Facile Hydrothermal Synthesis and Supercapacitor Performance of Mesoporous Necklace-Type ZnCo2O4 Nanowires
Abstract
:1. Introduction
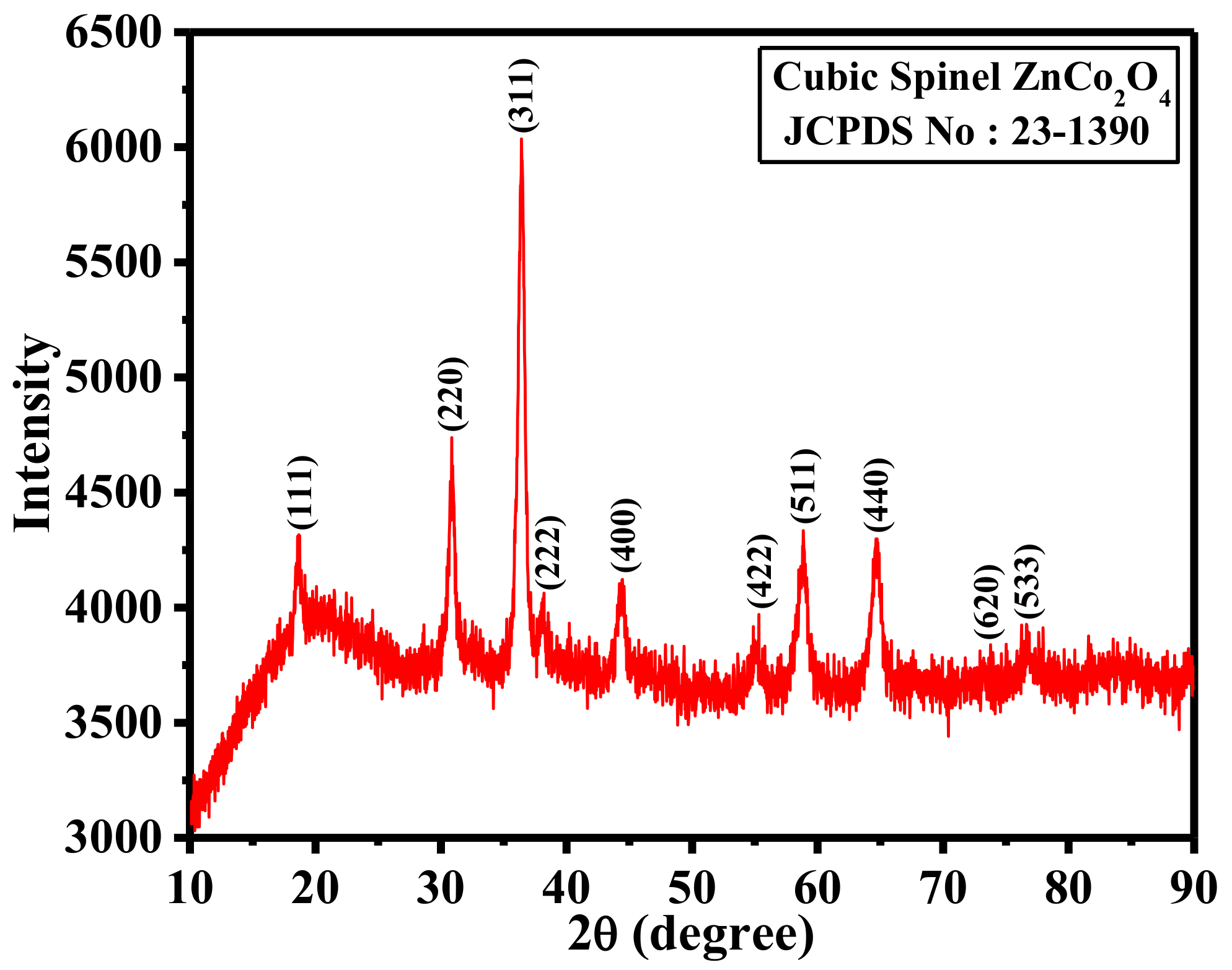
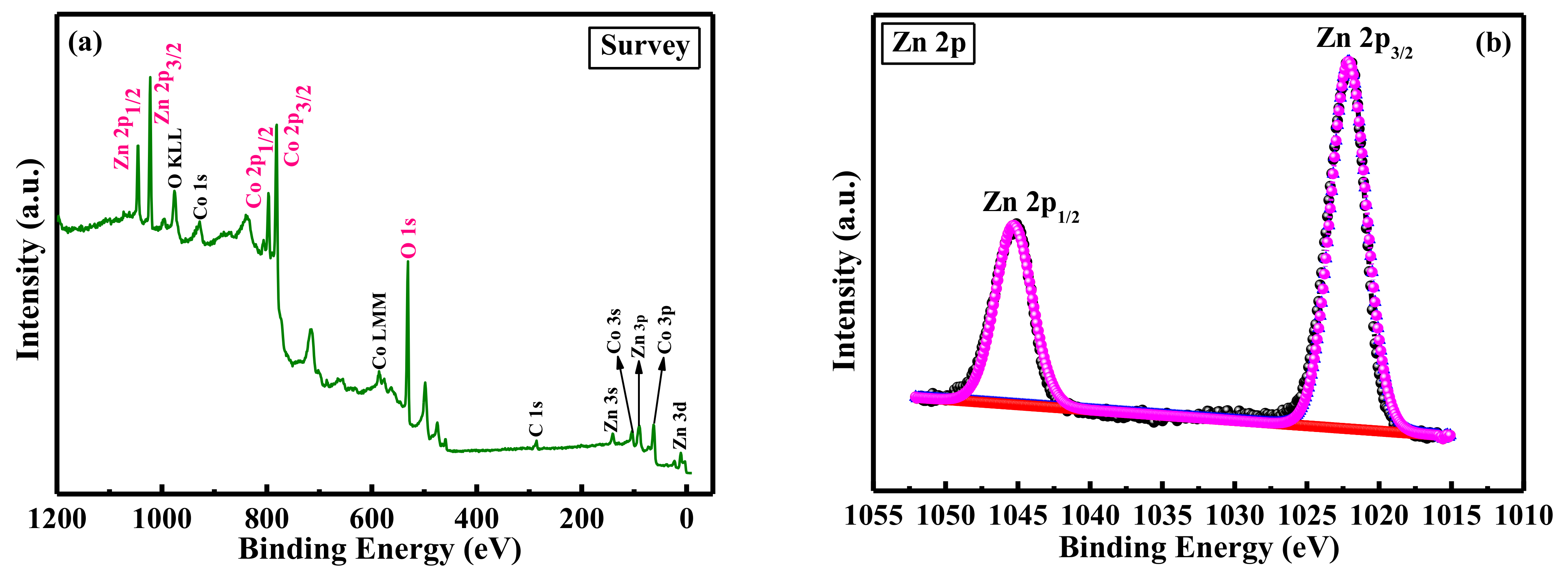
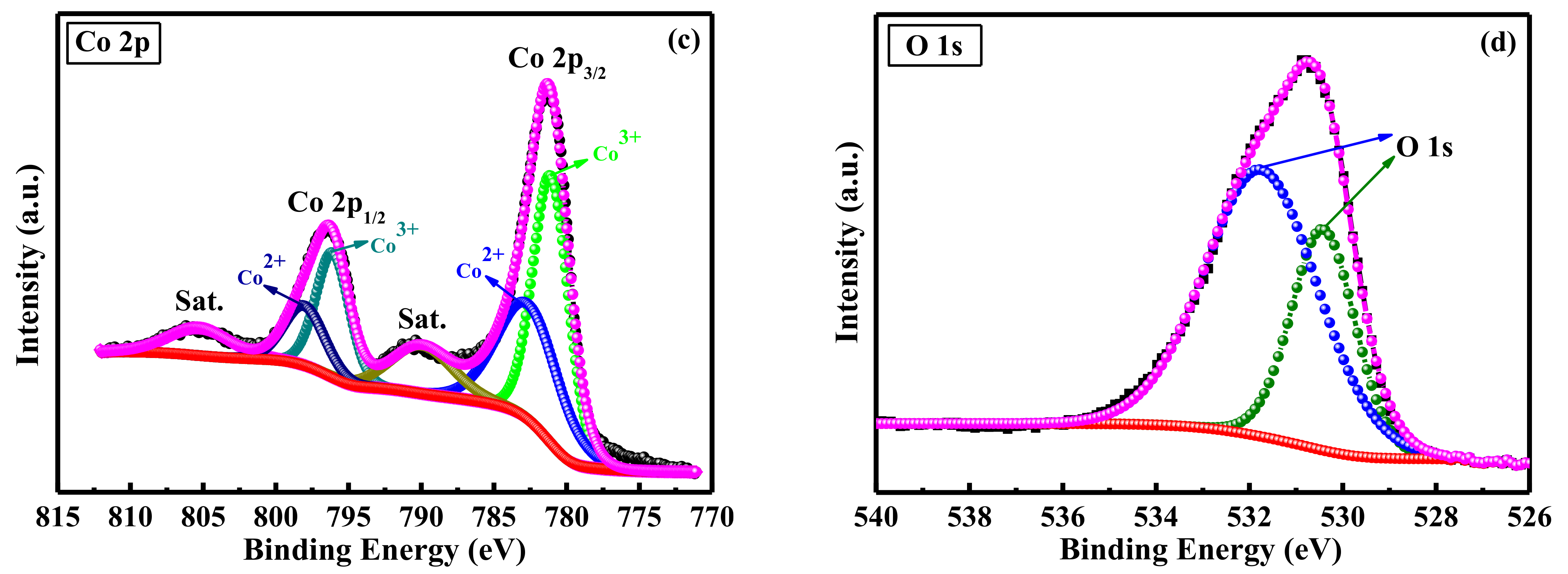
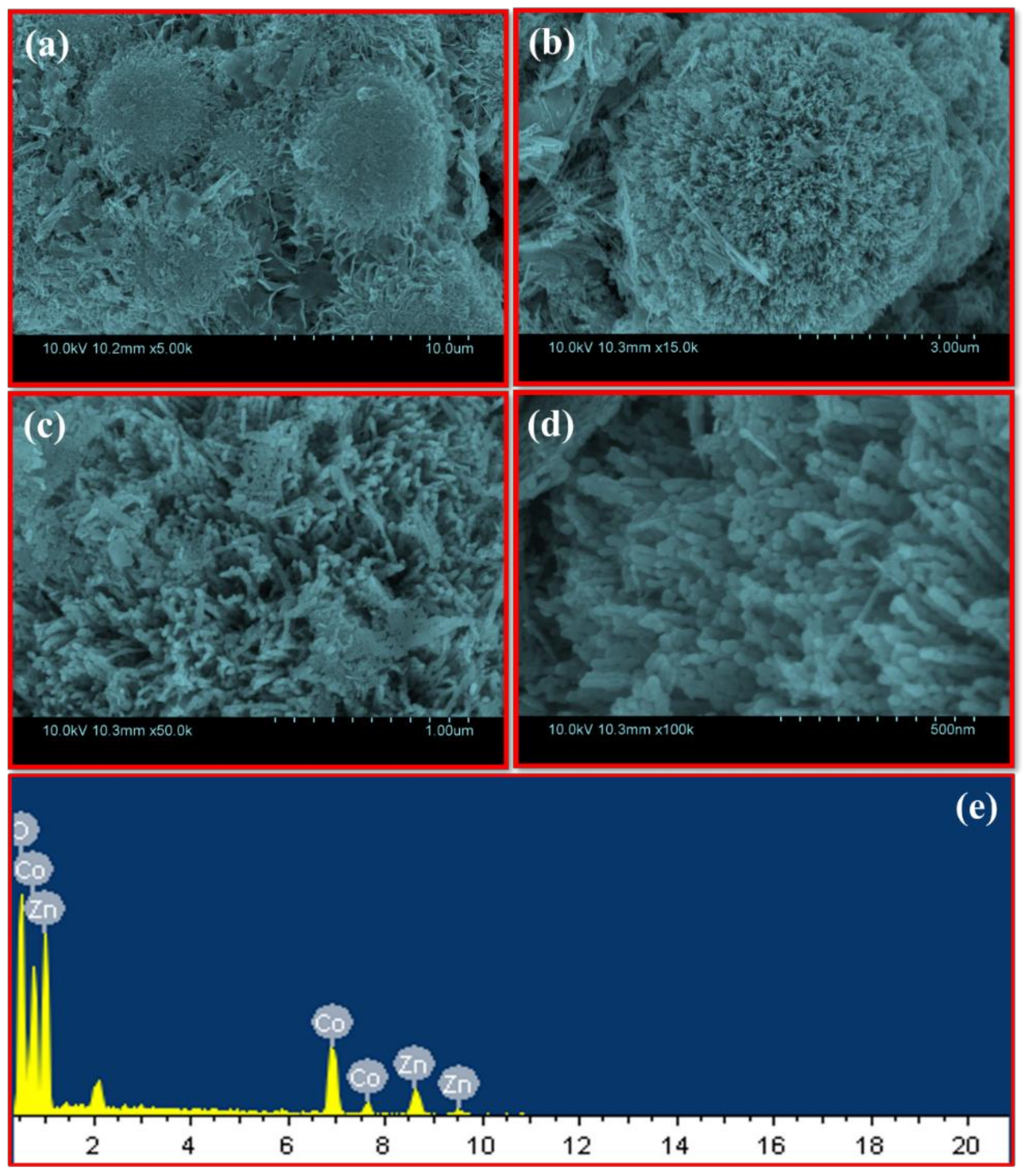
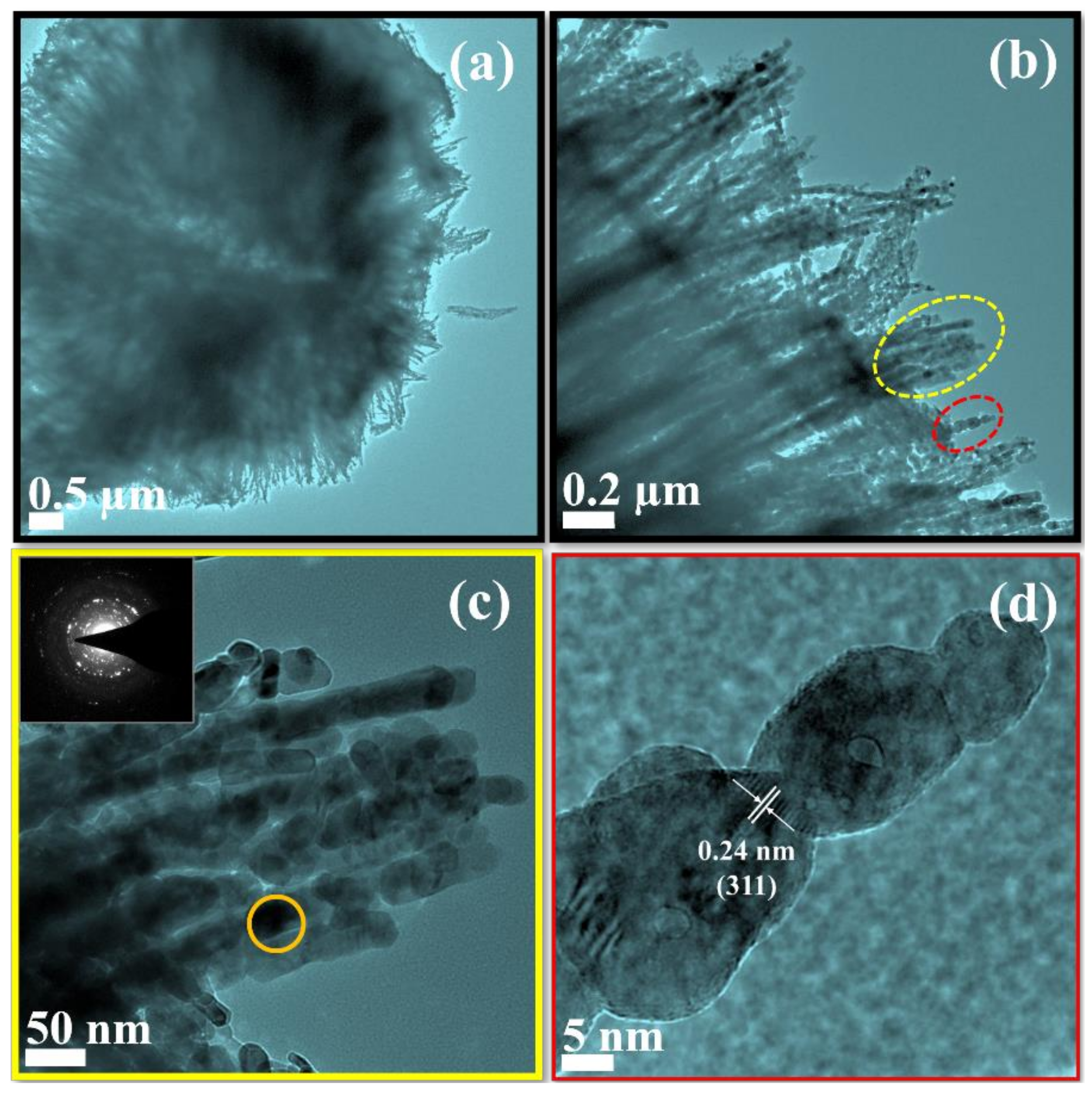
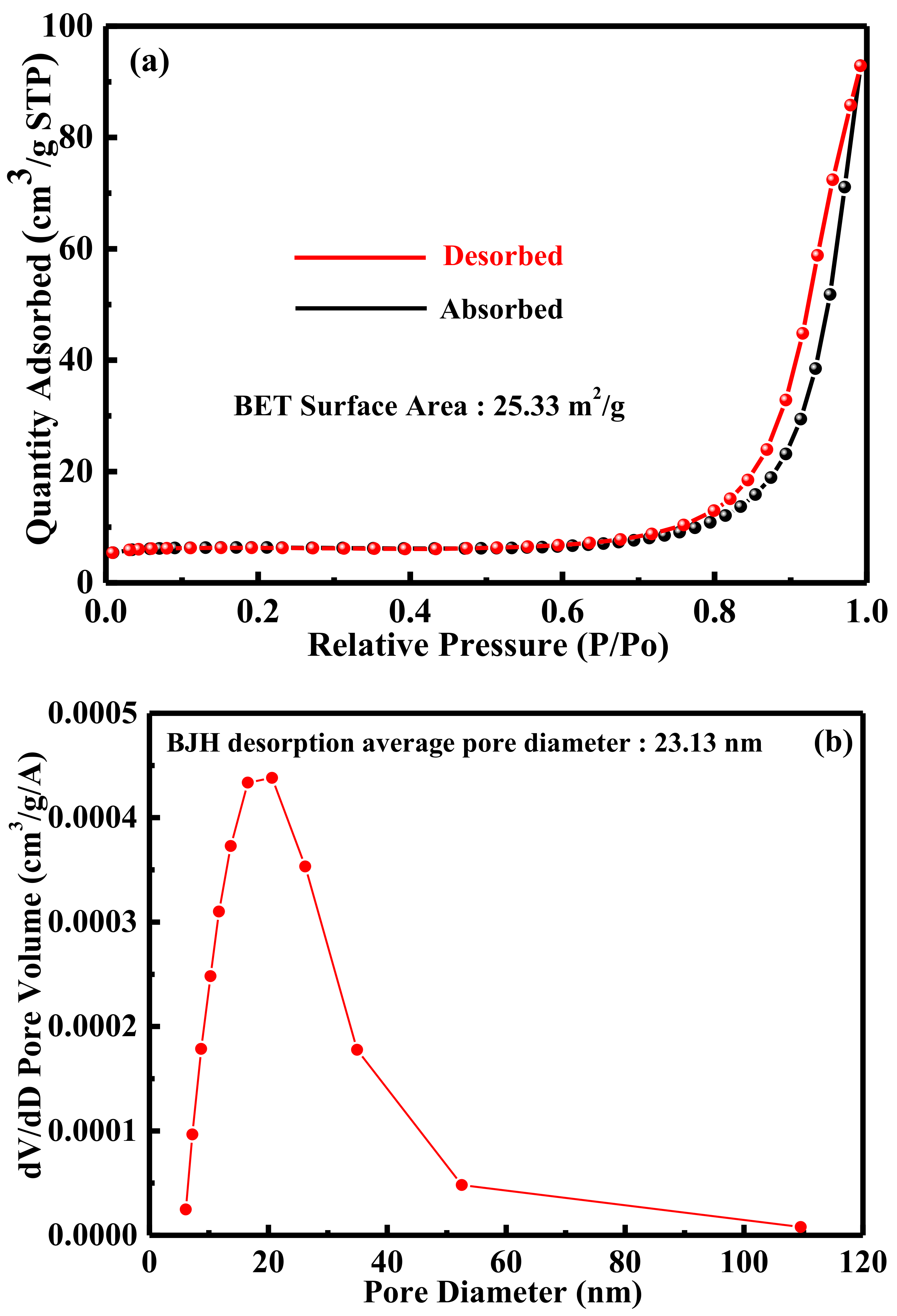
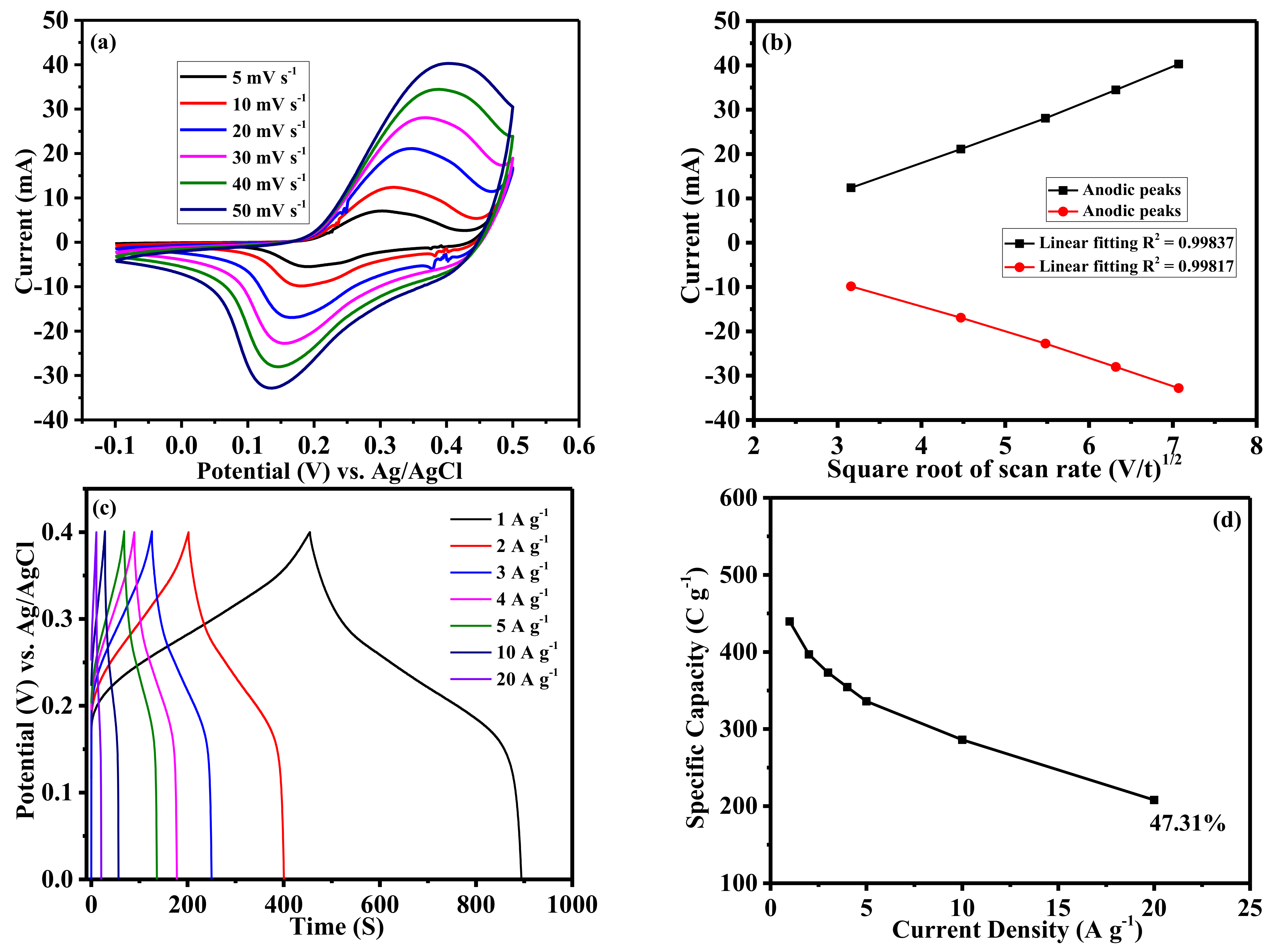
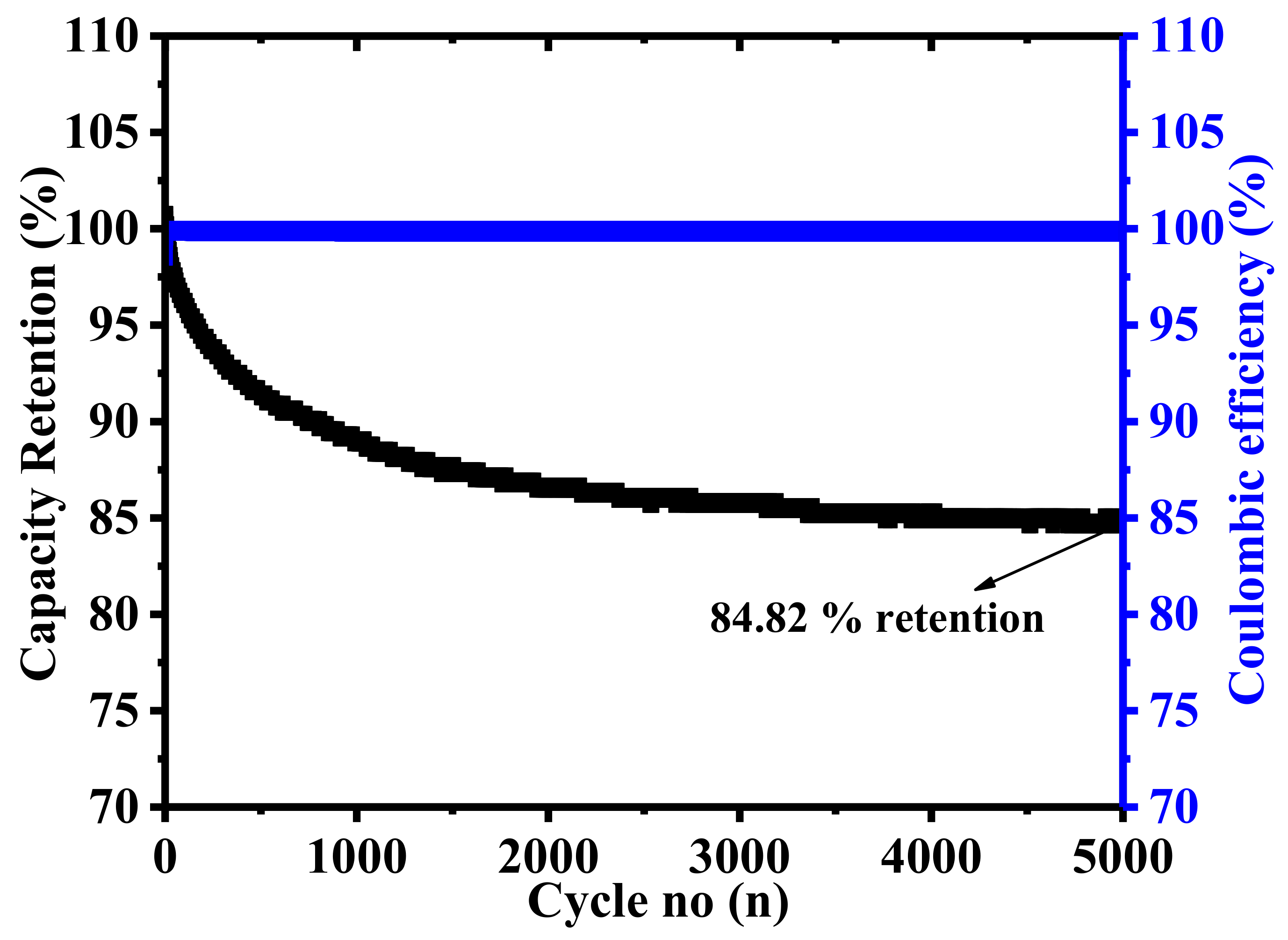
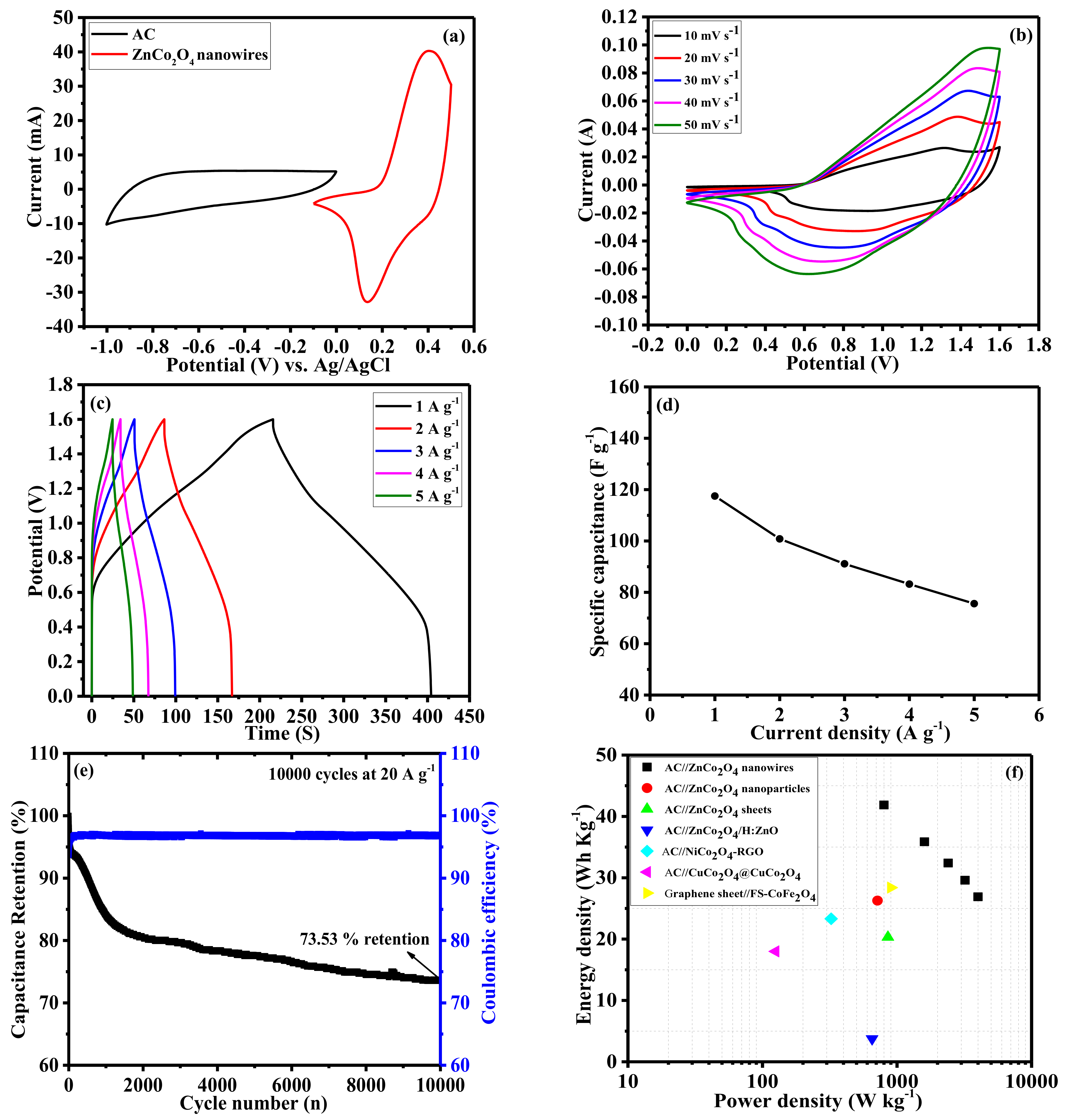
2. Results and Discussion
3. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.; Zheng, W. A review for aqueous electrochemical supercapacitors. Front. Energy Res. 2015, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Gur, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large–scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, K.C.; Lin, L.Y. A review of electrode materials based on core–shell nanostructures for electrochemical supercapacitors. J. Mater. Chem. A 2019, 7, 3516–3530. [Google Scholar] [CrossRef]
- Guan, L.; Yu, L.; Chen, G.Z. Capacitive and non–capacitive faradaic charge storage. Electrochim. Acta. 2016, 206, 464–478. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Dunn, B.; Gogotsi, Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 2016, 7, 12647. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition–metal oxides: Design, synthesis, and energy–related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Cheng, J.P.; Zhang, J.; Liu, F. Recent development of metal hydroxides as electrode material of electrochemical capacitors. RSC Adv. 2014, 4, 38893–38917. [Google Scholar] [CrossRef]
