Catalyst Stability—Bottleneck of Efficient Catalytic Pyrolysis
Abstract
1. Introduction
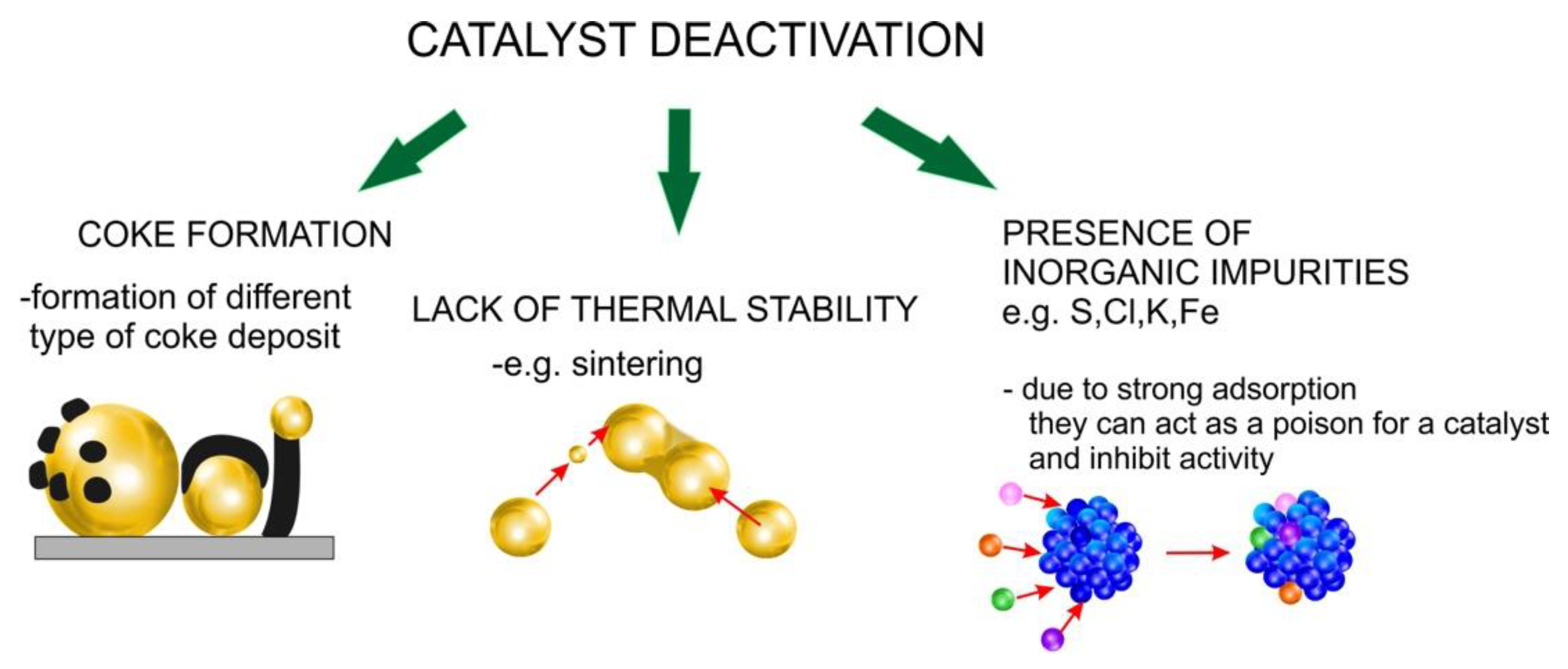
2. Formation of Coke Deposit
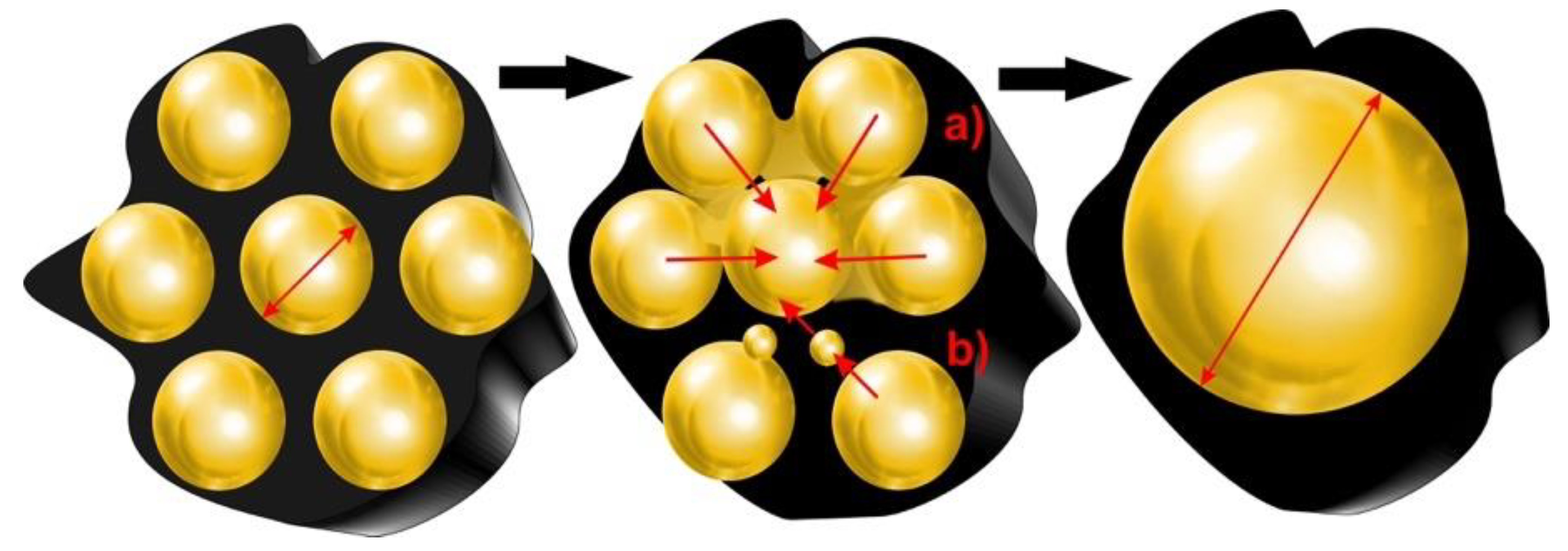
3. Sintering and Deterioration of Catalyst Structure
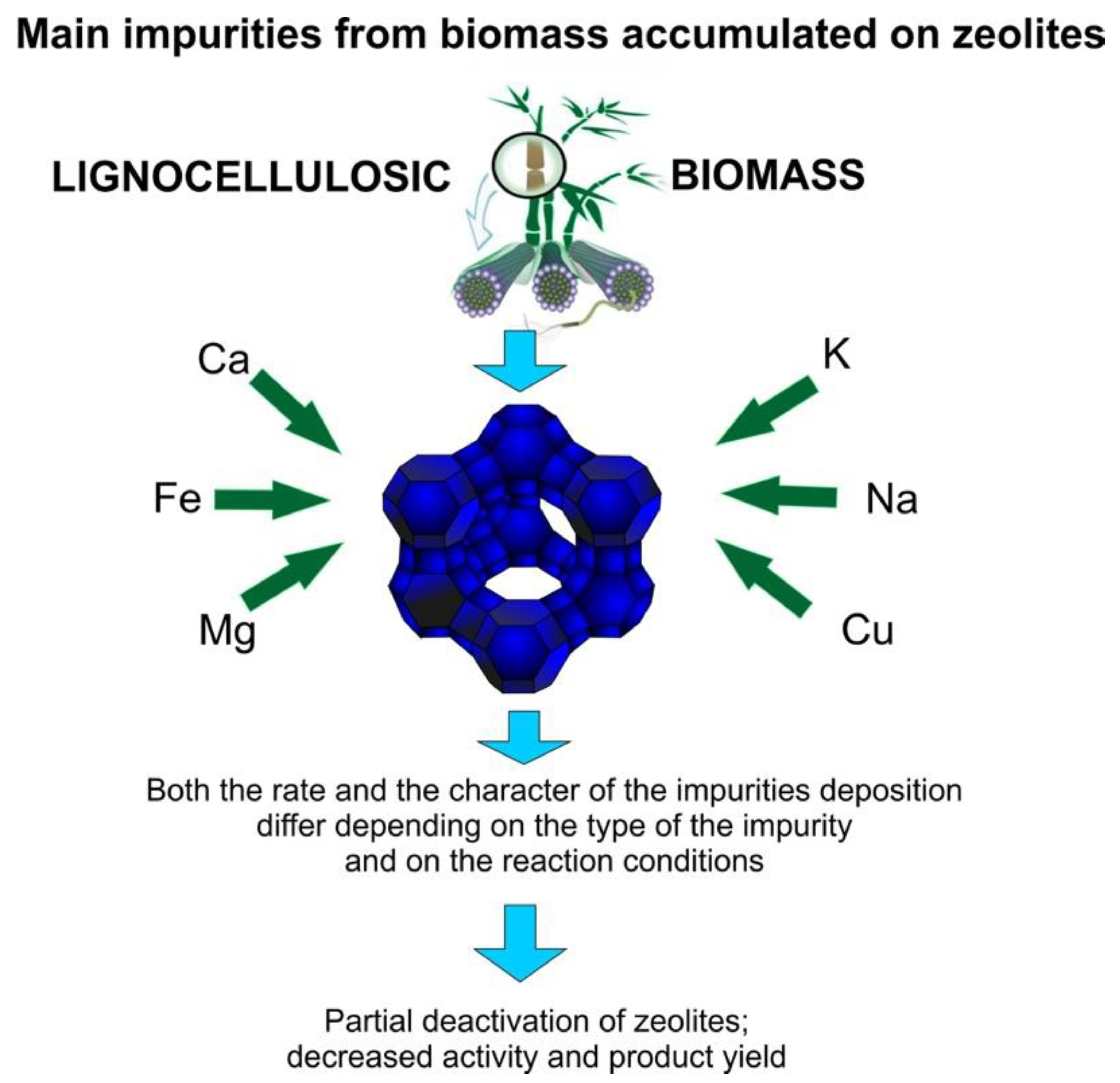
4. Contamination of Bio-Oil during Pyrolysis
5. Effect of Impurities Presence on the Catalytic Performance
5.1. Metal-Based Catalysts
5.2. Zeolites Used as Catalysts
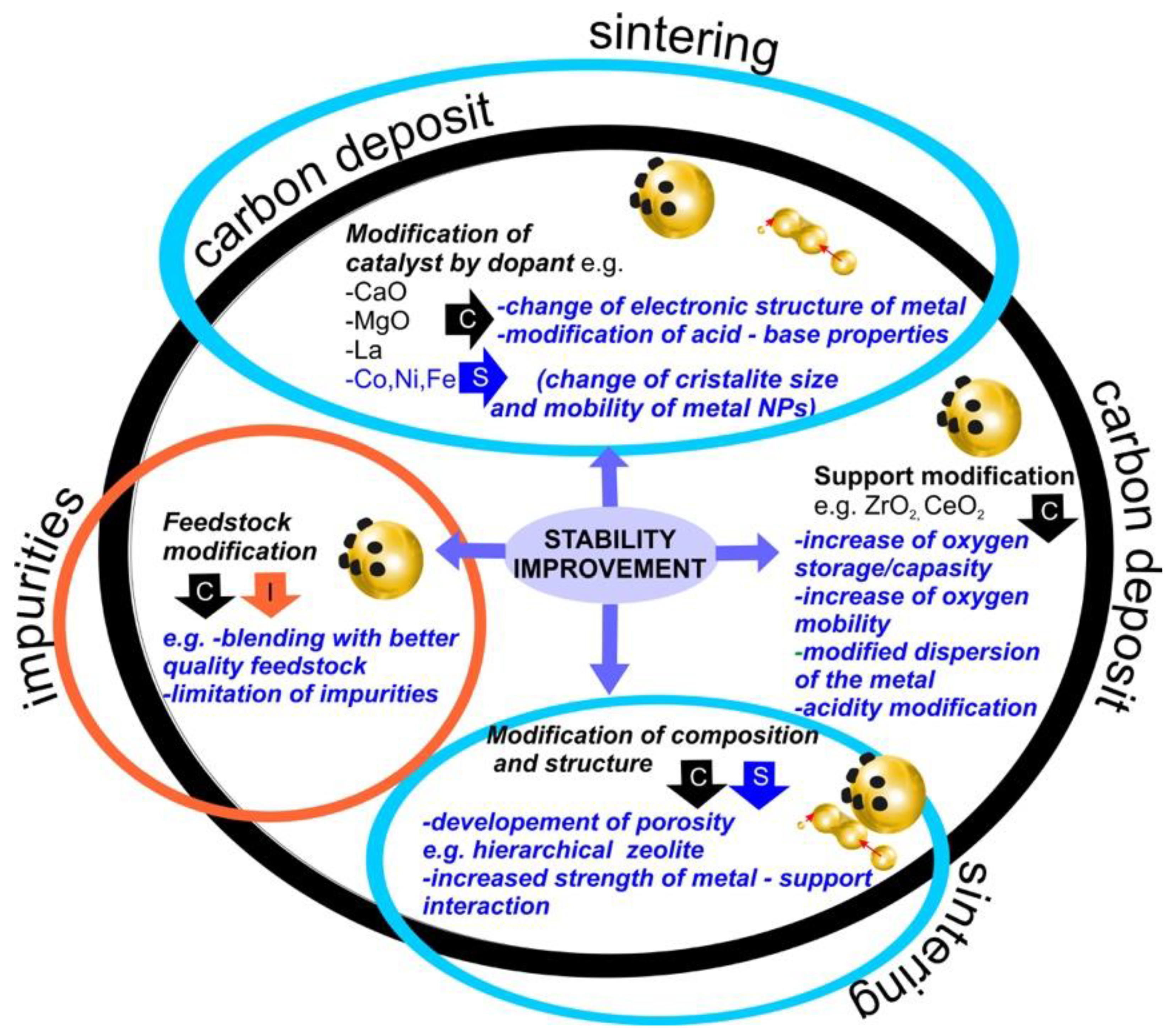
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Rahman, M.; Liu, R.; Cai, J. Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil—A review. Fuel Process. Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A. Production of aromatic hydrocarbons via catalytic pyrolysis of biomass over fe-modified HZSM-5 zeolites. ACS Sustain. Chem. Eng. 2015, 3, 1623–1631. [Google Scholar] [CrossRef]
- Yildiz, G.; Ronsse, F.; Van Duren, R.; Prins, W. Challenges in the design and operation of processes for catalytic fast pyrolysis of woody biomass. Renew. Sustain. Energy Rev. 2016, 57, 1596–1610. [Google Scholar] [CrossRef]
- Navarro, R.M.; Peña, A.M.A.; Fierro, J.L.G. Hydrogen production reactions from carbon feedstocks: Fossil fuels and biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef] [PubMed]
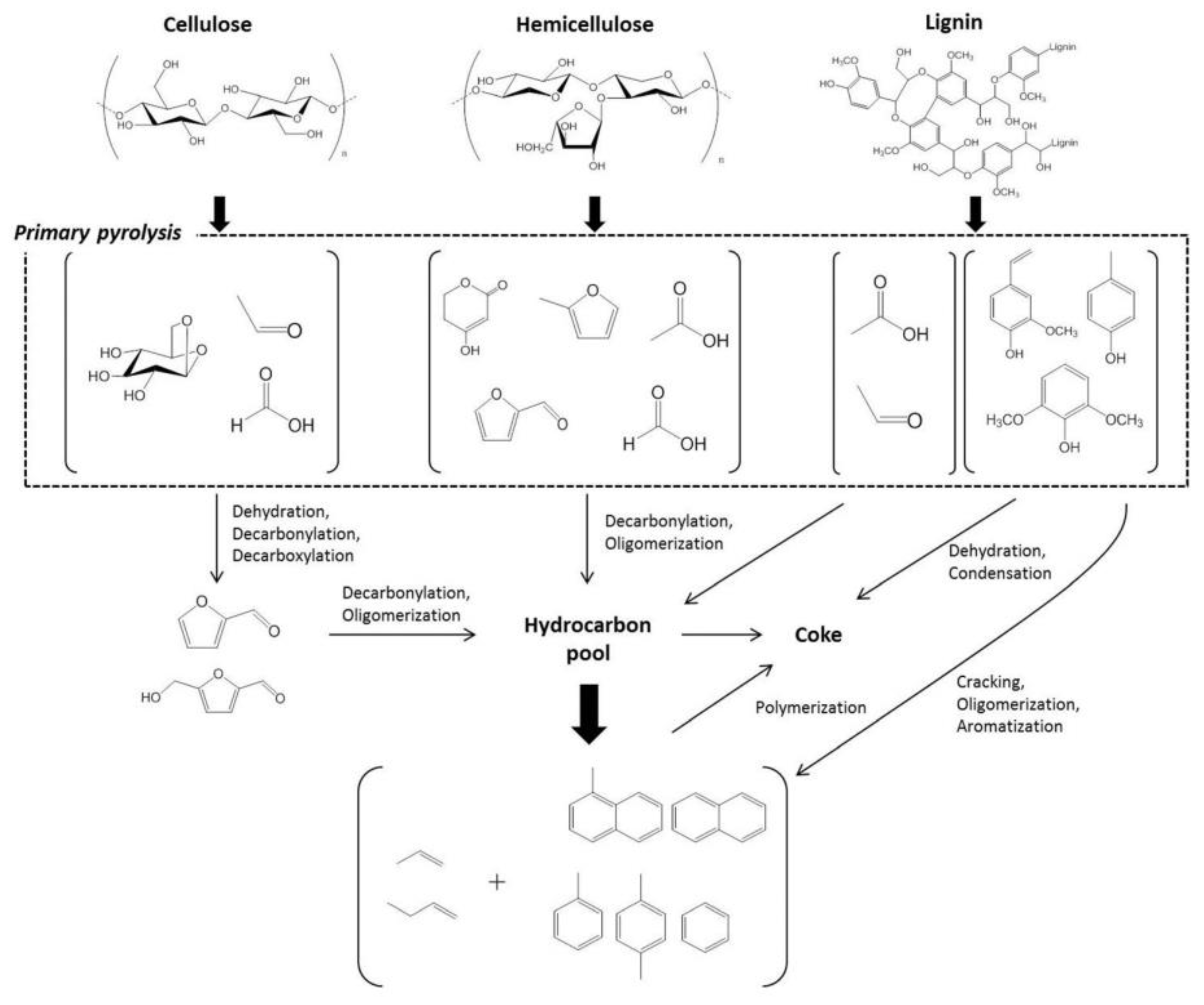
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Wang, K.; Kim, K.H.; Brown, R.C. Catalytic pyrolysis of individual components of lignocellulosic biomass. Green Chem. 2014, 16, 727–735. [Google Scholar] [CrossRef]
- Kabir, G.; Hameed, B. Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew. Sustain. Energy Rev. 2017, 70, 945–967. [Google Scholar] [CrossRef]
- Tanksale, A.; Beltramini, J.; Lu, G.Q.M. A review of catalytic hydrogen production processes from biomass. Renew. Sustain. Energy Rev. 2010, 14, 166–182. [Google Scholar] [CrossRef]
- Fan, Y.; Cai, Y.; Li, X.; Yin, H.; Xia, J. Coking characteristics and deactivation mechanism of the HZSM-5 zeolite employed in the upgrading of biomass-derived vapors. J. Ind. Eng. Chem. 2017, 46, 139–149. [Google Scholar] [CrossRef]
- Grams, J.; Ruppert, A.M. Development of heterogeneous catalysts for thermo-chemical conversion of lignocellulosic biomass. Energies 2017, 10, 545. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R. Catalysis for conversion of biomass to fuels via pyrolysis and gasification: A review. Catal. Today 2011, 171, 1–13. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.; Duman, I.; Wang, S.; Pettersson, L.J.; Yang, W. Catalytic pyrolysis over transition met-al-modified zeolites: A comparative study between catalyst activity and deactivation. J. Anal. Appl. Pyrol. 2019, 138, 54–61. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, X.; Guo, H.; Wei, T.; Dong, D.; Hu, G.; Hu, S.; Xiang, J.; Liu, Q.; Wang, Y. Pyrolysis of poplar, cellulose and lignin: Effects of acidity and alkalinity of the metal oxide catalysts. J. Anal. Appl. Pyrolysis 2018, 134, 590–605. [Google Scholar] [CrossRef]
- Ryczkowski, R.; Chałupka, K.; Kwapiński, W.; Przybysz, K.; Fridrichová, D.; Grams, J. Modification of Ni/ZrO2 catalyst by selected rare earth metals as a promising way for increase in the efficiency of thermocatalytic conversion of lignocellulosic biomass to hydrogen-rich gas. Fuel 2020, 276, 118110. [Google Scholar] [CrossRef]
- Ochoa, A.; Bilbao, J.; Gayubo, A.G.; Castaño, P. Coke formation and deactivation during catalytic reforming of biomass and waste pyrolysis products: A review. Renew. Sustain. Energy Rev. 2020, 119, 109600. [Google Scholar] [CrossRef]
