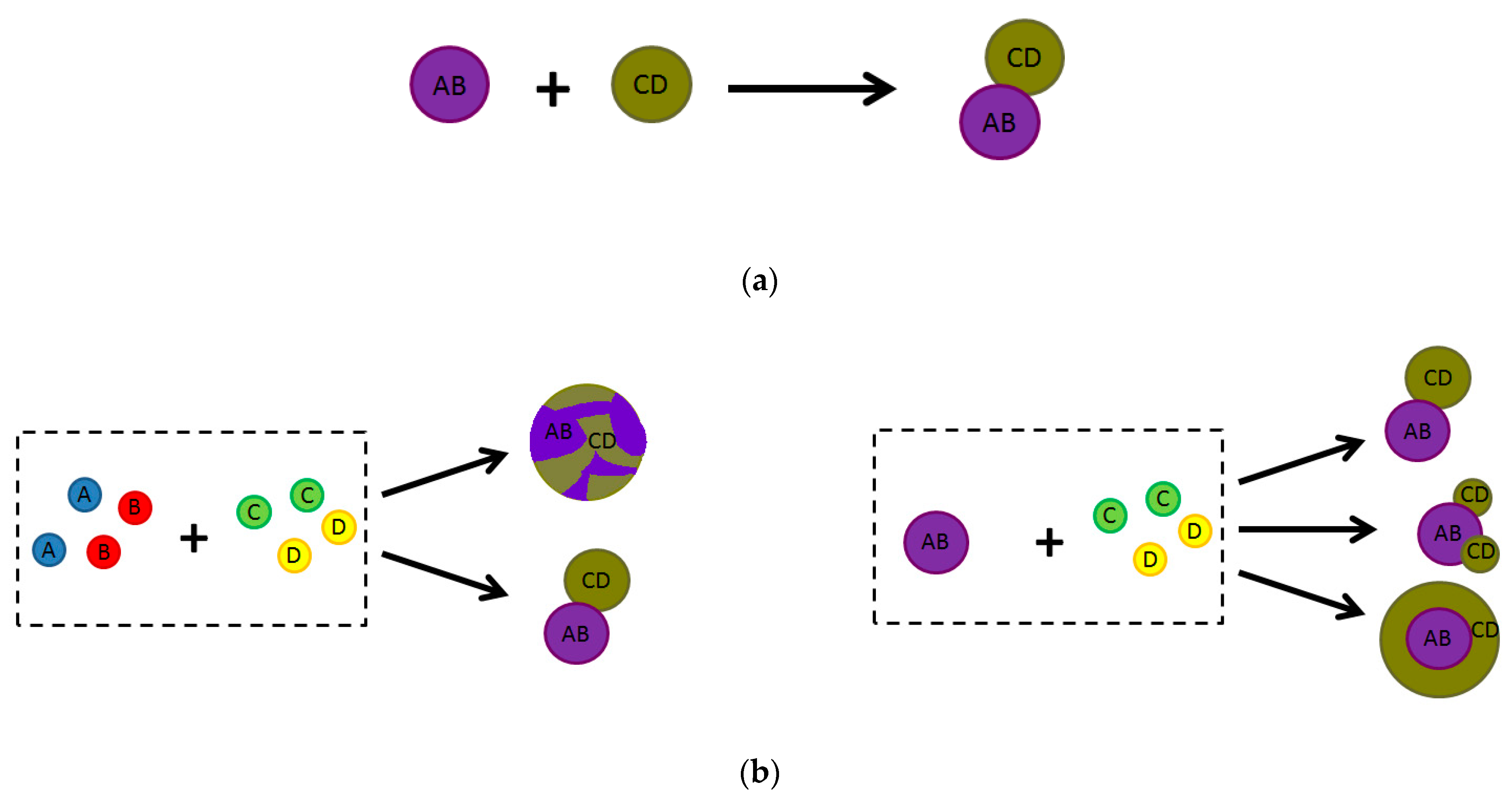
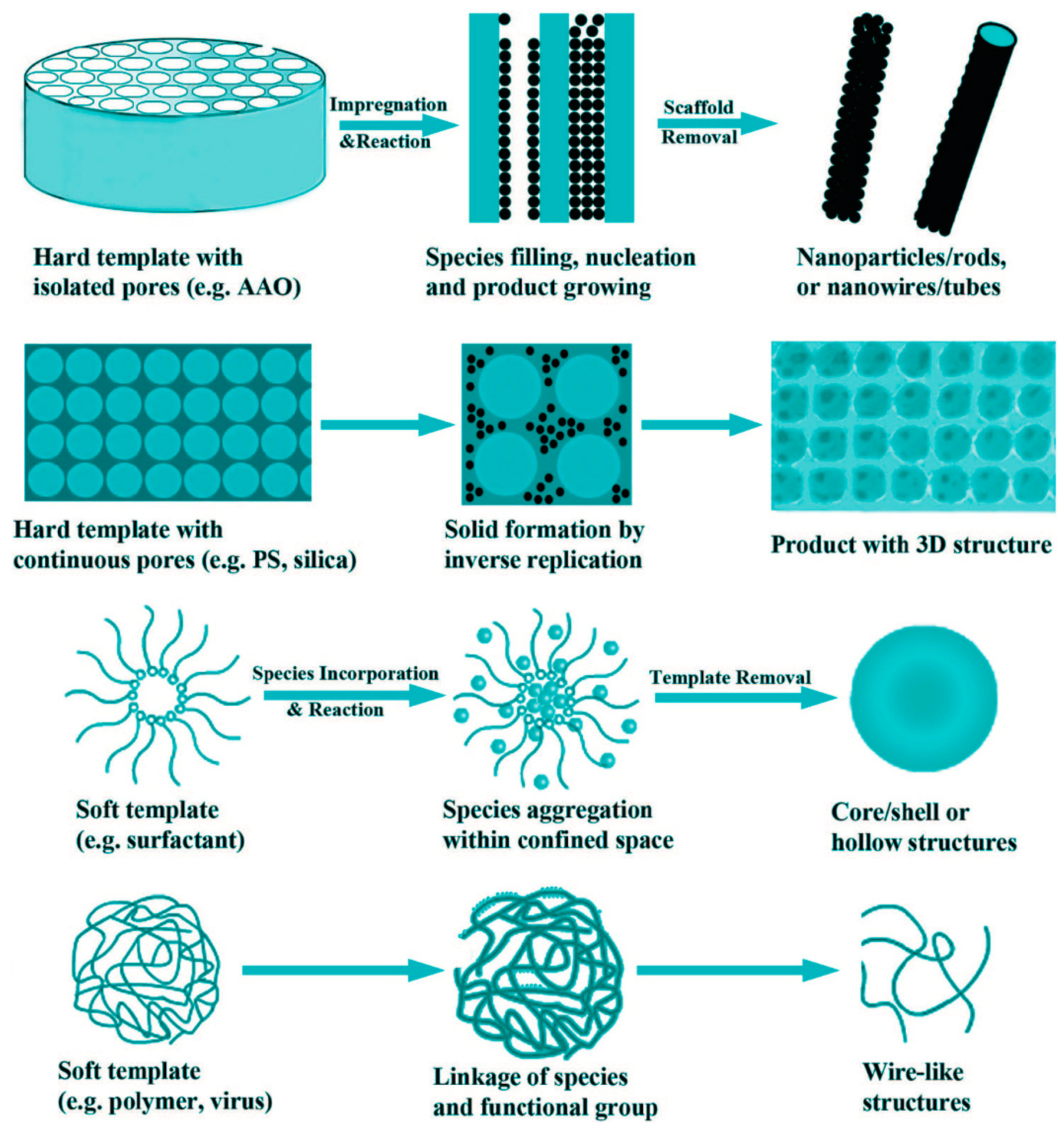
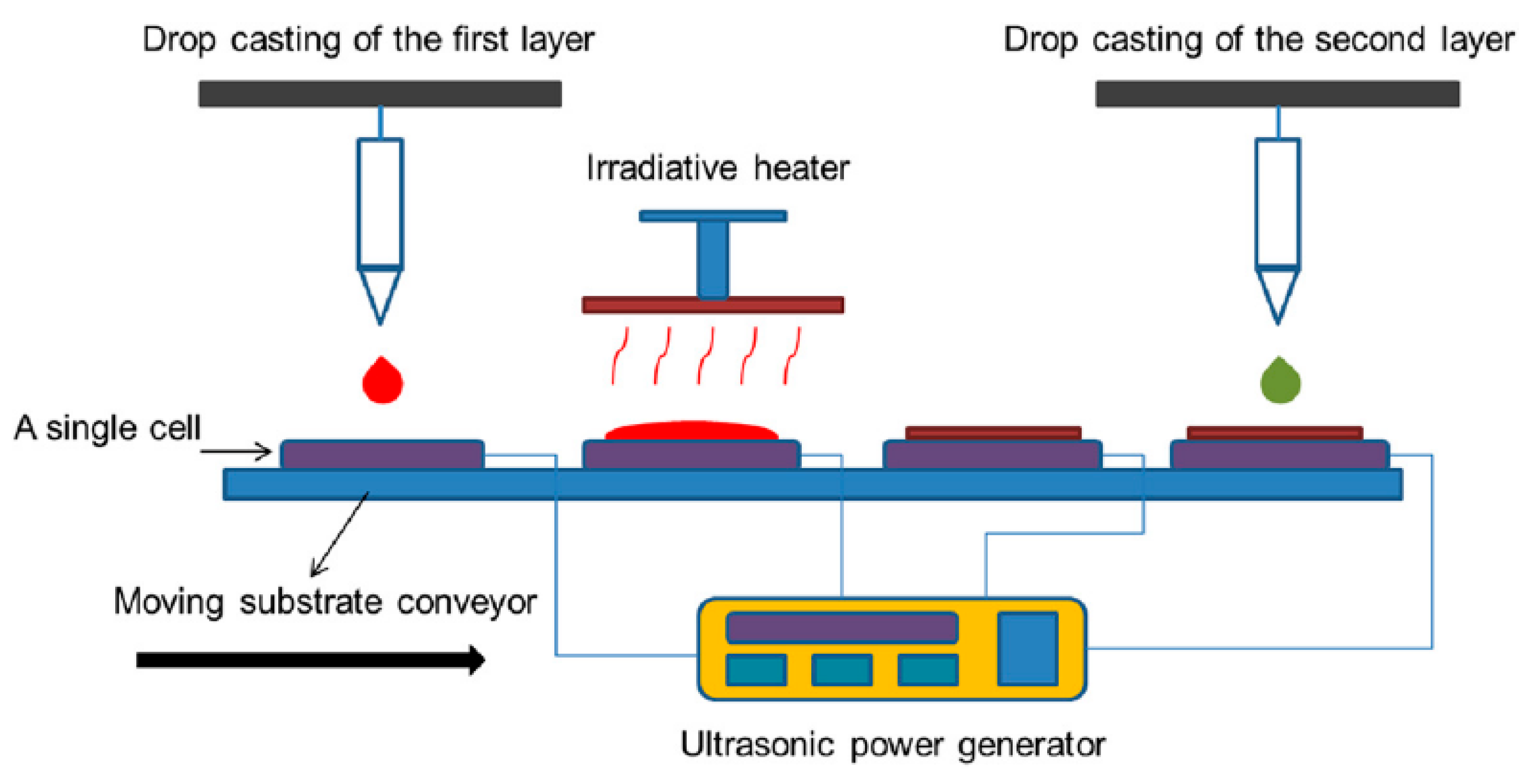
2. How Photoactive Heterostructures Are Made
3. How They Are Explored
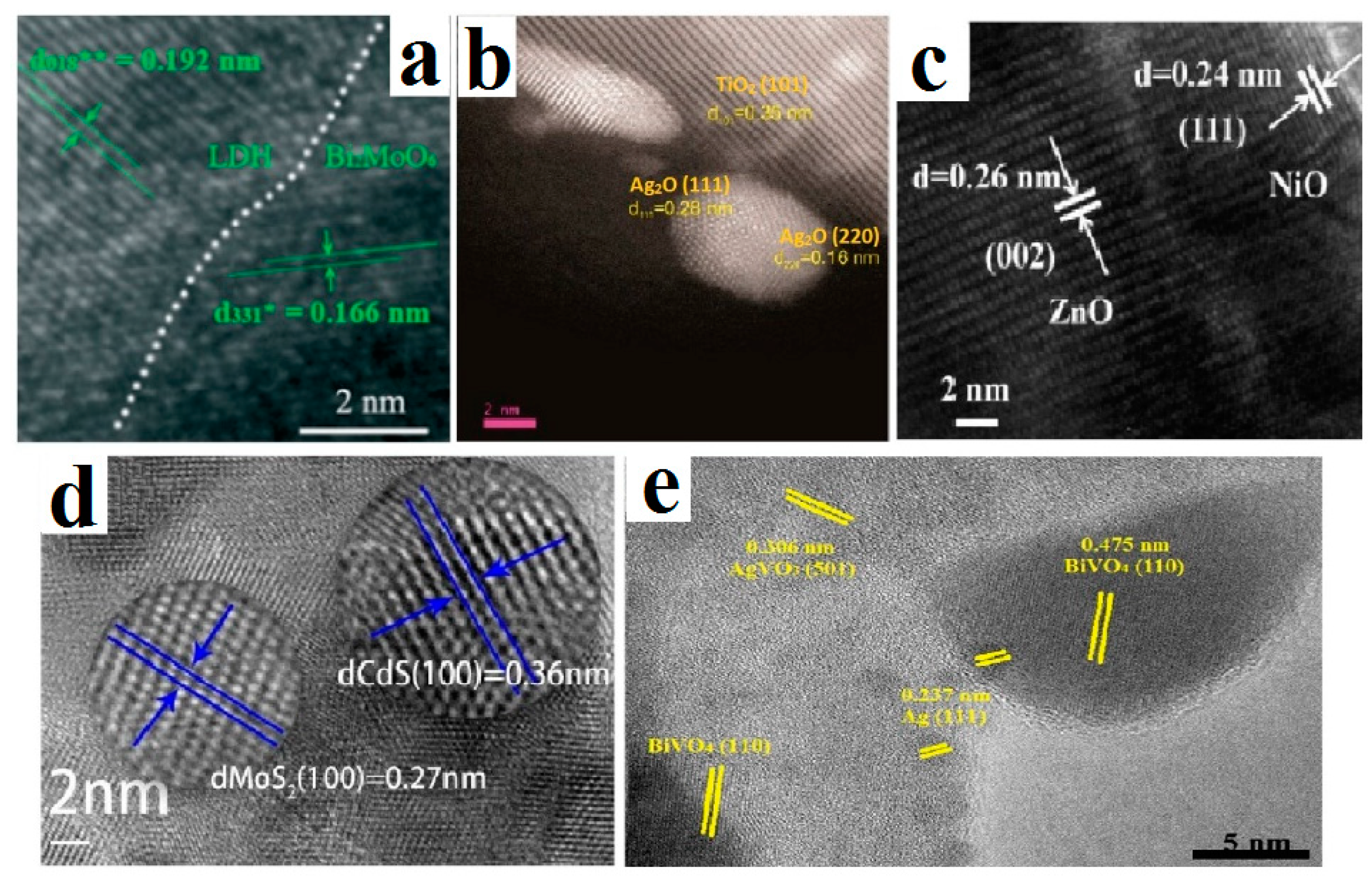
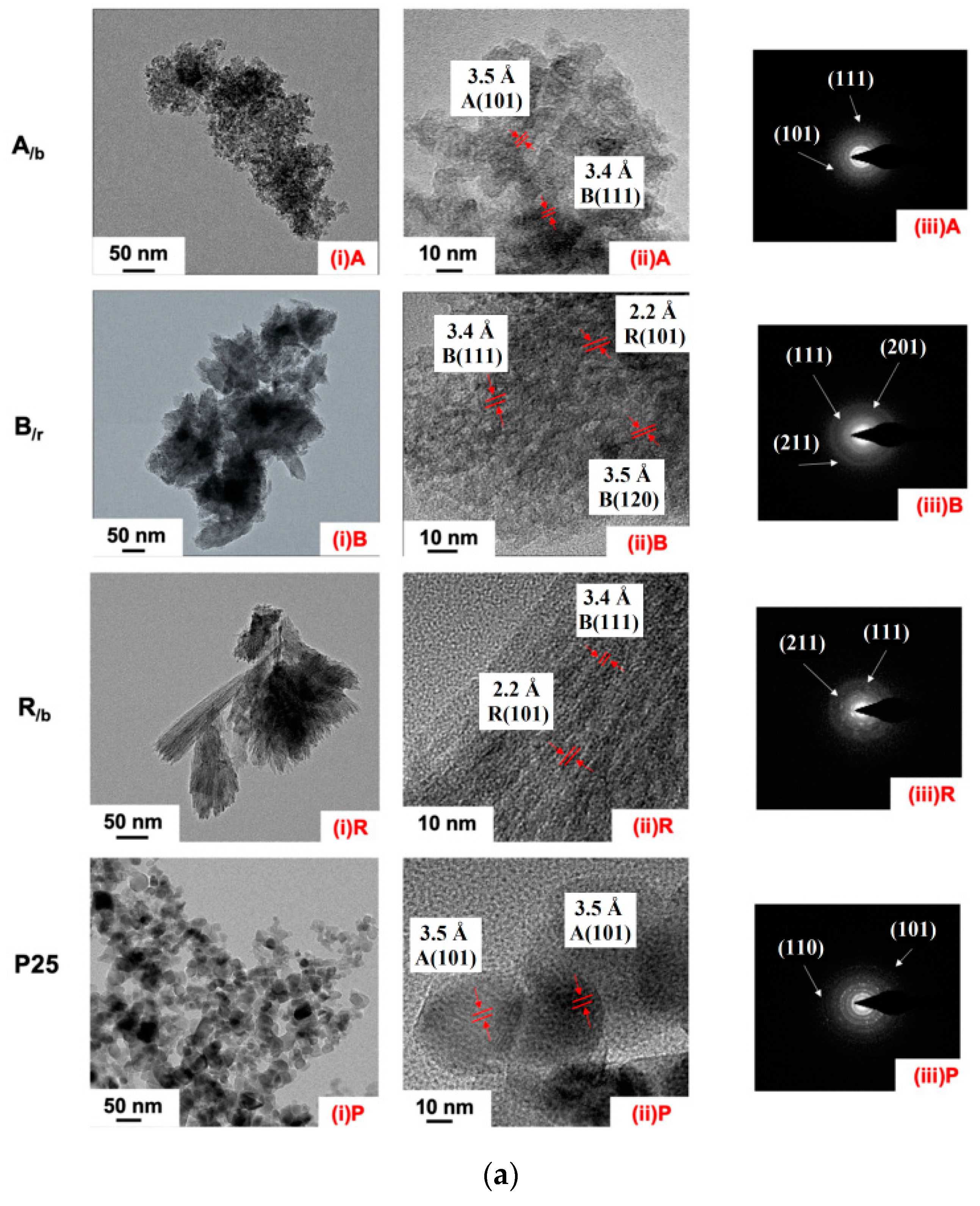
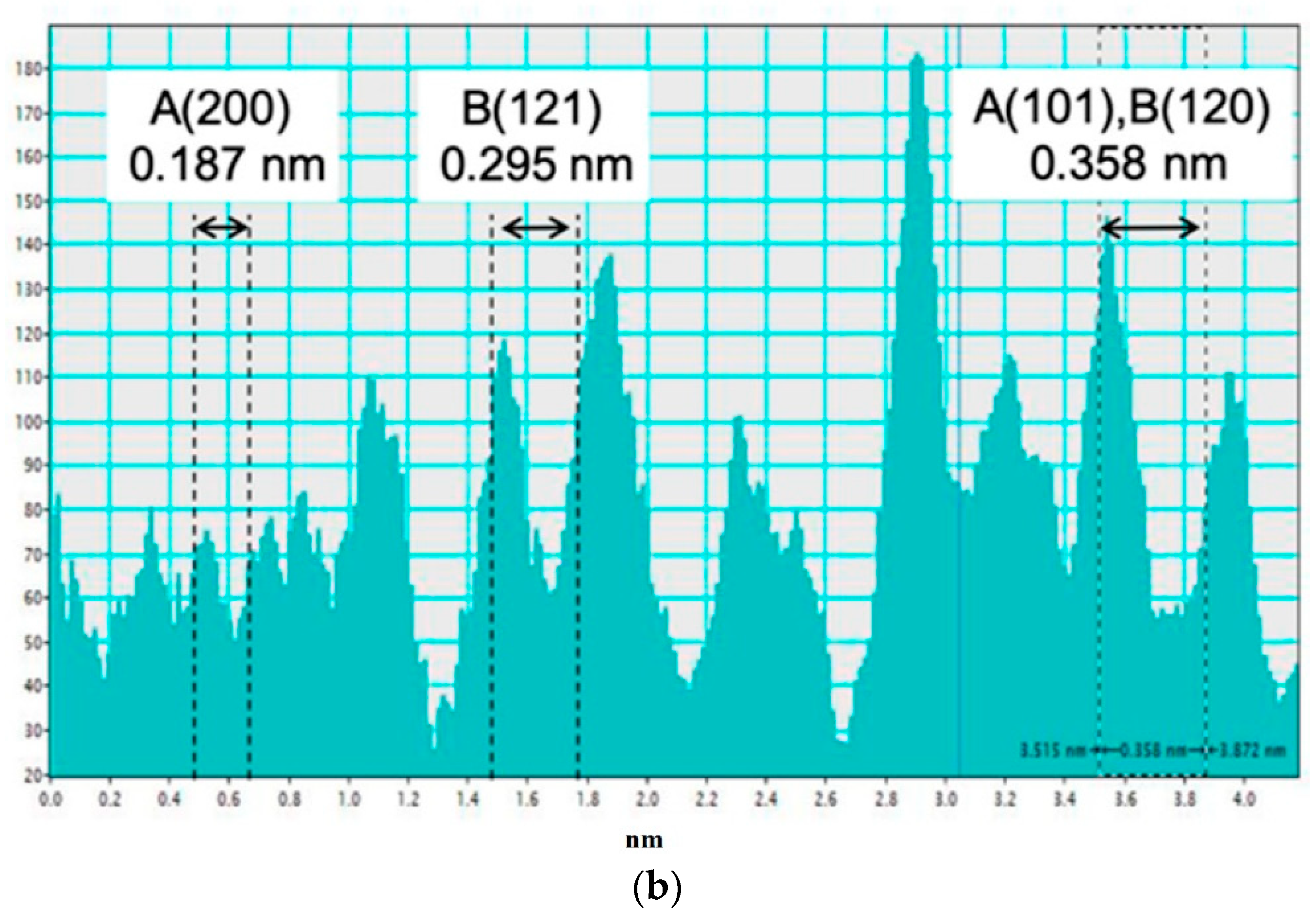
3.1. Electron Microscopy
3.2. Optical Characterization
- The absorption in the given spectral range corresponds to the edge of the fundamental absorption and not to another type of light absorption by the solids (such as surface or extrinsic absorption);
- The electronic transition between valence and conduction bands is either direct or indirect and a dependence of density of states on the energy near the top of the valence band and bottom of the conduction band is a square root dependence (that is characteristic for crystalline solids);
- The conditions for the application of the KFM approach are completely fulfilled for the treatment of the experimental diffuse reflectance spectra (this is especially true for the requirement of the optical uniformity of the dispersed samples).
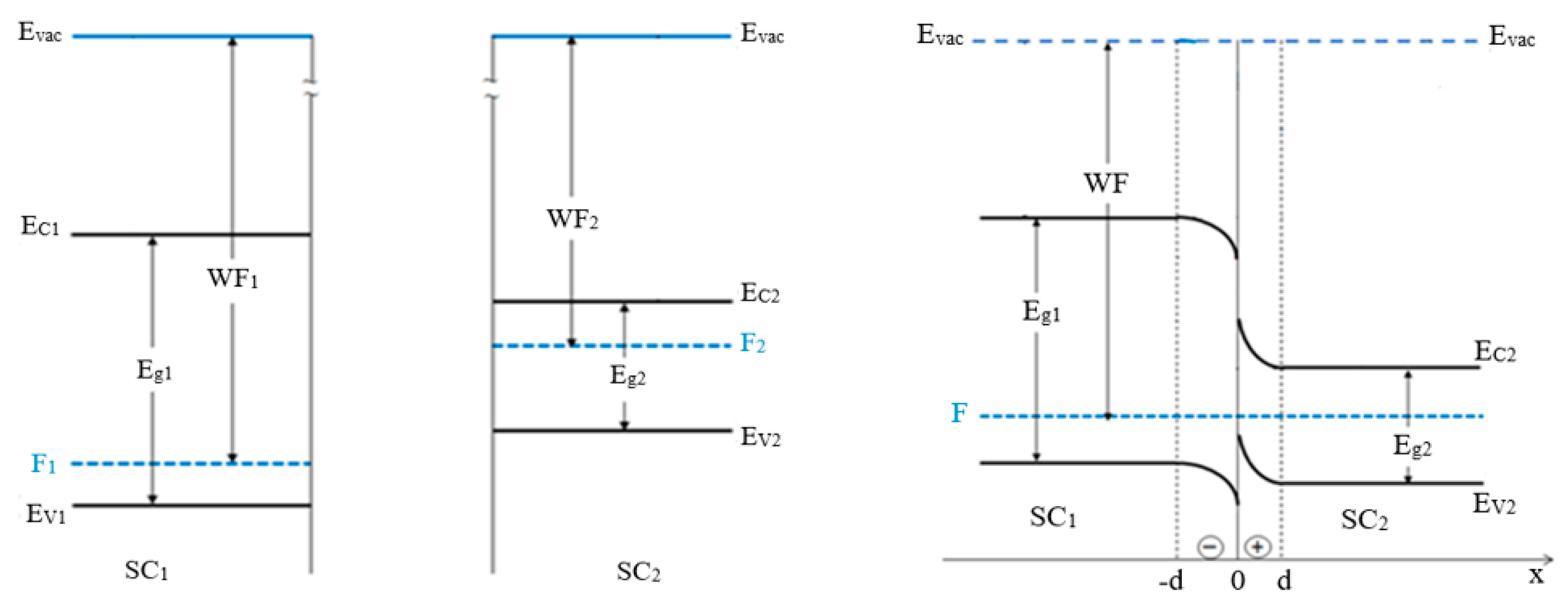
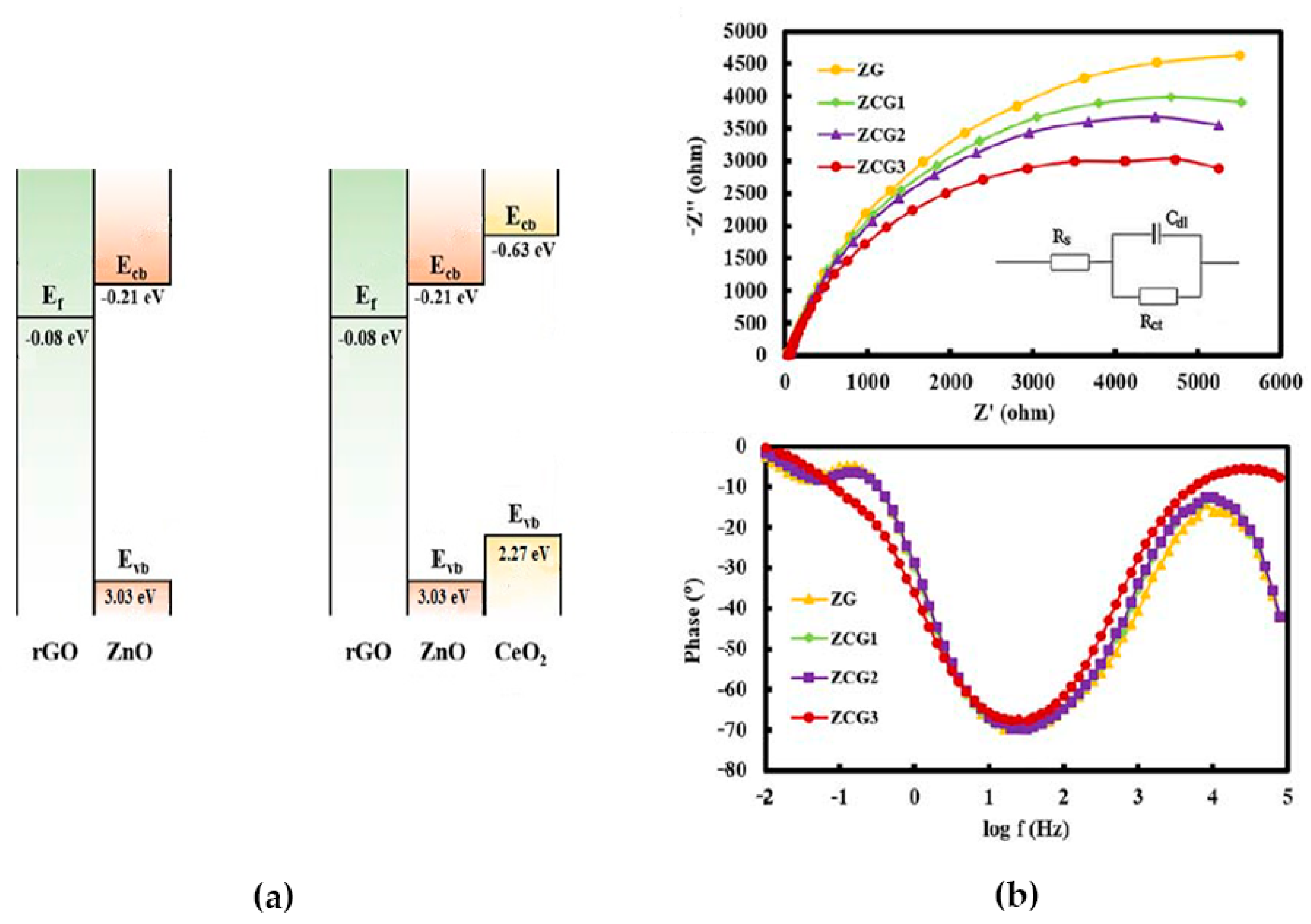
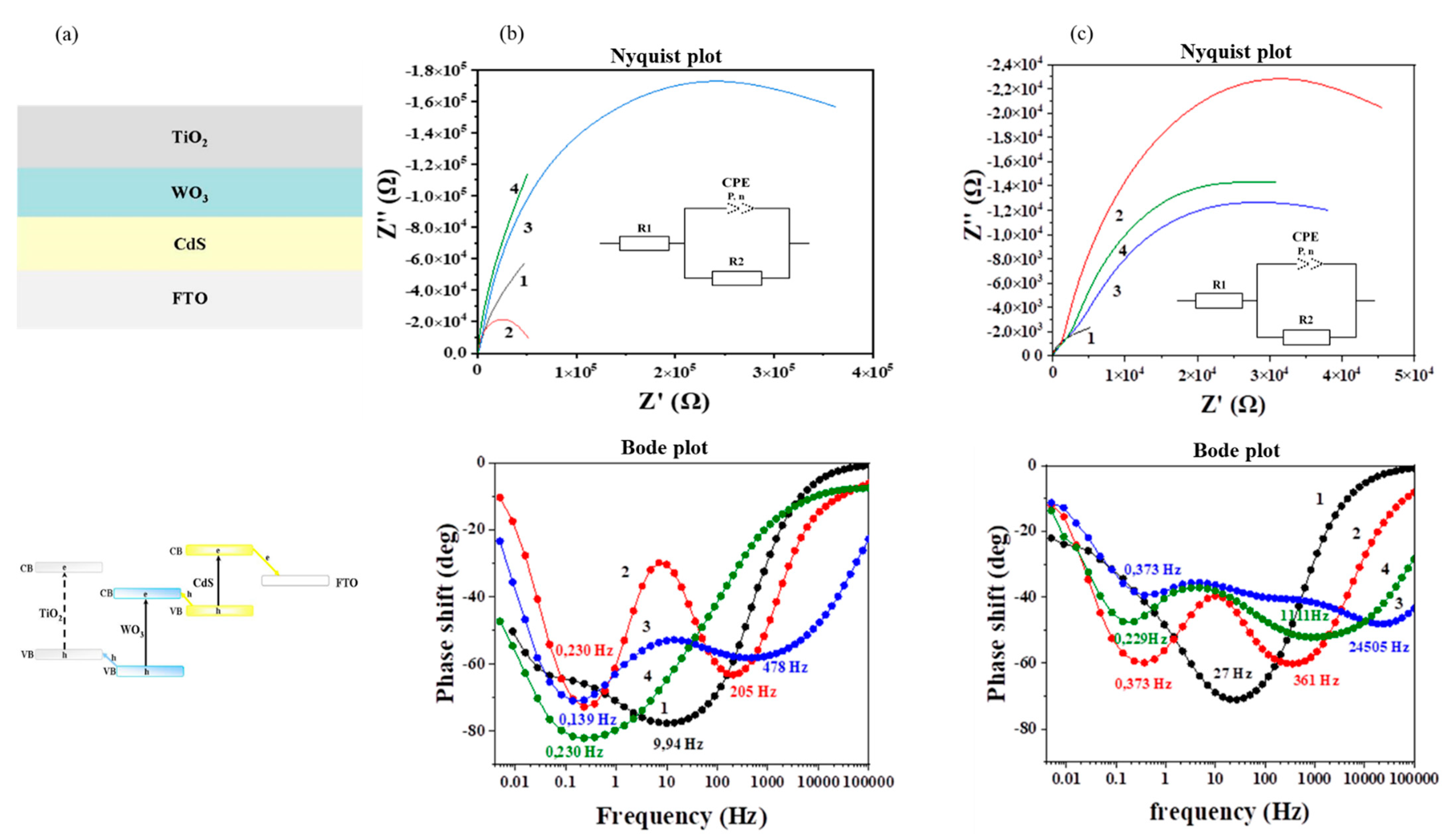
3.3. Electrophysical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 7, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Hashimoto, K.; Watanabe, T. TiO2 Photocatalysis: Fundamentals and Applications; BKC Publ.: Tokyo, Japan, 1999. [Google Scholar]
- Graetzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Fundamentals in metal-oxide heterogeneous photocatalysis. In Nanostructured and Photoelectrochemical Systems for Solar Photon Conversion; Archer, M.D., Nozik, A.J., Eds.; Imperial College Press: London, UK, 2008; pp. 275–381. [Google Scholar]
- Nakata, K.; Ochiai, T.; Murakami, T.; Fujishima, A. Photoenergy conversion with TiO2 photocatalysis: New materials and recent applications. Electrochim. Acta 2012, 84, 103–111. [Google Scholar]
- Serpone, N.; Lawless, D.; Disdier, J.; Herrmann, J.M. Spectroscopic, Photoconductivity, and Photocatalytic Studies of TiO2 Colloids: Naked and with the Lattice Doped with Cr3+, Fe3+, and V5+ Cations. Langmuir 1994, 10, 643–652. [Google Scholar] [CrossRef]
- Nagaveni, K.; Hedge, M.S.; Madras, G. Structure and photocatalytic activity of Ti1-xM xO2×δ (M = W, V, Ce, Zr, Fe, and Cu) synthesized by solution combustion method. J. Phys. Chem. B 2004, 108, 20204–20212. [Google Scholar] [CrossRef]
- Gracia, F.; Holgado, J.P.; Caballero, A.; Gonzalez-Elipe, A.R. Structural, Optical, and Photoelectrochemical Properties of Mn+−TiO2 Model Thin Film Photocatalyst. J. Phys. Chem. B 2004, 108, 17466–17476. [Google Scholar] [CrossRef]
- Torres, G.R.; Lindgren, T.; Lu, J.; Granqvist, C.-G.; Lindquist, S.-E. Photoelectrochemical study of nitrogen-doped titanium dioxide for water oxidation. J. Phys. Chem. B 2004, 108, 5995–6003. [Google Scholar] [CrossRef]
- Nakamura, R.; Tanaka, T.; Nakato, Y. Mechanism for visible light responses in anodic photocurrents at N-doped TiO2 film electrodes. J. Phys. Chem. B 2004, 108, 10617–10620. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, J.; Chen, S.H.; Zhang, S.; Huang, W. Perspective on construction of heterojunction photocatalysts and the complete utilization of photogenerated charge carriers. Appl. Surf. Sci. 2019, 476, 982–992. [Google Scholar] [CrossRef]
- Lianos, P. Review of recent trends in photoelectrocatalytic conversion of solar energy to electricity and hydrogen. Appl. Catal. B Environ. 2017, 210, 235–254. [Google Scholar] [CrossRef]
