1. Introduction
Titanium dioxide (TiO
2) nanoparticles (NPs) are photoactive to combat air and water pollutants under ultraviolet (UV) activation. To date, several studies have been conducted to enable the sensitization of visible light by TiO
2 NP [
1]. The prevalent approaches for extending TiO
2 absorption edge are doping with metals/non-metals [
2,
3] and coupling with metal oxides [
4] or noble metals [
5,
6]. The effect of coupling 3-composites was also studied and reported to show that TiO
2 forms heterojunctions in the presence of coupling TiO
2/g-C
3N
4/rGO compounds [
7]. One of the cost-effective practical techniques of doping and coupling TiO
2 is the wet process method. The disadvantage of coupling two or more NP composites is uncontrollable agglomeration in wet processes. This results in the incorporated phases being dominant and even deterioration of photocatalytic efficiencies.
In metal doping of TiO
2, Ag is among the most studied materials owing to its antibacterial property and 3d energy level, which induces low energy states in the TiO
2 conduction band. Doping TiO
2 with Ag NP to improve TiO
2 sensitivity to visible light has been reported to be effective in enhancing photocatalytic properties [
8]. However, several studies were conducted in the slurry type of reactor, where detachment of Ag NP and leaching problems limited the reusability of the TiO
2-Ag NP in the slurry reactor [
9]. Much research focuses on doping Ag on the TiO
2 NP surface [
10].
Nowadays, many researchers in this field are working towards the commercialization of TiO
2 nanoparticles (NPs) in water and air purification systems [
11,
12,
13]. However, owing a to high specific surface-to-volume ratio, TiO
2 NPs require immobilization on substrates for practical application [
14,
15]. There are studies where TiO
2 NPs are practically immobilized on stainless steel mesh [
16], glass slides [
17], glass mesh [
18], quartz substrate [
19], concrete [
20], coconut fibers [
21], and Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads [
22]. One merit for immobilizing TiO
2 is reusability and a less tedious recovery for the catalysts, even in slurry reactors. Furthermore, TiO
2 on the substrate is applicable for continuous airflow type of reactors facilitating air cleaning performances [
23]. Coating TiO
2 on substrates eliminates several processes to reduce particle agglomeration, which is cost-effective for the catalyst. Most importantly, crystalline phases are confined on the support surface and this improves the formation of inter-particle heterojunction. However, in hydraulic systems, catalysts suffer from hydro-abrasion during photoreaction mixing [
24]. Meanwhile, sampling the photocatalysts in air environments minimizes the antifouling of the catalyst [
25]. There has been little research on evaluating the photocatalytic performance of TiO
2 supported on a substrate decorated with Ag NP.
The reusability of a catalyst depends on physical and chemical stability after the photocatalytic reaction. Crystalline substrates such as long-lasting Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads have been reported to show stable support and photocatalytic activity in benzene [
22]. Long-lasting phosphors are reported to enhance light harvesting and improve photocatalytic efficiencies through heterojunctions with TiO
2 NP. There is a need to determine the optimum coating conditions on long-lasting phosphors owing to the afterglow characteristic, which is affected by the presence of other elements on the surface [
26,
27]. The afterglow property may aid the recovery of catalyst supports from photocatalytic reaction mixtures in large-scale applications. Up to now, there have been no reports of the photocatalytic performance of a TiO
2 NP support on long-lasting phosphor beads doped with varying amounts of Ag.
In this study, long-lasting phosphor beads were synthesized from inorganic binder Na2SiO3 and decorated with Ag/TiO2. The photocatalytic performance was evaluated at various Ag-molar concentrations and the effect of reducing atmospheres. The mechanical strength of phosphor bead substrates was evaluated to confirm whether the beads are mechanically stable for reusability.