- Liu, N.; Xie, H.; Cao, H.; Shi, L.; Meng, X. Multi-technique characterization of recycled acetylene carbonylation catalyst CuY: Deactivation and coke analysis. Fuel 2019, 242, 617–623. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Yang, Z.; He, Z. An experiment study of biomass steam gasification over NiO/Dolomite for hydrogen-rich gas production. Int. J. Hydrog. Energy 2017, 42, 76–85. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Pilavachi, P.A.; Fougret, C.M.; Jordan, E.; Lappas, A.A. Catalyst hydrother-mal deactivation and metal contamination during the in situ catalytic pyrolysis of biomass. Catal. Sci. Technol. 2016, 6, 2807–2819. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Liang, W.; Yan, H.; Chen, C.; Lin, D.; Tan, K.; Feng, X.; Liu, Y.; Chen, X.; Yang, C.; Shan, H. Revealing the ef-fect of nickel particle size on carbon formation type in the methane decomposition reaction. Catalysts 2020, 10, 890. [Google Scholar] [CrossRef]
- Veses, A.; Puértolas, B.; Callén, M.; García, T. Catalytic upgrading of biomass derived pyrolysis vapors over metal-loaded ZSM-5 zeolites: Effect of different metal cations on the bio-oil final properties. Microporous Mesoporous Mater. 2015, 209, 189–196. [Google Scholar] [CrossRef]
- Xu, M.; Mukarakate, C.; Iisa, K.; Budhi, S.; Menart, M.; Davidson, M.; Robichaud, D.J.; Nimlos, M.R.; Trewyn, B.G.; Richards, R.M. Deactivation of multilayered MFI nanosheet zeolite during upgrading of biomass pyrolysis vapors. ACS Sustain. Chem. Eng. 2017, 5, 5477–5484. [Google Scholar] [CrossRef]
- Yu, L.; Farinmade, A.; Ajumobi, O.; Su, Y.; John, V.T.; Valla, J.A. MCM-41/ZSM-5 composite particles for the catalytic fast pyrolysis of biomass. Appl. Catal. A Gen. 2020, 602, 117727. [Google Scholar] [CrossRef]
- Zheng, Y.; Tao, L.; Huang, Y.; Liu, C.; Wang, Z.; Zheng, Z. Improving aromatic hydrocarbon content from catalytic pyrolysis upgrading of biomass on a CaO/HZSM-5 dual-catalyst. J. Anal. Appl. Pyrolysis 2019, 140, 355–366. [Google Scholar] [CrossRef]
- Karnjanakom, S.; Suriya-Umporn, T.; Bayu, A.; Kongparakul, S.; Samart, C.; Fushimi, C.; Abudula, A.; Guan, G. High selectivity and stability of Mg-doped Al-MCM-41 for in-situ catalytic upgrading fast pyrolysis bio-oil. Energy Convers. Manag. 2017, 142, 272–285. [Google Scholar] [CrossRef]
- Mullen, C.A.; Dorado, C.; Boateng, A.A. Catalytic co-pyrolysis of switchgrass and polyethylene over HZSM-5: Catalyst deactivation and coke formation. J. Anal. Appl. Pyrolysis 2018, 129, 195–203. [Google Scholar] [CrossRef]
- Arregi, A.; Lopez, G.; Amutio, M.; Artetxe, M.; Barbarias, I.; Bilbao, J.; Olazar, M. Role of operating conditions in the catalyst deactivation in the in-line steam reforming of volatiles from biomass fast pyrolysis. Fuel 2018, 216, 233–244. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Artetxe, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Hydrogen-rich gas production by continuous pyrolysis and in-line catalytic reforming of pine wood waste and HDPE mixtures. Energy Convers. Manag. 2017, 136, 192–201. [Google Scholar] [CrossRef]
- Santamaria, L.; Lopez, G.; Arregi, A.; Amutio, M.; Artetxe, M.; Bilbao, J.; Olazar, M. Stability of different Ni supported catalysts in the in-line steam reforming of biomass fast pyrolysis volatiles. Appl. Catal. B Environ. 2019, 242, 109–120. [Google Scholar] [CrossRef]
- Grams, J.; Niewiadomski, M.; Ruppert, A.M.; Kwapiński, W. Influence of Ni catalyst support on the product distribution of cellulose fast pyrolysis vapors upgrading. J. Anal. Appl. Pyrolysis 2015, 113, 557–563. [Google Scholar] [CrossRef]
- Matras, J.; Niewiadomski, M.; Ruppert, A.M.; Grams, J. Activity of Ni catalysts for hydrogen production via biomass pyrolysis. Kinet. Catal. 2012, 53, 565–569. [Google Scholar] [CrossRef]
- Santamaria, L.; Arregi, A.; Alvarez, J.; Artetxe, M.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Performance of a Ni/ZrO2 catalyst in the steam reforming of the volatiles derived from biomass pyrolysis. J. Anal. Appl. Pyrolysis 2018, 136, 222–231. [Google Scholar] [CrossRef]
