Comparison of Three Catalytic Processes in Degradation of HPAM by tBu-TPyzPzCo
Abstract
1. Introduction
2. Results and Discussions
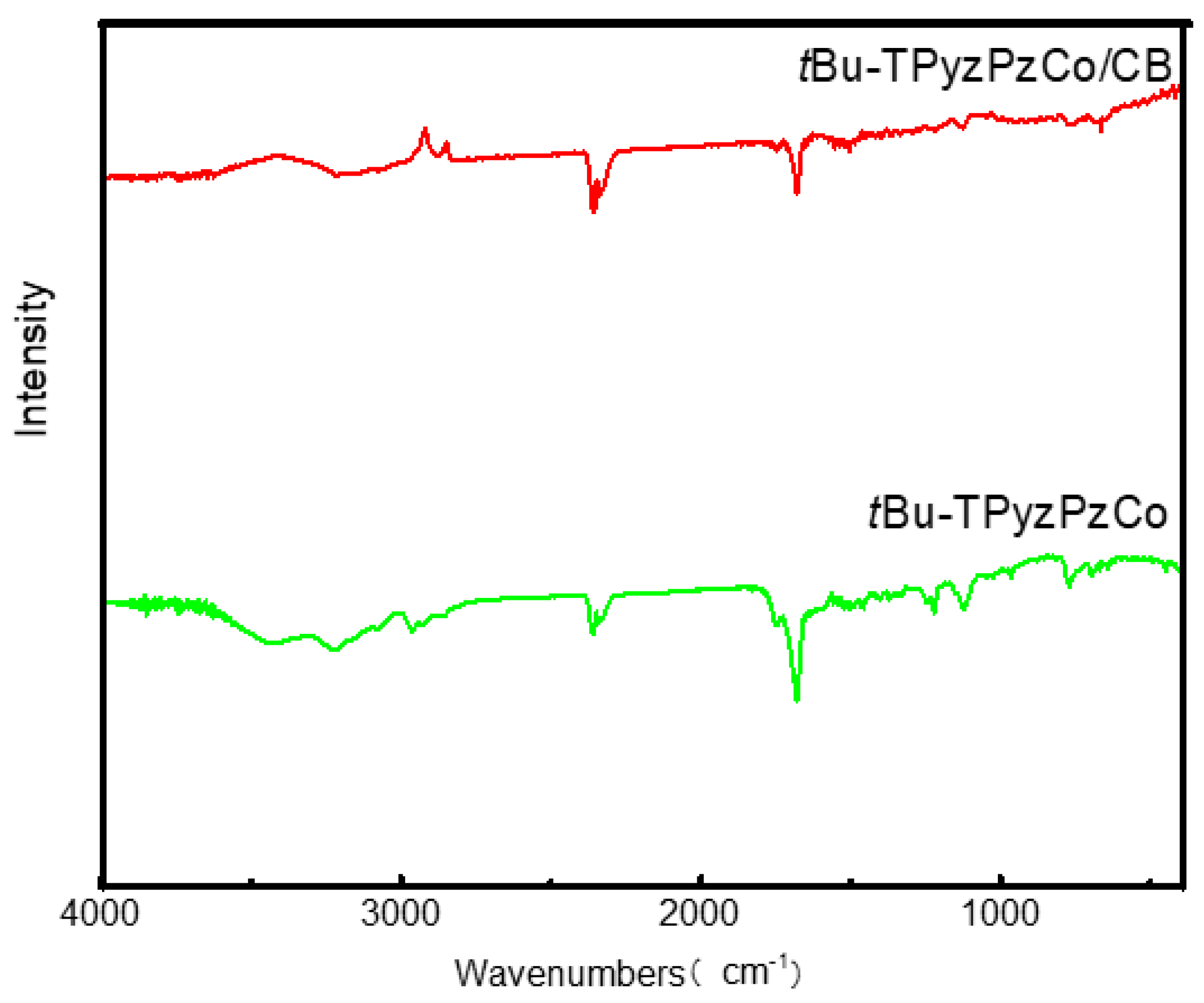
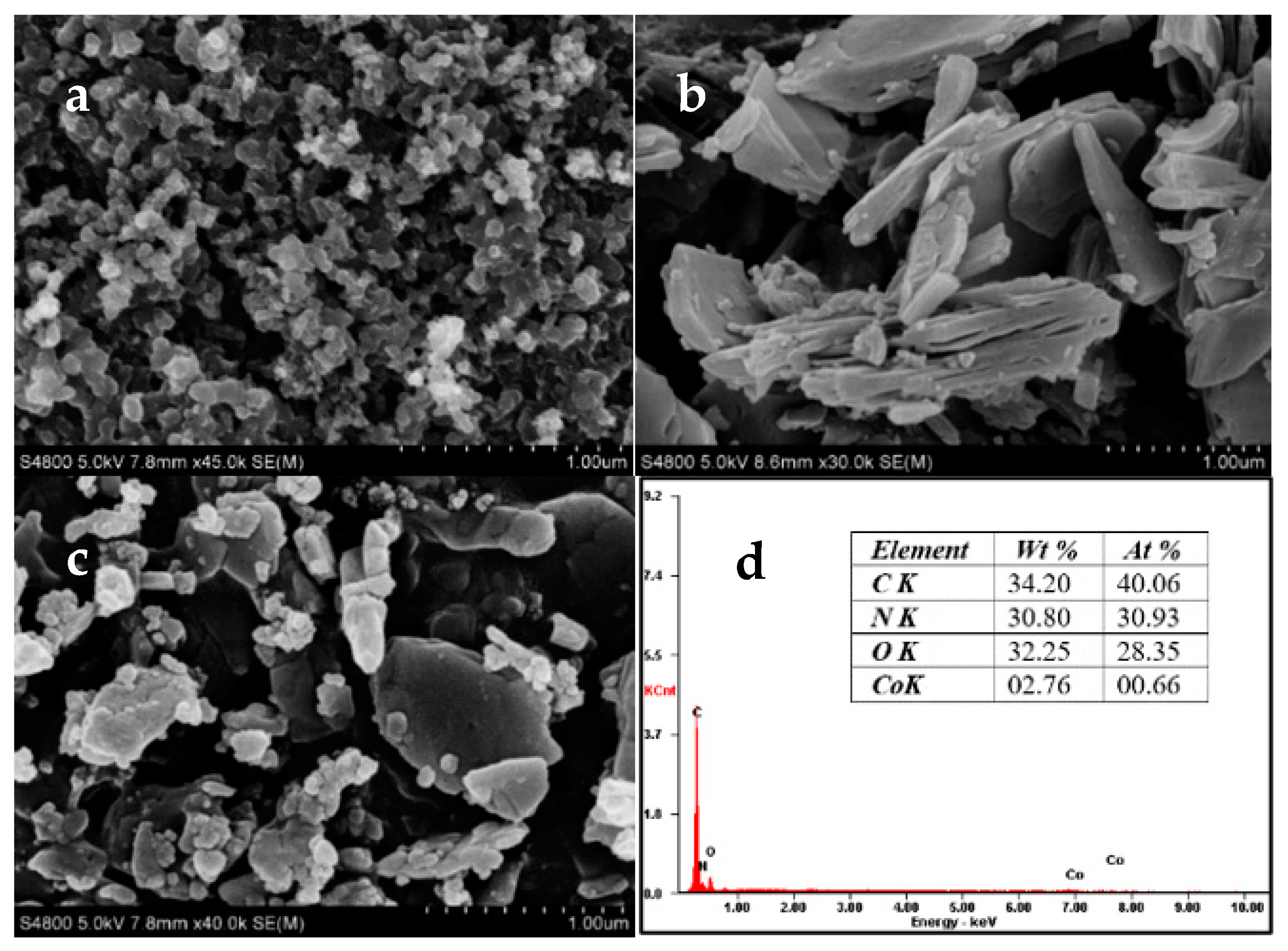
2.1. Catalyst Characterization
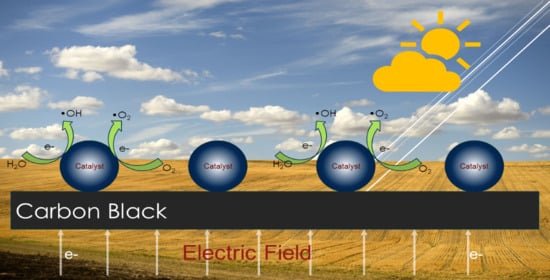
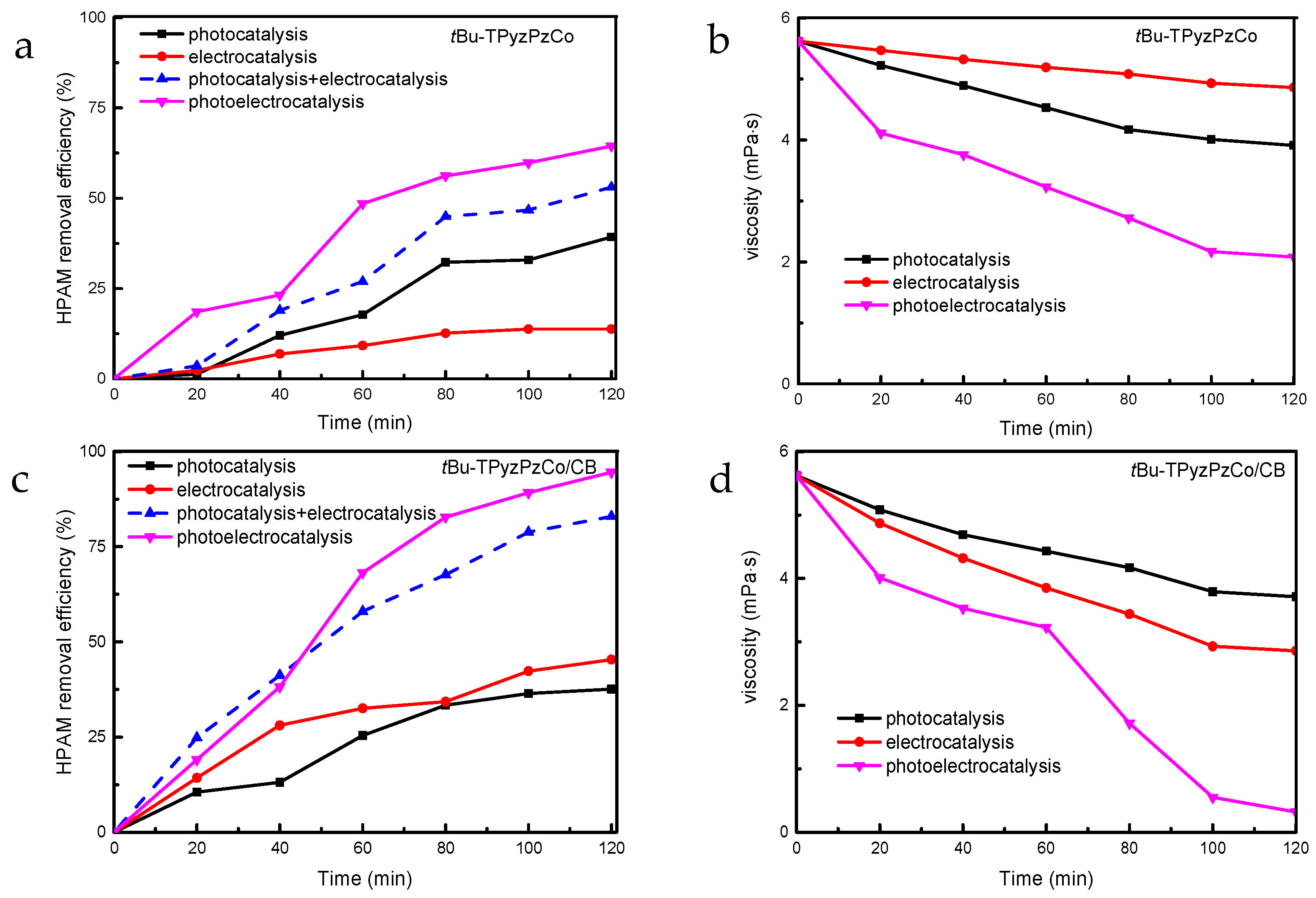
2.2. Photoelectrocatalytic Performance
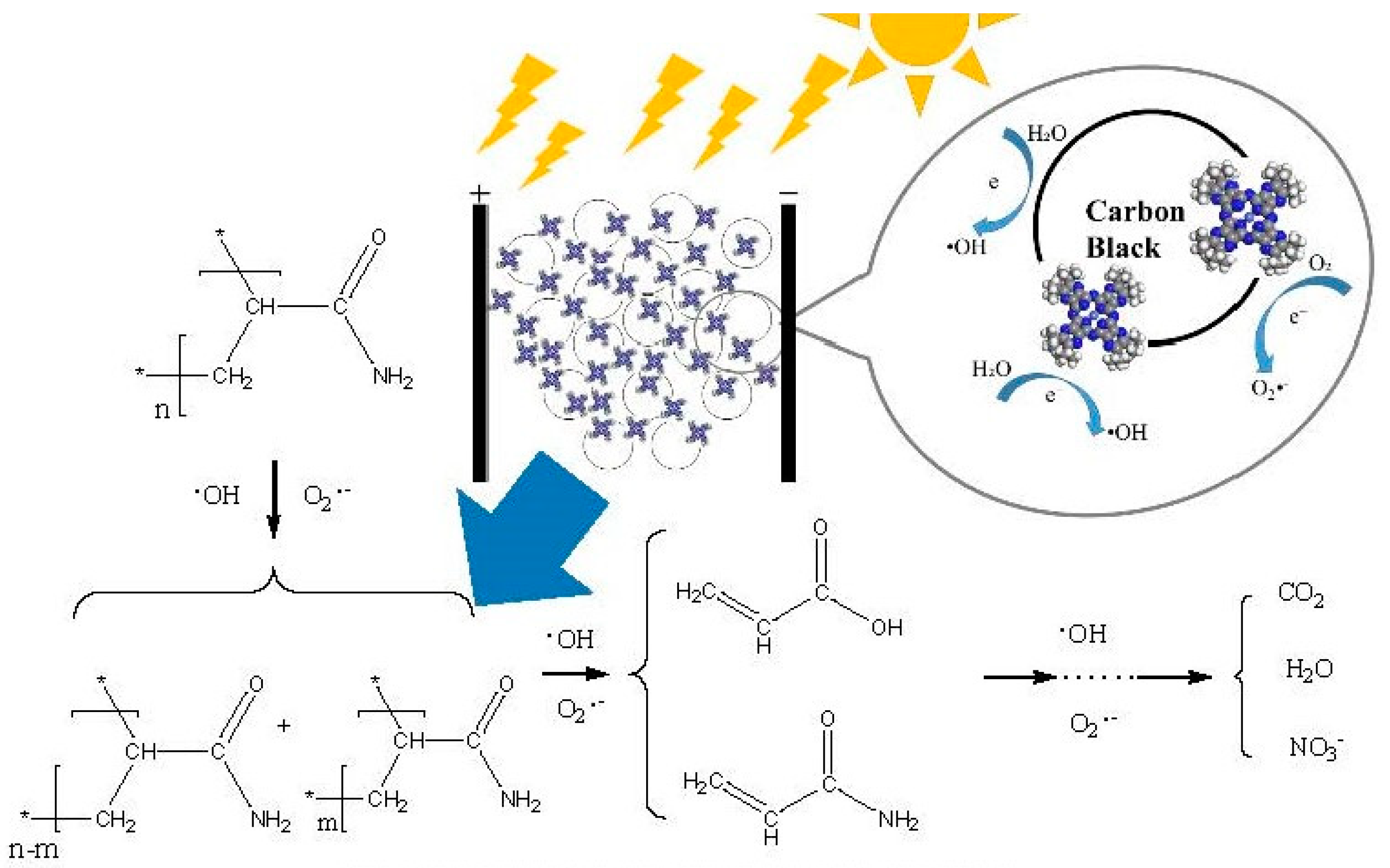
2.3. Synergy Mechanism Analysis
2.4. Energy Consumption Analysis
3. Materials and Methods
3.1. Materials
3.2. Procedure for the Preparation of tBu-TPyzPzCo/CB
3.3. Catalytic Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Zhou, F.; Zhu, Z.; Wu, F. Inactivating marine microorganisms for photoelectrocatalysis by ZnWO4 electrode obtained by surfactant-assisted synthesis. Appl. Surf. Sci. 2019, 467–468, 819–824. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Arotiba, O.A. Towards visible light driven photoelectrocatalysis for water treatment: Application of a FTO/BiVO4/Ag2S heterojunction anode for the removal of emerging pharmaceutical pollutants. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Zhou, G.; Zhao, T.; Wang, O.; Xia, X.; Pan, J.H. Bi2Se3, Bi2Te3 quantum dots-sensitized rutile TiO2 nanorod arrays for enhanced solar photoelectrocatalysis in azo dye degradation. J. Phys. Energy 2020, 3, 014003–014010. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Hong, M.; Chen, J.; Suo, G. Separable and recyclable meso-carbon@TiO2/carbon fiber composites for visible-light photocatalysis and photoelectrocatalysis. Sustain. Mater. Technol. 2019, 21, e00105. [Google Scholar] [CrossRef]