- Yu, X.Y.; Lou, X.W. Mixed metal sulfides for electrochemical energy storage and conversion. Adv. Energy Mater. 2018, 8, 1701592. [Google Scholar] [CrossRef]
- Lu, T.; Dong, S.; Zhang, C.; Zhang, L.; Cui, G. Fabrication of transition metal selenides and their applications in energy storage. Coord. Chem. Rev. 2017, 332, 75–99. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Durai, G.; Karuppasamy, K.; Arunachalam, P.; Elakkiya, V.; Kuppusami, P.; Maiyalagan, T.; Kim, H.S. Recent advances in 2–D nanostructured metal nitrides, carbides, and phosphides electrodes for electrochemical supercapacitors—A brief review. J. Ind. Eng. Chem. 2018, 67, 12–27. [Google Scholar] [CrossRef]
- Liu, C.F.; Liu, Y.C.; Yi, T.Y.; Hu, C.C. Carbon materials for high–voltage supercapacitors. Carbon 2019, 145, 529–548. [Google Scholar] [CrossRef]
- Suriyakumar, S.; Bhardwaj, P.; Grace, A.N.; Stephan, A.M. Role of polymers in enhancing the performance of electrochemical supercapacitors: A review. Batter. Supercaps 2021, 4, 571–584. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Su, H.; Huang, W.; Dong, X. Binary metal oxide: Advanced energy storage materials in supercapacitors. J. Mater. Chem. A 2015, 3, 43–59. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.; Wang, R.; Shen, G. Ternary oxide nanostructured materials for supercapacitors: A review. J. Mater. Chem. A 2015, 3, 10158–10173. [Google Scholar] [CrossRef]