- He, X.; Zhang, C. Recent advances in structure design for enhancing photocatalysis. J. Mater. Sci. 2019, 54, 8831–8851. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V.; Ryabchuk, V.K.; Kuznetsov, V.N.; Artem’ev, Y.M.; Horikoshi, S. Why do Hydrogen and Oxygen Yields from Semiconductor-Based Photocatalyzed Water Splitting Remain Disappointingly Low? Intrinsic and Extrinsic Factors Impacting Surface Redox Reactions. ACS Energy Lett. 2016, 1, 931–948. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Lowa, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor Photocatalysis - Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Enesca, A.; Andronic, L. The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review. Nanomaterials 2020, 10, 1766. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, M.; Zhao, G.; Yin, G.; Liu, B. Hierarchical Fe2O3 nanorods/TiO2 nanosheets heterostructure: Growth mechanism, enhanced visible-light photocatalytic and photoelectrochemical performances. Appl. Surf. Sci. 2019, 475, 380–388. [Google Scholar] [CrossRef]
- Ghosh, M.; Liu, J.; Chuang, S.S.C.; Jana, S.C. Fabrication of Hierarchical V2O5 Nanorods on TiO2 Nanofibers and Their Enhanced Photocatalytic Activity under Visible Light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Lin, Y.-Z.; Wang, K.; Zhang, Y.; Dou, Y.; Yang, Y.; Xu, M.; Wang, Y.; Liu, F.; Li, K. Metal–organic framework-derived CdS–NiO heterostructures with modulated morphology and enhanced photocatalytic hydrogen evolution activity in pure water. J. Mater. Chem. C 2020, 8, 10071–10077. [Google Scholar] [CrossRef]
- Kaur, N.; Zappa, D.; Ferroni, M.; Poli, N.; Campanini, M.; Negrea, R.; Comini, E. Branch-like NiO/ZnO heterostructures for VOC sensing. Sens. Actuators B Chem. 2018, 262, 477–485. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, B.; Lin, J.; Lv, D.; Shi, J.; Sun, L.; Zeng, Q.; Niu, L.; Liu, F.; Wang, X.; et al. Morphology Engineering in Monolayer MoS2-WS2 Lateral Heterostructures. Adv. Funct. Mater. 2018, 28, 1801568. [Google Scholar] [CrossRef]
- van de Voorde, M.; Tulinski, M.; Jurczyk, M. Engineered Nanomaterials: A Discussion of the Major Categories of Nanomaterials. In Metrology and Standardization of Nanotechnology: Protocols and Industrial Innovations; Mansfield, E., Kaiser, D.L., Fujita, D., van de Voorde, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 49–74. [Google Scholar]
- Vattikuti, S.V.P. Heterostructured Nanomaterials: Latest Trends in Formation of Inorganic Heterostructures. In Synthesis of Inorganic Nanomaterials; Bhagyaraj, S.M., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–120. [Google Scholar]
- Kulkarni, S.K. Nanotechnology: Principles and Practices; Springer: Berlin/Heidelberg, Germany, 2015; 403p. [Google Scholar]
- Grabowska, E.; Marchelek, M.; Paszkiewicz-Gawron, M.; Zaleska-Medynska, A. Metal Oxide-Based Photocatalysis; Zaleska-Medynska, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 51–207. [Google Scholar]
- Šutka, A.; Järvekülg, M.; Gross, K.A. Photocatalytic Nanoheterostructures and Chemically Bonded Junctions Made by Solution-Based Approaches. Crit. Rev. Solid State Mater. Sci. 2019, 44, 239–263. [Google Scholar] [CrossRef]
- Luo, B.; Liu, G.; Wang, L. Recent advances in 2D materials for photocatalysis. Nanoscale 2016, 8, 6904–6920. [Google Scholar] [CrossRef]
- Yuan, Y.-P.; Ruan, L.-W.; Barber, J.; Loo, S.C.J.; Xue, C. Hetero-nanostructured suspended photocatalysts for solar-to-fuel conversion. Energy Environ. Sci. 2014, 7, 3934–3951. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, X.; Feng, Z.; Jia, W.; Zheng, X.; Li, C. Facile Synthesis of Heterojunctioned ZnO/Bi2S3 Nanocomposites for Enhanced Photocatalytic Reduction of Aqueous Cr(VI) under Visible-Light Irradiation. Catalysts 2019, 9, 624. [Google Scholar] [CrossRef]
