Catalytic Pyrolysis of Lignin Model Compound (Ferulic Acid) over Alumina: Surface Complexes, Kinetics, and Mechanisms
Abstract
1. Introduction
2. Results and Discussion
2.1. FT-IR Spectroscopy
2.2. In-Situ FTIR Spectroscopic Study of FA Thermal Transformations over an Alumina Surface
2.3. Quantum Chemical Calculation
2.4. Thermogravimetric Analysis
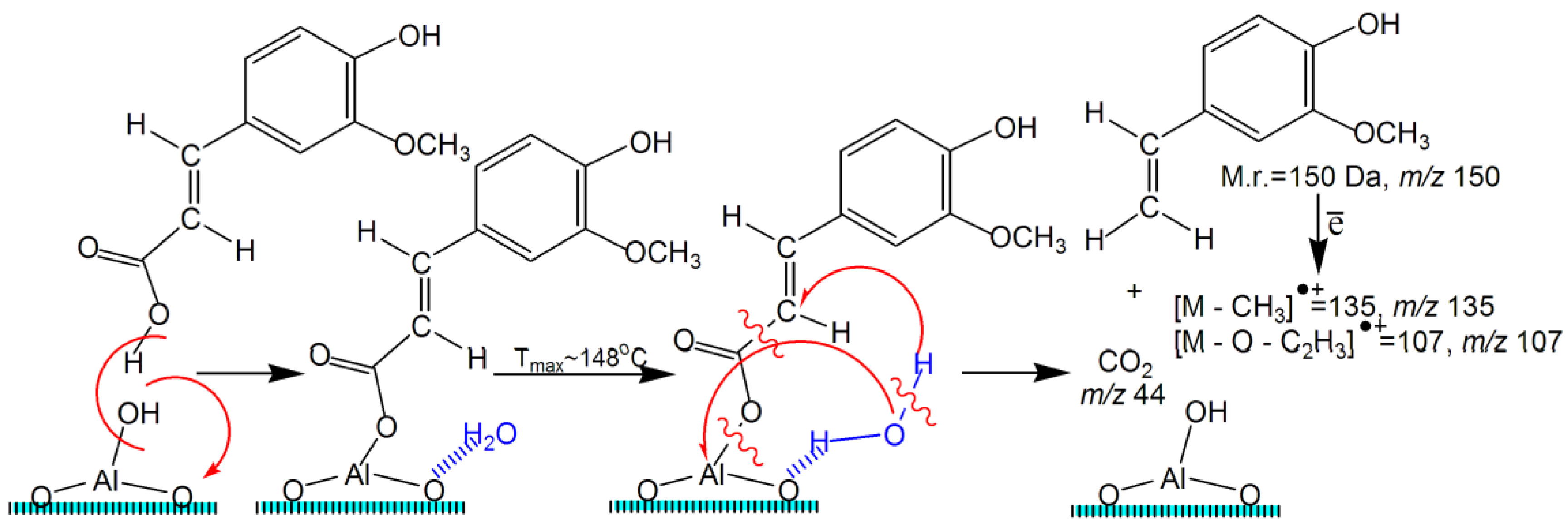
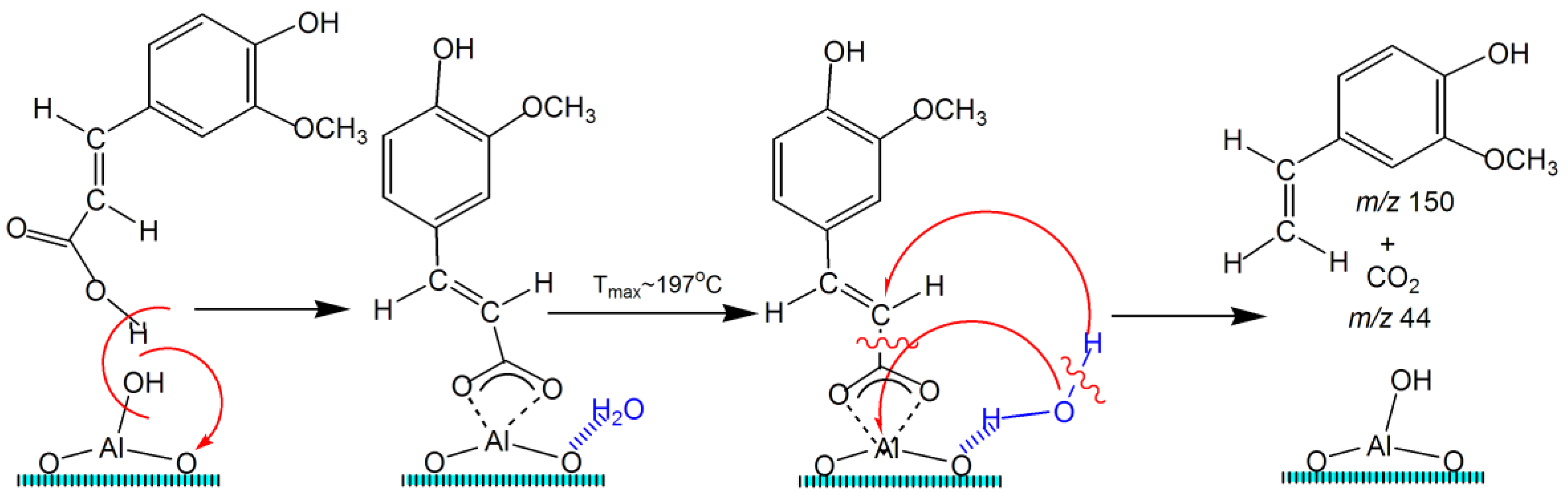
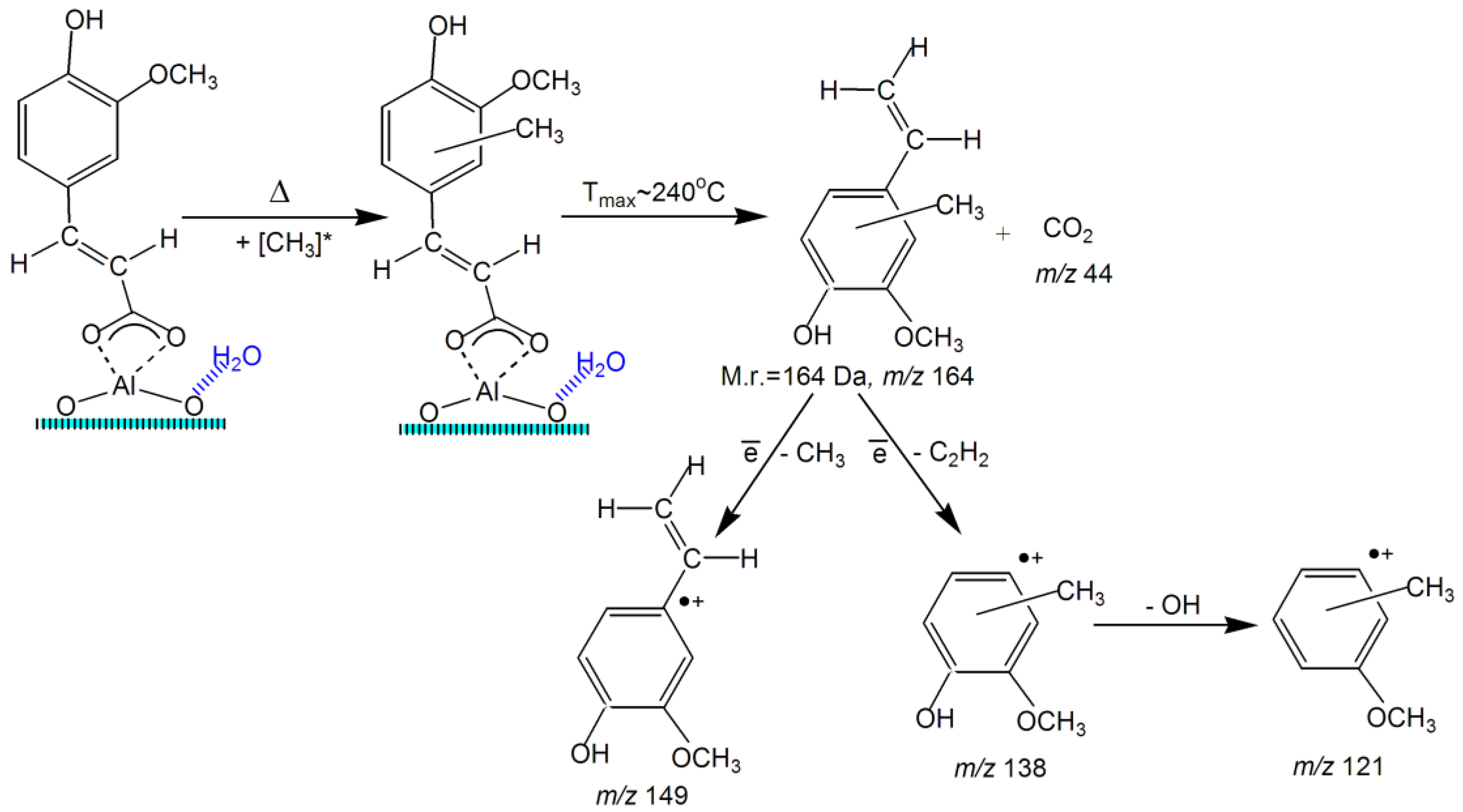
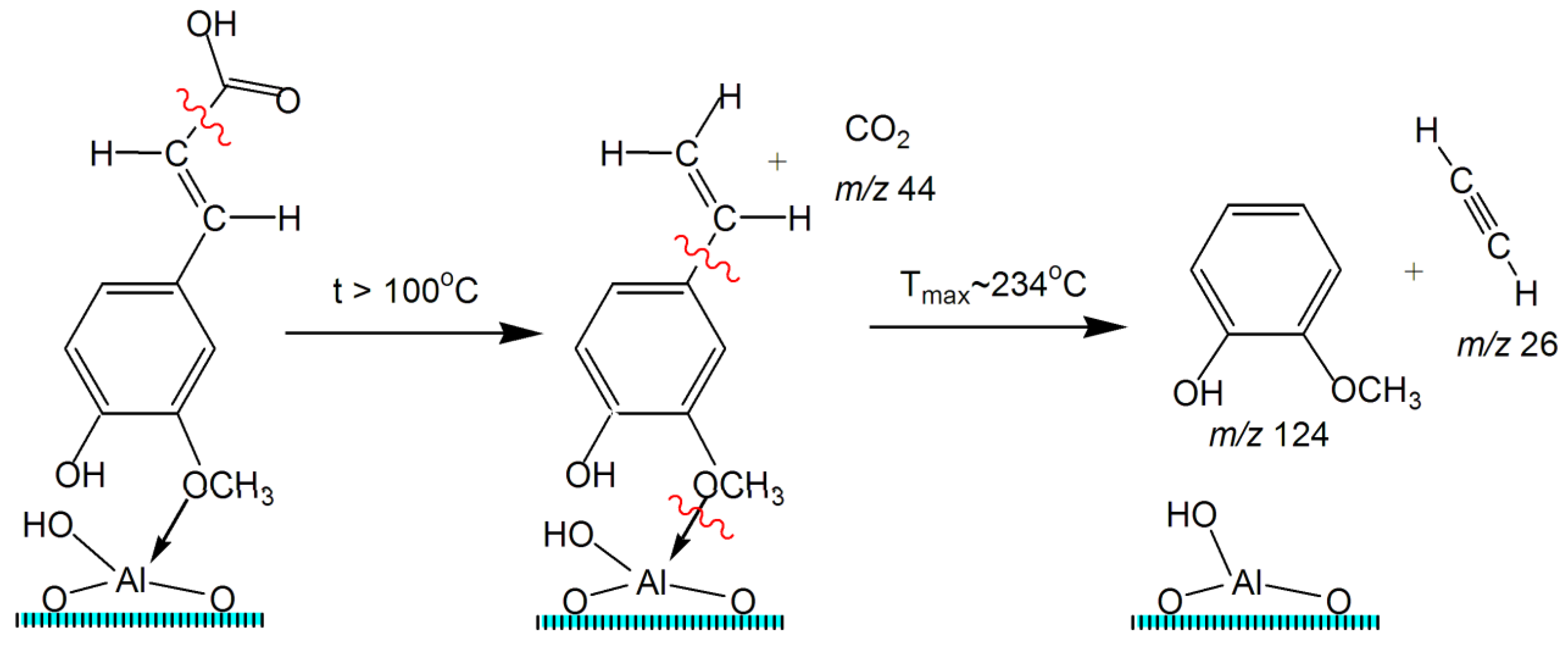
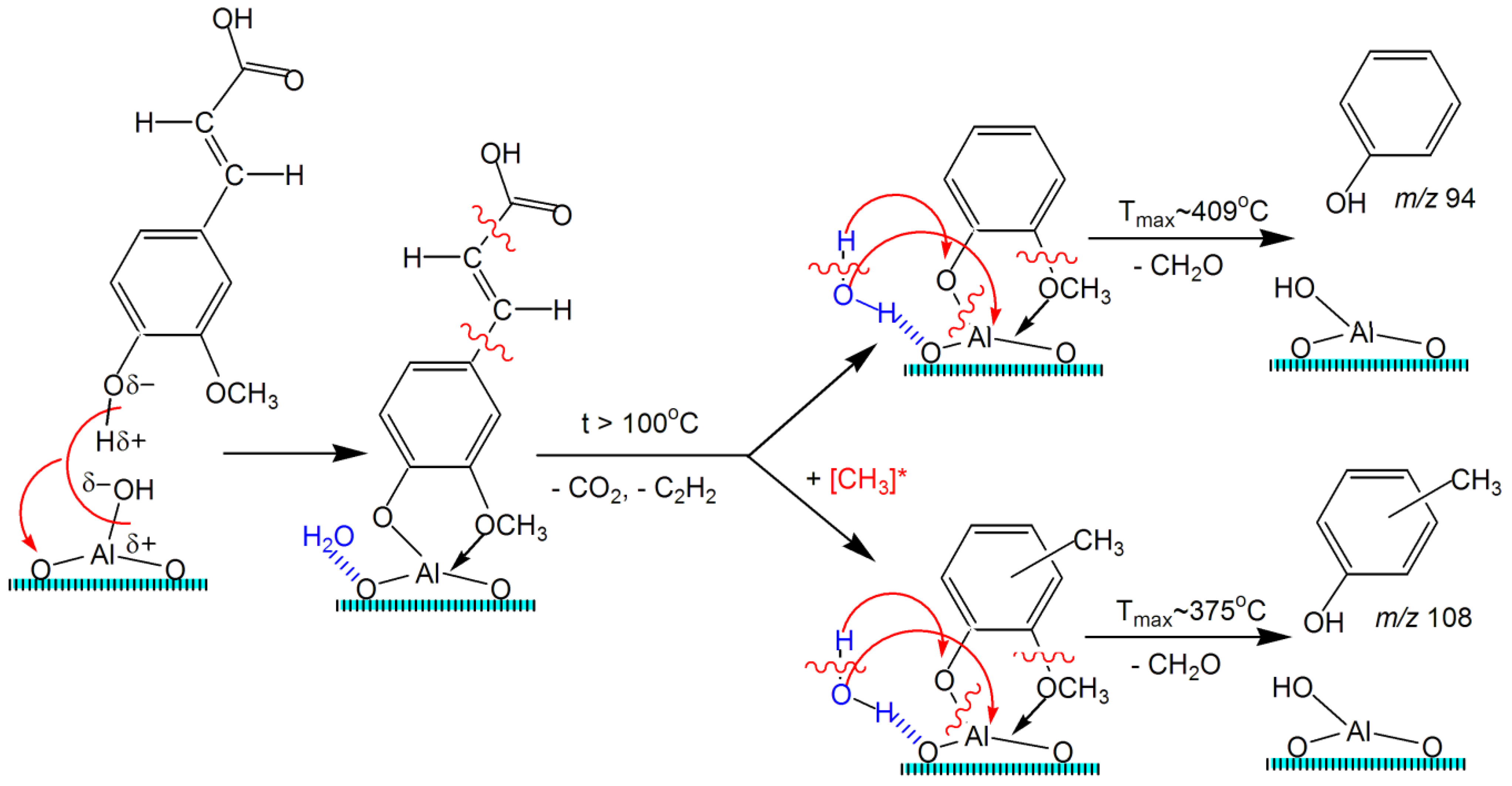
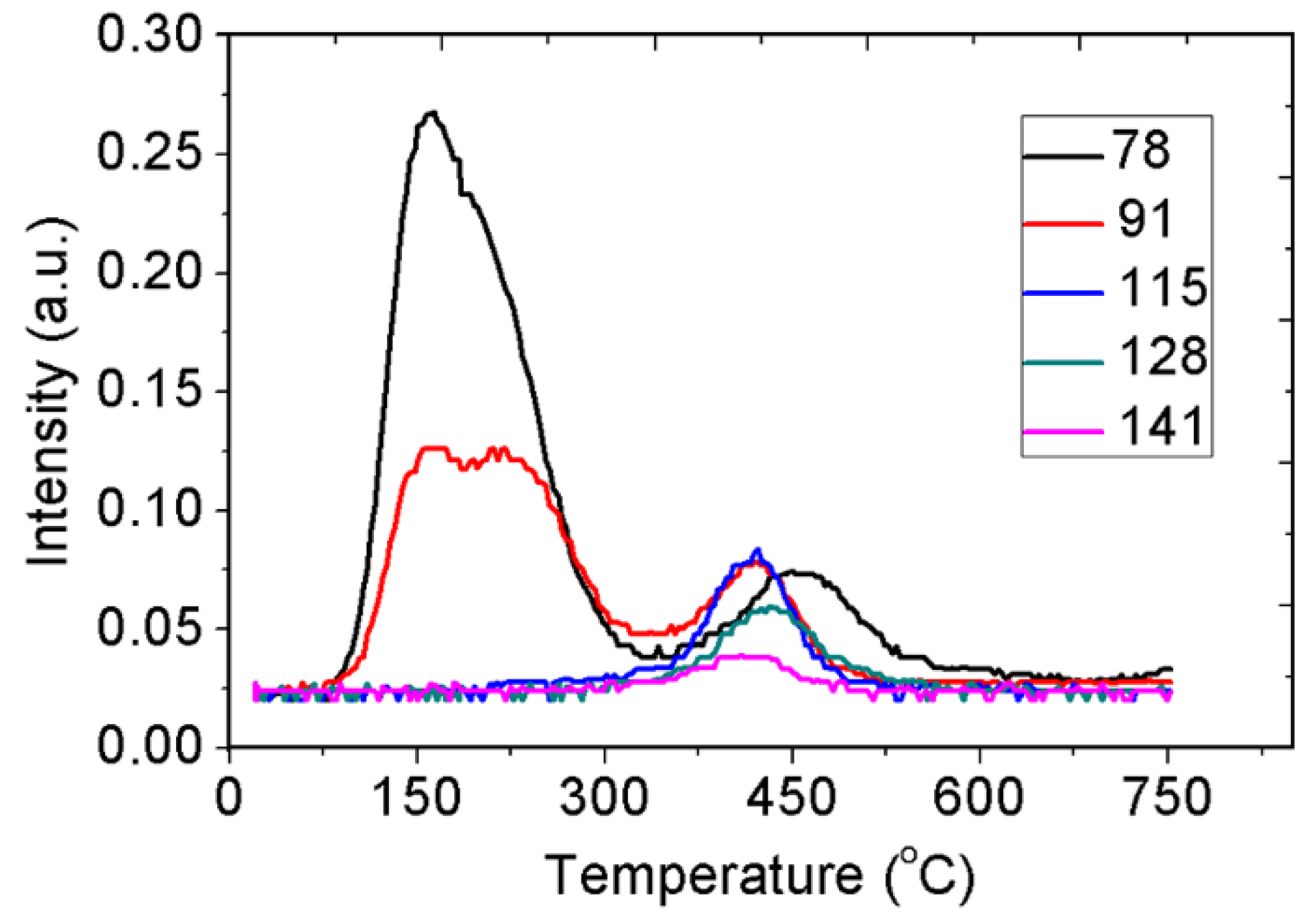
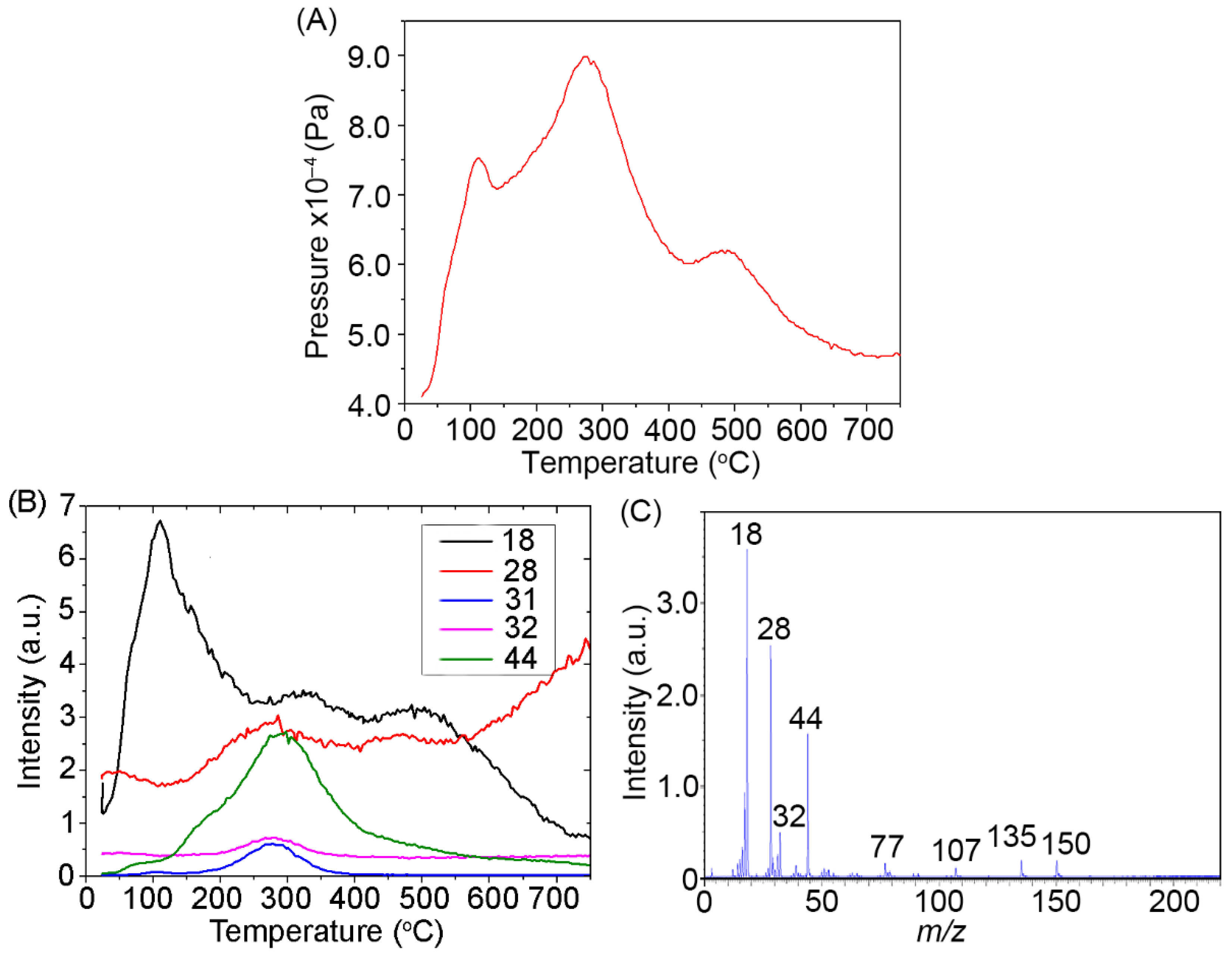
2.5. TPD MS Study of FA Catalytic Pyrolysis
3. Materials and Methods
3.1. Materials
3.2. Loading of FA on the Alumina Surface
3.3. FT-IR Spectroscopic Studies
3.4. TPD MS Study
3.5. Thermogravimetric Analysis
3.6. Density Functional Theory (DFT) Calculations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Kataki, R.; Chutia, R.S.; Mishra, M.; Bordoloi, N.; Saikia, R.; Bhaskar, T. Feedstock suitability for thermochemical processes. In Recent Advancesin Thermo-Chemical Conversion of Biomass, 1st ed.; Pandey, A., Bhaskar, T., Stöcker, M., Sukumaran, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 31–74. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Zhang, H.; Chu, C.; Zheng, K.; Ju, M.; Liu, L. Liquefaction of biomass and upgrading of bio-oil: A review. Molecules 2019, 24, 2250. [Google Scholar] [CrossRef]
- Lin, Y.C.; Huber, G.W. The critical role of heterogeneous catalysis in lignocellulosic biomass conversion. Energy Environ. Sci. 2009, 2, 68–80. [Google Scholar] [CrossRef]
- Albrecht, K.O.; Olarte, M.V.; Wang, H. Upgrading Fast Pyrolysis Liquids. In Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power, 2nd ed.; Brown, R.C., Ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green chemistry, catalysis and valorization of waste biomass. J. Mol. Catal. A Chem. 2016, 422, 3–12. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Effect of low-cost catalysts on yield and properties of fuel from waste biomass for hydrocarbon-rich oil production. Mater. Sci. Energy Technol. 2020, 3, 526–535. [Google Scholar]
- De Wit, M.; Faaij, A. European biomass resource potential and costs. Biomass Bioenergy 2010, 34, 188–202. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Kozinski, J.A.; Dalai, A.K. Hydrothermal and Thermochemical Synthesis of Bio-Oil from Lignocellulosic Biomass: Composition, Engineering and Catalytic Upgrading. In Industrial Biotechnology, 1st ed.; Thangadurai, D., Sangeetha, J., Eds.; Apple Academic Press: New York, NY, USA, 2017; pp. 345–390. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Kozinski, J.A.; Dalai, A.K. Physico-Chemical Properties of Bio-Oils from Pyrolysis of Lignocellulosic Biomass with High and Slow Heating Rate. Energy Environ. Res. 2014, 21, 21–32. [Google Scholar] [CrossRef]
- Novakovskiy, D.J.; Jones, J.M. Uncatalysed and potassium-catalysed pyrolysis of the cell-wall constituents of biomass and their model compounds. J. Anal. Appl. Pyrolysis 2008, 83, 12–25. [Google Scholar] [CrossRef]
- Ren, X.Y.; Feng, X.B.; Cao, J.P.; Tang, W.; Wang, Z.H.; Yang, Z.; Zhao, J.P.; Zhang, L.Y.; Wang, Y.J.; Zhao, X.Y. Catalytic conversion of coal and biomass volatiles: A review. Energy Fuels 2020, 34, 10307–10363. [Google Scholar] [CrossRef]
- Bardalai, M.; Mahanta, D.K. Characterisation of pyrolysis oil derived from teak tree saw dust and rice husk. J. Eng. Sci. Technol. 2018, 13, 242–253. [Google Scholar]
- Andrade, L.A.; Batista, F.R.X.; Lira, T.S.; Barrozo, M.A.S.; Vieira, L.G.M. Characterization and product formation during the catalytic and non-catalytic pyrolysis of the green microalgae Chlamydomonas reinhardtii. Renew. Energy 2018, 119, 731–740. [Google Scholar] [CrossRef]
