Nanobiocatalysts for Biodiesel Synthesis through Transesterification—A Review
Abstract
:1. Introduction
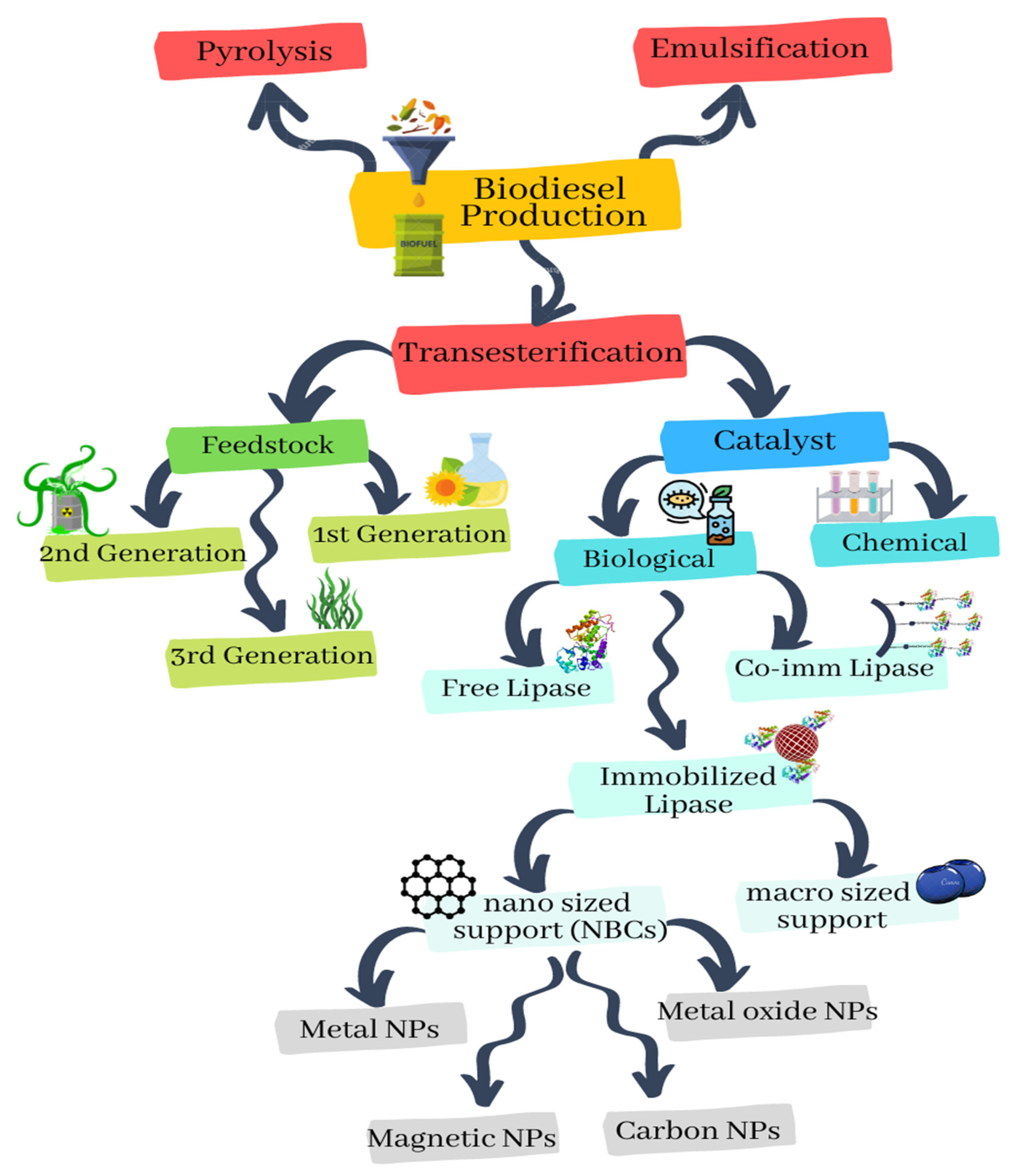
2. Resources of Biodiesel Production
2.1. First-Generation Feedstock Oils
2.2. Second-Generation Feedstock Oils
2.3. Third-Generation Feedstock Oils
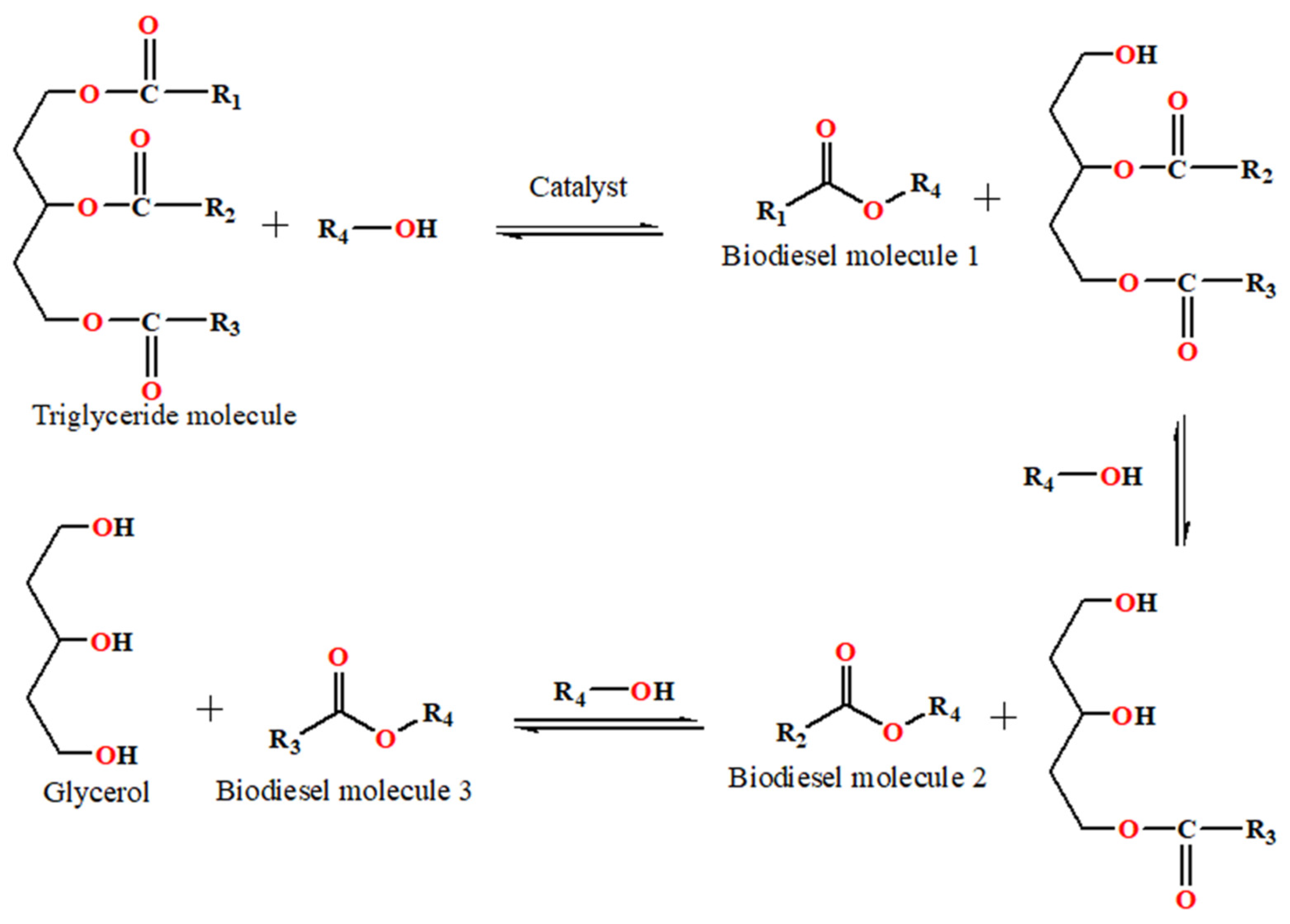
3. Biodiesel Production Strategies
4. Nanobiocatalysts (NBCs)
4.1. Nanocatalytic Component of NBCs
4.1.1. NBCs Containing Magnetic NMs
4.1.2. NBCs Containing Inorganic Nanoscaffolds
4.1.3. NBCs Containing Metal Oxide NMs
4.1.4. NBCs Containing Metal-Organic Frameworks (MOFs)
4.2. Biological Component of NBCs
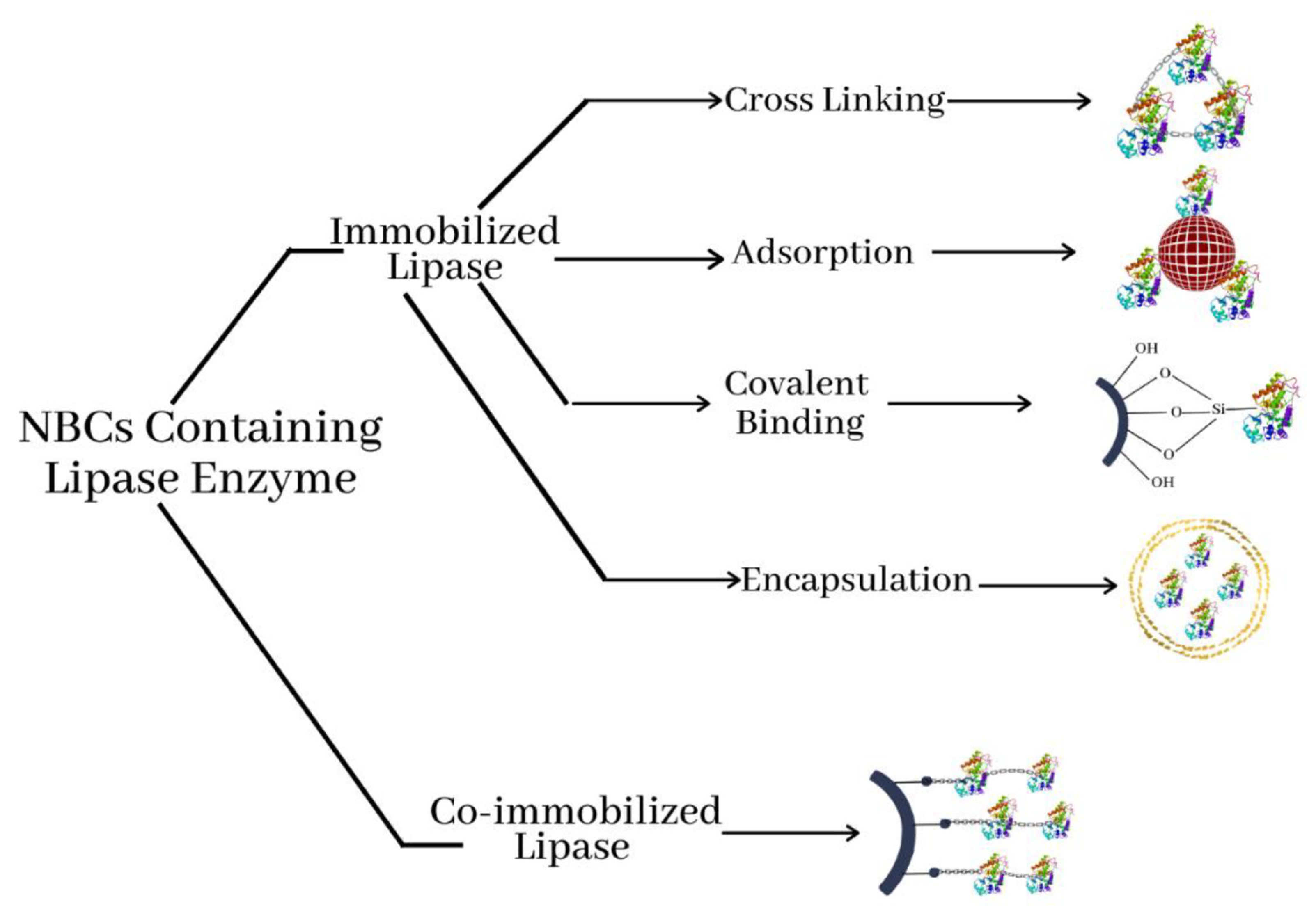
4.2.1. Physical Adsorption
4.2.2. Entrapment
4.2.3. Covalent Bonding
4.2.4. Cross-Linking
5. Fundamentals Associated with the Biodiesel Production via NBCs
5.1. Characterization of NBCs
5.2. Monitoring/Optimization of the Biodiesel Process
5.3. Reuse and Recovery Experiments
5.4. Physicochemical Parameter Study for the Synthesized Biodiesel
6. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NBCs | Nanobiocatalysts |
| NMs | Nanomaterials |
| SOx | Sulfur oxides |
| NOx | Nitrogen oxides |
| Fe3O4 | Magnetite |
| γ-Fe2O3 | Maghemite |
| AP | 3-aminopropyl triethoxysilane |
| GA | Glutaraldehyde |
| GO | Graphene oxide |
| CNTs | Carbon nanotubes |
| SiO2 | Silica |
| Al2O3 | Alumina |
| MWCNTs | Multi-walled Carbon nanotubes |
| H2SO4 | Sulfuric acid |
| HNO3 | Nitric acid |
| SEM | Scanning Electron Microscopy |
| EDX | Energy dispersive X-ray analysis |
| BET | Brunauer-Emmett-Teller analysis |
| FTIR | Fourier Transform Infrared Spectroscopy |
| UV-Vis | Ultraviolet Visible Spectroscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| TEM | Transmission Electron Spectroscopy |
| NRs | Nanorods |
| NPs | Nanoparticles |
| PDA | Polydopamine |
| PAA | Polyacrylic acid |
| PEI | Polyethyleneamine |
| TEOS | Tetraethyl orthosilicate |
| FESEM | Field Emission Scanning Electron Microscope |
| VSM | Vibrating Sample Magnetometer |
| ZPM | Zeta potential Measurements |
| AFM | Atomic Force Microscopy |
| CLEAs | Cross-linked Lipase Enzyme Aggregates |
| [BMIN]BF4 | 1-Butyl-3-methylimidazolium tetrafluoroborate |
| SQUID | Superconducting Quantum Interference Device |
| MWCNTs | Multi-walled carbon nanotubes |
| PMAM | Polyamidoamine |
| CLSM | Confocal laser scanning microscopy |
| MP | 3-mercaptopropyltrimethoxysilane |
| P(GMA-co-MAA) | Poly(glycidyl methacrylate-co-methacrylic acid) |
| GMA | Glycidylmethahacrylate |
| DVB | Divinylbenezene |
| MAA | Methacrylic acid |
| AIBN | 2,2′-azodiisobutyronitrile |
| TGA | Thermogravimetric analysis |
| DTA | Differential thermal analysis |
| CD | Circular dichroism spectroscopy |
| ATR | Attenuated total reflection |
| DLS | Dynamic Light Scattering |
| AFM | Atomic force microscopy |
| MOFs | Metal organic frameworks |
| ZIF | Zeoliticimidazolate framework |
| NFs | Nanoflowers |
| PANI | Polyaniline |
| PPY | Polypyrrole |
| MA | Methyl anthranilate |
| UiO-66 | Universitetet i Oslo |
| PDMS | Polydimethylsiloxane |
| ZrCl4 | Zirconium chloride |
| BDC | 1,4-benzenedicarboxylic acid |
| CVD | Chemical vapour deposition |
| DMF | N,N’-dimethylformamide |
| TiO2 | Titanium dioxide |
| CeO2 | Cerium dioxide |
| L | Lipase |
| FAAE | Fatty acid alkyl esters |
| GC-MS | Gas chromatography-Mass Spectrometry |
| RSM | Response Surface Methodology |
| 1H NMR | Proton nuclear magnetic resonance spectroscopy |
| 13C NMR | Carbon-13 nuclear magnetic spectroscopy |
| TLC | Thin-layer chromatography |
| HPLC | High-performance liquid chromatography |
| NMR | Nuclear magnetic resonance spectroscopy |
| ASTM | American Society for Testing and Material |
| ES | European Standard |
| ESOs | European Standardization Organizations |
| NA | Not applicable |
| ICP-MS | Inductively coupled plasma- Mass spectrometry |
| WDXRF | Wavelength Dispersive X-Ray Fluorescence Spectroscopy |
| MG | Monoglycerides |
| DG | Diglycerides |
| TG | Triglycerides |
| GC | Gas Chromatography |
References
- Armah, P.; Archer, A.; Phillips, G.C. Drivers leading to higher food prices: Biofuels are not the main factor. In Biofuels; Springer: Berlin/Heidelberg, Germany, 2011; pp. 19–36. [Google Scholar]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Demirbas, A. Relationships derived from physical properties of vegetable oil and biodiesel fuels. Fuel 2008, 87, 1743–1748. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. Safflower biodiesel: Improvement of its oxidative stability by using BHA and TBHQ. Energies 2019, 12, 1940. [Google Scholar] [CrossRef] [Green Version]
- Encinar, J.M.; Pardal, A.; Sánchez, N.; Nogales, S. Biodiesel by transesterification of rapeseed oil using ultrasound: A kinetic study of base-catalysed reactions. Energies 2018, 11, 2229. [Google Scholar] [CrossRef] [Green Version]
- Rechnia-Gorący, P.; Malaika, A.; Kozłowski, M. Effective conversion of rapeseed oil to biodiesel fuel in the presence of basic activated carbon catalysts. Catal. Today 2020, 357, 102–112. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Sánchez, N.; Encinar, J.M. Valorization of Cynara Cardunculus L. Oil as the Basis of a Biorefinery for Biodiesel and Biolubricant Production. Energies 2020, 13, 5085. [Google Scholar] [CrossRef]
- Barku, V.A.; Nyarko, H.; Dordunu, P. Studies on the physicochemical characteristics, microbial load and storage stability of oil from Indian almond nut (Terminalia catappa L.). Studies 2012, 8, 9–17. [Google Scholar]
- Ghani, N.A.A.; Channip, A.A.; Chok Hwee Hwa, P.; Ja’afar, F.; Yasin, H.M.; Usman, A. Physicochemical properties, antioxidant capacities, and metal contents of virgin coconut oil produced by wet and dry processes. Food Sci. Nutr. 2018, 6, 1298–1306. [Google Scholar] [CrossRef]
- Thirumarimurugan, M.; Sivakumar, V.; Xavier, A.M.; Prabhakaran, D.; Kannadasan, T. Preparation of biodiesel from sunflower oil by transesterification. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 441. [Google Scholar] [CrossRef]
- Demirbas, A.; Bafail, A.; Ahmad, W.; Sheikh, M. Biodiesel production from non-edible plant oils. Energy Explor. Exploit. 2016, 34, 290–318. [Google Scholar] [CrossRef] [Green Version]
- Kassem, Y.; Çamur, H.; Alassi, E. Biodiesel production from four residential waste frying oils: Proposing blends for improving the physicochemical properties of methyl biodiesel. Energies 2020, 13, 4111. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales, S.; González, J.F. Biodiesel and biolubricant production from different vegetable oils through transesterification. Eng. Rep. 2020, e12190. [Google Scholar] [CrossRef]
- Pinzi, S.; Garcia, I.; Lopez-Gimenez, F.; Luque de Castro, M.; Dorado, G.; Dorado, M. The ideal vegetable oil-based biodiesel composition: A review of social, economical and technical implications. Energy Fuels 2009, 23, 2325–2341. [Google Scholar] [CrossRef]
- Marulanda, V.F.; Anitescu, G.; Tavlarides, L.L. Investigations on supercritical transesterification of chicken fat for biodiesel production from low-cost lipid feedstocks. J. Supercrit. Fluids 2010, 54, 53–60. [Google Scholar] [CrossRef]
