Abstract
Vinylogous enolate and enolate-type carbanions, generated by deprotonation of α,β-unsaturated compounds and characterized by delocalization of the negative charge over two or more carbon atoms, are extensively used in organic synthesis, enabling functionalization and C–C bond formation at remote positions. Similarly, reactions with electrophiles at benzylic and heterobenzylic position are performed through generation of arylogous and heteroarylogous enolate-type nucleophiles. Although widely exploited in metal-catalysis and organocatalysis, it is only in recent years that the vinylogy and arylogy principles have been translated fruitfully in phase-transfer catalyzed processes. This review provides an overview of the methods developed to date, involving vinylogous and (hetero)arylogous carbon nucleophiles under phase-transfer catalytic conditions, highlighting main mechanistic aspects.
1. Introduction
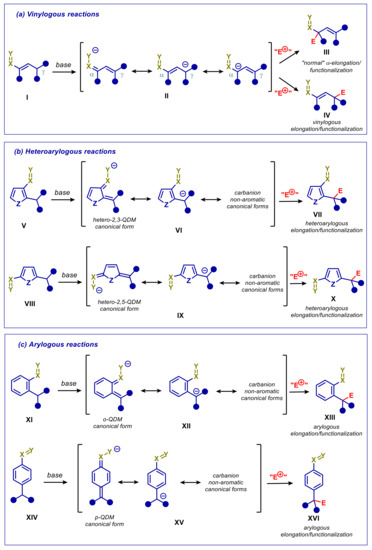
It is well-known that the resonance stabilization effect of a functional group is propagated through the π-electrons of one or more conjugated multiple bonds, according to the principle of vinylogy [1,2]. This basic concept has found several applications in organic synthesis, enabling the extension of reactivity to remote positions. One of the most popular declination of the principle of vinylogy concerns the enolate-based and closely related chemistry, as depicted in Scheme 1a. The deprotonation of the enolizable allylic γ-carbon of α,β-unsaturated carbonyl compounds under basic conditions, generates a resonance-stabilized dienolate which presents a pronounced electron density both at α and γ positions. Similarly, deprotonation of conjugated nitroalkenes, α,β-unsaturated nitriles, α,β-unsaturated sulfones and other electronpoor alkenes I, gives rise to π-extended enolate-type carbanions II, characterized by ambident reactivity towards a number of electrophiles, depending on experimental conditions and reacting partner. If, on one hand, reaction at α position is the same as expected for normal saturated pronucleophile (product III), on the other, involvement of the γ-carbon offers the opportunity for elongation or functionalization at remote position (vinylogous reaction leading to product IV). Notable examples of such reactivity are vinylogous aldol, Mannich, and Michael reactions.
Scheme 1.
General outline of processes involving vinylogous enolates and related π-extended carbanions: (a) Vinylogous reactions; (b) heteroarylogous reactions; (c) arylogous reactions. Group X=Y is an unsaturated electron-withdrawing group: e.g., (R)C=O, (RO)C=O, CHO, NO2, CN, (R)SO2. Z is a heteroatom. E+ is a generic electrophile.
Special cases of vinylogous systems are molecules in which the electronic effect of the unsaturated electron-withdrawing group is transmitted through an aromatic or an heteroaromatic moiety, thus stabilizing a negative charge at (hetero)benzylic positions (Scheme 1b,c). For the resonance effect to take place, the electron-withdrawing group and the reactive benzylic carbon should be ortho or para-positioned (XI and XIV in Scheme 1c), whereas in five-membered heterocycles they should be 1,2 or 1,4 mutually positioned (V and VIII in Scheme 1b). In order to emphasize the substantial difference with “normal” vinylogous processes, in this review we will refer to such processes as arylogous and heteroarylogous reactions. It should indeed be pointed out that deprotonation at such activated (hetero)benzylic carbons entails sacrificing the aromatic stabilization energy, as evidenced by the representative non-aromatic o-quinodimethane (oQDM) or p-quinodimethane (pQDM) canonical forms (VI, IX, XII, and XV in Scheme 1). This is especially true for carbocyclic aromatic substrates XI and XIV compared to heteroaromatic substrates V and VIII, due to the higher aromatic stabilization energy for the former. The “enolization” at the allylic γ-carbon of α,β-unsaturated compounds is therefore expected to be much easier than at the benzylic position of functionalized aromatic compounds XI and XIV, whereas an intermediate difficulty should be anticipated for the “enolization” of heteroaromatic precursors V and VIII. To confirm this, vinylogous transformations of alkene substrates I to products IV, basing on metal- and organocatalysis, are widely reported [3,4,5], and heteroarylogous transformations of V/VIII to give products VII/X are increasingly growing during recent years [3,6], as opposed to arylogous reactions, which are much rarer and mainly feasible with strongly activated substrates [7,8,9,10,11,12] or under special conditions, such as photocatalysis [13,14,15,16,17,18].
Phase transfer catalysis (PTC) has been a well-established methodology for over half a century, promoting reactions in two-phase immiscible systems [19,20,21,22,23]. Simple operational procedures, ease of catalyst separation and recycling, and absence of metal impurities and water sensitive reagents are some of the advantages provided by PTC, that have led to broad application both in small scale and industrial low cost, high performing, and environmentally benign processes [24,25,26,27,28]. The beginning of XXI century, in particular, has seen the explosion of the asymmetric PTC [29,30,31,32,33,34,35]. The inorganic base initiated reactions, involving the deprotonation of a moderately acidic substrate with the generation of a reacting organic anion, are the most commonly used phase transfer catalyzed processes in organic synthesis. However, this technique started to be applied to vinylogous precursors only recently. Such a developmental delay is quite surprising if one takes into account the unique benefits of PTC. A very important aspect, for instance, is the exceptional efficacy of base-initiated PTC in activating very weakly acidic pronucleophiles (pKa up to 23) despite non anhydrous conditions [36], matching or even exceeding the performances of homogeneous organic superbases. The presence of solid or highly concentrated aqueous alkali bases, makes it possible to generate strongly basic carbanions in non-hydrated form into the organic phase. This characteristic turns out to be useful in reactions of arylogous substrates which, as already mentioned, are hardly enolizable. In addition, to remain in the field of organocatalysis, PTC normally requires smaller catalyst loadings than aminocatalysis and N-heterocyclic carbene catalysis. Since it is well known that ion-pairing plays a key role in the mechanism of phase-transfer catalyzed processes, the regio- and stereoselectivity under such conditions are expected to be governed by interactions between the cation catalyst and the vinylogous enolate-type anion (II, VI, IX, XII, XV in Scheme 1) [37,38,39,40]. In fact, although site-selectivity of vinylogous reactions is often affected by intrinsic stereoelectronic features of reagents, the role played by the catalyst’s control in processes involving ion-pair intermediates has been demonstrated in many cases [41].
This review article aims to specifically focus on stereoselective methodologies involving vinylogous and arylogous carbon nucleophiles under PTC conditions, with a mechanistic discussion, wherever possible. Vinylogous, heteroarylogous, and arylogous reactions will be examined into three separate sections. In Figure 1 are summarized the pronucleophiles surveyed herein.
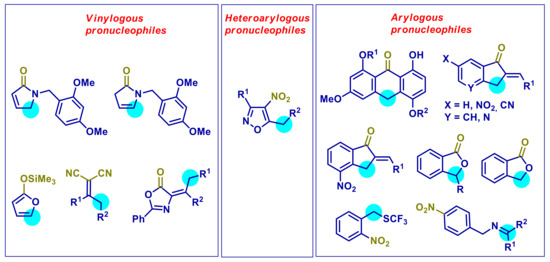
Figure 1.
Vinylogous and (hetero)arylogous pronucleophiles employed in PTC processes. Blue halos indicate the preferential nucleophilic site. The leading electron-withdrawing group is marked in green.
2. Reactions Involving Vinylogous Nucleophiles
2.1. γ-Butenolides and 2-Pyrrolinones
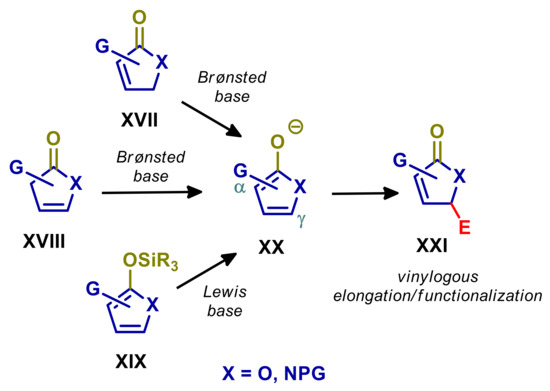
Both the γ-butyrolactone and γ-butyrolactam moieties are ubiquitous structural motifs in biologically relevant natural products and synthetic analogues, stimulating the development of a large number of stereoselective synthetic methodologies over the years [42,43,44,45,46]. One of the most widely used strategies involves the vinylogous reaction of conjugated (XVII) or deconjugated (XVIII) butenolides and pyrrolinones, or alternatively, of the corresponding silyloxydienes (XIX) [3,5,47,48,49,50]. Treating XVII or XVIII with Brønsted bases, or XIX with Lewis bases, leads to common dienolate intermediate (XX), exhibiting ambident α/γ nucleophilicity, with a normal preference for reaction at γ-carbon with most of electrophiles (Scheme 2). Silyloxyfurans and silyloxypyrroles are also used under Lewis acid catalysis or iminium ion catalysis [51,52,53,54].
Scheme 2.
Vinylogous reactions of butenolides, pyrrolidones, and the corresponding silyloxydienes with electrophiles.
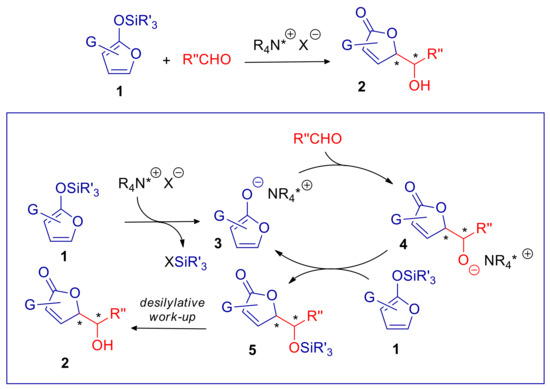
Silyloxyfurans 1 can be activated by ammonium salt catalysts R4N+X− presenting a Lewis base anion X− (e.g., F−, ArO−, RCOO−). This species carries out the desilylative formation of nucleophilic dienolate intermediate 3. If the reaction of 3 with the electrophile leads to an oxyanion species, as for Mukaiyama aldol (4 in Scheme 3) and Mukaiyama–Michael additions, this can desilylate the substrate 1 continuing the catalytic cycle (Scheme 3; the example of Mukayama aldol process is depicted). Basically, R4N+X− is indeed an initiator, while the cyclically generated chiral ammonium dienolate 3 is involved in the asymmetric addition. For instance, enantioselective vinylogous Mukaiyama aldol reaction (VMAR) of 2-trimethylsilyloxy furan (6), promoted by Cinchona alkaloid derived ammonium phenoxide or carboxylates, in CH2Cl2 have been described [55,56]. Tetrabutyl ammonium fluoride (TBAF) in THF proved to promote the anti-diastereoselective vinylogous Mukaiyama–Michael reaction of 6 [57,58].
Scheme 3.
Stereoselective vinylogous aldol reaction of silyloxy furans catalyzed by a (chiral) ammonium salt. X− is a Lewis base anion (e.g., F−, ArO−, RCOO−). The general mechanism is outlined in the box. Chirality centers are indicated with *.
Although the above-mentioned methods do not involve a phase-transfer mechanism, the underlying counterion directed stereocontrol suggests the feasibility of analogous processes, in which an insoluble (or water-soluble) Lewis base species X− is activated by a phase-transfer catalyst. With this idea in mind, our group developed a vinylogous Mukaiyama–Michael reaction (VMMR) of 6 with α,β-unsaturated ketones, using crown ethers as phase-transfer catalyst, in place of the ammonium salt, in the presence of stoichiometric amounts of KF suspended in an organic solvent (Scheme 4) [59]. The reaction works well in various solvents at low temperature (typically −78 °C), but interestingly, an extremely variable diastereoselectivity was observed depending on the medium dielectric constant. In fact, the very high anti-preference achieved in highly (e.g., DMF and CH3CN) and moderately polar (e.g., CH2Cl2) solvents switched to high syn-selectivity moving to a strongly non-polar solvent such as toluene. A similar diastereo-switching from syn to anti adduct was also observed moving from 18-crown-6 derivatives to 15-crown-5 derivatives in the same reaction medium. A strong anti-selectivity also resulted from reaction with catalytic amounts of TBAF under homogeneous conditions in many solvents, as well as from the phase-transfer catalyzed process with [2,2,2]-cryptand. On these grounds, we developed a diastereodivergent VMMR of 6 and α,β-unsaturated ketones, achieving anti or syn selectivity upon appropriate selection of the crown ether catalyst and the solvent (Scheme 4).
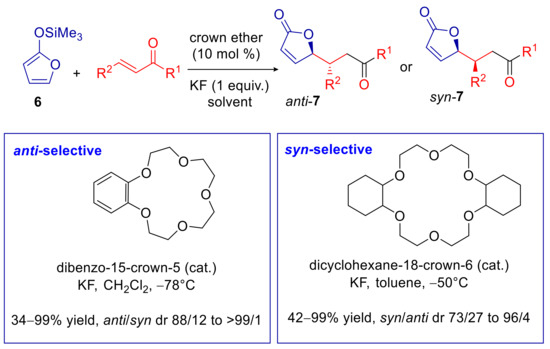
Scheme 4.
Diasteoreodivergent VMMR of 2-trimethylisilyloxy furan catalyzed by crown ethers.
The above mentioned diastereo-switching behavior was rationalized by assuming the involvement of a nucleophilic dienolate/K+ ⊂ crown ether ion pair (analogous to dienolate/R4N+ ion pair 3, depicted in Scheme 3). Since the space above and below the positively charged K+ ⊂ 18-crown-6 ether is easily accessible, we proposed a very tight ion pair with these macrocycles, provided that a low dielectric constant solvent is used. DFT calculations of such contact ion pair, in which both the dienolate anion and the carbonyl group of the α,β-unsaturated ketone are coordinated to K+, demonstrated a strong preference for the exo-type approach, leading to the syn adduct (Figure 2a), in agreement with the experimental results [59,60]. On the other hand, we suggested that catalytic quaternary ammonium salts, 15-crown-5 derivatives, and [2,2,2]-cryptand, entail separated ion pairs, as a result of the hard accessibility of positive charge for R4N+, the sandwich K+ ⊂ (15-crown-5)2, and the cryptate K+ ⊂ [2,2,2]-crypt. High dielectric constant solvents should also give rise to well-separated ion pairs. Consequently, under those conditions, we suggested that the cation chelation effect no longer occurs, and the dienolate anion reacts as a “naked” nucleophile. DFT calculations of the reaction transition state between the “naked” dienolate and α,β-unsaturated ketone revealed a preference for the endo-type approach, leading to the anti-adduct, that was again in agreement with the experimental results (Figure 2b) [59,60].
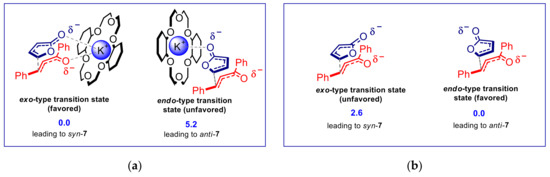
Figure 2.
DFT calculations of the transition states for the VMMR of 2-trimethylsilyloxy furan and trans-chalcone. Relative energies of the transition states, expressed in kcal/mol, are shown in blue: (a) Chelated model of the transition state involving the dienolate/K+ ⊂ dicyclohexane-18-crown-6 tight ion pair; (b) Model of the transition state involving the “naked” dienolate anion.
An asymmetric direct vinylogous aldol reaction of (5H)-furan-2-ones catalyzed by in situ generated cinchona alkaloids derived 4-methoxy-phenoxide salts was also reported [61]. Despite the analogies with the VMMR of 6 described above, given the involvement of a catalytic Lewis base salt, this process is not performed under phase-transfer conditions, being all the components soluble in the reaction medium, and therefore not further discussed herein.