2. Results
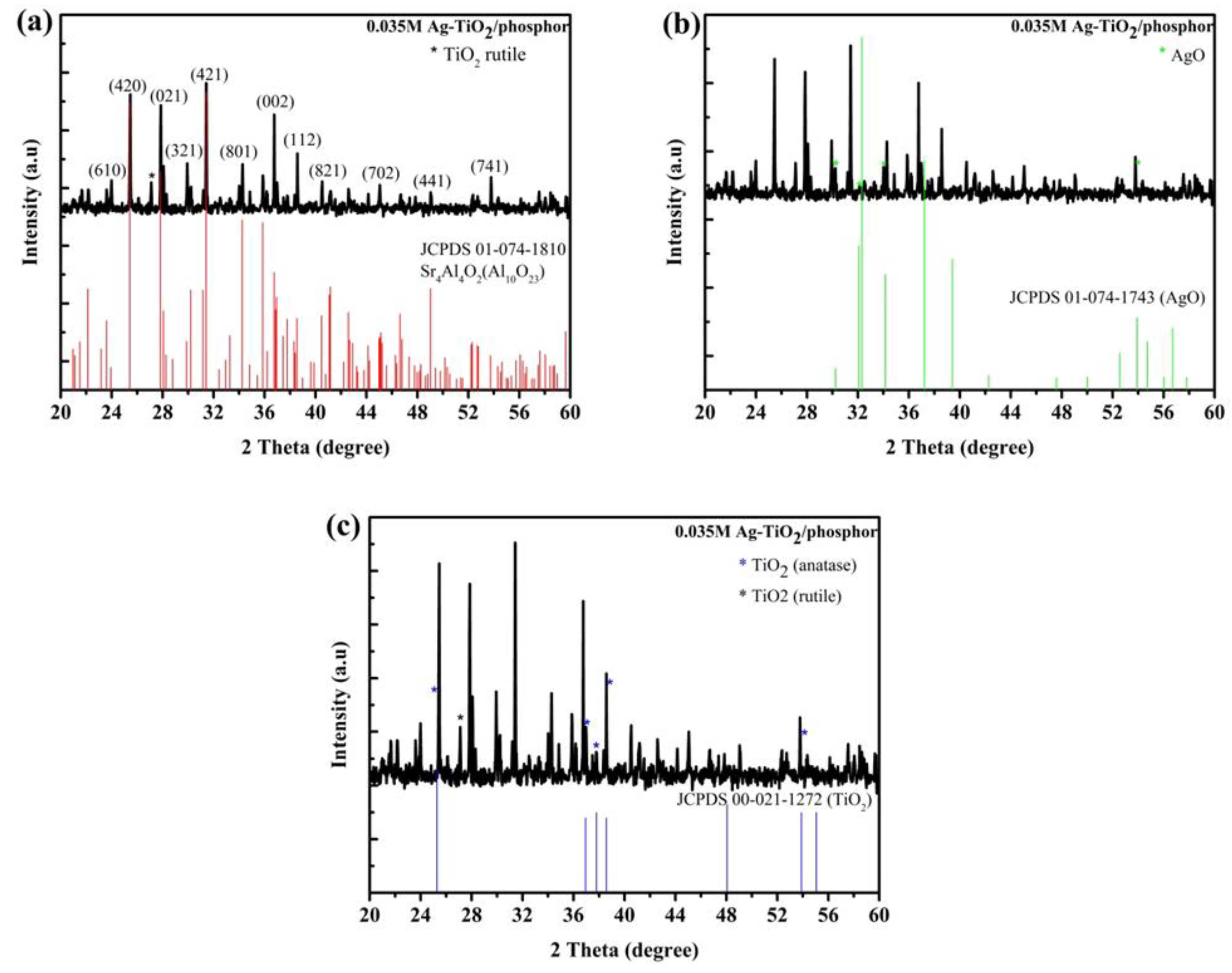
X-ray diffraction spectra (XRD) of 0.035 M Ag-TiO
2/phosphor is shown in
Figure 1 as compared to Joint Committee on Powder Diffraction Standards (JCPDS) cards for (a) Sr
4Al
4O
2(Al
10O
23), (b) AgO, and (c) TiO
2. The Ag-doped TiO
2/phosphor exhibited high crystallinity as indicated by intense XRD spectra. All the major peaks were matched and indexed in reference to Sr
4Al
4O
2(Al
10O
23). However, only the peak at 27.0° is referenced to TiO
2 rutile JCPDS 75-1753. While the AgO and TiO
2 peaks coincided with the Sr
4Al
4O
2(Al
10O
23) peaks (
Figure 1b,c).
Scanning electron microscopy (SEM) images of (a) Sr
4Al
14O
25:Eu
2+,Dy
3+, (b) TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+, (c) Ag-TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+, and (d) AgO on TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ photocatalyst/phosphor beads are shown in
Figure 2. Phosphor bead morphology after drying at 100 °C consists of angular submicron particles of irregular sizes ranging from 1 to 10 μm with pore space. The TiO
2-coated beads exhibit a spherical shape owing to the TiO
2 coating. With Ag-doping, silver oxide appears as white spots decorated on the TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ beads. After a two-step coating of Ag and TiO
2, the bead surface inter-particle porosity is covered over. However, thermal cracks and confined micro-pores result from heat treatments at 450 °C after the Ag and TiO
2 coating processes.
Figure 2d shows a high-resolution SEM image of AgO nanoparticles on phosphor and the respective
supplementary images for energy dispersion spectroscopy (EDS) are shown in
Figure S1a,b. The AgO nanoparticles were the brightest particles in
Figure 2d (as indicated by arrows) after scanning EDS map with the highest Ag in back-scattering mode.
Mapping analysis for the distribution of the elements on the bead surface and energy dispersion spectroscopy (EDS) spectra is shown in
Figure S1c,d, respectively. Details of the elemental composition of the Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor bead and the atomic percent confirming the presence of O, Na, Al, Si, and Sr are shown in
Table 1. The silicon content of 14.81 atom percent originated from the Na
2SiO
3 inorganic binder. However, Eu and Dy were undetected because they are embedded in the core matrix and their atomic percent is also small when compared with the bulky bead surface.
Elemental compositions for TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ photocatalyst/phosphor beads are shown in
Table 2. The composition of 2.71 atom percent Ti merely confirms that the coating process impregnates the phosphor surfaces with TiO
2 particles. The remaining atomic composition is for the bead surface, namely O, Na, Al, Si, and Sr. Eu and Dy were below the detection limit, as also observed in
Table 1, because of the bulky structure of the bead. The elemental mapping and EDS spectra for this sample are shown in
Figure S1e,f, respectively.
Elemental compositions of Ag-TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ photocatalyst/phosphor beads are shown in
Table 3. The amount of Ag-doped and Ti on phosphor was 1.30 and 2.21 atom percent, respectively. As mentioned in
Table 1 and
Table 2, the rare earth ions Eu and Dy were below the detection limit. The EDS mapping for Ag-doped TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ beads and the corresponding spectra are shown in
Figure S1g,h, respectively.
Compressive stress averages for the Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor photocatalyst/beads are shown in
Table 4. The phosphor beads show the highest average compressive strength of 15.36 kgf compared with the TiO
2-coated and Ag/TiO
2-beads with 8.71 and 5.80 kgf, respectively. This is a result of the thermal cracks observed in the SEM images in
Figure 2 that were caused by heat treatment procedures conducted after the coating processes. Despite the stress strain curves shown in
Figure S2a–c, the beads withstood compressive strength above 5 kgf. Therefore, the beads are reusable because mechanically they are able to endure fragmentation during the handling and packaging process.