- Rahman, M.M.; Liu, R.; Cai, J. Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil—A review. Fuel Process. Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Wang, K.; Johnston, P.A.; Brown, R.C. Comparison of in-situ and ex-situ catalytic pyrolysis in a micro-reactor system. Biores. Technol. 2014, 173, 124–131. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, B.; Qiu, Q.; Wang, B.; Yang, H.; Dinga, Y.; Wang, C. Pyrolysis of microalgae: A critical review. Fuel Process. Technol. 2019, 186, 53–72. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Rosillo-Calleet, F.; De Groot, P.; Hemstock, S.L.; Woods, J. Non-woody biomass and secondary fuels. In The Biomass Assesment Handbook: Bioenergy for a Sustainable Environment; Rosillo-Calle, F., de Groot, P., Hemstock, S.L., Woods, J., Eds.; Earthscan: Sterling, VA, USA, 2007. [Google Scholar]
- Hodasova, L.; Jablonský, M.; Škulcová, A.; Ház, A. Lignin, potential products and their market value. Wood Res. 2015, 60, 973–986. [Google Scholar]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Deepa, A.K.; Dhepe, P.L. Solid acid catalyzed depolymerization of lignin into value added aromatic monomers. RSC Adv. 2014, 4, 12625–12629. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, J.; Wang, X.; Wang, J.; Fan, W. Alcoholysis: A promising technology for conversion of lignocellulose and platform chemicals. ChemSusChem 2017, 10, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Nagel, C.W. Occurrence and content of hydroxycinnamic and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347. [Google Scholar] [CrossRef] [PubMed]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Karp, E.M.; Nimlos, C.T.; Deutch, S.; Salvachúa, D.; Cywar, R.M.; Beckham, G.T. Quantification of acidic compounds in complex biomass-derived streams. Green Chem. 2016, 18, 4750–4760. [Google Scholar] [CrossRef]
- Fiddler, W.; Parker, W.E.; Wasserman, A.E.; Doerr, R.C. Thermal decomposition of ferulic acid. J. Agric. Food Chem. 1967, 15, 757–761. [Google Scholar] [CrossRef]
- Steinke, R.D.; Paulson, M.C. Phenols from grain, production of steam-volatile phenols during cooking and alcoholic fermentation of grain. J. Agric. Food Chem. 1964, 12, 381–387. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Terao, J.; Karasawa, H.; Arai, H.; Nagao, A.; Suzuki, T.; Takama, K. Peroxyl radical scavenging activity of caffeic acid and its related phenolic compounds in solution. Biosci. Biotechnol. Biochem. 1973, 57, 1204–1205. [Google Scholar] [CrossRef]
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic Acid Stabilizes a Solution of Vitamins C and E and Doubles its Photoprotection of Skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef]
- Zang, L.W.; Al-Suwayeh, S.A.; Hsiesh, P.W.; Fang, J.Y. A comparison of skin delivery of ferulic acid and its derivatives: Evaluation of their efficacy and safety. Int. J. Pharm. 2010, 399, 44–51. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.; Menon, V. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K. Ferulic acid: Pharmaceutical function, preparation and applications in foos. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Aragno, M.; Parola, S.; Tamagno, E.; Brignardello, E.; Manti, R.; Danni, O.; Boccuzzi, G. Oxidative derangement in rat synaptosomes induced by hyperglycaemia: Restorative effect of dehydroepiandrosterone treatment. Biochem. Pharmacol. 2000, 60, 389–395. [Google Scholar] [CrossRef]
- Kulik, T.V.; Barvinchenko, V.N.; Palyanytsya, B.B.; Lipkovska, N.A.; Dudik, O.O. Thermal transformations of biologically active derivatives of cinnamic acid by TPD MS investigation. J. Anal. Appl. Pyrolysis 2011, 90, 219–223. [Google Scholar] [CrossRef]
- Kulik, T.V.; Lipkovska, N.A.; Barvinchenko, V.N.; Palyanytsya, B.B.; Kazakova, O.A.; Dovbiy, O.A.; Pogorelyi, V.K. Interactions between bioactive ferulic acid and fumed silica by UV–visspectroscopy, FT–IR, TPDMS investigation and quantum chemical methods. J. Colloid Interface Sci. 2009, 339, 60–68. [Google Scholar] [CrossRef]
- Kulik, T.V.; Barvinchenko, V.N.; Palyanitsa, B.B.; Smirnova, O.V.; Pogorelyi, V.K.; Chuiko, A.A. A desorption mass spectrometry study of the interaction of cinnamic acid with a silica surface. Russ. J. Phys. Chem. 2007, 8, 83–90. [Google Scholar] [CrossRef]
