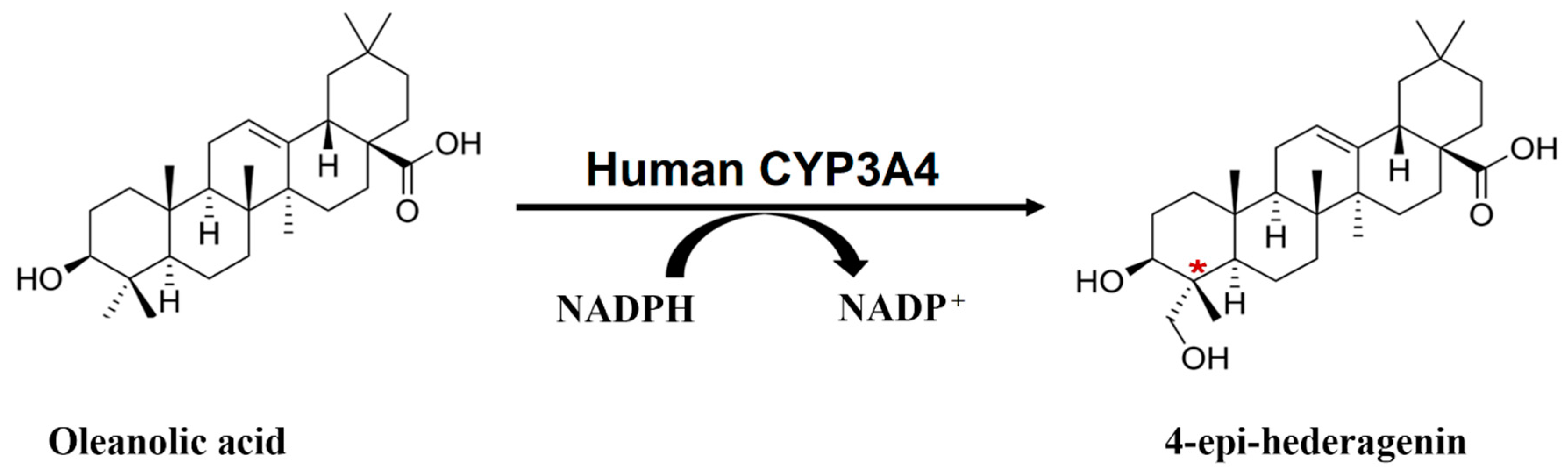
Regioselective Hydroxylation of Oleanolic Acid Catalyzed by Human CYP3A4 to Produce Hederagenenin, a Chiral Metabolite
Abstract
1. Introduction
2. Results and Discussion
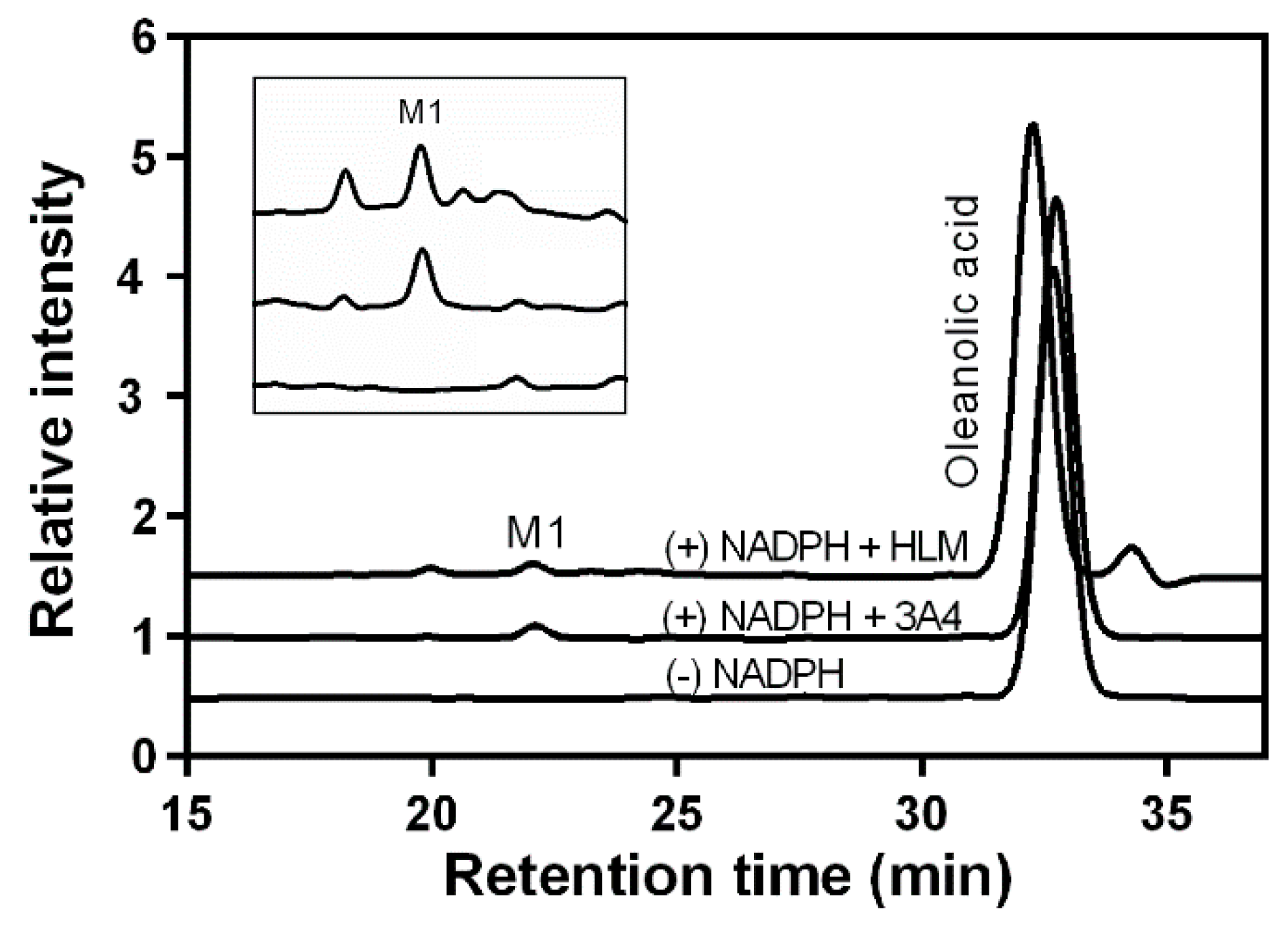
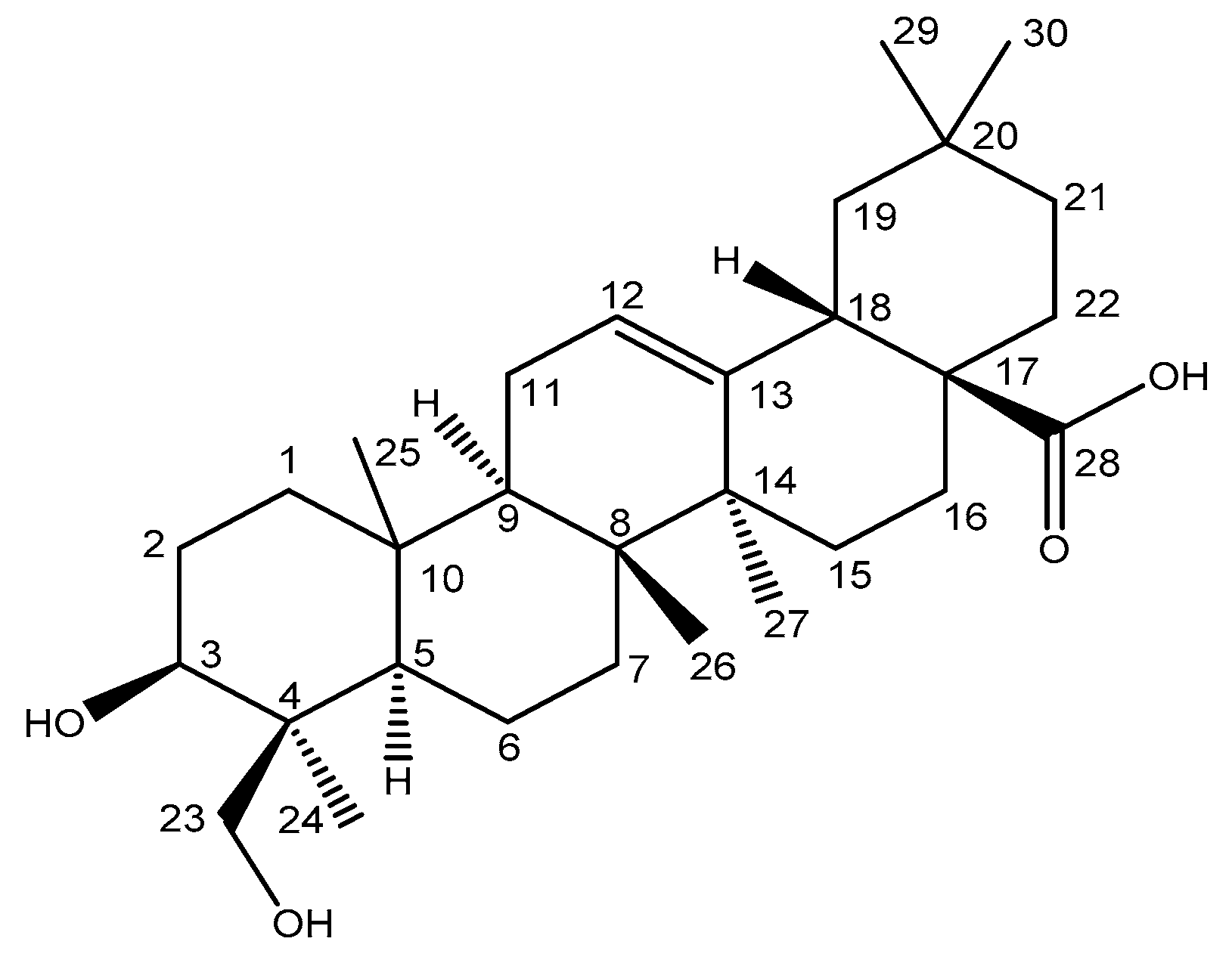
2.1. OA Metabolism by Human Liver Microsomes and Identification of the Major Metabolite
2.2. Kinetics Parameters and Total Turnover Numbers of OA Hydroxylation by CYP3A4 and HLMs
2.3. Inhibition of OA Hydroxylation Activity by Antibodies in HLMs