- Kwon, E.E.; Jeon, E.-C.; Yi, H.; Kim, S. Transforming duck tallow into biodiesel via noncatalytic transesterification. Appl. Energy 2014, 116, 20–25. [Google Scholar] [CrossRef]
- Teixeira, L.S.; Assis, J.C.; Mendonça, D.R.; Santos, I.T.; Guimarães, P.R.; Pontes, L.A.; Teixeira, J.S. Comparison between conventional and ultrasonic preparation of beef tallow biodiesel. Fuel Process. Technol. 2009, 90, 1164–1166. [Google Scholar] [CrossRef]
- Avhad, M.; Marchetti, J. A review on recent advancement in catalytic materials for biodiesel production. Renew. Sustain. Energy Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Akram, S.; Mumtaz, M.W.; Danish, M.; Mukhtar, H.; Irfan, A.; Raza, S.A.; Wang, Z.; Arshad, M. Impact of cerium oxide and cerium composite oxide as nano additives on the gaseous exhaust emission profile of waste cooking oil based biodiesel at full engine load conditions. Renew. Energy 2019, 143, 898–905. [Google Scholar] [CrossRef]
- Touqeer, T.; Mumtaz, M.W.; Mukhtar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Fe3O4-PDA-Lipase as surface functionalized nano biocatalyst for the production of biodiesel using waste cooking oil as feedstock: Characterization and process optimization. Energies 2020, 13, 177. [Google Scholar] [CrossRef] [Green Version]
- Hossain, N.; Zaini, J.; Mahlia, T.; Azad, A.K. Elemental, morphological and thermal analysis of mixed microalgae species from drain water. Renew. Energy 2019, 131, 617–624. [Google Scholar] [CrossRef]
- Muhammad, G.; Alam, M.A.; Mofijur, M.; Jahirul, M.; Lv, Y.; Xiong, W.; Ong, H.C.; Xu, J. Modern developmental aspects in the field of economical harvesting and biodiesel production from microalgae biomass. Renew. Sustain. Energy Rev. 2020, 135, 110209. [Google Scholar] [CrossRef]
- Leong, W.-H.; Lim, J.-W.; Lam, M.-K.; Uemura, Y.; Ho, Y.-C. Third generation biofuels: A nutritional perspective in enhancing microbial lipid production. Renew. Sustain. Energy Rev. 2018, 91, 950–961. [Google Scholar] [CrossRef]
- Akubude, V.; Nwaigwe, K.; Dintwa, E. Production of biodiesel from microalgae via nanocatalyzed transesterification process: A review. Mater. Sci. Energy Technol. 2019, 2, 216–225. [Google Scholar] [CrossRef]
- Nasreen, S.; Nafees, M.; Qureshi, L.A.; Asad, M.S.; Sadiq, A.; Ali, S.D. Review of catalytic transesterification methods for biodiesel production. Biofuels State Dev. 2018, 93–119. [Google Scholar]
- Mofijur, M.; Masjuki, H.; Kalam, M.; Atabani, A. Evaluation of biodiesel blending, engine performance and emissions characteristics of Jatropha curcas methyl ester: Malaysian perspective. Energy 2013, 55, 879–887. [Google Scholar] [CrossRef]
- Kumar, D.; Das, T.; Giri, B.S.; Verma, B. Preparation and characterization of novel hybrid bio-support material immobilized from Pseudomonas cepacia lipase and its application to enhance biodiesel production. Renew. Energy 2020, 147, 11–24. [Google Scholar] [CrossRef]
- Pollardo, A.A.; Lee, H.-s.; Lee, D.; Kim, S.; Kim, J. Solvent effect on the enzymatic production of biodiesel from waste animal fat. J. Clean. Prod. 2018, 185, 382–388. [Google Scholar] [CrossRef]
- Ferreira, L.F.P.; de Oliveira, T.M.; Toma, S.H.; Toyama, M.M.; Araki, K.; Avanzi, L.H. Superparamagnetic iron oxide nanoparticles (SPIONs) conjugated with lipase Candida antarctica A for biodiesel synthesis. Rsc Adv. 2020, 10, 38490–38496. [Google Scholar] [CrossRef]
- Banerjee, S.; Rout, S.; Banerjee, S.; Atta, A.; Das, D. Fe2O3 nanocatalyst aided transesterification for biodiesel production from lipid-intact wet microalgal biomass: A biorefinery approach. Energy Convers. Manag. 2019, 195, 844–853. [Google Scholar] [CrossRef]
- Fotiadou, R.; Patila, M.; Hammami, M.A.; Enotiadis, A.; Moschovas, D.; Tsirka, K.; Spyrou, K.; Giannelis, E.P.; Avgeropoulos, A.; Paipetis, A. Development of effective lipase-hybrid nanoflowers enriched with carbon and magnetic nanomaterials for biocatalytic transformations. Nanomaterials 2019, 9, 808. [Google Scholar] [CrossRef] [Green Version]
- Shaker, M.; Elhamifar, D. Sulfonic acid supported on magnetic methylene-based organosilica as an efficient and recyclable nanocatalyst for biodiesel production via esterification. Front. Energy Res. 2020, 8, 78. [Google Scholar] [CrossRef]
- Costa, V.; de Souza, M.; Fechine, P.; Macedo, A.; Gonçalves, L. Nanobiocatalytic systems based on lipase-Fe3O4 and conventional systems for isoniazid synthesis: A comparative study. Braz. J. Chem. Eng. 2016, 33, 661–673. [Google Scholar] [CrossRef]
- Badoei-dalfard, A.; Karami, Z.; Malekabadi, S. Construction of CLEAs-lipase on magnetic graphene oxide nanocomposite: An efficient nanobiocatalyst for biodiesel production. Bioresour. Technol. 2019, 278, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fan, F.; Wang, Y.; Feng, W.; Ji, P. Enzyme immobilization on carboxyl-functionalized graphene oxide for catalysis in organic solvent. Ind. Eng. Chem. Res. 2013, 52, 6343–6348. [Google Scholar] [CrossRef]
- Xie, W.; Huang, M. Immobilization of Candida rugosa lipase onto graphene oxide Fe3O4 nanocomposite: Characterization and application for biodiesel production. Energy Convers. Manag. 2018, 159, 42–53. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, G.; Su, F.; Li, K.; Xu, L.; Han, X.; Yan, Y. Lipase oriented-immobilized on dendrimer-coated magnetic multi-walled carbon nanotubes toward catalyzing biodiesel production from waste vegetable oil. Fuel 2016, 178, 172–178. [Google Scholar] [CrossRef]
- Mohamad, N.; Buang, N.A.; Mahat, N.A.; Jamalis, J.; Huyop, F.; Aboul-Enein, H.Y.; Wahab, R.A. Simple adsorption of Candida rugosa lipase onto multi-walled carbon nanotubes for sustainable production of the flavor ester geranyl propionate. J. Ind. Eng. Chem. 2015, 32, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Gardy, J.; Osatiashtiani, A.; Céspedes, O.; Hassanpour, A.; Lai, X.; Lee, A.F.; Wilson, K.; Rehan, M. A magnetically separable SO4/Fe-Al-TiO2 solid acid catalyst for biodiesel production from waste cooking oil. Appl. Catal. B Environ. 2018, 234, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Gao, X.; Yang, N.; Tantai, X.; Xiao, X.; Jiang, B.; Zhang, L. Morphology-controlled synthesis of three-dimensional hierarchical flowerlike Mg–Al layered double hydroxides with enhanced catalytic activity for transesterification. Ind. Eng. Chem. Res. 2019, 58, 7937–7947. [Google Scholar] [CrossRef]
- Fathi, Z.; Doustkhah, E.; Ebrahimipour, G.; Darvishi, F. Noncovalent immobilization of Yarrowia lipolytica lipase on dendritic-like amino acid-functionalized silica nanoparticles. Biomolecules 2019, 9, 502. [Google Scholar] [CrossRef] [Green Version]
- Ashjari, M.; Garmroodi, M.; Asl, F.A.; Emampour, M.; Yousefi, M.; Lish, M.P.; Habibi, Z.; Mohammadi, M. Application of multi-component reaction for covalent immobilization of two lipases on aldehyde-functionalized magnetic nanoparticles; production of biodiesel from waste cooking oil. Process Biochem. 2020, 90, 156–167. [Google Scholar] [CrossRef]