- Kulik, T.V.; Lipkovska, N.O.; Barvinchenko, V.M.; Palyanytsya, B.B.; Kazakova, O.A.; Dudik, O.O.; Menyhárd, A.; László, K. Thermal transformation of bioactive caffeic acid on fumed silica seen by UV-spectroscopy, thermogtavimetric analysis, temperature programmed desorption mass spectrometry and quantum chemical methods. J. Colloid Interface Sci. 2016, 470, 132–141. [Google Scholar] [CrossRef]
- Nastasiienko, N.; Palianytsia, B.; Kartel, M.; Larsson, M.; Kulik, T. Thermal Transformation of Caffeic Acid on the Nanoceria Surface Studied by Temperature Programmed Desorption Mass-Spectrometry, Thermogravimetric Analysis and FT-IR Spectroscopy. Colloid Interfaces 2019, 3, 34. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Sudheesh, S. Rapid conversion of ferulic acid to 4-vinyl guaiacol and vanillin metabolites by Debaryomyces hansenii. J. Mol. Catal. B Enzym. 2007, 44, 48–52. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Chemistry natural sources, dietayintake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Patto, M.C.V.; do Rosário Bronze, M. Relevance structure and and analysis of ferulic acid in maize cell walls. Food Chem. 2018, 246, 360–378. [Google Scholar] [CrossRef]
- Zhang, P.H.; Yu, X.Y.; Weng, L.X.; Sun, L.L.; Mao, Z.C.; Zhang, Y.L. Degradation of Ferulic Acid by the Endophytic Fungus Colletotrichum gloeosporioides TMTM-13 Associated with Ostrya rehderiana Chun. ACS Omega 2019, 4, 21000–21004. [Google Scholar] [CrossRef]
- Pazo-Cepeda, V.; Benito-Román, Ó.; Navarrete, A.; Alonso, E. Valorization of Wheat Bran: Ferulic Acid Recovery Using Pressurized Aqueous Ethanol Solutions. Waste Biomass Valorization 2020, 11, 4701–4710. [Google Scholar] [CrossRef]
- Dupoiron, S.; Lameloise, M.L.; Bedu, M.; Lewandowski, R.; Fargues, C.; Allais, F.; Rémond, C. Recovering ferulic acid from wheat bran enzymatic hydrolysate by a novel and non-thermal process associating weak anion-exchange and electrodialysis. Sep. Purif. Technol. 2018, 200, 75–83. [Google Scholar] [CrossRef]
- Gopalan, N.; Rodríguez-Duran, L.V.; Saucedo-Castaneda, G.; Nampoothiri, K.M. Review on technological and scientific aspects of feruloyl esterases; a versatile enzyme for biorefining of biomass. Bioresour. Technol. 2015, 193, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.; Flores, J.; Cruz, A.S.; Leyva-Gómez, G.; Krötzsch, E. Controlled release of ferulic acid from a hybrid hydrotalcite and its application as an antioxidant for human fibroblasts. Microporous Mesoporous Mater. 2013, 181, 1–7. [Google Scholar] [CrossRef]
- Yefremova, S.; Zharmenov, A.; Sukharnikov, Y.; Bunchuk, L.; Kablanbekov, A.; Anarbekov, K.; Kulik, T.; Nikolaichuk, A.; Palianytsia, B. Rice Husk Hydrolytic Lignin Transformation in Carbonization Process. Molecules 2019, 24, 3075. [Google Scholar] [CrossRef] [PubMed]
- Palianytsia, B.B.; Kulik, T.V.; Dudik, O.O.; Toncha, O.L.; Cherniavska, T.V. Study of the thermal decomposition of some components of biomass by desorption mass spectrometry. In Proceedings of the International Congress on Energy Efficiency and Energy Related Materials (ENEFM 2013), Antalya, Turkey, 9–12 October 2013; Volume 155, pp. 19–25. [Google Scholar] [CrossRef]
- Kabakcı, S.B.; Hacıbektaşoğlu, Ş. Catalytic Pyrolysis of Biomass; InTech.: London, UK, 2017. [Google Scholar]
- Mandal, S.; Bandyopadhyay, R.; Das, A.K. Thermo-catalytic process for conversion of lignocellulosic biomass to fuels and chemicals: A review. Int. J. Petrochem. Sci. Eng. 2018, 3, 78–89. [Google Scholar] [CrossRef][Green Version]
- Sharanda, L.F.; Shimansky, A.P.; Kulik, T.V.; Chuiko, A.A. Study of acid-base surface properties of pyrogenic γ-aluminium oxide. Colloids Surf. A Physicochem. Eng. Asp. 1995, 105, 167–172. [Google Scholar] [CrossRef]
- do Carmo, J.V.C.; Oliveira, A.C.; Araújo, J.C.; Campos, A.; Duarte, G.C.S. Synthesis of highly porous alumina-based oxides with tailored catalytic properties in the esterification of glycerol. J. Mater. Res. 2018, 33, 3625–3633. [Google Scholar] [CrossRef]
- Van Den Brand, J.; Blajiev, O.; Beentjes, P.C.J.; Terryn, H.; De Wit, J.H.W. Interaction of anhydride and carboxylic acid compounds with aluminum oxide surfaces studied using infrared reflection absorption spectroscopy. Langmuir 2004, 20, 6308–6317. [Google Scholar] [CrossRef] [PubMed]
