Synthesis of Indigo-Dyes from Indole Derivatives by Unspecific Peroxygenases and Their Application for In-Situ Dyeing
Abstract
:1. Introduction
2. Results
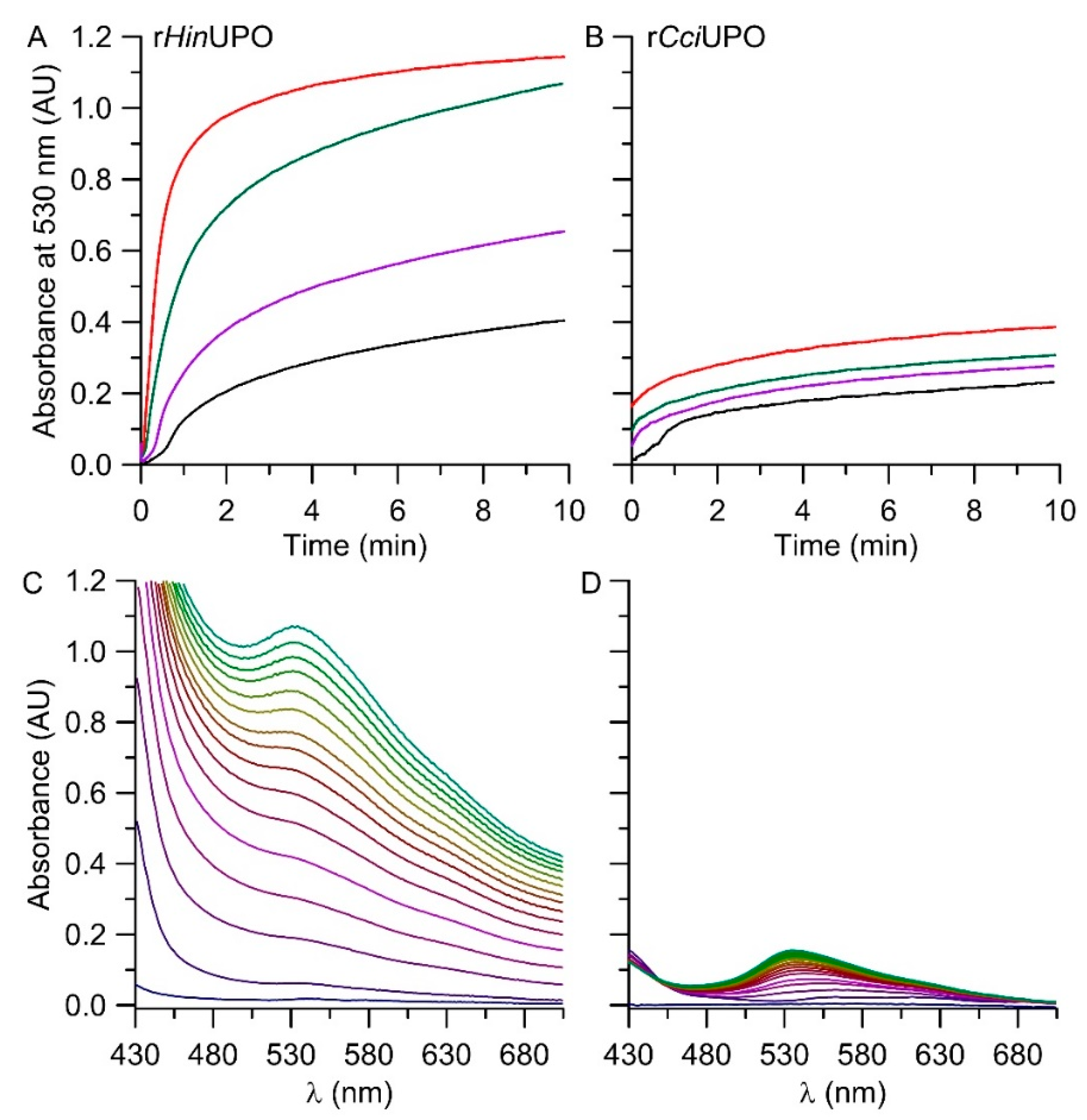