3. Materials and Methods
3.1. Materials
3.2. Hydroxylation of OA by CYP3A4 and HLMs
3.3. LC–MS Analysis of OA Metabolite
3.4. NMR Spectroscopy Analysis of OA Metabolite
3.5. Immunoinhibition of Antibodies on OA Hydroxylation Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ramana, K.V.; Singhal, S.S.; Reddy, A.B. Therapeutic Potential of Natural Pharmacological Agents in the Treatment of Human Diseases. Available online: https://www.hindawi.com/journals/bmri/2014/573452/ (accessed on 30 November 2020).
- Luchnikova, N.A.; Grishko, V.V.; Ivshina, I.B. Biotransformation of Oleanane and Ursane Triterpenic Acids. Molecules 2020, 25, 5526. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.R.; Rasbery, J.M.; Bartel, B.; Matsuda, S.P. Biosynthetic Diversity in Plant Triterpene Cyclization. Curr. Opin. Plant Biol. 2006, 9, 305–314. [Google Scholar] [CrossRef]
- Yumpu.com A Review of Presence of Oleanolic Acid in—Natura Proda Medica. Available online: https://www.yumpu.com/en/document/read/4492852/a-review-of-presence-of-oleanolic-acid-in-natura-proda-medica (accessed on 25 November 2020).