- Asmat, S.; Husain, Q. Exquisite stability and catalytic performance of immobilized lipase on novel fabricated nanocellulose fused polypyrrole/graphene oxide nanocomposite: Characterization and application. Int. J. Biol. Macromol. 2018, 117, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Celik, C.; Tasdemir, D.; Demirbas, A.; Katı, A.; Gul, O.T.; Cimen, B.; Ocsoy, I. Formation of functional nanobiocatalysts with a novel and encouraging immobilization approach and their versatile bioanalytical applications. Rsc Adv. 2018, 8, 25298–25303. [Google Scholar] [CrossRef] [Green Version]
- Rathour, R.K.; Bhatia, R.K.; Rana, D.S.; Bhatt, A.K.; Thakur, N. Fabrication of thermostable and reusable nanobiocatalyst for dye decolourization by immobilization of lignin peroxidase on graphene oxide functionalized MnFe2O4 superparamagnetic nanoparticles. Bioresour. Technol. 2020, 317, 124020. [Google Scholar]
- Fatima, A.; Mumtaz, M.W.; Mukhtar, H.; Akram, S.; Touqeer, T.; Rashid, U.; Ul Mustafa, M.R.; Nehdi, I.A.; Saiman, M.I. Synthesis of lipase-immobilized CeO2 nanorods as heterogeneous nano-biocatalyst for optimized biodiesel production from Eruca sativa seed oil. Catalysts 2020, 10, 231. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef]
- Rafiei, S.; Tangestaninejad, S.; Horcajada, P.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I.; Kardanpour, R.; Zadehahmadi, F. Efficient biodiesel production using a lipase@ ZIF-67 nanobioreactor. Chem. Eng. J. 2018, 334, 1233–1241. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, L.; Liu, D.; Du, W. Rationally designing hydrophobic UiO-66 support for the enhanced enzymatic performance of immobilized lipase. Green Chem. 2018, 20, 4500–4506. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Bai, S.; Shao, X.; Jiang, L.; Li, Q. Immobilized lipase in bio-based metal-organic frameworks constructed by biomimetic mineralization: A sustainable biocatalyst for biodiesel synthesis. Colloids Surf. B Biointerfaces 2020, 188, 110812. [Google Scholar] [CrossRef]
- Baskar, G.; Aiswarya, R. Lipase functionalized magnetic nanobiocatalyst for transesterification of waste cooking oil into biodiesel. Inter. J. Modern Sci. Technol. 2019, 4, 260–268. [Google Scholar]
- Nematian, T.; Salehi, Z.; Shakeri, A. Conversion of bio-oil extracted from Chlorella vulgaris micro algae to biodiesel via modified superparamagnetic nano-biocatalyst. Renew. Energy 2020, 146, 1796–1804. [Google Scholar] [CrossRef]
- Badoei-dalfard, A.; Malekabadi, S.; Karami, Z.; Sargazi, G. Magnetic cross-linked enzyme aggregates of Km12 lipase: A stable nanobiocatalyst for biodiesel synthesis from waste cooking oil. Renew. Energy 2019, 141, 874–882. [Google Scholar] [CrossRef]
- Miao, C.; Yang, L.; Wang, Z.; Luo, W.; Li, H.; Lv, P.; Yuan, Z. Lipase immobilization on amino-silane modified superparamagnetic Fe3O4 nanoparticles as biocatalyst for biodiesel production. Fuel 2018, 224, 774–782. [Google Scholar] [CrossRef]
- Xie, W.; Huang, M. Fabrication of immobilized Candida rugosa lipase on magnetic Fe3O4-poly (glycidyl methacrylate-co-methacrylic acid) composite as an efficient and recyclable biocatalyst for enzymatic production of biodiesel. Renew. Energy 2020, 158, 474–486. [Google Scholar] [CrossRef]
- Andrade, M.F.; Parussulo, A.L.; Netto, C.G.; Andrade, L.H.; Toma, H.E. Lipase immobilized on polydopamine-coated magnetite nanoparticles for biodiesel production from soybean oil. Biofuel Res. J. 2016, 3, 403. [Google Scholar] [CrossRef]
- Picó, E.A.; López, C.; Cruz-Izquierdo, Á.; Munarriz, M.; Iruretagoyena, F.J.; Serra, J.L.; Llama, M.J. Easy reuse of magnetic cross-linked enzyme aggregates of lipase B from Candida antarctica to obtain biodiesel from Chlorella vulgaris lipids. J. Biosci. Bioeng. 2018, 126, 451–457. [Google Scholar] [CrossRef]
- Asmat, S.; Husain, Q.; Khan, M.S. A polypyrrole–methyl anthranilate functionalized worm-like titanium dioxide nanocomposite as an innovative tool for immobilization of lipase: Preparation, activity, stability and molecular docking investigations. New J. Chem. 2018, 42, 91–102. [Google Scholar] [CrossRef]
- Esmaeilnejad Ahranjani, P.; Kazemeini, M.; Arpanaei, A. Green biodiesel production from various plant oils using nanobiocatalysts under different conditions. Bioenerg. Res. 2020, 13, 552–562. [Google Scholar] [CrossRef]
- Nematian, T.; Shakeri, A.; Salehi, Z.; Saboury, A.A. Lipase immobilized on functionalized superparamagnetic few-layer graphene oxide as an efficient nanobiocatalyst for biodiesel production from Chlorella vulgaris bio-oil. Biotechnol. Biofuels 2020, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Su, F.; Li, K.; Ke, C.; Yan, Y. Carbon nanotube filled with magnetic iron oxide and modified with polyamidoamine dendrimers for immobilizing lipase toward application in biodiesel production. Sci. Rep. 2017, 7, 45643. [Google Scholar] [CrossRef] [Green Version]
- Thangaraj, B.; Jia, Z.; Dai, L.; Liu, D.; Du, W. Lipase NS81006 immobilized on functionalized ferric-silica magnetic nanoparticles for biodiesel production. Biofuels 2020, 11, 811–819. [Google Scholar]
- Heidarizadeh, M.; Doustkhah, E.; Rostamnia, S.; Rezaei, P.F.; Harzevili, F.D.; Zeynizadeh, B. Dithiocarbamate to modify magnetic graphene oxide nanocomposite (Fe3O4-GO): A new strategy for covalent enzyme (lipase) immobilization to fabrication a new nanobiocatalyst for enzymatic hydrolysis of PNPD. Int. J. Biol. Macromol. 2017, 101, 696–702. [Google Scholar] [PubMed]
- Asmat, S.; Anwer, A.H.; Husain, Q. Immobilization of lipase onto novel constructed polydopamine grafted multiwalled carbon nanotube impregnated with magnetic cobalt and its application in synthesis of fruit flavours. Int. J. Biol. Macromol. 2019, 140, 484–495. [Google Scholar] [PubMed]
- Asmat, S.; Husain, Q.; Azam, A. Lipase immobilization on facile synthesized polyaniline-coated silver-functionalized graphene oxide nanocomposites as novel biocatalysts: Stability and activity insights. RSC Adv. 2017, 7, 5019–5029. [Google Scholar]
- Adnan, M.; Li, K.; Xu, L.; Yan, Y. X-shaped ZIF-8 for immobilization rhizomucor miehei lipase via encapsulation and its application toward biodiesel production. Catalysts 2018, 8, 96. [Google Scholar]
- Jiang, W.; Wang, X.; Yang, J.; Han, H.; Li, Q.; Tang, J. Lipase-inorganic hybrid nanoflower constructed through biomimetic mineralization: A new support for biodiesel synthesis. J. Colloid Interface Sci. 2018, 514, 102–107. [Google Scholar]
- Al-Qodah, Z.; Al-Shannag, M.; Al-Busoul, M.; Penchev, I.; Orfali, W. Immobilized enzymes bioreactors utilizing a magnetic field: A review. Biochem. Eng. J. 2017, 121, 94–106. [Google Scholar]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar]
- Shuai, W.; Das, R.K.; Naghdi, M.; Brar, S.K.; Verma, M. A review on the important aspects of lipase immobilization on nanomaterials. Biotechnol. Appl. Biochem. 2017, 64, 496–508. [Google Scholar]