The group of Maruoka developed in 2017 the first enantioselective vinylogous Michael reaction (VMR) of 5-arylpyrrol-2-ones, catalyzed by binaphthyl-based chiral ammonium salts [62]. A distinctive feature of this methodology is the applicability to mixtures of α,β and β,γ-unsaturated lactams 8 and 9, via formation of a common dienolate intermediate (see XX in Scheme 2), without affecting the enantioselectivity to a significant extent. This is particularly convenient, taking into account the difficulty to synthetize selectively each of the two isomers. The best results were obtained using N-(2,4-dimethoxybenzyl)-γ-lactams 8,9 and the catalyst 10a, with very good γ-regioselectivity, good yields and high ee’s (Scheme 5). Fine tuning of catalyst structure and inorganic base also enabled the extension to 3- and 4-substituted substrates with good results (products 12–15 in Scheme 5). Apparently, the presence of 5-aryl, or heteroaryl, group is of paramount importance to make γ-carbon acidic enough. Indeed, 5-alkyl substrates reacted sluggishly and with poor yields and enantioselectivity. Finally, the authors demonstrated that the 2,4-dimethoxybenzyl group can be easily removed by treating with TFA, providing N-unprotected products without loss of enantiomeric purity.
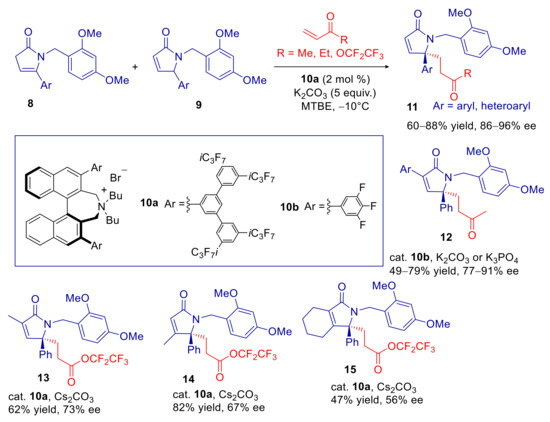
Scheme 5.
Enantioselective VMR of 5-arylpyrrol-2-ones.
2.2. α,α-Dicyanoalkylidenes
Compounds containing the α,α-dicyanolkylidene functionality are easily enolizable at the γ-position, as long as this is not a quaternary carbon atom (Scheme 6). Such pronucleophiles are among the most used substrates in vinylogous processes, with many type of catalysis [4,63,64]. Part of their success is also due to the easy cleavage of α,α-dicyanolkylidene, affording a carbonyl group. In other words, compounds XXII may be regarded as a “masked” ketone, and its vinylogous reaction considered as a viable alternative to the α-elongation/functionalization of less acidic carbonyl compounds. In addition, the α,α-dicyanovinylidene also presents electrophilic sites at cyano group and β-carbon, being thus suitable for further elaborations and tandem reactions.
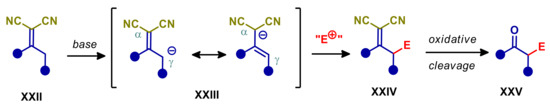
Scheme 6.
Vinylogous reaction of α,α-dicyanoalkylidene compounds under base conditions.
Jørgensen and Niess first demonstrated that substrates XXII are compatible with phase-transfer conditions [65]. They developed a vinylogous enantio- and diastereoselective Mannich reaction of α,α-dicyanolkylidenes 16 with in situ generated N-Boc imines from α-amidosulfones 17, using liquid–liquid conditions and only 3 mol% of Lygo’s catalyst 18 (Scheme 7). Adducts 19 were obtained in high yields, d.r. and ee starting from aromatic six-membered derivatives 16a. The five-membered derivative 16b furnished similar stereoselectivities but lower yields. Apparently the increased flexibility of substrate structure entailed lower diastereomeric ratio, but no fall in enantioselectivity (products 19c,d). Very good results were also achieved with cyclic aliphatic substrates (products 19d,e), even though the lower reactivity required double amount of catalyst. The corresponding enantioenriched aminoketones were obtained by oxidative cleavage with KMnO4 without erosion of ee. Although adducts 19 have also been obtained in extraordinarily high yields and enantioselectivity, starting from preformed N-Boc imines, by using bifunctional organocatalysis [66], the present phase-transfer methodology offers the remarkable advantage of employing much more stable and easily manipulated α-amidosulfones 18.
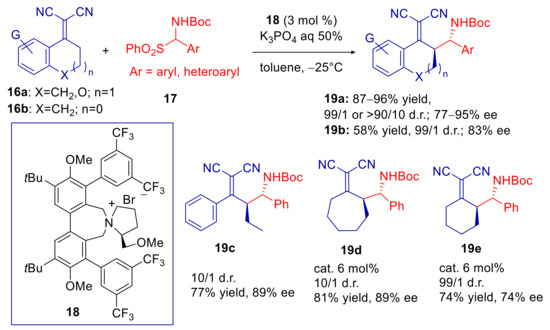
Scheme 7.
Vinylogous Mannich reaction of α,α-dicyanoalkylidenes promoted by Lygo’s catalyst 18.
Lin, Duan, and coworkers have recently tried, with a fair degree of success, to combine the above-mentioned advantages of phase-transfer catalysis and better performances of bifunctional catalysis, in this Mannich reaction [67]. A general improvement of stereoselectivities, compared to catalyst 18, were indeed achieved with both cyclic and acyclic dicyanoolefins and α-amidosulfones 17, by using the thiourea-functionalized cinchona alkaloid derived ammonium salt 20 (Scheme 8). These good results were preserved across a wide range of aromatic substituted substrates. Previous studies with N-benzylcinchonidinium chloride, on the other hand, furnished decreased level of enantioselectivity [68].
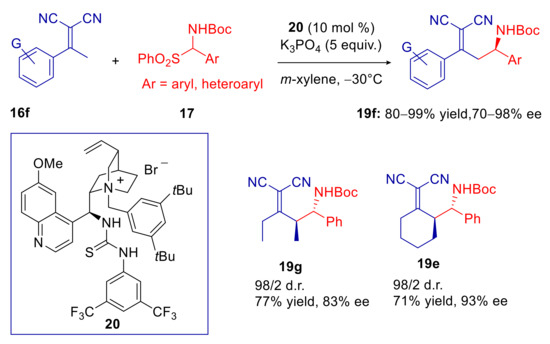
Scheme 8.
Vinylogous Mannich reaction of α,α-dicyanoalkylidenes catalyzed by the cinchona alkaloid derived thiourea-ammonium salt 20.
Cao, Wu, and coworkers demonstrated that only 1% mol of chiral phosphonium salt 22, could catalyze the vinylogous Mannich addition of acyclic α,α-dicyanoolefins 16f to N-Boc imine isatins 21 in high yields and ee’s, under base phase-transfer conditions (Scheme 9) [69]. It should be noted that the reaction conducted with the same substrates under homogenous bifunctional organocatalysis, furnished comparable results, but with much higher catalyst loading (10 mol%) [70]. Products 23 were submitted to mild oxidative cleavage with KMnO4, affording formal Mannich adducts of the corresponding acetophenone (24) with preservation of enantiomeric purity (Scheme 9). This phase-transfer catalyzed protocol was successfully applied for the enantioselective synthesis of the tert-butyl derivative 25. The products resulting from addition of propiophenone and tetralone derived α,α-dicyanoalkylidenes, however, turned out to be unstable under such conditions, undergoing subsequent attack of NHBoc group on one cyano group; such cyclization products were isolated in good yields and moderate enantioselectivities (26 and 27 in Scheme 9).
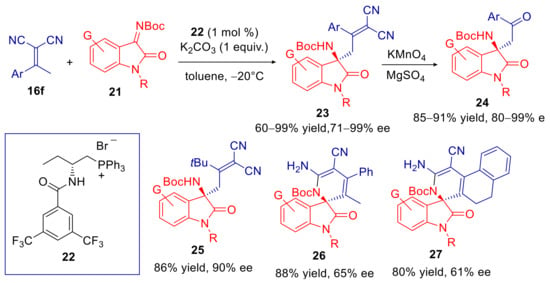
Scheme 9.
Vinylogous Mannich reaction of α,α-dicyanoalkylidenes and N-Boc isatins catalyzed by chiral phosphonium salt 22.
2.3. α-Alkylidene Azlactones
Oxazol-5(4H)-ones, or azlactones, XXVI are versatile amino acid derived synthetic building blocks, thanks to their multisite reactivity (Scheme 10a) [71,72,73,74]. Deprotonation of the α-carbon gives birth to a vinylogous enolate XXVII that may react with electrophiles at oxygen, C-α and C-γ sites. On the other hand, electrophilic nature of carbonyl group sets the stage for tandem or cascade processes [72]. Substituted α-vinylidene oxazolones XXVIII, also known as Erlenmayer azlactones, are commonly regarded as synthetically useful Michael acceptors due to the presence of the additional β’ electrophilic site. However, α-alkylidene azlactones presenting an enolizable γ’-carbon may generate exocyclic vinylogous enolates XXIX after deprotonation (Scheme 10b). Such reactivity pattern was put in practice using base organocatalysts [75,76]. In addition, exploiting the electrophilicity of carbonyl group, organocatalyzed vinylogous aldol reaction/azlactone opening cascade processes were realized [77,78].
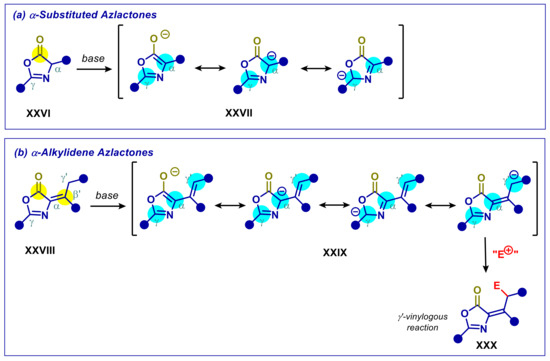
Scheme 10.
Multisite reactivity of azlactones: (a) Vinylogous enolate derived from α-substituted azlactones; (b) vinylogous enolate derived from α-alkylidene azlactones. Yellow halos indicate electrophilic sites, blue halos indicate nucleophilic sites. E+ is a generic electrophile.
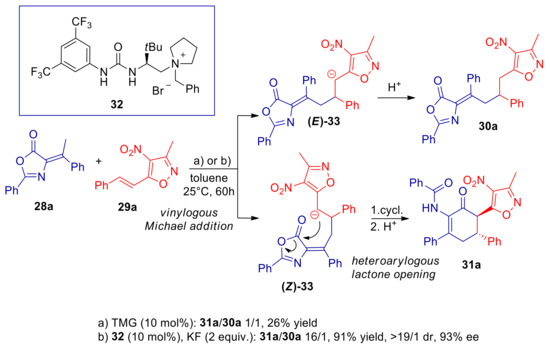
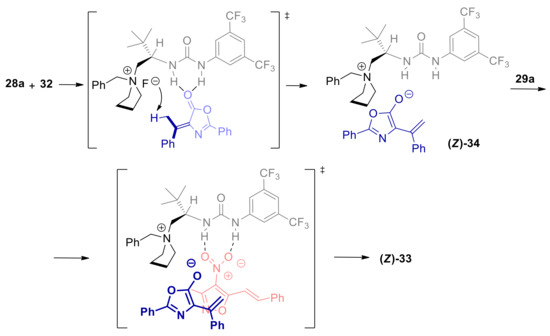
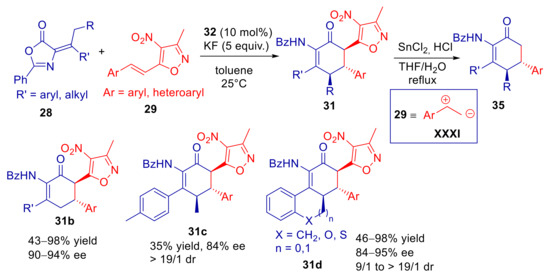
Recently, Jiang, Chang and co-workers studied the γ’-vinylogous Michael addition of α-alkylidene azlactones to 4-nitro-5-styrylisoxazoles, which are heteroarylogous Michael acceptors [79]. Reaction of 28a and 29a, conducted with catalytic amounts of organic superbase 1,1,3,3-tetramethylguanidine (TMG), was sluggish at room temperature, and furnished a 1:1 mixture of products 30a and 31a (Scheme 11). While the former product was the E-configured γ’-vinylogous Michael adduct, 30a was suggested to result from trapping of heteroarylogous Z-configured α-carbanion of 5-alkyl-4-nitroisoxazole (33) by the electrophilic carbonyl group, with subsequent azlactone opening. Moving to asymmetric PTC base conditions, using bifunctional urea ammonium salt 32, cyclization product 31a was obtained almost exclusively with high yield and ee. The authors proposed that γ’-vinylogous Michael/heteroarylogous azlactone opening cascade process is driven to completion due to better Z/E selectivity in the carbanion formation step, compared to the TMG catalyzed reaction. However, the higher basicity ensured by PTC, compared to superbase catalyzed process, plays a major role in speeding up the vinylogous Michael addition reaction (see also Section 3.1). According to the authors, the steric hindrance and the H-bonding interactions of the bifunctional ammonium catalyst 32, determines the Z-selectivity in the formation of dienolate 34, and also imparts stereocontrol in the subsequent conjugated addition (Scheme 12). A survey of different substrates provided good yield and excellent ee’s with several β-aryl and β-heteroaryl 4-nitro-5-vinylisoxazoles (31b, Scheme 13). The replacement of the olefinic methyl substituent with an ethyl group caused a sluggish transformation and a poor yield, with a small decrease of ee (31c, Scheme 13). A longer alkyl group was not tolerated. Moderate to high yields and very high enantioselectivities were achieved with substrates with cycloalkylidene substituted substrates, with remarkable enantiocontrol of three contiguous stereocenters (31d, Scheme 13). What is extremely useful is the easy removal of the 4-nitroxazole group, making electrophilic alkenes 29 the synthetic equivalent for the 1,2-dipolar synthon XXXI.
Scheme 11.
Vinylogous Michael/Heteroarylogous lactone opening cascade reaction of 28a and 29a catalyzed by TMG or by the bifunctional ammonium salt 32 under phase-transfer conditions.
Scheme 12.
Plausible origin of the stereoselectivity in the vinylogous Michael/Heteroarylogous lactone opening cascade reaction of α-alkylidene azlactones catalyzed by 32.
Scheme 13.
Plausible origin of the stereoselectivity in the vinylogous Michael/Heteroarylogous lactone opening cascade reaction of α-alkylidene azlactones and 4-nitro-5-styrylisoxazoles catalyzed by 32.
3. Reactions Involving Heteroarylogous Nucleophiles
As mentioned before, base catalyzed heteroarylogous reactions are generally more difficult to perform than ordinary vinylogous processes, since the stabilization of the heterobenzylic carbanion VI/IX is less effective compared to the functionalized allyl carbanion II (Scheme 1a,b). Such transformations are better conducted with aminocatalysis [3,6]. However, few examples using base promoted PTC have been reported and described in the following section.
3.1. 5-Alkyl-4-nitroisoxazoles
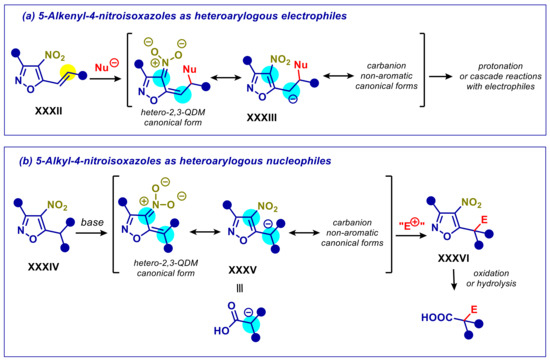
5-Alkyl-4-nitroisoxazoles have emerged as synthetically useful building blocks in organic synthesis in the recent years, due to the pharmacophoric properties of isoxazole unit and the facile transformation of the heterocyclic ring. The 4-nitroisoxazolyl group has electron-withdrawing features enabling moderate stabilization of negatively charged α-carbon. This aspect has been mainly exploited with the introduction of 5-alkenyl-4-nitroisoxazoles XXXII as Michael acceptors and dipolarophiles (Scheme 14a) [80,81,82,83,84,85,86]. On this principle, 5-alkyl-4-nitroisoxazoles XXXIV can behave as nucleophiles at heterobenzylic position, after deprotonation (Scheme 14b). The synthetic value is even more striking if one considers the facile cleavage of the nitroisoxazole framework to the carboxyl group, making intermediate XXXV a surrogate of a carboxylic acid enolate. However, such strategy proved to be challenging with homogeneous base catalysts, presumably due to the limited acidity of the heterobenzylic carbon, leading to long reaction times and low yields. Efficient transformations were only achieved turning to an electrophile activation strategy, by means of nucleophilic or iminium ion catalysis [87,88]. However, the pronucleophile-activation approach, through the action of a base, has been successfully implemented under PTC conditions, as shown below.