- Grams, J.; Ryczkowski, R.; Chalupka, K.A.; Sobczak, I.; Rzeznicka, I.I.; Przybysz, K. Impact of support (MCF, ZrO2, ZSM-5) on the efficiency of ni catalyst in high-temperature conversion of lignocellulosic biomass to hydrogen-rich gas. Materials 2019, 12, 3792. [Google Scholar] [CrossRef]
- Dong, L.; Wu, C.; Ling, H.; Shi, J.; Williams, P.T.; Huang, J. Promoting hydrogen production and minimizing catalyst deactivation from the pyrolysis-catalytic steam reforming of biomass on nanosized NiZnAlOx catalysts. Fuel 2017, 188, 610–620. [Google Scholar] [CrossRef]
- Bimbela, F.; Ábrego, J.; Puerta, R.; García, L.; Arauzo, J. Catalytic steam reforming of the aqueous fraction of bio-oil using Ni-Ce/Mg-Al catalysts. Appl. Catal. B Environ. 2017, 209, 346–357. [Google Scholar] [CrossRef]
- Grams, J.; Niewiadomski, M.; Ryczkowski, R.; Ruppert, A.M.; Kwapinski, W. Activity and characterization of Ni catalyst supported on CeO2–ZrO2 for thermo-chemical conversion of cellulose. Int. J. Hydrog. Energy 2016, 41, 8679–8687. [Google Scholar] [CrossRef]
- Santamaria, L.; Artetxe, M.; Lopez, G.; Cortazar, M.; Amutio, M.; Bilbao, J.; Olazar, M. Effect of CeO2 and MgO promoters on the performance of a Ni/Al2O3 catalyst in the steam reforming of biomass pyrolysis volatiles. Fuel Process. Technol. 2020, 198, 106223. [Google Scholar] [CrossRef]
- Bizkarra, K.; Bermudez, J.; Arcelus-Arrillaga, P.; Barrio, V.; Cambra, J.; Millan, M. Nickel based monometallic and bimetallic catalysts for synthetic and real bio-oil steam reforming. Int. J. Hydrog. Energy 2018, 43, 11706–11718. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Zhang, L.; Wang, Y.; Li, X.; Zhang, S.; Liu, Q.; Wei, T.; Gao, G.; Hu, X. Steam reforming of guaiacol over Ni/SiO2 catalyst modified with basic oxides: Impacts of alkalinity on properties of coke. Energy Convers. Manag. 2020, 205, 112301. [Google Scholar] [CrossRef]
- Santamaria, L.; Arregi, A.; Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Effect of La2O3 promotion on a Ni/Al2O3 catalyst for H2 production in the in-line biomass pyrolysis-reforming. Fuel 2020, 262, 116593. [Google Scholar] [CrossRef]
- Valle, B.; Aramburu, B.; Olazar, M.; Bilbao, J.; Gayubo, A.G. Steam reforming of raw bio-oil over Ni/La2O3-αAl2O3: Influence of temperature on product yields and catalyst deactivation. Fuel 2018, 216, 463–474. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Liu, J.; Chen, D. Catalytic characteristics of innovative Ni/slag catalysts for syngas production and tar removal from biomass pyrolysis. Int. J. Hydrog. Energy 2019, 44, 11848–11860. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Liu, J.; Chen, D. High catalytic performance of an innovative Ni/magnesium slag catalyst for the syngas production and tar removal from biomass pyrolysis. Fuel 2019, 254, 115622. [Google Scholar] [CrossRef]
- Jahromi, H.; Agblevor, F.A. Hydrodeoxygenation of pinyon-juniper catalytic pyrolysis oil using red mud-supported nickel catalysts. Appl. Catal. B Environ. 2018, 236, 1–12. [Google Scholar] [CrossRef]
- Al-Rahbi, A.S.; Williams, P.T. Waste ashes as catalysts for the pyrolysis–catalytic steam reforming of biomass for hydrogen-rich gas production. J. Mater. Cycles Waste Manag. 2019, 21, 1224–1231. [Google Scholar] [CrossRef]
- Ryczkowski, R.; Goscianska, J.; Panek, R.; Franus, W.; Przybysz, K.; Grams, J. Sustainable nickel catalyst for the conversion of lignocellulosic biomass to H2-rich gas. Int. J. Hydrog. Energy 2021, in press. [Google Scholar] [CrossRef]
