Photocatalytic Nanocomposite Materials Based on Inorganic Polymers (Geopolymers): A Review
Abstract
:1. Introduction
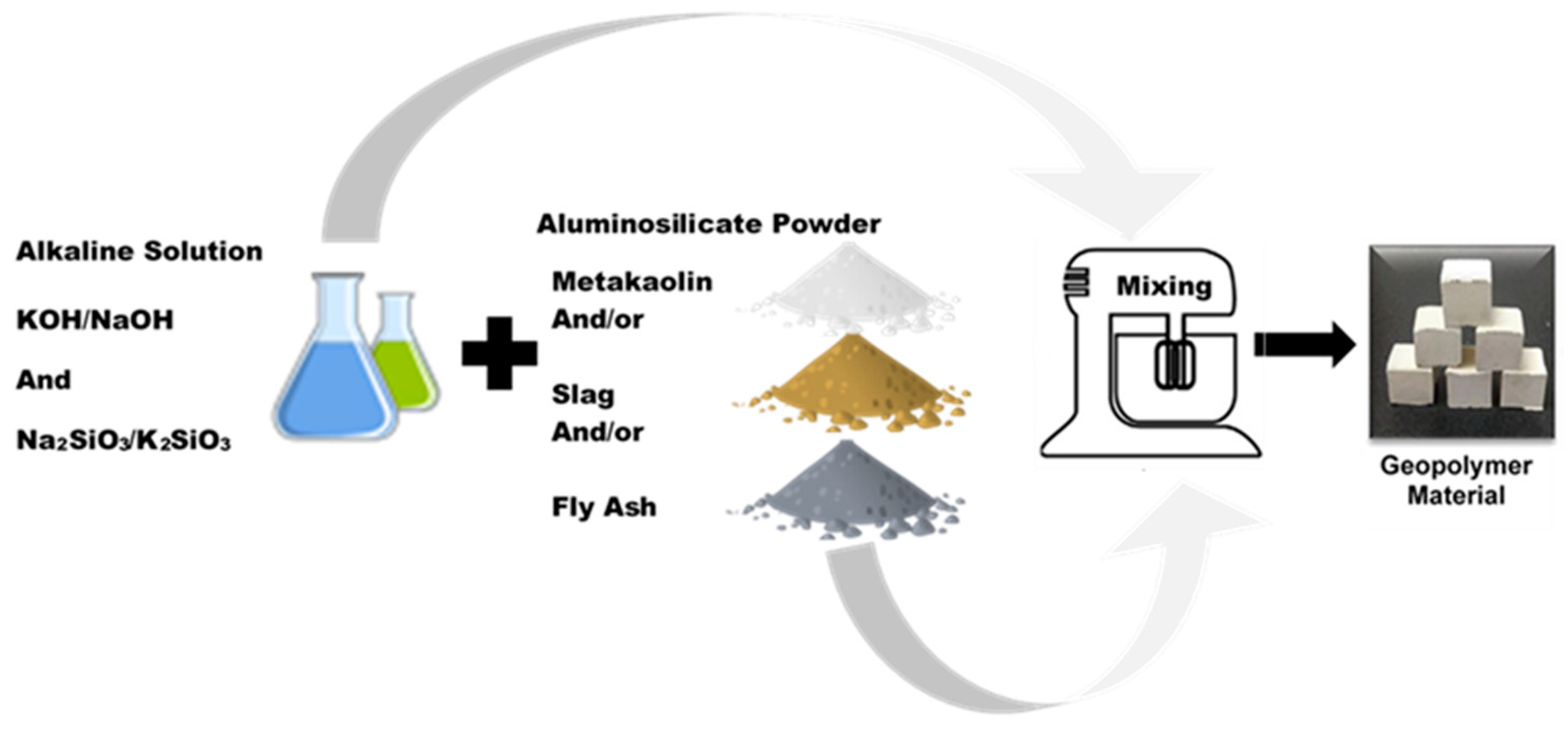
2. Aluminosilicate Geopolymers: Composition, Synthesis and Structure
3. Mechanism of Photocatalysis in Geopolymers
4. Aluminosilicate Geopolymers with Photocatalytic Functionality
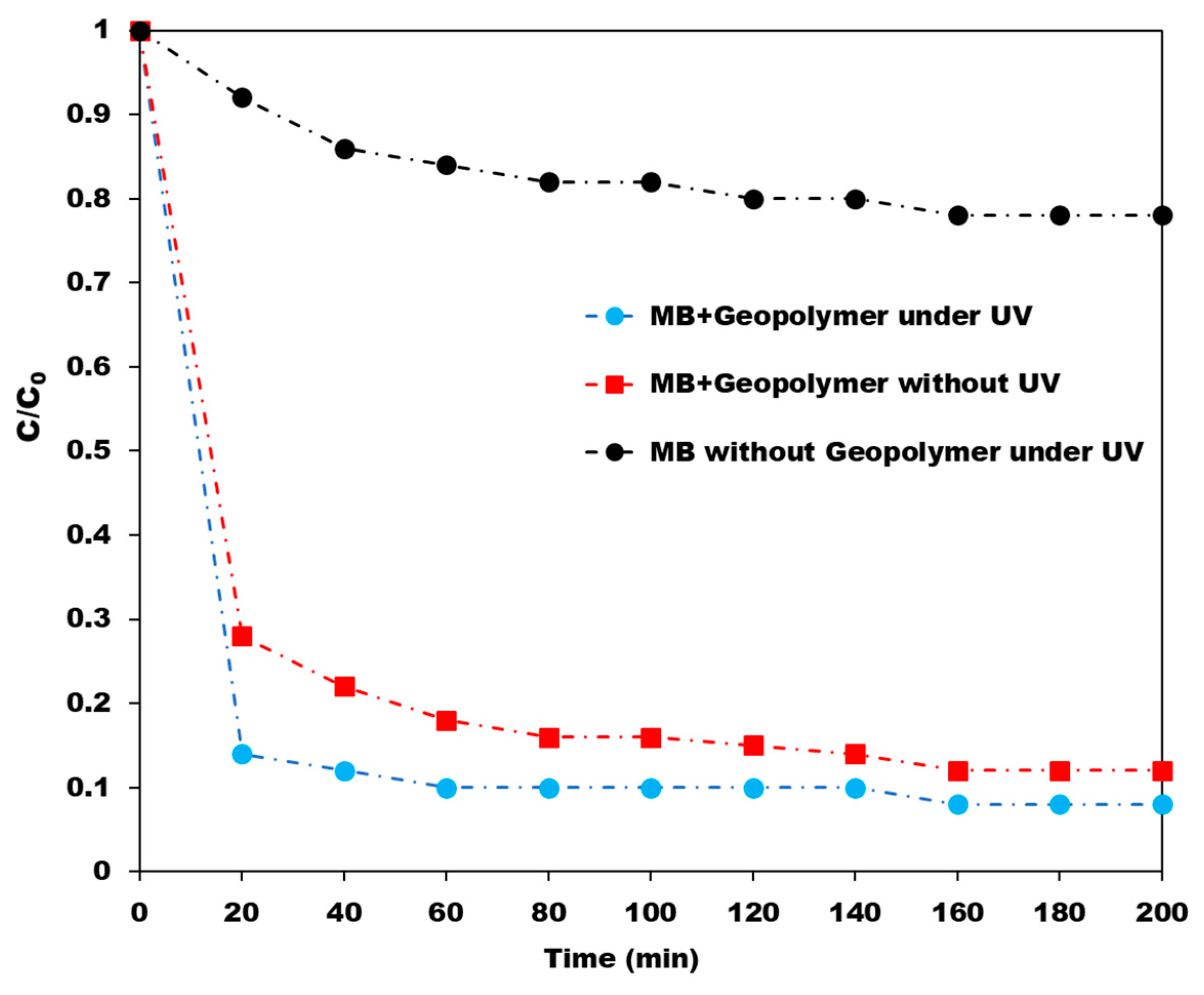
4.1. Geopolymer/TiO2 Photocatalysts
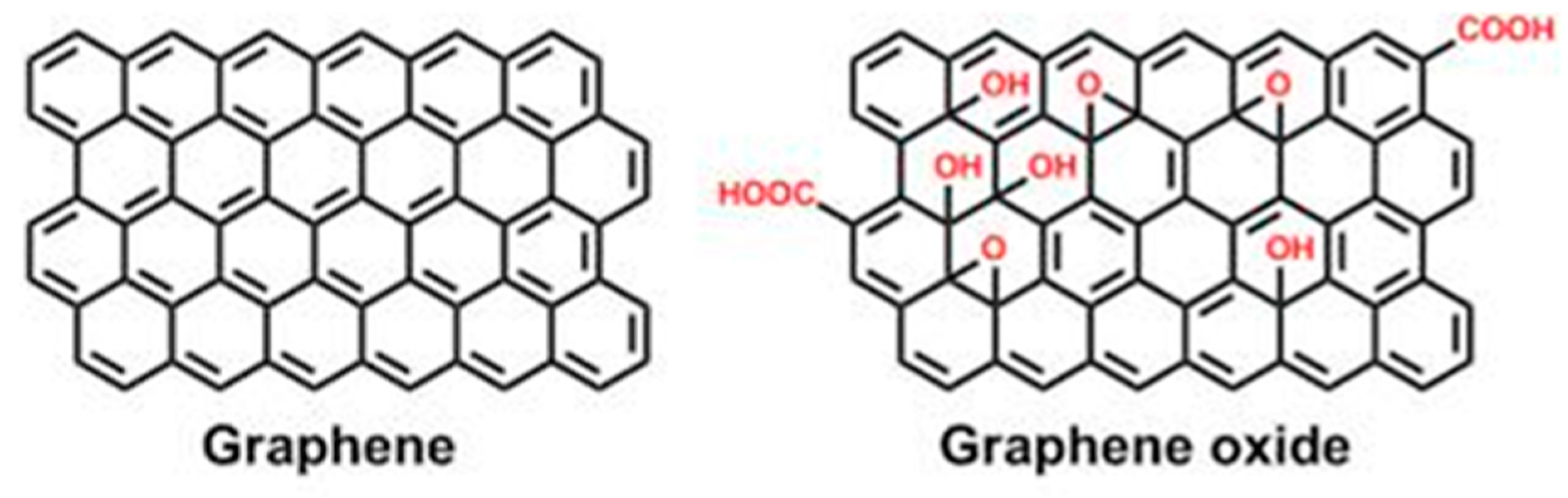
4.2. Geopolymer/Graphene Photocatalysts
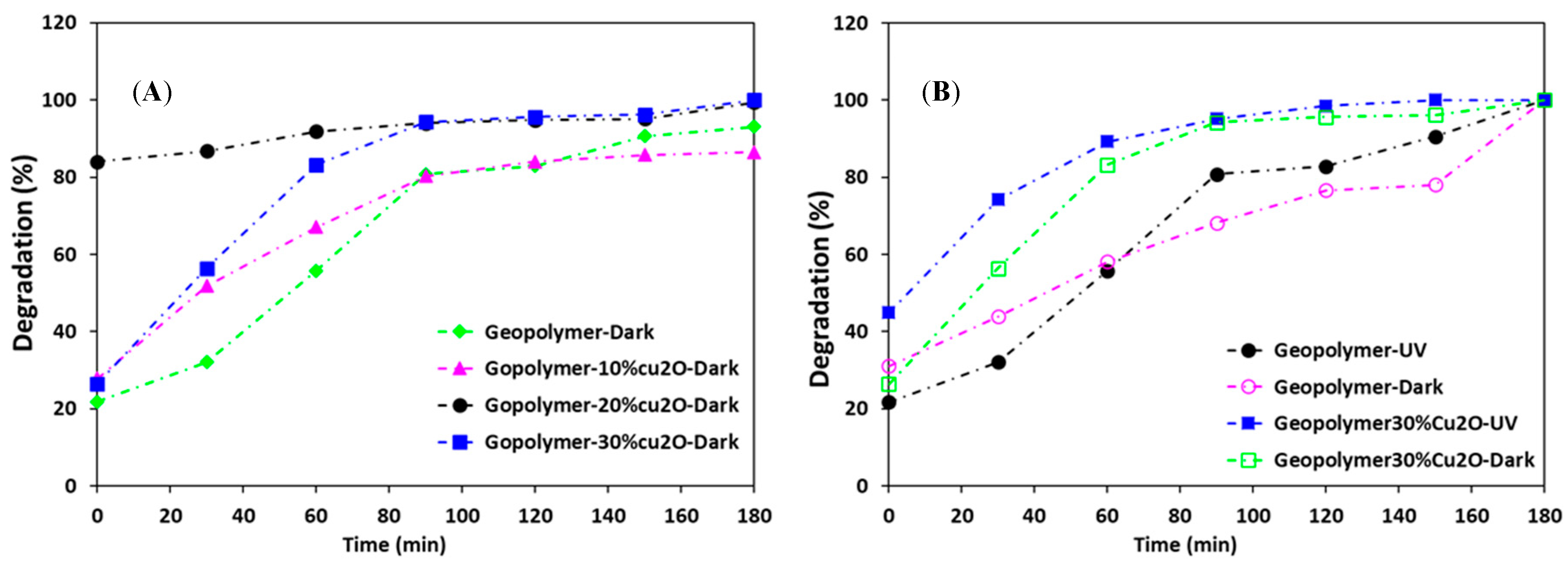
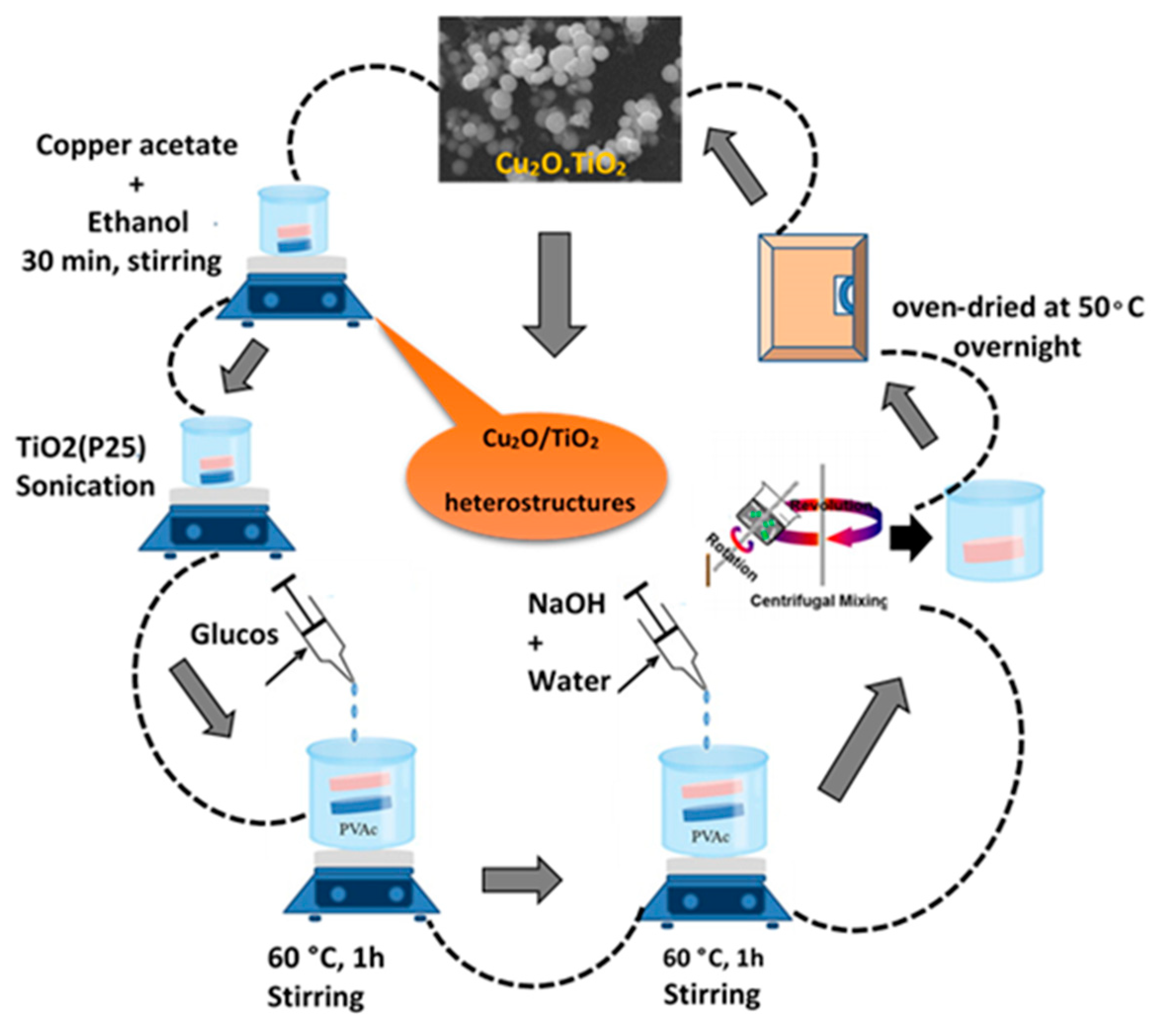
4.3. Geopolymer/Cu2O Photocatalysts
4.4. Geopolymer/Carbon Nanotube Photocatalysts
4.5. Other Geopolymer Photocatalysts
5. Conclusions
Funding
Conflicts of Interest
References
