Abstract
γ-Glutamyl transpeptidase (GGT) catalyzes the transfer of glutathione’s γ-glutamyl group and related γ-glutamyl amides to water, amino acids or peptides, and utilizes a conserved Thr residue to process its own polypeptide chain into a large and a small subunit that then assemble to produce a catalytically competent enzyme. In this study, the magnetic cross-linked enzyme aggregates (mCLEAs) of a transpeptidase-specialized variant (N450D) of Bacillus licheniformis GGT were successfully prepared with optimized process parameters viz.1.25:1 (v/v) of isopropanol to N450D (0.3 mg/mL) ratio/0.02:1 (w/w) of enzyme to 3-aminopropyl triethoxysilane (APTES)-coated magnetic nanoparticle ratio/20 mM of glutaraldehyde. The prepared magnetic nanoparticles and immobilized enzyme (N450D-mCLEAs) were characterized by X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy, field-emission scanning electron microscope integrated with energy dispersive X-ray spectroscopy (FESEM/EDS), and superparamagnetic analysis. As compared with free enzyme, N450D-mCLEAs displayed significantly higher heat resistance at temperatures of 55 and 60 °C, and had a greater stability over a storage period of one month. The immobilized enzyme could also be reused for 10 consecutive biocatalytic cycles with no significant reduction in the percent yield of l-theanine. Conclusively, this immobilization strategy surely provides a meaningful glance of developing N450D-mediated biocatalysis for the production of physiologically important γ-glutamyl compounds.
1. Introduction
One of the greatest challenges faced by various chemical and allied industries today is developing a more sustainable manufacturing base that is always preferable to avoid the generation of hazardous wastes and the use of toxic materials [1]. Biocatalysis offers substantial benefits in this respect and can be operated in conventional reactors under milder conditions with an environmentally friendly solvent (mainly water) [2]. Reactions catalyzed by enzymes proceed with high regio- and stereoselectivity, and are generally without the need of functional group activation and the subsequent protection/deprotection steps required for organic syntheses [3]. Hence, enzyme-based processes usually have fewer reaction steps, can be even more energy efficient and cost effective, and provide high-purity products [4,5].
Enzymes are efficient biocatalysts designed by nature to work within a variety of physiological environments of living systems and most of them are complex, highly sensitive protein molecules with unique three-dimensional shapes that are essential for the desired biological activities. The exposure of enzymes to certain conditions may lead to changes in the polypeptide conformation and a concomitant loss of specific functional activities [6]. Furthermore, biotechnologically relevant enzymes are used principally as aqueous-based solutions [7], which makes their recovery and recycling problematical. These drawbacks can generally be overcome by the immobilization of enzymes [8,9,10,11]. In fact, immobilization has been shown to enhance the operational stability of a variety of enzymes [6,12] and to provide practical insights into the facile separation and reuse of immobilized biocatalysts [13].
Even though there are various methods that can be used for the immobilization of enzymes, the selection of the appropriate one is of paramount importance to prevent irreversible enzyme inactivation [14]. The well-established techniques for enzyme immobilization include encapsulation/entrapment, physical adsorption, covalent binding, and carrier free cross-linking [15,16]. Carrier-free immobilization does not need an extra support and is usually prepared by direct cross-linking of dissolved enzymes (CLEs), crystallized enzymes (CLECs), and aggregated enzymes (CLEAs). Among them, CLEAs have been receiving priority attention as a versatile carrier-free approach of immobilization [17]. In the preparation of CLEAs, enzymes are initially aggregated by adequate precipitating agents and the aggregates so formed are then covalently attached to each other by cross-linking aldehydes (e.g., formaldehyde, glutaraldehyde) [18]. An excellent review guide to the selection and proper application of CLEAs allows us to know that enzymes immobilized in this way can be used cost effectively to produce industrially important products [17]. However, this strategy is generally not applicable to enzymes with an unusually low lysine content since the intermolecular cross-linking reaction mostly involves interaction between the aldehyde group of cross-linkers and the side-chain amino group of lysine residues on the exterior surface of enzyme molecules [13]. Besides, small CLEAs may form clusters or clumps during centrifugation and filtration treatments, which consequently reduces the internal mass transfer, particularly the immobilized enzymes that act on macromolecular substrates [19]. Although CLEAs prepared from enzymes with low levels of lysine can be obtained by their coaggregation with some polymers (e.g., bovine serum albumin, polyethyleneimine and poly-ε-lysine) [20,21,22], such preparations may lead to clump formation due to low compression resistance and thereby cause substantial difficulties in the separation of CLEAs. Through an extensive research on the subject [18], magnetic CLEAs (mCLEAs) have been suggested as a proven-effective approach to overcome these constraints. Apparently, mCLEAs have recently attracted much attention in enzyme technology because of their nontoxic nature, nanometer length scale, high surface area, effective enzyme loading, and most importantly magnetic properties that enable an easy recovery of the biocatalyst by applying a magnetic field [23,24,25,26,27].
γ-Glutamyl transpeptidase (GGT; EC 2.3.2.2) is a bisubstrate enzyme conserved across the three domains of life and is involved in the hydrolysis of γ-glutamyl bonds in γ-glutamyl compounds and/or the transfer of the γ-glutamyl moiety to other amino acids and peptides [28]. The heterodimeric eukaryotic GGT enzymes are most commonly localized at the plasma membrane with their active site facing the extracellular space [29], whereas the bacterial counterparts are present either in the cytosol or in the periplasm [30,31] and can exist as a heterodimer [32,33,34,35] or heterotetramer [36]. Experimental evidence has demonstrated that the eukaryotic and prokaryotic precursor polypeptides must undergo the post-translational autocatalytic processing to yield the active mature enzymes composed of a large subunit and a small subunit [37,38,39]. In a previous study, the leaderless Bacillus licheniformis GGT (BlGGT) gene was PCR-amplified and cloned into the expression vector pQE-30 for the heterologous production of BlGGT in Escherichia coli M15 (pREP4) cells [33]. The recombinant enzyme has been shown to catalyze the synthesis of kokumi-tasting γ-glutamyl compounds, including γ-l-glutamyl-S-allyl-l-cysteine [40], γ-glutamyl-l-phenylalanine [41], and γ-glutamyl-l-leucine [42]. However, the intrinsic property of GGT enzymes to simultaneously carry out transpeptidation and hydrolysis reactions is always rather a serious drawback in the practical application of this kind of biocatalyst. With the help of site-specific mutagenesis techniques, a dramatic increase in the transpeptidation/hydrolysis ratio has been observed in two variants (N450D and N450Q) of BlGGT [43]. Surely, the peculiar characteristics of N450D had considerable superiority in the bioatalytic synthesis of l-theanine [44]. The objective of this study was therefore aimed at preparing the magnetic cross-linked enzyme aggregates of N450D (N450D-mCLEAs) and using this robust and recyclable biocatalyst system for the synthesis of l-theanine (Scheme 1), a potential nutraceutical ingredient for improving mental health literacy.
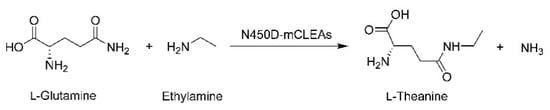
Scheme 1.