- Kulyk, K.; Palianytsia, B.; Alexander, J.D.; Azizova, L.; Borysenko, M.; Kartel, M.; Larsson, M.; Kulik, T. Kinetics of valeric acid ketonization and ketenization in catalytic pyrolysis on nanosized SiO2, γ-Al2O3, CeO2/SiO2, Al2O3/SiO2 and TiO2/SiO2. Chem. Phys. Chem. 2017, 18, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Alda-Onggar, M.; Maäki-Arvela, P.; Eraänen, K.; Aho, A.; Hemming, J.; Paturi, P.; Peurla, M.; Lindblad, M.; Simakova, I.L.; Murzin, D.Y. Hydrodeoxygenation of isoeugenol over alumina-supported Ir, Pt, and Re catalysts. ACS Sustain. Chem. Eng. 2018, 6, 16205–16218. [Google Scholar] [CrossRef] [PubMed]
- Mysore Prabhakara, H.; Bramer, E.A.; Brem, G. Biomass Fast Pyrolysis Vapor Upgrading over γ-Alumina, Hydrotalcite, Dolomite and Effect of Na2CO3 Loading: A Pyro Probe GCMS Study. Energies 2021, 14, 5397. [Google Scholar] [CrossRef]
- Kulyk, K.; Borysenko, M.; Kulik, T.; Mikhalovska, L.; Alexander, J.D.; Palianytsia, B. Chemisorption and thermally induced transformations of polydimethylsiloxane on the surface of nanoscale silica and ceria/silica. Polym. Degrad. Stab. 2015, 120, 203–211. [Google Scholar] [CrossRef]
- Kulyk, K.; Zettergren, H.; Gatchell, M.; Alexander, J.D.; Larsson, M.; Borysenko, M.; Palianytsia, B.; Kulik, T. Dimethylsilanone Generation from Pyrolysis of Polysiloxanes Filled with Nanosized Silica and Ceria/Silica. ChemPlusChem 2016, 81, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Li, X.; Frisch, M.J. Energy-represented direct inversion in the iterative subspace within a hybrid geometry optimization method. J. Chem. Theory Comput. 2006, 20, 835–839. [Google Scholar] [CrossRef]
- Pulay, P.; Fogarasi, G.; Pang, F.; Boggs, J.E. Systematic ab initio gradient calculation of molecular geometries, force constants, and dipole-moment derivatives. J. Am. Chem. Soc. 1979, 101, 2550–2560. [Google Scholar] [CrossRef]
- Podolyan, Y.; Leszczynski, J. MaSK: A visualization tool for teaching and research in computational chemistry. Int. J. Quantum Chem. 2009, 109, 8–16. [Google Scholar] [CrossRef]
- Foster, J.P.; Weinhold, F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980, 102, 7211–7218. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. Calculation of Small Molecular Interactions by Differences of Separate Total Energies—Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553. [Google Scholar] [CrossRef]
- Sebastian, S.; Sundaraganesan, N.; Manoharan, S. Molecular structure, spectroscopic studies and first-order molecularhyperpolarizabilities of ferulic acid by density functional study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 74, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, E.G.; Salinas, M.V.; Correa, M.J.; Vrdoljak, F.; Williams, P.A. ALP Inhibitors: Vanadyl (IV) Complexes of Ferulic and Cinnamic Acid. Z. Naturforsch. 2005, 60, 305–311. [Google Scholar] [CrossRef]
- Nakanishi, K. Infrared Adsorption Spectroscopy; Practical; Holden Day: San Francisco, CA, USA, 1962. [Google Scholar]
- Kalinowska, M.; Piekut, J.; Bruss, A.; Follet, C.; Sienkiewicz-Gromiuk, J.; Świsłocka, R.; Rzączyńska, Z.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman,1H,13C NMR, UV/VIS), thermogravimetric and antimicrobial studies of Ca(II), Mn(II),Cu(II), Zn(II) and Cd(II) complexes of ferulic acid. Spectrochim. Acta A Molec. Biomolec. Spectrosc. 2014, 122, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Kharlampovich, G.D.; Churkin, Y.V. Fenoly (Phenols); Khimiya: Moscow, Russia, 1974; 376p. (In Russian) [Google Scholar]
- Bobkova, E.Y.; Vasilieva, V.S.; Xenofontov, M.A.; Ostrovskaya, L.E.; Shundalov, M.B. Spectral energy characteristics of dyhydroxybenzene the crystalline state. Bull. Bgu. Phys. 2009, 3, 7–13. (In Russian) [Google Scholar]
- Wang, X.; Zhu, S.; Wang, S.; He, Y.; Liu, Y.; Wang, J.; Fan, W.; Lv, Y. Low temperature hydrodeoxygenation of guaiacol into cyclohexane over Ni/SiO catalyst combined with Hβ zeolite. RSC Adv. 2019, 9, 3868–3876. [Google Scholar] [CrossRef]