- Davidovits, J. Geopolymers. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Komphanchai, S.; Vagana, R. Formation of inorganic polymers (geopolymers) from 2:1 layer lattice aluminosilicates. J. Eur. Ceram. Soc. 2008, 28, 177–181. [Google Scholar] [CrossRef]
- Lemougna, P.N.; MacKenzie, K.J.D.; Melo, U.C. Synthesis and thermal properties of inorganic polymers (geopolymers) for structural and refractory applications from volcanic ash. Ceram. Int. 2011, 37, 3011–3018. [Google Scholar] [CrossRef]
- Xu, G.; Shi, X. Characteristics and applications of fly ash as a sustainable construction material: A state-of-the-art review. Resour. Conserv. Recycl. 2018, 136, 95–109. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Bayarzul, U.; Kim, D.S.; Lee, S.-H.; Lee, H.J.; Ruescher, C.H.; MacKenzie, K.J. Properties of geopolymer binders prepared from milled pond ash. Mater. Constr. 2017, 67, 134. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F. Carbon dioxide equivalent (CO2- e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D. Inorganic Polymers (Geopolymers). In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2017; pp. 1–31. ISBN 9780471440260. [Google Scholar] [CrossRef]
- Santhi, K.; Rani, C.; Karuppuchamy, S. Synthesis and characterization of a novel SnO/SnO2 hybrid photocatalyst. J. Alloys Compd. 2016, 662, 102–107. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A. Photocatalytic roads: From lab tests to real scale applications. Eur. Transp. Res. Rev. 2012, 5, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Falah, M. Synthesis of New Composites of Inorganic Polymers (Geopolymers) with Metal Oxide Nanoparticles and Their Photodegradation of Organic Pollutants. Ph.D. Thesis, Victoria University of Wellington, Wellington, New Zealand, 2015. Available online: http://researcharchive.vuw.ac.nz/handle/10063/4847 (accessed on 8 October 2020).
