Recent Progress in Plasmonic Hybrid Photocatalysis for CO2 Photoreduction and C–C Coupling Reactions
Abstract
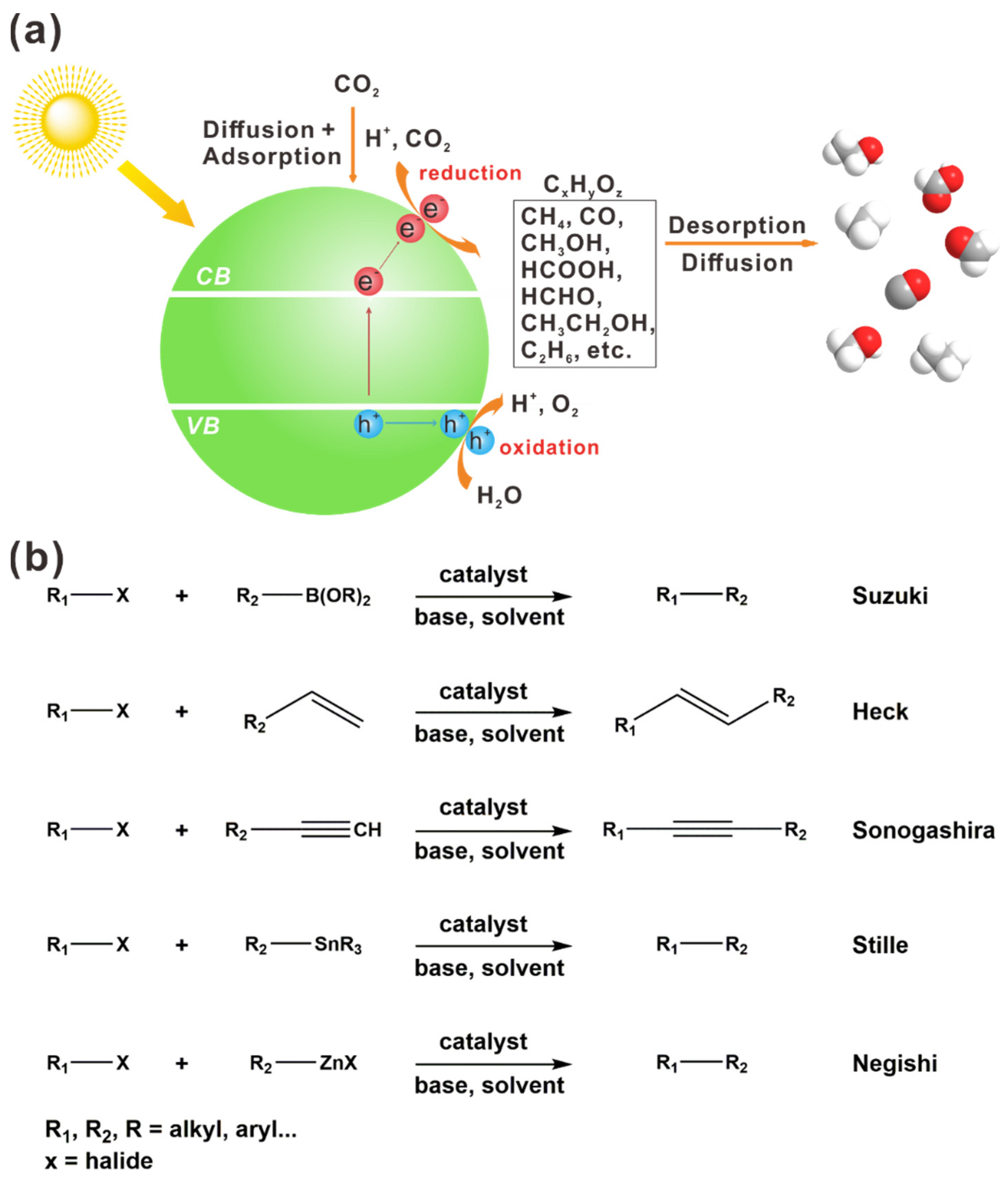
1. Introduction
2. Plasmonic Hybrid Photocatalysts for CO2 Reduction into Hydrocarbon with Multi-Carbon Products
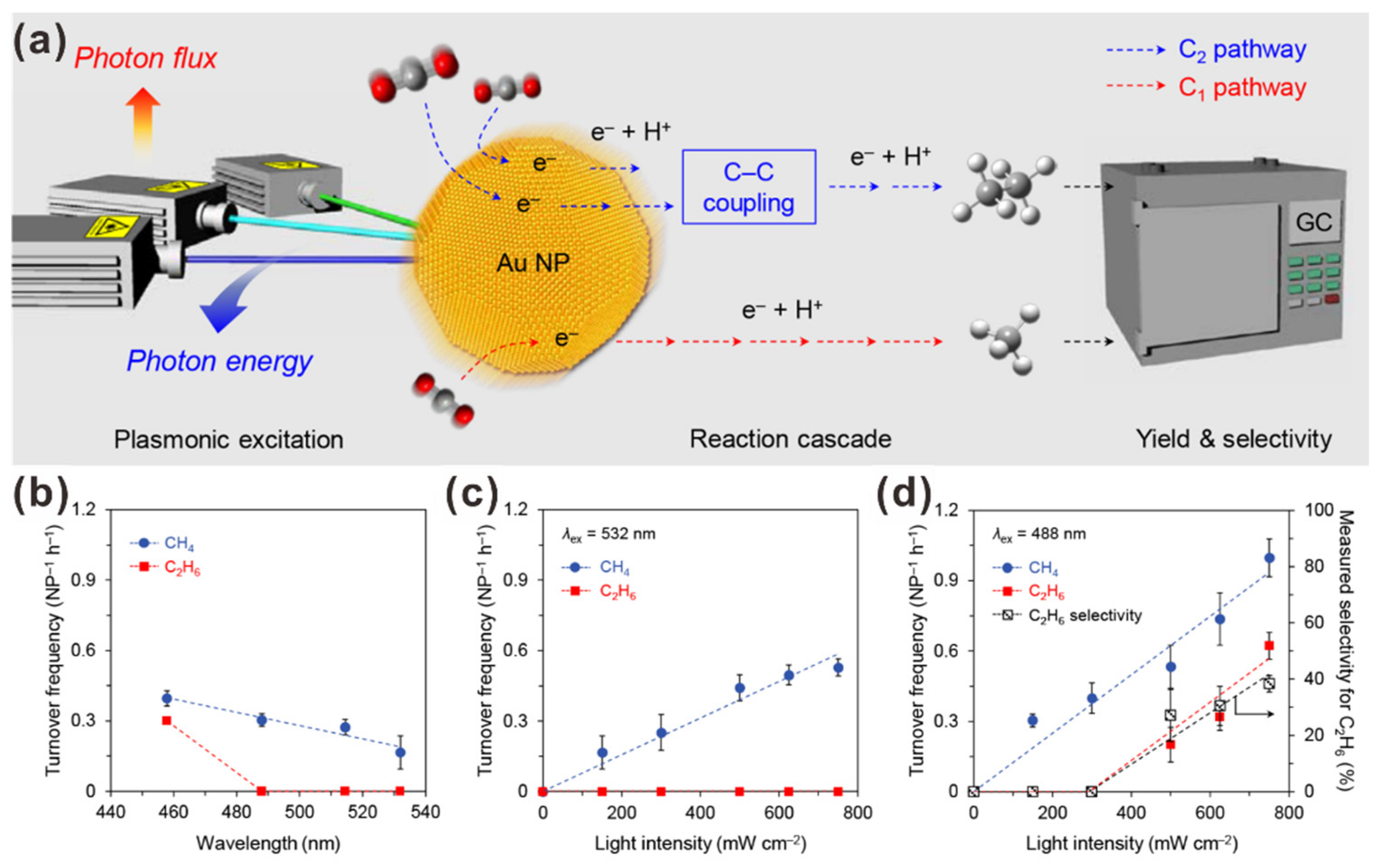
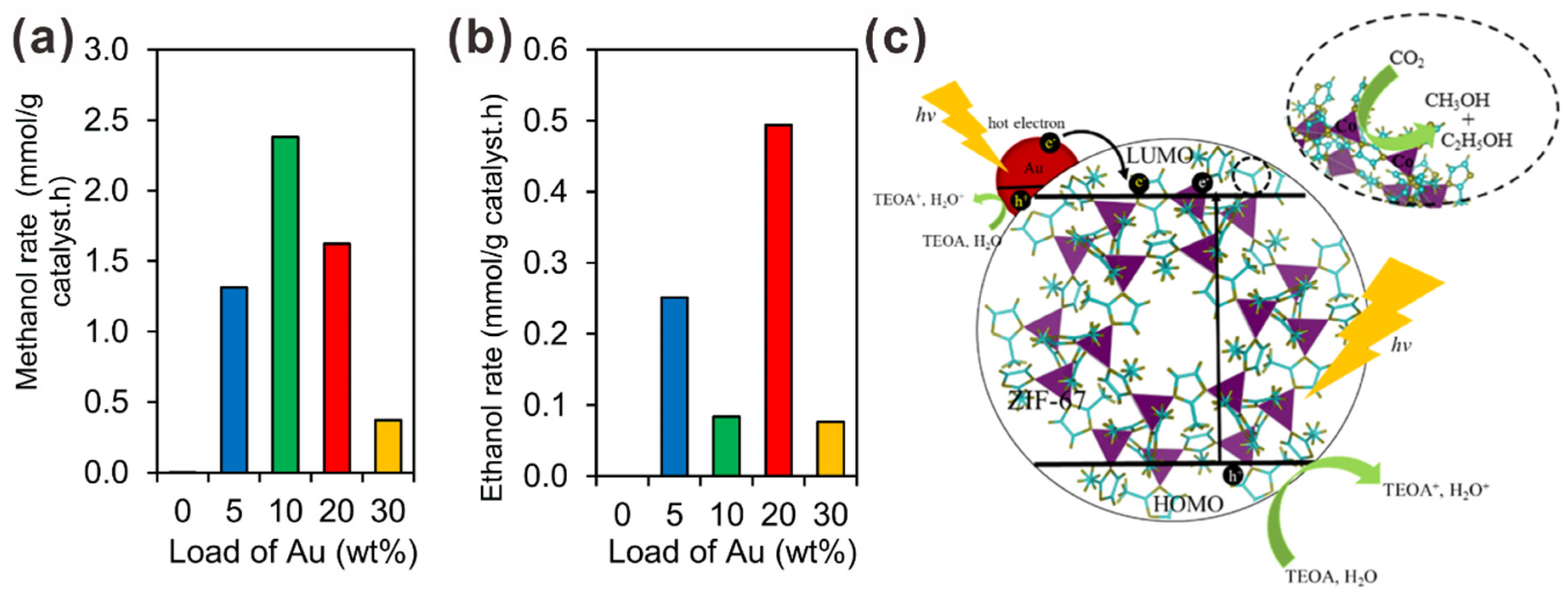
2.1. Gold Nanoparticle (AuNP)-Assisted Plasmonic Photocatalysts for CO2 Reduction to Multi-Carbon Products
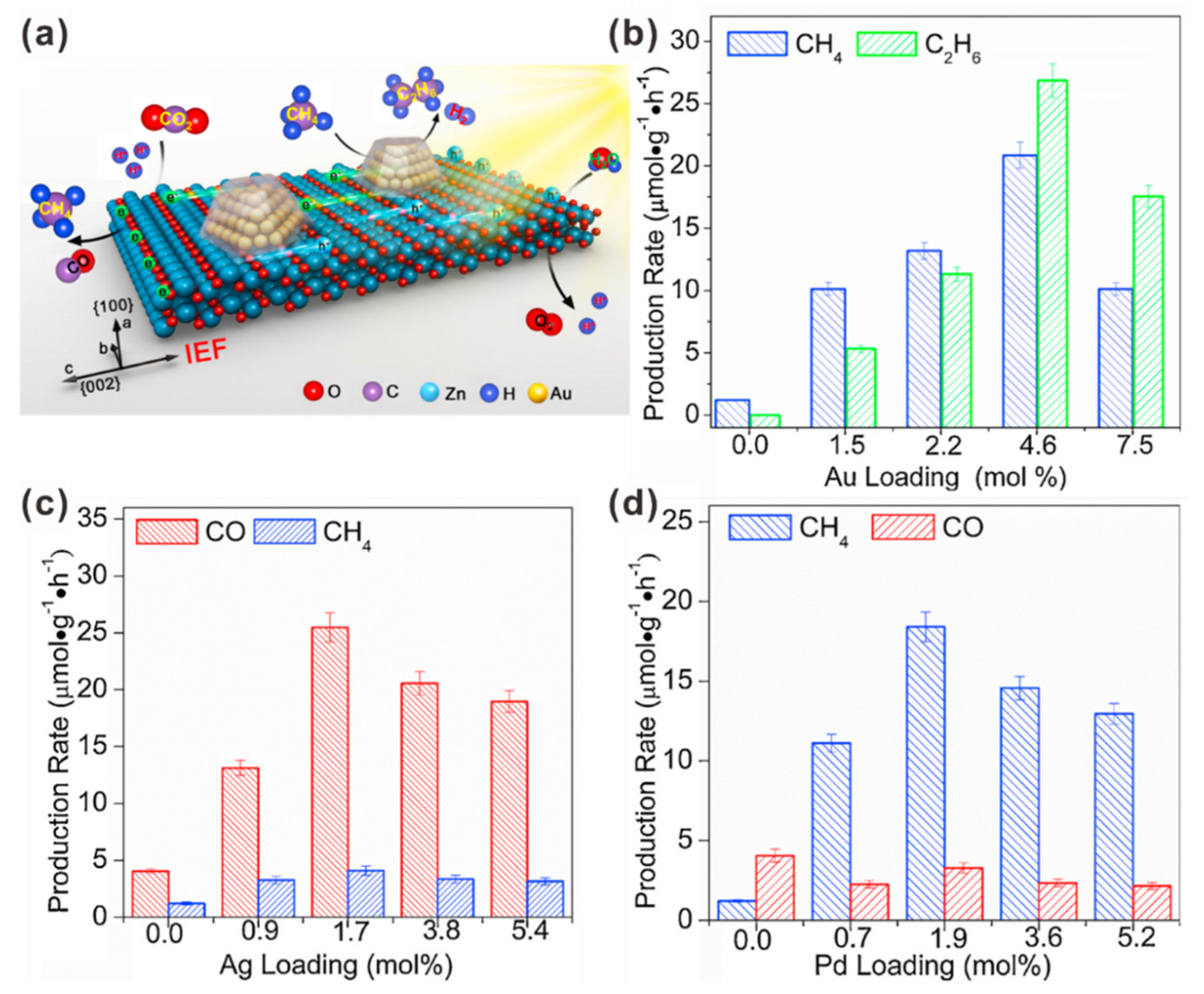
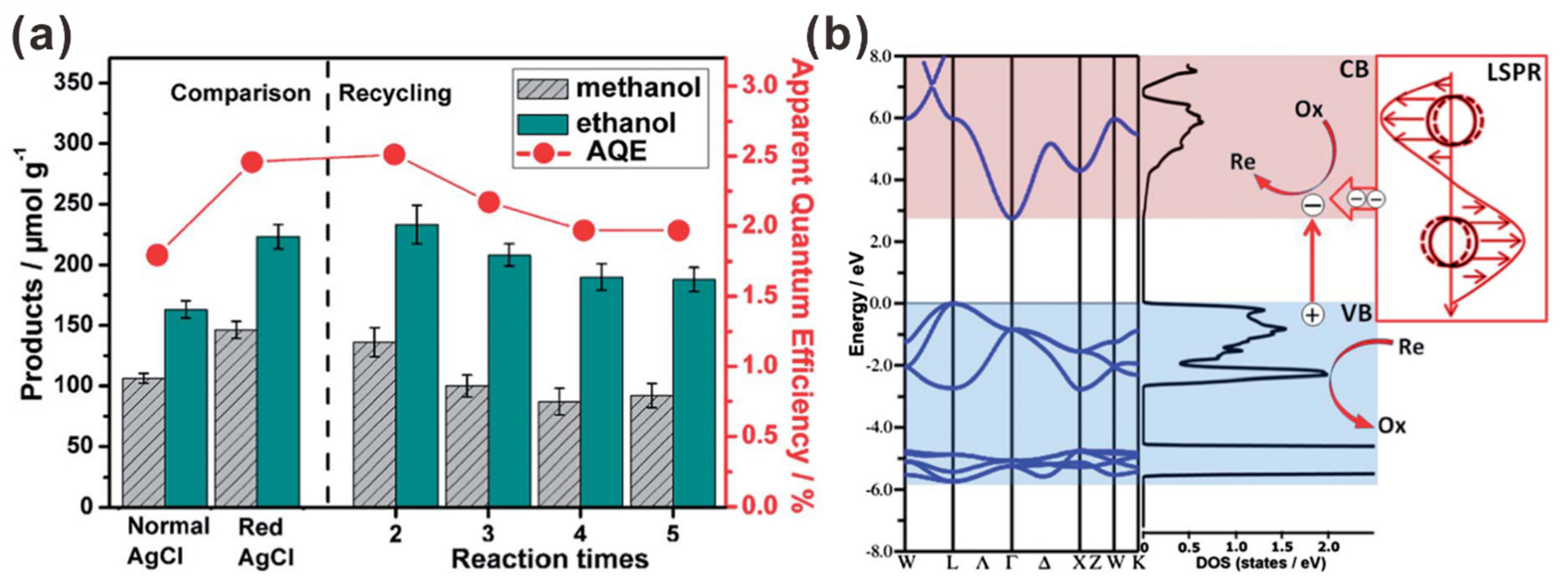
2.2. Silver Nanoparticle (AgNP)-Assisted Plasmonic Photocatalysts for CO2 Reduction to Multi-Carbon Products
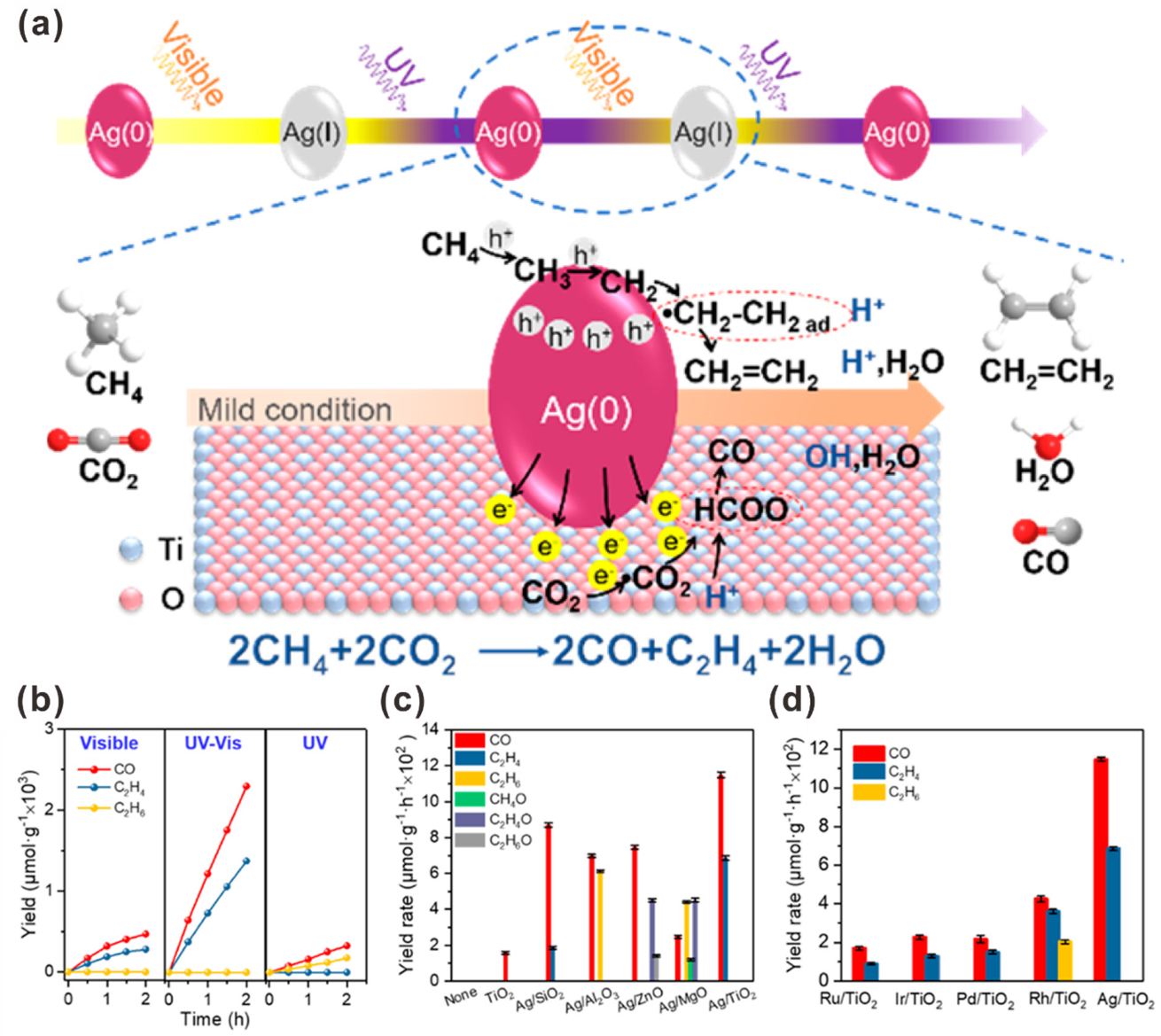
2.3. Copper Nanoparticle (CuNP)-Assisted Photocatalytic CO2 Reduction to Multi-Carbon Products
3. Plasmonic Hybrid Photocatalysts for C–C Cross-Coupling
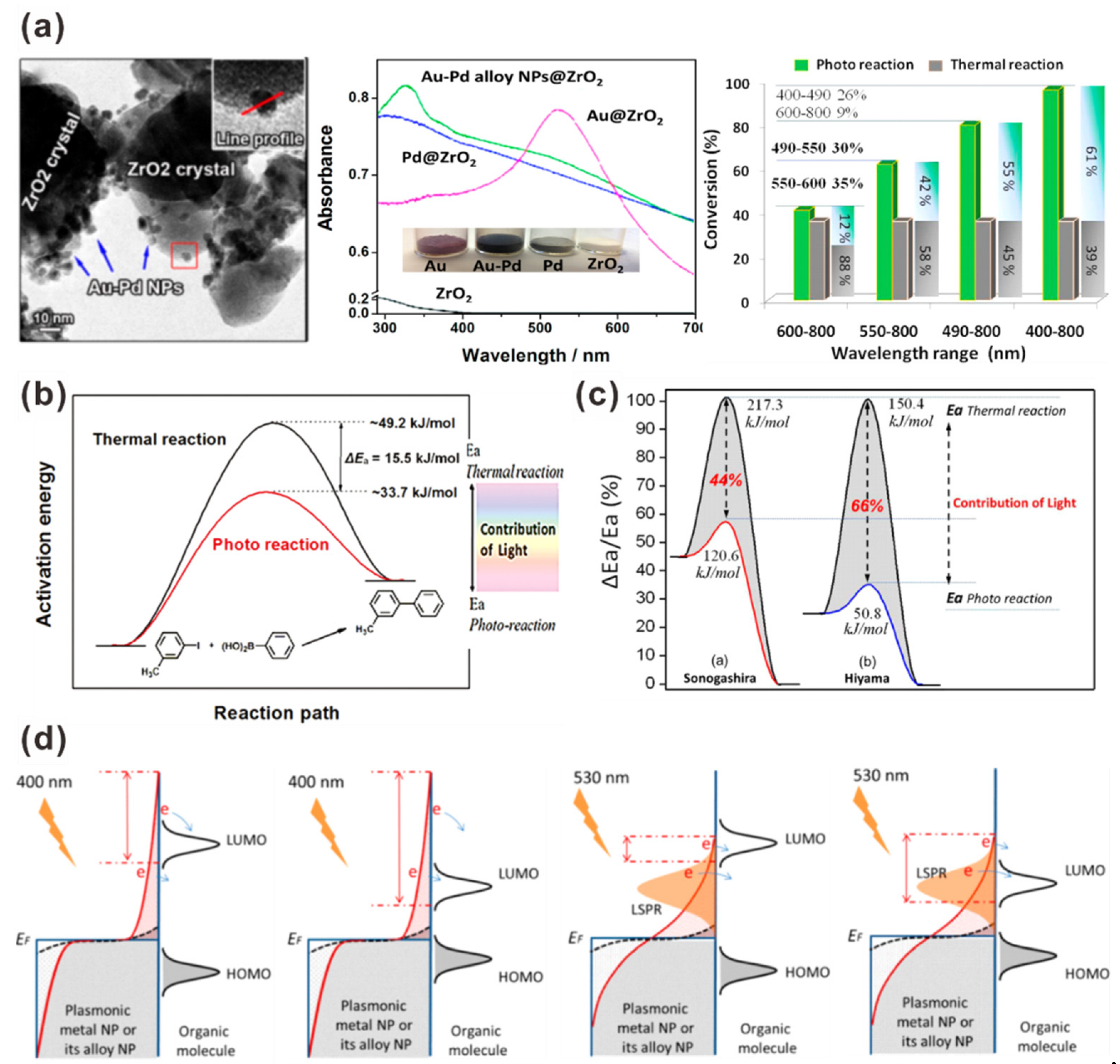
3.1. AuNP-Assisted Plasmonic Photocatalysts for C–C Cross-Coupling
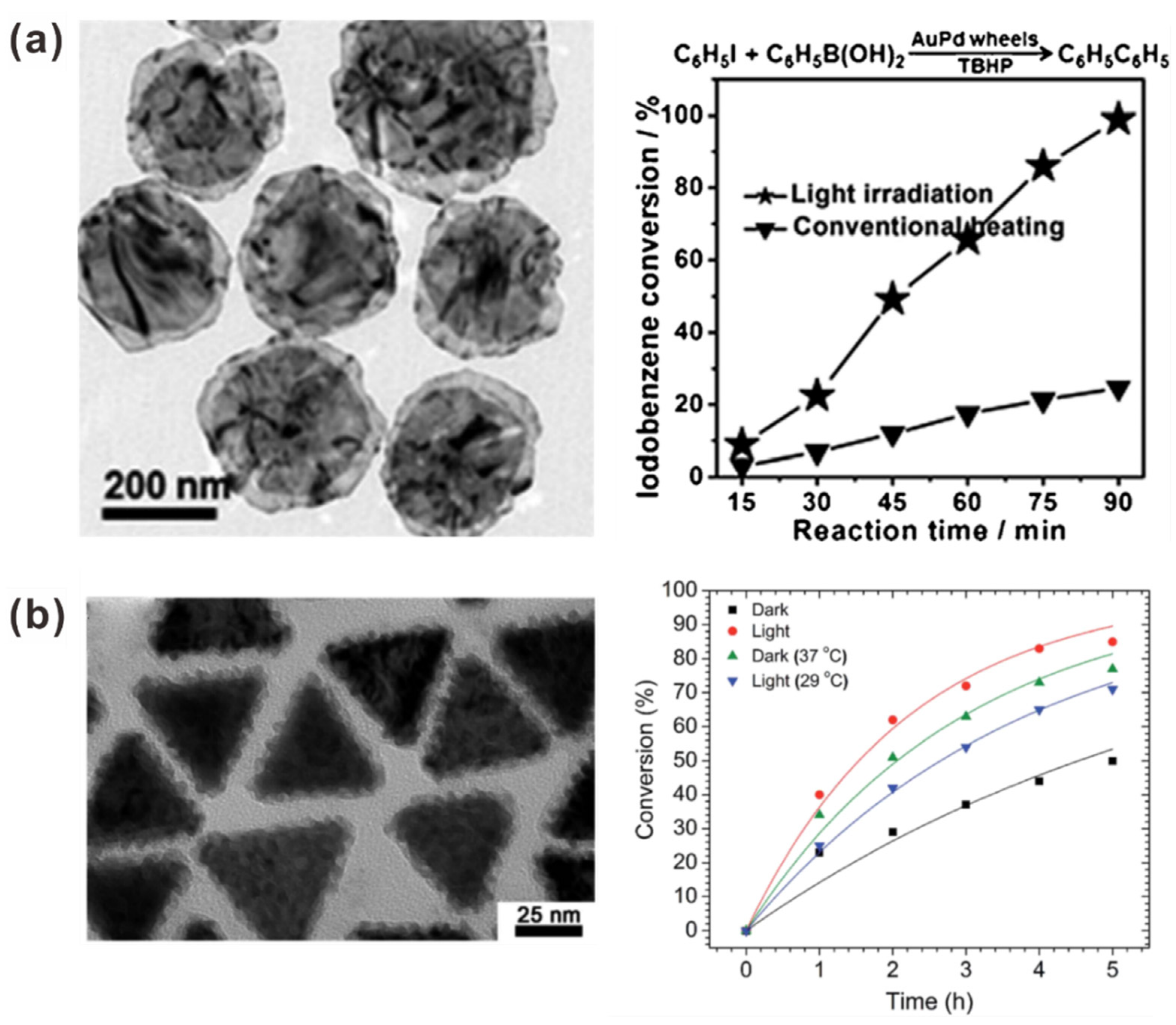
3.2. AgNP-Assisted Plasmonic Photocatalysts for C–C Cross-Coupling
3.3. CuNP-Assisted Plasmonic Photocatalysts for C–C Cross-Coupling
4. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Aman, M.M.; Solangi, K.H.; Hossain, M.S.; Badarudin, A.; Jasmon, G.B.; Mokhlis, H.; Bakar, A.H.A.; Kazi, S.N. A review of safety, health and environmental (SHE) issues of solar energy system. Renew. Sustain. Energy Rev. 2015, 41, 1190–1204. [Google Scholar] [CrossRef]
- Hosenuzzaman, M.; Rahim, N.A.; Selvaraj, J.; Hasanuzzaman, M.; Malek, A.B.M.A.; Nahar, A. Global prospects, progress, policies, and environmental impact of solar photovoltaic power generation. Renew. Sustain. Energy Rev. 2015, 41, 284–297. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. a comprehensive approach. Appl. Catal. B 2015, 170, 90–123. [Google Scholar] [CrossRef]
- Long, R.; Li, Y.; Song, L.; Xiong, Y. Coupling solar energy into reactions: Materials design for surface plasmon-mediated catalysis. Small 2015, 11, 3873–3889. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.G.; Faucheaux, J.A.; Jain, P.K. Plasmon resonances for solar energy harvesting: A mechanistic outlook. Nano Today 2015, 10, 67–80. [Google Scholar] [CrossRef]
- Goodenough, J.B. Energy storage materials: A perspective. Energy Storage Mater. 2015, 1, 158–161. [Google Scholar] [CrossRef]
- Halabi, M.A.; Al-Qattan, A.; Al-Otaibi, A. Application of solar energy in the oil industry—Current status and future prospects. Renew. Sustain. Energy Rev. 2015, 43, 296–314. [Google Scholar] [CrossRef]
- Alper, E.; Orhan, O.Y. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Du, C.; Wang, X.; Chen, W.; Feng, S.; Wen, J.; Wu, Y.A. CO2 transformation to multicarbon products by photocatalysis and electrocatalysis. Mater. Today Adv. 2020, 6, 100071. [Google Scholar] [CrossRef]
- Royer, M.E. Réduction de l’acide carbonique en acide formique. Comptes Rendus 1870, 1870, 731–732. [Google Scholar]
- Halmann, M. Photoelectrochemical reduction of aqueous carbon dioxide on p-type gallium phosphide in liquid junction solar cells. Nature 1978, 275, 115–116. [Google Scholar] [CrossRef]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Li, K.; Peng, B.; Peng, T. Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal. 2016, 6, 7485–7527. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Z.; Zheng, Y.; Hu, Y.H. Recent progress in visible light photocatalytic conversion of carbon dioxide. J. Mater. Chem. A 2019, 7, 865–887. [Google Scholar] [CrossRef]
- Kim, D.; Sakimoto, K.K.; Hong, D.; Yang, P. Artificial photosynthesis for sustainable fuel and chemical production. Angew. Chem. Int. Ed. 2015, 54, 3259–3266. [Google Scholar] [CrossRef]
- Marshall, J. Solar energy: Springtime for the artificial leaf. Nature 2014, 510, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Creel, E.B.; McCloskey, B.D. Scalable CO2-to-oxygenate production. Nat. Catal. 2018, 1, 6–7. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Tao, L.; Saur, G.; van de Lagemaat, J.; Kelley, S.O.; Sargent, E.H. What should we make with CO2 and how can we make it? Joule 2018, 2, 825–832. [Google Scholar] [CrossRef]
- Li, H.; Johansson Seechurn, C.C.C.; Colacot, T.J. Development of preformed Pd catalysts for cross-coupling reactions, beyond the 2010 Nobel prize. ACS Catal. 2012, 2, 1147–1164. [Google Scholar] [CrossRef]
- Shi, W.; Liu, C.; Lei, A. Transition-metal catalyzed oxidative cross-coupling reactions to form C-C bonds involving organometallic reagents as nucleophiles. Chem. Soc. Rev. 2011, 40, 2761–2776. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-coupling reactions of organoboranes: An easy way to construct C–C bonds (Nobel lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef] [PubMed]
- Bariwal, J.; Van der Eycken, E. C–N bond forming cross-coupling reactions: An overview. Chem. Soc. Rev. 2013, 42, 9283–9303. [Google Scholar] [CrossRef]
- Tamao, K.; Sumitani, K.; Kumada, M. Selective carbon–carbon bond formation by cross-coupling of grignard reagents with organic halides. catalysis by nickel-phosphine complexes. J. Am. Chem. Soc. 1972, 94, 4374–4376. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Heck, R.F.; Nolley, J.P., Jr. Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem. 1972, 37, 2320–2322. [Google Scholar] [CrossRef]