- Cao, D.; Zheng, L.; Li, Q.; Zhang, J.; Dong, Y.; Yue, J.; Wang, X.; Bai, Y.; Tan, G.; Wu, C. Crystal phase–controlled modulation of binary transition metal oxides for highly reversible Li−O2 batteries. Nano Lett. 2021, 21, 5225–5232. [Google Scholar] [CrossRef]
- Zheng, J.H.; Zhang, R.M.; Yu, P.F.; Wang, X.G. Binary transition metal oxides (BTMO) (Co–Zn, Co–Cu) synthesis and high supercapacitor performance. J. Alloys Compd. 2019, 772, 359–365. [Google Scholar] [CrossRef]
- Adamson, W.; Bo, X.; Li, Y.; Suryanto, B.H.R.; Chen, X.; Zhao, C. Co–Fe binary metal oxide electrocatalyst with synergistic interface structures for efficient overall water splitting. Catal. Today 2020, 351, 44–49. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Mukherjee, A.; Basu, S. RGO–MoS2 supported NiCo2O4 catalyst toward solar water splitting and dye degradation. ACS Sustain. Chem. Eng. 2018, 6, 5238–5247. [Google Scholar] [CrossRef]
- Nithya, J.S.M.; Do, J.Y.; Kang, M. Fabrication of flower–like copper cobaltite/graphitic–carbon nitride (CuCo2O4/g–C3N4) composite with superior photocatalytic activity. J. Ind. Eng. Chem. 2018, 57, 405–415. [Google Scholar]
- Wang, Q.; Du, J.; Zhu, Y.; Yang, J.; Chen, J.; Wang, C.; Li, L.; Jiao, L. Facile fabrication and supercapacitive properties of mesoporous zinc cobaltite microspheres. J. Power Sources 2015, 284, 138–145. [Google Scholar] [CrossRef]
- Mary, A.J.C.; Bose, A.C. Surfactant assisted ZnCo2O4 nanomaterial for supercapacitor application. Appl. Surf. Sci. 2018, 449, 105–112. [Google Scholar] [CrossRef]