X-ray photoelectron spectroscopy (XPS) spectra of (a) TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ and (b) Ag-TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ photocatalyst/phosphor beads are shown in
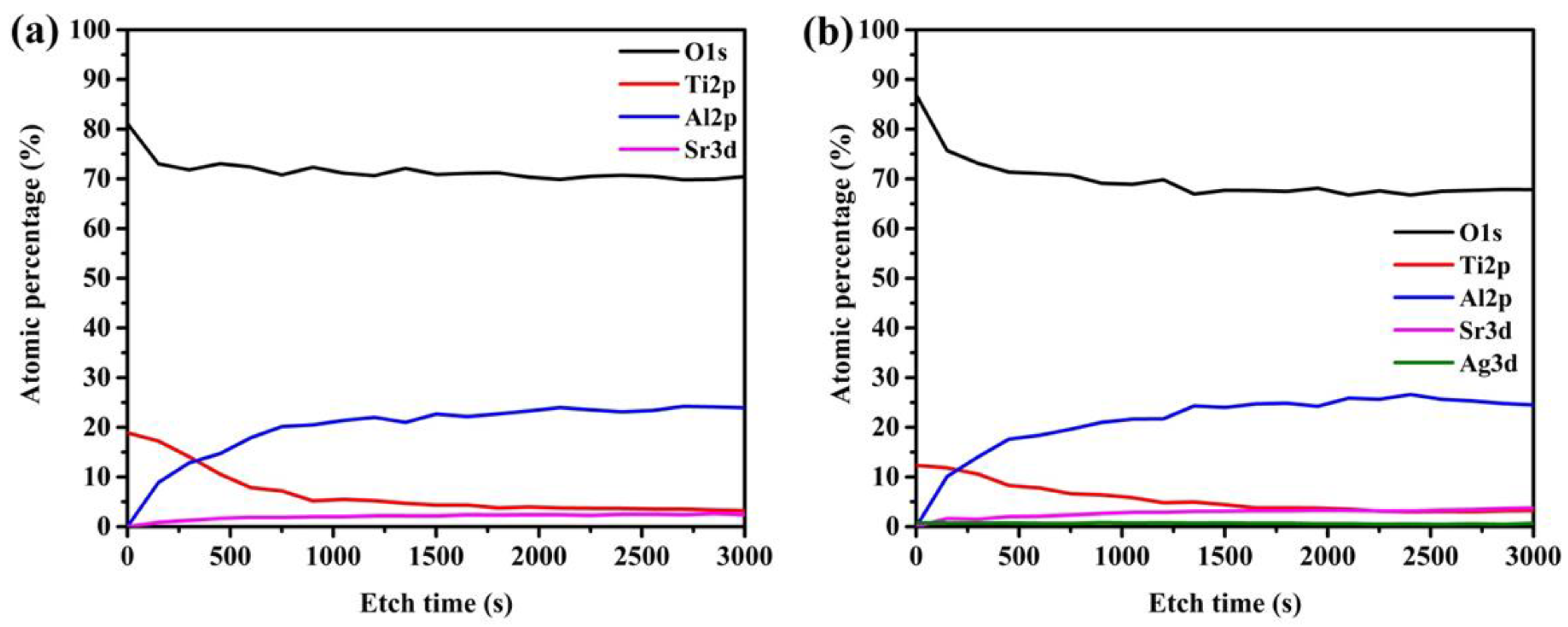
Figure 3. The depth profile of TiO
2/Sr
4Al
14O
25:Eu
2 +,Dy
3+ photocatalyst/phosphor beads exhibits the elements of O and Ti as the highest on the surface, while Al and Sr increase with etching time (
Figure 3a,b). This shows that the TiO
2 NPs are loaded at the surface while Sr and Al are in the core of the bead. The composition of Ag varied from 0.76 at the surface to 0.48 atomic percent after 3000 s etching (
supplementary Tables S1 and S2).