- Negishi, E.-I.; Baba, S. Novel stereoselective alkenyl–aryl coupling via nickel-catalysed reaction of alkenylanes with aryl halides. J. Chem. Soc. Chem. Commun. 1976, 596b–597b. [Google Scholar] [CrossRef]
- Ishiyama, T.; Matsuda, N.; Miyaura, N.; Suzuki, A. Platinum(0)-catalyzed diboration of alkynes. J. Am. Chem. Soc. 1993, 115, 11018–11019. [Google Scholar] [CrossRef]
- Ishiyama, T.; Murata, M.; Miyaura, N. Palladium(0)-catalyzed cross-coupling reaction of alkoxydiboron with haloarenes: A direct procedure for arylboronic esters. J. Org. Chem. 1995, 60, 7508–7510. [Google Scholar] [CrossRef]
- Guram, A.S.; Rennels, R.A.; Buchwald, S.L. A simple catalytic method for the conversion of aryl bromides to arylamines. Angew. Chem. Int. Ed. Engl. 1995, 34, 1348–1350. [Google Scholar] [CrossRef]
- Louie, J.; Hartwig, J.F. Palladium-catalyzed synthesis of arylamines from aryl halides. Mechanistic studies lead to coupling in the absence of tin reagents. Tetrahedron Lett. 1995, 36, 3609–3612. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Diederich, F.; Stang, P.J. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.-M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef] [PubMed]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: A critical review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Zayat, M.; Garcia-Parejo, P.; Levy, D. Preventing UV-light damage of light sensitive materials using a highly protective UV-absorbing coating. Chem. Soc. Rev. 2007, 36, 1270–1281. [Google Scholar] [CrossRef]
- Andrady, A.L.; Hamid, H.S.; Torikai, A. Effects of climate change and UV-B on materials. Photochem. Photobiol. Sci. 2003, 2, 68–72. [Google Scholar] [CrossRef]
- Andrady, A.L.; Hamid, H.; Torikai, A. Effects of solar UV and climate change on materials. Photochem. Photobiol. Sci. 2011, 10, 292–300. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Nanogold plasmonic photocatalysis for organic synthesis and clean energy conversion. Chem. Soc. Rev. 2014, 43, 7188–7216. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.J.; Chang, S.-H.; Schatz, G.C.; Van Duyne, R.P.; Wiley, B.J.; Xia, Y. Localized surface plasmon resonance spectroscopy of single silver nanocubes. Nano Lett. 2005, 5, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-plasmon-driven hot electron photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.J.; Avanesian, T.; Christopher, P. Direct photocatalysis by plasmonic nanostructures. ACS Catal. 2014, 4, 116–128. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Li, Q.; Wang, J. Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 2013, 42, 2679–2724. [Google Scholar] [CrossRef] [PubMed]
- Nehl, C.L.; Hafner, J.H. Shape-dependent plasmon resonances of gold nanoparticles. J. Mater. Chem. 2008, 18, 2415–2419. [Google Scholar] [CrossRef]
- Farokhnezhad, M.; Esmaeilzadeh, M.; Nourian, M.; Jalaeikhoo, H.; Rajaeinejad, M.; Iravani, S.; Majidzadeh-A, K. Silica–gold nanoshell@graphene: A novel class of plasmonic nanoagents for photothermal cancer therapy. J. Phys. D Appl. Phys. 2020, 53, 405401. [Google Scholar] [CrossRef]
- Oldenburg, S.J.; Averitt, R.D.; Westcott, S.L.; Halas, N.J. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Chen, J.; Saeki, F.; Wiley, B.J.; Cang, H.; Cobb, M.J.; Li, Z.-Y.; Au, L.; Zhang, H.; Kimmey, M.B.; Li, X.; et al. Gold nanocages: Bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 2005, 5, 473–477. [Google Scholar] [CrossRef]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human domination of Earth’s ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-H.; Himeda, Y.; Muckerman, J.T.; Manbeck, G.F.; Fujita, E. CO2 hydrogenation to formate and methanol as an alternative to photo- and electrochemical CO2 reduction. Chem. Rev. 2015, 115, 12936–12973. [Google Scholar] [CrossRef]
- Nielsen, C.J.; Herrmann, H.; Weller, C. Atmospheric chemistry and environmental impact of the use of amines in carbon capture and storage (CCS). Chem. Soc. Rev. 2012, 41, 6684–6704. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Y.; Ye, W.; Li, Y. CO2 reduction: From the electrochemical to photochemical approach. Adv. Sci. 2017, 4, 1700194. [Google Scholar] [CrossRef]
- Dahl, S.; Chorkendorff, I. Towards practical implementation. Nat. Mater. 2012, 11, 100–101. [Google Scholar] [CrossRef]
- Schneider, J.; Jia, H.; Muckerman, J.T.; Fujita, E. Thermodynamics and kinetics of CO2, CO, and H+ binding to the metal centre of CO2 reduction catalysts. Chem. Soc. Rev. 2012, 41, 2036–2051. [Google Scholar] [CrossRef]
- Hu, B.; Guild, C.; Suib, S.L. Thermal, electrochemical, and photochemical conversion of CO2 to fuels and value-added products. J. CO2 Util. 2013, 1, 18–27. [Google Scholar] [CrossRef]
- Li, X.; Wen, J.; Low, J.; Fang, Y.; Yu, J. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Sci. China Mater. 2014, 57, 70–100. [Google Scholar] [CrossRef]