Scheme 14.
Heteroarylogous reactivity of 5-alkenyl and 5-alkyl-4-nitrooxazoles. Yellow halos indicate electrophilic sites, blue halos indicate nucleophilic sites. E+ is a generic electrophile. Nu− is a generic nucleophile.
Coote, Jiang and coworkers described the heteroarylogous amination of 5-alkyl-4-nitroisoxazoles 36 with azodicarboxylates 37 catalyzed by the polyfunctional dipeptide-based guanidinium phase-transfer catalyst 38 (Scheme 15a) [89]. Under optimized conditions, with solid sodium acetate as the base in CPME (cyclopentyl methyl ether), hydrazides 39 were obtained with uniformly high yields and ee’s with diethyl or diisopropyl azodicarboxylates, regardless the electronic nature of substituents in 36. A lower level of enantioselectivity was instead observed with di-t-butyl azodicarboxylate. It should be stressed that reaction catalyzed by structurally related amino-thiourea and amino-urea organocatalysts under homogeneous conditions resulted in low yields and enantioselectivities. Synthetic elaboration of hydrazide 39a gave access to the protected α-aminoalcohol 41 without loss of enantiomeric purity (Scheme 15b). DFT calculations and NCI analysis revealed a preference for a transition state in which 37 is activated and suitably oriented by specific hydrogen bonding with both catalyst’s thiourea and amide N-H functional groups, whereas the nitroisoxazole α-carbanion/guanidinium ion pair is held by coulombic interaction as well as NH/NO2 hydrogen bonding (Figure 3). The model is in agreement with the attack on the Si-face of the (Z)-configured heterobenzylic carbanion which is experimentally observed.
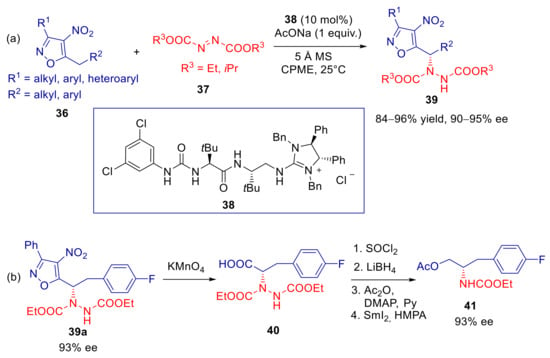
Scheme 15.
Heteroarylogous amination of 5-alkyl-4-nitroisoxazoles catalyzed by 38. (a) Scope of reaction; (b) synthetic elaboration.
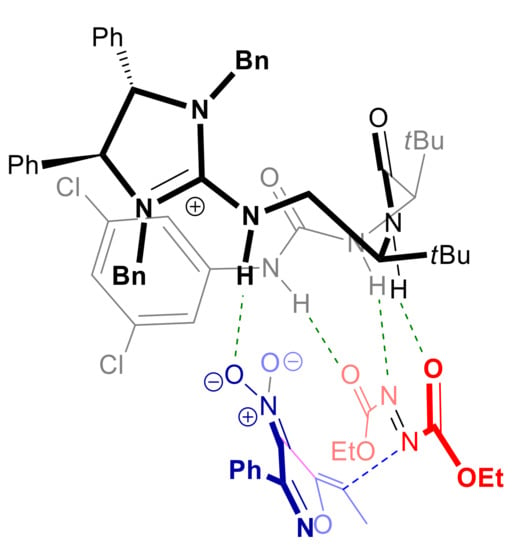
Figure 3.
Model of the favorite transition state in the heteroarylogous amination of 5-alkyl-4-nitroisoxazoles catalyzed by 38, resulting from DFT calculations and NCI analysis. Green spotted lines represent hydrogen bonds, blue spotted line represents forming C-N bond.
The same polyfunctional guanidinium catalyst 38 showed to be effective in the heteroarylogous aldol reaction of 36 with paraformaldehyde [90]. Under similar conditions to those adopted for the amination reaction, aldol products 42 were formed in high yields and ee’s in most cases with a broad range of substrates 36, bearing alkyl, benzyl, aryl or heteroaryl substituents, except for some cases (e.g., for 3-phenyl-5-benzyl-4-nitroisoxazole, for which 48% ee and 73% yield was detected) (Scheme 16a). As expected, Si-selectivity was attained, suggesting a transition state very close to that assumed for amination reaction and depicted in Figure 3. Better performances of PTC over homogeneous Brønsted base catalysis were also established for this process. Despite the higher reactivity of paraformaldehyde compared to 37, amino-thiourea and amino-urea bifunctional bases proved again to be ineffective catalysts, whereas good conversions were achieved with superbase TMG. Fungicide 45 was synthetized by transformation of the nitroisoxazole ring into an aminopyrazole unit (Scheme 16b).
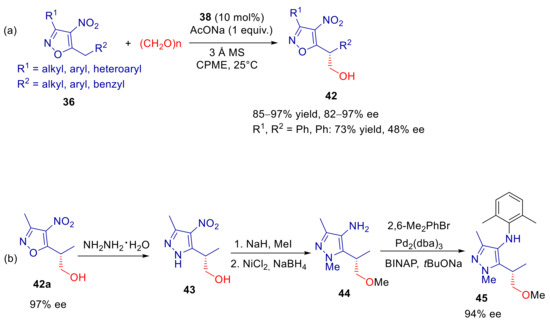
Scheme 16.
Heteroarylogous hydroxymethylation of 5-alkyl-4-nitroisoxazoles catalyzed by 38. (a) Scope of reaction; (b) synthetic elaboration.
In the same year, Zhu, Wu, and co-workers extended the use of β-acylaminophosphonium salts, previously introduced for the vinylogous Mannich addition of α,α-dicyanoolefins to N-Boc imine isatins (Scheme 9), to the related process involving 5-methyl- and 5-benzyl-4-nitroisoxazoles [91]. The sec-butyl derivative 46, structural analogue of 23, proved to be the most enantioselective catalyst, using K3PO4 as the inorganic base in chlorobenzene at −10 °C. Under such optimized conditions, a quite wide range of products, containing both the isoxazole and aminooxindole pharmacophores, were delivered in high to excellent yields and enantioselectivities (Scheme 17a). The authors speculated that the amide functional group might play a key role as a hydrogen bond donor in orienting and activating the N-Boc isatin electrophile (Scheme 17b). Some useful synthetic elaborations were also presented. Boc group has been substituted with an acyl group in two steps with minimal erosion of enantiomeric purity. Moreover, hydrolytic cleavage of isoxazole moiety of the derivative 47a, followed by esterification of the resulting carboxyl group, gave access to the synthetic precursor of AG-041R, a potent gastrin/CCK-B receptor agonist (Scheme 18).
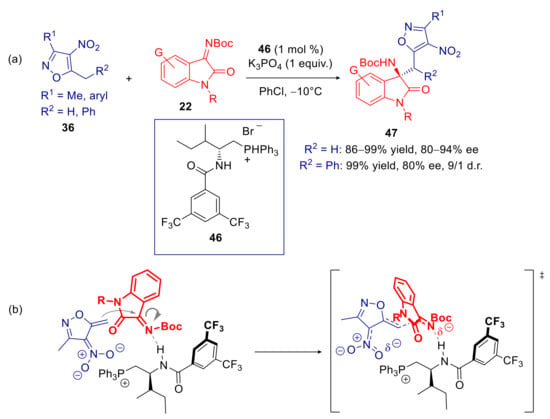
Scheme 17.
Heteroarylogous Mannich reaction 5-alkyl-4-nitroisoxazoles and N-Boc isatins catalyzed by chiral phosphonium salt 46. (a) Scope of reaction; (b) Plausible transition state. ‡ identifies the transition state.
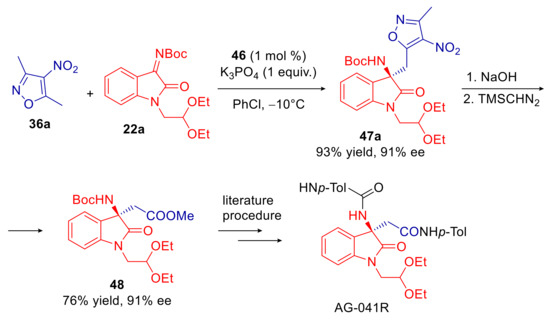
Scheme 18.
Formal synthesis of AG-041R.
4. Reactions Involving Arylogous Nucleophiles
While several organocatalytic methods involving vinylogous and heteroarylogous nucleophiles are now available in organic synthesis, the activation of arylmethyl nucleophiles is still a quite challenging task. The few examples reported are typically restricted to most enolizable substrates, bearing one or more electron-withdrawing groups on the aromatic ring [7,8,9,10,11,12]. However, PTC, due to the ease of generating poorly stabilized carbanions under non anhydrous conditions, appears to be an ideal complement to address this strategic gap. It should be stressed, indeed, that for most of arylogous reactions reported herein, organocatalytic equivalent approaches have not been described yet.
4.1. Anthrones
9-Anthrones are relevant molecules, due to their biological activity and applications in chemistry of materials [92,93], which represent a special case of arylogous substrates. The facile enolization at C-10, generating fully aromatic intermediate 9-anthrolate XXXVIII, enables straightforward functionalization or elongation promoted by weak base organocatalysts [94,95,96,97,98,99] (Scheme 19).
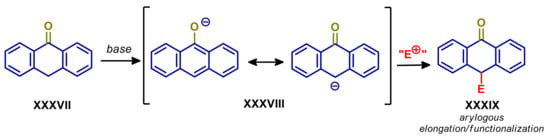
Scheme 19.
Arylogous reactivity of 9-anthrones. E+ is a generic electrophile.
However, phase transfer catalysis remains the preferred strategy for reaction with alkylating agents [100,101]. In particular, asymmetric PTC is particularly recommended to achieve mono-alkylation products enantioselectively, avoiding racemization of the newly generated stereocenter. En route to the synthesis of the anthracycline antibiotic (+)-viridicatumtoxin B, Nicolaou and co-workers realized the diastereoselective C-alkylation of the polyoxygenated 9-anthrone 49 with the chiral allylic bromide 50 [102]. After an extensive screening of reaction conditions, intermediate 51 was obtained in 73% yield and 95/5 diastereomeric ratio with the cupreine derived phase-transfer catalyst 52 (Scheme 20). The authors demonstrated that no epimerization occurred during the process. Lower stereoselectivity was obtained with the chiral Brønsted base β-isocupreidine. Diastereomerically pure 51 was achieved after recrystallization and submitted to a 14 steps synthetic sequence to provide (+)-viridicatumtoxin B, so determining unambiguously its absolute configuration. The optical antipode (−)-viridicatumtoxin B was obtained in analogous way, through the intermediate ent-51, in the alkylation of 49 with ent-50 catalyzed by the pseudoenantiomer of salt 54 derived from cupreidine. In addition to the natural products, synthetic intermediates (+) and (−)-53 proved useful to prepare a library of synthetic analogues with comparable or even higher antimicrobial activity.
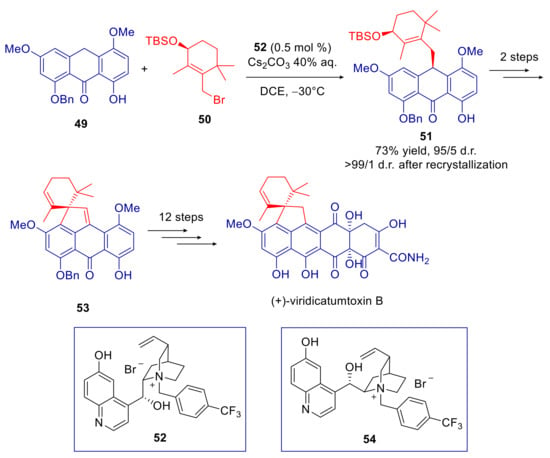
Scheme 20.
Synthesis of enantiomerically pure viridicatumtoxin B, employing diastereoselective C-alkylation of the anthrone 49.
4.2. 2-Alkylidene-1-indanones
α-Alkylidene-indanones XL have been long neglected as arylogous nucleophiles in organic synthesis. The reason for this could be that deprotonation of endocyclic 3-carbon, generating a π-extended carbanion with partial dearomative 1-hydroxyl character XLI (Scheme 21), has proven difficult. For instance, while asymmetric aminocatalysis turned out to be an effective tool in generating 8-amino isobenzofulvene intermediates from indene 2-carbaldeydes, resulting in enantioselective cascade processes [103,104], it failed when applied to substrates XL [105]. In contrast, non-aromatic 4-amino fulvene intermediates are easily formed starting from vinylogous α-alkylidene-cyclopentenones, furnishing formal [4+2] cycloadducts in reaction with electrophilic alkenes [106]. However, the diastereoselective dimerization of 1-alkylidene-indanones, via anionic intermediate XLI, has been reported heating with alkoxide or inorganic bases in polar solvents [107,108]. The anion XLI resulting by deprotonation of XL is a resonance hybrid represented by a dearomative 1-enolate isobenzofulvene canonical form, along with aromatic C-3 and C-8 carbanion forms. However, despite the potential ambident nucleophilicity, reactions with Michael acceptors have been reported to occur selectively at the C-3 site resulting in a formal [10+2] cascade process (Scheme 21).
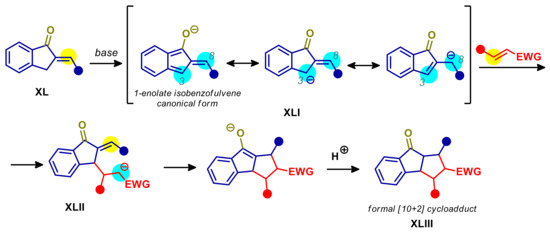
Scheme 21.
Arylogous [10+2] cascade process of 2-alkylidene-1-indanones with Michael acceptors. Blue halos indicate potential nucleophilic sites. Yellow halos indicate electrophilic sites.
The sole example of asymmetric formal [10+2] cycloaddition of 2-alkylidene-1-indanones has been reported by Chen and coworkers, exploiting chiral phase-transfer catalysis [105]. Chiral amines failed to promote reaction of indanone 55a with 3-alkylidene oxindole 56a. Moderate conversion (30% yield) to the expected spiro cycloadduct 57a were instead achieved under PTC conditions, employing TBAB (tetrabutyl ammonium bromide). Unfortunately cinchonidine derived ammonium salts provided only traces of 57a. However, better results were attained with the more acidic nitro-substituted indanones 55b. In the presence of catalytic amount of quinidine derived ammonium salt 58 and Cs2CO3, cycloadducts 57b were released in good to excellent diastereoselectivities and high enantioselectivities (Scheme 22a). The opposite enantiomer was produced with the corresponding quinine derived catalyst. This methodology was also extended to other electron poor substituted 2-alkylidene-1-indanones and electrophilic alkenes with generally good results, with the exception of β-nitrostyrene and 4,4,4-Trifluoro-1-phenyl-2-buten-1-one, providing products 59–66. In addition, substitution of 4-nitro group by palladium catalyzed Suzuki coupling or via diazonium salt, gave access to further derivatives. Models for the ion pair involved and the transition states of both steps were also proposed by the authors (Scheme 22b). The ion pair 67 would be stabilized by hydrogen bonding and π-stacking interactions, orienting 55a in such a way that Si face is more exposed to the electrophilic attack of 56a. The observed diastereoselectivity would be governed by steric hindrance minimization in the transition state 68, with the aromatic ring of 56a far away from the substituted benzyl group of the catalyst. In the resulting ternary intermediate 69, the reactants would be arranged so that Re face attack would be favored, bringing to product 57a. However, whatever the true mechanism, the free OH group of catalyst 58 should play a key role, since the employment of the protected O-benzyl derivative led to a prevalence of the opposite enantiomer.