- Shen, S.; Kronawitter, C.; Kiriakidis, G. An overview of photocatalytic materials. J. Materiomics 2017, 3, 1–2. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J. Geopolymerisation kinetics. Reaction kinetic modelling. Chem. Eng. Sci. 2007, 62, 2318–2329. [Google Scholar] [CrossRef]
- Rowles, M.R.; Hanna, J.V.; Pike, K.J.; Smith, M.E.; O’Connor, B.H. 29 Si, 27 Al, 1 H and 23 Na MAS NMR study of the bonding character in aluminosilicate inorganic polymers. Appl. Magn. Reson. 2007, 32, 663–689. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Smith, M.E. Multinuclear Solid State NMR of Inorganic Materials, Pergamon Materials Series; Pergamon/Elsevier: Oxford, UK, 2002; Volume 6, p. 304. [Google Scholar]
- O’Connor, S.J.; MacKenzie, K.J.D.; Smith, M.E.; Hanna, J.V. Ion exchange in the charge-balancing sites of aluminosilicate inorganic polymers. J. Mater. Chem. 2010, 20, 10234. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Galindo, C.; Jacques, P.; Kalt, A. Photodegradation of the aminoazobenzene acid orange 52 by three advanced oxidation processes: UV/H2O2, UV/TiO2 and VIS/TiO2. J. Photochem. Photobiol. A Chem. 2000, 130, 35–47. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, C.; Ma, W. Photocatalytic degradation of organic pollutants under visible light irradiation. Top. Catal. 2005, 35, 269–278. [Google Scholar] [CrossRef]
- Maragatha, J.; Jothivenkatachalam, K.; Karuppuchamy, S. Synthesis and characterization of visible light-responsive carbon doped Ti4O7 photocatalyst. J. Mater. Sci. Mater. Electron. 2016, 27, 9233–9239. [Google Scholar] [CrossRef]
- Nagalakshmi, M.; Karthikeyan, C.; Anusuya, N.; Brundha, C.; Basu, M.J.; Karuppuchamy, S. Synthesis of TiO2 nanofiber for photocatalytic and antibacterial applications. J. Mater. Sci. Mater. Electron. 2017, 28, 15915–15920. [Google Scholar] [CrossRef]
- Mills, A.; Davies, R.H.; Worsley, D. Water purification by semiconductor photocatalysis. Chem. Soc. Rev. 1993, 22, 417. [Google Scholar] [CrossRef]
- Wilke, K.; Breuer, H. The influence of transition metal doping on the physical and photocatalytic properties of titania. J. Photochem. Photobiol. A Chem. 1999, 121, 49–53. [Google Scholar] [CrossRef]
- Falah, M.; MacKenzie, K.J.D.; Hanna, J.V.; Page, S.J. Novel photoactive inorganic polymer composites of inorganic polymers with copper (I) oxide nanoparticles. J. Mater. Sci. 2015, 50, 7374–7383. [Google Scholar] [CrossRef]