- Heracleous, E.; Pachatouridou, E.; Hernández-Giménez, A.; Hernando, H.; Fakin, T.; Paioni, A.; Baldus, M.; Serrano, D.; Bruijnincx, P.; Weckhuysen, B.; et al. Characterization of deactivated and regenerated zeolite ZSM-5-based catalyst extrudates used in catalytic pyrolysis of biomass. J. Catal. 2019, 380, 108–122. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Li, Q.; Wang, Y.; Liu, Q.; Wei, T.; Dong, D.; Salavati, S.; Gholizadeh, M.; Hu, X. Catalytic pyrolysis of poplar wood over transition metal oxides: Correlation of catalytic behaviors with physiochemical properties of the oxides. Biomass- Bioenergy 2019, 124, 125–141. [Google Scholar] [CrossRef]
- Yi, L.; Liu, H.; Li, M.; Man, G.; Yao, H. Prevention of CaO deactivation using organic calcium precursor during multicyclic catalytic upgrading of bio-oil. Fuel 2020, 271, 117692. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Stefanidis, S.D.; Karakoulia, S.A.; Triantafyllidis, K.S.; Yiannoulakis, H.; Michailof, C.; Lappas, A.A. First pilot scale study of basic vs. acidic catalysts in biomass pyrolysis: Deoxygenation mecha-nisms and catalyst deactivation. Appl. Catal. B Environ. 2018, 238, 346–357. [Google Scholar] [CrossRef]
- Li, M.; Hu, Y.; Fang, Y.; Tan, T. Coating mesoporous ZSM-5 by thin microporous Silicalite-1 shell: Formation of core/shell structure, improved hydrothermal stability and outstanding catalytic performance. Catal. Today 2020, 339, 312–320. [Google Scholar] [CrossRef]
- Paluch, P.; Potrzebowska, N.; Ruppert, A.M.; Potrzebowski, M.J. Application of 1H and 27Al magic angle spinning solid state NMR at 60 kHz for studies of Au and Au-Ni catalysts supported on boehmite/alumina. Solid State Nucl. Magn. Reson. 2017, 84, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, Y.; Gao, C.; Zhao, Y. Structural and textural evolution of Ni/γ-Al2O3 catalyst under hydrothermal conditions. Catal. Today 2010, 158, 475–480. [Google Scholar] [CrossRef]
- Sehested, J.; Gelten, J.A.P.; Helveg, S. Sintering of nickel catalysts: Effects of time, atmosphere, temperature, nickel–carrier interactions, and dopants. Appl. Catal. A Gen. 2006, 309, 237–246. [Google Scholar] [CrossRef]
- Liu, X. DRIFTS Study of Surface of γ-Alumina and Its Dehydroxylation. J. Phys. Chem. C 2008, 112, 5066–5073. [Google Scholar] [CrossRef]
- Ochoa, A.; Arregi, A.; Amutio, M.; Gayubo, A.G.; Olazar, M.; Bilbao, J.; Castaño, P. Coking and sintering progress of a Ni supported catalyst in the steam reforming of biomass pyrolysis volatiles. Appl. Catal. B Environ. 2018, 233, 289–300. [Google Scholar] [CrossRef]
- Arregi, A.; Lopez, G.; Amutio, M.; Barbarias, I.; Santamaria, L.; Bilbao, J.; Olazar, M. Regenerability of a Ni catalyst in the catalytic steam reforming of biomass pyrolysis volatiles. J. Ind. Eng. Chem. 2018, 68, 69–78. [Google Scholar] [CrossRef]
- Santamaria, L.; Lopez, G.; Arregi, A.; Amutio, M.; Artetxe, M.; Bilbao, J.; Olazar, M. Effect of calcination conditions on the performance of Ni/MgO–Al2O3 catalysts in the steam reforming of biomass fast pyrolysis volatiles. Catal. Sci. Technol. 2019, 9, 3947–3963. [Google Scholar] [CrossRef]
- Arandia, A.; Remiro, A.; Valle, B.; Bilbao, J.; Gayubo, A.G. Deactivation of Ni spinel derived catalyst during the oxidative steam reforming of raw bio-oil. Fuel 2020, 276, 117995. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Chen, S.; Liu, Y. Co–Ni bimetal catalyst supported on perovskite-type oxide for steam reforming of ethanol to produce hydrogen. Int. J. Hydrog. Energy 2014, 39, 5644–5652. [Google Scholar] [CrossRef]
- Remiro, A.; Ochoa, A.; Arandia, A.; Castaño, P.; Bilbao, J.; Gayubo, A.G. On the dynamics and reversibility of the deactivation of a Rh/CeO2ZrO2 catalyst in raw bio-oil steam reforming. Int. J. Hydrog. Energy 2019, 44, 2620–2632. [Google Scholar] [CrossRef]
- Dai, L.; Zeng, Z.; Yang, Q.; Yang, S.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Yu, Z.; Jiang, L. Synthesis of iron nanoparticles-based hydrochar catalyst for ex-situ catalytic microwave-assisted pyrolysis of lignocellulosic biomass to renewable phenols. Fuel 2020, 279, 118532. [Google Scholar] [CrossRef]