- Nelson, N.C.; Manzano, J.S.; Sadow, A.D.; Overbury, S.H.; Slowing, I.I. Slowing Selective Hydrogenation of Phenol Catalyzed by Palladium on High-Surface-Area Ceria at Room Temperature and Ambient Pressure. ACS Catal. 2015, 5, 2051–2061. [Google Scholar] [CrossRef]
- Khvan, A.M.; Kristallovich, E.L.; Abduazimov, K.A. Abduazimov Complexation of Caffeic and Ferulic Acids by Transition-Metal Ions. Chem. Nat. Compd. 2001, 37, 72–75. [Google Scholar] [CrossRef]
- Singh, V.; Naka, T.; Takami, S.; Sahraneshin, A.; Togashi, T.; Aoki, N.; Hojo, D.; Arita, T.; Adschiri, T. Hydrothermal synthesis of inorganic–organic hybrid gadolinium hydroxide nanoclusters with controlled size and morphology. Dalton Trans. 2013, 42, 16176–16184. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Jiang, P.; Wei, C.; Zhuang, D.; Shi, J. Low-temperature one-step synthesis of covalently chelated ZnO/dopamine hybrid nanoparticles and their optical properties. J. Mater. Res. 2008, 23, 1946–1952. [Google Scholar] [CrossRef]
- Hachani, R.; Lowdell, M.; Birchall, M.; Hervault, A.; Mertz, D.; Begin-Colin, S.; Thanh, N.T.K. ThanhPolyol synthesis, functionalisation, and biocompatibility studies of superparamagnetic iron oxide nanoparticles as potential MRI contrast agents. Nanoscale 2016, 8, 3278–3287. [Google Scholar] [CrossRef]
- Togashi, T.; Naka, T.; Asahina, S.; Sato, K.; Takami, S.; Adschiri, T. Adschiri, Surfactant-assisted one-pot synthesis of superparamagnetic magnetite nanoparticle clusters with tunable cluster size and magnetic field sensitivity. Dalton Trans. 2011, 40, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L. The Infrared Spectra of Complex. Molecules, 2nd ed.; Chapman and Hall: London, UK, 1980. [Google Scholar]
- Palacios, E.G.; Juárez-López, G.; Monhemius, A.J. Monhemius, Infrared spectroscopy of metal carboxylates: II. Analysisof Fe(III), Ni and Zn carboxylate solutions. Hydrometallurgy 2004, 72, 139–148. [Google Scholar] [CrossRef]
- Yost, E.C.; Tejedor-Tejedor, M.I.; Anderson, M.A. In Situ CIR-FTIR Characterization of Salicylate Complexes at the Goethite/Aqueous Solution Interface. Environ. Sci. Technol. 1990, 24, 822–828. [Google Scholar] [CrossRef]
- Tunesi, S.; Anderson, M.A. Surface Effects in Photochemistry: An in Situ Cylindrical Internal Reflection Fourier Transform Infrared Investigation of the Effect of Ring Substituents on Chemisorption onto TiO2 Ceramic Membranes. Langmuir 1992, 8, 487–495. [Google Scholar] [CrossRef]
- Parrino, F.; Augugliaro, V.; Camera-Roda, G.; Loddo, V.; López-Muñoz, M.J.; Márquez-Álvarez, C.; Palmisano, G.; Palmisano, L.; Puma, M.A. Visible-light-induced oxidation of trans-ferulic acid by TiO2 photocatalysis. J. Catal. 2012, 295, 254–260. [Google Scholar] [CrossRef]
- Savić, T.D.; Janković, I.A.; Šaponjić, Z.V.; Čomor, M.I.; Veljković, D.Ž.; Zarić, S.D.; Nedeljković, J.M. Surface modification of anatase nanoparticles with fused ring catecholate type ligands: A combined DFT and experimental study of optical properties. Nanoscale 2012, 4, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Dobson, K.D.; McQuillan, A.J. In situ infrared spectroscopic analysis of the adsorption of aromatic carboxylic acids to TiO2, ZrO2, Al2O3, and Ta2O5 from aqueous solutions. Spectrochim. Acta A 2000, 56, 557–565. [Google Scholar] [CrossRef]
- Ding, S.; Zhao, J.; Yu, Q. Effect of Zirconia Polymorph on Vapor-Phase Ketonization of Propionic Acid. Catalysts 2019, 9, 768. [Google Scholar] [CrossRef]
- Kowczyk-Sadowy, M.; Świsłocka, R.; Lewandowska, H.; Piekut, J.; Lewandowski, W. Theoretical and Microbiological Study of trans o-Coumaric Acid and Alkali Metal o-Coumarates. Molecules 2015, 20, 3146–3169. [Google Scholar] [CrossRef]
- Świsłocka, R.; Kowczyk-Sadowy, M.; Kalinowska, M.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H and 13 C NMR) and theoretical studies of p-coumaric acid and alkali metal p-coumarates. Spectroscopy 2012, 27, 35–48. [Google Scholar] [CrossRef]
- Stein, S.E. IR and Mass Spectra, Database Number 69; Mallard, W.G., Linstrom, P.J., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000. Available online: http://webbook.nist.gov (accessed on 5 October 2021).