Synthesis of l-theanine from l-glutamine and ethylamine by N450D-mCLEAs.
2. Results and Discussion
2.1. Preparation of N450D-mCLEAs
In the preparation process of CLEAs, the enzyme molecules are normally aggregated into supramolecular structures by an appropriate precipitating agent, including organic solvents [19,20,27], inorganic salts [18,23,24,25,26], and some nonionic polymers [21,45]. The physical aggregates are then cross-linked with a bifunctional reagent, usually glutaraldehyde [17], to form more robust structure. In this study, several protein precipitants were evaluated in a search for one which would give maximum recovery of the enzymatic activity. Of these tested, isopropanol was found to be the most satisfactory, with the highest percent recovery (94.6%) of initial GGT activity (Figure 1a). An earlier investigation on the immobilization of Candida rugosa lipase as CLEAs has also shown that isopropanol is the best precipitant [46]. Acetone was also found preferable to precipitate the enzyme molecule; however, the use of other organic precipitants obtained less than 5.4% of initial GGT activity in aggregates (Figure 1a). This outcome may be caused by deep penetration of the nonpreferred precipitants, which in turn affect the conformational flexibility of an enzyme molecule [47]. Another reason for the loss of GGT activity is probably that the enzyme experiences unfolding due to changes in medium polarity by the nonpreferred precipitants [48]. Subsequently, the concentration effect of isopropanol on the immobilization was studied by varying the ratio of this preferred precipitant to enzyme from 0.25:1 to 2:1. As shown in Figure 1b, isopropanol-to-enzyme in a 1.25:1 ratio appeared to be the best one to physically aggregate N450D. The activity recovery was still stagnant up to a ratio of 1.75:1 and then declined marginally upon a further increase in the ratio to 2:1. As concluded by Li et al. [47], the decline in activity recovery could have resulted from a conformational change in the enzyme’s structure.
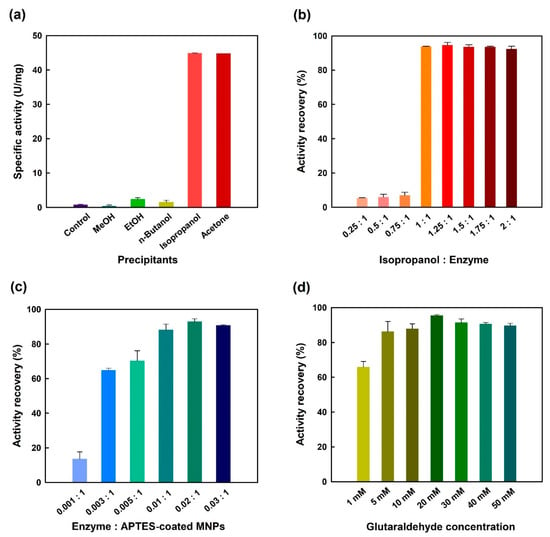
Figure 1.
Effects of different precipitants (a), isopropanol/enzyme ratio (b), enzyme/APTES-coated MNPs ratio (c) and glutaraldehyde concentration (d) on the immobilization of N450D. Control group in panel (a) represents enzyme sample without the addition of precipitating agent.
The next logical step in this study is to investigate the influence of enzyme to 3-aminopropyl triethoxysilane (APTES)-coated magnetic nanoparticles (MNPs) ratio and cross-linker concentration on the preparation of N450D-mCLEAs. As shown in Figure 1c, the enzyme ratio of N450D to APTES-coated MNPs was determined to have an optimal ratio of 0.02:1, and a significant reduction in the activity recovery was observed at a ratio of 0.03:1. This phenomenon can be partly attributed to the steric hindrance caused by the immobilization of a higher amount of enzyme on the surface of APTES-coated MNPs [49]. It is noteworthy that glutaraldehyde concentrations (1–50 mM) did significantly affect the recovery of GGT activity with a maximum recovery (95.2%) at 20 mM (Figure 1d), and beyond that concentration there was no obvious difference in activity recovery. As has been previously reported in the literature [50], higher concentrations of glutaraldehyde may induce a conformational change that alters the shape of an enzyme’s active site and eventually inactivates it. Moreover, the lower recovery of GGT activity of N450D-mCLEAs at glutaraldehyde concentrations below 5 mM is probably due to the insufficient covalent cross-linking between the enzyme molecules and APTES-coated MNPs. Previous studies have already shown that glutaraldehyde at concentrations exceeding 5 mM is more often appropriate for the preparation of cross-linking enzymatic aggregates [21,27,47,48,49,50]. In this respect, the best conditions for the preparation of N450D-mCLEA are an enzyme–APTES-coated MNPs ratio of 0.02:1 and the covalent cross-linker at a final concentration of 20 mM.
2.2. Physiochemical Properties of MPNs, APTES-Coated MNPs and N450D-mCLEAs
The crystallinity and crystallite size of MNPs are important properties that interact strongly with the crystallization process itself as well as having a great influence on the end-use applications [51,52]. In this regard, XRD was employed to study the crystalline structure of MNPs before and after the immobilization process of N450D. Powder XRD patterns of as-synthesized samples showed the position and relative intensities of all diffraction peaks matching well with the reference magnetite Fe3O4 pattern (JCPDS card 19-0629) (Figure 2). The results suggest that MNPs with cubic inverse spinel structure were successfully synthesized. As can be noted in XRD diffractograms, the coating and cross-linking steps did not change the core-shell structure of MNPs. However, the characteristic diffraction peaks of MNPs were attenuated in the pattern of N450D-mCLEAs. This observation can be explained by shielding effect of enzyme immobilization. Confirmation analyses of the APTES coating and enzyme immobilization were performed using FTIR analysis. As shown in Figure 3a, the FTIR spectrum of MNPs demonstrated a strong absorption characteristic peak at 577 cm−1 corresponding to the intrinsic stretching vibrations of metal-oxygen (Fe-O). The spectrum of APTES-coated MNPs showed a characteristic peak at 997 cm−1 due to the stretching vibrations of Si-O and a prominent peak at 1625 cm−1 corresponding to the bending vibrations of C-H (CH2) (Figure 3b) [53]. Furthermore, the FTIR spectrum of N450D-mCLEAs exhibited characteristic peaks at 1653 and 1531 cm−1 corresponding to the stretching vibrations of C=O (amide I band) and N-H (amide II band), respectively, and an intense peak at 3429 cm−1 corresponding to the stretching vibrations of O-H (-COOH) (Figure 3c). All these observations suggest the successful immobilization of N450D on the APTES-coated MNPs.
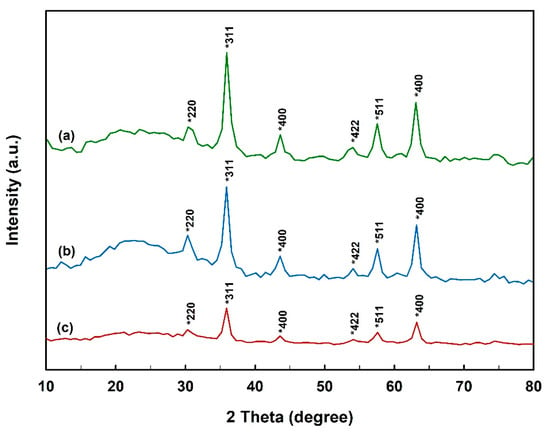
Figure 2.