- Peng, B.; Xia, M.; Li, C.; Yue, C.; Diao, P. Network Structured CuWO4/BiVO4/Co-Pi Nanocomposite for Solar Water Splitting. Catalysts 2018, 8, 663. [Google Scholar] [CrossRef]
- Ju, P.; Wang, Y.; Sun, Y.; Zhang, D. In-Situ Green Topotactic Synthesis of a Novel Z-Scheme Ag@AgVO3/BiVO4 Heterostructure with Highly Enhanced Visible-Light Photocatalytic Activity. J. Colloid Interface Sci. 2020, 579, 431–447. [Google Scholar] [CrossRef]
- Mi, F.; Chen, X.; Ma, Y.; Yin, S.; Yuan, F.; Zhang, H. Facile Synthesis of Hierarchical Core–Shell Fe3O4@MgAl–LDH@Au as Magnetically Recyclable Catalysts for Catalytic Oxidation of Alcohols. Chem. Commun. 2011, 47, 12804–12806. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Zhang, L.; Sun, S.; Wang, L.; Zhou, L. 3D Bi2WO6/TiO2 Hierarchical Heterostructure: Controllable Synthesis and Enhanced Visible Photocatalytic Degradation Performances. J. Phys. Chem. C 2009, 113, 14727–14731. [Google Scholar] [CrossRef]
- Kim, H.; Tak, Y.; Senthil, K.; Joo, J.; Jeon, S.; Yong, K. Novel Heterostructure of CdS Nanoparticle/WO3 Nanowhisker: Synthesis and Photocatalytic Properties. J. Vac. Sci. Technol. 2009, 27, 2182. [Google Scholar] [CrossRef]
- Handoko, C.T.; Moustakas, N.G.; Peppel, T.; Springer, A.; Oropeza, F.E.; Huda, A.; Bustan, M.D.; Yudono, B.; Gulo, F.; Strunk, J. Characterization and Effect of Ag(0) vs. Ag(I) Species and Their Localized Plasmon Resonance on Photochemically Inactive TiO2. Catalysts 2019, 9, 323. [Google Scholar] [CrossRef]
- Wang, C.; Lin, H.; Xu, Z.; Cheng, H.; Zhang, C. One-Step Hydrothermal Synthesis of Flowerlike MoS2/CdS Heterostructures for Enhanced Visible-Light Photocatalytic Activities. RSC Adv. 2015, 5, 15621. [Google Scholar] [CrossRef]
- Rogé, V.; Didierjean, J.; Crêpellière, J.; Arl, D.; Michel, M.; Fechete, I.; Dinia, A.; Lenoble, D. Tuneable Functionalization of Glass Fibre Membranes with ZnO/SnO2 Heterostructures for Photocatalytic Water Treatment: Effect of SnO2 Coverage Rate on the Photocatalytic Degradation of Organics. Catalysts 2020, 10, 733. [Google Scholar] [CrossRef]
- He, G.; Zhang, Y.; He, Q. MoS2/CdS Heterostructure for Enhanced Photoelectrochemical Performance under Visible Light. Catalysts 2019, 9, 379. [Google Scholar] [CrossRef]
- Luo, Y.; Kong, D.; Luo, J.; Wang, Y.; Zhang, D.; Qiu, K.; Cheng, C.; Li, C.M.; Yu, T. Seed-Assisted Synthesis of Co3O4@α-Fe2O3 Core–Shell Nanoneedle Arrays for Lithium-Ion Battery Anode with High Capacity. RSC Adv. 2014, 4, 13241. [Google Scholar] [CrossRef]
- Wang, L.; Su, Z.; Yuan, J. The Influence of Materials, Heterostructure, and Orientation for Nanohybrids on Photocatalytic Activity. Nanoscale Res. Lett. 2019, 14, 20. [Google Scholar] [CrossRef]
- Qiao, S.-Z.; Liu, J.; Lu, G.Q.M. Synthetic Chemistry of Nanomaterials. In Modern Inorganic Synthetic Chemistry; Xu, R., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 613–640. [Google Scholar]
- Butler, S.Z.; Hollen, S.M.; Cao, L.Y.; Cui, Y.; Gupta, J.A.; Gutierrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.X.; Ismach, A.F.; et al. Progress, Challenges, and Opportunities in Two-Dimensional Materials Beyond Graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef]
- Chen, D.; Chen, W.; Ma, L.; Ji, G.; Chang, K.; Lee, J.Y. Graphene-Like Layered Metal Dichalcogenide/Graphene Composites: Synthesis and Applications in Energy Storage and Conversion. Mater. Today 2014, 17, 184–193. [Google Scholar] [CrossRef]