- Nastasiienko, N.; Kulik, T.; Palianytsia, B.; Laskin, J.; Cherniavska, T.; Kartel, M.; Larsson, M. Catalytic Pyrolysis of Lignin Model Compounds (Pyrocatechol, Guaiacol, Vanillic and Ferulic Acids) over Nanoceria Catalyst for Biomass Conversion. Appl. Sci. 2021, 11, 7205. [Google Scholar] [CrossRef]
- Kulyk, K.; Ishchenko, V.; Palyanytsya, B.; Khylya, V.; Borysenko, M.; Kulyk, T. A TPD-MS study of the interaction of coumarins and their heterocyclic derivatives with a surface of fumed silica and nanosized oxides CeO2/SiO2, TiO2/SiO2, Al2O3/SiO2. J. Mass Spectrom. 2010, 45, 750–761. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L. Catalyst deactivation. Catal. Today 1999, 52, 165–181. [Google Scholar] [CrossRef]
- Astafan, A.; Sachse, A.; Batiot-Dupeyrat, C.; Pinard, L. Impact of the Framework Type on the Regeneration of Coked Zeolites by Non-Thermal Plasma in a Fixed Bed Dielectric Barrier Reactor. Catalysts 2019, 9, 985. [Google Scholar] [CrossRef]
- Kulik, T.; Palianytsia, B.; Larsson, M. Catalytic Pyrolysis of Aliphatic Carboxylic Acids into Symmetric Ketones over Ceria-Based Catalysts: Kinetics, Isotope Effect and Mechanism. Catalysts 2020, 10, 179. [Google Scholar] [CrossRef]
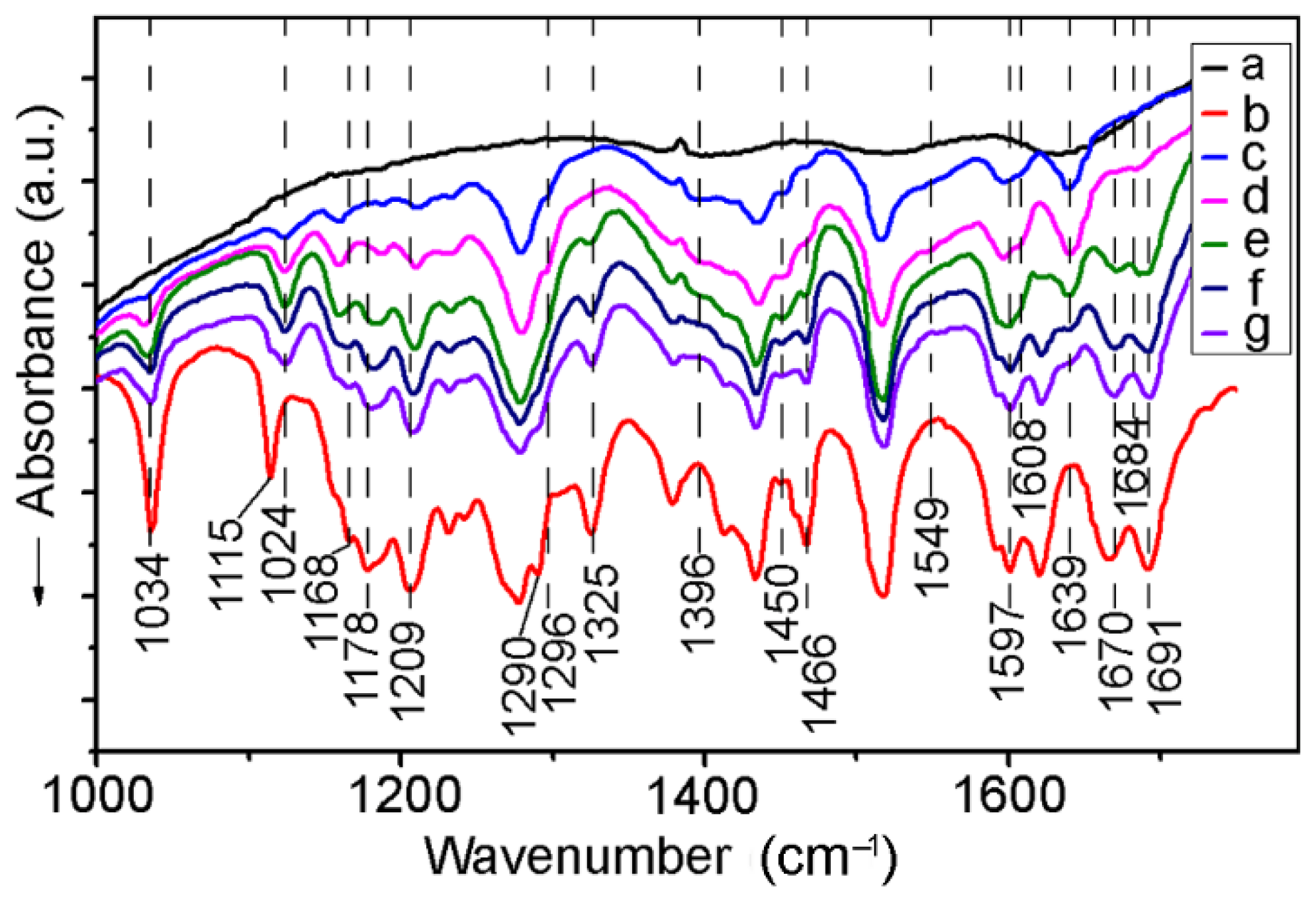
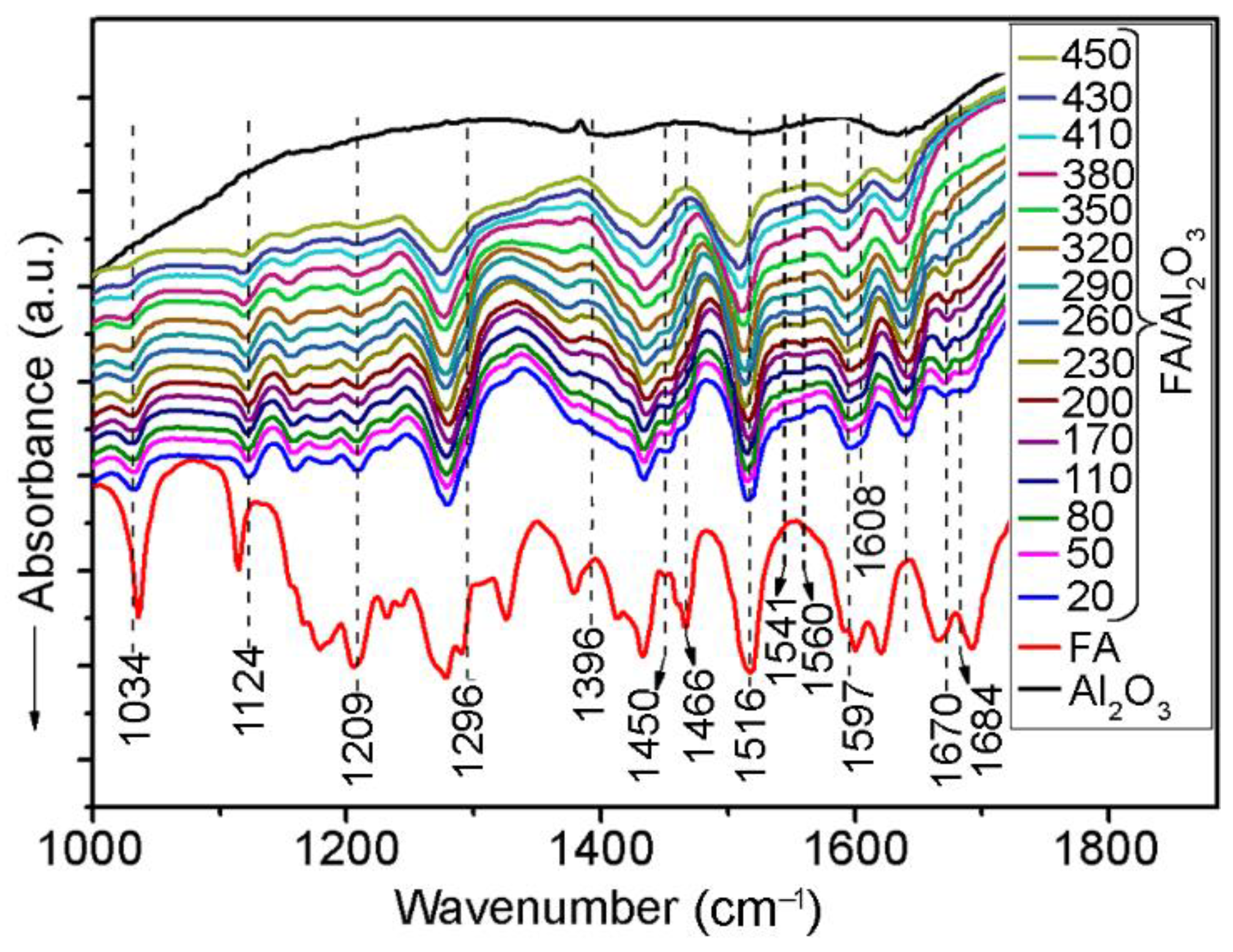

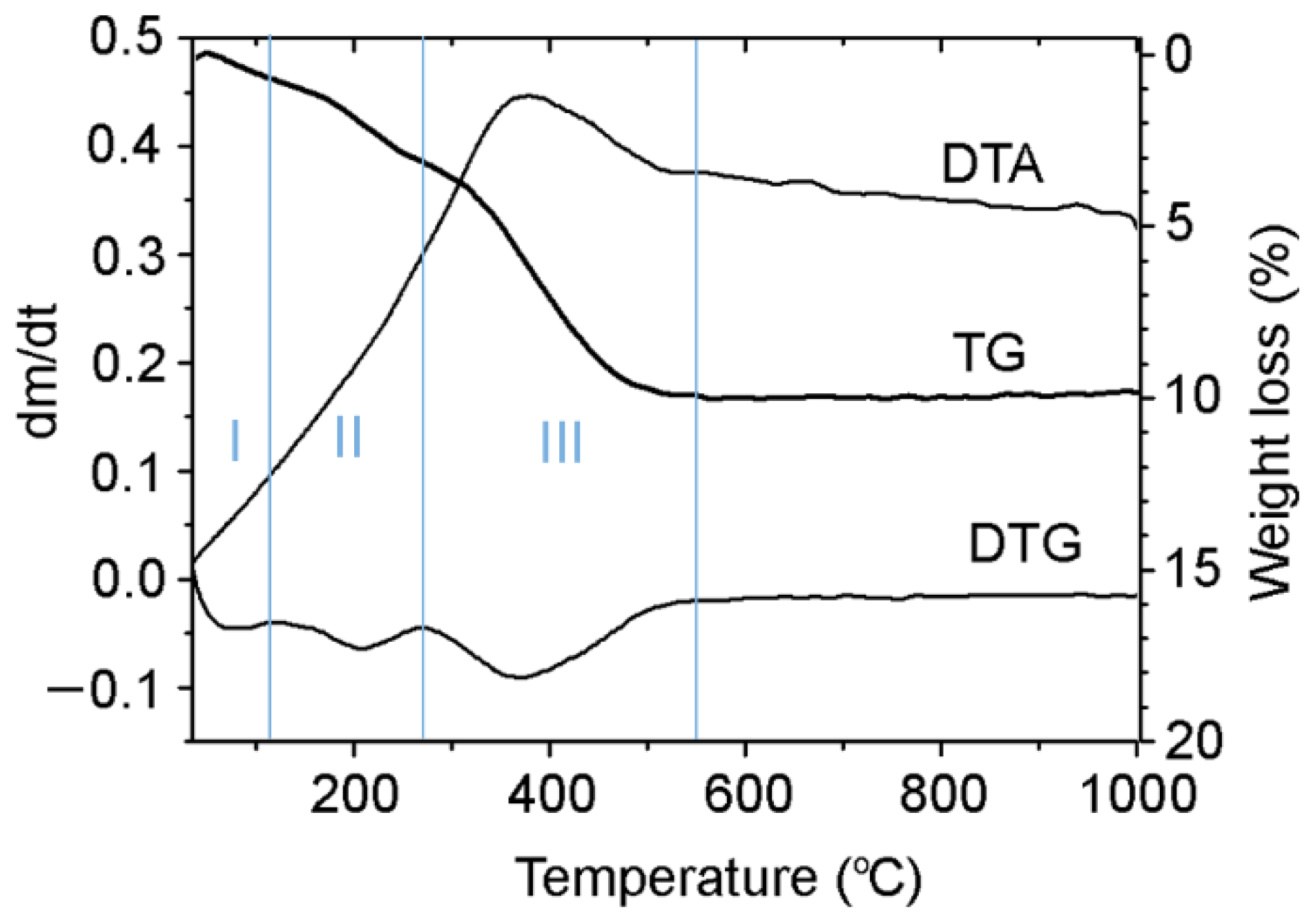
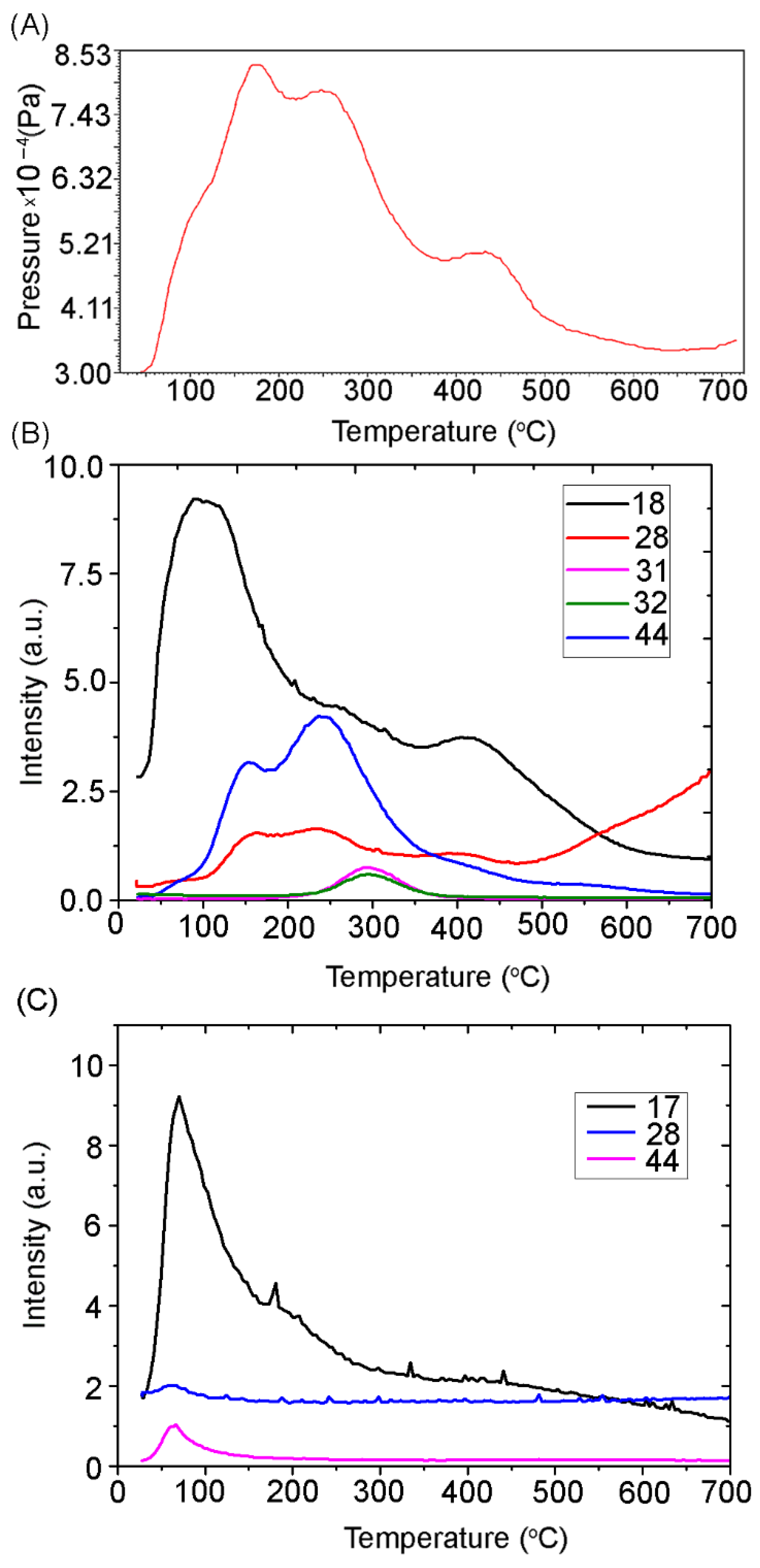



| Assignments | Frequency (cm−1) | Ref. | |
|---|---|---|---|
| FA | FA/Al2O3 | ||
| δ (CH3) | 1115 | – | [69] |
| δ (CH3) | – | 1124 | |
| δ (CH3) | 1178 | – | [69] |
| δ (CH3) | 1379 | 1379 | [69] |
| δ(CH3) | 1466 | 1464 | [69] |
| νs(COCH3) | 1036 | 1030 | [69,70,71,72] |
| νas(COCH3) | 1205 | 1211 | [69,70] |
| β(OHap) | 1167 | – | [69] |
| β(CHap) | 1155 | 1159 | [69] |
| ν(COap) | 1290 | – | [69,70] |
| ν(CO–) | – | 1296 | - |
| ν(CCap) | 1518 | 1518 | [69,72] |
| 1601 | 1597 | [69,72] | |
| ν(C=C) | 1620 | 1639 | [69,72] |
| ν(C=O) | 1666 | – | [69,70,71,72] |
| ν(C=O) | 1691 | 1691 * | [69,70,71,72] |
| ν(C=O) | – | 1670 | [69,70,71,72] |
| ν(C=O) | – | 1684 ** | [69,70,71,72] |