2.1. Screening of UPOs for the Conversion of Indole in the Presence of H2O2
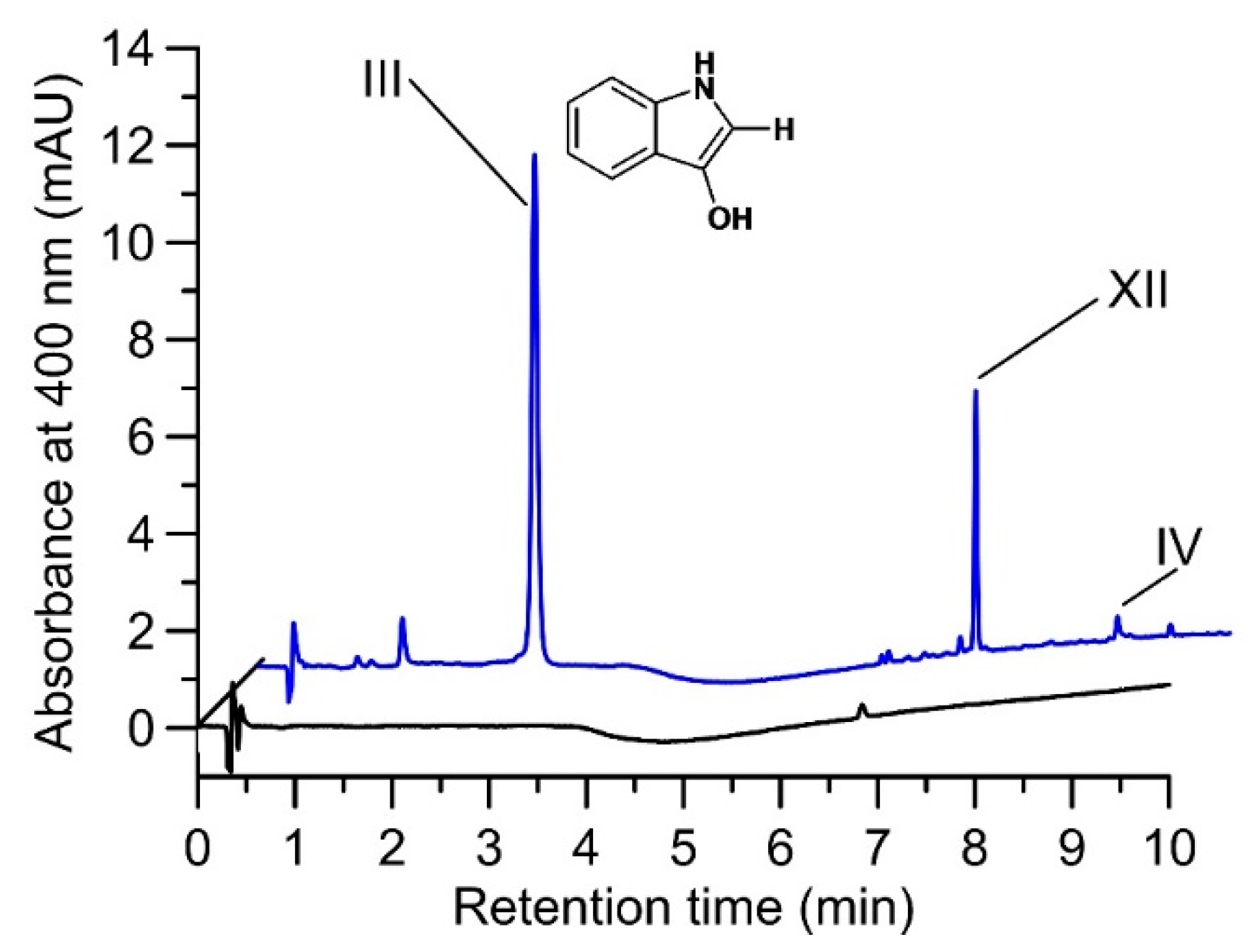
2.2. Product Identification and Verification of Indoxyl Formation