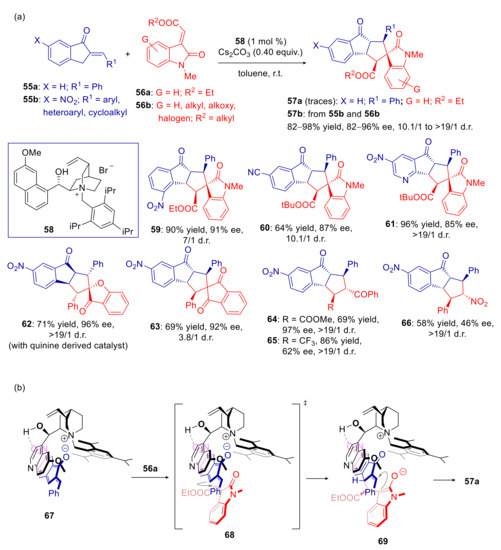
Scheme 22.
Cascade [10+2] cycloaddition of 2-alkylidene-1-indanones to Michael acceptors catalyzed by the ammonium salt 58. (a) Scope of reaction; (b) plausible models for the ion pair and transition states of the two steps. ‡ identifies the transition state.
4.3. Phthalides
1(3H)-Isobenzofuranones XLIV, more commonly known as phthalides, are aromatic lactones widespread in natural sources and exhibiting a broad range of biological activities, capturing the interest of synthetic community [109,110]. A possible strategy aiming at the introduction of a stereocenter at C-3, is the generation of an arylogous enolate XLV, under the action of a sufficiently strong base, followed by the treatment with the appropriate electrophile (Scheme 23). Normally this approach requires the use of strong bases, such as metal amides, with poor control of the stereoselectivity [111,112,113,114]. Alternatively, the acidity of C-3 may be enhanced by directly introducing an additional unsaturated electron-withdrawing group at this site (e.g., COOR and CN), thus making possible enantioselective and diastereoselective reactions, even with catalytic amounts of moderately strong bases [115,116,117,118,119,120,121,122,123]. However, since these activated substrates are not strictly arylogous pronucleophiles, they will not be covered in this review.
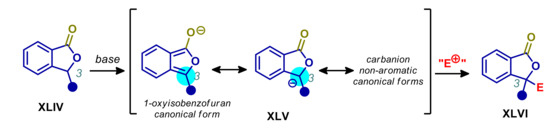
Scheme 23.
Arylogous 3-elongation/functionalization of phthalides.
In 2017, taking advantage of the strong basicity of alkali hydroxides in non-polar solvents under PTC conditions, we realized the first true arylogous reaction of 3-arylphthalides [124]. In particular, a diastereoselective arylogous Michael reaction (AMR) of substrates 70 with α,β-unsaturated carbonyl compounds was developed, using catalytic amounts of solid KOH or K3PO4 and crown ethers (Scheme 24). After screening of catalysts and solvents, we achieved syn adducts 71 as single diastereomers in good yields in almost all cases examined using catalytic dibenzo-18-crown-6 in mesitylene, whereas a small decline of d.r. was observed for products 72 and 73. The use of stronger base KOH ensured shorter reaction times, although generally higher yields and occasionally improved d.r. were attained with K3PO4. Similar results were observed in other non-polar solvents such as toluene, and diverse 18-crown-6 derived catalysts. Interestingly, use of more polar solvents and 15-crown-5 derivatives or tetrabutyl ammonium bromide (TBAB) as the catalysts, led to a significant decrease of diastereoselectivity. This effect, already noted in the previously described VMMR of 7 (Scheme 4, Figure 2) [59], has been studied in detail for 3-unsubstituted phthalide derivatives (see below) and must be attributed to the involvement of a chelated model, which favors the exo approach between reactants and the consequent formation of the syn diastereomer. Another factor that should be stressed for this reaction is the superiority of PTC compared to the use of catalytic organobases. In particular, we found that catalytic amounts of triethylamine failed to promote the AMR [124], whereas superbases such as DBU and phosphazene P1-t-Bu-tris(tetramethylene) (BTPP) provided complete conversion after several days and with slightly lower d.r. compared to the metal hydroxide/crown ether system [125].
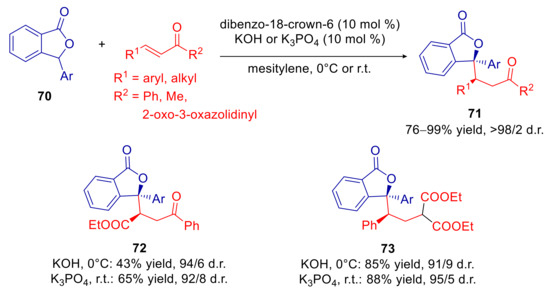
Scheme 24.
Diastereoselective arylogous Michael reaction of 3-aryl phthalides with α,β-unsaturated carbonyl compounds, catalyzed by KOH/dibenzo-18-crown-6.
3-unsubstituted and 3-alkyl phthalides are less reactive than 3-aryl derivatives due to the less effective stabilization of the arylogous 1-oxyisobenzofuran enolate LV. As evidence, a reaction of 1(3H)isobenzofuranone with trans-chalcone, conducted under conditions illustrated in Scheme 24, furnished only traces of expected adduct [60]. In addition, using stoichiometric amounts of KOH and 20 mol % of dicyclohexane-18-crown-6, led to capricious results, with a maximum yield of 55% and 93/7 syn diastereoselectivity, mainly due to competing lactone opening. A suitable protocol for such inactivated substrates was devised using catalytic amounts of KOH and crown ether, along with stoichiometric N,O-bis(trimethylsilyl)acetamide (BSA, 75), at low temperature. The best balance between reaction times, yields and diastereoselectivity was attained by employing 18-crown-6 at −40 °C in toluene, with a few exceptions of less reactive substrates requiring higher temperatures (Scheme 25a). Under these conditions, good to excellent yields and syn diastereoselectivities were obtained in the arylogous addition to α,β-unsaturated ketones, esters and N-acyloxazolidinones. Also noteworthy was the application to 3-alkyl substrate, affording 77 in high yield and d.r., although the lower reactivity required to conduct the addition at −20 °C. The role of BSA in speeding up the process and reducing byproducts is probably the generation of base intermediate amide salt 78 and the enolate adduct trapping (Scheme 25b).
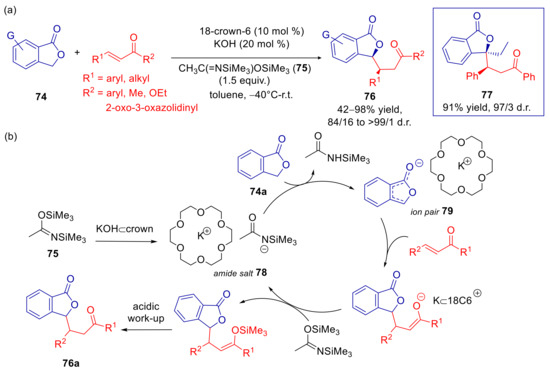
Scheme 25.
Diastereoselective arylogous Michael reaction of 3-unsubstituted and 3-alkyl substituted phthalides with α,β-unsaturated carbonyl compounds, catalyzed by KOH/18-crown-6. (a) Scope of reaction; (b) Plausible mechanism.
The effect of solvent and catalyst structure, as well as DFT calculations, supported a transition state model involving chelation to the alkali metal cation [60]. A key point to consider is that K+⊂18-crown-6 and analogous complexes are characterized by a positive charge which is readily accessible on the open faces of the macrocycle plane. This makes it very easy, under appropriate conditions (e.g., low dielectric constant solvents, such as toluene), the establishment of contact ion pairs and coordination complexes. In toluene, DFT calculations predict a preference for chelated transition state model characterized by an exo approach of the reactants, leading to the syn adduct (Figure 4a). As the dielectric constant of the solvent increases, causing a larger degree of ion pair separation and inhibiting coordination, the syn-anti diastereomeric ratio decreases accordingly. A small prevalence of the anti-adduct was indeed observed in DMF, as a result of a slight preference for the endo approach between reactants, correctly predicted also by DFT calculations performed on the “naked” 1-oxyisobenzofuran enolate in DMF (Figure 4b). In corroboration of this hypothesis, reaction performed with phase-transfer catalyst which generate hardly accessible cations, such as bulky quaternary ammonium salts, K+⊂cryptand and K+⊂15-crown-5 sandwich complexes, led to a low syn-anti ratio, determined by a weaker ion-pairing. Moderately accessible benzyl(trimethyl)ammonium salts furnished intermediate diastereoselectivity.
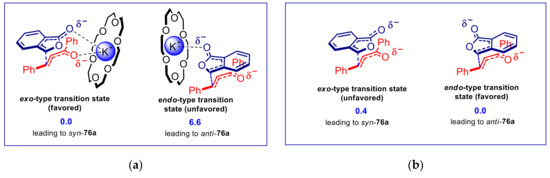
Figure 4.
DFT calculations of the transitions states for the AMR of 1(3H)isobenzofuranone and trans-chalcone. Relative energies of the transition states, expressed in kcal/mol, are shown in blue. (a) Chelated model of the transition state involving the dienolate/K+ ⊂ 18-crown-6 tight ion pair in toluene; (b) Model of the transition state involving the “naked” dienolate anion in DMF.
4.4. 2- and 4-Alkylnitroarenes
The generation and reaction of arylmethyl carbanions without using organometal chemistry is an extremely challenging task. The poor effectiveness of organobases is easy to understand if one takes into consideration, for instance, the low acidity of the benzylic carbon in alkylarenes monofunctionalized with one electron-withdrawing group at 2- or 4- position. In fact, the interposition of an arylene moiety between the leading electron-withdrawing group and the acidic carbon results in a conspicuous decrease of its acidity. By way of example, the pKa values of nitromethane in DMSO is 17.2, whereas the pKa of 4-nitrotoluene is 20.4 [126]. Accordingly, examples of aminocatalyzed and organobase catalyzed reaction of alkylnitroarenes are quite rare, and normally restricted to polyfunctional electronpoor substrates [7,8,9,11,12]. However, hydroxides in polar aprotic solvents, such as DMSO [127,128,129], or under phase-transfer conditions [130,131,132,133,134], are sufficiently strong bases to promote C-C bond formation or functionalization reaction at the benzylic position of 2- and 4-alkylnitroarenes.
Considering the unique properties of SCF3 group in enhancing the membrane permeability of drug candidates, there was recently and extraordinary interest in the asymmetric introduction of this functionality at chiral center [135,136,137]. However, an alternative strategy is the prior introduction of SCF3 at the prochiral center of the substrate, followed by an enantioselective reaction. In addition, the electron-withdrawing properties of this functional group contribute to enhance the reactivity of the carbon attached to it in base promoted processes. In this view, Zhao and coworkers recently described the phase-transfer catalyzed arylogous Mannich reaction of 2-(trifluoromethylthiomethyl)nitroarenes 80 [138]. Moderate to good yields, good diastereoselectivities, and a generally high level of enantioselectivity were attained with diversely functionalized substrates, by employing bifunctional phosphonium salt 81 (Scheme 26a). Similar results were also obtained by replacing the SCF3 with a SCF2CF3 in the substrate. The presence and the position of o-NO2 group proved to be crucial for activating the benzylic carbon site. In fact, no reaction occurred when the nitro group was removed or replaced by cyano or ester functionalities. m-Nitro derivative was also unreactive. Similar reactivity was observed with the p-nitro derivative, but stereoselectivity was disappointing. Moreover, organic base catalysis was also ineffective to promote the formation of 82. Transformation of the products to tetrahydrobenzodiazepin-2-ones was accomplished by deprotection of N-Boc, reduction of the nitro group, and final treatment with triphosgene. In the proposed transition state, catalyst 81 has been supposed to orient the reactants both through electrostatic interactions and hydrogen bonding (Scheme 26b).
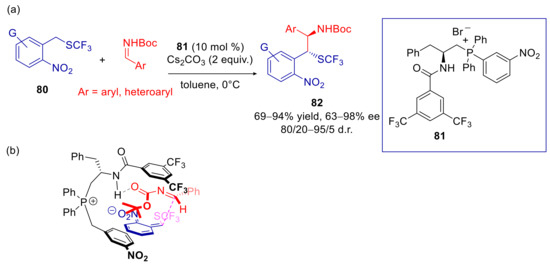
Scheme 26.
Arylogous Mannich reaction of 2-(trifluoromethylthiomethyl)nitroarenes catalyzed by chiral phosphonium salt 81. (a) Scope of reaction; (b) Plausible transition state.
An exceptionally interesting class of arylogous pronucleophiles are p-nitrobenzyl imines XLVII (Scheme 27). The deprotonation of these substrates generates an (aza-allyl)p-nitrobenzyl anion XLVIII, with extensive π-delocalization, which possesses two main nucleophilic sites at C-1 and C-3. Reaction with electrophiles at the C-3 benzylic site would lead to a normal arylogous C-C bond formation product XLIX. Even more intriguing is the product L arising from reaction at C-1, involving isomerization of C=N bond; this is actually a styrylogous transformation, since the electronic effect of NO2 is transmitted both through arylene and C=N π-systems. The most relevant aspect from the synthetic point of view, is that XLVIII features a reversal of polarity and reactivity at C-1 when compared to imines, behaving as a nucleophile instead of as an electrophile. As demonstrated by Wu and Deng, the presence of an electron-withdrawing substituent on the benzyl moiety is of paramount importance for activating such substrates, being 2-NO2 or 4-NO2 much more effective than 4-COOMe and 4-CF3, enabling the use of catalytic organobases [139]. In contrast, the formation of semistabilized aza-allyl anions starting from benzyl imines devoid of electron-withdrawing functional groups generally requires strong bases (e.g., metal amides or alkoxides) or transition metals activation [140].
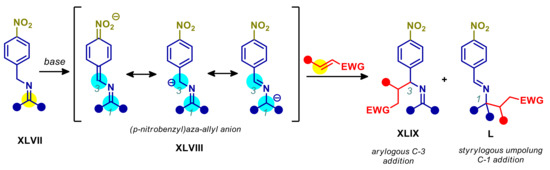
Scheme 27.
Ambident reactivity of p-nitrobenzyl imines.
In their first application of PTC, Deng and coworkers described the arylogous Michael addition of p-nitrobenzyl imines to α,β-unsaturated aldehydes [141]. In an attempt to perform the umpolung Michael addition of 1,1,1-trifluoroacetone derived p-nitrobenzyl imine (83a, R1 = Me) to crotonaldehyde, cinchona alkaloids derived Brønsted base catalysts were explored, obtaining no traces of C-C bond formation, but only the tautomerization product 88a, likely arising from the rapid C-1 protonation of the corresponding aza-allyl anion carried out by the catalyst’s conjugate acid. The expected Michael adduct 85a was instead achieved in variable amount, depending on temperature and catalyst’s structure, adopting phase-transfer conditions. Tautomerization product 88a prevailed at room temperature using N-benzyl cinchonine derived ammonium salts, whereas higher amounts of 85a were attained at −20 °C and using more hindered N-terphenylmethyl ammonium salt derivatives. The best results in terms of enantioselectivity, diastereoselectivity and 85/88 ratio were achieved with ammonium salt 84, with as low as 0.2 mol % of catalyst loading (Scheme 28a). Ee’s > 90% ee and d.r. > 90/10 were achieved with aryl, alkyl and alkenyl ketimines in the addition to aliphatic and aromatic β-substituted enals as well as to acrolein. The umpolung adducts 85 were transformed in situ to the corresponding aminoalcohol 86 or N-debenzylated aminoalcohol 87. Only minor amounts of isomerization products 88 were formed. Noteworthy, no traces of regioisomer 89 were detected in almost all the cases, except for adduct to cinnamaldehyde, which was accompanied by substantial amount of this byproduct. Products were also converted to pyrrolidine derivatives after removal of the p-nitrobenzyl group and subsequent reductive cyclization.