| ν(CO) | – | 1396 | [69] |
| νs(COO–) | – | 1450 | [56] |
| νas(COO–) | – | 1549 | [70,71,72] |
| ν(C=O) | – | 1608 | [89] |
| Structure | H298, a.u. | G298, a.u. | ∆G, kcal/mol |
|---|---|---|---|
| 2A | −1627.939549 | −1628.024516 | 0.0 |
| 2B | −1627.940484 | −1628.023856 | 0.41 * |
| 2C | −1627.937973 | −1628.022237 | 0.99 ** |
| 3A | −1627.911185 | −1628.000011 | 0.0 |
| 3B | −1627.903850 | −1627.988411 | 7.28 * |
| 3C | −1627.931414 | −1628.014775 | −9.26 ** |
| Stage | Tmax, °C | Volatiles (%) | Char (%) |
|---|---|---|---|
| I | 80 | 6.8 | |
| II | 210 | 20.6 | |
| III | 360 | 57.6 | |
| Σ(I + II + III) | 85 | 15 |
| Product or Its Fragment Ion | Scheme | m/z | I, a.u. | Trange, °C | Tmax, °C | n | E≠, kJ/mol | ν0, s−1 | ∆S≠, cal/(K × mol) | R2 * |
|---|---|---|---|---|---|---|---|---|---|---|
| Decarboxylation | ||||||||||
| CO2 | - | 44 | 3.1 | ~70–250 | 155 | - | - | - | - | - |
| - | 44 | 4.2 | ~100–400 | 240 | - | - | - | - | - | |
| Demethoxylation | ||||||||||
| MeOH | - | 31 | 0.739 | ~220–390 | ~300 | 1 | 88 | 3.64 × 106 | −29 | 0.936 |
| - | 32 | 0.583 | ~220–390 | ~300 | 1 | 85 | 1.75 × 106 | −31 | 0.952 | |
| Decarbonylation | ||||||||||
| CO | - | 28 | 1.4 | ~100–220 | 160 | - | - | - | - | - |
| - | 28 | 1.5 | ~180–300 | 235 | - | - | - | - | - | |
| - | 28 | 1.0 | ~300–500 | 400 | - | - | - | - | - | |
| Dehydration | ||||||||||
| H2O | - | 18 | 9.3 | ~20–200 | 100 | - | - | - | - | - |
| - | 18 | 4.2 | ~180–350 | 265 | - | - | - | - | - | |
| - | 18 | 4.1 | ~300–600 | 410 | - | - | - | - | - | |
| Decomposition of carboxylate complexes | ||||||||||
| 4-Vinylguaiacol | 1 | 150 | ~1.0 | ~70–270 | ~148 | - | - | - | - | - |
| 4-Vinylguaiacol | 2 | 150 | ~0.7 | ~100–300 | ~197 | - | - | - | - | - |
| Decomposition of carboxylate complexes with desorption of methylated products | ||||||||||
| 4-Vinyl-methylguaiacol | 3 | 164 | 0.077 | 164–324 | 240 | 1 | 84 | 1.12 × 106 | −32 | 0.943 |
| 138 | 0.040 | 183–292 | 234 | 1 | 85 | 2.11 × 106 | −31 | 0.932 | ||
| 121 | 0.054 | 180–292 | 250 | 1 | 90 | 5.91 × 106 | −29 | 0.963 | ||