- Kabra, K.; Chaudhary, R.; Sawhney, R.L. Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: A review. Ind. Eng. Chem. Res. 2004, 43, 7683–7696. [Google Scholar] [CrossRef]
- Hanus, M.J.; Harris, A.T. Nanotechnology innovations for the construction industry. Prog. Mater. Sci. 2013, 58, 1056–1102. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Nanotechnology: Advantages and drawbacks in the field of construction and building materials. Constr. Build. Mater. 2011, 25, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Chen, L.; Gbozee, M. Thermal stability of geopolymers used as supporting materials for TiO2 film coating through sol-gel process: Feasibility and improvement. Constr. Build. Mater. 2016, 125, 1114–1126. [Google Scholar] [CrossRef]
- Fan, W.; Lai, Q.; Zhang, Q.; Wang, Y. Nanocomposites of TiO2 and reduced graphene oxide as efficient photocatalysts for hydrogen evolution. J. Phys. Chem. C 2011, 115, 10694–10701. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, K.; Liu, Y. Geopolymer-supported photocatalytic TiO2 film: Preparation and characterization. Constr. Build. Mater. 2017, 151, 63–70. [Google Scholar] [CrossRef]
- Strini, A.; Roviello, G.; Ricciotti, L.; Ferone, C.; Messina, F.; Schiavi, L.; Corsaro, D.; Cioffi, R. TiO2-based photocatalytic geopolymers for nitric oxide degradation. Materials 2016, 9, 513. [Google Scholar] [CrossRef]
- Bravo, P.I.; Malenab, R.A.; Shimizu, E.; Yu, D.E.; Promentilla, M.A. Synthesis of geopolymer spheres with photocatalytic activity. MATEC Web Conf. 2019, 268, 04007. [Google Scholar] [CrossRef]
- Samuneva, B.; Kozhukharov, V.; Trapalis, C.; Kranold, R. Sol-gel processing of titanium- containing thin coatings-Part I. Preparation and structure. J. Mater. Sci. 1993, 28, 2353–2360. [Google Scholar] [CrossRef]
- Gasca-Tirado, J.; Manzano-Ramírez, A.; Villaseñor-Mora, C.; Muñiz-Villarreal, M.; Zaldivar-Cadena, A.; Rubio-Avalos, J.; Borrás, V.A.; Mendoza, R.N. Incorporation of photoactive TiO2 in an aluminosilicate inorganic polymer by ion exchange. Microporous Mesoporous Mater. 2012, 153, 282–287. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Yan, C.; Peng, R.; Wang, H. Geopolymer-TiO2 nanocomposites for photocsatalysis: Synthesis by one-step adding treatment versus two-step acidification calcination. Minerals 2019, 9, 658. [Google Scholar] [CrossRef] [Green Version]