2.3. The pH Profile of rHinUPO for the Conversion of Indole
2.4. Screening of Rational Design Variants of rHinUPO
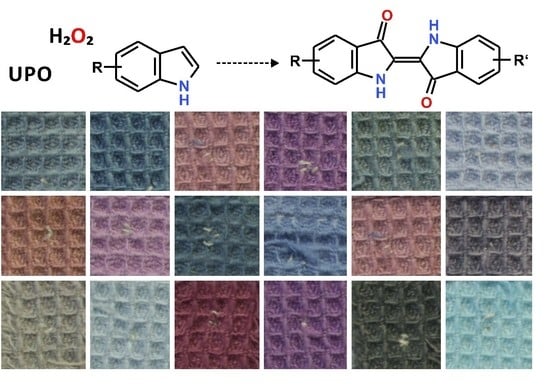
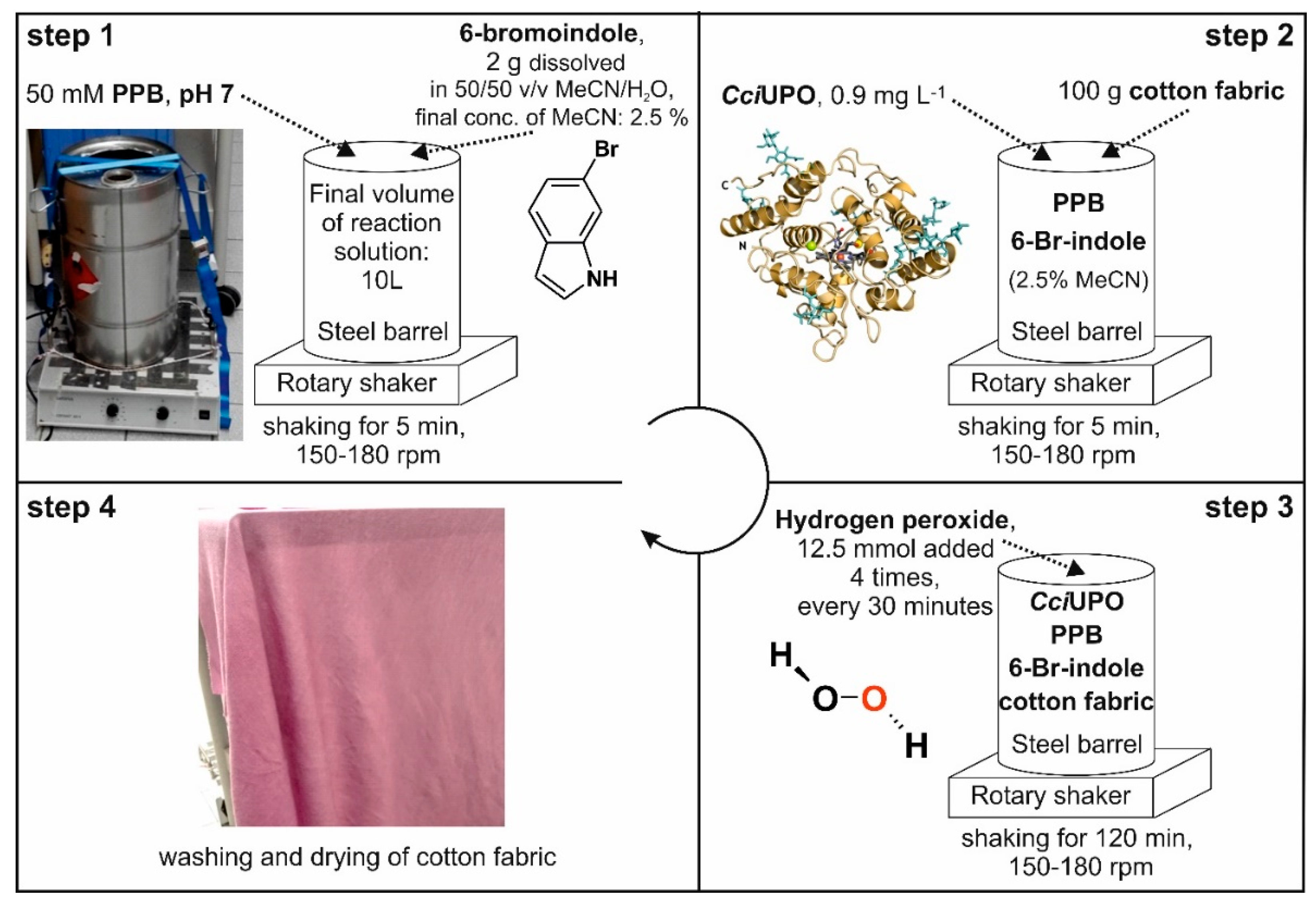
2.5. In-Situ Dyeing Process and Its Scale up