- Chena, H.; Wang, J.; Han, X.; Liao, F.; Zhang, Y.; Han, X.; Xu, C. Simple growth of mesoporous zinc cobaltite urchin–like microstructures towards high–performance electrochemical capacitors. Ceram. Int. 2019, 45, 4059–4066. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Lian, J.; Xu, Y.; Bao, J.; Qiu, J.; Xu, L.; Xu, H.; Hua, M.; Li, H. Morphology controlled preparation of ZnCo2O4 nanostructures for asymmetric supercapacitor with ultrahigh energy density. Energy 2017, 123, 296–304. [Google Scholar] [CrossRef]
- Chen, H.; Du, X.; Sun, J.; Mao, H.; Wu, R.; Xu, C. Simple preparation of ZnCo2O4 porous quasi–cubes for high performance asymmetric supercapacitors. Appl. Surf. Sci. 2020, 515, 146008. [Google Scholar] [CrossRef]
- Bhagwan, J.; Hussain, S.K.; Yu, J.S. Aqueous asymmetric supercapacitors based on ZnCo2O4 nanoparticles via facile combustion method. J. Alloys Compd. 2020, 815, 152456. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Lee, S.H.; Ryu, K.S. Porous thin layered nanosheets assembled ZnCo2O4 grown on Ni–foam as an efficient electrode material for hybrid supercapacitor applications. Int. J. Hydrog. Energy. 2017, 42, 3122–3129. [Google Scholar] [CrossRef]
- Wu, H.; Lou, Z.; Yang, H.; Shen, G. A flexible spiral–type supercapacitor based on ZnCo2O4 nanorod electrodes. Nanoscale 2015, 7, 1921–1926. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Y.; Wang, Z. Facile synthesis of a ZnCo2O4 electrocatalyst with three–dimensional architecture for methanol oxidation. J. Alloys Compd. 2019, 810, 151879. [Google Scholar] [CrossRef]