- Lertcumfu, N.; Jaita, P.; Thammarong, S.; Lamkhao, S.; Tandorn, S.; Randorn, C.; Tunkasiri, T.; Rujijanagul, G. Influence of graphene oxide additive on physical, microstructure, adsorption, and photocatalytic properties of calcined kaolinite-based geopolymer ceramic composites. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 602, 125080. [Google Scholar] [CrossRef]
- Gupta, K.; Khatri, O.P. Reduced graphene oxide as an effective adsorbent for removal of malachite green dye: Plausible adsorption pathways. J. Colloid Interface Sci. 2017, 501, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, J.-C.; Chen, C.; Tan, X.; Hayat, T.; Alsaedi, A.; Cheng, C.; Chen, C. Impact of graphene oxide on the antibacterial activity of antibiotics against bacteria. Environ. Sci. Nano 2017, 4, 1016–1024. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Krishnan, R.; Thangavel, S.; Venugopal, G.; Kim, S.-J.; Arthi, G.; Rajasekar, K.; Sakthivel, T.; Gunasekaran, V. Removal of heavy metal ions from pharma-effluents using graphene-oxide nanosorbents and study of their adsorption kinetics. J. Ind. Eng. Chem. 2015, 30, 14–19. [Google Scholar] [CrossRef]
- Shamsaei, E.; De Souza, F.B.; Yao, X.; Benhelal, E.; Akbari, A.; Zhuang, J. Graphene-based nanosheets for stronger and more durable concrete: A review. Constr. Build. Mater. 2018, 183, 642–660. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yang, M.Y.; Zhang, L.; Zhang, K.; Kang, L. A new graphene/geopolymer nanocomposite for degradation of dye wastewater. Integr. Ferroelectr. 2016, 171, 38–45. [Google Scholar] [CrossRef]
- Zhang, Y.J.; He, P.Y.; Zhang, Y.X.; Chen, H. A novel electroconductive graphene/fly ash-based geopolymer composite and its photocatalytic performance. Chem. Eng. J. 2018, 334, 2459–2466. [Google Scholar] [CrossRef]
- Huang, L.; Peng, F.; Yu, H.; Wang, H. Preparation of cuprous oxides with different sizes and their behaviors of adsorption, visible-light driven photocatalysis and photocorrosion. Solid State Sci. 2009, 11, 129–138. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Wang, Z.; Guo, M.; Qin, X.; Zhang, X.; Wang, P.; Dai, Y. Crystal Faces of Cu2O and Their stabilities in photocatalytic reactions. J. Phys. Chem. C 2009, 113, 14448–14453. [Google Scholar] [CrossRef]
- Saleh, T.A. Syntheses and Applications of Carbon Nanotubes and Their Composites; InTech Open: London, UK, 2013. [Google Scholar] [CrossRef]
- Kumar, M. Effect of Nanomaterials on the Properties of Geopolymer Mortars and Concrete; Elsevier BV: Amsterdam, The Netherlands, 2018; Volume 5, pp. 9035–9040. [Google Scholar]