- Jamoussi, B.; Chakroun, R.; Timoumi, A.; Essalah, K. Synthesis and Characterization of New Imidazole Phthalocyanine for Photodegradation of Micro-Organic Pollutants from Sea Water. Catalysts 2020, 10, 906. [Google Scholar] [CrossRef]
- Kusmierek, E. A CeO2 Semiconductor as a Photocatalytic and Photoelectrocatalytic Material for the Remediation of Pollutants in Industrial Wastewater: A Review. Catalysts 2020, 10, 1435. [Google Scholar] [CrossRef]
- Kusmierek, E. Semiconductor Electrode Materials Applied in Photoelectrocatalytic Wastewater Treatment—an Overview. Catalysts 2020, 10, 439. [Google Scholar] [CrossRef]
- Papagiannis, I.; Koutsikou, G.; Frontistis, Z.; Konstantinou, I.; Avgouropoulos, G.; Mantzavinos, D.; Lianos, P. Photoelectrocatalytic vs. Photocatalytic Degradation of Organic Water Born Pollutants. Catalysts 2018, 8, 455. [Google Scholar]
- Franz, S.; Falletta, E.; Arab, H.; Murgolo, S.; Bestetti, M.; Mascolo, G. Degradation of carbamazepine by photo (electro) catalysis on nanostructured TiO2 meshes: Transformation products and reaction pathways. Catalysts 2020, 10, 169. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double perovskites in catalysis, electrocatalysis, and photo (electro) catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Zul, A.; Muhammad, N.; Buchari, B. Photoelectrocatalysis performance of la2O3 doped TiO2/Ti electrode in degradation of rhodamine B organic compound. Int. J. Chemtech Res. 2016, 9, 113–130. [Google Scholar]