- Ozer, L.Y.; Garlisi, C.; Oladipo, H.; Pagliaro, M.; Sharief, S.A.; Yusuf, A.; Almheiri, S.; Palmisano, G.J. Inorganic semiconductors-graphene composites in photo (electro) catalysis: Synthetic strategies, interaction mechanisms and applications. Photochem. Photobiol. C Photochem. Rev. 2017, 33, 132–164. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Mechanochemistry and Mechanical Activation of Solids. Rus. Chem. Rev. 2006, 75, 177–189. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for New and Cleaner Synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Tulinski, M.; Jurczyk, M. Nanomaterials Synthesis Methods. In Metrology and Standardization of Nanotechnology: Protocols and Industrial Innovations; Mansfield, E., Kaiser, D.L., Fujita, D., van de Voorde, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 75–98. [Google Scholar]
- Xu, R.-R.; Su, Q. High Temperature Synthesis. In Modern Inorganic Synthetic Chemistry; Xu, R., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 9–43. [Google Scholar]
- Rani, A.; Reddy, R.; Sharma, U.; Mukherjee, P.; Mishra, P.; Kuila, A.; Sim, L.C.; Saravanan, P. A Review on the Progress of Nanostructure Materials for Energy Harnessing and Environmental Remediation. J. Nanostruct. Chem. 2018, 8, 255–291. [Google Scholar] [CrossRef]
- Colson, P.; Henrist, C.; Cloots, R. Nanosphere Lithography: A Powerful Method for the Controlled Manufacturing of Nanomaterials. J. Nanomater. 2013, 948510, 19. [Google Scholar] [CrossRef]
- Xia, Y.N.; Whitesides, G.M. Soft Lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Phonthammachai, N.; Rumruangwong, M.; Gulari, E.; Jamieson, A.M.; Jitkarnka, S.; Wongkasemjit, S. Synthesis and Rheological Properties of Mesoporous Nanocrystalline CeO2 via Sol–Gel Process. Coll. Surf. A Physicochem. Eng. Asp. 2004, 247, 61–68. [Google Scholar] [CrossRef]
- Tyrpekl, V.; Vejpravová, J.P.; Roca, A.G.; Murafa, N.; Szatmary, L.; Nižňanský, D. Magnetically Separable Photocatalytic Composite γ-Fe2O3@TiO2 Synthesized by Heterogeneous Precipitation. Appl. Surf. Sci. 2011, 257, 4844–4848. [Google Scholar] [CrossRef]
- Sánchez-Martínez, D.; la Cruz, A.M.; López-Cuéllar, E. Synthesis of WO3 Nanoparticles by Citric Acid-Assisted Precipitation and Evaluation of Their Photocatalytic Properties. Mater. Res. Bull. 2013, 48, 691–697. [Google Scholar] [CrossRef]
- Acharya, K.P.; Hewa-Kasakarage, N.N.; Alabi, T.R.; Nemitz, I.; Khon, E.; Ullrich, B.; Anzenbacher, P.; Zamkov, M. Synthesis of PbS/TiO2 Colloidal Heterostructures for Photovoltaic Applications. J. Phys. Chem. C 2010, 114, 12496–12504. [Google Scholar] [CrossRef]
- Aniskevich, Y.M.; Malashchonak, M.V.; Chulkin, P.V.; Ragoisha, G.A.; Streltsov, E.A. Cadmium Underpotential Deposition on CdSe and CdS Quantum Dot Films: Size Dependent Underpotential Shift. Electrochim. Acta 2016, 220, 493–499. [Google Scholar] [CrossRef]
- Cao, S.-W.; Zhu, Y.-J. Iron Oxide Hollow Spheres: Microwave–Hydrothermal Ionic Liquid Preparation, Formation Mechanism, Crystal Phase and Morphology Control and Properties. Acta Mater. 2009, 57, 2154–2165. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef]
- Feng, S.-H.; Li, G.-H. Hydrothermal and Solvothermal Syntheses. In Modern Inorganic Synthetic Chemistry; Xu, R., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–104. [Google Scholar]
- Rao, V.N.; Reddy, N.L.; Kumari, M.M.; Cheralathan, K.K.; Ravi, P.; Sathish, M.; Neppolian, B.; Reddy, K.R.; Shetti, N.P.; Prathap, P.; et al. Sustainable Hydrogen Production for the Greener Environment by Quantum Dots-Based Efficient Photocatalysts: A Review. J. Environ. Manag. 2019, 248, 109246. [Google Scholar] [CrossRef] [PubMed]
- Türk, M.; Erkey, C. Synthesis of Supported Nanoparticles in Supercritical Fluids by Supercritical Fluid Reactive Deposition: Current State, Further Perspectives and Needs. J. Supercrit. Fluids 2018, 134, 176–183. [Google Scholar] [CrossRef]
- He, X.; Wang, W.-N. Rational Design of Efficient Semiconductor-Based Photocatalysts via Microdroplets: A Review. KONA Powder Part. J. 2019, 36, 201–214. [Google Scholar] [CrossRef]