- Bilal, M.; Asgher, M.; Cheng, H.; Yan, Y.; Iqbal, H.M. Multi-point enzyme immobilization, surface chemistry, and novel platforms: A paradigm shift in biocatalyst design. Crit. Rev. Biotechnol. 2019, 39, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.R.; Baek, K.-H. Lipase immobilization with support materials, preparation techniques, and applications: Present and future aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Lee, I.; Kwon, S.-J.; Wee, Y.; Kwon, K.Y.; Jeon, C.; An, H.J.; Jung, H.-T.; Ha, S.; Dordick, J.S. Fabrication of enzyme-based coatings on intact multi-walled carbon nanotubes as highly effective electrodes in biofuel cells. Sci. Rep. 2017, 7, 40202. [Google Scholar] [CrossRef] [PubMed]
- Bourkaib, M.C.; Guiavarc’h, Y.; Chevalot, I.; Delaunay, S.; Gleize, J.; Ghanbaja, J.; Valsaque, F.; Berrada, N.; Desforges, A.; Vigolo, B. Non-covalent and covalent immobilization of Candida antarctica lipase B on chemically modified multiwalled carbon nanotubes for a green acylation process in supercritical CO2. Catal. Today 2020, 348, 26–36. [Google Scholar] [CrossRef]
- Piligaev, A.; Sorokina, K.; Samoylova, Y.; Parmon, V. Lipid production by microalga Micractinium sp. IC-76 in a flat panel photobioreactor and its transesterification with cross-linked enzyme aggregates of Burkholderia cepacia lipase. Energy Convers. Manag. 2018, 156, 1–9. [Google Scholar] [CrossRef]
- Khan, N.; Maseet, M.; Basir, S.F. Synthesis and characterization of biodiesel from waste cooking oil by lipase immobilized on genipin cross-linked chitosan beads: A green approach. Int. J. Green Energy 2020, 17, 84–93. [Google Scholar] [CrossRef]
- Wang, C.; Han, H.; Jiang, W.; Ding, X.; Li, Q.; Wang, Y. Immobilization of thermostable lipase QLM on core-shell structured polydopamine-coated Fe3O4 nanoparticles. Catalysts 2017, 7, 49. [Google Scholar] [CrossRef]
- Ren, Y.; Rivera, J.G.; He, L.; Kulkarni, H.; Lee, D.-K.; Messersmith, P.B. Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotechnol. 2011, 11, 63. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, K.; He, Y.; Wang, Y.; Han, X.; Yan, Y. Enhanced performance of lipase immobilized onto Co2+-chelated magnetic nanoparticles and its application in biodiesel production. Fuel 2019, 255, 115794. [Google Scholar] [CrossRef]
- Gao, J.; Yin, L.; Feng, K.; Zhou, L.; Ma, L.; He, Y.; Wang, L.; Jiang, Y. Lipase immobilization through the combination of bioimprinting and cross-linked protein-coated microcrystal technology for biodiesel production. Ind. Eng. Chem. Res. 2016, 55, 11037–11043. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M. Highly active and stable Fe3O4/Au nanoparticles supporting lipase catalyst for biodiesel production from waste tomato. Appl. Surf. Sci. 2019, 474, 135–146. [Google Scholar] [CrossRef]
- Baskar, G.; Selvakumari, I.A.E.; Aiswarya, R. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, T.; Kahrizi, D.; Feyzi, M.; Ahmadvandi, H.R.; Mostafaei, M. Catalytic performance of MgO/Fe2O3-SiO2 core-shell magnetic nanocatalyst for biodiesel production of Camelina sativa seed oil: Optimization by RSM-CCD method. Ind. Crop. Prod. 2021, 159, 113065. [Google Scholar] [CrossRef]
- Bencze, L.C.; Bartha-Vári, J.H.; Katona, G.; Toşa, M.I.; Paizs, C.; Irimie, F.-D. Nanobioconjugates of Candida antarctica lipase B and single-walled carbon nanotubes in biodiesel production. Bioresour. Technol. 2016, 200, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, M.; Yu, M.; Jambhrunkar, M.; Liu, Y.; Yang, Y.; Huang, X.; Yu, C. Designed synthesis of organosilica nanoparticles for enzymatic biodiesel production. Mater. Chem. Front. 2018, 2, 1334–1342. [Google Scholar] [CrossRef]
- Shafiq, F.; Mumtaz, M.W.; Mukhtar, H.; Touqeer, T.; Raza, S.A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Response surface methodology approach for optimized biodiesel production from waste chicken fat oil. Catalysts 2020, 10, 633. [Google Scholar] [CrossRef]
- Ngo, T.P.; Li, A.; Tiew, K.W.; Li, Z. Efficient transformation of grease to biodiesel using highly active and easily recyclable magnetic nanobiocatalyst aggregates. Bioresour. Technol. 2013, 145, 233–239. [Google Scholar] [CrossRef]
- Jambulingam, R.; Shalma, M.; Shankar, V. Biodiesel production using lipase immobilised functionalized magnetic nanocatalyst from oleaginous fungal lipid. J. Clean. Prod. 2019, 215, 245–258. [Google Scholar] [CrossRef]
- Chandran, D. Compatibility of diesel engine materials with biodiesel fuel. Renew. Energy 2020, 147, 89–99. [Google Scholar] [CrossRef]
- Hoseini, S.; Najafi, G.; Ghobadian, B.; Ebadi, M.; Mamat, R.; Yusaf, T. Biodiesels from three feedstock: The effect of graphene oxide (GO) nanoparticles diesel engine parameters fuelled with biodiesel. Renew. Energy 2020, 145, 190–201. [Google Scholar] [CrossRef]
- Jeyalakshmi, P. Characterization of Simarouba glauca seed oil biodiesel. J. Therm. Anal. Calorim. 2019, 136, 267–280. [Google Scholar] [CrossRef]
- Islami, H.R.; Assareh, R. Enhancement effects of ferric ion concentrations on growth and lipid characteristics of freshwater microalga Chlorococcum oleofaciens KF584224. 1 for biodiesel production. Renew. Energy 2020, 149, 264–272. [Google Scholar] [CrossRef]
- Kaisan, M.; Anafi, F.; Nuszkowski, J.; Kulla, D.; Umaru, S. Calorific value, flash point and cetane number of biodiesel from cotton, jatropha and neem binary and multi-blends with diesel. Biofuels 2017, 8, 1–7. [Google Scholar] [CrossRef]
| Feed Stock | Oil Sources | Benefits | Challenges |
|---|---|---|---|
| First Generation | Edible oil |
|
|
| Second Generation | Non-edible oil |
|
|
| Third Generation | Algae Microorganisms yeast |
|
|
| Nanomaterials | Biomaterials/ Stabilizing Agents | Formation Process | Formation Conditions | Characterization Techniques | Size of the Nanomaterials | Nature of Lipase Attachment with NMs | Reference |
|---|---|---|---|---|---|---|---|
| NBCs containing magnetic NMs | |||||||
| GA-Fe3O4 NPs | Lipase from Candida rugosa | NPs by co-precipitation method | NaOH was used as precipitating agent for the mixture of iron salts | AFM XRD FTIR VSM | 80–100 nm | Covalent attachment | [51] |
| Chemical grafting for lipase immobilization | 100 mM phosphate buffer, pH = 7, 25% GA, nanocatalyst and lipase addition at T = 35 °C with continuous stirring for 2 h | ||||||
| Fe3O4 NPs AP-Fe3O4 NPs GA-AP-Fe3O4 NPs | Lipase from Rhizopus oryzae | Fe3O4 NPs by co-precipitation method Functionalization by chemical grafting | For the functionalization with AP and GA, the NPs were sonicated in the solution of functionalizing moiety | FESEM EDX XRD FTIR VSM | 20–30 nm | Physical attachment in case of Fe3O4 NPs. | [52] |
| Chemical grafting for lipase immobilization | 5 mg of catalyst, 1 cm3 100 mM phosphate buffer, pH = 7.5, 0.300 cm3 enzyme solution at 4 °C and 120 rpm shaking speed for 17 h in incubator shaker. | Covalent attachment and electrostatic attachment in case of AP-Fe3O4 NPs and GA-AP-Fe3O4 NPs | |||||
| AP-Fe3O4 NPs | CLEAs from Candida Antarctica | Fe3O4 NPs by co-precipitation method Functionalization with AP by chemical grafting | NH4OH added in the FeCl3.6H2O and FeSO4.7H2O for acquiring precipitates followed by functionalization with AP in presence of CH3OH and glycerol. | SEMFTIR | -- | Covalent attachment of CLEAs with the AP-Fe3O4 NPs | [53] |
| Formation of CLEAs | Purified lipase solution was mixed with GA solution at T = 30 °C for 3 h to get CLEAs | ||||||