- Gao, D.; Arán-Ais, R.M.; Jeon, H.S.; Cuenya, B.R. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019, 2, 198–210. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.J.; Meyer, G.J.; Fujita, E. Molecular approaches to the photocatalytic reduction of carbon dioxide for solar fuels. Acc. Chem. Res. 2009, 42, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Kiesgen de_Richter, R.; Ming, T.; Caillol, S. Fighting global warming by photocatalytic reduction of CO2 using giant photocatalytic reactors. Renew. Sustain. Energy Rev. 2013, 19, 82–106. [Google Scholar] [CrossRef]
- Marszewski, M.; Cao, S.; Yu, J.; Jaroniec, M. Semiconductor-based photocatalytic CO2 conversion. Mater. Horiz. 2015, 2, 261–278. [Google Scholar] [CrossRef]
- Razzaq, A.; In, S.-I. TiO2 based nanostructures for photocatalytic CO2 conversion to valuable chemicals. Micromachines 2019, 10, 326. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.-H.; Le, T.-H.; Vo, D.-V.N.; Trinh, Q.T.; Bae, S.-R.; Chae, S.Y.; Kim, S.Y.; Le, Q.V. Recent advances in TiO2-based photocatalysts for reduction of CO2 to fuels. Nanomaterials 2020, 10, 337. [Google Scholar] [CrossRef]
- Kumar, D.; Lee, A.; Lee, T.; Lim, M.; Lim, D.-K. Ultrafast and efficient transport of hot plasmonic electrons by graphene for Pt Free, highly efficient visible-light responsive photocatalyst. Nano Lett. 2016, 16, 1760–1767. [Google Scholar] [CrossRef]
- Yu, S.; Wilson, A.J.; Kumari, G.; Zhang, X.; Jain, P.K. Opportunities and challenges of solar-energy-driven carbon dioxide to fuel conversion with plasmonic catalysts. ACS Energy Lett. 2017, 2, 2058–2070. [Google Scholar] [CrossRef]
- Liu, E.; Hu, Y.; Li, H.; Tang, C.; Hu, X.; Fan, J.; Chen, Y.; Bian, J. Photoconversion of CO2 to methanol over plasmonic Ag/TiO2 nano-wire films enhanced by overlapped visible-light-harvesting nanostructures. Ceram. Int. 2015, 41, 1049–1057. [Google Scholar] [CrossRef]
- Louis, C.; Pluchery, O. Gold Nanoparticles for Physics, Chemistry, and Biology; Imperial College Press: London, UK, 2012. [Google Scholar]
- Sarina, S.; Waclawik, E.R.; Zhu, H. Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chem. 2013, 15, 1814–1833. [Google Scholar] [CrossRef]
- Cao, L.; Sahu, S.; Anilkumar, P.; Bunker, C.E.; Xu, J.; Fernando, K.A.S.; Wang, P.; Guliants, E.A.; Tackett, K.N.; Sun, Y.-P. Carbon nanoparticles as visible-light photocatalysts for efficient CO2 conversion and beyond. J. Am. Chem. Soc. 2011, 133, 4754–4757. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Moss, J.A.; Baum, M.M. Artificial photosynthesis: Semiconductor photocatalytic fixation of CO2 to afford higher organic compounds. Dalton Trans. 2011, 40, 5151–5158. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhou, Y.; Li, H.; Li, P.; Zou, Z. Au@TiO2 yolk–shell hollow spheres for plasmon-induced photocatalytic reduction of CO2 to solar fuel via a local electromagnetic field. Nanoscale 2015, 7, 14232–14236. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wilson, A.J.; Heo, J.; Jain, P.K. Plasmonic control of multi-electron transfer and C-C coupling in visible-light-driven CO2 reduction on Au nanoparticles. Nano Lett. 2018, 18, 2189–2194. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, B.; Meng, L.; He, S.; Yuan, R.; Hou, Y.; Ding, Z.; Lin, H.; Zhang, Z.; Wang, X.; et al. Plasmonic control of solar-driven CO2 conversion at the metal/ZnO interfaces. Appl. Catal. B 2019, 256, 117823. [Google Scholar] [CrossRef]
- Becerra, J.; Nguyen, D.-T.; Gopalakrishnan, V.-N.; Do, T.-O. Plasmonic Au nanoparticles incorporated in the zeolitic imidazolate framework (ZIF-67) for the efficient sunlight-driven photoreduction of CO2. ACS Appl. Energy Mater. 2020, 3, 7659–7665. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Cai, B.; Wang, J.; Han, D.; Gan, S.; Zhang, Q.; Wu, Z.; Niu, L. Ternary alloyed AgClxBr1−x nanocrystals: Facile modulation of electronic structures toward advanced photocatalytic performance. Nanoscale 2013, 5, 10989–10995. [Google Scholar] [CrossRef]
- Cai, B.; Wang, J.; Gan, S.; Han, D.; Wu, Z.; Niu, L. A distinctive red Ag/AgCl photocatalyst with efficient photocatalytic oxidative and reductive activities. J. Mater. Chem. A 2014, 2, 5280–5286. [Google Scholar] [CrossRef]
- Li, N.; Jiang, R.; Li, Y.; Zhou, J.; Ma, Q.; Shen, S.; Liu, M. Plasma-assisted photocatalysis of CH4 and CO2 into ethylene. ACS Sustain. Chem. Eng. 2019, 7, 11455–11463. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; LaTempa, T.J.; Grimes, C.A. High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett. 2009, 9, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Tseng, I.-H.; Wu, J.C.-S. Chemical states of metal-loaded titania in the photoreduction of CO2. Catal. Today 2004, 97, 113–119. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.; Lee, J. Electrocatalytic production of C3-C4 compounds by conversion of CO2 on a chloride-induced Bi-phasic Cu2O-Cu catalyst. Angew. Chem. 2015, 127, 14914–14918. [Google Scholar] [CrossRef]
- Rawat, K.S.; Mahata, A.; Pathak, B. Thermochemical and electrochemical CO2 reduction on octahedral Cu nanocluster: Role of solvent towards product selectivity. J. Catal. 2017, 349, 118–127. [Google Scholar] [CrossRef]