- Ma, L.; Xiao, T.; Ning, Z.; Liu, Y.; Chen, H.; Peng, J. Pollution and health risk assessment of toxic metal(loid)s in soils under different land use in sulphide mineralized areas. Sci. Total. Environ. 2020, 724, 138176. [Google Scholar] [CrossRef]
- Velghe, I.; Carleer, R.; Yperman, J.; Schreurs, S. Study of the pyrolysis of municipal solid waste for the production of valuable products. J. Anal. Appl. Pyrol. 2011, 92, 366–375. [Google Scholar] [CrossRef]
- Wise, J.; Vietor, D.; Provin, T.; Capareda, S.C.; Munster, C.; Boateng, A. Mineral nutrient recovery from pyrolysis systems. Environ. Prog. Sustain. Energy 2012, 31, 251–255. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A.; Goldberg, N.M.; Lima, I.M.; Laird, D.A.; Hicks, K.B. Bio-oil and bio-char pro-duction from corn cobs and stover by fast pyrolysis. Biomass Bioenerg. 2010, 34, 67–74. [Google Scholar] [CrossRef]
- Olsson, J.G.; Jäglid, U.; Pettersson, J.B.C.; Hald, P. Alkali metal emission during pyrolysis of biomass. Energy Fuels 1997, 11, 779–784. [Google Scholar] [CrossRef]
- Jensen, P.A.; Frandsen, F.J.; Dam-Johansen, A.K.; Sander, B. Experimental investigation of the transformation and release to gas phase of potassium and chlorine during straw pyrolysis. Energy Fuels 2000, 14, 1280–1285. [Google Scholar] [CrossRef]
- Davidsson, K.O.; Stojkova, B.J.; Pettersson, J.B.C. Alkali emission from birchwood particles during rapid py-rolysis. Energy Fuels 2002, 16, 1033–1039. [Google Scholar] [CrossRef]
- Kowalski, T.; Ludwig, C.; Wokaun, A. Qualitative Evaluation of Alkali Release during the Pyrolysis of Bio-mass. Energy Fuels 2007, 21, 3017–3022. [Google Scholar] [CrossRef]
- Tu, Y.; Tian, S.; Kong, L.; Xiong, Y. Co-catalytic effect of sewage sludge-derived char as the support of Fen-ton-like catalyst. Chem. Eng. J. 2012, 185–186, 44–51. [Google Scholar] [CrossRef]
- Lup, A.N.K.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on reactivity and stability of heterogeneous metal catalysts for deoxygenation of bio-oil model compounds. J. Ind. Eng. Chem. 2017, 56, 1–34. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Wiliams, P.T. Thermochemical conversion of sewage sludge: A critical re-view. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Yang, T.; Huang, H.; Lai, F.-Y. Pollution hazards of heavy metals in sewage sludge from four wastewater treatment plants in Nanchang, China. Bioresour. Technol. 2016, 200, 991–998. [Google Scholar] [CrossRef]
- Mansur, D.; Yoshikawa, T.; Norinaga, K.; Hayashi, J.-I.; Tago, T.; Masuda, T. Production of ketones from py-roligneous acid of woody biomass pyrolysis over an iron-oxide catalyst. Fuel 2013, 103, 130–134. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Gardini, D.; De Carvalho, H.W.P.; Damsgaard, C.D.; Grunwaldt, J.-D.; Jensen, P.A.; Wagner, J.B.; Jensen, A.D. Stability and resistance of nickel catalysts for hydrodeoxygenation: Carbon deposition and effects of sulfur, potassium, and chlorine in the feed. Catal. Sci. Technol. 2014, 4, 3672–3686. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Steam reforming. In Handbook of Heterogeneous Catalysis; John Wiley & Sons, Inc.: New York, NY, USA, 2008; pp. 2882–2905. [Google Scholar]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Xu, J. Hydrogenolysis of lignosulfonate into phenols over heterogeneous nickel catalysts. Chem. Commun. 2012, 48, 7019–7021. [Google Scholar] [CrossRef]
- Soszka, E.; Reijneveld, H.M.; Jędrzejczyk, M.; Rzeznicka, I.I.; Grams, J.; Ruppert, A.M. Chlorine influence on palladium doped nickel catalysts in levulinic acid hydrogenation with formic acid as hydrogen source. ACS Sustain. Chem. Eng. 2018, 6, 14607–14613. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Gardini, D.; Damsgaard, C.D.; Grunwaldt, J.-D.; Jensen, P.A.; Wagner, J.B.; Jensen, A.D. Deactivation of Ni-MoS2 by bio-oil impurities during hydrodeoxygenation of phenol and octanol. Appl. Catal. A Gen. 2016, 523, 159–170. [Google Scholar] [CrossRef]