| Formation of CLEAs-AP-Fe3O4 NPs by chemical grafting | 5 mg NPs were mixed with the CLEAs solution in the presence of the GA (precipitating agent) for acquiring complex system | ||||||
| AP-GO-Fe3O4 NPs | CLEAs from Enterobacter cloacae | GO by Hummers method Fe3O4 NPs by co-precipitation method Functionalization with AP by chemical grafting | -- | SEM EDX FTIR | -- | Covalent attachment of CLEAs with the AP-GO-Fe3O4 NPs | [34] |
| Formation of CLEAs | Purified lipase solution was mixed with GA solution at T = 30 °C for 4 h to get CLEAs | ||||||
| Formation of CLEAs-AP-GO-Fe3O4 NPs by chemical grafting | 3 mg/mL CLEAs were mixed with the NPs in the presence of the GA (precipitating agent) for acquiring complex system | ||||||
| PDA-Fe3O4 NPs | Lipase from Aspergillusterreus AH-F2 | Fe3O4 NPs by solvothermal method PDA coating by grafting method | Mixture of 1 g of FeCl3.6H2O, 20 mL ethylene glycol, 3 g C2H3NaO2 and 10 mL ethylene diamine followed by the autoclave at 180 °C for 8 h. Functionalization with 0.1 g of dopamine hydrochloride. | XRD FTIR TEM | 24–27 nm | Covalent attachment of Lipase with NPs | [20] |
| Chemical grafting for lipase immobilization | 0.4 g Lipase, 40 mL phosphate buffer, drop wise addition of NPs at 4 °C and continuous stirring for 3 h | ||||||
| GA-AP-Fe3O4 NPs | Lipase from Candida antarctic | Fe3O4 by co-precipitation Functionalization with AP and GA by grafting | Ionic liquid of ([BMIN]BF4) was used as template during co-precipitation. | XRD FTIR TEM EDX SQUID | 150–220 nm | Covalent attachment of lipase with NMs | [54] |
| Chemical grafting for lipase immobilization | 800 mg Lipase, 25 mL of phosphate buffer, pH = 7.0 and 2 g GA-activated NMs was incubated for 2 h. | ||||||
| P(GMA-co- MAA)-Fe3O4 NPs | Lipase from Candida rugosa | Fe3O4 NPs by solvothermal method P(GMA-co-MAA)-Fe3O4 NPs by blending | 0.1 g of Fe3O4, 60 mL of acetonitrile, 0.4 g of GMA, 0.4 g of MAA, 0.2 g DVB and 0.02 g of AIBN was reflexed for 20 min. | FTIR XRD SEM TEM XPS VSM N2 adsorption-desorption studies | 200–400 nm | Covalent attachment of lipase with NMs | [55] |
| Chemical grafting for lipase immobilization | 0.1 g NBCs, lipase enzyme, 100 mL phosphate buffer, pH = 7, T = 30 °C and incubation reaction time = 5 h | ||||||
| Fe3O4@PDA NPs | Lipase from Pseudomonas cepacia | Fe3O4 NPs by co-precipitation Coating with the PDA to get core@shell morphology | 200 mL NaOH solution, 0.2 mol/L FeCl3 and 0.1 mol/L FeCl2 solutions were magnetically stirred at 1100 rpm. | DLS AFM FTIR TEM | 11 nm Fe3O4 NPs | Covalent attachment of lipase NPs | [56] |
| Chemical grafting for lipase immobilization | 10 mL phosphate buffer, pH = 7, T = 4 °C, 200 mg lipase solution, 4 mL NBCs dispersion and reaction time = 1 h | ||||||
| GA-Fe3O4 NMs | Lipase B from Candida antarctica | Fe3O4 NMs by co-precipitation method | GA was utilized as the precipitating and activating agent | SEM DLS | 293 ± 87 nm | Covalent attachment of lipase | [57] |
| Formation of CLEAs by cross-linking | Purified lipase solution was cross-linked in the presence of GA | ||||||
| Immobilization of CLEAs by | Both dispersions were mixed in the presence of ammonium sulphate and GA as the cross-linkers/precipitating agent. 150 mM phosphate buffer, pH = 4.4 and stored at 4 °C | ||||||
| NBCs containing metal oxide NMs | |||||||
| PDA-CeO2 NRs | Lipase from Aspergillus terreus | Hydrothermal process for PDA-CeO2 NPs | Hydrothermal process: autoclave for 24 h at 120 °C, calcination in furnace for 2 h at 500 °C followed by coating of NRs with PDA by grafting method/covalent attachment | SEM XRD FTIR EDX | Diameter 50–60 nm and length 150–200 nm | Chemical attachment of Lipase with NRs | [46] |
| Chemical grafting for lipase immobilization | 0.4 g Lipase, 40 mL phosphate buffer, drop wise addition of NRs at 4 °C and continuous stirring for 3 h | ||||||
| PPy/MA-TiO2 NPs | Lipase from Rhizopus oryzae | PPy/MA-TiO2 NPs are synthesized by in-situ chemical polymerization | In TiO2 dispersion, pyrrole and methyl anthranilate was added into the suspension to get the matrix containing embedded NPs | FTIR XRD TGA DTA TEM SEM EDX | 38–50 nm functionalized NMs | Chemical attachment when immobilization is performed in the presence of GA. | [58] |
| Lipase grafting by chemical method | 5 mL lipase solution, 5 mL NBCs dispersion, 0.1 M TrisHCl-buffer with pH = 8 and incubation time = 24 h | Physical attachment in the absence of GA | |||||
| NBCs containing inorganic nanoscaffolds | |||||||
| Fe3O4@SiO2 NPs PEI-Fe3O4@SiO2 NPs PAA-Fe3O4@SiO2 NPs | Lipase from Pseudomonas cepacia | Co-precipitation for Fe3O4 NPs and Stober Method for Fe3O4@SiO2 NPs | TEOS, ethanol and NH3 for preparation followed by drying at 50 °C for NPs | - | - | Physical attachment for Fe3O4@SiO2 NPs | [59] |
| Chemical grafting for lipase immobilization | 2 mg/L lipase, 2.5 mL phosphate buffer, addition of dispersed NPs at 4 °C with continuous stirring for 2 h. | Chemical attachment for PAA and PEI modified NPs | |||||
| Fe3O4 NPs GO/Fe3O4 NPs AP-GO/Fe3O4 NPs GA-GO/Fe3O4 NPs | Lipase from Rhizopus oryzae | GO by Hummer’s Method Fe3O4 NPs by co-precipitation method GO/Fe3O4 NPs by in-situ deposition of NPs on GO surface | T = 80 °C, N2 flow, 25% NH3 solution was utilized for in-situ deposition For the functionalization with AP and GA, the NPs were sonicated for 2 h in the solution of functionalizing moiety | AFM FTIR XRD EDX FESEM VSM ZPM BET | 20–30 nm for Fe3O4 NPs | Physical attachment for Fe3O4 NPs and GO/Fe3O4 NPs | [60] |
| Chemical grafting for lipase immobilization | 1 mL phosphate buffer solution, 5 mg of each NPs, pH = 7.5 followed by the addition into lipase solution. Mixture was shaken at 4 °C for 17 h in shaking incubator at a stirring speed of 120 rpm. | Electrostatic attractions and covalent attachment for AP-GO/Fe3O4 NPs and GA-GO/Fe3O4 NPs | |||||
| GA-PMAM-Fe3O4/MWCNTs | Lipase from Burkholderiacepacia | MWCNTs-NH2 was prepared by chemical grafting. Fe3O4 NMs was incorporated in the MWCNTs-NH2 by deposition | MWCNTs were purchased from Nanotech Port Corporation Limited. Functionalization was carried out in the order: MWCNTs-COOH, MWCNTs-NH2, Fe3O4-MWCNTs, PMAM-Fe3O4-MWCNTs and GA- PMAM-Fe3O4-MWCNTs | TEM SQUID XRD CLSM XPS | 40–60 nm for MWCNTs | Chemical attachment of lipase with the NBCs | [61] |
| Chemical grafting for lipase immobilization | 0.1 g of nanocomposite, 25% GA, 5 mL phosphate buffer, pH = 7, shaker speed 200 rpm at 30 °C and reaction time of 10 h. | ||||||
| Fe3O4@SiO2 NPs AP-Fe3O4@SiO2 NPsMP- Fe3O4@SiO2 NPs | Lipase (NS81006) from the genetically modified Aspergillus niger | Fe3O4 NPs by co-precipitation Functionalization with AP and MP by Stober Method | Fe3O4 NPs synthesis: stirring = 600 rpm, T = 30 °C, reaction time = 30 min, 25% ammonia solution followed by heating at 85 °C for 30 min. Ultrasonication for 30 min for functionalization. | XRD SEM TEM FTIR VSM | 20.05 nm | Chemical attachment of Lipase with the NBCs | [62] |
| Chemical grafting for lipase immobilization | 0.5 g NBCs, 10 mL ethanol, 10% GA, T = 25 °C, reaction time = 2 h, phosphate buffer solution and pH = 7. | ||||||