XRD diffractograms of bare (a) and APTES-coated (b) MNPs, and N450D-mCLEAs (c).
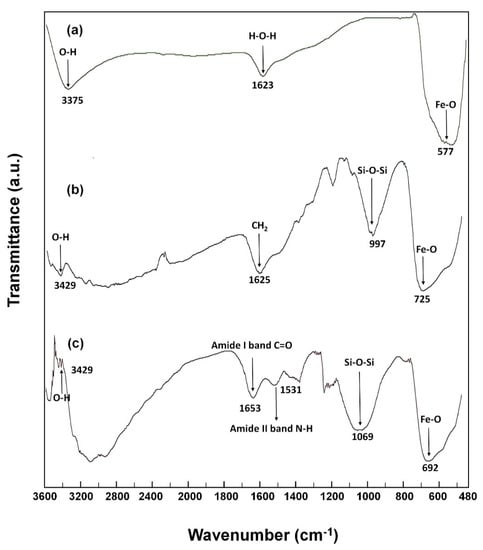
Figure 3.
FTIR spectra of bare (a) and APTES-coated (b) MNPs, and N450D-mCLEAs (c).
The comparison of FESEM images of bare and APTES-coated MNPs, and N450D-mCLEAs illustrated a surface morphology change of the nanoparticles after the immobilization process (Figure 4). It was seen that the majority of APTES-coated MNPs are of nanoscale size and have a morphology similar to MNPs, with high roughness and porosity (Figure 4a,c). After the incorporation of N450D into APTES-coated MNPs, the micrograph illustrated a huge change in the particle size and surface roughness due to the formation of agglomerates that can confidently be assigned to the apparent presence of the enzyme (Figure 4e). In order to determine the surface chemical composition of these samples, elemental mapping was carried out by using EDS (Figure 4b,d,f). From the EDS spectra, we can calculate the weight percentage of a typical element on the surface of the nanoparticle samples. As shown in Figure 4b,d, the surface of MNPs and APTES-coated MNPs is rich in iron with high homogeneity. It is also worth mentioning that the surface of APTES-coated MNPs contains a certain amount of Si. For N450D-mCLEAs, no iron was observed in the EDS spectra and the percentage of carbon in this sample is obviously higher than APTES-coated MNPs (Figure 4f). The absence of iron in the surface of N450D-mCLEAs is probably due to the performance of SEM-EDS elemental microanalysis at a specific location, where iron is covered by the enzyme. Besides, nitrogen, sulfur, and sodium were also found in N450D-mCLEAs. Thus, the EDS spectra suggest that the mCLEAs of N450D are successfully prepared.
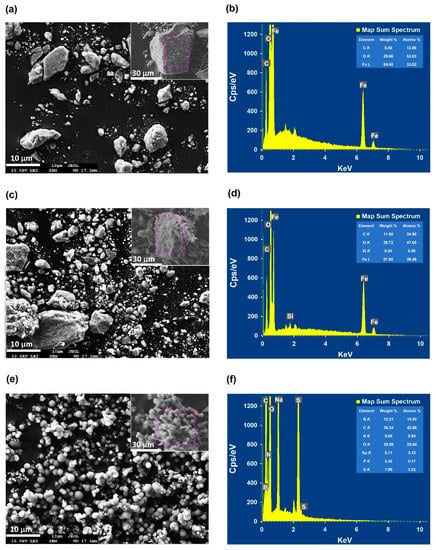
Figure 4.
FESEM images and EDS spectra of bare (a,b) and APTES-coated (c,d) MNPs, and N450D-mCLEAs (e,f).
The magnetic properties of bare and APTES-coated MNPs, and N450D-mCLEAs were characterized by the magnetization curves depicted in Figure 5a. Since remanent magnetization and coercivity were not observed, all these samples seem to confer a superparamagnetic character. The saturation magnetization value at the 10 kOe applied field was found to be 76.9, 72.5, and 65.1 emu g−1 for bare and APTES-coated MNPs, and N450D-mCLEAs, respectively. This fact can be explained by the binding of APTES on the surface of MNPs and enzyme immobilization that in turn hampers the alignment of magnetic dominions in the material. Consistent observations have been reported for mCLEAs of some other enzymes [24,27,47]. It is noteworthy that N450D-mCLEAs could be easily dispersed in an aqueous solution via shaking or ultrasonic vibration (Figure 5b). In order to demonstrate the magnetic performance of N450D-mCLEAs in a liquid phase, a magnet was placed outside the glass bottle. It can be clearly seen that N450D-mCLEAs were magnetically separable under the magnetic field (Figure 5c). This further indicates that N450D-mCLEAs are endowed with intrinsic superparamagnetic properties.
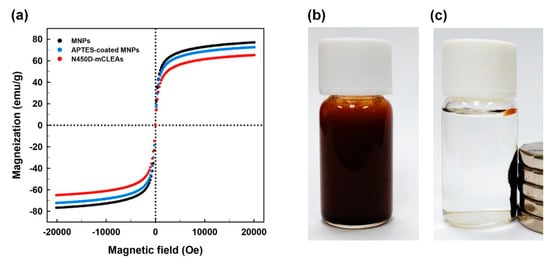
Figure 5.
Magnetic hysteresis curves of bare and APTES-coated MNPs, and N450D-mCLEAs (a), dispersivity of N450D-mCLEAs in Tris-HCl buffer (b), and the collection of N450D-mCLEAs by an external magnetic field (c).
2.3. Biochemical Characterization of N450D and N450D-mCLEAs
As compared with free enzyme, N450D-mCLEAs were active in the range of 8–9 and had an optimum pH of 9.0 (Figure 6a). The significant shift in the pH range of N450D-mCLEAs can result from the changes in the ionization states of acidic and basic residues around the active site during the cross-linking. This observation is in line with an earlier report on the pH shift for acrylamidase-CLEAs [27]. Moreover, N450D-mCLEAs displayed relatively greater stability than free enzyme at each pH (Figure 6b). As in the case of acrylamidase-CLEAs [27], such a result can be attributed to the increased structural rigidity of N450D upon immobilization.
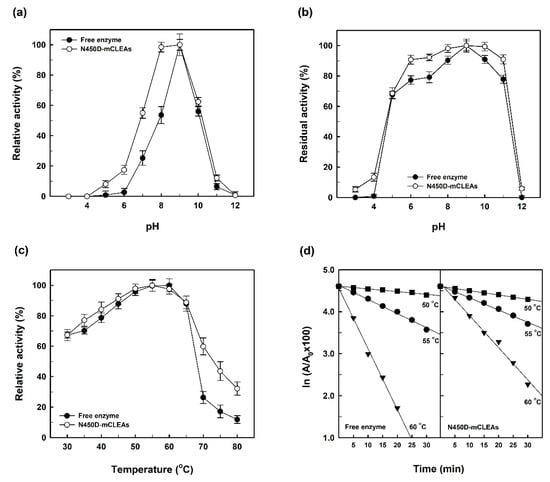
Figure 6.