- Mahmoodi, N.M. Synthesis of magnetic carbon nanotube and photocatalytic dye degradation ability. Environ. Monit. Assess. 2014, 186, 5595–5604. [Google Scholar] [CrossRef]
- Bi, S.; Liu, M.; Shen, J.; Hu, X.; Zhang, L. Ultrahigh self-sensing performance of geopolymer nanocomposites via unique interface engineering. ACS Appl. Mater. Interfaces 2017, 9, 12851–12858. [Google Scholar] [CrossRef]
- Huang, L.; Peng, F.; Wang, H.; Yu, H.; Li, Z. Preparation and characterization of Cu2O/TiO2 nano-nano heterostructure photocatalysts. Catal. Commun. 2009, 10, 1839–1843. [Google Scholar] [CrossRef]
- Falah, M.; MacKenzie, K.J.D. Synthesis and properties of novel photoactive composites of P25 titanium dioxide and copper (I) oxide with inorganic polymers. Ceram. Int. 2015, 41, 13702–13708. [Google Scholar] [CrossRef]
- Falah, M.; MacKenzie, K.J.D.; Knibbe, R.; Page, S.J.; Hanna, J.V. New composites of nanoparticle Cu (I) oxide and titania in a novel inorganic polymer (geopolymer) matrix for destruction of dyes and hazardous organic pollutants. J. Hazard. Mater. 2016, 318, 772–782. [Google Scholar] [CrossRef]
- Zailan, S.N.; Bouaissi, A.; Mahmed, N.; Abdullah, M.M.A.B. Influence of ZnO Nanoparticles on Mechanical Properties and Photocatalytic Activity of Self-cleaning ZnO-Based Geopolymer Paste. J. Inorg. Organomet. Polym. Mater. 2019, 30, 2007–2016. [Google Scholar] [CrossRef]
- Zhang, Y.J.; He, P.Y.; Yang, M.Y.; Chen, H.; Liu, L.C. Renewable conversion of slag to graphene geopolymer for H2 production and wastewater treatment. Catal. Today 2019. [Google Scholar] [CrossRef]
- Zhang, Y.J.; He, P.Y.; Chen, H. A novel CdO/graphene alkali-activated steel slag nanocomposite for photocatalytic degradation of dye wastewater. Ferroelectrics 2018, 522, 1–8. [Google Scholar] [CrossRef]
- Saufi, H.; El Alouani, M.; Alehyen, S.; El Achouri, M.; Aride, J.; Taibi, M. Photocatalytic degradation of methylene blue from aqueous medium onto perlite-based geopolymer. Int. J. Chem. Eng. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, L. Fly ash-based geopolymer as a novel photocatalyst for degradation of dye from wastewater. Particuology 2013, 11, 353–358. [Google Scholar] [CrossRef]
- He, P.Y.; Zhang, Y.J.; Chen, H.; Liu, L.C. Development of an eco-efficient CaMoO4/electroconductive geopolymer composite for recycling silicomanganese slag and degradation of dye wastewater. J. Clean. Prod. 2019, 208, 1476–1487. [Google Scholar] [CrossRef]