- Schmitt, C.C.; Reolon, M.B.G.; Zimmermann, M.; Raffelt, K.; Grunwaldt, J.-D.; Dahmen, N. Synthesis and regeneration of nickel-based catalysts for hydrodeoxygenation of beech wood fast pyrolysis bio-oil. Catalysts 2018, 8, 449. [Google Scholar] [CrossRef]
- Boscagli, C.; Morgano, M.T.; Raffelt, K.; Leibold, H.; Grunwaldt, J.-D. Influence of feedstock, catalyst, pyrol-ysis and hydrotreatment temperature on the composition of upgraded oils from intermediate pyrolysis. Biomass Bioenergy 2018, 116, 236–248. [Google Scholar] [CrossRef]
- Gastelu, N.; Lopez-Urionabarrenechea, A.; Acha, E.; Caballero, B.M.; de Marco, I. Evaluation of HZSM-5 zeolite as cracking catalyst for upgrading the vapours generated in the pyrolysis of an epoxy-carbon fibre waste composite. Top. Catal. 2019, 62, 479–490. [Google Scholar] [CrossRef]
- Carlson, T.R.; Cheng, Y.-T.; Jae, J.; Huber, G.W. Production of green aromatics and olefins by catalytic fast pyrolysis of wood sawdust. Energy Environ. Sci. 2010, 4, 145–161. [Google Scholar] [CrossRef]
- Paasikallio, V.; Lindfors, C.; Kuoppala, E.; Solantausta, Y.; Oasmaa, A.; Lehto, J.; Lehtonen, J. Product quality and catalyst deactivation in a four day catalytic fast pyrolysis production run. Green Chem. 2014, 16, 3549–3559. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A. Accumulation of inorganic impurities on HZSM-5 zeolites during catalytic fast pyrolysis of switchgrass. Ind. Eng. Chem. Res. 2013, 52, 17156–17161. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Meeldijk, J.D.; Kuipers, B.W.M.; Erné, B.H.; Weckhuysen, B.M. Glycerol etherification over highly active CaO-based materials: New mechanistic aspects and related colloidal particle formation. Chem. Eur. J. 2008, 14, 2016–2024. [Google Scholar] [CrossRef] [PubMed]



| Catalyst | Process/Feedstock | Comments | Ref. |
|---|---|---|---|
| Multilayered ZSM-5 nanosheet, HZSM-5 | Upgrading of pyrolysis vapors/Cellulose | Deposition of coke blocks access to active sites in the micropores of the catalysts, reduction in deactivation rate by creation of mesopores. | [22] |
| MCM-41/ZSM-5 composite | Catalytic fast pyrolysis/Miscanthus | Presence of MCM-41 reduced deactivation rate of ZSM-5 and protected the zeolite against severe coking. | [23] |
| HZSM-5, Fe/ZSM-5, Ni/ZSM-5 and FeNi/ZSM-5 | Pyrolysis and catalytic upgrading of vapors/Softwood sawdust | Introduction of metals increases the concentration of aromatic hydrocarbons in the liquid product; Rate of carbon deposit formation was dependent on the strength of acid sites and the choice of metal; FeNi/ZSM-5 allowed for a high level of aromatization of formed products together with moderate catalyst coking. | [14] |
| CaO/HZSM-5 | Catalytic pyrolysis/Yunnan pine | Order of relative content of coking: HZSM-5 > CaO > CaO/HZSM-5. | [24] |
| ZrO2-promoted ZSM-5 catalyst extrudates | Pyrolysis and catalytic upgrading of vapors/Oak | Eggshell spatial distribution of the coke deposits within the catalyst extrudates; At the beginning, coke is formed on the strong Brønsted acid sites, which promote deep deoxygenation and cracking; then, carbon deposition slows down and occurs mainly on the external surface; more hydrogen-rich coke is formed on ZrO2 domains. | [49] |
| Mg-doped Al-MCM-41 | In-situ catalytic upgrading of bio-oils/Cellulose, lignin, and sunflower stalk | Mg/AlMCM-41 showed high selectivity towards production of aromatics; the reduction in coke deposition was related to the amount of introduced Mg. | [25] |