- Shown, I.; Hsu, H.-C.; Chang, Y.-C.; Lin, C.-H.; Roy, P.K.; Ganguly, A.; Wang, C.-H.; Chang, J.-K.; Wu, C.-I.; Chen, L.-C.; et al. Highly efficient visible light photocatalytic reduction of CO2 to hydrocarbon fuels by Cu nanoparticle decorated graphene oxide. Nano Lett. 2014, 14, 6097–6103. [Google Scholar] [CrossRef]
- Park, H.; Ou, H.-H.; Colussi, A.J.; Hoffmann, M.R. Artificial photosynthesis of C1–C3 hydrocarbons from water and CO2 on titanate nanotubes decorated with nanoparticle elemental copper and CdS quantum dots. J. Phys. Chem. A 2015, 119, 4658–4666. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Ma, Y.; Li, Y.; Cai, J.; Li, Z. Coupling photocatalytic CO2 reduction with benzyl alcohol oxidation to produce benzyl acetate over Cu2O/Cu. Catal. Sci. Technol. 2018, 8, 2218–2223. [Google Scholar] [CrossRef]
- Maluenda, I.; Navarro, O. Recent developments in the Suzuki–Miyaura reaction: 2010–2014. Molecules 2015, 20, 7528–7557. [Google Scholar] [CrossRef]
- Teranishi, T.; Eguchi, M.; Kanehara, M.; Gwo, S. Controlled localized surface plasmon resonance wavelength for conductive nanoparticles over the ultraviolet to near-infrared region. J. Mater. Chem. 2011, 21, 10238–10242. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A. Progress, challenge, and perspective of bimetallic TiO2-based photocatalysts. J. Nanomater. 2014, 2014, 208920. [Google Scholar] [CrossRef]
- Wali, L.A.; Alwan, A.M.; Dheyab, A.B.; Hashim, D.A. Excellent fabrication of Pd-Ag NPs/PSi photocatalyst based on bimetallic nanoparticles for improving methylene blue photocatalytic degradation. Optik 2019, 179, 708–717. [Google Scholar] [CrossRef]
- Lee, S.; Wy, Y.; Lee, Y.W.; Ham, K.; Han, S.W. Core–shell nanoparticle clusters enable synergistic integration of plasmonic and catalytic functions in a single platform. Small 2017, 13, 1701633. [Google Scholar] [CrossRef]
- Furube, A.; Hashimoto, S. Insight into plasmonic hot-electron transfer and plasmon molecular drive: New dimensions in energy conversion and nanofabrication. NPG Asia Mater. 2017, 9, e454. [Google Scholar] [CrossRef]
- Shan, H.; Yu, Y.; Wang, X.; Luo, Y.; Zu, S.; Du, B.; Han, T.; Li, B.; Li, Y.; Wu, J.; et al. Direct observation of ultrafast plasmonic hot electron transfer in the strong coupling regime. Light Sci. Appl. 2019, 8, 9. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Chen, Y.; Zhou, H.; Duan, X.; Huang, Y. Plasmonic and catalytic AuPd nanowheels for the efficient conversion of light Into chemical energy. Angew. Chem. Int. Ed. 2013, 52, 6063–6067. [Google Scholar] [CrossRef]
- Gangishetty, M.K.; Fontes, A.M.; Malta, M.; Kelly, T.L.; Scott, R.W.J. Improving the rates of Pd-catalyzed reactions by exciting the surface plasmons of AuPd bimetallic nanotriangles. RSC Adv. 2017, 7, 40218–40226. [Google Scholar] [CrossRef]
- Sarina, S.; Zhu, H.; Jaatinen, E.; Xiao, Q.; Liu, H.; Jia, J.; Chen, C.; Zhao, J. Enhancing catalytic performance of palladium in gold and palladium alloy nanoparticles for organic synthesis reactions through visible light irradiation at ambient temperatures. J. Am. Chem. Soc. 2013, 135, 5793–5801. [Google Scholar] [CrossRef]
- Xiao, Q.; Sarina, S.; Jaatinen, E.; Jia, J.; Arnold, D.P.; Liu, H.; Zhu, H. Efficient photocatalytic Suzuki cross-coupling reactions on Au-Pd alloy nanoparticles under visible light irradiation. Green Chem. 2014, 16, 4272–4285. [Google Scholar] [CrossRef]
- Xiao, Q.; Sarina, S.; Bo, A.; Jia, J.; Liu, H.; Arnold, D.P.; Huang, Y.; Wu, H.; Zhu, H. Visible light-driven cross-coupling reactions at lower temperatures using a photocatalyst of palladium and gold alloy nanoparticles. ACS Catal. 2014, 4, 1725–1734. [Google Scholar] [CrossRef]
- Sarina, S.; Jaatinen, E.; Xiao, Q.; Huang, Y.M.; Christopher, P.; Zhao, J.C.; Zhu, H.Y. Photon energy threshold in direct photocatalysis with metal nanoparticles: Key evidence from the action spectrum of the reaction. J. Phys. Chem. Lett. 2017, 8, 2526–2534. [Google Scholar] [CrossRef]
- Zhang, S.; Chang, C.; Huang, Z.; Ma, Y.; Gao, W.; Li, J.; Qu, Y. Visible-light-activated Suzuki–Miyaura coupling reactions of aryl chlorides over the multifunctional Pd/Au/porous nanorods of CeO2 catalysts. ACS Catal. 2015, 5, 6481–6488. [Google Scholar] [CrossRef]
- Movahed, S.K.; Miraghaee, S.; Dabiri, M. AuPd alloy nanoparticles decorated graphitic carbon nitride as an excellent photocatalyst for the visible-light-enhanced Suzuki–Miyaura cross-coupling reaction. J. Alloys Compd. 2020, 819, 152994. [Google Scholar] [CrossRef]
- Subudhi, S.; Mansingh, S.; Tripathy, S.P.; Mohanty, A.; Mohapatra, P.; Rath, D.; Parida, K. The fabrication of Au/Pd plasmonic alloys on UiO-66-NH2: An efficient visible light-induced photocatalyst towards the Suzuki–Miyaura coupling reaction under ambient conditions. Catal. Sci. Technol. 2019, 9, 6585–6597. [Google Scholar] [CrossRef]
- Kataria, M.; Kumar, M.; Singh, Z.; Bhalla, V. Gold nanoparticles immobilized polymeric PBI derivative: Productive, portable, and photocatalytic system for Heck coupling. ACS Sustain. Chem. Eng. 2018, 6, 8223–8229. [Google Scholar] [CrossRef]