- Liu, J. Pharmacology of Oleanolic Acid and Ursolic Acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Liu, J. Oleanolic Acid and Ursolic Acid: Research Perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial Activity of Oleanolic and Ursolic Acids: An Update. Evid. Based Complement. Altern. Med. ECAM 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, Q.; Zhang, Z.; Peng, Y.; Qiu, Y.; Shi, Y.; Zheng, Y.; Xiao, S.; Wang, H.; Huang, X.; et al. Development of Oleanane-Type Triterpenes as a New Class of HCV Entry Inhibitors. J. Med. Chem. 2013, 56, 4300–4319. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.-W.; Dou, T.-Y.; Wang, P.; Lei, W.; Weng, Z.-M.; Hou, J.; Wang, D.-D.; Fan, Y.-M.; Zhang, W.-D.; Ge, G.-B.; et al. Structure-Activity Relationships of Pentacyclic Triterpenoids as Potent and Selective Inhibitors against Human Carboxylesterase 1. Front. Pharmacol. 2017, 8, 435. [Google Scholar] [CrossRef]
- Villar, V.H.; Vögler, O.; Barceló, F.; Gómez-Florit, M.; Martínez-Serra, J.; Obrador-Hevia, A.; Martín-Broto, J.; Ruiz-Gutiérrez, V.; Alemany, R. Oleanolic and Maslinic Acid Sensitize Soft Tissue Sarcoma Cells to Doxorubicin by Inhibiting the Multidrug Resistance Protein MRP-1, but Not P-Glycoprotein. J. Nutr. Biochem. 2014, 25, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Sieben, A.; Prenner, L.; Sorkalla, T.; Wolf, A.; Jakobs, D.; Runkel, F.; Häberlein, H. α-Hederin, but Not Hederacoside C and Hederagenin from Hedera Helix, Affects the Binding Behavior, Dynamics, and Regulation of Β2-Adrenergic Receptors. Biochemistry 2009, 48, 3477–3482. [Google Scholar] [CrossRef]
- Zeng, J.; Huang, T.; Xue, M.; Chen, J.; Feng, L.; Du, R.; Feng, Y. Current Knowledge and Development of Hederagenin as a Promising Medicinal Agent: A Comprehensive Review. RSC Adv. 2018, 8, 24188–24202. [Google Scholar] [CrossRef]
- Hasler, J.A.; Estabrook, R.; Murray, M.; Pikuleva, I.; Waterman, M.; Capdevila, J.; Holla, V.; Helvig, C.; Falck, J.R.; Farrell, G.; et al. Human Cytochromes P450. Mol. Aspects Med. 1999, 20, 1–137. [Google Scholar] [CrossRef]
- Guengerich, F.P. Human Cytochrome P450 Enzymes. In Cytochrome P450: Structure, Mechanism, and Biochemistry; de Montellano, P.R.O., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 523–785. ISBN 978-3-319-12108-6. [Google Scholar]
- Nelson, D.R.; Kamataki, T.; Waxman, D.J.; Guengerich, F.P.; Estabrook, R.W.; Feyereisen, R.; Gonzalez, F.J.; Coon, M.J.; Gunsalus, I.C.; Gotoh, O.; et al. The P450 Superfamily: Update on New Sequences, Gene Mapping, Accession Numbers, Early Trivial Names of Enzymes, and Nomenclature. DNA Cell Biol. 1993, 12, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Hirosue, S.; Fujii, T.; Matsumoto, N.; Agematu, H.; Arisawa, A. Hydroxylation of Oleanolic Acid to Queretaroic Acid by Cytochrome P450 from Nonomuraea Recticatena. Biosci. Biotechnol. Biochem. 2006, 70, 2299–2302. [Google Scholar] [CrossRef]
- Molina-García, L.; Martínez-Expósito, R.; Fernández-de Córdova, M.L.; Llorent-Martínez, E.J. Determination of the Phenolic Profile and Antioxidant Activity of Leaves and Fruits of Spanish Quercus Coccifera. Available online: https://www.hindawi.com/journals/jchem/2018/2573270/ (accessed on 25 January 2021).