| HZSM-5 | Catalytic co-pyrolysis/Switchgrass and polyethylene | Polyethylene-derived hydrocarbon vapors contributed to the reduction in coke formation. | [26] |
| Commercial Ni catalyst (G90-LDP) | Pyrolysis and in-line catalytic steam reforming/Wood sawdust | Coke deposition results from decomposition of the oxygenates derived from biomass pyrolysis and the repolymerization of phenolic oxygenates; amount of carbon can be limited by selection of optimal reaction conditions enhancing WGS and reforming. | [27] |
| Commercial Ni catalyst (G90-LDP) | Pyrolysis and in-line catalytic reforming/Mixture of pine wood waste and HDPE | Deactivation rate of the catalyst depends on the type of formed coke; encapsulation of Ni active sites by amorphous carbon leads to faster deactivation than the presence of filamentous coke. | [28] |
| Ni supported on Al2O3, SiO2, MgO, TiO2 and ZrO2 | In-line steam reforming of biomass fast pyrolysis volatiles/Pine | Initially active Ni/Al2O3 suffers from carbon deposit formation, Ni/ZrO2 and Ni/MgO less active and more resistant to coke deposition. | [29] |
| Ni/ZrO2 | Pyrolysis and in-line catalytic reforming/Pine sawdust | Coke deposition leads to the blockage of Ni active sites, with oxygenates being the main coke precursors; Application of ZrO2 limits coke formation and decreases coke combustion temperatures. | [32] |
| NiZnAlOx | Pyrolysis-catalytic steam reforming/Wood sawdust | Introduction of Zn to the catalyst structure suppressed formation of carbon deposit; Presence of amorphous carbon and filamentous carbon was confirmed. | [34] |
| Ni-Ce/Mg-Al | Catalytic steam reforming of aqueous fraction of bio-oil/Pine, poplar | Introduction of small content of Ce by impregnation method allowed for the most effective reduction in carbon deposit formation. | [35] |
| Ni/Al2O3, Ni/CeO2-Al2O3 and Ni/MgO-Al2O3 | Pyrolysis and in-line steam reforming/Pine wood | Similar initial activity for all catalysts; improved stability of CeO2-doped sample connected with gasification of coke precursors; Ni/MgO-Al2O3 less stable than Ni/Al2O3 due to the formation of MgAl2O4 spinel phase. | [37] |
| Ni/CeO2-Al2O3, Ni/La2O3-Al2O3, Ni-Rh/CeO2-Al2O3, | Steam reforming of bio-oil | Ni/CeO2-Al2O3 more resistant to deactivation due to higher oxygen mobility leading to the limitation of carbon deposit formation; Increased stability of Ni-Rh/CeO2-Al2O3 related to enhancement of carbon oxidation over C-C bond formation. | [38] |
| Ni/SiO2 modified with Na, Mg or La | Steam reforming/Guaiacol | Modification of Ni/SiO2 by La led to highest resistivity towards coking among studied catalysts. | [39] |
| Ni/La2O3-Al2O3, Ni/Al2O3 and commercial Ni catalyst (G90-LDP) | Pyrolysis and in-line catalytic steam reforming/Pine wood waste | Amorphous structure of coke; La2O3 inhibited carbon deposition due to its basicity and water adsorption capacity during reforming reaction, which led to the gasification of deposited coke and prevented catalyst deactivation. | [40] |
| Ni/La2O3-Al2O3 | Pyrolysis and steam reforming/Pine sawdust | Presence of two types of carbon deposit (encapsulating coke and filamentous coke); their contribution depends on temperature and space-time used during the reaction. | [41] |
| Ni/slag (magnesium slag steel slag, blast furnace slag, pyrite cinder and calcium silicate slag) | Pyrolysis and catalytic reforming/Pine sawdust | Ni supported on magnesium slag revealed the highest activity among tested catalysts; amorphous and graphite-like carbons were formed during reaction. | [42] |
| Ni/magnesium slag and Ni/γ-Al2O3 | Pyrolysis and catalytic reforming/Pine sawdust | Lower coke formation rate and lower graphitization degree of deposited carbon on the surface of Ni/magnesium slag than in the case of Ni/γ-Al2O3; interaction of Ni with slag components (Mg, Fe, or Ca) increases resistance against coke. | [43] |