- Kopecky, K.; Zimcik, P.; Novakova, V.; Miletin, M.; Musil, Z.; Stribna, J. The synthesis and characterization of metal-free, unsymmetrical azaphthalocyanines with hydroxy groups and their complex formation with pyridine. Dye. Pigment. 2008, 78, 231–238. [Google Scholar] [CrossRef]
- Makhseed, S.; Samuel, J.; Ibrahim, F. Synthesis and characterization of non-aggregating octa-substituted azaphthalocyanines bearing bulky phenoxy substituents. Tetrahedron 2008, 64, 8871–8877. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Zhang, F.; Zhao, F.; Tang, Y.; Song, X.; Yao, G. Dimerization of Metal-free Sulfonated Phthalocyanines in Aqueous Methanol Solution. Acta Phys. Chim. Sin. 1996, 12, 163–167. [Google Scholar]
- Sidorov, A.N.; Kotlyar, I.P. Infrared Spectra of Phthalocyanines. I. The Effect of Crystalline Structure and of the Central Metallic Atom on the Phthalocyanine Molecule in the Solid State. Opt. Spectrosc. 1961, 11, 92. [Google Scholar]
- Feng, Z.; Wenqi, L.; Chuanping, F.; Xu, G. Study on synergetic degradation of phenol by independent electrochemical and photocatalytic processes. Chin. J. Environ. Eng. 2011, 5, 2161–2166. [Google Scholar]
- Ayoubi-Feiz, B.; Mashhadizadeh, M.H.; Sheydaei, M. Degradation of diazinon by new hybrid nanocomposites N-TiO2/Graphene/Au and N-TiO2/Graphene/Ag using visible light photo-electro catalysis and photo-electro catalytic ozonation: Optimization and comparative study by Taguchi method. Sep. Purif. Technol. 2019, 211, 704–714. [Google Scholar] [CrossRef]