Effects of pH and temperature on the catalytic activity (a,c) and stability (b,d) of N450D and N450D-mCLEAS.
As shown in Figure 6c, both N450D and N450D-mCLEAs had an optimum temperature of around 55 °C, but the immobilized enzyme was more active at temperatures above 70 °C. The shifting of reaction temperature towards higher values for the immobilized enzyme is probably caused by the formation of intermolecular covalent cross links between N450D and APTES-coated MNPs. This phenomenon is consistent with the previous report on α-amylase-mCLEAs [18].
Given that the thermal inactivation profiles of N450D and N450D-mCLEAs will provide unique insights into the structure–function relationship of the enzyme at a specific temperature, the temperature-dependent loss of GGT activity in both forms was determined and the results are shown in Figure 6d. It can be seen that the thermal stability of N450D-mCLEAs was superior to free enzyme at 55 and 60 °C. Based on the thermal inactivation profiles, the inactivation rate constant (Kd), half-life (t1/2) and D-value of both forms of N450D were calculated and presented in Table 1. The thermal inactivation kinetics of N450D and N450D-mCLEAs revealed a significant decrease in the transpeptidation activity during the extended incubation period. The Kd value of N450D-mCLEAs was 15.7 and 94.4% lower than that of free enzyme at 55 and 60 °C, respectively, indicating that the thermal stability was profoundly improved by enzyme immobilization. Moreover, the t1/2 and D-value of N450D-mCLEAs were superior to free enzyme at 55 and 60 °C (Table 1). The increased half-life and D-values of N450D-mCLEAs under these two temperatures may be attributed to the covalent cross-links between N450D and glutaraldehyde, which are helpful for retaining the active tertiary structure of the enzyme under high temperatures [18,27].

Table 1.
Inactivation parameters of N450D and N450D-mCLEAs.
2.4. Storage Stability of Free Enzyme and N450D-mCLEAs
Several previous studies have already noted that free enzymes can generally lose their activities fairly quickly during the storage period [54,55,56,57]. Considering that immobilization is one of the important ways for enzymes to become stable [58], the storage stability of free enzyme and N450D-mCLEAs was evaluated and the experimental results are shown in Figure 7. In contrary to the above-mentioned reports, both N450D and N450D-mCLEAs retained greater than 87% of their initial GGT activity after one month of storage at 4 °C. The storage stability data are consistent with those of the immobilization of BlGGT in surface-functionalized hyperbranched poly(amido acids) magnetic nanocarriers [59] and graphene oxide nanosheets [60]. It is also noteworthy to mention that no GGT activity was found in the clarified solution of N450D-mCLEAs. This observation clearly indicates that N450D is stably present in the matrix of mCLEAs.
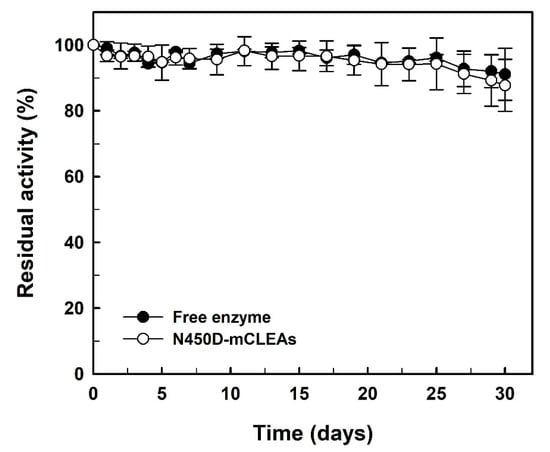
Figure 7.
Storage stability of N450D and N450D-mCLEAs. The data are the average of three independent experiments with standard deviations.
2.5. Reusability of N450D-mCLEAs
N450D-mCLEAs were recycled in ten consecutive batches and the residual activity was determined under the standard assay conditions. As shown in Figure 8, the immobilized enzyme retained an activity of almost 100% after being reused 10 times. These observations suggest that the immobilized enzyme is quite stable during its consecutive operations.
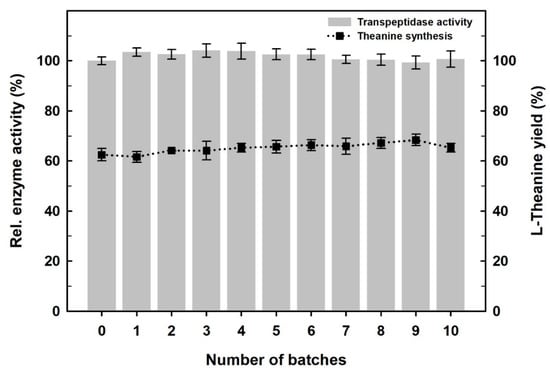
Figure 8.
Reusability of N450D-mCLEAs as monitored by activity assay (left scale) and the synthesis of l-theanine (right scale). The data are the average of three independent experiments with standard deviations.
The bioactive compounds produced by natural species are of great importance for modern therapeutics [61]. l-Theanine is such a bioactive nonproteinic amino acid presented in a variety of plants and fungi, especially in tea tree [62,63]. It has a high impact on preventing lifestyle-related diseases, such as cardiovascular disorders, hypertension, diabetes, liver injury and tumor suppression [63,64]. This bioactive amide can also help to maintain healthy sleep, improve mental clarity, and nullify the harmful effects of neurotoxins [64,65]. Due to the important contribution of l-theanine to a wide range of significant health benefits, there is an increasing demand for its use in the fields of dietary supplements, functional food ingredients, and nutraceuticals [64,66,67]. Over the past few years, extensive efforts have been devoted to establishing a biocatalytic process for the facile and efficient synthesis of l-theanine using bacterial GGT enzymes [68]. In our earlier study, the biocatalytic synthesis of l-theanine from inexpensive substrates, l-glutamine and ethylamine, by N450D was achieved at molar scale with a conversion rate of approximately 94% [44]. Considering that l-theanine can be efficiently synthesized by N450D-mediated reaction, N450D-mCLEAs were recycled ten consecutive times using l-glutamine as the donor substrate and ethylamine as the acceptor substrate. As shown in Figure 8, N450D-mCLEAs earned a 63.2% conversion rate for the first run of biocatalytic synthesis and the conversion efficiency did not decrease significantly in the successive cycles. The amazing reusability of N450D-mCLEAs absolutely renders the immobilized enzyme with prosperous prospects in practical applications.
3. Materials and Methods
3.1. Materials
Ferric chloride hexahydrate (FeCl3·6H2O), ferrous chloride tetrahydrate (FeCl2·4H2O), APTES, glutaraldehyde (25%, v/v), and enzyme assay reagents, l-γ-glutamyl-p-nitroanilide (l-γ-Glu-p-NA), p-nitroaniline (p-NA), and Gly-Gly, were acquired from Sigma-Aldrich Fine Chemicals (St. Louis, MO, USA). Nickel-nitrilotriacetic acid (Ni-NTA) Superflow Cartridges (1 × 5 mL) for the affinity purification of His6-tagged fusion protein was purchased from Qiagen Inc. (Valencia, CA, USA). Avantor’s J.T. Baker® solvents, including methanol, ethanol, isopropanol, n-butanol and acetone, for protein precipitation were supplied by a local vendor (Uni-Onward Corp., New Taipei City, Taiwan). l-Theanine reference standard ≥98% (HPLC) was a commercially available product of Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All other chemicals and media components were of ready-to-use high-purity and obtained from reliable commercial sources.