- Kizu, H.; Tomimori, T. Studies on the Constituents of Clematis Species. V. On the Saponins of the Root of Clematis Chinensis OSBECK (5). Chem. Pharm. Bull. 1982, 30, 3340–3346. [Google Scholar] [CrossRef]
- Kim, E.H.; Baek, S.; Shin, D.; Lee, J.; Roh, J.-L. Hederagenin Induces Apoptosis in Cisplatin-Resistant Head and Neck Cancer Cells by Inhibiting the Nrf2-ARE Antioxidant Pathway. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Kivistö, K.T.; Kroemer, H.K.; Eichelbaum, M. The Role of Human Cytochrome P450 Enzymes in the Metabolism of Anticancer Agents: Implications for Drug Interactions. Br. J. Clin. Pharmacol. 1995, 40, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Lee, J.-S.; Park, H.-J.; Kim, J.-W.; Kim, C.-J.; Shim, I.-S.; Kim, N.-J.; Han, S.-M.; Lim, S. Inhibition of Cytochrome P450 Activities by Oleanolic Acid and Ursolic Acid in Human Liver Microsomes. Life Sci. 2004, 74, 2769–2779. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.-H.; Ahn, T.; Guengerich, F.P.; Yamazaki, H.; Shimada, T. Phospholipase D Activity of Cytochrome P450 in Human Liver Endoplasmic Reticulum. Arch. Biochem. Biophys. 1999, 367, 81–88. [Google Scholar] [CrossRef]
- Yun, C.-H.; Yim, S.-K.; Kim, D.-H.; Ahn, T. Functional Expression of Human Cytochrome P450 Enzymes in Escherichia Coli. Curr. Drug Metab. 2006, 7, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-K.; Jang, H.-H.; Nguyen, H.T.H.; Doan, T.T.M.; Lee, G.-Y.; Park, K.D.; Ahn, T.; Joung, Y.H.; Kang, H.-S.; Yun, C.-H. Highly Regioselective Hydroxylation of Polydatin, a Resveratrol Glucoside, for One-Step Synthesis of Astringin, a Piceatannol Glucoside, by P450 BM3. Enzyme Microb. Technol. 