| NHCS2H-Fe3O4/GO NMs | Lipase from Porcine pancreas | GO by Hummer’s methodFe3O4 prepared via co-precipitation Functionalization of GO and Fe3O4/GO NMs | GO dispersion, 1.29 g FeCl2, 3.51 g FeCl3, 3%wt. acetic acid, T = 80 °C, 20 mL NH3 solution and oven drying of the Fe3O4/GO NMs at 60 °C for 12 h. Functionalization with dithiocarbamate by doing reflex in the presence of AP and CS2 | SEM TEM EDX UV-VIS | 10–25 nm | Chemical attachment of Lipase with GO as well as Fe3O4/GO NMs | [63] |
| Chemical grafting for lipase immobilization | 3 mg/mL Lipase solution, pH = 7.5, phosphate buffer solution, incubation at T = 38 °C for reaction time 10 h | ||||||
| PDA@Co-MWCNTs | Lipase from Candida rugosa | Co-MWCNTs by in-situ oxidative polymerization Functionalization of MWCNTs with PDA | Co doped MWCNTs were generated by using the precursor salt of CoCl2. Polymerization of the aniline was used to stabilize MWCNTs while PDA coating was carried out to stabilize the Co-MWCNTs. | FTIR TEM TGA DTA CLSM CD | 70–90 nm | Chemical attachment of lipase with the PDA@Co/MWCNTs | [64] |
| Chemical grafting for lipase immobilization | 1 mg/mL lipase solution, 2.5% GA solution, pH = 7.4, phosphate buffer solution 50 mM and incubation time of 6 h | ||||||
| Fe3O4/GO NMs | Lipase from Candida rugosa | GO by Hummer’s method Fe3O4 prepared via co-precipitation Functionalization of GO and Fe3O4/GO NMs | 500 mg GO suspension, 3 g FeCl3.6H2O, 2.1 g FeSO4.7H2O and NH3 solution was stirred at 80 °C followed by ultrasonication for 45 min. Vacuum drying at 60 °C | TEM XRD XPS FTIR VSM N2 absorption-desorption studies | 10–20 nm | Covalent attachment of lipase with Fe3O4/GO NMs | [36] |
| Chemical grafting for lipase immobilization | Powdered lipase, phosphate buffer 100 mL, pH = 7 and NMs suspension was incubated at 30 °C for 5 h. | ||||||
| PANI/Ag/GO NMs | Lipase from Aspergillus niger | Formation of PANI/Ag/GO by cross-linking method | In-situ polymerization by combining GO (obtained by Hummer’s method) and PANI/Ag by crosslinking via ammonium sulphate in hot air incubator. | FTIR TEM SEM XRD DLS AFM TGA | 50 nm | Covalent attachment of lipase with PANI-Ag/GO NMs | [65] |
| Chemical grafting for lipase immobilization | Activation of nanocatalyst via GA. 10 mg of lipase solution, 100 mL phosphate buffer, pH = 7 and NMs suspension was incubated at 1800 rpm at 4 °C | ||||||
| NBCs with advanced nanocatalytic systems | |||||||
| ZIF-8 (Zn-MOFs) | Lipase from Rhizomucor miehei | one-step encapsulation method for synthesizing X-shaped L@ZIF-8 | Coordination between zinc (Zn2+) ions and 2-methylimidazole generated ZIF-8 | FESEM EDX TEM FTIR Powder XRD BET | - | Encapsulation | [66] |
| (MOFs) Cu-NFs | Lipase from Porcine pancreas | L@Cu-NFs by biomimetic mineralization strategy | 1 mL CuSO4, 20 mL phosphate buffer and 20 mg lipase enzyme mixture was incubated for 3 days at 4 °C. Centrifugation was used for the collection of NBCs. | FTIR TGA SEM XRD EDX BET CLSM N2 absorption-desorption studies | - | Encapsulation | [67] |
| ZIF-67 (Co-MOFs) | Lipase from Candida rugose | in situ encapsulation of lipase with cobalt 2-methylimidazolate framework ZIF-67 | 4 mL lipase solution, 2 mL cobalt nitrate and 5 mL 2-methylimidazole were stirred at T = 25 °C. Centrifugation at 6000 rpm for 20 min and was washed with phosphate buffer solution. | Powder XRD TGA FESEM NMR EDX FTIR BET UV-VIS | 5.03 nm MOFs after the lipase encapsulation | Encapsulation | [48] |
| UiO-66 (Zr-MOFs) | Lipase from Aspergillus niger | UiO-66 was synthesized by the hydrothermal methodology Modification with PDMS was done by CVD | ZrCl4 was ultra-sonicated in the presence of DMF and BDC and was autoclaved to get the UiO-66. Modification of the UiO-66 was done by placing the UiO-66 powder on thin glass placed under fresh PDMS stamp | Powder XRD BET N2 adsorption-desorption studies Contact angle studies | - | Physical adsorption | [49] |
| Immobilization by physical adsorption | 30 mg UiO-66, 22.5 mg/mL lipase, 0.05 mol/L phosphate buffer solution, pH = 5.6, incubation at 45 °C with the shaker speed of 200 rpm carried out for 4 h. | ||||||
| Zn-MOFs | thermophilic lipase from Alcaligenes sp. | L@Zn-MOFs by biomimetic mineralization strategy | Centrifugation at 6000 rpm at 4 °C. 50 mg lipase and zinc acetate 200 mg incubation at room temperature for 48 h, the immobilizedL@Bi-MOF was collected by the centrifugation 8000 rpm for 5 min at 4 °C. | SEM TEM PXRD TGA FTIR CLSM CD BET | 800 nm | Encapsulation | [50] |
| Analytical Technique | Explanation of Analytical Technique in Case of NBCs | Reference |
|---|---|---|
| FTIR | Detection of functional groups on the surface of NMs. Immobilization methods particularly covalent bond based immobilization is studied by using this technique. | [77] |
| DLS | The size and disperse nature of the NBCs are investigated by using the DLS technique. The increment in the hydrodynamic radius, as well as the polydispersity values of the NMs after the conjugation with the enzyme, is used as a tool for the validation of the formation of NBCs. | [65] |
| N2 adsorption-desorption studies | The information regarding physical absorption based immobilization of the enzymes of the NMs in NBCs is investigated by this technique. The adsorption mechanism and model followed for the adsorption is validated by this technique. Selective separation of the required crystalline MOFs from the sample carried out for the synthesis of the NBCs is also performed by using this technique. | [49] |
| EDX | The elemental composition and purity of NMs are estimated by this technique. Moreover, the surface modifications and immobilization of lipase on NMs are also affirmed by EDX. | [43] |
| XRD/Powder XRD | The insights regarding size, composition, and crystallinity of NMs (before and after immobilization of lipase) are obtained by this technique. Generally, lipase immobilization or surface modifications does not change the structure of core NMs. | [78] |
| Contact angle study | The hydrophobic nature of the NBCs and its interaction with the hydrophobic lipase enzyme is investigated by carrying out contact angle studies. This property can provide useful insights regarding the stability by investigating the interaction between lipase and the NMs present in the NBCs. | [49] |
| SEM | Morphology of NMs and NBCs is confirmed by using the SEM micrographs. An increase in the size and variation in surface morphology of the NMs after the lipase attachment is considered an indication of successful immobilization. | [79] |
| TEM | The morphology and more importantly surface of NMs is characterized by TEM. The NMs modified with polymers exhibits typical core-shell morphology, while after immobilization of enzyme relatively aggregative structures appear. | [78] |
| VSM | To detect the magnetic potential of the synthesized NBCs by developing the hysteresis loop. High saturation magnetization values of the NBCs also provide information regarding the separation potential of the NBCs under the influence of the external fields. Comparison of the saturation magnetization values of pure NMs and functionalized NMs (in NBCs) provide validation regarding the immobilization and modification procedures associated with the NMs. | [80] |