- Wang, J.-T. Chemical Vapor Deposition and Its Applications in Inorganic Synthesis. In Modern Inorganic Synthetic Chemistry; Xu, R., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 167–188. [Google Scholar]
- Korotcenkov, G.; Tolstoy, V.; Schwank, J. Successive Ionic Layer Deposition (SILD) as a New Sensor Technology: Synthesis and Modification of Metal Uxides. Meas. Sci. Technol. 2006, 17, 1861–1869. [Google Scholar] [CrossRef]
- Tolstoi, V.P. New Routes for the Synthesis of Nanocomposite Layers of Inorganic Compounds by the Layer-by-Layer Scheme. Rus. J. Gen. Chem. 2009, 79, 2578–2583. [Google Scholar] [CrossRef]
- Chalastara, K.; Guo, F.; Elouatik, S.; Demopoulos, G.P. Tunable Composition Aqueous-Synthesized Mixed-Phase TiO2 Nanocrystals for Photo-Assisted Water Decontamination: Comparison of Anatase, Brookite and Rutile Photocatalysts. Catalysts 2020, 10, 407. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Nguyen, V.-H.; Nanda, S.; Vo, D.-N.; Nguyen, V.H.; Tran, T.V.; Xuan, L.N.; Nguyen, T.T.; Bach, L.-G.; Abdullah, B.; et al. BiVO4 Photocatalysis Design and Applications to Oxygen Production and Degradation of Organic Compounds: A Review. Environ. Chem. Lett. 2020. [Google Scholar] [CrossRef]
- Sudha, D.; Sivakumar, P. Review on the Photocatalytic Activity of Various Composite Catalysts. Chem. Eng. Process. 2015, 97, 112–133. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.; Roy, V.A.L. Recent Progress in Magnetic Iron Oxide–Semiconductor Composite Nanomaterials as Promising Photocatalysts. Nanoscale 2015, 7, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.; Tang, H.; Liu, Q.Q.; Zulfiqar, S.; Shah, S.; Bahadur, I. An Overview of Semiconductors/Layered Double Hydroxides Composites: Properties, Synthesis, Photocatalytic and Photoelectrochemical Applications. J. Molec. Liquids 2019, 289, 111114. [Google Scholar] [CrossRef]
- Chu, K.-W.; Lee, S.L.; Chang, C.-J.; Liu, L. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymers 2019, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.-L.; Zboril, R.; Varma, R.S. Core–Shell Nanoparticles: Synthesis and Applications in Catalysis and Electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zang, S.-Q.; Lou, X.W. Hierarchical Hollow Heterostructures for Photocatalytic CO2 Reduction and Water Splitting. Small Methods 2019, 4, 1900586. [Google Scholar] [CrossRef]
- Cheng, F.; Tao, Z.; Liang, J.; Chen, J. Template-Directed Materials for Rechargeable Lithium-Ion Batteries. Chem. Mater. 2008, 20, 667–681. [Google Scholar] [CrossRef]
- Eslamian, M.; Zabihi, F. Ultrasonic Substrate Vibration-Assisted Drop Casting (SVADC) for the Fabrication of Photovoltaic Solar Cell Arrays and Thin-Film Devices. Nanoscale Res. Let. 2015, 10, 462. [Google Scholar] [CrossRef]
- Tolstoy, V.P. Successive Ionic Layer Deposition. The Use in Nanotechnology. Rus. Chem. Rev. 2006, 75, 161–175. [Google Scholar] [CrossRef]
- Erkov, V.G.; Devyatova, S.F.; Molodstova, E.L.; Malsteva, T.V.; Yanovskii, U.A. Si–TiO2 Interface Evolution at Prolonged Annealing in Low Vacuum or N2O Ambient. Appl. Surf. Sci. 2000, 166, 51–56. [Google Scholar] [CrossRef]
- Chen, B.; Meng, Y.; Sha, J.; Zhong, C.; Hua, W.; Zhao, N. Preparation of MoS2/TiO2 Based Nanocomposites for Photocatalysis and Rechargeable Batteries: Progress, Challenges, and Perspective. Nanoscale 2018, 10, 34–68. [Google Scholar] [CrossRef]
- Zori, M.H. Synthesis of TiO2 Nanoparticles by Microemulsion/Heat Treated Method and Photodegradation of Methylene Blue. J. Inorg. Organomet. Polym. 2011, 21, 81–90. [Google Scholar] [CrossRef]