3.2. Enzyme Production, Protein Analysis, and Activity Assay
The cultivation of E. coli M15 (pQE-BlGGT/N450D) cells and IPTG-induced overexpression of the His6-tagged enzyme were carried out as described previously [69]. The heterologously expressed N450D was purified to near homogeneity from the culture-free extract of E. coli M15 (pQE-BlGGT/N450D) using Ni-NTA Superflow Cartridges from Qiagen. Sequential washing and eluting procedures were performed according to the manufacturer’s recommended protocols. The protein concentration of the eluted fractions was routinely determined by PierceTM Coomassie Plus Bradford Assay Kit (Thermo Fisher Scientific Inc., Taipei, Taiwan) and reference to a standard curve of bovine serum albumin. The purity of affinity-purified N450D was checked under reducing conditions using 12% (w/v) polyacrylamide gels. Transpeptidation activity was measured in triplicate as described previously with the donor substrate, l-γ-Glu-p-NA, and the acceptor substrate, Gly-Gly [45]. One unit of transpeptidation activity is defined as the amount of enzymes required to release 1 μmol of p-NA from the chromogenic substrate per minute.
3.3. Preparation of APTES-Coated Magnetic Nanoparticles
MPNs were essentially synthesized by coprecipitating Fe2+ and Fe3+ ions in an aqueous ammonia solution under hydrothermal conditions [70]. Initially, 2.0 g of FeCl2·4H2O and 5.4 g of FeCl3·6H2O were dissolved in 100 mL water to give a final iron ion concentration of 0.3 M. Chemical precipitation was accomplished at 25 °C by dropwise addition of a 75 mL ammonium hydroxide (NH4OH) solution (29.6%, w/v) under vigorous stirring. Ideally, the pH was maintained at about 10 during the precipitation process. The resultant precipitate was heated at 80 °C for 30 min, washed several times with an adequate amount of water and ethanol, dissolved in 100 mL ethanol and then directly used for the surface coating of APTES.
The preparation of APTES-coated MPNs was carried out according to previously described protocols [71]. Typically, the above-prepared nanoparticles (~0.42 g) were thoroughly dispersed in 9.7 mL ethanol by sonication. APTES (0.3 mL) was subsequently added to this suspension and then homogenized with an ultrasonic bath for 10 min, followed by rolling the sample bottle overnight at ambient temperature. The APTES-coated MNPs so obtained were easily recovered manually from the reaction mixture by placing the sample bottle on a permanent magnet with a surface magnetization of 3000 G. The surface-modified MNPs were washed with distilled water (thrice) and ethanol (once) to remove unreacted APTES. Thereafter, the APTES-coated MNPs were dried at ambient temperature and their surface functionalization was verified by Fourier Transform Infrared Spectrometer (FTIR) and Field Emission Scanning Electron Microscope (FESEM) with energy dispersive X-ray spectroscopy (EDS).
3.4. Immobilization of N450D
Various protein precipitants, including methanol, ethanol, isopropanol, n-butanol and acetone, were employed to test the possible maximum precipitation of N450D. Initially, 0.5 mL of the ice-cold solvents were individually added dropwise to a stirred buffer solution of N450D (0.3 mg/mL) to give the precipitant/buffer volume ratio of 1.25:1. To remove the supernatant, the turbid suspension was centrifuged at 12,000× g for 1 h at 4 °C and the resultant pellet was redissolved in 0.1 mL of 50 mM Tris-HCl buffer (pH 9.0). The discard supernatant and resuspended sample were all subjected to the analyses of protein concentration and transpeptidation activity. The precipitant that allowed the highest recovery of the enzymatic activity was selected for further studies. To aggravate activity recovery of N450D, mass ratio of the desired precipitant to enzyme (0.25:1–2:1) was optimized. The optimal ratio of precipitant/enzyme was used in the subsequent design of experiments.
Effect of mass ratio of enzyme to APTES-coated MNPs (0.001:1 to 0.03:1) on the activity recovery of immobilized enzyme was studied with isopropanol/N450D ratio of 1.25:1. Briefly, 1 mg of APTES-coated MNPs were thoroughly dispersed in 1 mL of 50 mM Tris-HCl buffer (pH 9.0) containing previously precipitated N450D, followed by the addition of glutaraldehyde to a final concentration of 20 mM under constant stirring (150 rpm) at 30 °C for 1.5 h. After cross-linking, the magnetic cross-linked enzyme aggregates of N450D (N450D-mCLEAs) were recovered from the reaction mixture by using an external magnetic field. The obtained N450-mCLEAs were then washed thrice with a suitable amount of 50 mM Tris-HCl buffer (pH 9.0) and stored at 4 °C in a refrigerator until use. To ensure a maximum recovery of N450D, glutaraldehyde (0–50 mM) concentrations were also optimized using one-at-a-time experimentation. The activity recovery (%) of N450D-mCLEAs was computed using Equation (1).
3.5. Instrumental Characterization
The crystal structures of bare and APTES-coated MNPs, and N450D-mCLEAs as well were individually obtained from X-ray diffraction (XRD) pattern recorded on a XRD-6000 powder diffractometer (Shimadzu Co., Kyoto, Japan) using a monochromatic X-ray beam with nickel-filtered Cu Kα radiation (λ = 1.54 Å). A continuous-scanning mode was used to collect the 2-theta values from 25 to 90°. Infrared spectra of bare and APTES-coated MNPs, and N450D-mCLEAs were recorded, respectively, by a Shimadzu IR Prestige-21/FTIR-8400S spectrophotometer (Shimadzu Co., Kyoto, Japan) over the wavelength range of 4000 to 400 cm−1 using the KBr pellet technique. The surface morphology and size of bare and APTES-coated MNPs, and N450D-mCLEAs were determined by FESEM (JeoL JSM-7800F Prime, Hitachi High-Technologies, Tokyo, Japan). EDS attached to FESEM was used to analyze the elemental composition of the above-mentioned samples. The magnetic properties of bare and APTES-coated MNPs, and N450D-mCLEAs were determined under a varying magnetic field by a Superconducting Quantum Interference Device (SQUID) Vibrating Sample Magnetometer using a physical property measurement system 9 T DynaCool (Quantum Design North America, San Diego, CA, USA).