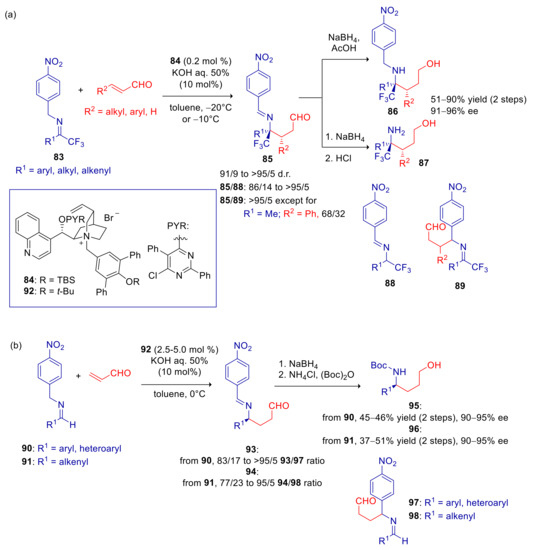
Scheme 28.
Umpolung Michael reaction of p-nitrobenzyl imines with α,β-unsaturated aldehydes catalyzed by 84 and 92. (a) Reaction of imines derived from 1,1,1-trifluoromethyl ketones; (b) Reaction of imines derived from aldehydes.
Despite their lower reactivity, p-nitrobenzyl imines 90 and 91, respectively derived from aromatic and α,β-unsaturated aldehydes, were also applied successfully to this umpolung Michael addition, although modification of the catalyst’s structure (92) and raising of catalytic amount to 2.5 mol % and temperature to 0 °C were needed (Scheme 28b). Umpolung products 93,94 were formed in excellent ee’s and directly converted into N-Boc aminoalcohols 95,96. However, with these substrates, especially 91, significantly higher amounts of undesired regioisomers 97 and 98 were detected. Products 96 were hydrogenated without any loss of ee.
Reaction with α-substituted enals, conducted under previously described conditions, led mostly to the formation of pyrrolidines 103, resulting from tandem Michael/intramolecular Mannich process [142]. The formation of this undesired byproduct was suppressed by addition of variable amounts of 4-chloro-2,6-dimethylphenol, which rapidly protonates the enolate adduct preventing its subsequent intramolecular addition to the imine group. Optimized stereoselectivity was reached with catalyst 99 (Scheme 29). The umpolung tandem Michael/protonation reaction of CF3-substituted p-nitrobenzyl imines 83 afforded products 100, that were converted in situ into amines 101 or 102 in good to high total yields, high d.r. and ee (Scheme 29a). Aryl-substituted substrates required stoichiometric amounts of 4-chloro-2,6-dimethylphenol to limit pyrrolidine byproducts 103. Lower amounts of 103 were instead detected with alkyl- and alkenyl-substituted imines, even with catalytic amounts of 4-chloro-2,6-dimethylphenol. Trace amounts of isomerization byproduct 88 were formed in all the cases. Good diastereoselectivities and excellent ee’s were also achieved with aromatic and heteroaromatic aldehyde derived imines 90, notably without any detectable amount of pyrrolidine byproducts even in the absence of phenol additive, although some minor quantities of C-3 regioisomers 106 were formed (Scheme 29b).
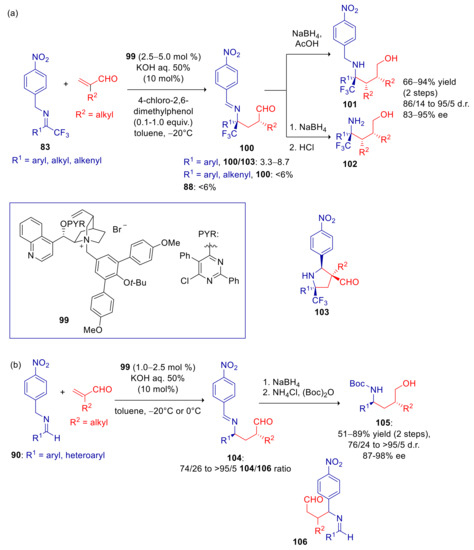
Scheme 29.
Umpolung Michael reaction of p-nitrobenzyl imines with α-substituted enals catalyzed by 99. (a) Reaction of imines derived from 1,1,1-trifluoromethyl ketones; (b) Reaction of imines derived from aldehydes.
This methodology proved successful also using α,β-unsaturated ketones, after appropriate modification of the catalyst’s structure [143]. Both imines 83 and 90 reacted with high to excellent enantioselectivities with methyl vinyl ketone and 2-cyclopentenone using cinchoninium catalyst 107, whereas somewhat lower ee’s were observed in the addition to ethyl vinyl ketone (Scheme 30a,b). Yields obtained with 83 were high, and only minor amounts of tautomerization product 88 were detected (Scheme 30a). Michael addition of less reactive 90, carried out with larger quantity of catalyst, furnished moderate yields, and variable amounts of secondary regioisomer 110 were formed (Scheme 30b). Both with 83 and 90, the corresponding pseudo-enantiomeric cinchonidinium salt, led to the opposite enantiomers with slightly diminished yields and ee’s. The synthetic usefulness of this transformation was demonstrated in the enantioselective synthesis of indolizidine 111, a potent antinociceptive agent (Scheme 30c).
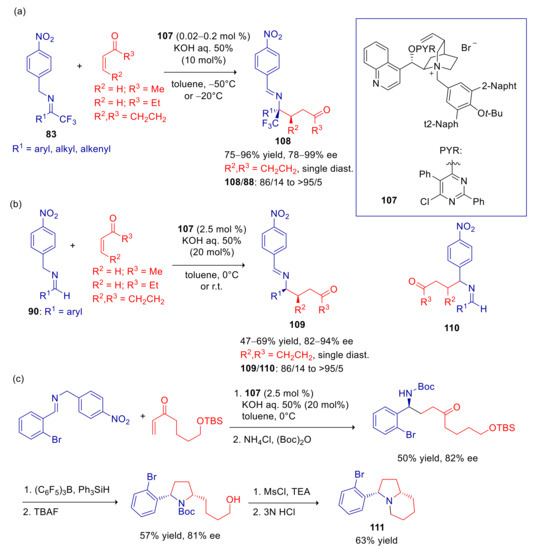
Scheme 30.
Umpolung Michael reaction of p-nitrobenzyl imines with α,β-unsaturated ketones catalyzed by 107. (a) Reaction of imines derived from 1,1,1-trifluoromethyl ketones; (b) reaction of imines derived from aldehydes; (c) enantioselective synthesis of antinociceptive agent 111.
Yoshida and coworkers later showed that α(p-nitrobenzylimino)esters 112 can be employed as nucleophiles in Michael addition to acrolein for the asymmetric synthesis of α-amino acids [144]. These substrates are expected to be more reactive than imines 83, since the benzyl carbon is doubly activated by the arylogous effect of NO2 and the vinylogous effect of ester group. Thus the weaker aqueous base K2CO3 turned out to be sufficient in promoting the reaction, whereas KOH, CsOH, and K2CO3 led to partial decomposition of the starting materials. With optimal cinchoninium catalyst 113 (structurally related to 84, 92, 99, and 107), products 114 were formed in moderate to excellent enantioselectivity and acceptable yields, after in situ reduction of the aldehyde (Scheme 31). The addition occurred exclusively at the benzylic carbon, that is the most nucleophilic site of the aza-allyl anion intermediate, as also demonstrated by deuteration experiments. The opposite enantiomer was obtained with similar enantiopurity, by using the pseudo-enantiomeric cinchonidinium derived catalyst. In aryl substituted substrates (R1 = aryl), lower ee values were observed with electron-withdrawing substituents. The authors attributed this effect to a greater stabilization of the negative charge in the aza-allyl anion, that would result in a looser ion pair with the chiral ammonium catalyst. 3-hydroxypropyl amino esters were accessed by hydrogenolysis of the p-nitrobenzyl group. On the other hand, acidic treatment afforded a α-amino lactone 115.
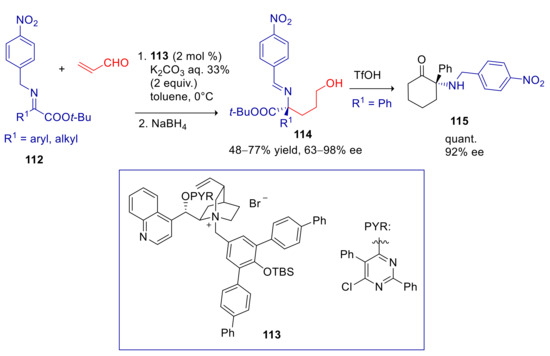
Scheme 31.
Umpolung Michael reaction of α(p-nitrobenzylimino)esters with acrolein catalyzed by 113.
Deng and Hu, with the aim to find a viable synthetic strategy to trifluoromethylated γ-amino acid derivatives, extended their previously developed umpolung Michael reaction to α,β-unsaturated acyl pirroles, that are synthetic surrogates of α,β-unsaturated esters [145]. With N-acryloyl pyrrole, N-terphenylmethyl catalysts, such as 84 and 92, were ineffective, with low level of enantioselectivity and moderate C-1/C-3 regioselectivity. Use of N-anthracenylmethyl quinidininium salt 116 (0.5–5 mol % of catalyst loading, depending on the substrate reactivity), in the presence of mesitol as additive, gave instead excellent results (Scheme 32a). The only exception was 1,1,1-trifluoroacetaldehyde derived imine, that turned out very reactive, but leading to a moderately enantiopure product. Products obtained from aryl substituted imines were isolated in lower yields, and accompanied by small amounts of isomerization and C-3-regioisomer byproducts 88 and 120a. The easy transformation of 117 into fluorinated γ-amino acid esters 118 and γ-lactam 119 were also performed. N-crotonoyl- and cinnamoyl pyrroles proved to be more challenging Michael acceptors. In fact, suitable reactivity could be reached only by increasing the electron-withdrawing character of the pyrrole ring, with the introduction of 2-CN substituent (Scheme 32b). Analyzing the X-ray structure of the N-anthracenylmethyl quinidininium phenoxide salt derived from 116, the authors clearly identified a π–π-stacking interaction between anthracenyl and nitrophenyl moieties. This furnishes useful insight on the enantioselective discrimination, suggesting that similar interactions are also involved in the N-anthracenylmethyl quinidininium/aza-allyl anion ion pair.
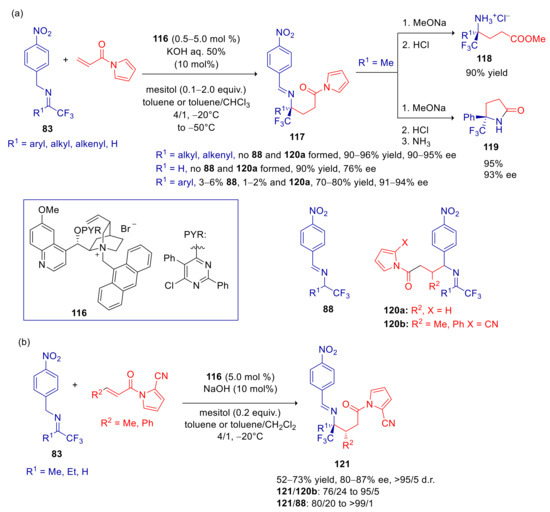
Scheme 32.
Umpolung Michael reaction of p-nitrobenzyl imines with N-alkenoyl pyrroles catalyzed by 116. (a) Use of acryloyl pyrroles; (b) use of crotonoyl and cinnamoyl pyrroles.
The frequent occurrence of tautomerization byproducts in the phase-transfer catalyzed Michael umpolung addition of p-nitrobenzyl imines, gave rise to the prospect of achieving an enantioselective isomerization process in the absence of the electrophilic partner. Following this aim, Deng and coworkers realized the asymmetric tautomerization of trifluoromethyl p-nitrobenzylimines 83 to α-trifluoromethyl(p-nitrobenzylbenzylidene)amines 88, promoted by cinchona alkaloid derived betaine 122 and catalytic K2CO3, with high to excellent levels of ee and low catalyst loading (Scheme 33a) [146]. Slightly lower enantioselectivities were obtained with the quinine-derived pseudoenantiomer of 122. Isotopic labelling studies supported the pivotal role of phenoxide group which carries out an internal deprotonation/protonation process following the prior generation of π-π imine-catalyst complex 124 (Scheme 33b). Control experiments revealed the paramount importance of the arylogous activation of the NO2 group, since benzylimines proved to be unreactive, whereas (p-methoxycarbonyl)imines were isomerized slowly and with lower ee (Scheme 33c). It is important to stress the superiority of this PTC methodology over the previously described Brønsted base catalyzed tautomerization of 83, which showed lower turnover efficiency and disappointingly different enantioselectivity in the production of the two optical antipodes [139].
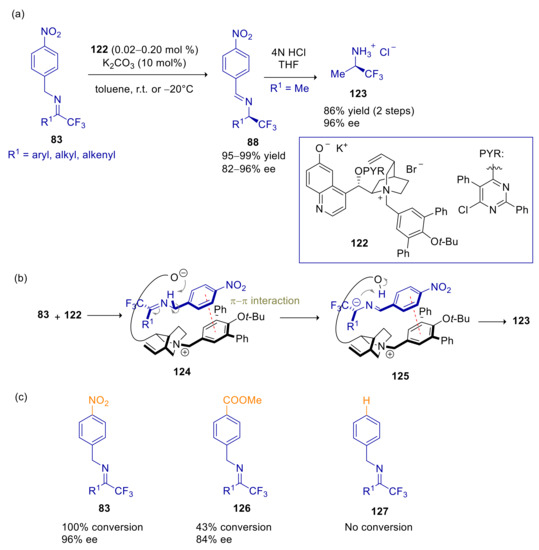
Scheme 33.
Asymmetric tautomerization of p-nitrobenzyl imines catalyzed by 122. (a) Scope of the reaction; (b) suggested mechanism involving internal proton transfer; (c) effect of the arylogous electron-withdrawing group after 24 h.
One of the most evident drawbacks in the arylogous umpolung Michael addition and tautomerization of p-nitrobenzylimines 83 and 90, is the different effectiveness in the production of two enantiomers. In fact, while cinchoninium- and quinidinium-derived catalysts consistently led to Si face attack in >80% ee, pseudo-enantiomeric cinchonidinium- and quininium-derived salts generally results in Re face attack with significantly lower efficiency. This gap is especially pronounced with less reactive electrophiles such as N-alkenoyl pyrroles. A structural analysis of pseudoenantiomeric N-anthracenylmethyl catalysts proved helpful to clarify the origin of such discrepancy [147]. These studies demonstrated that cinchoninium and quinidinium cations feature a very accessible pocket which serves as a preferential coordination site for the aza-allyl anion, resulting in high catalytic efficiency and enantiodiscrimination. This pocket, in pseudo-enantiomeric cinchonidinium and quininium derivatives, is partially congested by the orientation of the vinyl group (Figure 5), giving rise to less stable and diverse modes of ion interaction, thereby reducing catalytic activity and enantioselectivity. To overcome this issue, Deng and coworkers synthetized a series of cinchonidinium and quininium 3-epimer derivatives possessing a quasi-enantiomeric cavity which serves as a preferential coordination site for the aza-allyl anion (Figure 5) [147]. These compounds, as anticipated, provided catalytic activities, product selectivity and stereoselectivity very similar or even higher than the corresponding cinchoninium and quinidinium salts for transformations reported in Scheme 28,Scheme 30,Scheme 32, and Scheme 33, with opposite enantioselection (Scheme 34).
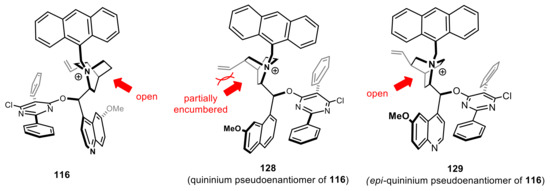
Figure 5.
Three-dimensional structures of the N-anthracenyl-O-PYR quinidinium, quininium, and 3-epi-quininium cations obtained from X-ray analysis (the red arrows show the cavity which serves as a preferential coordination site for the aza-allyl anion.