| CLSM | Superposed field images provided the visual confirmation of the immobilization of the enzyme on the NMs. Two and three-dimensional fluorescence intensity further confirm the immobilization of the enzyme. | [37,80] |
| XPS | Elemental composition, surface chemistry, and electronic state of nano-material are determined by XPS. The difference in XPS spectra before and after immobilization of lipase on NMs validates the conjugation of lipase with NM. Generally, carbon/nitrogen ratio verifies the attachment of lipase. | [79] |
| AFM | This technique when utilized with the tapping mode is used to analyze the surface roughness of the NMs. The distance between peak to peak valley values in the micrographs is indicative of the roughness of the NMs. The immobilization of the enzyme also influences the roughness of NMs and the change in the roughness values of NMs is utilized as evidence for the formation of the NBCs. | [65] |
| DTA | The thermal stability imparted to the enzyme via immobilization on the NMs to generate NBCs is investigated by using this technique. | [58] |
| CD | To investigate the secondary structure of the lipase enzyme in its immobilized form, this technique is utilized. | [50] |
| Nanobiocatalyst | Transesterification Process | Raw Material | FAME Monitoring Technique/ Optimization Technique | Transesterification Conditions/ Optimized Parameter | FAME Yield | Remarks | Reference |
|---|---|---|---|---|---|---|---|
| L-PDA-CeO2 NRs | Enzymatic transesterification | Eruca sativa oil | GC-MS | -- | C16:0 (1.448%) C18:0 (0.186%) C18:1 (28.181%) C22:0 (65.111%) C20:1 (4.712%) | The utilization of the RSM for the optimization of the transesterification processes allowed numerous advantages to this study. According to RSM, the transesterification process followed the quadratic model with R2 value and adjusted R2 value of 0.9802 and 0.9903 respectively. Reusability studies revealed that the NCBs were efficient for performing the transesterification up to five cycles without any significant loss in the activity of the NBCs | [46] |
| RSM | 10% NBCs, 6:1 methanol to oil ratio, T = 35 °C, 0.6% water content and reaction time = 30 h. | Maximum biodiesel yield 89.3% | |||||
| L-Fe3O4 NPs L-Fe3O4/GO NPs L-AP-Fe3O4/GO NPs L-GA-Fe3O4/GO NPs | Whole cell transesterification | Chlorella vulgaris oil | GC-MS | 8.6 mg of catalyst, T = 45 °C, reaction time = 24 h, 0.5 mL of extracted lipid, 5 mg cm-3 n-Hexane and 0.15 mL methanol | L-Fe3O4 NPs (54.14%), L-GO-Fe3O4 NPs (57.05%), L-AP-GO-Fe3O4 NPs (~62%), L-GA-GO- Fe3O4 NPs (~70%) | The functionalization of GO-Fe3O4 NPs by GA and AP improved efficacy of the transesterification process by effectively immobilizing lipase enzyme. The amine group in AP and aldehyde group in GA facilitated lipase attachment via electrostatic interaction and covalent bond formation (C=N) respectively. Therefore, functionalized NPs were more stable and exhibited better results in comparison. | [60] |
| L-GA-Fe3O4 NPs | Enzymatic transesterification | Waste cooking oil | GC-MS | 36% w/w NPs, 200 rpm stirring rate, reaction time = 90 min, T = 40 °C and 1:5 oil to methanol ratio | 93.58% | Extremely high yield of biodiesel was observed in case of the synthesized NBCs. However, the physicochemical characters of biodiesel were not studied. | [51] |
| L-Fe3O4 NPs L-AP-Fe3O4 NPs L-GA-AP-Fe3O4 NPs | Whole cell transesterification | Chlorella vulgaris oil | GC-MS | 8.6 mg of catalyst, T = 45 °C, reaction time = 24 h, 0.5 cm3 of extracted lipid, 5 mg cm-3 n-Hexane and 0.15 cm3 methanol | L-Fe3O4 NPs (54.14%), L-GA-Fe3O4 NPs (~58%) L-GA-AP- Fe3O4 NPs (69.8%) | Among the NBCs, the system containing both the moieties responsible for the covalent and electrostatic interactions based attachment of lipase enzyme with the NMs yielded the maximum results in terms of transesterification process. | [52] |
| CLEAs-AP-Fe3O4 NPs | Enzymatic transesterification | Waste cooking oil | GC-MS | 2.2 g of waste cooking oil, 1:3 oil to methanol ratio, 0.3% NBCs, 170 rpm stirring rate, T = 35 °C. for 72 h. | 49% | The CLEAs although exhibited better results in terms of stability and reusability in comparison to the lipase immobilized without the process of cross linking or precipitation. However, yield was found to be quite low in comparison to the recent literature which may be attributed to the reduction in the surface area of the NBCs owing to the process of crystallization. | [53] |
| CLEAs-AP-GO-Fe3O4 NPs | Enzymatic transesterification | Ricinus communis oil | GC-MS | 0.4 g oil, 1:3 oil to methanol ratio, 0.2% w/w NBCs, T = 25 °C and reaction time 24 h | 78% | Comparison of the NBCs with the free lipase indicated that the yield of biodiesel production increased 3 folds owing to the immobilization and formation of CLEAs. | [34] |
| L-PDA-Fe3O4 NPs | Enzymatic transesterification | waste cooking oil | RSM | 10% catalyst concentration, 1:6 oil to methanol ratio, reaction time 30 h, T = 37 °C and water concentration 0.6% | 92% | Optimization of the biodiesel transesterification by RSM revealed that the quadratic model was found to be best fitted in case of this reaction. The synthesized biodiesel was found to be comparable with the ASTM international reference for biodiesel. | [20] |
| L-PDA-Fe3O4 NPs | Enzymatic transesterification | Waste chicken fat oil | GC-MS | -- | C16:0 (17.96%) C18:0 (20.85%) C18:1 (42.92%) C18:2 (16.54%) | Comparison between actual and predicted FAME values affirms that the quadratic model was the best fit for the transesterification process. The results of the FTIR analysis were used as the confirmation technique for the immobilization of the enzyme on the NPs. Recovery from the medium was carried out by using the magnetic properties of the NBCs. Reuse studies further confirm that no decrease in the activity of the NBCs was observed for three reuse cycles. | [87] |
| RSM | 6% catalyst concentration, T = 42 °C, 1:6 oil to methanol ratio and Reaction time 36 h | 90.6% | |||||
| L-GA-PA-Fe3O4 NPs | Enzymatic transesterification | Rapeseed oil | GC-MS | 20% NBCs, 1:6 oil to methanol ratio, 2%water content, T = 45 °C, reaction time = 24 h and 250 rpm agitation speed. | 89.4% | FAME conversion of greater than 70% was acquired after the 5 rerun cycles of transesterification carried out via NBCs. The external magnetic field was utilized as the recovery tool in case of NBCs. Utilization of ionic liquids as major stabilization medium provided an alternative route for the preparation of NMs. | [54] |
| L-AP-Fe3O4@SiO2 NPsL-MP-Fe3O4@SiO2 NPs | Enzymatic transesterification | Soybean oil | GC-MS | oil 10 mL, T = 45 °C, 1:3 oil to methanol, catalyst 0.5 g, stirring = 600 rpm and reaction time = 12 h | L-AP-Fe3O4@SiO2 NPs (76%) L-MP-Fe3O4@SiO2 NPs (72%) | The maximum immobilization efficacy for L-AP-Fe3O4@SiO2NPs and L-MP-Fe3O4@SiO2 NPs was found to be 78.7% and 77% respectively. The NBCs could maintain the catalytic efficacy up to five cycles | [62] |