- Chun, H.; Yizhong, W.; Hongxiao, T. Kinetics of Photocatalytic Oxidation of Phenol on TiO2. Chin. J. Environ. Sci. 1997, 18, 1–4. [Google Scholar]
- Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochem. Acta 1994, 39, 1857–1862. [Google Scholar] [CrossRef]
- Comninellis, C.; Pulgarin, C. Anodic oxidation of phenol for waste water treatment. J. Appl. Electrochem. 1991, 21, 703–708. [Google Scholar] [CrossRef]
- Rajkumar, D.; Palanivelu, K. Electrochemical treatment of industrial wastewater. J. Hazard. Mater. 2004, 113, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ozer, L.Y.; Garlisi, C.; Oladipo, H.; Pagliaro, M.; Sharief, S.A.; Yusuf, A.; Almheiri, S.; Palmisano, G. Inorganic semiconductors-graphene composites in photo (electro) catalysis: Synthetic strategies, interaction mechanisms and applications. J. Photochem. Photobiol. C Photochem. Rev. 2017, 33, 132–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, W.; An, W.; Liu, L.; Liang, Y.; Zhu, Y. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure. Appl. Catal. B Environ. 2018, 221, 36–46. [Google Scholar] [CrossRef]
- Sanjuan, A.; Aguirre, G.; Alvaro, M.; García, H. 2, 4, 6-Triphenylpyrylium ion encapsulated within Y zeolite as photocatalyst for the degradation of methyl parathion. Water Res. 2000, 34, 320–326. [Google Scholar]
- Sanjuán, A.; Alvaro, M.; Aguirre, G.; García, H.; Scaiano, J. Intrazeolite photochemistry. 21. 2, 4, 6-Triphenylpyrylium encapsulated inside zeolite Y supercages as heterogeneous photocatalyst for the generation of hydroxyl radical. J. Am. Chem. Soc. 1998, 120, 7351–7352. [Google Scholar]