| Decomposition of phenolate complexes | ||||||||||
| Guaiacol | 4 | 124 | 0.057 | 160–295 | 234 | 1 | 106 | 3.48 × 108 | −21 | 0.961 |
| Cresol | 5 | 108 | 0.057 | 317–453 | 375 | 1 | 140 | 6.41 × 108 | −20 | 0.959 |
| Phenol | 5 | 94 | 0.082 | 301–491 | 409 | 1 | 135 | 8.14 × 107 | −24 | 0.961 |
| Desorption of aromatic/polycyclic hydrocarbons | ||||||||||
| Naphthalene | - | 128 | 0.060 | 333–530 | 432 | 1 | 125 | 4.77 × 106 | −30 | 0.94 |
| Methylnaphthalene | - | 142 | 0.038 | 326–486 | 409 | 1 | 99 | 1.10 × 105 | −37 | 0.97 |
| Indene | - | 115 | 0.084 | 349–487 | 422 | 1 | 177 | 9.22 × 1010 | −10 | 0.932 |
| Benzene | - | 78 | 0.268 | 353–536 | 447 | 1 | 129 | 5.32 × 106 | −29 | 0.943 |
| Toluene | - | 92 | 0.053 | 350–490 | 428 | 1 | 151 | 7.65 × 108 | −19 | 0.959 |
| Tropylium ion, C7H7+ | - | 91 | 0.130 | 100–570 | ~170 ~230 ~430 | - | - | - | - | - |
| 0.125 | ||||||||||
| 0.07 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulik, T.; Nastasiienko, N.; Palianytsia, B.; Ilchenko, M.; Larsson, M. Catalytic Pyrolysis of Lignin Model Compound (Ferulic Acid) over Alumina: Surface Complexes, Kinetics, and Mechanisms. Catalysts 2021, 11, 1508. https://doi.org/10.3390/catal11121508
Kulik T, Nastasiienko N, Palianytsia B, Ilchenko M, Larsson M. Catalytic Pyrolysis of Lignin Model Compound (Ferulic Acid) over Alumina: Surface Complexes, Kinetics, and Mechanisms. Catalysts. 2021; 11(12):1508. https://doi.org/10.3390/catal11121508
Chicago/Turabian StyleKulik, Tetiana, Nataliia Nastasiienko, Borys Palianytsia, Mykola Ilchenko, and Mats Larsson. 2021. "Catalytic Pyrolysis of Lignin Model Compound (Ferulic Acid) over Alumina: Surface Complexes, Kinetics, and Mechanisms" Catalysts 11, no. 12: 1508. https://doi.org/10.3390/catal11121508
APA StyleKulik, T., Nastasiienko, N., Palianytsia, B., Ilchenko, M., & Larsson, M. (2021). Catalytic Pyrolysis of Lignin Model Compound (Ferulic Acid) over Alumina: Surface Complexes, Kinetics, and Mechanisms. Catalysts, 11(12), 1508. https://doi.org/10.3390/catal11121508