| L-P(GMA-co- MAA)-Fe3O4 NPs | Enzymatic transesterification | Soybean oil | GC-MS | T = 40 °C, 1:4 oil to methanol, NBCs 25% and reaction time 54 h. | 92.8% | The leaching test performed in case of NBCs revealed that the lipase immobilization imparted long term stability to the NBCs. | [55] |
| L-Fe3O4@PDA NPs | Enzymatic transesterification | Soybean oil | ATR-FTIR | 200 mg NBCs, 1:1 oil to methanol, T = 37 °C and reaction time 12 h | 90% | Immobilization was achieved via Michael addition and aldolic condensation at the specific catechol sites eliminated the need for the use of extensive coupling agents. Denaturing owing to methanol medium limited the use of NBCs to three runs. | [56] |
| L@Cu-NFs | Enzymatic transesterification | sunflower oil | NMR | T = 45 °C, 1:2 oil: methanol, 12 mL oil, 0.1 g NBCs, | 96.5% | MOFs possess favorable catalytic activity and stability in the ester hydrolysis. Further, the hybrid nanoflower was used as a catalyst for biodiesel production. It can be reused for 5 cycles. So, the hybrid nanoflower exhibited potential for acting as economically viable biocatalyst for the production of biofuel. | [67] |
| L@ZIF-8 | Enzymatic transesterification | Soybean oil | GC-MS | 2.19 g soybean oil, 1:4 oil to ethanol, 9% wt. water content, reaction time = 24 h, T = 45 °C and 8% wt. lipase dosage. | 95.6% | In terms of the recovery studies and retention of efficacy after reruns, the synthesized NBCs exhibited excellent results. 26-fold recovery rate was observed in case of NBCs in comparison to free lipase. | [66] |
| L@ZIF-67 | Enzymatic transesterification | Soybean oil | NMR | 0.5 g Soybean oil, 100 mg NBCs, 10% water content, 1:6 oil to methanol, reaction time = 6 h and T = 45 °C. | 78% | The rerun experiments indicate that the NBCs can be effectively utilized for 6 runs. The activity loss of 78.5% to 56% was observed in case of utilizing NBCs for 6 runs. Enzyme leaching was not observed during the studies. | [48] |
| L- Fe3O4/GO NBCs | Enzymatic transesterification | Soybean oil | GC | 48.5 g oil, 1:4 oil to methanol ratio, 20%wt. NBCs, reaction time = 60 h and T = 40 °C. | 92.8% | The NBCs can be effectively separated by using external magnetic field. The NBCs do not exhibit any significant decrease in the catalytic efficacy up to five cycles in terms of biodiesel production. | [36] |
| L-PMAM-Fe3O4/MWCNTs NBCs | Enzymatic transesterification | Waste vegetable oil | GC | 1.98 g oil, 1:4 oil to methanol, lipase dosage 6% wt., reaction time = 10 h, T = 50 °C and water content 8%wt. | >93% | Esterification activities of NBCs were found to be 27 fold more in comparison to free lipase enzyme. | [37] |
| RSM | |||||||
| L@UiO-66 | Enzymatic transesterification | Soybean oil | GC | 10g of oil, 0.2–0.5 mg NBCs and reaction time = 12 h | 93% | Physical adsorption was utilized as a mode of immobilization owing to the complementary hydrophobic nature of the lipase and the modified UiO-66. | [49] |
| L@Zr-MOFs | Enzymatic transesterification | Sunflower oil | 1H NMR | 100 mg NBCs,15.9 mg lipase dispersion, 12 mL of sunflower oil,1 M NaOH solution for adjusting the pH. | Catalytic activity, stability, recyclability, and reusability potential of synthesized NBCs were increased by using biomimetic mineralization strategy for the preparation of NBCs. | [50] |
| Properties | Units | Standard and Corresponding ASTM Method | Standard and Corresponding ASTM Method | Standard and Corresponding EN Method | Test Requirements and Comments | |||
|---|---|---|---|---|---|---|---|---|
| ASTM D0975 | ASTM Method | ASTM D6751 | ASTM Method | EN 14214 | EN Method | |||
| Density * | g/cm3 | 0.876 | - | 0.875 to 0.90 | - | 0.86 to 0.90 | EN ISO 3675 or 12185 | Densitometer Hydrometer |
| Kinematic Viscosity ** | mm2/s | 1.9 to 4.1 | D445 | 1.9 to 6 | D445 | 3.5 to 5 | EN ISO 3104 | Methods are equivalent |
| Specific gravity | -- | 0.850 | D1298 or D4052 | 0.88 | - | NA | -- | |
| Flash Point | °C | 60 to 80 | D93 | 100 to 170 | D93 | >120 | EN ISO 3679 | EN method (Rapid Equilibrium closed cup) and ASTM method (Pensky–Martens closed cup) |
| Cloud Point | °C | −15 to 5 | D2500 | −3 to 12 | D2500 | Location and season dependent | EN ISO 23015 | Wax Appearance Temperature |
| Cetane number | - | 40 | D613 | 47 | D613 | 75 | EN ISO 5165 | Cetane Engine, Methods are equivalent |
| Acid number | mg KOH/g | - | - | 0.5 | D664 | 0.5 | EN 14104 | EN method (calorimetric titration), ASTM method (Potentiometric titration) |
| Iodine value | g I2/100g | - | - | - | - | 120 | EN 14111 and EN 16300 | Titration method |
| Ash content | % | 0.01 | D2709 | <0.02 | D 2709 | - | - | Centrifugation |
| Sulphur | mg/kg | 15 mg/kg | D5453 or D2622 | 15 mg/kg | D5453 | 10 mg/kg | EN ISO 20846 or 20884 or 13032 | UV fluorescence WDXRF |
| Water content | % | 0.02 | D2709 | 0.03 | D2709 | - | - | Centrifugation |
| 90% recovered Distillation | % | 90% 338 °C max | D86 | 90% 360 °C max | D1160 | - | - | Vacuum distillation |
| Total contamination, max | mg/kg | - | - | - | - | 24 | EN ISO 12262 | - |
| Copper Strip corrosion | - | No. 03 | D130 | No. 03 | D130 | Class 1 | EN 1SO 2160 | Methods are equivalent |
| Linolenic acid methyl ester, max | % wt. | - | - | - | - | 12 | EN ISO 14103 | GC |
| Polyunstatured methyl esters, max | % wt. | - | - | - | - | 0.2 | EN ISO 15779 | GC |
| MG, DG and TG | % wt. | 0.4 MG | D6584 | - | - | 0.70 MG 0.20 DG 0.20 TG | EN ISO 14105 | GC |
| Free Glycerine | % wt. | 0.020 | D6584 | - | - | 0.020 | EN ISO 14105 And 14106 | GC |
| Total Glycerine | % wt. | 0.240 | D6584 | - | - | 0.250 | EN ISO 14105 | GC |
| Phosphorus | % wt. and mg/kg | 0.001 | D4951 | - | - | 4mg/kg | EN ISO 14107 | ICP-MS |
| Lubricity | μm | 520 | D6079 | - | - | - | - | Lubricity tester |
| Conductivity | pS/m | 25 | D2624 D4308 | - | - | - | - | Electrical Conductivity meter |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najeeb, J.; Akram, S.; Mumtaz, M.W.; Danish, M.; Irfan, A.; Touqeer, T.; Rashid, U.; Ghani, W.A.W.A.K.; Choong, T.S.Y. Nanobiocatalysts for Biodiesel Synthesis through Transesterification—A Review. Catalysts 2021, 11, 171. https://doi.org/10.3390/catal11020171
Najeeb J, Akram S, Mumtaz MW, Danish M, Irfan A, Touqeer T, Rashid U, Ghani WAWAK, Choong TSY. Nanobiocatalysts for Biodiesel Synthesis through Transesterification—A Review. Catalysts. 2021; 11(2):171. https://doi.org/10.3390/catal11020171
Chicago/Turabian StyleNajeeb, Jawayria, Sadia Akram, Muhammad Waseem Mumtaz, Muhammad Danish, Ahmad Irfan, Tooba Touqeer, Umer Rashid, Wan Azlina Wan Ab Karim Ghani, and Thomas Shean Yaw Choong. 2021. "Nanobiocatalysts for Biodiesel Synthesis through Transesterification—A Review" Catalysts 11, no. 2: 171. https://doi.org/10.3390/catal11020171
APA StyleNajeeb, J., Akram, S., Mumtaz, M. W., Danish, M., Irfan, A., Touqeer, T., Rashid, U., Ghani, W. A. W. A. K., & Choong, T. S. Y. (2021). Nanobiocatalysts for Biodiesel Synthesis through Transesterification—A Review. Catalysts, 11(2), 171. https://doi.org/10.3390/catal11020171