- Sanjuán, A.; Aguirre, G.; Alvaro, M.; García, H. 2, 4, 6-Triphenylpyrylium ion encapsulated in Y zeolite as photocatalyst. A co-operative contribution of the zeolite host to the photodegradation of 4-chlorophenoxyacetic acid using solar light. Appl. Catal. B Environ. 1998, 15, 247–257. [Google Scholar]
- Hoffmann, N. ChemInform Abstract: Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 39, 1052–1103. [Google Scholar]
- Jackson, C.V.; Mickelson, J.K.; Stringer, K.; Rao, P.S.; Lucchesi, B.R. Electrolysis-induced myocardial dysfunction: A novel method for the study of free radical mediated tissue injury. J. Pharmacol. Methods 1986, 15, 305–320. [Google Scholar] [CrossRef]
- Li, D.; Ge, S.; Huang, J.; Gong, J.; Wang, T.; Yan, P.; Li, G.; Ding, L. Photocatalytic chromogenic identification of chlorophenol pollutants by manganese phthalocyanine under sunlight irradiation. Sep. Purif. Technol. 2014, 125, 216–222. [Google Scholar] [CrossRef]
- Bao, M.; Chen, Q.; Li, Y.; Jiang, G. Biodegradation of partially hydrolyzed polyacrylamide by bacteria isolated from production water after polymer flooding in an oil field. J. Hazard. Mater. 2010, 184, 105–110. [Google Scholar] [CrossRef]
- Rong, X.; Qiu, F.; Zhang, C.; Fu, L.; Wang, Y.; Yang, D. Preparation of Ag–AgBr/TiO2–graphene and its visible light photocatalytic activity enhancement for the degradation of polyacrylamide. J. Alloys Compd. 2015, 639, 153–161. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, Z.; Zhao, Y.; Zhang, H.; Feng, Y. Biodegradation of polyacrylamide by bacteria isolated from activated sludge and oil-contaminated soil. J. Hazard. Mater. 2010, 175, 955–959. [Google Scholar]