- Nemygina, N.A.; Nikoshvili, L.Z.; Tiamina, I.Y.; Bykov, A.V.; Smirnov, I.S.; LaGrange, T.; Kaszkur, Z.; Matveeva, V.G.; Sulman, E.M.; Kiwi-Minsker, L. Au core–Pd shell bimetallic nanoparticlesi within hyper-cross-linked polystyrene for mechanistic study of Suzuki cross-coupling: Homogeneous or heterogeneous catalysis? Org. Process Res. Dev. 2018, 22, 1606–1613. [Google Scholar] [CrossRef]
- Han, D.; Bao, Z.; Xing, H.; Yang, Y.; Ren, Q.; Zhang, Z. Fabrication of plasmonic Au-Pd alloy nanoparticles for photocatalytic Suzuki–Miyaura reactions under ambient conditions. Nanoscale 2017, 9, 6026–6032. [Google Scholar] [CrossRef]
- Tyagi, A.; Yamamoto, A.; Yoshida, H. Photocatalytic Ullmann coupling of aryl halides by a novel blended catalyst consisting of a TiO2 photocatalyst and an Al2O3 supported Pd-Au bimetallic catalyst. Catal. Sci. Technol. 2018, 8, 6196–6203. [Google Scholar] [CrossRef]
- Tyagi, A.; Yamamoto, A.; Yoshida, H. Novel blended catalysts consisting of a TiO2 photocatalyst and an Al2O3 supported Pd-Au bimetallic catalyst for direct dehydrogenative cross-coupling between arenes and tetrahydrofuran. RSC Adv. 2018, 8, 24021–24028. [Google Scholar] [CrossRef]
- Rohani, S.; Ziarati, A.; Ziarani, G.M.; Badiei, A.; Burgi, T. Engineering of highly active Au/Pd supported on hydrogenated urchin-like yolk@shell TiO2 for visible light photocatalytic Suzuki coupling. Catal. Sci. Technol. 2019, 9, 3820–3827. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Pd/Ag and Pd/Au bimetallic nanocatalysts on mesoporous silica for plasmon-mediated enhanced catalytic activity under visible light irradiation. J. Mater. Chem. A 2016, 4, 10142–10150. [Google Scholar] [CrossRef]
- Li, X.-H.; Baar, M.; Blechert, S.; Antonietti, M. Facilitating room-temperature Suzuki coupling reaction with light: Mott-Schottky photocatalyst for C-C coupling. Sci. Rep. 2013, 3, 1743. [Google Scholar] [CrossRef]
- Shin, H.H.; Kang, E.; Park, H.; Han, T.; Lee, C.-H.; Lim, D.-K. Pd-nanodot decorated MoS2 nanosheets as a highly efficient photocatalyst for the visible-light-induced Suzuki–Miyaura coupling reaction. J. Mater. Chem. A 2017, 5, 24965–24971. [Google Scholar] [CrossRef]
- Raza, F.; Yim, D.; Park, J.H.; Kim, H.-I.; Jeon, S.-J.; Kim, J.-H. Structuring Pd nanoparticles on 2H-WS2 nanosheets induces excellent photocatalytic activity for cross-coupling reactions under visible light. J. Am. Chem. Soc. 2017, 139, 14767–14774. [Google Scholar] [CrossRef]
- Chen, J.-H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S. Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nat. Nanotechnol. 2008, 3, 206–209. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Kang, E.; Shin, H.H.; Lim, D.-K. Interface-controlled Pd nanodot-Au nanoparticle colloids for efficient visible-light-induced photocatalytic Suzuki–Miyaura coupling reaction. Catalysts 2018, 8, 463. [Google Scholar] [CrossRef]
- Sahoo, M.; Mansingh, S.; Subudhi, S.; Mohapatra, P.; Parida, K. A plasmonic AuPd bimetallic nanoalloy decorated over a GO/LDH hybrid nanocomposite via a green synthesis route for robust Suzuki coupling reactions: A paradigm shift towards a sustainable future. Catal. Sci. Technol. 2019, 9, 4678–4692. [Google Scholar] [CrossRef]
- Shin, H.H.; Han, T.; Yang, W.; Lim, D.-K. Pd2+-conjugated reduced graphene oxide as a highly efficient visible-light-induced photocatalytic Suzuki–Miyaura coupling. Carbon 2019, 143, 362–370. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Cheng, Q.; Dou, S.X.; Du, Y. Near-infrared-driven photocatalysts: Design, construction, and applications. Small 2019, 1904107. [Google Scholar] [CrossRef]
- Murphy, C.J.; Sau, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 2005, 109, 13857–13870. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Khlebtsov, N.G. Multipole plasmons in metal nanorods: Scaling properties and dependence on particle size, shape, orientation, and dielectric environment. J. Phys. Chem. C 2007, 111, 11516–11527. [Google Scholar] [CrossRef]
- Encina, E.R.; Coronado, E.A. Resonance conditions for multipole plasmon excitations in noble metal nanorods. J. Phys. Chem. C 2007, 111, 16796–16801. [Google Scholar] [CrossRef]
- Wang, J.-H.; Chen, M.; Luo, Z.-J.; Ma, L.; Zhang, Y.-F.; Chen, K.; Zhou, L.; Wang, Q.-Q. Ceria-coated gold nanorods for plasmon-enhanced near-infrared photocatalytic and photoelectrochemical performances. J. Phys. Chem. C 2016, 120, 14805–14812. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Chen, H.; Jiang, R.; Sun, L.-D.; Li, Q.; Wang, J.; Yu, J.C.; Yan, C.-H. Plasmonic harvesting of light energy for Suzuki coupling reactions. J. Am. Chem. Soc. 2013, 135, 5588–5601. [Google Scholar] [CrossRef]
- Wen, M.; Takakura, S.; Fuku, K.; Mori, K.; Yamashita, H. Enhancement of Pd-catalyzed Suzuki–Miyaura coupling reaction assisted by localized surface plasmon resonance of Au nanorods. Catal. Today 2015, 242, 381–385. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Shi, L.; Zhu, Y.; Mideksa, M.F.; Hou, K.; Zhao, W.; Wang, D.; Zhao, M.; Zhang, X.; et al. Boosting hot electrons in hetero-superstructures for plasmon-enhanced catalysis. J. Am. Chem. Soc. 2017, 139, 17964–17972. [Google Scholar] [CrossRef]