- Rajesh, J.A.; Min, B.K.; Kim, J.H.; Kim, H.; Ahn, K.S. Cubic spinel AB2O4 type porous ZnCo2O4 microspheres: Facile Hydrothermal synthesis and their electrochemical performances in pseudocapacitor. J. Electrochem. Soc. 2016, 163, A2418–A2427. [Google Scholar] [CrossRef]
- Rajesh, J.A.; Min, B.K.; Kim, J.H.; Kang, S.H.; Kim, H.; Ahn, K.S. Facile hydrothermal synthesis and electrochemical supercapacitor performance of hierarchical coral–like ZnCo2O4 nanowires. J. Electroanal. Chem. 2017, 785, 48–57. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Liu, D.; Zhen, J.; Li, J.; Wang, J.; Zhang, H. High–performance ZnCo2O4@CeO2 core@shell microspheres for catalytic CO oxidation. ACS Appl. Mater. Interfaces 2014, 6, 22216–22223. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, J.; Liu, Q.; Song, D.; Zhang, H.; Zhang, H.; Wang, J. Synthesis, characterization and enhanced gas sensing performance of porous ZnCo2O4 nano/microspheres. Nanoscale 2015, 7, 19714–19721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhu, J.; Chen, Y.; Mei, L.; Duan, X.; Zhang, G.; Chen, L.; Wang, T.; Lu, B. Simple method for the preparation of highly porous ZnCo2O4 nanotubes with enhanced electrochemical property for supercapacitor. Electrochim. Acta 2014, 123, 450–455. [Google Scholar] [CrossRef]
- Bai, J.; Wang, K.; Feng, J.; Xiong, S. ZnO/CoO and ZnCo2O4 hierarchical bipyramid nanoframes: Morphology control, formation mechanism, and their lithium storage properties. ACS Appl. Mater. Interfaces 2015, 7, 22848–22857. [Google Scholar] [CrossRef]