- Steinebrunner, D.; Schnurpfeil, G.; Wichmann, A.; Wöhrle, D.; Wittstock, A. Synergistic Effect in Zinc Phthalocyanine-Nanoporous Gold Hybrid Materials for Enhanced Photocatalytic Oxidations. Catalysts 2019, 9, 555. [Google Scholar] [CrossRef]
- Krakowiak, R.; Musial, J.; Frankowski, R.; Spychala, M.; Mielcarek, J.; Dobosz, B.; Krzyminiewski, R.; Sikorski, M.; Bendzinska-Berus, W.; Tykarska, E. Phthalocyanine-Grafted Titania Nanoparticles for Photodegradation of Ibuprofen. Catalysts 2020, 10, 1328. [Google Scholar] [CrossRef]
- Yang, S.; Feng, Y.; Gao, D.; Wang, X.; Suo, N.; Yu, Y.; Zhang, S. Electrocatalysis degradation of tetracycline in a three-dimensional aeration electrocatalysis reactor (3D-AER) with a flotation-tailings particle electrode (FPE): Physicochemical properties, influencing factors and the degradation mechanism. J. Hazard. Mater. 2020, 407, 124361. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Lu, Y.; Zhu, X. Degradation of disperse dye wastewater by electrocatalysis and descaling effect by reversing electrodes. J. Environ. Eng. Technol. 2017, 7, 587–593. [Google Scholar]
- Mazierski, P.; Borzyszkowska, A.F.; Wilczewska, P.; Białk-Bielińska, A.; Zaleska-Medynska, A.; Siedlecka, E.M.; Pieczyńska, A. Removal of 5-fluorouracil by solar-driven photoelectrocatalytic oxidation using Ti/TiO2 (NT) photoelectrodes. Water Res. 2019, 157, 610–620. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Wu, G.; Guo, R.; Li, L.; Zhao, C.; Liu, F.; Wang, Y. Photoelectrocatalytic degradation of partially hydrolyzed polyacrylamide using a novel particle electrode. Rsc Adv. 2017, 7, 55496–55503. [Google Scholar] [CrossRef]
- Newman, M.S.; Arkell, A. New Reactions on Decomposition of a Hindered α-Diazoketone1. J. Org. Chem. 1959, 24, 385–387. [Google Scholar]

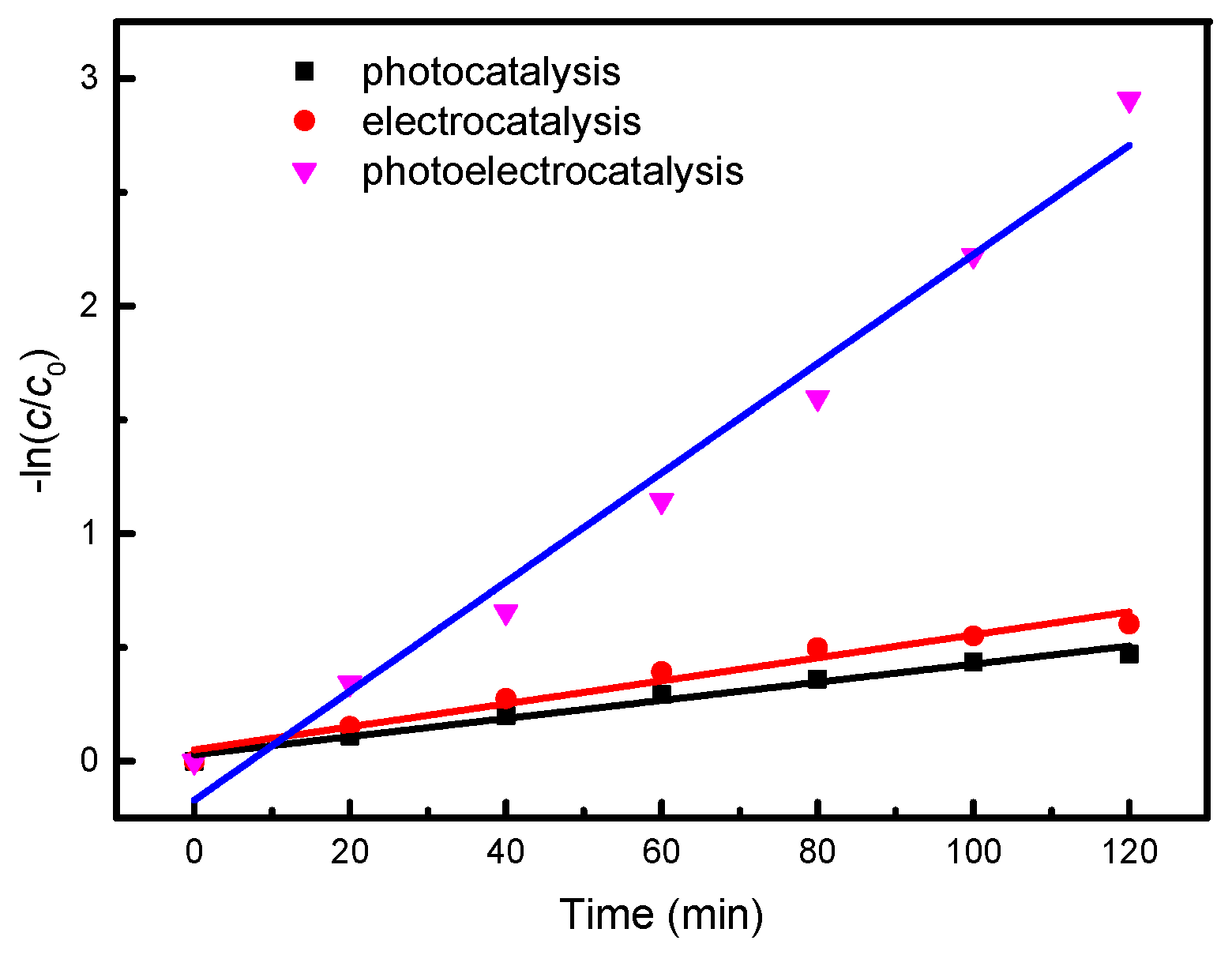

| No. | Cell Voltage (V) | Na2SO4 (mol/L) | Reaction Rate (min−1) | R2 | Process |
|---|---|---|---|---|---|
| 1 | - | 0.1 | 3.98 × 10−3 | 0.9789 | Photocatalytic |
| 2 | 40 | 0.1 | 5.05 × 10−3 | 0.9614 | Electrocatalytic |
| 3 | 40 | 0.1 | 2.4 × 10−2 | 0.9771 | Photoelectrocatalytic |
| Catalyst | Process | Time Required to Remove 40% HPAM | Energy Consumption | Energy Efficiency |
|---|---|---|---|---|
| h | kW·h | mg HPAM/(kW·h) | ||
| Photocatalysis | 4 | 1.32 | 22.73 | |
| CoTPyzPz | Electrocatalysis | 6 | 1.38 | 21.74 |
| Photoelectrocatalysis | 0.67 | 0.36 | 84.48 | |
| Photocatalysis | 4 | 1.32 | 22.73 | |
| CoTPyzPz/CB | Electrocatalysis | 3.5 | 0.81 | 37.27 |
| Photoelectrocatalysis | 0.51 | 0.27 | 110.99 | |
| Photocatalysis | 2.1 | 0.69 | 43.29 | |
| tBu-CoTPyzPz | Electrocatalysis | 6 | 1.38 | 21.74 |
| Photoelectrocatalysis | 0.8 | 0.42 | 70.75 | |
| Photocatalysis | 2.1 | 0.69 | 43.29 | |
| tBu-CoTPyzPz/CB | Electrocatalysis | 1.5 | 0.35 | 86.96 |
| Photoelectrocatalysis | 0.4 | 0.21 | 141.51 |
| Catalyst | Process | Pollutant | Degradation Rate | Reference |
|---|---|---|---|---|