- Verkaaik, M.; Grote, R.; Meulendijks, N.; Sastre, F.; Weckhuysen, B.M.; Buskens, P. Suzuki-Miyaura cross-coupling using plasmonic Pd-decorated Au nanorods as catalyst: A study on the contribution of laser illumination. ChemCatChem 2019, 11, 4974–4980. [Google Scholar] [CrossRef]
- Yoshii, T.; Kuwahara, Y.; Mori, K.; Yamashita, H. Design of Pd–graphene–Au nanorod nanocomposite catalyst for boosting Suzuki–Miyaura coupling reaction by assistance of surface plasmon resonance. J. Phys. Chem. C 2019, 123, 24575–24583. [Google Scholar] [CrossRef]
- Gao, S.; Shang, N.; Feng, C.; Wang, C.; Wang, Z. Graphene oxide–palladium modified Ag-AgBr: A visible-light-responsive photocatalyst for the Suzuki coupling reaction. RSC Adv. 2014, 4, 39242–39247. [Google Scholar] [CrossRef]
- Verma, P.; Kuwahara, Y.; Mori, K.; Yamashita, H. Synthesis and characterization of a Pd/Ag bimetallic nanocatalyst on SBA-15 mesoporous silica as a plasmonic catalyst. J. Mater. Chem. A 2015, 3, 18889–18897. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, M.; Bhalla, V. Aggregates of the pentacenequinone derivative as reactors for the preparation of Ag@Cu2O core-shell NPs: An active photocatalyst for Suzuki and Suzuki type coupling reactions. Chem. Commun. 2015, 51, 12529–12532. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.; Kumar, M.; Bhalla, V. Development of a supramolecular ensemble of an AIEE active hexaphenylbenzene derivative and Ag@Cu2O Ccore-shell NPs: An efficient photocatalytic system for C-H activation. Chem. Commun. 2016, 52, 10179–10182. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, L. Silver nanoparticles doped TiO2 catalyzed Suzuki coupling of bromoaryl with phenylboronic acid under visible light. J. Photochem. Photobiol. B 2020, 205, 111807. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.H.; Zhao, J.; Hicks, E.M.; Schatz, G.C.; Van Duyne, R.P. Plasmonic properties of copper nanoparticles fabricated by nanosphere lithography. Nano Lett. 2007, 7, 1947–1952. [Google Scholar] [CrossRef]
- Liu, P.; Wang, H.; Li, X.; Rui, M.; Zeng, H. Localized surface plasmon resonance of Cu nanoparticles by laser ablation in liquid media. RSC Adv. 2015, 5, 79738–79745. [Google Scholar] [CrossRef]
- Gezgin, S.Y.; Kepceoğlu, A.; Kılıç, H.Ş. An experimental investigation of localised surface plasmon resonance (LSPR) for Cu nanoparticles depending as a function of laser pulse number in pulsed laser deposition. AIP Conf. Proc. 2017, 1815, 030020. [Google Scholar]
- Kim, J.H.; Ehrman, S.H.; Germer, T.A. Influence of particle oxide coating on light scattering by submicron metal particles on silicon wafers. Appl. Phys. Lett. 2004, 84, 1278–1280. [Google Scholar] [CrossRef]
- DeSario, P.A.; Pietron, J.J.; Brintlinger, T.H.; McEntee, M.; Parker, J.F.; Baturina, O.; Stroud, R.M.; Rolison, D.R. Oxidation-stable plasmonic copper nanoparticles in photocatalytic TiO2 nanoarchitectures. Nanoscale 2017, 9, 11720–11729. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z.; Gao, G.; Xiao, G.; Du, A.; Bottle, S.; Sarina, S.; Zhu, H. Stable copper nanoparticle photocatalysts for selective epoxidation of alkenes with visible light. ACS Catal. 2017, 7, 4975–4985. [Google Scholar] [CrossRef]
- Zhang, P.; Song, T.; Wang, T.; Zeng, H. Plasmonic Cu nanoparticle on reduced graphene oxide nanosheet support: An efficient photocatalyst for improvement of near-infrared photocatalytic H2 evolution. Appl. Catal. B 2018, 225, 172–179. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Li, J.; Guo, X.; Bai, G.; Tong, X.; Jin, G.; Guo, X. Photocatalytic Sonogashira reaction over silicon carbide supported Pd-Cu alloy nanoparticles under visible light irradiation. Catal. Sci. Technol. 2018, 8, 3357–3362. [Google Scholar] [CrossRef]
- Sun, D.; Xu, M.; Jiang, Y.; Long, J.; Li, Z. Small-sized bimetallic CuPd nanoclusters encapsulated inside cavity of NH2-UiO-66(Zr) with superior performance for light-induced Suzuki coupling reaction. Small Methods 2018, 2, 1800164. [Google Scholar] [CrossRef]
- Guo, X.; Hao, C.; Jin, G.; Zhu, H.-Y.; Guo, X.-Y. Copper nanoparticles on graphene support: An efficient photocatalyst for coupling of nitroaromatics in visible light. Angew. Chem. Int. Ed. 2014, 53, 1973–1977. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.-L.; Guo, X.-N.; Wang, Y.-Y.; Guo, X.-Y. Visible-light-driven photocatalytic N-arylation of imidazole derivatives and arylboronic acids on Cu/graphene catalyst. Sci. Rep. 2015, 5, 12005. [Google Scholar] [CrossRef]
- Kaur, S.; Kumar, M.; Bhalla, V. Supramolecular ensemble of PBI derivative and copper nanoparticles: A light harvesting antenna for photocatalytic C(sp2)–H functionalization. Green Chem. 2016, 18, 5870–5883. [Google Scholar] [CrossRef]
- Li, M.; Xing, X.; Ma, Z.; Lv, J.; Fu, P.; Li, Z. Synthesis of composition tunable and (111)-faceted Cu/Cu2O nanoparticles toward photocatalytic, ligand-free, and solvent-free C-N Ullmann coupling reactions. ACS Sustain. Chem. Eng. 2018, 6, 5495–5503. [Google Scholar] [CrossRef]