- Rivera-Gonzalez, N.; Chauhan, S.; Watson, D.F. Aminoalkanoic Acids as Alternatives to Mercaptoalkanoic Acids for the Linker-Assisted Attachment of Quantum Dots to TiO2. Langmuir 2016, 32, 9206–9215. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, L.; Nasi, L.; Grillo, V. Transmission Electron Microscopy Techniques for Imaging and Compositional Evaluation in Semiconductor Heterostructures. In Characterization of Semiconductor Heterostructures and Nanostructures; Lamberti, C., Agostini, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 413–465. [Google Scholar]
- Li, H.; Deng, Q.; Liu, J.; Hou, W.; Du, N.; Zhang, R.; Tao, X. Synthesis, Characterization and Enhanced Visible Light Photocatalytic Activity of Bi2MoO6/Zn–Al Layered Double Hydroxide Hierarchical Heterostructures. Catal. Sci. Technol. 2014, 4, 1028–1037. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Mi, R.; Deng, C.; Gao, P. An Environment-Benign Method for the Synthesis of p-NiO/n-ZnO Heterostructure with Excellent Performance for Gas Sensing and Photocatalysis. Sens. Actuators B 2014, 191, 537–544. [Google Scholar] [CrossRef]
- Gemmi, M.; Mugnaioli, E.; Gorelik, T.E.; Kolb, U.; Palatinus, L.; Boullay, P.; Hovmoller, S.; Abrahams, J.P. 3D Electron Diffraction: The Nanocrystallography Revolution. ACS Cent. Sci. 2019, 5, 1315–1329. [Google Scholar] [CrossRef]
- Hens, Z.; de Roo, J. Atomically Precise Nanocrystals. J. Am. Chem. Soc. 2020, 142, 15627–15637. [Google Scholar] [CrossRef]
- Beanland, R.; Thomas, P.J.; Woodward, D.I.; Thomas, P.A.; Roemer, R.A. Digital Electron Diffraction-Seeing the Whole Picture. Acta Cryst. 2013, 69, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Molaei, R.; Bayati, R.; Wu, F.; Narayan, J. A Microstructural Approach toward the Effect of Thickness on Semiconductor-to-Metal Transition Characteristics of VO2 Epilayers. J. Appl. Phys. 2014, 115, 164311. [Google Scholar] [CrossRef]
- Kortum, G. Reflectance Spectroscopy: Principles, Methods, Applications; Lohr, J.E., Ed.; Springer: New York, NY, USA, 1969. [Google Scholar]
- Klier, K. Reflectance spectroscopy as a tool for investigating dispersed solids and their surfaces. Catal. Rev. Sci. Eng. 1968, 1, 207–232. [Google Scholar] [CrossRef]
- Kortum, G.; Braun, W.; Herzog, G. Principles and Techniques of Diffuse-Reflectance Spectroscopy. Angew. Chem. Internat. Edit. 1963, 2, 333–341. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag zur Optik der Farbanstriche. Z. Tech. Phys. (Leipzig) 1931, 12, 593–601. [Google Scholar]
- Steele, F.A. The optical characteristics of paper. I. The mathematical relationships between basis weight, reflectance, contrast ratio and other optical properties. Paper Trade, J. 1935, 100, 37–42. [Google Scholar]
- Kubelka, P.J. New contribution to the optics of intensely light-scattering materials. Part I. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef]
- Smith, T. The hiding power of diffusing media. Trans. Opt. Soc. 1931, 33, 150–159. [Google Scholar] [CrossRef]
- Gurevich, M. Über eine Rationelle Klassifikation der Lichtenstreuenden Medien. Phys. Z. 1930, 31, 753. [Google Scholar]
- Amy, L.; Sannié, C.; Sarrat, J.M. Sur la couleur des par reflexion. Rev. Optique 1937, 16, 81–86. [Google Scholar]
- Judd, D.B. Opacity Standards. J. Res. Nat. Bur. Stand. 1934, 13, 281–291. [Google Scholar] [CrossRef]
- Bruce, H.D. A Photometric method for determining the Hiding Power of Paints. Nat. Bur. Stand 1926, 306, 173–190. [Google Scholar] [CrossRef]
- Simmons, E.L. Relation of the diffuse reflectance remission function to the fundamental optical parameters. Optica Acta 1972, 19, 845–851. [Google Scholar] [CrossRef]
- Kubelka, P.J. New contribution to the optics of intensely light-scattering materials. Part II. Opt. Soc. Am. 1954, 44, 330–335. [Google Scholar] [CrossRef]
- Bodo, Z. Some optical properties of luminescent powders. Acta Phys. Acad. Sci. Hung. 1951, 1, 135–150. [Google Scholar] [CrossRef]