2017, 97, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.-H.; Shimada, T.; Guengerich, F.P. Roles of Human Liver Cytochrome P4502C and 3A Enzymes in the 3-Hydroxylation of Benzo(a)Pyrene. Cancer Res. 1992, 52, 1868–1874. [Google Scholar] [PubMed]

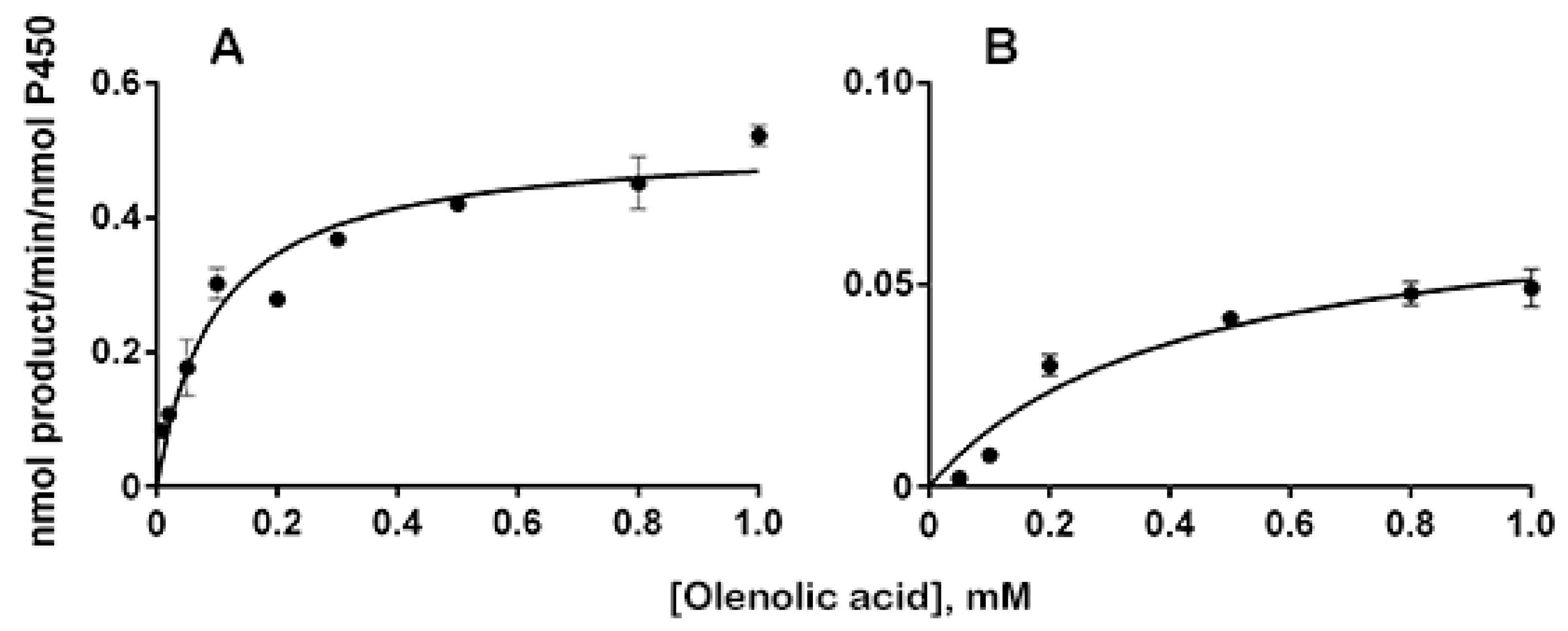


| Enzymes | kcat (min−1) | Km (μM) | kcat/Km (min−1μM−1) |
|---|---|---|---|
| HLMs | 0.072 ± 0.010 | 420 ± 140 | 0.00017 ± 0.00006 |
| CYP3A4 | 0.51 ± 0.06 | 98 ± 19 | 0.0052 ± 0.0012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, N.T.; Nguyen, N.A.; Le, T.-K.; Cha, G.S.; Park, K.D.; Yun, C.-H. Regioselective Hydroxylation of Oleanolic Acid Catalyzed by Human CYP3A4 to Produce Hederagenenin, a Chiral Metabolite. Catalysts 2021, 11, 267. https://doi.org/10.3390/catal11020267
Cao NT, Nguyen NA, Le T-K, Cha GS, Park KD, Yun C-H. Regioselective Hydroxylation of Oleanolic Acid Catalyzed by Human CYP3A4 to Produce Hederagenenin, a Chiral Metabolite. Catalysts. 2021; 11(2):267. https://doi.org/10.3390/catal11020267
Chicago/Turabian StyleCao, Ngoc Tan, Ngoc Anh Nguyen, Thien-Kim Le, Gun Su Cha, Ki Deok Park, and Chul-Ho Yun. 2021. "Regioselective Hydroxylation of Oleanolic Acid Catalyzed by Human CYP3A4 to Produce Hederagenenin, a Chiral Metabolite" Catalysts 11, no. 2: 267. https://doi.org/10.3390/catal11020267
APA StyleCao, N. T., Nguyen, N. A., Le, T.-K., Cha, G. S., Park, K. D., & Yun, C.-H. (2021). Regioselective Hydroxylation of Oleanolic Acid Catalyzed by Human CYP3A4 to Produce Hederagenenin, a Chiral Metabolite. Catalysts, 11(2), 267. https://doi.org/10.3390/catal11020267