- Trinh, T.T.; Sato, R.; Sakamoto, M.; Fujiyoshi, Y.; Haruta, M.; Kurata, H.; Teranishi, T. Visible to near-infrared plasmon-enhanced catalytic activity of Pd hexagonal nanoplates for the Suzuki coupling reaction. Nanoscale 2015, 7, 12435–12444. [Google Scholar] [CrossRef]
- Cui, J.; Li, Y.; Liu, L.; Chen, L.; Xu, J.; Ma, J.; Fang, G.; Zhu, E.; Wu, H.; Zhao, L.; et al. Near-infrared plasmonic-enhanced solar energy harvest for highly efficient photocatalytic reactions. Nano Lett. 2015, 15, 6295–6301. [Google Scholar] [CrossRef]
- Lou, Z.; Gu, Q.; Liao, Y.; Yu, S.; Xue, C. Promoting Pd-catalyzed Suzuki coupling reactions through near-infrared plasmon excitation of WO3−x nanowires. Appl. Catal. B 2016, 184, 258–263. [Google Scholar] [CrossRef]
- Sarhid, I.; Abdellah, I.; Martini, C.; Huc, V.; Dragoe, D.; Beaunier, P.; Lampre, I.; Remita, H. Plasmonic catalysis for the Suzuki–Miyaura cross-coupling reaction using palladium nanoflowers. New J. Chem. 2019, 43, 4349–4355. [Google Scholar] [CrossRef]
- Koohgard, M.; Hosseini-Sarvari, M. Enhancement of Suzuki–Miyaura coupling reaction by photocatalytic palladium nanoparticles anchored to TiO2 under visible light irradiation. Catal. Commun. 2018, 111, 10–15. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Bazyar, Z. Visible light driven photocatalytic cross-coupling reactions on nano Pd/ZnO photocatalyst at room-temperature. ChemistrySelect 2018, 3, 1898–1907. [Google Scholar] [CrossRef]
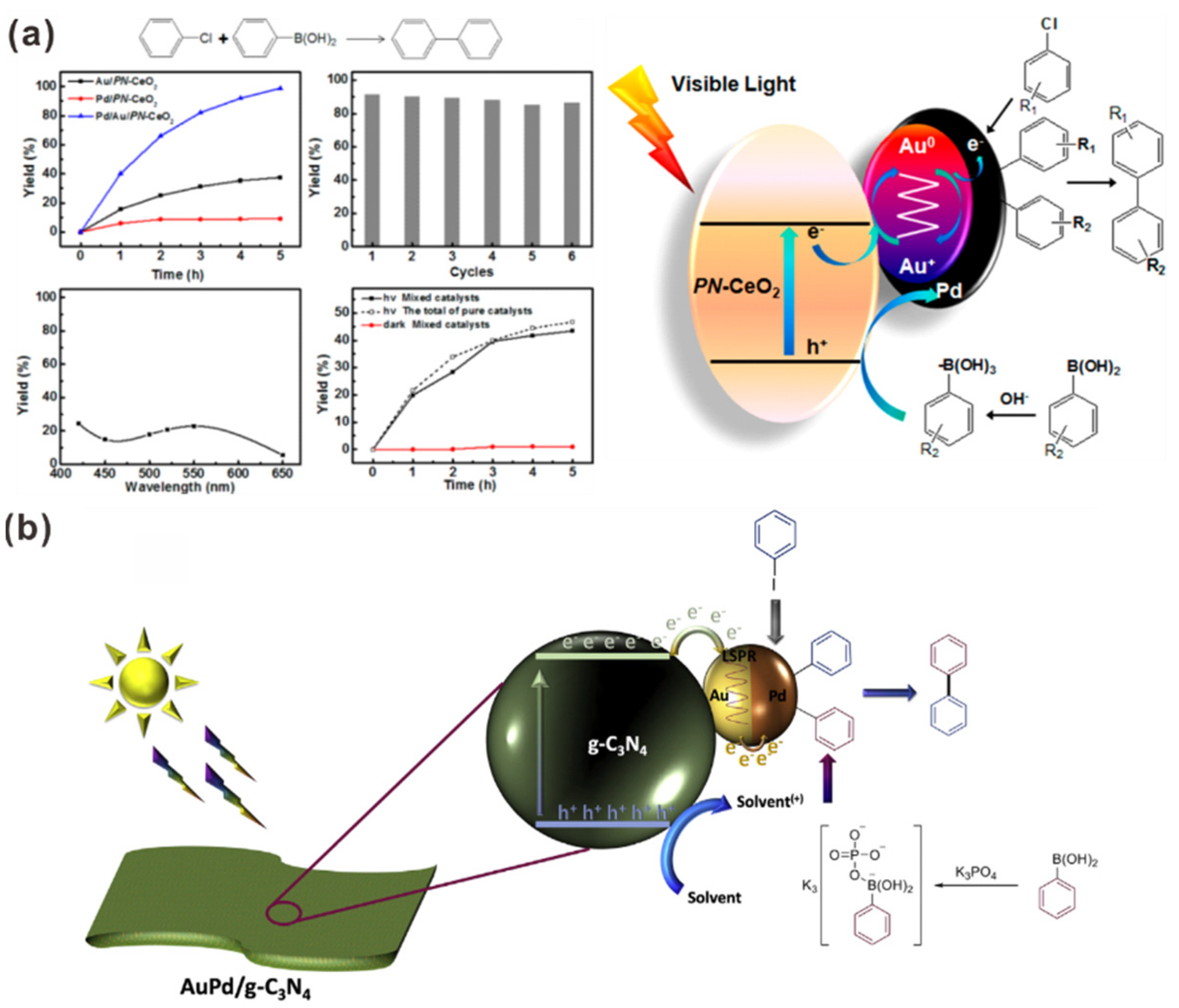
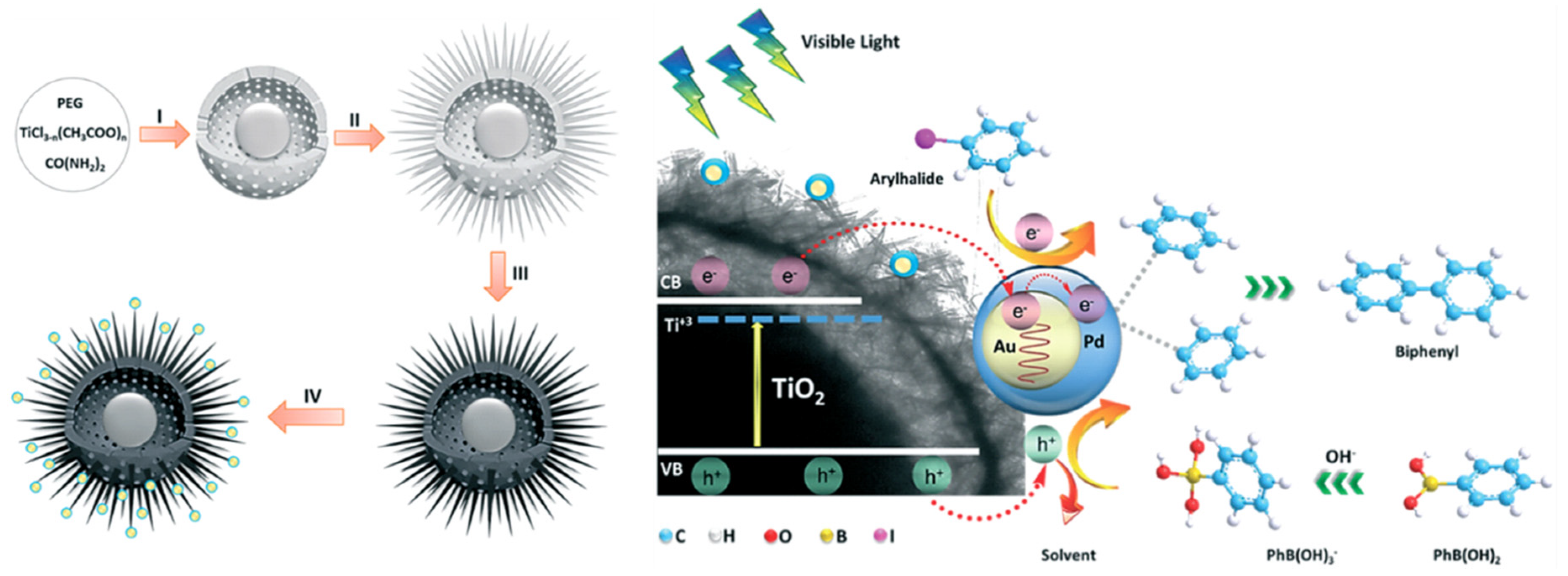
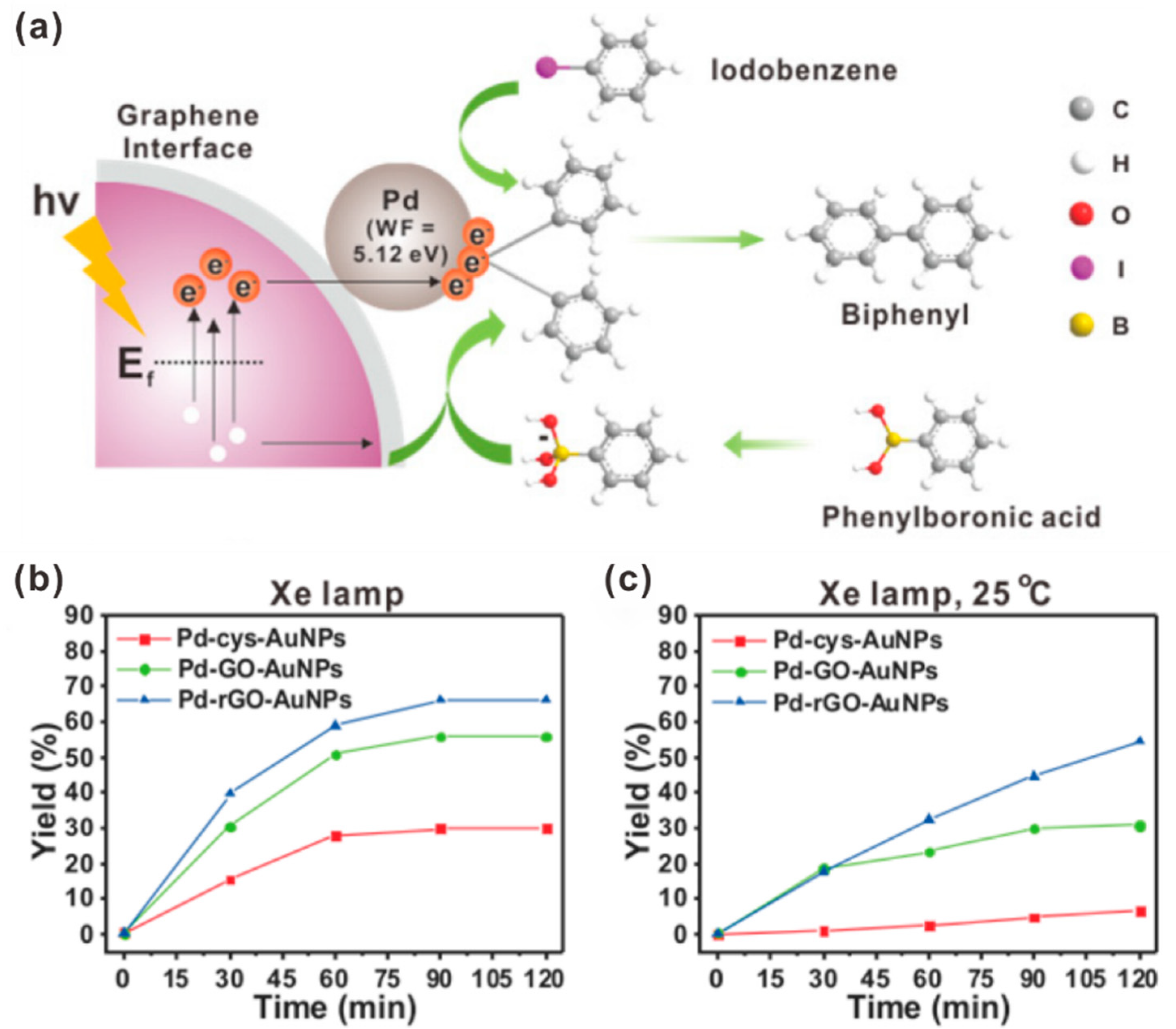
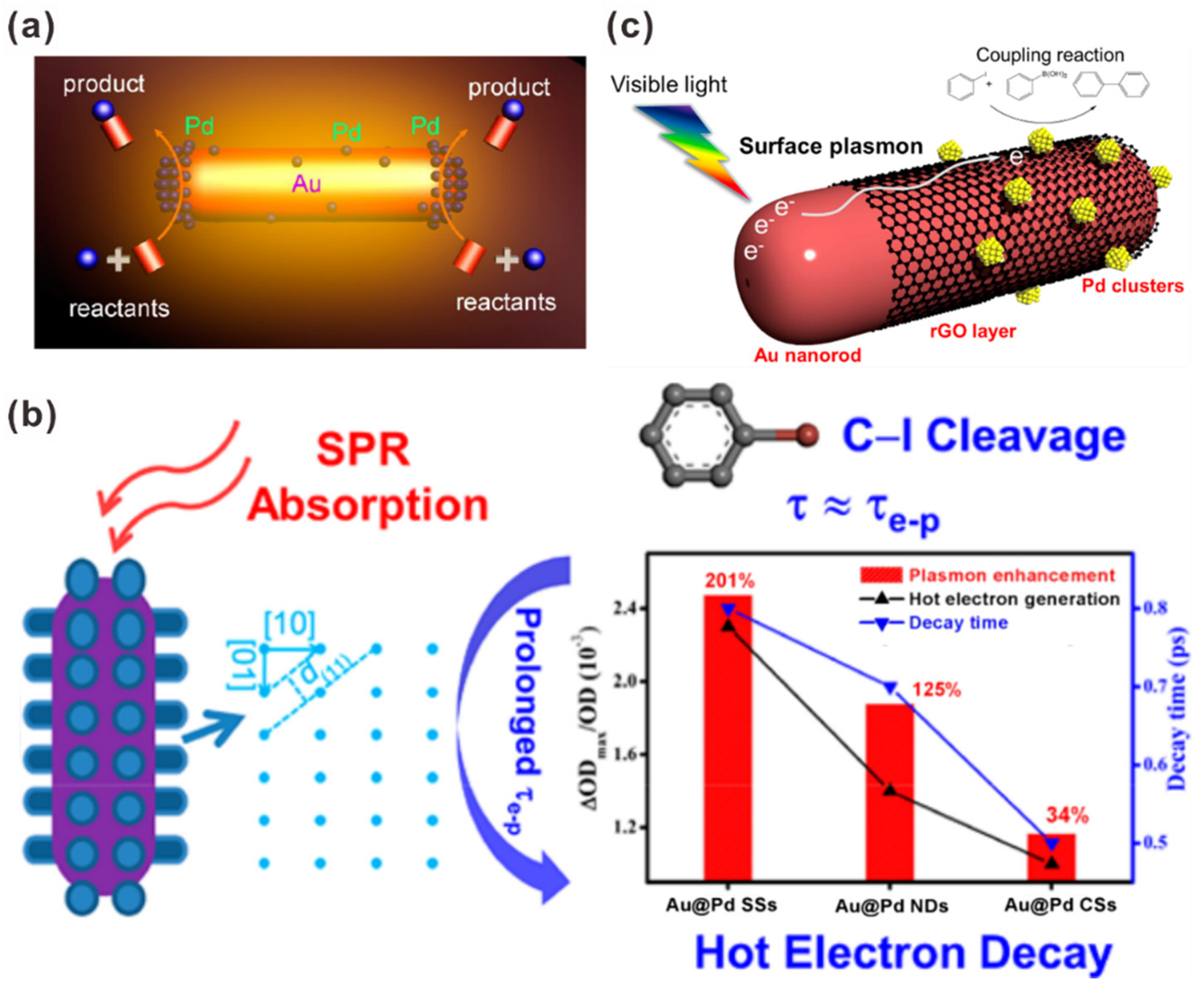
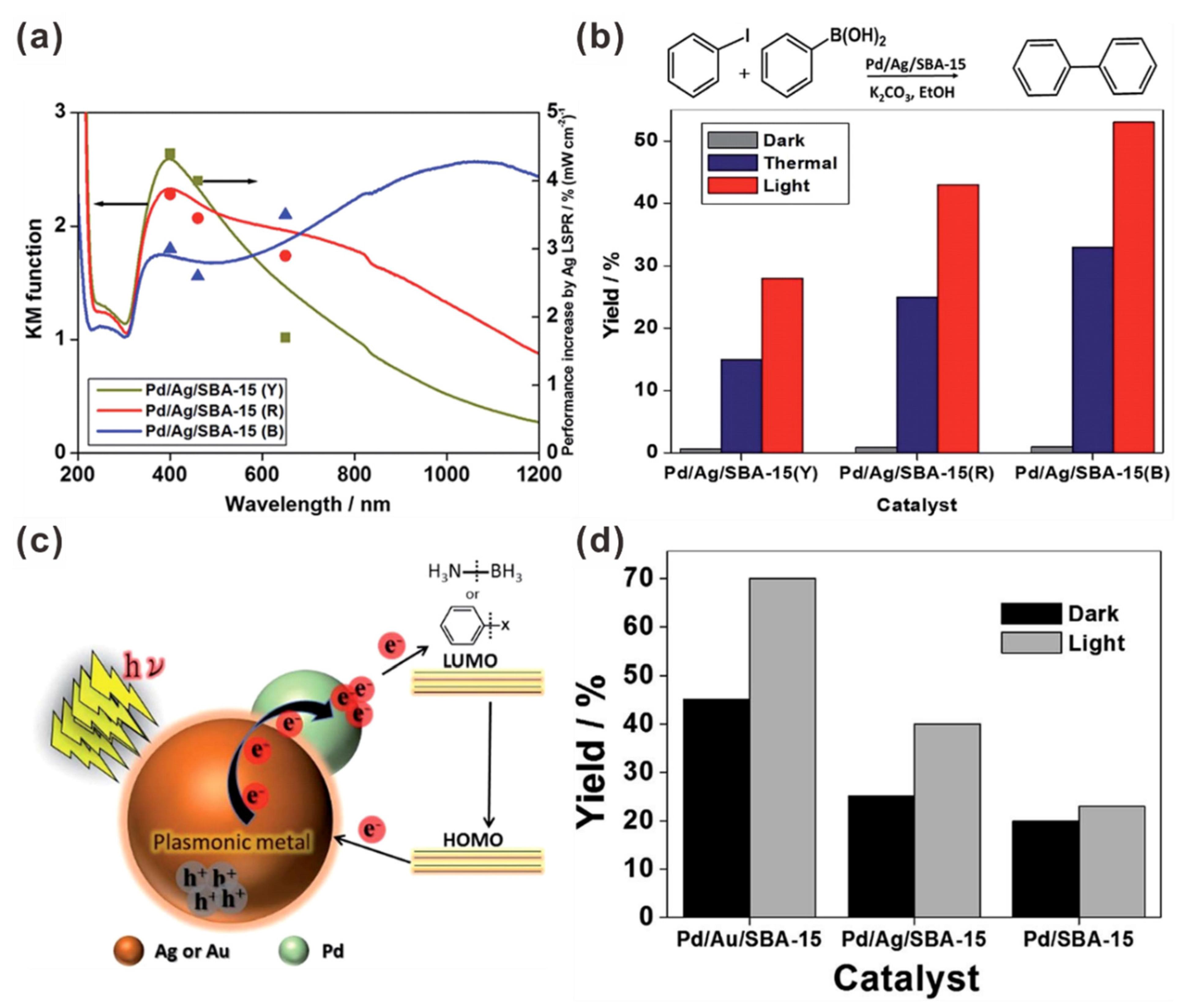
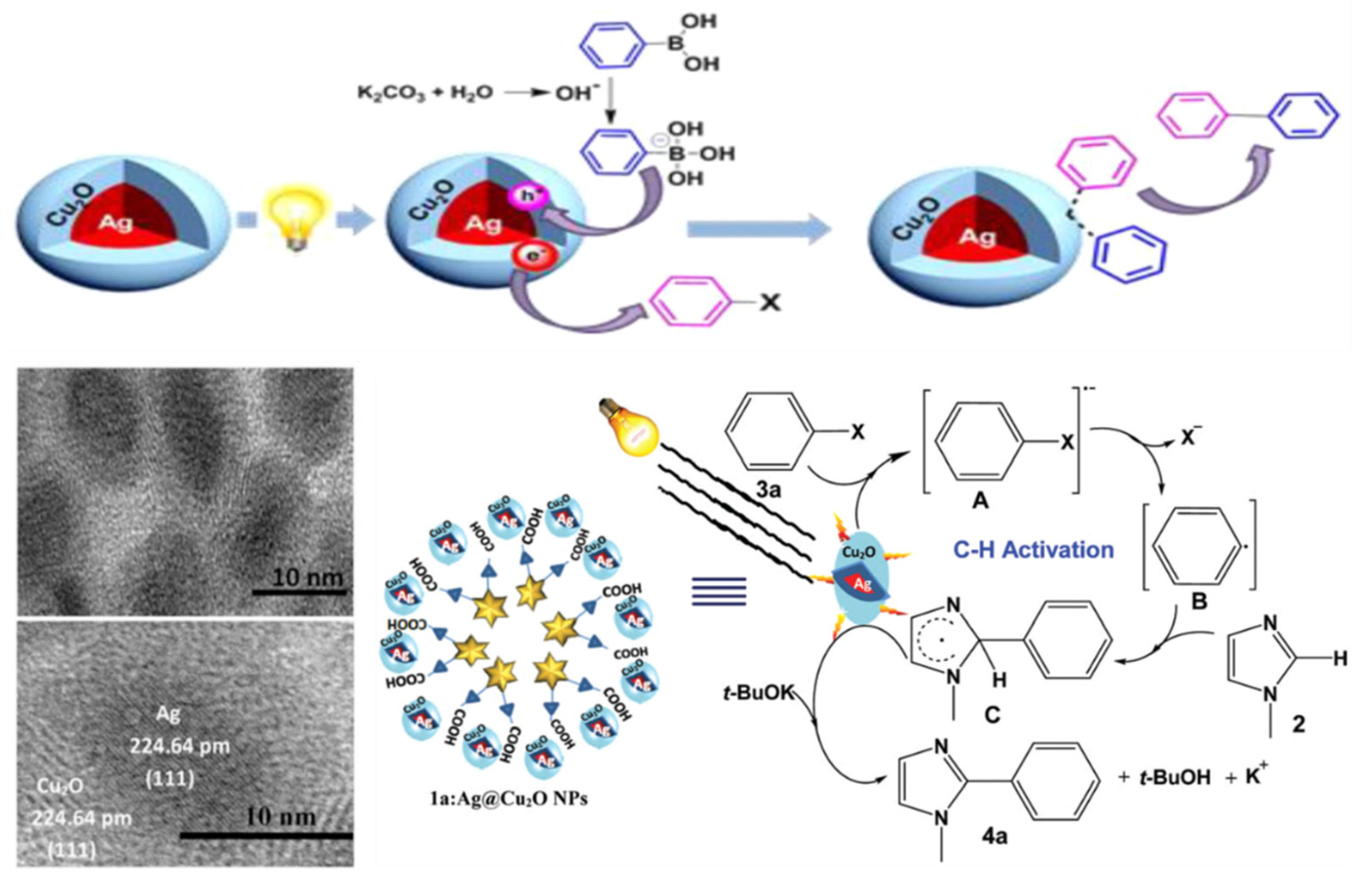
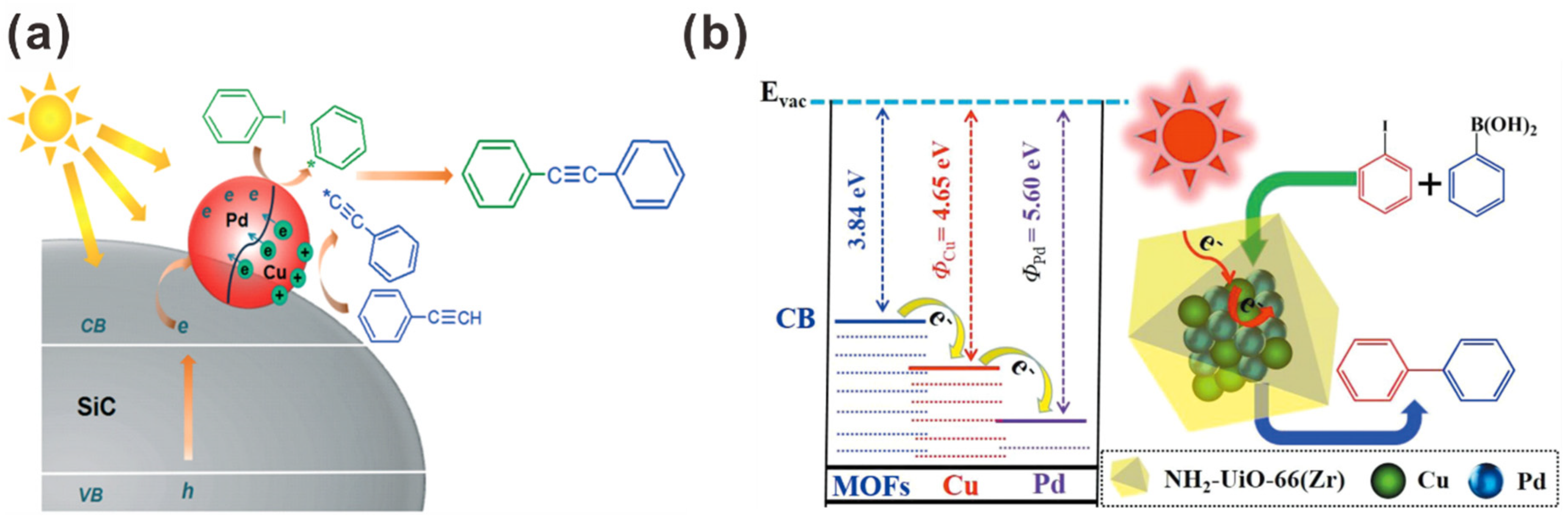
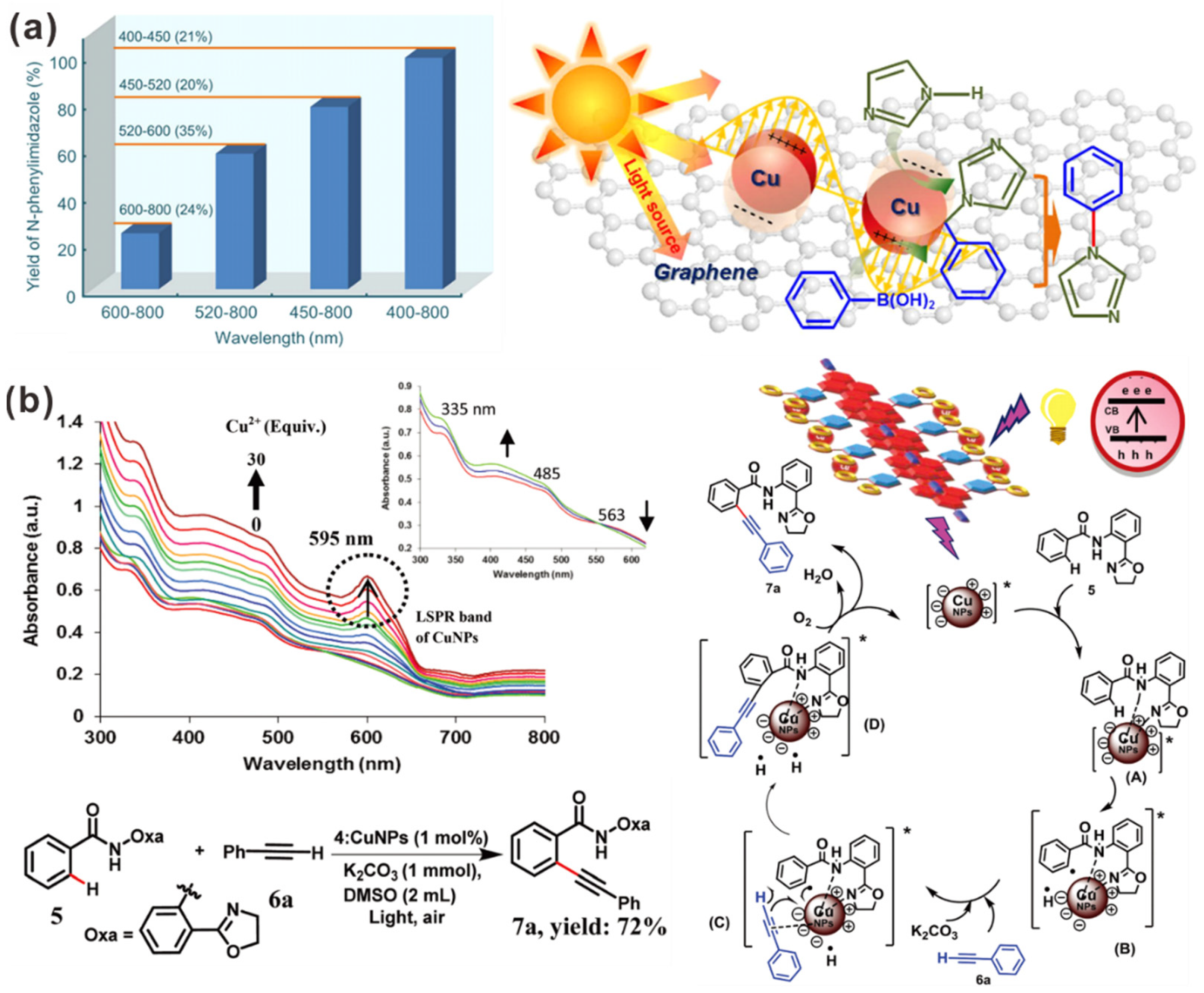
| Photocatalyst | Size and Shape | Product | Light Source | Reaction Condition | Time | Yield (μmoL g−1 h−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Au@TiO2 | Spherical core: 45 nm Spherical shell: 200–250 nm (50 nm thickness) | CH4, C2H6 | 300 W Xe arc lamp | H2O | 10 h | 2.52 (CH4), 1.67 (C2H6) | [76] |
| AuNPs | 11.8 ± 2.3 nm spherical NPs | CH4, C2H6 | 300 W Xe lamp | 10% IPA | 10 h | 0.65 (CH4), 0.56 (C2H6) as TOF (NP−1h−1) | [77] |
| Au/ZnO | AuNPs: 7 nm ZnO sheets: >μm (1.6 nm thickness, 10–100 nm pores) | CH4, C2H6 | 300 W Xe lamp (>320 nm) | H2O | 1–6 h | 21.0 (CH4), 27.0 (C2H6) | [78] |
| Au20@ZIF-67 | AuNPs: 30–40 nm ZIF-67: ~μm | CH3OH C2H5OH | Solar simulator (150 mW cm−2) | 10 wt.% TEOA, 0.08 M NaHCO3 | 4 h | 1623 (CH3OH), 495 (C2H5OH) | [79] |
| AgCl0.75Br0.25 | Cubic nanocrystals 150–260 nm | CH3OH C2H5OH | 500 W Xe arc lamp (>420 nm) | 0.1 M NaHCO3 | 5 h | 36.2 (CH3OH), 72.4 (C2H5OH) | [81] |
| Ag/AgCl | Coaxial tri-cubic 500–600 nm | CH3OH C2H5OH | 500 W Xe arc lamp (>420 nm) | 0.1 M NaHCO3 | 5 h | 29.2 (CH3OH), 44.6 (C2H5OH) | [82] |
| Ag/TiO2 | AgNPs: 4 nm TiO2: 25 nm | CO C2H4 | Xe lamp | Quartz cotton, micro- autoclave | 2 h | 1149 (CO) 686 (C2H4) | [83] |
| Cu/GO | Cu (111) NPs: 5 nm GO: >μm | CH3OH CH3CHO | Halogen lamp (300 W) | Continuous gas flow reactor | 2 h | 2.94 (CH3OH), 3.88 (CH3CHO) | [88] |
| CdS/(Cu-TNTs) | Hexagonal CdS | CH4 C2H6 C3H8 | 450 W Xe lamp (>420 nm) | H2O | 5 h | ~28 (CH4), ~17 (C2H6), ~9 (C3H8) μL g−1 h−1 | [89] |
| Cu2O/Cu | Irregular porous structures 100 nm | Benzyl acetate | 300 W Xe lamp (420–800 nm) | MeCN, benzyl alcohol | 20 h | 116.7 | [90] |
| Photocatalyst | Size and Shape | Reaction | Light Source | Reaction Temp. | Solvent, Base | Time | Yield | TOF (h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| AuPd wheels | Nano-wheels 290 nm (6 nm thickness) | Suzuki | Xe lamp | 50 °C | EtOH/H2O (9:1), K2CO3 | 1 h | 65.8% | - | [98] |
| AuPd nanotriangles | Nanotriangles 43 ± 4 nm | Suzuki | Green LED (450–600 nm) | - | EtOH/H2O (1:6), K2CO3 | 5 h | >80% | - | [99] |
| Au–Pd alloy NPs/ZrO2 | Au-Pd NPs: <8 nm | Suzuki | 500 W halogen lamp (400–750 nm) | 30 °C | DMF/H2O (3:1), K2CO3 | 6 h | 96% | 14.5 | [100] |
| Au–Pd alloy NPs/ZrO2 | Au-Pd NPs: <7 nm | Sonogashira or Stille | Halogen lamp (400–750 nm) | 45 °C | H2O, K3PO4 or NaOH | 24 h | 80% −81% | 4.7 −4.8 | [102] |
| Au–Pd alloy NPs/ZrO2 | Au-Pd NPs: <7 nm | Suzuki | Halogen lamp (400–750 nm) | 30 °C | DMF/H2O (3:1), K2CO3 | 6 h | 96% | 14.5 | [101] |
| Pd/Au/CeO2 | AuNPs (111): 4.28 ± 1.05 nm PdNPs (111): 5.14 ± 1.01 nm CeO2 nanorods: ~5 nm (width), ~30 nm (length) | Suzuki | 150 W Xe lamp (>400 nm) | 25 °C | DMF/H2O (1:1), K2CO3 | 5 h | 98.8% | - | [104] |
| Pd/Au/SBA-15 | Pd/Au NPs: 4.9 nm SBA-15: >μm | Suzuki | Xe lamp | Room Temp. | EtOH K2CO3 | 2 h | 70% | - | [113] |
| Au–Pd alloy NPs/TiO2 | Au-Pd NPs: 3 nm | Suzuki | 5 W blue LED lamp | 25 °C | EtOH/H2O (1:1), K2CO3 | 5 h | 98% | - | [109] |
| Pd-rGO-AuNPs | Pd nanodots: 2–3 nm AuNPs: ~30 nm | Suzuki | Xe lamp (400–800 nm) | 25 °C | EtOH/H2O (1:1), K2CO3 | 2 h | 54.5% | - | [119] |
| Au–Pd/HPS | AuNPs core: ~4 nm Pd shell: <1 nm HPS: 15–50 nm | Suzuki | 300 W filament lamp | 60 °C | EtOH/H2O (5:1), NaOH | 3 h | 71.6% | 130.7 | [108] |