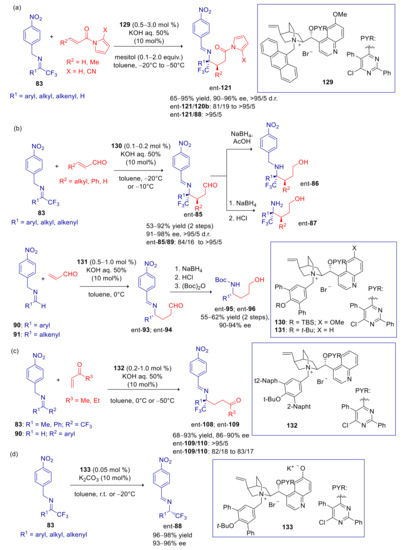
Scheme 34.
Asymmetric Michael additions and tautomerization reaction of p-nitrobenzyl imines cata-lyzed by 3-epi-cinchonidinium and 3-epi-quininium salts. (a) Use of α,β-unsaturated N-acyl pyrroles; (b) use of substituted or unsubstituted enals; (c) use of various enones; (d) asymmetric isomerization of trifluoromethyl imines.
α,β-Unsaturated esters are less reactive but more readily accessible substrates than alkenoyl pyrroles. In the attempt to perform the arylogous umpolung addition of 83 with these convenient substrates, Hu and Deng, found that catalysis with previously employed cinchoninium and quinidinium salts (e.g., the N-3,5-diphenyl-tBuO-benzyl and the N-anthracenylmethyl ammonium salts) led to major amounts of isomerization and tandem Michael/Mannich addition byproducts. Fortunately, a significant improvement of the enantioselectivity and a major suppression of undesirable reaction pathway was achieved by introducing a bulkier group at C-6′ quinoline position and by adding 4-chloro-2,6-dimethyl phenol [148]. With catalyst 134, products deriving from addition to various acrylic esters were delivered in good yields and high ee values (Scheme 35a). In spite of the disappointing catalytic activity and enantioselectivity of the quinine pseudoenantiomer of 134, the optical antipodes of adducts 135 could be obtained by employing its 3-epimer 136, with even higher yields and ee’s. The addition to unsaturated lactones required the adjustment of the catalyst’s and additive’s structures, ultimately resulting in excellent level of diastereo- and enantioselectivity with salt 137 and mesitol. In facts, the adducts to 2(5H)furanone were formed in almost or totally enantiopure form, and the reaction with homologous 5,6-dihydro-2H-pyran-2-one resulted in only minor decrease of enantioselectivity (Scheme 35b). Similar results and reversal of enantioselection were achieved with the 3-epi-quininium salt 140.
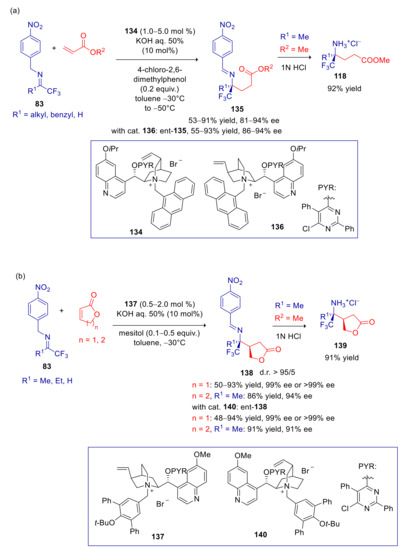
Scheme 35.
Umpolung Michael reaction of p-nitrobenzyl imines with α,β-unsaturated esters catalyzed by 134/136 and 137/140. (a) Use of acrylic esters; (b) Use of α,β-unsaturated lactones.
5. Conclusions and Outlook
Stereoselective vinylogous processes are gaining prominence in organic synthesis, making possible the construction of remotely polyfunctionalized molecules in a stereoselective manner. Particularly relevant are the reactions involving vinylogous enolate and related carbanions, in which the stabilizing effect of the unsaturated electron withdrawing groups is propagated through a conjugated π-system. Conjugated olefinic substrates are the most commonly used and reactive nucleophilic substrates. Although in early years vinylogous synthesis was dominated by metal promoted processes, asymmetric organocatalysis has assumed an increasing pivotal role in this field, as witnessed by the most recent review papers on this topic. Amines and Brønsted base organic catalysts are normally reactive enough to enable the activation of easily enolizable conjugated olefinic substrates. On the other hand, despite the advantages offered by PTC in terms of low environmental impact, easy process scalability and handling procedures, its application in this context has long been overlooked. However, in recent years, there have been an increasing number of phase-transfer catalyzed reactions involving vinylogous nucleophiles. The examples reported in this review demonstrate the competitiveness of onium and crown ether catalysts under non anhydrous phase transfer conditions, often resulting in similar or higher stereochemical outcomes combined with smaller catalytic loadings compared to organocatalysts (e.g., the Mannich addition of acyclic α,α-dicyanoolefins to N-Boc imine isatins and the Michael addition/lactone opening tandem reaction of α-alkylidene azlactones to 4-nitro-5-styrylisoxazoles). In addition, the rapid progress of computational studies applied to PTC has made it possible to shed light on the origin of stereoselectivity, thus boosting the design of novel and more effective catalysts. This will certainly bring to a progressive expansion of the scope of vinylogous pronucleophiles in the near future.
Deserving special mention are the vinylogous systems in which the electron-withdrawing effect of the leading group is propagated through an heteroaromatic or an aromatic moiety, that we call, for convenience, heteroarylogous and arylogous systems, respectively. Since the stabilizing effect of the anion is weaker, the enolization and subsequent reaction of such substrates is significantly more challenging, especially for arylogous ones. Considerable progress has been made in the organocatalyzed heteroarylogous reactions, of which several examples have been recently reported. On the other hand, a generation of arylogous carbanions promoted by organocatalysts is especially difficult, and the few examples described to date are restricted to very reactive polyfunctionalized aromatic substrates. In this regard PTC provides big advantages, being able to generate reactive carbanions from weakly acidic substrates under non anhydrous conditions, whereas the use of moisture and air sensitive metal bases, inert atmosphere and anhydrous solvents are usually required in homogeneous systems. Our review showcases examples of phase-transfer processes involving heteroarylogous and arylogous nucleophiles where homogeneous organocatalysis proved to be unsuccessful. From this point of view, it is no accident that the range of (hetero)arylogous carbon nucleophiles used in PTC reactions is wider than vinylogous substrates to date, and it is destined to further increase in the next few years. In our opinion, in facts, the application of novel weakly acidic pronucleophiles in arylogous reactions under environmentally low impact and undangerous non anhydrous conditions, represents one of the more promising frontier areas to be explored.
Author Contributions
Writing—review and editing A.D. and G.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fuson, R.C. The principle of vinylogy. Chem. Rev. 1935, 16, 1–27. [Google Scholar] [CrossRef]
- Krishnamurthy, J. The principle of vinylogy. J. Chem. Ed. 1982, 59, 543–547. [Google Scholar] [CrossRef]
- Curti, C.; Battistini, L.; Sartori, A.; Zanardi, F. New Developments of the Principle of Vinylogy as Applied to π-Extended Enolate-Type Donor Systems. Chem. Rev. 2020, 120, 2448–2612. [Google Scholar] [CrossRef]
- Battistini, L.; Curti, C.; Rassu, G.; Sartori, A.; Zanardi, F. Enolizable Alkylidene Heterocyclic and Carbocyclic Carbonyl Systems: Valuable Vinylogous Donor Substrates in Synthesis. Synthesis 2017, 49, 2297–2336. [Google Scholar] [CrossRef]
- Casiraghi, G.; Battistini, L.; Curti, C.; Rassu, G.; Zanardi, F. The Vinylogous Aldol and Related Addition Reactions: Ten Years of Progress. Chem. Rev. 2011, 111, 3076–3154. [Google Scholar] [CrossRef]
- Przydacz, A.; Skrzyńska, A.; Albrecht, Ł. Breaking Aromaticity with Aminocatalysis: A Convenient Strategy for Asymmetric Synthesis. Angew. Chem. Int. Ed. 2019, 58, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Cheng, Y.; Cheng, J.; Li, R.; Li, P. Organocatalytic Asymmetric Benzylation and Aldol-Hemiacetalization of α,β-Unsaturated Trifluoromethyl Ketones: Efficient Enantioselective Construction of 3,4-Dihydroisocoumarins. Chem. Eur. J. 2017, 23, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Leng, H.J.; Yang, X.Y.; Han, B.; Rao, C.L.; Liu, L.; Peng, C.; Huang, W. Efficient Synthesis of Tetrahydronaphthalene- or Isochroman-Fused Spirooxindoles Using Tandem Reactions. RSC Adv. 2015, 5, 88272–88276. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Li, T.; Li, J.; Li, H.; Wang, W. Formation of Dihydronaphthalenes via Organocatalytic Enatioselective Michael-Aldol Cascade Reactions with Arylalkanes. Org. Lett. 2013, 15, 5634–5637. [Google Scholar] [CrossRef]
- Raja, A.; Hong, B.-C.; Lee, G.-H. Organocatalytic Enantioselective Michael−Michael−Michael−Aldol Condensation Reactions: Control of Five Stereocenters in a Quadruple-Cascade Asymmetric Synthesis of Highly Functionalized Hexahydrophenanthrenes. Org. Lett. 2014, 16, 5756–5759. [Google Scholar] [CrossRef]
- Dell’Amico, L.; Companyõ, X.; Naicker, T.; Brauer, T.M.; Jørgensen, K.A. Asymmetric Organocatalytic Benzylation of α,β-Unsaturated Aldehydes with Toluenes. Eur. J. Org. Chem. 2013, 2013, 5262–5265. [Google Scholar] [CrossRef]
- Li, T.; Zhu, J.; Wu, D.; Li, X.; Wang, S.; Li, H.; Li, J.; Wang, W. A Strategy Enabling Enantioselective Direct Conjugate Addition of Inert Aryl Methane Nucleophiles to Enals with a Chiral Amine Catalyst under Mild Conditions. Chem. Eur. J. 2013, 19, 9147–9150. [Google Scholar] [CrossRef]
- Hiltebrandt, K.; Elies, K.; D’hooge, D.R.; Blinco, J.P.; Barner-Kowollik, C. A Light-Activated Reaction Manifold. J. Am. Chem. Soc. 2016, 138, 7048–7054. [Google Scholar] [CrossRef]
- Cuadros, S.; Dell’Amico, L.; Melchiorre, P. Forging Fluorine-Containing Quaternary Stereocenters by a Light-Driven Organocatalytic Aldol Desymmetrization Process. Angew. Chem. Int. Ed. 2017, 56, 11875–11879. [Google Scholar] [CrossRef]
- Dell’Amico, L.; Vega-Peñaloza, A.; Cuadros, S.; Melchiorre, P. Enantioselective Organocatalytic Diels-Alder Trapping of Photochemically Generated Hydroxy-o-Quinodimethanes. Angew. Chem. Int. Ed. 2016, 55, 3313–3317. [Google Scholar] [CrossRef]
- Dell’Amico, L.; Fernández-Alvarez, V.M.; Maseras, F.; Melchiorre, P. Light-Driven Enantioselective Organocatalytic β-Benzylation of Enals. Angew. Chem. Int. Ed. 2017, 56, 3304–3308. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Dong, S.; Liu, Z.; Wu, G.; Zou, C.; Ye, J. Enantioselective Michael Addition of Photogenerated o-Quinodimethanes to Enones Catalyzed by Chiral Amino Acid Esters. Org. Lett. 2017, 19, 2322–2325. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Ishida, N.; Murakami, M. Light-Driven Carboxylation of o-Alkylphenyl Ketones with CO2. J. Am. Chem. Soc. 2015, 137, 14063–14066. [Google Scholar] [CrossRef]
- Starks, C.M.; Liotta, C.L.; Halpern, M.E. Phase-Transfer Catalysis, Fundamentals, Applications, and Industrial Perspectives; Chapman & Hall: New York, NY, USA, 1994. [Google Scholar] [CrossRef]
- Halpern, M.E. (Ed.) Phase-Transfer Catalysis, Fundamentals, Mechanisms and Syntheses; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar] [CrossRef]
- Sasson, Y.; Neumann, R. (Eds.) Handbook of Phase-Transfer Catalysis; Chapman & Hall: London, UK, 1997. [Google Scholar] [CrossRef]
- Albanese, D. Liquid–Liquid Phase Transfer Catalysis: Basic Principles and Synthetic Applications. Catal. Rev. Sci. Eng. 2003, 45, 369–395. [Google Scholar] [CrossRef]
- Dehmlow, E.V.; Dehmlow, S.S. Phase Transfer Catalysis, 3rd ed.; Wiley-VCH: New York, NY, USA, 1993; ISBN 3527284087. [Google Scholar]
- Ikunaka, M. PTC in OPRD: An Illustrative Overview. Org. Process Res. Dev. 2008, 12, 698–709. [Google Scholar] [CrossRef]
- Freedman, H.H. Industrial applications of phase transfer catalysis (PTC): Past, present and future. Pure Appl. Chem. 1986, 58, 857–868. [Google Scholar] [CrossRef][Green Version]
- Sharma, M. Application of phase transfer catalysis in the chemical industry. In Handbook of Phase-Transfer Catalysis; Sasson, Y., Neumann, R., Eds.; Chapman & Hall: London, UK, 1997; pp. 168–199. [Google Scholar] [CrossRef]
- Mąkosza, M. Phase-transfer catalysis. A general green methodology in organic synthesis. Pure Appl. Chem. 2000, 72, 1399–1403. [Google Scholar] [CrossRef]
- Tan, J.; Yasuda, N. Contemporary Asymmetric Phase Transfer Catalysis: Large-Scale Industrial Applications. Org. Process Res. Dev. 2015, 19, 1731–1746. [Google Scholar] [CrossRef]
- Maruoka, K. (Ed.) Asymmetric Phase Transfer Catalysis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]
- Li, S.; Ma, J.-A. Asymmetric Phase-Transfer Catalysis in Organic Synthesis. In Bridging Heterogeneous and Homogeneous Catalysis: Concepts, Strategies, and Applications; Li, C., Liu, Y., Eds.; Wiley-VCH: Weinheim, Germany, 2014; pp. 425–468. [Google Scholar] [CrossRef]
- Shirakawa, S.; Maruoka, K. Chiral Onium Salts (Phase-Transfer Reactions). In Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions and Applications; Dalko, P., Ed.; Wiley-VCH: Weinheim, Germany, 2013; Volume 2, pp. 365–379. [Google Scholar] [CrossRef]
- Shirakawa, S.; Maruoka, K. Recent Developments in Asymmetric Phase-Transfer Reactions. Angew. Chem. Int. Ed. 2013, 52, 4312–4348. [Google Scholar] [CrossRef]
- Kaneko, S.; Kumatabara, Y.; Shirakawa, S. A new generation of chiral phase-transfer catalysts. Org. Biomol. Chem. 2016, 14, 5367–5376. [Google Scholar] [CrossRef]
- Schettini, R.; Sicignano, M.; De Riccardis, F.; Izzo, I.; Della Sala, G. Macrocyclic Hosts in Asymmetric Phase-Transfer Catalyzed Reactions. Synthesis 2018, 50, 4777–4795. [Google Scholar] [CrossRef]
- Gururaja, G.N.; Waser, M. Asymmetric Phase-Transfer Catalysis as a Powerful Tool in the Synthesis of Biologically Active Chiral Complex Natural Products. In Studies of Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Oxford, UK, 2014; Volume 43, pp. 409–435. [Google Scholar] [CrossRef]