| Imidazole Phthalocyanine | Photocatalysis | 2,3,4,5-Tetrachlorophenol | 80–90% | [5] |
| Zinc Phthalocyanine— Nanoporous Gold | Photocatalysis | 1,3-diphenylisobenzofuran | 92% | [33] |
| Metal phthalocyanines-TiO2 nanoparticles | Photocatalysis | Ibuprofen | 90% | [34] |
| Flotation tailings particle electrode | Electrocatalysis | tetracycline | 75% | [35] |
| cerium doped Ti/nano-TiO2/PbO2 | Electrocatalysis | COD | 96.6% | [36] |
| Ti/TiO2 nanotube | Photoelectrocatalysis | 5-fluorouracil | 100% | [37] |
| Nanopararticulate titania films/FTO | Photoelectrocatalysis | azo dye Basic Blue 41 | 95% | [8] |
| tBu-CoTPyzPz/CB | Photoelectrocatalysis | HPAM | 94.55% | This paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Li, H.; Jiang, X.; Zhao, C.; Zhao, Y. Comparison of Three Catalytic Processes in Degradation of HPAM by tBu-TPyzPzCo. Catalysts 2021, 11, 181. https://doi.org/10.3390/catal11020181
Wang D, Li H, Jiang X, Zhao C, Zhao Y. Comparison of Three Catalytic Processes in Degradation of HPAM by tBu-TPyzPzCo. Catalysts. 2021; 11(2):181. https://doi.org/10.3390/catal11020181
Chicago/Turabian StyleWang, Dejun, Hui Li, Xiren Jiang, Chaocheng Zhao, and Yuhui Zhao. 2021. "Comparison of Three Catalytic Processes in Degradation of HPAM by tBu-TPyzPzCo" Catalysts 11, no. 2: 181. https://doi.org/10.3390/catal11020181
APA StyleWang, D., Li, H., Jiang, X., Zhao, C., & Zhao, Y. (2021). Comparison of Three Catalytic Processes in Degradation of HPAM by tBu-TPyzPzCo. Catalysts, 11(2), 181. https://doi.org/10.3390/catal11020181