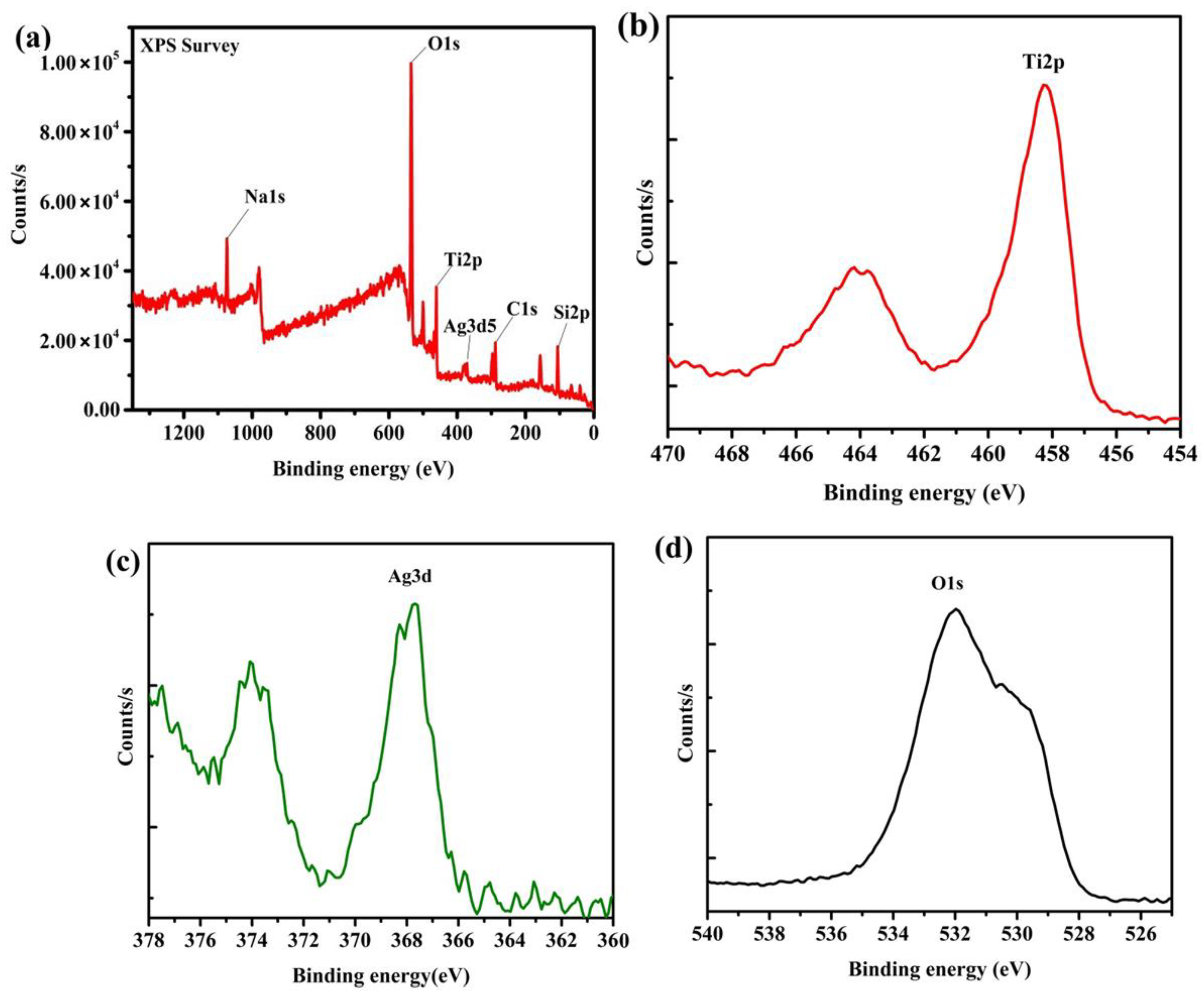
XPS spectra of the photocatalyst/phosphor bead surface of 0.035 M Ag decorated TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ are shown in
Figure 4 as (a) survey, (b) Ti 2p, (c) Ag 3d, and (d) O 1s. The spectra show peaks of Na 1s, O 1s, Ti 2p, Ag 3d5, C 1s, and Si 2p (
Figure 4a). Except for C impurity from the instruments and the environment, all the elements are part of the Sr
4Al
14O
25:Eu
2+,Dy
3+ bead/TiO
2/Ag composite. The atomic compositions of the respective elements are provided in
supplementary Tables S3 and S4. Ti 2p spectra for the Ag-TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ bead are shown in
Figure 4b. The binding energy of 464 and 458 eV confirms the bonding of Ti to O. Atomic percent for TiO
2 is 2.7% (
supplementary Table S2). The spectrum has two peaks at 369.5 and 375.7 eV, which correspond to the core level of Ag 3d
3/2 and Ag 3d
5/2 (
Figure 4c). These peaks are referenced to Ag
2O compounds owing to Ag and O bonds, where at Ag
0 and Ag
+ exist in the deconvoluted peak of 360 to 370 eV [
28]. The spectra for O 1s with a maximum binding energy peak at 532 eV are shown in
Figure 4d.
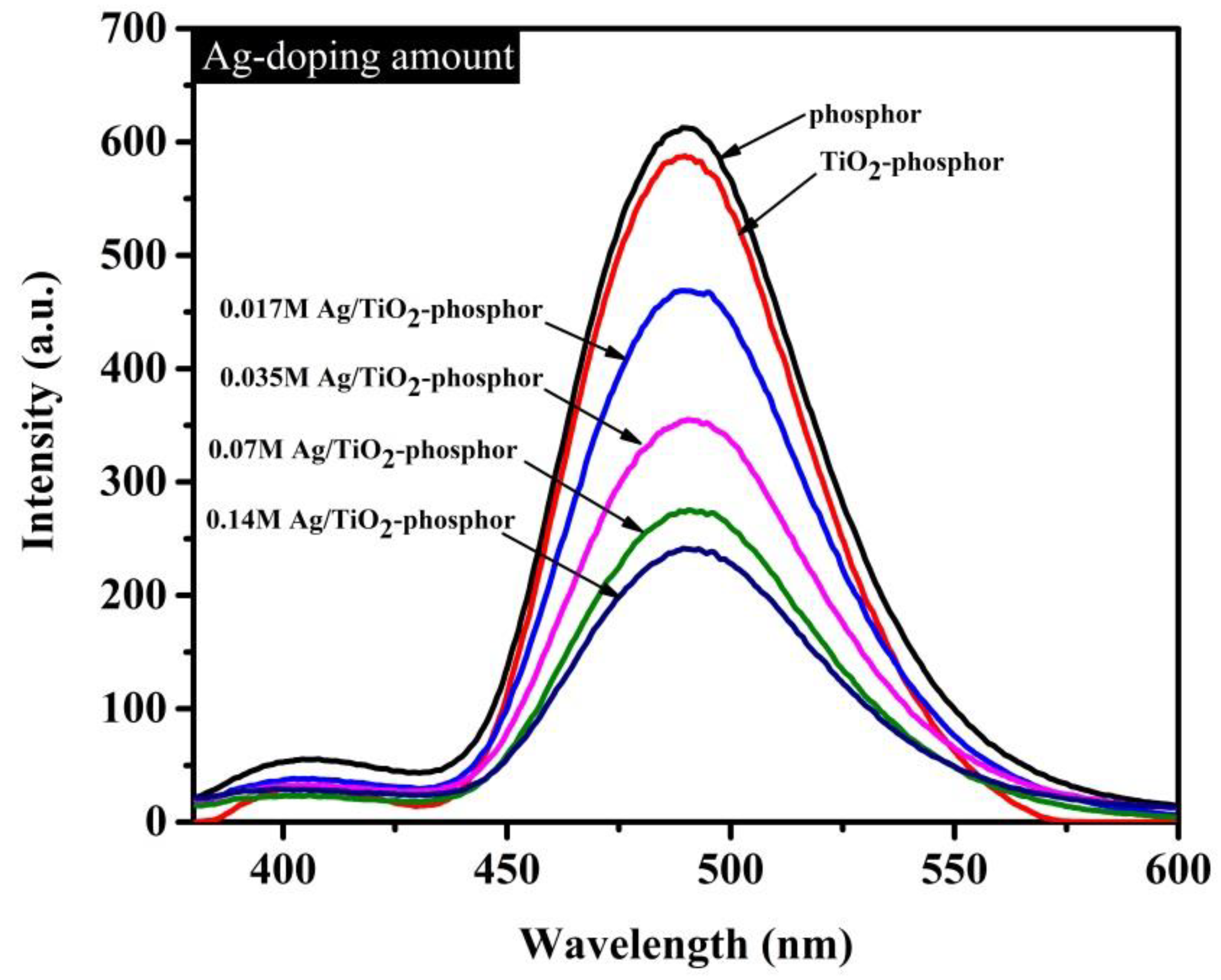
Photoluminescence (PL) spectra of the TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ beads containing varying amounts of Ag-doping in
Figure 5 show that the maximum emission from the phosphor beads peaks at 490 nm. TiO
2 without Ag on Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads exhibits about a 5% decrease in luminescence intensity compared with Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads. The phosphor beads with and without TiO
2 coating were inserted as control samples. The emission peak intensity decreased as the doping amount of Ag increased from 0.017 M to 0.14 M. Phosphor emission centers are localized at the surface, such that only transparent materials effectively emit light. However, TiO
2 and AgO seem to partially absorb and simultaneously hinder the propagation of emitted light from phosphor photons.
The PL spectra of the Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads coated with/without Ag and TiO
2 calcined in (air, N
2 or N
2-H
2) unique atmospheres are shown in