- Starks, C.M.; Liotta, C.L.; Halpern, M.E. Phase-Transfer Catalysis Reaction with Strong Bases. In Phase-Transfer Catalysis, Fundamentals, Applications, and Industrial Perspectives; Chapman & Hall: New York, NY, USA, 1994; pp. 383–451. [Google Scholar] [CrossRef]
- Brak, K.; Jacobsen, E.N. Asymmetric Ion-Pairing Catalysis. Angew. Chem. Int. Ed. 2013, 52, 534–561. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Sun, J. Recent Progress in Asymmetric Ion-Pairing Catalysis with Ammonium Salts. Chem. Eur. J. 2019, 25, 3740–3751. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Tan, C.-H. Phase-Transfer and Ion-Pairing Catalysis of Pentanidiums and Bisguanidiniums. Acc. Chem. Res. 2017, 50, 842–856. [Google Scholar] [CrossRef]
- Waser, M.; Novacek, J.; Gratzer, K. Cooperative Catalysis Involving Chiral Ion Pair Catalysts. In Cooperative Catalysis: Designing Efficient Catalysts for Synthesis; Peters, R., Ed.; Wiley-VCH: Weinheim, Germany, 2015; pp. 197–226. [Google Scholar] [CrossRef]
- Uraguchi, D.; Ooi, T. Site-Selective Conjugate Addition through Catalytic Generation of Ion-Pairing Intermediates. In Site-Selective Catalysis; Kawabata, T., Ed.; Topics in Current Chemistry; Springer: Cham, Switzerland, 2016; Volume 372. [Google Scholar] [CrossRef]
- Mao, B.; Fañanás-Mastral, M.; Feringa, B.L. Catalytic Asymmetric Synthesis of Butenolides and Butyrolactones. Chem. Rev. 2017, 117, 10502–10566. [Google Scholar] [CrossRef] [PubMed]
- Kitson, R.R.A.; Millemaggi, A.; Taylor, R.J.K. The Renaissance of α-Methylene-γ-butyrolactones: New Synthetic Approaches. Angew. Chem. Int. Ed. 2009, 48, 9426–9451. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.-D.; Ye, L.-W. Chiral γ-lactam synthesis via asymmetric C–H amidation. Nat. Catal. 2019, 2, 182–183. [Google Scholar] [CrossRef]
- Lebedev, A. Preparation of Chiral 4-Substituted γ-Lactams and the Corresponding γ-Aminobutyric Acids. Chem. Heterocycl. Compd. 2007, 43, 673–684. [Google Scholar] [CrossRef]
- Mardjan, M.I.D.; Parrain, J.L.; Commeiras, L. Strategies to Access γ-Hydroxy-γ-butyrolactams. Synthesis 2018, 50, 1175–1198. [Google Scholar] [CrossRef]
- Choudhury, A.R.; Mukjerjee, S. Deconjugated butenolide: A versatile build block for asymmetric catalysis. Chem. Soc. Rev. 2020, 49, 6755–6788. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X.; Liu, H.; Xie, L.; Jiang, Z. Catalytic Asymmetric Synthesis of γ-Butenolides by Direct Vinylogous Reactions. Mini-Rev. Med. Chem. 2013, 13, 845–853. [Google Scholar] [CrossRef]
- Schneider, C.; Abels, F. Catalytic, enantioselective vinylogous Michael reactions. Org. Biomol. Chem. 2014, 12, 3531–3543. [Google Scholar] [CrossRef] [PubMed]
- Jusseau, X.; Chabaud, L.; Guillou, C. Synthesis of γ-butenolides and α,β-unsaturated γ-butyrolactams by addition of vinylogous nucleophiles to Michael acceptors. Tetrahedron 2014, 70, 2595–2615. [Google Scholar] [CrossRef]
- Casiraghi, G.; Zanardi, F.; Battistini, L.; Rassu, G. Advances in Exploring Heterocyclic Dienoxysilane Nucleophiles in Asymmetric Synthesis. Synlett 2009, 10, 1525–1542. [Google Scholar] [CrossRef]
- Rassu, G.; Zanardi, F.; Battistini, L.; Casiraghi, G. The synthetic utility of furan-, pyrrole- and thiophene-based 2-silyloxy dienes. Chem. Soc. Rev. 2000, 29, 109–118. [Google Scholar] [CrossRef]
- Rassu, G.; Zanardi, F.; Battistini, L.; Casiraghi, G. The Vinylogous Aldol Addition of Heterocyclic Silyloxy Dienes: Application in Synthesis. Synlett 1999, 1999, 1333–1350. [Google Scholar] [CrossRef]
- Yin, Y.; Jiang, Z. Organocatalytic Asymmetric Vinylogous Michael Reactions. ChemCatChem 2017, 9, 4306–4318. [Google Scholar] [CrossRef]
- Nagao, H.; Yamane, Y.; Mukaiyama, T. Effective Synthesis of 5-Substituted Butenolide Derivatives by Using Cinchonidine-derived Quaternary Ammonium Phenoxide Catalyst. Chem. Lett. 2007, 36, 8–9. [Google Scholar] [CrossRef]
- Singh, R.P.; Foxman, B.M.; Deng, L. Asymmetric Vinylogous Aldol Reaction of Silyloxy Furans with a Chiral Organic Salt. J. Am. Chem. Soc. 2010, 132, 9558–9560. [Google Scholar] [CrossRef]
- Fukuyama, T.; Goto, S. Synthetic Approaches Toward FR-900482. I. Stereoselective Synthesis of a Pentacyclic Model Compound. Tetrahedron Lett. 1989, 30, 6491–6494. [Google Scholar] [CrossRef]
- Fukuyama, T.; Yang, L. Synthetic Approaches Toward Mitomycins. I. Stereoselective Synthesis of a Tetracyclic Intermediate. Tetrahedron Lett. 1986, 27, 6299–6300. [Google Scholar] [CrossRef]
- Della Sala, G.; Sicignano, M.; Schettini, R.; De Riccardis, F.; Cavallo, L.; Minenkov, Y.; Batisse, C.; Hanquet, C.; Leroux, F.; Izzo, I. Switchable Diastereoselectivity in the Fluoride-Promoted Vinylogous Mukaiyama−Michael Reaction of 2-[(Trimethylsilyl)oxy]furan Catalyzed by Crown Ethers. J. Org. Chem. 2017, 82, 6629–6637. [Google Scholar] [CrossRef]
- Sicignano, M.; Schettini, R.; Sica, L.; Pierri, G.; De Riccardis, F.; Izzo, I.; Maity, B.; Minenkov, Y.; Cavallo, L.; Della Sala, G. Unprecedented Diastereoselective Arylogous Michael Addition of Unactivated Phthalides. Chem. Eur. J. 2019, 25, 7131–7141. [Google Scholar] [CrossRef]
- Claraz, A.; Oudeyer, S.; Levacher, V. Chiral Quaternary Ammonium Aryloxide/N,O-Bis(trimethylsilyl)acetamide Combination as Efficient Organocatalytic System for the Direct Vinylogous Aldol Reaction of (5H)-Furan-2-one Derivatives. Adv. Synth. Catal. 2013, 55, 841–846. [Google Scholar] [CrossRef]
- Arlt, A.; Toyama, H.; Takada, K.; Hashimoto, T.; Maruoka, K. Phase-transfer catalyzed asymmetric synthesis of α,β-unsaturated γ, γ-disubstituted γ-lactams. Chem. Commun. 2017, 53, 4779–4782. [Google Scholar] [CrossRef]
- Cui, H.-L.; Chen, Y.-C. α,α-Dicyanoalkenes: Versatile vinylogous nucleophiles for organic synthesis. Chem. Commun. 2009, 4479–4486. [Google Scholar] [CrossRef]
- Curti, C.; Sartori, A.; Battistini, L.; Zanardi, F. Exploring the Remote Reactivity of π-Extended Carbonyl Compounds: The Vinylogous Alkylidene Malononitrile Activation Strategy. Synlett 2018, 29, 266–281. [Google Scholar] [CrossRef]
- Niess, B.; Jørgensen, K.A. The asymmetric vinylogous Mannich reaction of dicyanoalkylidenes with α-amido sulfones under phase-transfer conditions. Chem. Commun. 2007, 1620–1622. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-Y.; Cui, H.-L.; Long, J.; Li, B.-J.; Wu, Y.; Ding, L.-S.; Chen, Y.-C. Organocatalytic and Highly Stereoselective Direct Vinylogous Mannich Reaction. J. Am. Chem. Soc. 2007, 129, 1878–1879. [Google Scholar] [CrossRef]
- Fang, Y.; Wei, Z.; Wang, Y.; Liu, S.; Cao, J.; Liang, D.; Lin, Y.; Duan, H. The asymmetric vinylogous Mannich reaction of noncyclic dicyanoolefins catalyzed by a bifunctional thiourea–ammonium salt phase transfer catalyst. New J. Chem. 2019, 43, 10012–10016. [Google Scholar] [CrossRef]
- Lu, J.; Chen, W.-Y. Organocatalytic and Enantioselective Mannich Reaction of Dicyanoolefins with α-Amido Sulfones. Bull. Korean Chem. Soc. 2012, 33, 3175–3176. [Google Scholar] [CrossRef][Green Version]
- Cheng, C.; Lu, X.; Ge, L.; Chen, J.; Cao, W.; Zhao, G. Effective asymmetric vinylogous Mannich reaction of isatin imines with α,α-dicyanoolefins in the presence of a simple chiral amide phosphonium bifunctional phase transfer catalyst. Org. Chem. Front. 2017, 4, 101–114. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Meng, Q.; Li, X. An organocatalytic enantioselective vinylogous Mannich reaction of α,α-dicyanoolefins with isatin N-Boc ketimines. Org. Chem. Front. 2016, 3, 709–713. [Google Scholar] [CrossRef]
- De Castro, P.P.; Carpanez, A.G.; Amarante, G.W. Azlactone Reaction Developments. Chem. Eur. J. 2016, 22, 10294–10318. [Google Scholar] [CrossRef] [PubMed]
- Marra, I.F.S.; de Castro, P.P.; Amarante, G.W. Recent Advances in Azlactone Transformations. Eur. J. Org. Chem. 2019, 5830–5855. [Google Scholar] [CrossRef]
- Alba, A.-N.R.; Rios, R. Oxazolones in Organocatalysis, New Tricks for an Old Reagent. Chem. Asian J. 2011, 6, 720–734. [Google Scholar] [CrossRef]
- Fisk, J.S.; Mosey, R.A.; Tepe, J.J. The diverse chemistry of oxazol-5-(4H)-ones. Chem. Soc. Rev. 2007, 36, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Dell’Amico, L.; Albrecht, Ł.; Naicker, T.; Poulsen, P.H.; Jørgensen, K.A. Beyond Classical Reactivity Patterns: Shifting from 1,4- to 1,6-Additions in Regio- and Enantioselective Organocatalyzed Vinylogous Reactions of Olefinic Lactones with Enals and 2,4-Dienals. J. Am. Chem. Soc. 2013, 135, 8063–8070. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, Y.-Y.; Lin, J.-B.; Xie, T.; Liang, Y.-M.; Xu, P.-F. Organocatalyzed Asymmetric Vinylogous Allylic−Allylic Alkylation of Morita−Baylis−Hillman Carbonates with Olefinic Azlactones: Facile Access to Chiral Multifunctional α-Amino Acid Derivatives. Org. Lett. 2015, 17, 3206–3209. [Google Scholar] [CrossRef]
- Gao, T.-P.; Lin, J.-B.; Hu, X.-Q.; Xu, P.-F. A catalytic asymmetric hetero-Diels–Alder reaction of olefinic azlactones and isatins: Facile access to chiral spirooxindole dihydropyranones. Chem. Commun. 2014, 50, 8934–8936. [Google Scholar] [CrossRef]
- Gao, T.-P.; Liu, D.; Lin, J.-B.; Hu, X.-Q.; Wang, Z.-Y.; Xu, P.-F. Direct construction of chiral quaternary dihydropyranones through highly enantioselective organocatalytic hetero-Diels–Alder reactions of olefinic azlactones. Org. Chem. Front. 2016, 3, 598–602. [Google Scholar] [CrossRef]
- Zhu, B.; Lu, B.; Zhang, H.; Xu, X.; Jiang, Z.; Chang, J. Phase-Transfer-Catalyzed, Enantioselective Vinylogous Conjugate Addition−Cyclization of Olefinic Azlactones To Access Multifunctionalized Chiral Cyclohexenones. Org. Lett. 2019, 21, 3271–3275. [Google Scholar] [CrossRef]
- Baschieri, A.; Bernardi, L.; Ricci, A.; Suresh, S.; Adamo, M.F.A. Catalytic Asymmetric Conjugate Addition of Nitroalkanes to 4-Nitro-5-styrylisoxazoles. Angew. Chem. Int. Ed. 2009, 48, 9342–9345. [Google Scholar] [CrossRef]
- Del Fiandra, C.; Piras, L.; Fini, F.; Disetti, P.; Moccia, M.; Adamo, M.F.A. Phase transfer catalyzed enantioselective cyclopropanation of 4-nitro-5-styrylisoxazoles. Chem. Commun. 2012, 48, 3863–3865. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xiong, Y.; Wang, Z.-F.; Tao, H.-Y.; Wang, C.-J. Ligand-controlled stereodivergent 1,3-dipolar cycloaddition of azomethine ylides with 3-methyl-4-nitro-5-styrylisoxazoles. Chem. Commun. 2016, 52, 9458–9461. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Ma, X.; Wang, R. Organocatalyzed asymmetric vinylogous Michael addition of α,β-unsaturated γ-butyrolactam. Chem. Commun. 2013, 49, 9329–9331. [Google Scholar] [CrossRef]
- Liu, X.-L.; Han, W.-Y.; Zhang, X.-M.; Wang, W.-C. Highly Efficient and Stereocontrolled Construction of 3,3′-Pyrrolidonyl Spirooxindoles via Organocatalytic Domino Michael/Cyclization Reaction. Org. Lett. 2013, 15, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; López-Delgado, F.J.; Jørgensen, D.K.B.; Nielsen, R.P.; Jiang, H.; Jørgensen, K.A. Trienamine-mediated asymmetric [4+2]-cycloaddition of α,β-unsaturated ester surrogates applying 4-nitro-5-styrylisoxazoles. Chem. Commun. 2014, 50, 15689–15691. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Pei, W.; Wang, J.; Liu, J.; Wang, J.; Zhang, M.-l.; Chen, Z.; Liu, L. Organocatalytic asymmetric synthesis of compounds bearing both isoxazole and pyrazole moieties via 1,6-addition of pyrazol-5-ones to 3-methyl-4-nitro-5-alkenylisoxazoles. Org. Chem. Front. 2018, 5, 1342–1347. [Google Scholar] [CrossRef]
- Kowalczyk-Dworak, D.; Kwit, M.; Albrecht, Ł. Allylic−Allylic Alkylation with 3,5-Dimethyl-4-nitroisoxazole: A Route to Dicarboxylic Acid Derivatives. J. Org. Chem. 2020, 85, 2938–2944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, B.; Lin, H.; Cui, W.; Zeng, X.; Fan, X. Organocatalyzed Asymmetric Vinylogous Michael Reactions of 3,5-Dialkyl-Substituted 4-Nitroisoxazoles: A Direct Method for the Synthesis of Chiral Isoxazole Derivatives. Adv. Synth. Catal. 2015, 357, 1299–1304. [Google Scholar] [CrossRef]
- Zhu, B.; Lee, R.; Yin, Y.; Li, F.; Coote, M.L.; Jiang, Z. Enantioselective Vinylogous Amination of 5-Alkyl-4-nitroisoxazoles with a Dipeptide-Based Guanidinium Phase-Transfer Catalyst. Org. Lett. 2018, 20, 429–432. [Google Scholar] [CrossRef]
- Zhu, B.; Li, F.; Lu, B.; Chang, J.; Jiang, Z. Organocatalytic Enantioselective Vinylogous Aldol Reaction of 5-Alkyl-4-Nitroisoxazoles to Paraformaldehyde. J. Org. Chem. 2018, 83, 11350–11358. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhu, Q.; Wang, J.; Chen, J.; Cao, W.; Zhu, B.; Wu, X. Direct Asymmetric Vinylogous Mannich Addition of 3,5-Disubstituted-4-nitroisoxazoles to Isatin-Derived Imines Catalyzed by a Bifunctional Phase-Transfer-Catalyst. J. Org. Chem. 2018, 83, 14617–14625. [Google Scholar] [CrossRef]
- Shaqura, I.I.; Bule, M.; Khan, F.; Niaz, K. Phloroglucinols, xanthones and anthrones. In Recent Advances in Natural Products Analysis, 1st ed.; Nabavi, S.M., Saeedi, M., Nabavi, S.F., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherland, 2020; pp. 175–197. [Google Scholar]