- Melamed, N.T. Optical Properties of Powders. Part I. Optical Absorption Coefficients and the Absolute Value of the Diffuse Reflectance. Part II. Properties of Luminescent Powders. J. Appl. Phys. 1963, 34, 560–570. [Google Scholar] [CrossRef]
- Johnson, P.D. Absolute Optical Absorption from Diffuse Reflectance. J. Opt. Soc. Am. 1952, 42, 978–981. [Google Scholar] [CrossRef]
- Simmons, E.L. Diffuse reflectance spectroscopy: a comparison of the theories. Appl. Optics 1975, 14, 1380–1386. [Google Scholar] [CrossRef]
- Karvaly, B. Investigation on the connections between the bulk absorption and diffuse reflectance spectra of powdered solids. Acta Phys. Acad. Sci. Hung. 1970, 28, 381–399. [Google Scholar] [CrossRef]
- Hecht, H.G. The Interpretation of Diffuse Reflectance Spectra. J. Res. Natl. Bureau Stand. A Phys. Chem. 1976, 80, 567–583. [Google Scholar] [CrossRef]
- Klier, K.J. Absorption and Scattering in Plane Parallel Turbid Media. Opt. Soc. Amer. 1972, 62, 882–885. [Google Scholar] [CrossRef]
- Brinkworth, B.J. On the theory of reflection by scattering and absorbing media. J. Phys. D: Appl. Phys. 1971, 4, 1105–1106. [Google Scholar] [CrossRef]
- Murphy, A.B. Modified Kubelka-Munk model for calculation of the reflectance of coatings with optically-rough surfaces. J. Phys. D Appl. Phys. 2006, 39, 3571–3581. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Ge and Si. Mat. Res. Bull., Pergamon Press 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Emeline, A.V.; Bahnemann, D.W. Effect of the TiO2–ZnO Heterostructure on the Photoinduced Hydrophilic Conversion of TiO2 and ZnO Surfaces. J. Phys. Chem. C 2019, 123, 14–8884. [Google Scholar] [CrossRef]
- Murzin, P.D.; Murashkina, A.A.; Emeline, A.V.; Bahnemann, D.W. Effect of Sc 3+/V 5+ Co-doping on photocatalytic activity of TiO2. Top. Catal. 2020. [Google Scholar] [CrossRef]
- Claessen, R.; Sing, M.; Paul, M.; Berner, G.; Wetscherek, A.; Müller, A.; Drube, W. Hard X-Ray Photoelectron Spectroscopy of Oxide Hybrid and Heterostructures: A New Method for the Study of Buried Interfaces. New J. Phys. 2009, 11, 125007. [Google Scholar] [CrossRef]
- Zhi, Y.; Liu, Z.; Wang, X.; Li, S.; Wang, X.; Chu, X.; Li, P.; Guo, D.; Wu, Z.; Tang, W.J. X-Ray Photoelectron Spectroscopy Study for Band alignments of BaTiO3/Ga2O3 and In2O3/Ga2O3 Heterostructures. Vac. Sci. Technol. A 2020, 38, 023202. [Google Scholar] [CrossRef]
- Sushko, P.V.; Chambers, S.A. Extracting Band Edge Profiles at Semiconductor Heterostructures from Hard-X-Ray Core-Level Photoelectron Spectra. Sci. Rep. 2020, 10, 13028. [Google Scholar] [CrossRef] [PubMed]
- Bauerle, J.E. Study of Solid Electrolyte Polarization by a Complex Admittance Method. J. Phys. Chem. Solids 1969, 30, 2657–2670. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy. In Theory, Experiment and Applications; Macdonald, J.R., Barsoukov, E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; 595p. [Google Scholar]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef]
- Mora-Sero, I.; Garcia-Belmonte, G.; Boix, P.P.; Vazquez, M.A. Bisquert, Impedance Spectroscopy Characterisation of Highly Efficient Silicon Solar Cells under Different Light Illumination Intensities. J. Energy Environ. Sci. 2009, 2, 678–686. [Google Scholar] [CrossRef]
- Bisquert, J.; Gimenez, S.; Bertoluzzi, L.; Herraiz-Cardona, I. Analysis of Photoelectrochemical Systems by Impedance Spectroscopy. In Photoelectrochemical Solar Fuel Production; Giménez, S., Bisquert, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 281–321. [Google Scholar]
- The Impedance Measurement Handbook: A Guide to Measurement Technology and Techniques; Agilent Technologies Co. Ltd.: USA, 2006; 126p.