| TiO2 + PdAu/Al2O3 | - | Ullmann | Xe lamp (≥350 nm) | Room Temp. | CH3CN | 0.5 h | 2.2% | - | [110] |
| TiO2 + PdAu/Al2O3 | PdAu NPs: 3–4 nm | Dehydrogenative cross-coupling | Xe lamp (≥350 nm) | Room Temp. | - | 1 h | 15.2 μmol | - | [111] |
| Supramolecular Polymer 5:AuNPs | Supramolecule: ~μm AuNPs: <30 nm | Heck | 100 W tungsten filament blub | Room Temp. | H2O, K2CO3 | 1 h | 89% | - | [107] |
| GO/LDH @AuPd | AuPd NPs: ~4.2 nm | Suzuki | 300 W Xe lamp (≥420 nm) | 25 °C | EtOH/H2O (3:1), K2CO3 | 2 h | 99.5% | - | [120] |
| HUY@S-TOH/AuPd | AuNPs core: 5 nm Pd shell: ~0.7 nm HUY@S-TOH: ~3 μm | Suzuki | 300 W Xe lamp | Room Temp. | EtOH/H2O (2:1), K2CO3 | 0.5 h | >99% | 7095 | [112] |
| Au/Pd@UiO-66-NH2 | Au/Pd NPs: 6.45 nm UiO-66-NH2: ~50 nm | Suzuki | 300 W Xe lamp (>420 nm) | 25 °C | EtOH/H2O (1:1), K2CO3 | 1 h | >99% | 433 | [106] |
| AuPd/g-C3N4 | AuPd NPs: 5 nm | Suzuki | 5 W Xe HID lamp | 25 °C | EtOH/H2O (1:1), K3PO4 | 0.5 h | 99% | 7920 | [105] |
| Au–Pd nanostructures | AuNRs: 25 ± 2 (diameter), 82 ± 6 nm (length) PdNPs: 3–5 nm | Suzuki | Continuous semiconductor laser (809 nm) | Room Temp. | H2O, NaOH | 1 h | 99% | 162 | [127] |
| Pd-Au/SiO2 | AuNRs: ~10 nm (diameter), ~40 nm (length) | Suzuki | 500 W Xe lamp (>420 nm) | Room Temp. | EtOH | 0.5 h | 78% | 334 | [128] |
| Au nanorod@Pd superstructures | AuNRs: ~20 nm (diameter), ~60 nm (length) Pd: 3.8 ± 0.1 nm | Suzuki | 300 W Xe lamp (>510 nm) | 40 °C | EtOH/H2O (1:3), NaOH | 0.5 h | - | ~2880 | [129] |
| Au@Pd NRs | AuNRs: 49 ± 5 nm (diameter), 107 ± 8 nm PdNPs: ~5 nm | Suzuki | Continuous 808-nm laser | -. | H2O, NaOH | 1 h | 97.6% | - | [130] |
| Pd/Au@rGO-10/SiO2 | AuNRs: 25 nm (diameter), 75 nm (length) rGO layer: 2.8 nm Pd: 1.4 nm | Suzuki | 500 W Xe lamp (>420 nm) | - | EtOH, K2CO3 | 0.5 h | 56% | - | [131] |
| GO-Pd@Ag-AgBr | >μm | Suzuki | 300 W Xe lamp (>400 nm) | 25 °C | EtOH/H2O (1:1), K2CO3 | 0.5 h | 97% | - | [132] |
| Pd/Ag/SBA-15 | Spherical AgNPs: ~4 nm Ag nanorods: ~10 nm | Suzuki | 500 W Xe lamp (>420 nm) | 35 °C | EtOH, K2CO3 | 6 h | 53% | 489 | [133] |
| Pd/Ag/SBA-15 | Pd/Ag NPs: 4.2 nm SBA-15: >μm | Suzuki | Xe lamp | Room Temp. | EtOH K2CO3 | 2 h | 40% | - | [113] |
| Supramolecular ensemble-based Ag@Cu2O NPs | AgNPs core: 10–15 nm Cu2O shell: 10–15 nm thickness | Suzuki | 100 W tungsten filament bulb | Room Temp. | EtOH/H2O (3:1), K2CO3 | 5 h | 75% | - | [134] |
| Supramolecular ensemble-based Ag@Cu2O NPs | AgNPs core: 7.5 nm Cu2O shell: 2.5 nm thickness | C-H activation | 100 W tungsten filament bulb | Room Temp. | Toluene/H2O (3:7), KOtBu | 5.5 h | 80% | - | [135] |
| Ag/TiO2 | AgNPs: 1.5–5 nm TiO2: 10-15 nm pores | Suzuki | 20 W white LED (>420 nm) | Room Temp. | Toluene | 24 h | 97% | - | [136] |
| Cu/graphene | CuNPs: ~15 nm | C-N cross-coupling | 300 W Xe lamp (400–800 nm) | Room Temp. | MeOH | 1 h | 99% | 25.4 | [147] |
| Supramolecular ensemble 3:CuNPs | CuNPs: 3–20 nm | C-H alkynylation | 60 W tungsten filament bulb | Room Temp. | DMSO, K2CO3 | 8 h | 72% | - | [148] |
| Pd-Cu/SiC | Pd-Cu NPs: <5 nm | Sonogashira | 300 W Xe lamp | 60 °C | DMF, Cs2CO3 | 8 h | 99% | - | [144] |
| CuPd@NH2-UiO-66(Zr) | CuPd alloy nanoclusters: ~0.9 nm | Suzuki | 300 W Xe lamp (420–800 nm) | Room Temp. | DMF/H2O (1:1), TEA | 4 h | 99% | - | [145] |
| Cu/Cu2O NPs | Tetrahexahedron: ~μm | Ullmann | Xe lamp | - | - | 12 h | 77% | - | [149] |
| Pd hexagonal nanoplates | 60.4 ± 19.3 nm (20.5 ± 3.7 nm thickness) | Suzuki | Xe lamp (300–1000 nm) | 25 °C | EtOH, K2CO3 | 3 h | - | ~288 | [150] |
| Cu7S4@Pd | Cu7S4: 14 nm Pd: 4.3 nm | Suzuki | 1500-nm diode laser | Room Temp. | H2O, NaOH | 0.5 h | 97% | - | [151] |
| Pd/WO3-x NWs | Pd: ~5 nm WO3-x NWs: >μm (length), ~10 nm (diameter) | Suzuki | 500 W Xe lamp (>650 nm) | - | EtOH, K2CO3 | 100 min | 68.75% | - | [152] |
| Pd nanoflowers | 150 nm | Suzuki | 300 W Xe lamp (>475 nm) | Room Temp. | EtOH Cs2CO3 | 4 h | 96% | - | [153] |
| Pd/TiO2 | - | Suzuki | 15 W white LED | 28 °C | H2O-PEG, NaOC(CH3)3 | 4 h | 93% | - | [154] |
| Pd/ZnO | ~25 nm | Suzuki | 11 W white LED lamp | Room Temp. | H2O, Cs2CO3 | 40 min | >99% | - | [155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.H.; Suh, Y.D.; Lim, D.-K. Recent Progress in Plasmonic Hybrid Photocatalysis for CO2 Photoreduction and C–C Coupling Reactions. Catalysts 2021, 11, 155. https://doi.org/10.3390/catal11020155
Shin HH, Suh YD, Lim D-K. Recent Progress in Plasmonic Hybrid Photocatalysis for CO2 Photoreduction and C–C Coupling Reactions. Catalysts. 2021; 11(2):155. https://doi.org/10.3390/catal11020155
Chicago/Turabian StyleShin, Hyeon Ho, Yung Doug Suh, and Dong-Kwon Lim. 2021. "Recent Progress in Plasmonic Hybrid Photocatalysis for CO2 Photoreduction and C–C Coupling Reactions" Catalysts 11, no. 2: 155. https://doi.org/10.3390/catal11020155
APA StyleShin, H. H., Suh, Y. D., & Lim, D.-K. (2021). Recent Progress in Plasmonic Hybrid Photocatalysis for CO2 Photoreduction and C–C Coupling Reactions. Catalysts, 11(2), 155. https://doi.org/10.3390/catal11020155