- Moon, I.K.; Yoon, S.; Oh, J. Three-dimensional hierarchically mesoporous ZnCo2O4 nanowires grown on graphene/sponge foam for high-performance, flexible, all-solid-state supercapacitors. Chem. Eur. J. 2017, 23, 597–604. [Google Scholar] [CrossRef]
- Cheng, J.; Lu, Y.; Qiu, K.; Yan, H.; Hou, X.; Xu, J.; Han, L.; Liu, X.; Kim, J.K.; Luo, Y. Mesoporous ZnCo2O4 nanoflakes grown on nickel foam as electrodes for high performance supercapacitors. Phys. Chem. Chem. Phys. 2015, 17, 17016–17022. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, F.; Gao, M.; Yang, W.; Yu, Y. L-Cysteine capped Mo2C/Zn0.67Cd0.33S heterojunction with intimate covalent bonds enables efficient and stable H2-Releasing photocatalysis. Chem. Eng. J. 2022, 428, 132628. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, F.; Lan, X.; Liu, Y.; Yang, W.; Zhang, J.; Yu, Y. Building P-doped MoS2/g-C3N4 layered heterojunction with a dual-internal electric field for efficient photocatalytic sterilization. Chem. Eng. J. 2022, 429, 132588. [Google Scholar] [CrossRef]
- Rajesh, J.A.; Park, J.-H.; Quy, V.H.V.; Kwon, J.M.; Chae, J.; Kang, S.-H.; Kim, H.; Ahn, K.-S. Rambutan–like cobalt nickel sulfide (CoNi2S4) hierarchitecture for high–performance symmetric aqueous supercapacitors. J. Ind. Eng. Chem. 2018, 63, 73–83. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Kang, J.S.; Jo, I.-K.; Sung, Y.-E.; Ahn, K.-S. Double–layer cobalt selenide/nickel selenide with web–like nanostructures as a high–performance electrode material for supercapacitors. J. Electroanal. Chem. 2021, 895, 115479. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, L.; Jiao, Y.T.; Li, Z.; Gao, Y. Controllable fabrication of ZnCo2O4 ultra–thin curved sheets on Ni foam for high–performance asymmetric supercapacitors. Electrochim. Acta 2019, 299, 388–394. [Google Scholar] [CrossRef]
- Boruah, B.D.; Maji, A.; Misra, A. Synergistic effect in the heterostructure of ZnCo2O4 and hydrogenated zinc oxide nanorods for high capacitive response. Nanoscale 2017, 9, 9411–9420. [Google Scholar] [CrossRef]
- Kuang, M.; Wen, Z.Q.; Guo, X.L.; Zhang, S.M.; Zhang, Y.X. Engineering firecracker–like beta–manganese dioxides@spinel nickel cobaltates nanostructures for high–performance supercapacitors. J. Power Sources. 2014, 270, 426–433. [Google Scholar] [CrossRef]
- Lu, X.-F.; Wu, D.-J.; Li, R.-Z.; Li, Q.; Ye, S.-H.; Tong, Y.-X.; Li, G.-R. Hierarchical NiCo2O4 nanosheets@hollow microrod arrays for high–performance asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 4706–4713. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.S.; Lu, X.; Lee, P.S. Dodecyl sulfate–induced fast faradic process in nickel cobalt oxide–reduced graphite oxide composite material and its application for asymmetric supercapacitor device. J. Mater. Chem. 2012, 22, 23114–23119. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhang, L.; Qi, T.; Xia, D.; Wan, H. Facilely synthesized porous NiCo2O4 flowerlike nanostructure for high–rate supercapacitors. J. Power Sources 2014, 248, 28–36. [Google Scholar] [CrossRef]