3.6. Effects of Temperature and pH on The enzymatic Activity of Free Enzyme and N450D-mCLEAs
The effect of temperature on the enzymatic activity of N450D and N450D-mCLEAs was investigated by incubating a 0.5-mL reaction mixture consisting of either N450D (68.5 U/mL) or N450D-mCLEAs (ca. 20 mg; wet weight), 1 mM l-γ-Glu-p-NA, 60 mM Gly-Gly, 75 mM NaCl, and 50 mM Tris-HCl buffer (pH 9.0) at various temperatures (30–80 °C) for 10 min. GGT activity was determined by monitoring the absorbance at 410 nm using a UV-visible spectrophotometer (ChromTech® CT-3800, Great Tide Instrument Co., Ltd., Taipei, Taiwan). The thermal stability of N450D (68.5 U/mL) and N450D-mCLEAs (ca. 20 mg; wet weight) was also tested in 0.5 mL of 50 mM Tris-HCl buffer (pH 9.0) over a wide temperature range of 30 to 80 °C. After 5 to 30 minutes of incubation at the specified temperatures, residual GGT activity was determined under the standard assay conditions. All assays were performed in triplicate and the data were expressed as the mean ± standard deviation.
To understand the effect of pH on the GGT activity of free and immobilized enzymes, N450D (68.5 U/mL) and N450D-mCLEAs (ca. 35 mg; wet weight) in a l-mL reaction mixture were prepared with either 50 mM sodium acetate buffer (pH 3.0–6.0), 50 mM Tris-HCl buffer (pH 7.0–9.0), or 50 mM glycine-NaOH buffer (pH 9.0–12.0). Their transpeptidation activity was determined under the standard assay conditions. For measurements of pH-stability, N450D (68.5 U/mL) and N450D-mCLEAs (ca. 35 mg; wet weight) were maintained in the above-mentioned buffer systems at 4 °C for 20 min. Residual GGT activity was determined under the standard assay conditions and calculated by using Equation (2).
where A is the GGT activity at time t and A0 represents the GGT activity at time zero.
The deactivation rate constant (Kd), half-life (t1/2), D-value (time required for reducing its initial GGT activity by 90% under a specific temperature), and activation energy (Ed) of free enzyme and mCLEAs were calculated as described elsewhere [27].
3.7. Storage Stability of N450D and N450D-mCLEAs
Storage stability of N450D (66.5 U/mL) and N450D-mCLEAs (ca. 90 mg, wet weight) preserved at 4 °C in 90 mL of 50 mM NaH2PO4 buffer (pH 8.0) was assessed over a storage period of one month. Aliquots (1 mL) were withdrawn at intervals of up to every two days by means of a pipette to determine the residual GGT activity under the standard assay conditions. The experiments were performed in triplicate and all data were expressed as the mean ± standard deviation.
3.8. Reusability of N450D-mCLEAs
The reusability of N450D-mCLEAs in aqueous system was investigated either with the enzyme assay mixture or with a reaction mixture (1 mL) containing 250 mM l-glutamine, 600 mM ethylamine, N450D-mCLEAs (40 μg/mL), and 50 mM borate buffer (pH 10.5). The enzyme assay mixture was incubated at 40 °C with constant shaking (150 rpm) for 10 min and its transpeptidation activity was determined by measuring the absorbance at 410 nm [69], while the synthesis of l-theanine was carried out at 37 °C for 4 h and quantified as previously described [44]. In the last step of each repeated cycle, N450D-mCLEAs were recovered through an external magnet, washed twice with 1 mL of 50 mM ice-cold NaH2PO4 buffer (pH 8.0), and then reused in the next cycle. The experiments were performed in triplicate and all data were expressed as the mean ± standard deviation.
4. Conclusions
In this work we describe a facile, efficient, and economical method for preparing mCLEAs of a transpeptidase-specialized variant of B. licheniformis GGT by cross-linking its physical aggregates onto the surface of APTES-coated MNPs. The prepared biocatalyst displays an enhanced stability as compared with free enzyme and maintains the typical behavior of superparamagnetic materials, which enables easy magnetic separation from the reaction mixture and can be reused several times without apparent loss of GGT activity. The repeated use of N450D-mCLEAs for the efficient production of l-theanine has been demonstrated although it could be also used to synthesize other γ-glutamyl compounds with potential applications in the food and pharmaceutical industries. The findings of this study would thus help us to lay the groundwork towards the utilization of the carrier free immobilization of GGT enzymes in the field of biocatalysis.
Author Contributions
M.-C.C., Y.-F.H., B.-Y.L., M.-G.L. performed the experiments, analyzed the data, and provided feedback on the interpretation of results. T.-F.W. participated in the design of the study and wrote the original draft. L.-L.L. acted as the project administrator to supervise the study and revised the draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the research grants (MOST 109-2313-B-415-006; MOST 109-2320-B-415-003) from the Ministry of Science and Technology of Taiwan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are greatly indebted to Tzong-Yuan Juang of China Medical University for his help with the instrumentation characterization.
Conflicts of Interest
The authors have declared that there are no conflicts of interest related to this article.
References
- De Marco, B.A.; Rechelo, B.S.; Gandolpho, E.G.; Kogawa, A.G.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Leitão, V.S.; Cammarota, M.C.; Aguieiras, E.C.G.; Vasconcelos de Sá, L.R.; Fernandez-Lafuente, R.; Freire, D.M.G. The protagonism of biocatalysis in green chemistry and its environmental benefits. Catalysts 2017, 7, 9. [Google Scholar] [CrossRef]
- De María, P.D.; de Gonzalo, G.; Alcántara, A.R. Biocatalysis as useful tool in asymmetric synthesis: An assessment of recently granted patents (2014–2019). Catalysts 2019, 9, 802. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial enzymes: Industrial progress in 21st century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef]
- Abdelraheem, E.M.M.; Busch, H.; Hanefeld, U.; Tonin, F. Biocatalysis explained: From pharmaceutical to bulk chemical production. React. Chem. Eng. 2019, 4, 1878–1894. [Google Scholar] [CrossRef]
- Silva, C.; Martins, M.; Jing, S.; Fu, J.; Cavaco-Paulo, A. Practical insights on enzyme stabilization. Crit. Rev. Biotechnol. 2017, 38, 335–350. [Google Scholar] [CrossRef]