| Metal oxides: CoO, Cr2O3, CuO, Fe2O3, Mn2O3, NiO, TiO2, V2O5 and CeO2 | Catalytic pyrolysis/Poplar | V-, Mn-, Cu-, and Co-based catalysts with the highest tendency towards coke formation; Presence of oxygen vacancies played important role in the polymerization of the volatile compounds formed during pyrolysis. | [50] |
| CaO | Catalytic fast pyrolysis/Jatropha seeds de-oil cake | Effect of precursor on catalyst deactivation, differences in coking mechanism. | [51] |
| Catalyst | Process/Feedstock | Comments | Ref. |
|---|---|---|---|
| ZSM-5 and MgO | Fast pyrolysis in pilot unit/Commercial lignocellulosic biomass (Lignocel HBS 150–500) | Hydrothermal deactivation of zeolite resulted from the presence of water vapor leading to dealumination of the zeolitic framework and due to that, reduction in both the strength and density of the acid sites; Magnesium oxide loses surface area and basicity due to the sintering of MgO crystallites at high temperature. | [47] |
| Mesoporous ZSM-5 coated by thin microporous silicalite shell | Pyrolysis in bench-scale fluidized bed pyrolyzer/Pine sawdust | Improved hydrothermal stability of core/shell structure even at 800 °C. | [48] |
| Commercial Ni catalyst (ReforMax® 330 and G90LDP) | Pyrolysis and in-line catalytic steam reforming/Pine wood | Deactivation of catalyst is mainly due to the encapsulation of Ni particles by coke and Ni sintering (increase in Ni particle size from 25 to 39 nm after the reaction). | [49] |
| Commercial Ni catalyst (G90LDP) | Pyrolysis and in-line steam reforming/Pine wood | Irreversible deactivation of Ni in successive reaction–regeneration cycles. | [50] |
| Ni/Al2O3, Ni/CeO2-Al2O3 and Ni/MgO-Al2O3 | Pyrolysis and in-line steam reforming/Pine wood | Sintering may contribute to catalyst deactivation, while the main reason for a decrease in activity is related to coking. | [37] |
| Ni/MgO–Al2O3 | Pyrolysis and in-line catalytic steam reforming/Pine wood | Loss of activity related to the catalyst pretreatment conditions; formation of the spinel structure decreased catalytic performance of Ni-based system. | [51] |
| NiAl2O4 | Pyrolysis and catalytic oxidative steam reforming/Pine sawdust | Ni sintering observed attributed to high reaction temperature and the presence of water in the reaction mixture. | [52] |
| Ni-Co/LaFeO3 | Steam reforming/Model compound—ethanol | Bimetallic Ni-Co particles possess much better anti-sintering ability than that of monometallic nickel and cobalt species in the reaction conditions. | [53] |
| Rh/CeO2-ZrO2 c | Steam reforming of bio-oil from fast pyrolysis/Pine sawdust | Catalyst undergoes structural changes during the reaction (irreversible support aging involving partial occlusion of Rh species); Observed Rh sintering did not contribute significantly to decrease in activity. | [54] |
| Fe/hydrochar | ex-situ catalytic microwave-assisted pyrolysis/Rice husk and corn cob | Sintering, oxidation of α-Fe and Fe3C phases, active site coverage, and pore blockage were the most important factors influencing catalytic activity. | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grams, J.; Ruppert, A.M. Catalyst Stability—Bottleneck of Efficient Catalytic Pyrolysis. Catalysts 2021, 11, 265. https://doi.org/10.3390/catal11020265
Grams J, Ruppert AM. Catalyst Stability—Bottleneck of Efficient Catalytic Pyrolysis. Catalysts. 2021; 11(2):265. https://doi.org/10.3390/catal11020265
Chicago/Turabian StyleGrams, Jacek, and Agnieszka M. Ruppert. 2021. "Catalyst Stability—Bottleneck of Efficient Catalytic Pyrolysis" Catalysts 11, no. 2: 265. https://doi.org/10.3390/catal11020265
APA StyleGrams, J., & Ruppert, A. M. (2021). Catalyst Stability—Bottleneck of Efficient Catalytic Pyrolysis. Catalysts, 11(2), 265. https://doi.org/10.3390/catal11020265