- Tang, C.; Tang, Z.; Gong, H. Hierarchically porous Ni–Co oxide for high reversibility asymmetric full–cell supercapacitors. J. Electroanal. Chem. 2012, 159, A651–A656. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zheng, Y.; Zhang, Y.; Hu, X.; Xu, T. Construction of CuCo2O4@CuCo2O4 hierarchical nanowire arrays grown on Ni foam for high–performance supercapacitors. RSC Adv. 2017, 7, 3983–3991. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Seong, K.-D.; Kang, J.; Hwang, M.; Kim, J.M.; Jin, X.; Piao, P. Fluoride ion–mediated morphology control of fluorine–doped CoFe2O4/graphene sheet composites for hybrid supercapacitors with enhanced performance. Electrochim. Acta 2018, 279, 241–249. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajesh, J.A.; Ahn, K.-S. Facile Hydrothermal Synthesis and Supercapacitor Performance of Mesoporous Necklace-Type ZnCo2O4 Nanowires. Catalysts 2021, 11, 1516. https://doi.org/10.3390/catal11121516
Rajesh JA, Ahn K-S. Facile Hydrothermal Synthesis and Supercapacitor Performance of Mesoporous Necklace-Type ZnCo2O4 Nanowires. Catalysts. 2021; 11(12):1516. https://doi.org/10.3390/catal11121516
Chicago/Turabian StyleRajesh, John Anthuvan, and Kwang-Soon Ahn. 2021. "Facile Hydrothermal Synthesis and Supercapacitor Performance of Mesoporous Necklace-Type ZnCo2O4 Nanowires" Catalysts 11, no. 12: 1516. https://doi.org/10.3390/catal11121516
APA StyleRajesh, J. A., & Ahn, K.-S. (2021). Facile Hydrothermal Synthesis and Supercapacitor Performance of Mesoporous Necklace-Type ZnCo2O4 Nanowires. Catalysts, 11(12), 1516. https://doi.org/10.3390/catal11121516