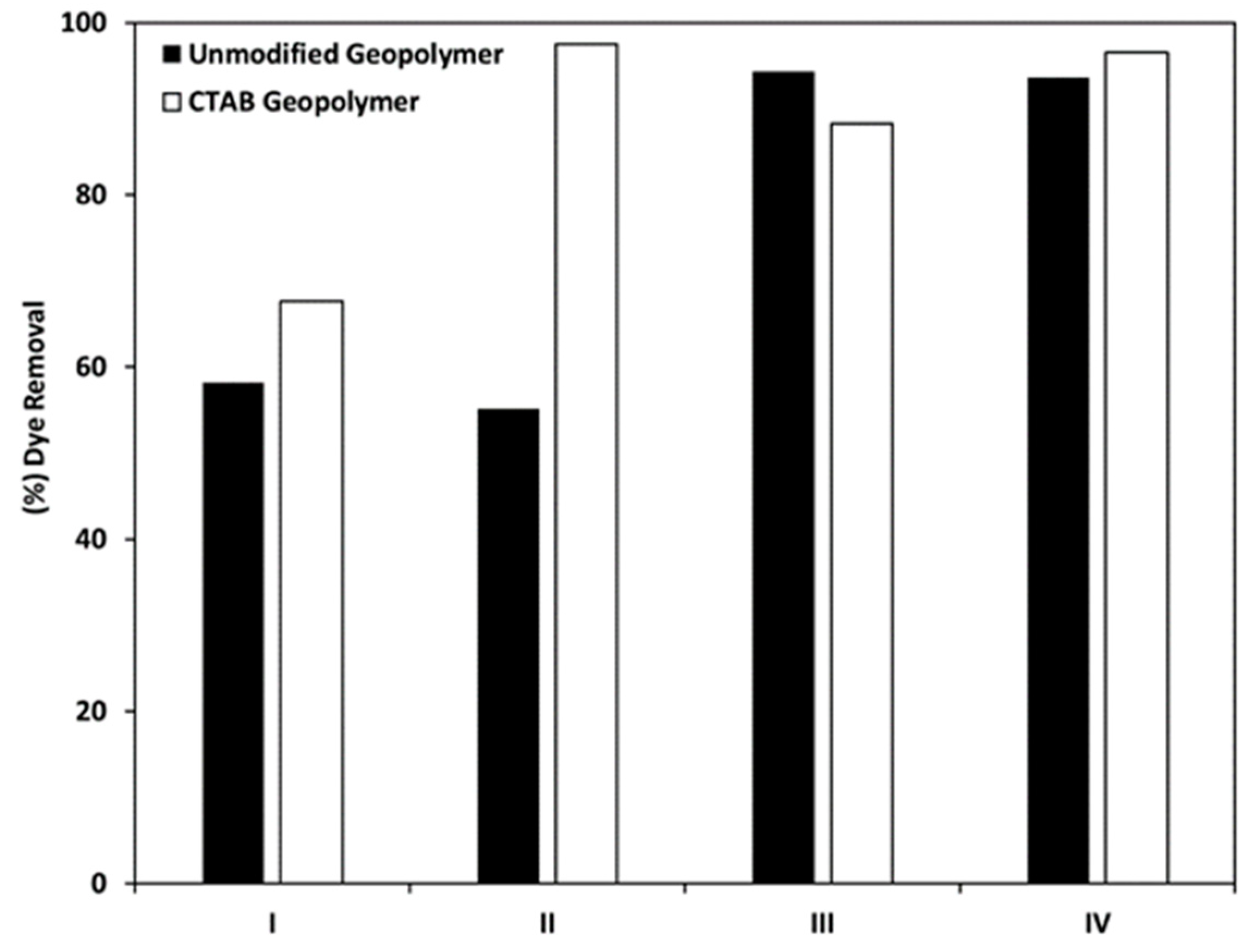

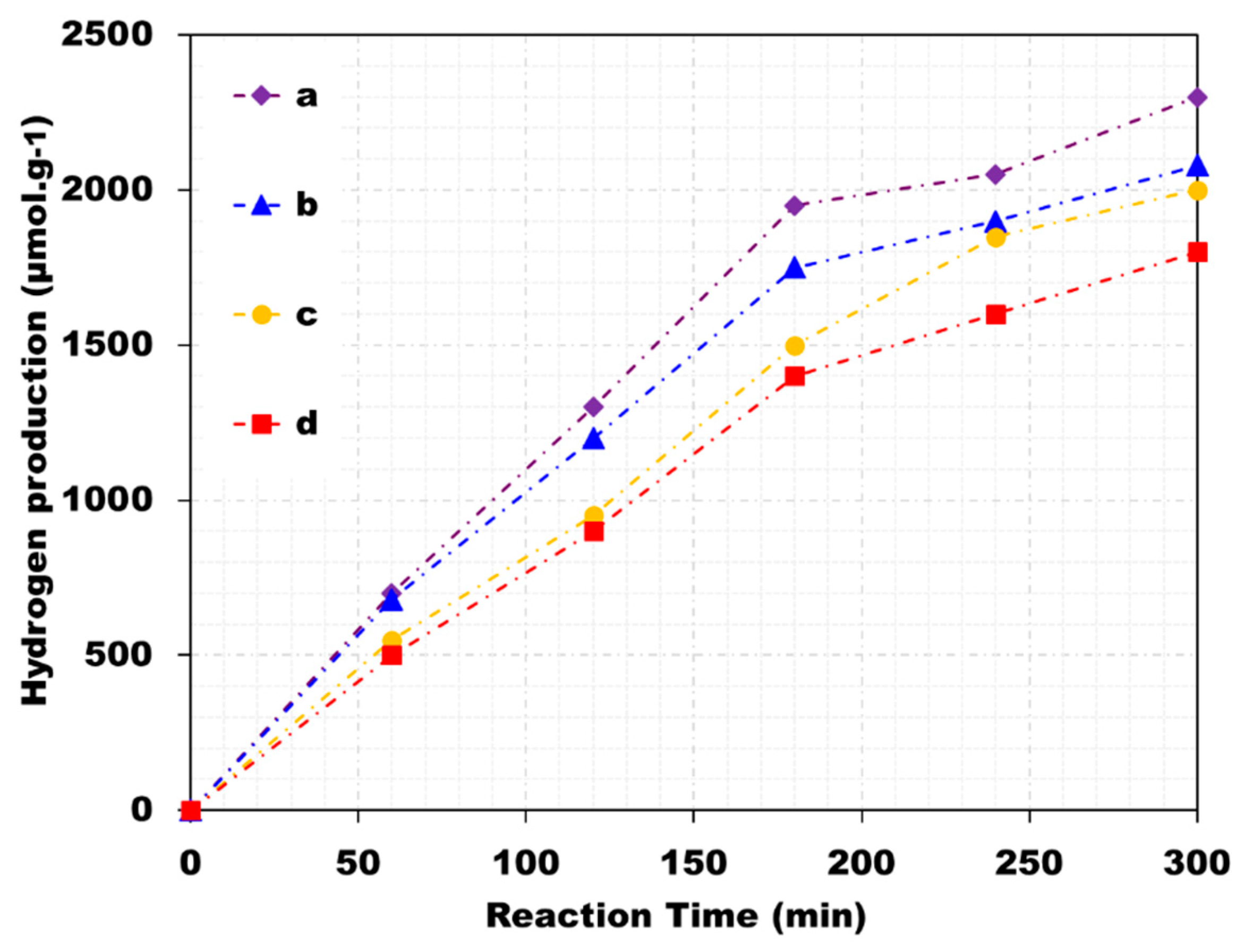
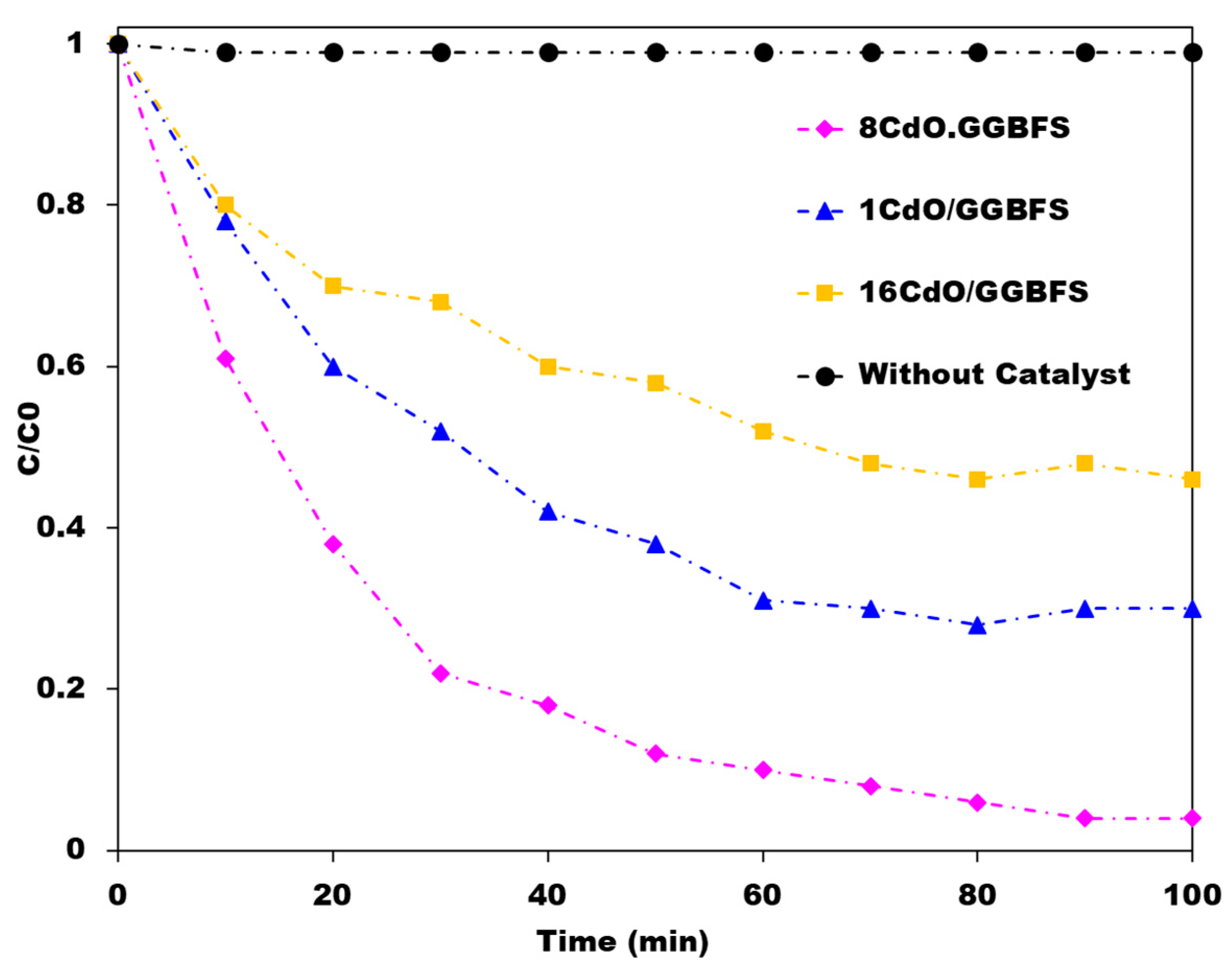
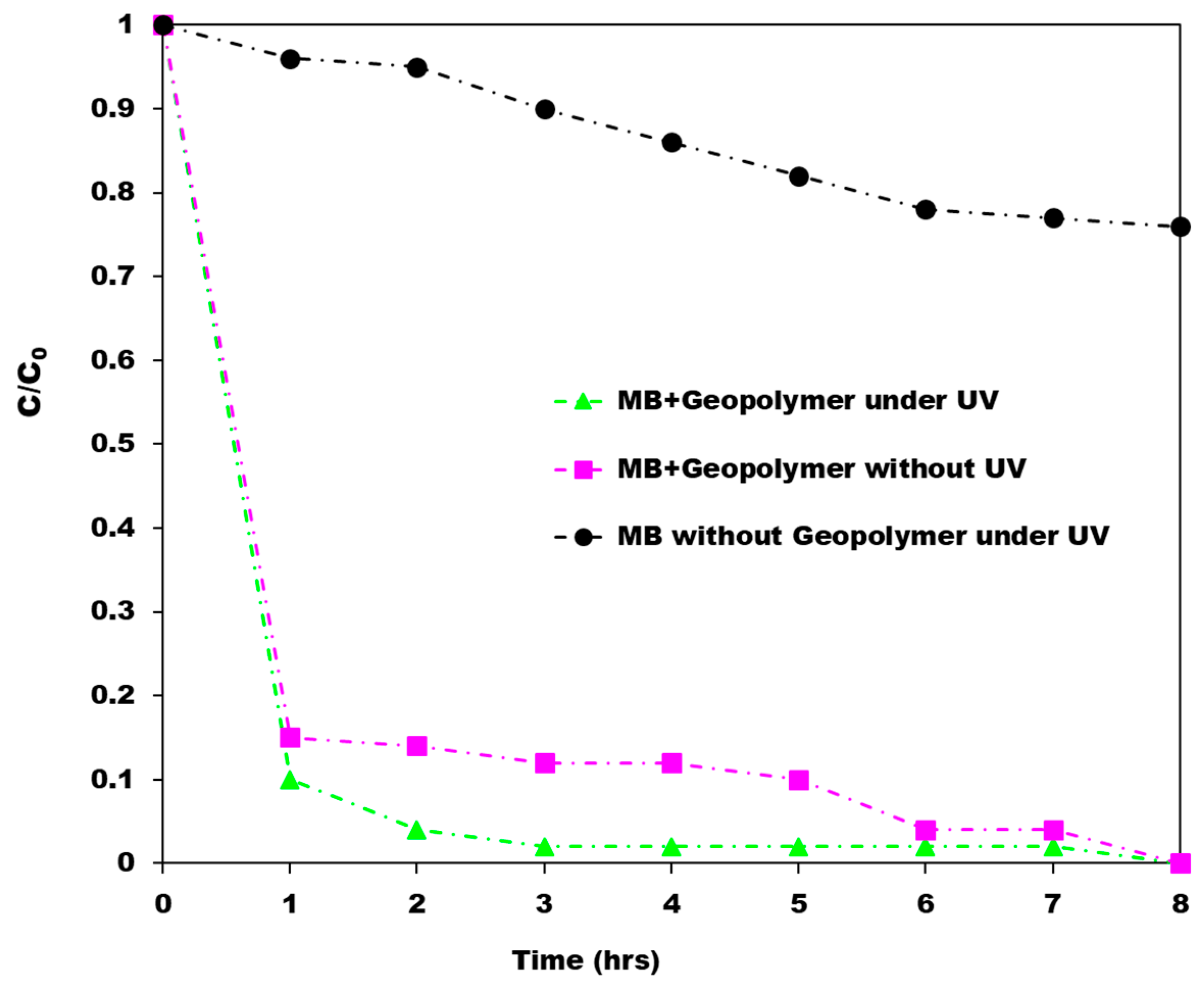
| Photocatalyst | Bandgap (eV) | Photocatalyst | Bandgap (eV) |
|---|---|---|---|
| Diamond | 5.4 | SnO2 | 3.8 |
| Cubic ZnS | 3.6 | SrTiO3 | 3.4 |
| ZnO | 3.3 | TiO2 (anatase) | 3.2 |
| α-Fe2O3 | 3.1 | TiO2 (rutile) | 3.0 |
| WO3 | 2.8 | CdS | 2.4 |
| Fe2O3 | 2.2 | Cu2O | 2.1 |
| CdSe | 1.7 | CdTe | 1.4 |
| WSe2 | 1.2 | Si | 1.1 |
| Adsorbent | Preparation Method | TiO2 Type | TiO2 Content | Adsorbate | Reference |
|---|---|---|---|---|---|
| TiO2/fly ash or metakaolin geopolymer | Mixing | P25 | 3% | NO and NOx | Strini 2016 [32] |
| TiO2/metakaolin geopolymer | Ion-Exchange | Anatase | 28% | MB | Gasca-Tirado 2012 [35] |
| TiO2/fly ash-metakaolin geopolymer | Sol-Gel dip coating | Anatase, Rutile | NA | MB | Chen 2017 [31] |
| TiO2/fly ash geopolymer | Mixing | P25 | 10% | MB | Yang 2019 [36] |
| TiO2/metakaolin geopolymer spheres | Inside quartz tube at high temperature | P25 | 10 mg | MB | Bravo 2019 [33] |
| Matrix | Preparation Method | Graphene Content (%) | Adsorbate | Reference |
|---|---|---|---|---|
| graphene/fly ash-based geopolymer | Mixing | 0.1, 0.4, 0.7 and 1 | Indigo carmine | Zhang 2018 [43] |
| graphene oxide/calcined kaolinite-based geopolymer | Mixing | 0, 2.5, 5, and 10 | Methylene blue | Lertcumfu 2020 [37] |
| graphene/blast furnace slag-based geopolymer | Mixing | 0.01 | Methyl violet | Zhang 2016 [42] |
| Cycle Number | Degradation (%) |
|---|---|
| 1 | 92.7 |
| 2 | 90.6 |
| 3 | 89.7 |
| 4 | 88.5 |
| 5 | 87.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falah, M.; MacKenzie, K.J.D. Photocatalytic Nanocomposite Materials Based on Inorganic Polymers (Geopolymers): A Review. Catalysts 2020, 10, 1158. https://doi.org/10.3390/catal10101158
Falah M, MacKenzie KJD. Photocatalytic Nanocomposite Materials Based on Inorganic Polymers (Geopolymers): A Review. Catalysts. 2020; 10(10):1158. https://doi.org/10.3390/catal10101158
Chicago/Turabian StyleFalah, Mahroo, and Kenneth J. D. MacKenzie. 2020. "Photocatalytic Nanocomposite Materials Based on Inorganic Polymers (Geopolymers): A Review" Catalysts 10, no. 10: 1158. https://doi.org/10.3390/catal10101158
APA StyleFalah, M., & MacKenzie, K. J. D. (2020). Photocatalytic Nanocomposite Materials Based on Inorganic Polymers (Geopolymers): A Review. Catalysts, 10(10), 1158. https://doi.org/10.3390/catal10101158