Figure 6. In comparison with other beads, Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor maintains the most extraordinary intensities, followed by the TiO
2-coated phosphor beads. Evidently, Ag-doped beads coated in an N
2 environment show a higher luminescence than ones treated in N
2-H
2 and air atmospheres. As can be observed in SEM images, the coating of phosphor with Ag-sol showed AgO nanoparticle clusters along the phosphor surface. On this account, AgO clusters cause light obstruction from the emitted phosphor photons. Ag calcined in air shows the least peak intensity, undoubtedly owing to possible significant amounts of AgO on the bead surface.
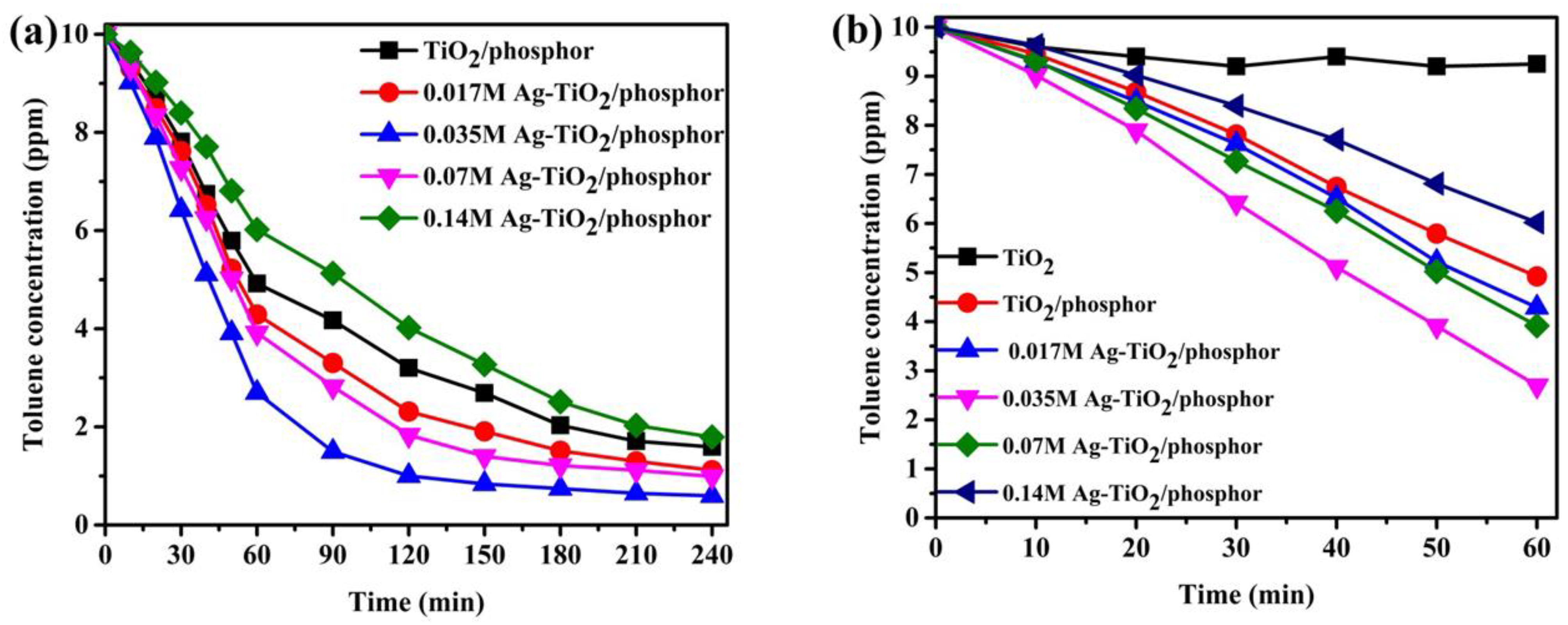
The variation of toluene photodegradation over the Ag-doped TiO
2/phosphor beads under a UV light source is shown in
Figure 7. These beads were calcined in the N
2 atmosphere. The photocatalysts exhibit photocatalytic activity reaching over 80% efficiency in 1 h. Although the 0.035 M doped samples displayed an outstanding photocatalytic performance, the 0.14 M doped TiO
2 phosphor beads exhibited less efficiency as an indication of the undesirable AgO barrier between the TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ interface (enlarged
Figure 7b, extracted from
Figure 7a). The variation of toluene degradation in the presence of commercial TiO
2 (for 1-h cycle) was also inserted in
Figure 7b. Clearly, commercial TiO
2 exhibits 70% efficiency as compared to phosphor/TiO
2 composites with above 80% efficiency in a 1-h cycle. The reason being the Ag-doping and phosphor bead support improves light sensitization.
The photodegradation of toluene with the Ag-doped TiO
2/phosphor beads under a visible light source is shown in
Figure 8. The photoreactions exhibited a degradation of toluene in a 240-min cycle, where the 0.035 M Ag doped sample shows the highest photocatalytic efficiency and the 0.14 M sample is the lowest. In detail, the 0.035 M degrades over 70% while the 0.14 M reaches only 40% efficiency in 120 min (enlarged
Figure 8b, extracted from
Figure 8a). Photodegradation of toluene in TiO
2 commercial powder (
Figure 8b) shows that TiO