- Adrio, J.L.; Demain, A.L. Microbial enzymes: Tools for biotechnological processes. Biomolecules 2014, 4, 117–139. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. Broadening the scope of biocatalysis in sustainable organic synthesis. ChemSusChem 2019, 12, 2859–2881. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Reis, C.L.B.; Sousa, E.Y.A.; Serpa, J.F.; Oliveira, R.C.; Santos, J.C.S. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Quim. Nova 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Bedade, D.K.; Sutar, Y.B.; Singhal, R.S. Chitosan coated calcium alginate beads for covalent immobilization of acrylamidase: Process parameters and removal of acrylamide from coffee. Food Chem. 2019, 275, 95–104. [Google Scholar] [CrossRef]
- Brady, D.; Jordaan, J. Advances in enzyme immobilization. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Mateos-Díaz, J.C.; Favela-Torres, E. Cross-linked enzyme aggregates (CLEA) in enzyme improvement—A review. Biocatalysis 2015, 1, 166–177. [Google Scholar] [CrossRef]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef]
- Talekar, S.; Ghodake, V.; Ghotage, T.; Rathod, P.; Deshmukh, P.; Nadar, S.; Mulla, M.; Ladole, M. Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of α-amylase. Bioresour. Technol. 2012, 123, 542–547. [Google Scholar] [CrossRef]
- Wang, M.; Jia, C.; Qi, W.; Yu, Q.; Peng, X.; Su, R.; He, Z. Porous-CLEAs of papain: Application to enzymatic hydrolysis of macromolecules. Bioresour. Technol. 2011, 102, 3541–3545. [Google Scholar] [CrossRef]
- Dong, T.; Zhao, L.; Huang, Y.; Tan, X. Preparation of cross-linked aggregates of aminoacylase from Aspergillus melleus by using bovine serum albumin as an inert additive. Bioresour. Technol. 2010, 101, 6569–6571. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.K.; Kuwar, S.S.; Golegaonkar, S.B.; Nene, S.N. Preparation of crosslinked enzyme aggregates of L-aminoacylase via co-aggregation with polyethyleneimine. J. Mol. Catal. B Enzym. 2012, 74, 184–191. [Google Scholar] [CrossRef]
- Bankar, S.B.; Jadhav, S.B.; Singhal, R.S. Poly-ε-lysine amylase conjugates to increase the stability of enzyme. Food Biosci. 2014, 5, 85–90. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Pletschke, B.I. Magnetic cross-linked enzyme aggregates (CLEAs): A novel concept towards carrier free immobilization of lignocellulolytic enzymes. Enzyme Microb. Technol. 2014, 61–62, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Izquierdo, A.; Picó, E.A.; López, C.; Serra, J.L.; Liama, M.J. Magnetic cross-linked enzyme aggregates (mCLEAs) of Candida antarctica lipase: An efficient and stable biocatalyst for biodiesel synthesis. PLoS ONE 2014, 9, e115202. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rathod, V.K. Magnetic macromolecular cross-linked enzyme aggregates (CLEAs) of glucoamylase. Enzyme Microb. Technol. 2016, 83, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Peirce, S.; Russo, M.E.; Isticato, R.; Lafuente, R.F.; Salatino, P.; Marzocchella, A. Structure and activity of magnetic cross-linked enzyme aggregates of bovine carbonic anhydrase as promoters of enzymatic CO2 capture. Biochem. Eng. J. 2017, 127, 188–195. [Google Scholar] [CrossRef]
- Bedade, D.K.; Muley, A.B.; Singhal, R.S. Magnetic cross-linked enzyme aggregates of acrylamidase from Cupriavidus oxalaticus ICTDB921 for biodegradation of acrylamide from industrial waste. Bioresour. Technol. 2019, 272, 137–145. [Google Scholar] [CrossRef]
- Tate, S.; Meister, A. γ-Glutamyl transpeptidase: Catalytic, structural and functional aspects. Mol. Cell. Biochem. 1981, 39, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, J.B. γ-Glutamyltranspeptidase. Crit. Rev. Clin. Lab. Sci. 2001, 38, 263–355. [Google Scholar] [CrossRef]
- Suzuki, H.; Hashimoto, W.; Kumagai, H. Glutathione metabolism in Escherichia coli. J. Mol. Catal. B Enzym. 1999, 6, 175–184. [Google Scholar] [CrossRef]
- Castellano, I.; Merlino, A. γ-Glutamyltranspeptidases: Sequence, structure, biochemical properties, and biotechnological applications. Cell. Mol. Life Sci. 2012, 69, 3381–3394. [Google Scholar] [CrossRef]
- Suzuki, H.; Kumagai, H.; Tochikura, T. γ-Glutamyltranspeptidase from Escherichia coli K-12: Purification and properties. J. Bacteriol. 1986, 168, 1325–1331. [Google Scholar] [CrossRef]
- Lin, L.L.; Chou, P.R.; Hua, Y.W.; Hsu, W.H. Overexpression, one-step purification, and biochemical characterization of a recombinant γ-glutamyltranspeptidase from Bacillus licheniformis. Appl. Microbiol. Biotechnol. 2006, 73, 103–112. [Google Scholar] [CrossRef]
- Castellano, I.; Di Salle, A.; Merlino, A.; Rossi, M.; La Cara, F. Gene cloning and protein expression of γ-glutamyltranspeptidases from Thermus thermophilus and Deinococcus radiodurans: Comparison of molecular and structural properties with mesophilic counterparts. Extremophiles 2011, 15, 259–270. [Google Scholar] [CrossRef]
- Murty, N.A.; Tiwary, E.; Sharma, R.; Nair, N.; Gupta, R. γ-Glutamyl transpeptidase from Bacillus pumilus KS 12: Decoupling autoprocessing from catalysis and molecular characterization of N-terminal region. Enzyme Microb. Technol. 2012, 50, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Castellano, I.; Merlino, A.; Rossi, M.; La Cara, F. Biochemical and structural properties of γ-glutamyl transpeptidase from Geobacillus thermodenitrificans: An enzyme specialized in hydrolase activity. Biochimie 2010, 92, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Suzuki, H.; Wada, K.; Kumagai, H.; Fukuyama, K. Crystal structures of γ-glutamyltranspeptidase from Escherichia coli, a key enzyme in glutathione metabolism, and its reaction intermediate. Proc. Natl. Acad. Sci. USA 2006, 103, 6471–6476. [Google Scholar] [CrossRef]
- Boanca, G.; Sand, A.; Barycki, J.J. Uncoupling the enzymatic and autoprocessing activities of Helicobacter pylori γ-glutamyltranspeptidase. J. Biol. Chem. 2006, 281, 19029–19037. [Google Scholar] [CrossRef] [PubMed]
- West, M.B.; Wickham, S.; Quinalty, L.M.; Pavlovicz, R.E.; Li, C.; Hanigan, M.H. Autocatalytic cleavage of human γ-glutamyltranspeptidase is highly dependent on N-glycosylation at asparagine 95. J. Biol. Chem. 2011, 286, 28876–28888. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Lo, H.F.; Wang, T.F.; Lin, M.G.; Lin, L.L.; Chi, M.C. Enzymatic synthesis of γ-L-glutamyl-S-allyl-L-cysteine, a naturally occurring organosulfur compound from garlic, by Bacillus licheniformis γ-glutamyltranspeptidase. Enzyme Microb. Technol. 2015, 75–76, 18–24. [Google Scholar] [CrossRef]