- Bandarenka, A.S. Exploring the Interfaces between Metal Electrodes and Aqueous Electrolytes with Electrochemical Impedance Spectroscopy. Analyst 2013, 138, 5540–5554. [Google Scholar] [CrossRef]
- Dincer, I.; Bicer, Y. Photoelectrochemical Energy Conversion. Compr. Energy Syst. 2018, 4, 816–855. [Google Scholar]
- Fleig, J.; Maier, J. The Impedance of Imperfect Electrode Contacts on Solid Electrolytes. Solid State Ionics 1996, 85, 17–24. [Google Scholar] [CrossRef]
- Lipilin, A.S.; Ivanov, V.V.; Shkerin, S.N.; Nikonov, A.V.; Khrustov, V.R. Fuel Cell Technologies: State and Perspectives; Sammes, N., Smirnova, A., Vasylyev, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 265–270. [Google Scholar]
- Braun, P.; Uhlmann, C.; Weber, A.; Störmer, H.; Gerthsen, D.; Ivers-Tiffée, E.J. Separation of the Bulk and Grain Boundary Contributions to the Total Conductivity of Solid Lithium-Ion Conducting Electrolytes. J. Electroceram 2017, 38, 157–167. [Google Scholar] [CrossRef]
- Shkerin, S.N. Fuel Cell Technologies: State and Perspectives; Sammes, N., Smirnova, A., Vasylyev, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 301–306. [Google Scholar]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: Berlin/Heidelberg, Germany, 2014; 367p. [Google Scholar]
- Balasubramani, V.; Chandraleka, S.; Rao, T.S.; Sasikumar, R.; Kuppusamy, M.R.; Sridhar, T.M. Recent Advances in Electrochemical Impedance Spectroscopy Based Toxic Gas Sensors Using Semiconducting Metal Oxides. J. Electrochem. Soc. 2020, 167, 037572. [Google Scholar] [CrossRef]
- Keddam, M.; Portail, N.; Trinh, D.; Vivier, V. Progress in Scanning Electrochemical Microscopy by Coupling with Electrochemical Impedance and Quartz Crystal Microbalance. ChemPhysChem 2009, 10, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Berkes, B.B.; Maljusch, A.; Schuhmann, W.; Bondarenko, A.S. Influence of Cs + and Na+ on Specific Adsorption of *OH, *O, and *H at Platinum in Acidic Sulfuric Medi. J. Phys. Chem. C 2011, 115, 9122–9130. [Google Scholar] [CrossRef]
- Bard, A.J. Inner-Sphere Heterogeneous Electrode Reactions. Electrocatalysis and Photocatalysis: The Challenge. J. Am. Chem. Soc. 2010, 132, 7559–7567. [Google Scholar] [CrossRef] [PubMed]
- Bertoluzzi, L.; Bisquert, J.J. Equivalent Circuit of Electrons and Holes in Thin Semiconductor Films for Photoelectrochemical Water Splitting Applications. Phys. Chem. Lett. 2012, 3, 2517–2522. [Google Scholar] [CrossRef]
- Quang, N.D.; Majumder, S.; Choi, G.S.; Kim, C.; Kim, D. Optimization strategy for CdSe@CdS Core–Shell Nanorod Structures Toward High Performance Water Splitting Photoelectrodes. Mater. Res. Bull. 2020, 129, 110914. [Google Scholar] [CrossRef]
- Shin, D.-M.; Choi, J.-R.; Oh, J.-W.; Kim, H.K.; Han, D.-W.; Kim, K.; Hwang, Y.-H. Exploring the Use of Impedance Spectroscopy in Relaxation and Electrochemical Studies. Appl. Spectrosc. Rev. 2018, 53, 157–176. [Google Scholar] [CrossRef]
- Kafizas, A.; Godin, R.; Durrant, J.R. Charge Carrier Dynamics in Metal Oxide Photoelectrodes for Water. In Semiconductors for Photocatalysis; Mi, Z., Wang, L., Jagadish, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 97, pp. 3–46. [Google Scholar]
- Murali, A.; Sarswat, P.K.; Free, M.L. Minimizing Electron-Hole Pair Recombination Through Band-Gap Engineering in novel ZnO-CeO2-rGO Ternary Nanocomposite for Photoelectrochemical and Photocatalytic Applications. Environ. Sci. Pollut. Res. 2020, 27, 25042–25056. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lu, C.; Liu, C.; Qi, M.; Xu, X.; Yang, D.; Xu, X.J. Electrophoretic Deposition of ZnSnO3/MoS2 Heterojunction Photoanode with Improved Photoelectric Response by Low Recombination Rate. J. Alloys Compds. 2019, 810, 151845. [Google Scholar] [CrossRef]
- Yi, S.-S.; Yan, J.-M.; Wulan, B.-R.; Li, S.-J.; Liu, K.-H.; Jiang, Q. Noble-Metal-Free Cobalt Phosphide Modified Carbon Nitride: An Efficient Photocatalyst for Hydrogen Generation. Appl. Catal. B Environ. 2017, 200, 477–483. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Yi, Z.; Xian, T.; Wang, X. Direct Z-scheme CaTiO3@BiOBr Composite Photocatalysts with Enhanced Photodegradation of Dyes. Environ. Sci. Pollut. Res. 2019, 26, 29020–29031. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Wan, D.; Xue, C.; Zhao, J.; Shao, G. Direct Evidence of 2D/1D Heterojunction Enhancement on Photocatalytic Activity Through Assembling MoS2 Nanosheets onto Super-Long TiO2 Nanofibers. Appl. Surf. Sci. 2020, 504, 144361. [Google Scholar] [CrossRef]
- Khalid, N.R.; Israr, Z.; Tahir, M.B.; Iqbal, T. Highly Efficient Bi2O3/MoS2 p-n Heterojunction Photocatalyst for H2 Evolution from Water Splitting. Int. J. Hydrog. Energy 2020, 45, 8479–8489. [Google Scholar] [CrossRef]
- Shabbir, A.; Ajmal, S.; Shahid, M.; Shakir, I.; Agboola, P.O.; Warsi, M.F. Zirconium Substituted Spinel Nano-ferrite Mg0.2Co0.8Fe2O4 Particles and Their Hybrids with Reduced Graphene Oxide for Photocatalytic and Other Potential Applications. Ceram. Int. 2019, 45, 16121–16129. [Google Scholar] [CrossRef]
- Murashkina, A.A.; Bakiev, T.V.; Artemev, Y.M.; Rudakova, A.V.; Emeline, A.V.; Bahnemann, D.W. Photoelectrochemical Behavior of the Ternary Heterostructured Systems CdS/WO3/TiO2. Catalysts 2019, 9, 999. [Google Scholar] [CrossRef]
- Randles, J.E.B. Kinetics of Rapid Electrode Reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Borisenko, V.E.; Ossicini, S. What Is What in the Nanoworld: A Handbook on Nanoscience and Nanotechnology; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Anderson, R.L. Germanium-Gallium Arsenide Heterojunctions. IBM J. Res. Dev. 1960, 4, 283–287. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Arunachalam, P.; Hezamm, M.; Al-Mayouf, A.M. Cooperative Catalytic Behavior of SnO2 and NiWO4 over BiVO4 Photoanodes for Enhanced Photoelectrochemical Water Splitting Performance. Catalysts 2019, 9, 879. [Google Scholar] [CrossRef]
- Lv, Y.-R.; Liu, C.-J.; He, R.-K.; Li, X.; Xu, Y.-H. BiVO4/TiO2 Heterojunction with Enhanced Photocatalytic Activities and Photoelectochemistry Performances under Visible Light Illumination. Mater. Res. Bull. 2019, 117, 35–40. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Ibrahim, I.; Salama, T.M. Rational Design of Manganese Ferrite-Graphene Hybrid Photocatalysts: Efficient Water Splitting and Effective Elimination of Organic Pollutants. Appl. Catal. A Gener. 2016, 524, 182–191. [Google Scholar] [CrossRef]
- Zhu, T.; Huang, L.; Song, Y.; Chen, Z.; Ji, H.; Li, Y.; Xu, Y.; Zhang, Q.; Xu, H.; Li, H. Modification of Ag3VO4 with Graphene-Like MoS2 for Enhanced Visible-Light Photocatalytic Property and Stability. New J. Chem. 2016, 40, 2168–2177. [Google Scholar] [CrossRef]
- Ng, C.H.; Lima, H.N.; Hayase, S.; Zainal, Z.; Huang, N.M. Photovoltaic Performances of Mono- and Mixed-Galide Structures for Perovskite Solar Cell: A Review. Renew. Sust. Energ. Rev. 2018, 90, 248–274. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Guerrero, A.; Bisquert, J.J. Elucidating Operating Modes of Bulk-Heterojunction Solar Cells from Impedance Spectroscopy Analysis. Phys. Chem. Lett. 2013, 4, 877–886. [Google Scholar] [CrossRef]





| Method Group | Synthesis Method | Type of Materials (Systems) |
|---|---|---|
| “bottom-up” | direct self-assembly | 1D, 2D (QDs, dispersed) |
| layer-by-layer assembly | 2D (dispersed) | |
| emulsion-based synthesis | ||
| colloid-chemical routes | 1D (nanosized dispersed) | |
| thermal decomposition | 2D, 3D (dispersed, planar) | |
| hydrothermal / solvothermal | 1D, 2D, 3D (dispersed, planar) | |
| sol–gel | 2D, 3D (dispersed, planar) | |
| template-directed synthesis | 3D (dispersed) | |
| microwave-assisted | 1D (QDs, dispersed) | |
| laser (photo) ablation | 1D, 2D (QDs, planar) | |
| deposition methods(ALD, PVD, CVD, magnetron sputtering, epitaxy, plasma-enhanced deposition, etc.) | 2D (planar) | |
| “top-down” | mechanical impact (ball milling, gridding, shaking, etc.) | 3D (dispersed) |
| exfoliation methods (in situ ion-exchange or ion intercalation assisted, ultrasonic-assisted, etc.) | 2D (nanosized planar) | |
| techniques based on photolithography, nanoparticle lithography | 1D, 2D (surface nanostructures) | |
| scanning probe lithographic techniques | 1D, 2D (surface nanostructures, up to subnanometers) | |
| chemical etching | 1D, 2D (surface nanostructures) |
| TiO2/FTO | TiO2/CdS/FTO | TiO2/WO3/FTO | TiO2/WO3/CdS/FTO | |||||
|---|---|---|---|---|---|---|---|---|
| f0, Hz | τ, s | f0, Hz | τ, s | f0, Hz | τ, s | f0, Hz | τ, s | |
| in the darkness | 9.94 | 1.6 × 10−2 | 0.23 | 0.692 | 0.139 | 1.145 | 0.23 | 0.692 |
| 205 | 7.76 × 10−4 | 478 | 3.33 × 10−4 | |||||
| under irradiation | 27.0 | 6.0 × 10−3 | 0.373 | 0.427 | 0.373 | 0.427 | 0.229 | 0.695 |
| 361 | 4.0 × 10−4 | 24505 | 6.49 × 10−6 | 1111 | 14.3 × 10−5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emeline, A.V.; Rudakova, A.V.; Mikhaylov, R.V.; Bulanin, K.M.; Bahnemann, D.W. Photoactive Heterostructures: How They Are Made and Explored. Catalysts 2021, 11, 294. https://doi.org/10.3390/catal11020294
Emeline AV, Rudakova AV, Mikhaylov RV, Bulanin KM, Bahnemann DW. Photoactive Heterostructures: How They Are Made and Explored. Catalysts. 2021; 11(2):294. https://doi.org/10.3390/catal11020294
Chicago/Turabian StyleEmeline, Alexei V., Aida V. Rudakova, Ruslan V. Mikhaylov, Kirill M. Bulanin, and Detlef W. Bahnemann. 2021. "Photoactive Heterostructures: How They Are Made and Explored" Catalysts 11, no. 2: 294. https://doi.org/10.3390/catal11020294
APA StyleEmeline, A. V., Rudakova, A. V., Mikhaylov, R. V., Bulanin, K. M., & Bahnemann, D. W. (2021). Photoactive Heterostructures: How They Are Made and Explored. Catalysts, 11(2), 294. https://doi.org/10.3390/catal11020294