2 on its own is not activated by visible light owing to the large band gap of 3.2 eV. However, by coupling with phosphor and with additional Ag-doping, the composites exhibit significant photocatalytic activity. The reason for the fastest photolysis rate in 0.035 M Ag-TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ photocatalyst/phosphor beads is enhanced sensitization by Ag ions causing the overall electron–hole generation and separation that occurs unexpectedly with visible light.
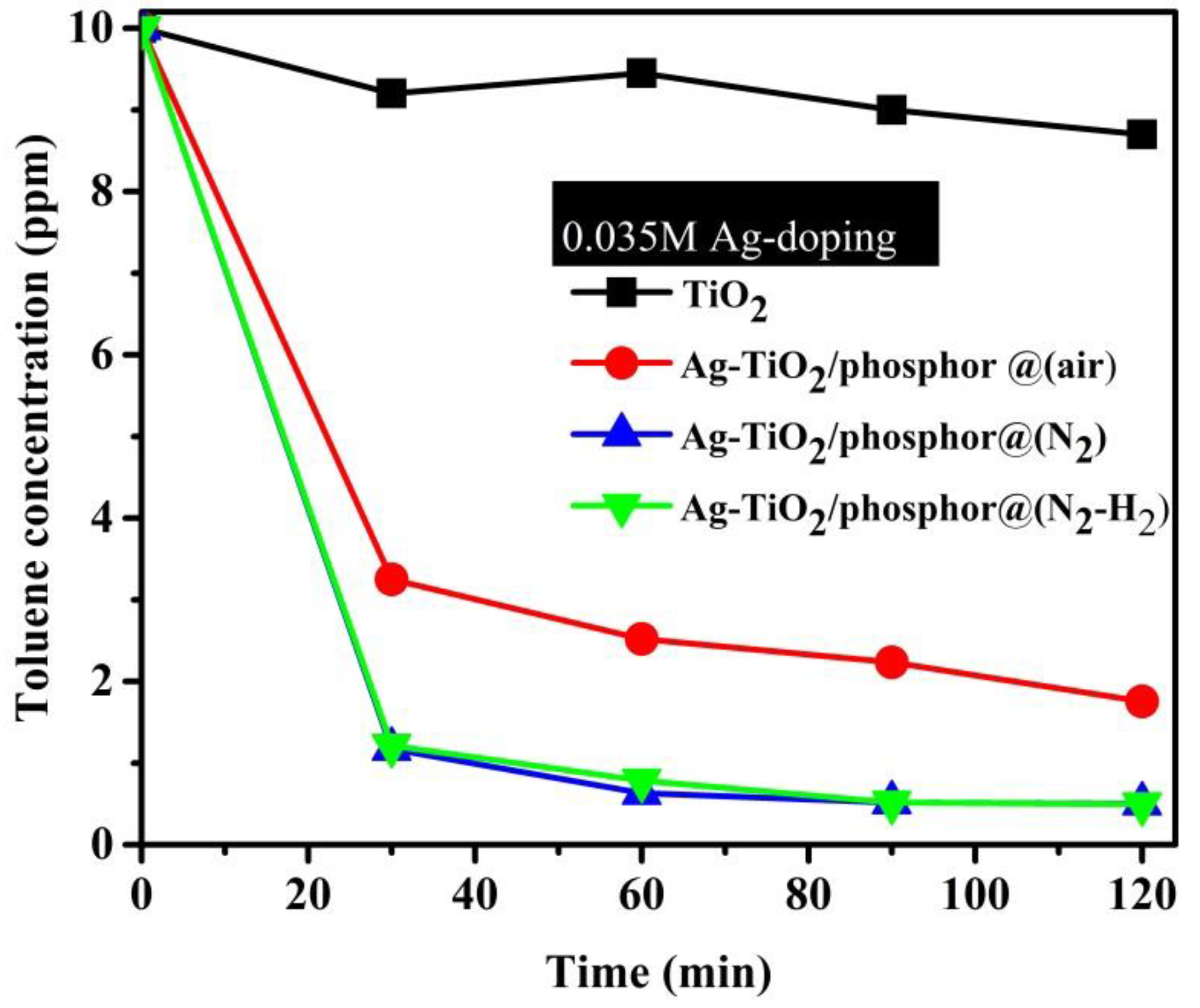
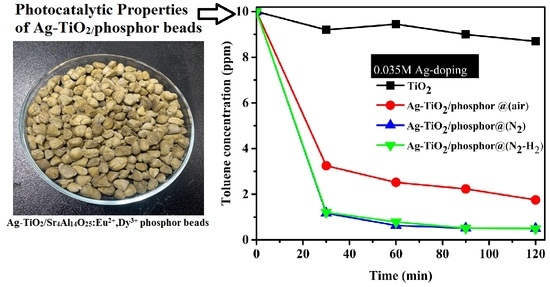
Photodegradation of toluene with the 0.035 M Ag-doped TiO
2/phosphor beads heat treated in air, N
2, and N
2-H
2 as activated by visible light is shown in
Figure 9. The photocatalytic performance in commercial TiO
2 powder was inserted for comparisons. As discussed in
Figure 8b, TiO
2 is not activated by visible light. Thus, only adsorption of the toluene volatile organic compound (VOC) occurs on the commercial TiO
2 nano powders. At the 30-min sampling intervals, samples calcined in N
2 and N
2-H
2 gas exhibited about 90% efficiency while heat treated in air samples maintained about 70% efficiency. The samples heat treated in air exhibited 20% points less efficiency than those in the reducing atmosphere. Consequently, the reducing atmosphere shows improved photocatalytic efficiencies while the oxidizing condition increases AgO oxides, which become barriers to photocatalytic enhancement.
3. Discussion
Sr4Al14O25:Eu2+,Dy3+ phosphor beads synthesized from sodium silicate binders revealed thermal cracks as an effect of heat treatment. This is prevalent with sodium silicate additive because the glass phases transform and cause inter-particle encapsulation. However, owing to different thermal expansions, cracks and voids form within sintered specimens. As observed, the detrimental effects of the cracks and pores were low compressive strength because the cracks and pores act as centers of weakness as fracture points. One advantage of porosity and micro-cracks is the possible active sites formed during incipient wetness impregnation. As observed in depth profiling for 3000 s, the Ag:Ti ratio was observed from 12:0.76 at the surface to 3.16:0.48. Therefore, the cracks and micro-pores were loaded with Ag/TiO2, which resulted in enhanced photocatalytic efficiencies.
The amount of Ag-TiO
2 on the phosphor surface and heat treatment in air, N
2, and N
2-H