- Chi, M.C.; Lo, H.F.; Lin, M.G.; Chen, Y.Y.; Lin, L.L.; Wang, T.F. Application of Bacillus licheniformis γ-glutamyltranspeptidase to the biocatalytic synthesis of γ-glutamyl-phenylalanine. Biocatal. Agric. Biotechnol. 2017, 10, 278–284. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chi, M.C.; Lin, M.G.; Chen, Y.Y.; Lin, L.L.; Wang, T.F. Biocatalytic synthesis of γ-glutamyl-L-leucine, a kokumi-imparting dipeptide, by Bacillus licheniformis γ-glutamyltranspeptidase. Food Biotechnol. 2018, 32, 130–147. [Google Scholar] [CrossRef]
- Lin, M.G.; Chi, M.C.; Chen, Y.Y.; Wang, T.F.; Lo, H.F.; Lin, L.L. Site-directed mutagenesis of a conserved Asn450 residue of Bacillus licheniformis γ-glutamyltranspeptidase. Int. J. Biol. Macromol. 2016, 91, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.C.; Lin, M.G.; Huang, Y.F.; Chen, Y.Y.; Wang, T.F.; Lin, L.L. Enzymatic synthesis of L-theanine from L-glutamine and ethylamine by Bacillus licheniformis γ-glutamyltranspeptidase and its mutants specialized in transpeptidase activity. Biocatal. Agric. Biotechnol. 2019, 22, 101393. [Google Scholar] [CrossRef]
- Talekar, S.; Shah, V.; Patil, S.; Nimbalkar, M. Porous cross linked enzyme aggregates (p-CLEAs) of Saccharomyces cerevisiae invertase. Catal. Sci. Technol. 2012, 2, 1575–1579. [Google Scholar] [CrossRef]
- Kartal, F.; Janssen, M.H.A.; Hollmann, F.; Sheldon, R.A.; Kilinc, A. Improved esterification activity of Candida rugosa lipase in organic solvent by immobilization as cross-linked enzyme aggregates (CLEAs). J. Mol. Catal. B Enzym. 2011, 71, 85–89. [Google Scholar] [CrossRef]
- Li, K.; Wang, J.; He, Y.; Cui, G.; Abdulrazaq, M.A.; Yan, Y. Enhancing enzyme activity and enantioselectivity of Burkholderia cepacia lipase via immobilization on melamine-glutaraldehyde dendrimer modified magnetic nanoparticles. Chem. Eng. J. 2018, 351, 258–268. [Google Scholar] [CrossRef]
- Matijošyte, I.; Arends, I.W.C.E.; de Vries, S.; Sheldon, R.A. Preparation and use of cross-linked enzyme aggregates (CLEAs) of laccases. J. Mol. Catal. B Enzym. 2010, 62, 142–148. [Google Scholar] [CrossRef]
- Ladole, M.R.; Muley, A.B.; Patil, I.D.; Talib, M.I.; Parate, V.R. Immobilization of tropizyme P on amino-functionalized magnetic nanoparticles for fruit juice clarification. J. Biochem. Technol. 2014, 5, 838–845. [Google Scholar]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 798–802. [Google Scholar] [CrossRef]
- McGrath, A.J.; Cheong, S.; Henning, A.M.; Gooding, J.J.; Tilley, R.D. Size and shape evolution of highly magnetic iron nanoparticles from successive growth reactions. Chem. Commun. 2017, 53, 11548–11551. [Google Scholar] [CrossRef]
- Sandler, S.E.; Fellows, B.; Mefford, O.T. Best practices for characterization of magnetic nanoparticles for biomedical applications. Anal. Chem. 2019, 91, 14159–14169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Li, X.; Li, T. Kinetics of (3-aminopropyl) triethoxylsilane (APTES) silanization of superparamagnetic iron oxide nanoparticles. Langmuir 2013, 29, 15275–15282. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Su, P.; Yang, Y.; Yang, Y. Efficient immobilization of enzymes onto magnetic nanoparticles by DNA strand displacement: A stable and high-performance biocatalyst. New J. Chem. 2017, 41, 6089–6097. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, G.; Wang, G.; Li, Y.; Tang, R. Robust glucose oxidase with a Fe3O4@C-silica nanohybrid structure. J. Mater. Chem. B 2016, 4, 4726–4731. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Xu, C.; Lin, Y.; Ni, H.; Zhu, Y.; Cai, H. Preparation and characterization of κ-carrageenase immobilized onto magnetic iron oxide nanoparticles. Electron. J. Biotechnol. 2016, 19, 1–7. [Google Scholar] [CrossRef]
- Li, C.; Jiang, S.; Zhao, X.; Liang, H. Co-immobilization of enzymes and magnetic nanoparticles by metal-nucleotide hydrogel nanofibers for improving stability and recycling. Molecules 2017, 22, 179. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Juang, T.Y.; Kan, S.J.; Chen, Y.Y.; Tsai, Y.L.; Lin, M.G.; Lin, L.L. Preparation of surface-functionized hyperbranched poly(amido acids) magnetic nanocarriers for the covalent immobilization of a bacterial γ-glutamyltranspeptidase. Molecules 2014, 19, 4997–5012. [Google Scholar] [CrossRef]
- Lin, L.L.; Chi, M.C.; Lan, E.G.; Lin, M.G.; Juang, T.Y.; Wang, T.F. Facile immobilization of Bacillus licheniformis γ-glutamyltranspeptidase onto graphene oxide nanosheets and its application to the biocatalytic synthesis of γ-l-glutamyl peptides. Int. J. Biol. Macromol. 2018, 117, 1326–1333. [Google Scholar] [CrossRef]
- Mushtag, S.; Abbasi, B.H.; Uzair, B.; Abbasi, R. Natural products as reservoirs of novel therapeutic agents. EXCLI J. 2018, 17, 420–451. [Google Scholar]
- Ashihara, H. Occurrence, biosynthesis and metabolism of theanine (γ-glutamyl-l-ethylamide) in plants: A comprehensive review. Nat. Prod. Commun. 2015, 10, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, P.; Liu, F. Production of theanine by Xerocomus badius (mushroom) using submerged fermentation. LWT 2008, 41, 883–889. [Google Scholar] [CrossRef]
- Adhikary, R.; Mandal, V. L-Theanine: A potential multifaceted natural bioactive amide as health supplement. Asian J. Trop. Biomed. 2017, 7, 842–845. [Google Scholar] [CrossRef]
- Liang, Y.R.; Liu, C.; Ziang, L.P.; Zheng, X.Q. Health benefits of theanine in green tea: A review. Trop. J. Pharm. Res. 2015, 14, 1943–1949. [Google Scholar] [CrossRef]
- Williams, J.; Kellett, J.; Roach, P.D.; McKune, A.; Mellor, D.; Thomas, J.; Naumovski, N. L-Theanine as a functional food additive: Its role in disease prevention and health promotion. Beverages 2016, 2, 13. [Google Scholar] [CrossRef]
- Eschenauer, G.; Sweet, B.V. Pharmacology and therapeutic uses of theanine. Am. J. Health Syst. Pharm. 2006, 63, 26–30. [Google Scholar] [CrossRef]
- Mu, W.; Zhang, T.; Jiang, B. An overview of biological production of L-theanine. Biotechnol. Adv. 2015, 33, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Liang, W.C.; Chen, Y.Y.; Chi, M.C.; Lo, H.F.; Chen, H.L.; Lin, L.L. Biophysical characterization of Bacillus licheniformis and Escherichia coli γ-glutamyltranspeptidases: A comparative analysis. Int. J. Biol. Macromol. 2011, 48, 414–422. [Google Scholar] [CrossRef]
- Khalil, M.I. Co-precipitation in aqueous solution synthesis of magnetite nanoparticles using iron(III) salts as precursors. Arab. J. Chem. 2015, 8, 279–284. [Google Scholar] [CrossRef]
- Shaw, S.Y.; Chen, Y.J.; Ou, J.J.; Ho, L. Preparation and characterization of Pseudomonas putida esterase immobilized on magnetic nanoparticles. Enzyme Microb. Technol. 2006, 39, 1089–1095. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).