- Kadarkaraisamy, M.; Sykes, A.G. The chemistry of constrained crown ring systems and fluorescence sensor applications. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 23–30. [Google Scholar] [CrossRef]
- Shen, J.; Nguyen, T.T.; Goh, Y.-P.; Ye, W.; Fu, X.; Xu, J.; Tan, C.-H. Chiral Bicyclic Guanidine-Catalyzed Enantioselective Reactions of Anthrones. J. Am. Chem. Soc. 2006, 128, 13692–13693. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lei, Z.-Y.; Zhao, M.-X.; Shi, J.-W. A highly efficient asymmetric Michael addition of anthrone to nitroalkenes with cinchona organocatalysts. Tetrahedron Lett. 2007, 48, 5743–5746. [Google Scholar] [CrossRef]
- Alba, A.-N.; Bravo, N.; Moyano, A.; Rios, R. Enantioselective addition of anthrones to α,β-unsaturated aldehydes. Tetrahedron Lett. 2009, 50, 3067–3069. [Google Scholar] [CrossRef]
- Wu, C.; Li, W.; Yang, J.; Liang, X.; Ye, J. Asymmetric organocatalytic Michael addition of anthrone to enone. Org. Biomol. Chem. 2010, 8, 3244–3250. [Google Scholar] [CrossRef]
- Zhao, H.; Xiao, M.; Xu, L.; Wang, L.; Xiao, J. Bifunctional thiourea catalyzed asymmetric Michael addition of anthrone to methyleneindolinones. RSC Adv. 2016, 6, 38558–38562. [Google Scholar] [CrossRef]
- Ceban, V.; Tauchman, J.; Meazza, M.; Gallagher, G.; Light, M.E.; Gergelitsová, I.; Veselý, J.; Rios, R. Expanding the scope of MetalFree enantioselective allylic substitutions: Anthrones. Sci. Rep. 2015, 5, 16886. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, K.C.; Chattopadhyay, S.K.; Khan, A.T. Phase-transfer-catalyzed Alkylation of Anthrone and 10-Propargylanthrone. Synthesis 1988, 552–553. [Google Scholar] [CrossRef]
- Nickel, H.C.; Schmidt, P.; Böhm, K.J.; Baasner, S.; Müller, K.; Gerlach, M.; Unger, E.; Günther, E.G.; Prinz, H. Synthesis, antiproliferative activity and inhibition of tubulin polymerization by 1,5- and 1,8-disubstituted 10H-anthracen-9-ones bearing a 10-benzylidene or 10-(2-oxo-2-phenylethylidene) moiety. Eur. J. Med. Chem. 2010, 45, 3420–3438. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Liu, G.; Beabout, K.; McCurry, M.D.; Shamoo, Y. Asymmetric Alkylation of Anthrones, Enantioselective Total Synthesis of (−)- and (+)-Viridicatumtoxins B and Analogues Thereof: Absolute Configuration and Potent Antibacterial Agents. J. Am. Chem. Soc. 2017, 139, 3736–3746. [Google Scholar] [CrossRef]
- Donslund, B.S.; Monleón, A.; Palazzo, T.A.; Christensen, M.L.; Dahlgaard, A.; Erickson, J.D.; Jørgensen, K.A. Organocatalytic Enantioselective Higher-Order Cycloadditions of In Situ Generated Amino Isobenzofulvenes. Angew. Chem. Int. Ed. 2018, 57, 1246–1250. [Google Scholar] [CrossRef]
- Donslund, B.S.; Jessen, N.I.; Bertuzzi, G.; Giardinetti, M.; Palazzo, T.A.; Christensen, M.L.; Jørgensen, K.A. Catalytic Enantioselective [10+4] Cycloadditions. Angew. Chem. Int. Ed. 2018, 57, 13182–13186. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, Y.; Du, W.; Chen, Y.-C. Asymmetric Cross [10+2] Cycloadditions of 2-Alkylidene-1-indanones and Activated Alkenes under Phase-Transfer Catalysis. Chem. Eur. J. 2020, 26, 1754–1758. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, Z.-X.; Zhou, Y.-C.; Xiao, W.; Ouwyang, Q.; Du, W.; Chen, Y.-C. Switchable regioselectivity in amine-catalysed asymmetric cycloadditions. Nat. Chem. 2017, 9, 590–594. [Google Scholar] [CrossRef]
- Berthelette, C.; McCooye, C.; Leblanc, Y.; Trimble, L.A.; Tsou, N.N. Studies on the Dimerization of 2-Benzylidene-1-indanone. J. Org. Chem. 1997, 62, 4339–4342. [Google Scholar] [CrossRef]
- Camps, P.; Domingo, L.R.; Formosa, X.; Galdeano, C.; González, D.; Muñoz-Torrero, D.; Segalés, S.; Font-Bardia, M.; Solans, X. Highly Diastereoselective One-Pot Synthesis of Spiro{cyclopenta[a]indene-2,2’-indene}diones from 1-Indanones and Aromatic Aldehydes. J. Org. Chem. 2006, 71, 3464–3471. [Google Scholar] [CrossRef]
- Karmakar, R.; Pahari, P.; Mal, D. Phthalides and Phthalans: Synthetic Methodologies and Their Applications in the Total Synthesis. Chem. Rev. 2014, 114, 6213–6284. [Google Scholar] [CrossRef]
- Awasthi, A.; Singh, M.; Rathee, G.; Chandra, R. Recent advancements in synthetic methodologies of 3-substituted phthalides and their application in the total synthesis of biologically active natural products. RSC Adv. 2020, 10, 12626–12652. [Google Scholar] [CrossRef]
- Janowski, W.K.; Prager, R.H. The Chemistry of Phthalide-3-carboxylic Acid. III Decarboxylation of Salts in the Presence of α,β-Unsaturated Ketones. Aust. J. Chem. 1985, 38, 921–929. [Google Scholar] [CrossRef]
- Ciufolini, M.A.; Browne, M.E. Efficient palladium-mediated synthesis of a spirocyclic model for fredericamycin A. Tetrahedron Lett. 1987, 28, 171–174. [Google Scholar] [CrossRef]
- Ogawa, Y.; Hosaka, K.; Chin, M.; Mitsuhashi, H. Synthesis of (Z)-3-Butylidene-4-hydroxyphthalide. Heterocycles 1991, 32, 1737–1744. [Google Scholar] [CrossRef]
- Mali, R.S.; Babu, K.N. Reactions of 3-(1-Hydroxyalkyl)phthalides with Acids: Synthesis of (Z)-3-Alkylidenephthalides and 3-Alkyl-8-hydroxyisocoumarins. J. Org. Chem. 1998, 63, 2488–2492. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, C.; Wang, H.; Xu, L.-W.; Lu, Y. Direct asymmetric Michael addition of phthalide derivatives to chalcones. Tetrahedron Lett. 2013, 54, 5261–5265. [Google Scholar] [CrossRef]
- Luo, J.; Wang, H.; Zhong, F.; Kwiatkowski, J.; Xu, L.-W.; Lu, Y. Highly diastereoselective and enantioselective direct Michael addition of phthalide derivatives to nitroolefins. Chem. Commun. 2013, 49, 5775–5777. [Google Scholar] [CrossRef]
- Luo, J.; Wang, H.; Zhong, F.; Kwiatkowski, J.; Xu, L.-W.; Lu, Y. Direct asymmetric Mannich reaction of phthalides: Facile access to chiral substituted isoquinolines and isoquinolinones. Chem. Commun. 2012, 48, 4707–4709. [Google Scholar] [CrossRef]
- Zhong, F.; Luo, J.; Chen, G.-Y.; Dou, X.; Lu, Y. Highly Enantioselective Regiodivergent Allylic Alkylations of MBH Carbonates with Phthalides. J. Am. Chem. Soc. 2012, 134, 10222–10227. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Cheng, J.-P. Quinine-derived thiourea promoted enantioselective Michael addition reactions of 3-substituted phthalides to maleimides. Sci. China Chem. 2019, 62, 649–652. [Google Scholar] [CrossRef]
- Liu, W.; Hu, Z.-P.; Yan, Y.; Liao, W.-W. Highly diastereo- and enantioselective construction of phthalideoxindole hybrids bearing vicinal quaternary chiral centers via an organocatalytic allylic alkylation. Tetrahedron Lett. 2018, 59, 3132–3135. [Google Scholar] [CrossRef]
- Zhuang, Z.; Hu, Z.-P.; Liao, W.-W. Asymmetric Synthesis of Functionalized Dihydronaphthoquinones Containing Quaternary Carbon Centers via a Metal-Free Catalytic Intramolecular Acylcyanation of Activated Alkenes. Org. Lett. 2014, 16, 3380–3383. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Zhuang, Z.; Liao, W.-W. Asymmetric Synthesis of Dihydronaphthoquinones Containing Adjacent Stereocenters via a Sulfa-Michael Addition Triggered Ring-Expansion Approach. J. Org. Chem. 2015, 80, 4627–4637. [Google Scholar] [CrossRef]
- Sicignano, M.; Schettini, R.; Pierri, G.; Marino, M.L.; Izzo, I.; De Riccardis, F.; Della Sala, G. An Entry to Enantioenriched 3,3-Disubstituted Phthalides through Asymmetric Phase-Transfer-Catalyzed γ-Alkylation. J. Org. Chem. 2020, 85, 7476–7484. [Google Scholar] [CrossRef] [PubMed]
- Sicignano, M.; Dentoni Litta, A.; Schettini, R.; De Riccardis, F.; Pierri, G.; Tedesco, C.; Izzo, I.; Della Sala, G. Highly Diastereoselective Crown Ether Catalyzed Arylogous Michael Reaction of 3-Aryl Phthalides. Org. Lett. 2017, 19, 4383–4386. [Google Scholar] [CrossRef]
- Autuori, G. Addizione di Michael di Ftalidi Promossa da Superbasi Organiche. Bachelor’s Thesis, Università degli Studi di Salerno, Fisciano, Italy, 17 May 2021. [Google Scholar]
- Ripin, D.H.B. pKa. In Practical Synthetic Organic Chemistry—Reactions, Principles, and Techniques; Caron, S., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 771–803. [Google Scholar]
- Specht, A.; Thomann, J.-S.; Alarcon, K.; Wittayanan, W.; Ogden, D.; Furuta, T.; Kurakawa, Y.; Goeldner, M. New Photoremovable Protecting Groups for Carboxylic Acids with High Photolytic Efficiencies at Near-UV Irradiation. Application to the Photocontrolled Release of L-Glutamate. ChemBioChem 2006, 7, 1690–1695. [Google Scholar] [CrossRef]
- Bléger, D.; Ciesielski, A.; Samorì, P.; Hecht, S. Photoswitching Vertically Oriented Azobenzene Self-Assembled Monolayers at the Solid–Liquid Interface. Chem. Eur. J. 2010, 16, 14256–14260. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Jwa, D.G.; You, A.; Park, S.; Kim, J.G.; Kang, S.M.; Kim, M. Photochemical Control of Polydopamine Coating in an Aprotic Organic Solvent. Asian J. Org. Chem. 2019, 8, 1610–1612. [Google Scholar] [CrossRef]
- Artamkina, G.A.; Grinfel’d, A.A.; Beletskaya, I.P. Oxidation of triaryl- and diarylmethanes by oxygen in the system KOH-dimethoxyethane-18-crown-6-ether and cleavage of triarylcarbinol and diaryl ketone intermediates. Russ. Chem. Bull. 1983, 32, 345–352. [Google Scholar] [CrossRef]
- Chupp, J.P.; Grabiak, R.C.; Leschinsky, K.L.; Neumann, T.L. Substituted Benzotrichloride Synthesis by Phase Transfer-Catalyzed Chlorination. Synthesis 1986, 224–226. [Google Scholar] [CrossRef]
- Taha, N.; Sasson, Y.; Chidambaram, M. Phase transfer methodology for the synthesis of substituted stilbenes under Knoevenagel condensation condition. Appl. Catal. A Gen. 2008, 350, 217–224. [Google Scholar] [CrossRef]
- Ye, F.; Li, Y.; Fu, Y.; Gao, S.; Zhao, L.-X. Microwave-assisted synthesis and crystal structure of novel 2-dichloromethyl-1,3-dioxolanes. Heterocycles 2013, 87, 407–415. [Google Scholar] [CrossRef]
- Ye, F.; Sun, C.-Y.; Fu, Y. Synthesis and Crystal Structure of 2-(Dichloromethyl)-2-(4-nitrophenyl)-1,3-dioxane. J. Chem. 2013, 2013, 974174. [Google Scholar] [CrossRef]
- Hardy, M.A.; Chachignon, H.; Cahard, D. Advances in Asymmetric Di- and Trifluoromethylthiolation, and Di- and Trifluoromethoxylation Reactions. Asian J. Org. Chem. 2019, 8, 591–609. [Google Scholar] [CrossRef]
- Yang, X.; Wu, T.; Phipps, R.J.; Toste, F.D. Advances in Catalytic Enantioselective Fluorination, Mono-, Di-, and Trifluoromethylation, and Trifluoromethylthiolation Reactions. Chem. Rev. 2015, 115, 826–870. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Puglisi, A.; Raimondi, L.; Benaglia, M. Synthesis of Alpha-trifluoromethylthio Carbonyl Compounds: A Survey of the Methods for the Direct Introduction of the SCF3 Group on to Organic Molecules. ChemCatChem 2018, 10, 2717–2733. [Google Scholar] [CrossRef]
- Xu, L.; Yu, L.; Liu, J.; Wang, H.; Zheng, C.; Zhao, G. Enantioselective Vinylogous Mannich-Type Reactions to Construct CF3S-Containing Stereocenters Catalysed by Chiral Quaternary Phosphonium Salts. Adv. Synth. Catal. 2020, 362, 1851–1857. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, L. Asymmetric Synthesis of Trifluoromethylated Amines via Catalytic Enantioselective Isomerization of Imines. J. Am. Chem. Soc. 2012, 134, 14334–14337. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, X.; Sun, J.; Niu, D.; Chruma, J.J. 2-Azaallyl Anions, 2-Azaallyl Cations, 2-Azaallyl Radicals, and Azomethine Ylides. Chem. Rev. 2018, 118, 10393–10457. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, L.; Li, Z.; Deng, L. Catalytic asymmetric umpolung reactions of imines. Nature 2015, 523, 445–450. [Google Scholar] [CrossRef]
- Li, Z.; Hu, B.; Wu, Y.; Fei, C.; Deng, L. Control of chemoselectivity in asymmetric tandem reactions: Direct synthesis of chiral amines bearing nonadjacent stereocenters. Proc. Natl. Acad. Sci. USA 2018, 115, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wu, Y.; Li, Z.; Deng, L. Catalytic Asymmetric Synthesis of Chiral γ-Amino Ketones via Umpolung Reactions of Imines. J. Am. Chem. Soc. 2016, 138, 15817–15820. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Mino, T.; Sakamoto, M. Organocatalytic Highly Regio- and Enantioselective Umpolung Michael Addition Reaction of α-Imino Esters. Chem. Eur. J. 2017, 23, 12749–12753. [Google Scholar] [CrossRef]
- Hu, B.; Deng, L. Catalytic Asymmetric Synthesis of Trifluoromethylated γ-Amino Acids through the Umpolung Addition of Trifluoromethyl Imines to Carboxylic Acid Derivatives. Angew. Chem. Int. Ed. 2018, 57, 2233–2237. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Y.; Deng, L. Cinchonium Betaines as Efficient Catalysts for Asymmetric Proton Transfer Catalysis: The Development of a Practical Enantioselective Isomerization of Trifluoromethyl Imines. J. Am. Chem. Soc. 2016, 138, 12297–12302. [Google Scholar] [CrossRef]
- Hu, B.; Bezpalko, M.W.; Fei, C.; Dickie, D.A.; Foxman, B.M.; Deng, L. Origin of and a Solution for Uneven Efficiency by Cinchona Alkaloid-Derived, Pseudoenantiomeric Catalysts for Asymmetric Reactions. J. Am. Chem. Soc. 2018, 138, 13913–13920. [Google Scholar] [CrossRef]
- Hu, B.; Deng, L. Direct Catalytic Asymmetric Synthesis of Trifluoromethylated γ-Amino Esters/Lactones via Umpolung Strategy. J. Org. Chem. 2019, 84, 994–1005. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).