2 affect photoluminescence emission. This is a common phenomenon on long-lasting phosphors coated with non-luminescence materials. Nanoparticles on the surface of phosphor block the emitted photons. The photocatalytic efficiencies were enhanced in 0.035 M as optimum Ag concentration, while the efficiencies were lower on 0.14 M. This is a possible indication of excess Ag-O on the phosphor surface, which acts as a barrier to luminescent light. Additionally, the inter-phosphor-TiO
2 junction is increased, which merely renders phosphor inefficient to TiO
2 performance [
23].
The effect of heat treatment condition on photocatalytic efficiencies of 0.035 M Ag (
Figure 9) is elaborated on as the best activity in N
2 and N
2-H
2 over-oxidizing atmospheres. The reducing atmospheres enhance oxygen deficient Ag, which easily migrates into Ti at interstitial sites. While in an oxidizing atmosphere, Ag crystallizes as oxygen-rich oxides that limit the chances of TiO
2 doping. Thus, sensitization of UV/visible light is enhanced because of lower Fermi levels imparted by Ag-doping. Therefore, separation of electron–hole pairs is improved with Ag-doping for more superior photocatalytic efficiencies [
10].
TiO
2 commercial powder showed no photocatalytic variation in visible light owing to its large band gap. While under UV light activation, significant photocatalytic decomposition of toluene in TiO
2 commercial powder was observed since irradiated photons activated the catalyst. Overall, the Ag-doped-TiO
2/phosphor showed better photocatalytic performances than commercial TiO
2 because of an improved light sensitization by phosphor support and the Ag-doping. The results in our research also confirm enhancement of photocatalytic efficiencies after Ag-doping compared to only TiO
2 coating. A similar trend was reported in Ag-doped calcium aluminate phosphor/TiO
2 beads [
23]. The test conditions in the reference were a 75-W mercury lamp UV light and 200-W visible light incandescent lamp over a 410 nm filter. However, the photocatalytic activity of Ag-doped/TiO
2-Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads has been reported for the first time with fast photoactivity completing in 60 to 240 min under UV and visible light, respectively. Therefore, Ag-doped/TiO
2-Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads are possible candidates for alleviating volatile organic compounds.
4. Materials and Methods
4.1. Experimental
Phosphor beads were prepared using the long-lasting phosphorescent Sr
4Al
14O
25:Eu
2+,Dy
3+ (Luminova BG-300M, Nemoto & Co., Ishioka-shi, Ibaraki-ken, Japan) and sodium alginate (C5H7O4·(COOH)n) solution. The alginate process for phosphor beads is described in detail elsewhere [
22]. Ag-doping sol was prepared from silver nitrate (AgNO
3 99.8%, Junsei Chemical Co., Tokyo, Japan). Four batches of 0.017 M, 0.035 M, 0.07 M, and 0.14 M concentration Ag-doping sol were coated batch-wise on Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads. Each AgNO
3 batch was dissolved in a beaker of 38 mL distilled H
2O, 2 mL ethyl alcohol (C
2H
5OH 99.9%, Duksan Pure Chemicals Co., Ansan-si, Kyunggi-do, Korea) and 2 mL NH
4OH (extra pure grade, Duksan Pure Chemicals Co., Ansan-si, Kyunggi-do, Korea) and stirred at 30 °C for 2 h. Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads weighing 40 g were dip-coated in 40 mL of Ag-doping sol and filtered through a funnel. After drying in an oven at 100 °C for 6 h, the beads were placed in a closed crucible for heat treatment. Heat treatment was performed on the 40 g batches in a tube furnace (AJ-MBT6, AHJEON Industrial Co., Namyangju-si, Kyunggi-do, Korea) at 450 °C for 2 h. The heat treatment atmosphere was adjusted per batch by air, N
2, and N
2-H
2.
TiO
2-sol stock solution was prepared from 40 mL titanium tetraisopropoxide (97%, Sigma Aldrich, St. Louis, MO, USA), 200 mL ethyl alcohol (C
2H
5OH 70%, Daejung Chemicals, Siheung-si, Gyeonggi-do, Korea), 4 mL nitric acid (HNO
3 70%, Daejung Chemicals, Siheung-si, Gyeonggi-do, Korea), and 40 mL distilled H
2O. Afterwards, 40 g of Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads were dip-coated in 40 mL of TiO
2 solution, filtered and dried at 100 °C for 6 h. Ag-doped Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads were also coated with TiO
2 using a similar process. TiO
2-coated beads were calcined at 450 °C for 2 h in a box furnace (DSF-7GFS, Daejung Science Co., Incheon, Korea). Images of (a) Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads, (b) TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads, and (c) Ag-TiO
2/Sr
4Al
14O
25:Eu
2+,Dy
3+ phosphor beads are shown in
Figure 10. The 4–6 mm diameter phosphor beads in
Figure 10a are off-whitish while TiO
2 and Ag-doped/TiO
2 samples in (b) and (c) are colored greyish and yellowish-olive, respectively. Thus, Ag coating shows that it imparts a deep olive color which may reduce the light-scattering phenomenon common in whitish TiO
2 surfaces.
4.2. Characterizations
The crystallinity of the 0.035 M Fe-TiO2/phosphor bead was analyzed by XRD with Cu Kα irradiation (Bruker AXS8 Advanced, D8 Discover, GmbH, Karlsruhe, Germany). Surface morphology and element composition were evaluated by SEM (Hitachi S4300, Tokyo, Japan), EDS (JEOL, JSM-6010PLUS, Peabody, MA, USA) and XPS (ThermoFisher Scientific, Nexsa, Waltham, MA, USA). A fluorescence spectrometer (Hitachi F4500, Tokyo, Japan) was used to measure the luminescence characteristics of the synthesized composite materials. The photodecomposition of toluene (C7H8 10.7 μmol/mol), one of the hazardous volatile organic compounds (VOCs) was analyzed by gas chromatography (GC, 7890A, Agilent Technologies Inc., Santa Clara, CA, USA).
The compressive strength of the phosphor beads was measured using a compression testing machine (Instron 3344, Instron Co., Norwood, MA, USA). The maximum compressive strength for each bead was measured using a tip of 7 cm in diameter at a constant crosshead speed of 0.5 mm/min. Two samples of Sr4Al14O25:Eu2+,Dy3+, TiO2/Sr4Al14O25:Eu2+,Dy3+, and Ag-TiO2/Sr4Al14O25:Eu2+,Dy3+ beads were evaluated for compressive strength measurements.
The toluene photodegradation experiment was performed in 1-L Teflon bags containing 40 g of TiO2/Sr4Al14O25:Eu2+,Dy3+ photocatalyst/phosphor beads and 10 ppm toluene under UV and visible light conditions. The 40 g phosphor beads were a mixture consisting of 4 mm and 6 mm diameter at 1:1 ratio, respectively. At intervals of UV (6 W, 2 fluorescent lamps, G6T5 Sankyo Denki, Sankyo Denki Co., Kanagawa, Japan) or 100 W visible light (ST55 L Ilkwang Lamp Co., Daegu, Korea) irradiation with 410 nm filters, 1 mL of toluene was sampled and injected into the GC analyzers. The lights were placed at 15 cm height illuminating the samples throughout the experiments. The light flux was measured by a Digital Lux Meter (Smart Sensor AR823). For the two photocatalytic conditions, one 100 W bulb (visible light) and two fluorescent lamps (UV light) of 6 W each emitted an average of 1200 and 30 lux at 15 cm height, respectively. For comparisons, 1 g of commercial TiO2 powder (Aeroxide TiO2 P25, Evonik, Essen, Germany) was spread on a 9.4 cm diameter quartz petri dish and tested for toluene photodegradation using similar conditions. The photocatalytic tests were conducted without any pH modifiers.