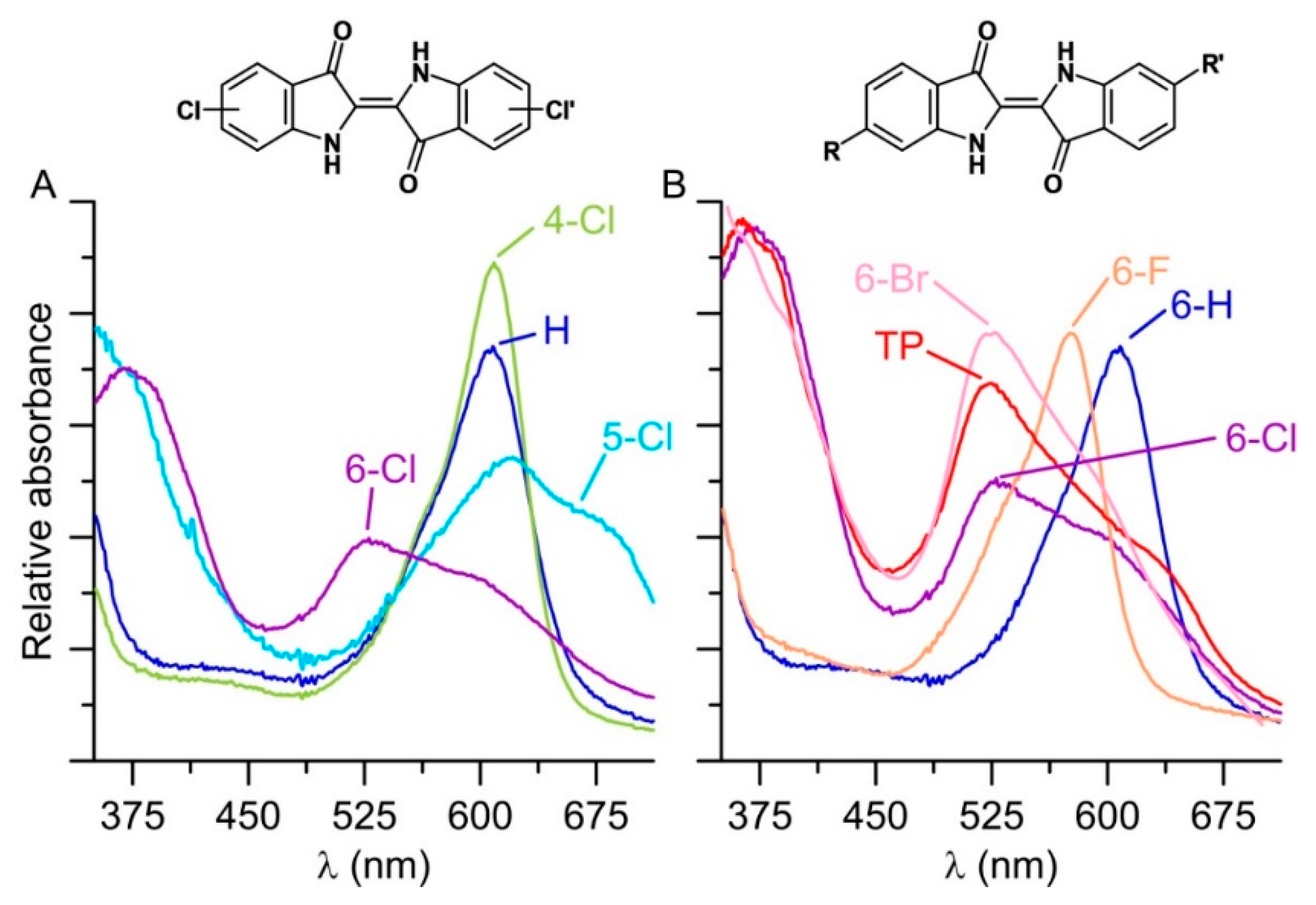
2.6. Synthesis of Further Halogenated Indigoids Using UPOs
2.7. Comparison of UPO-Catalyzed Conversion of 5-Bromoindole and 5-Bromoindoline
3. Discussion
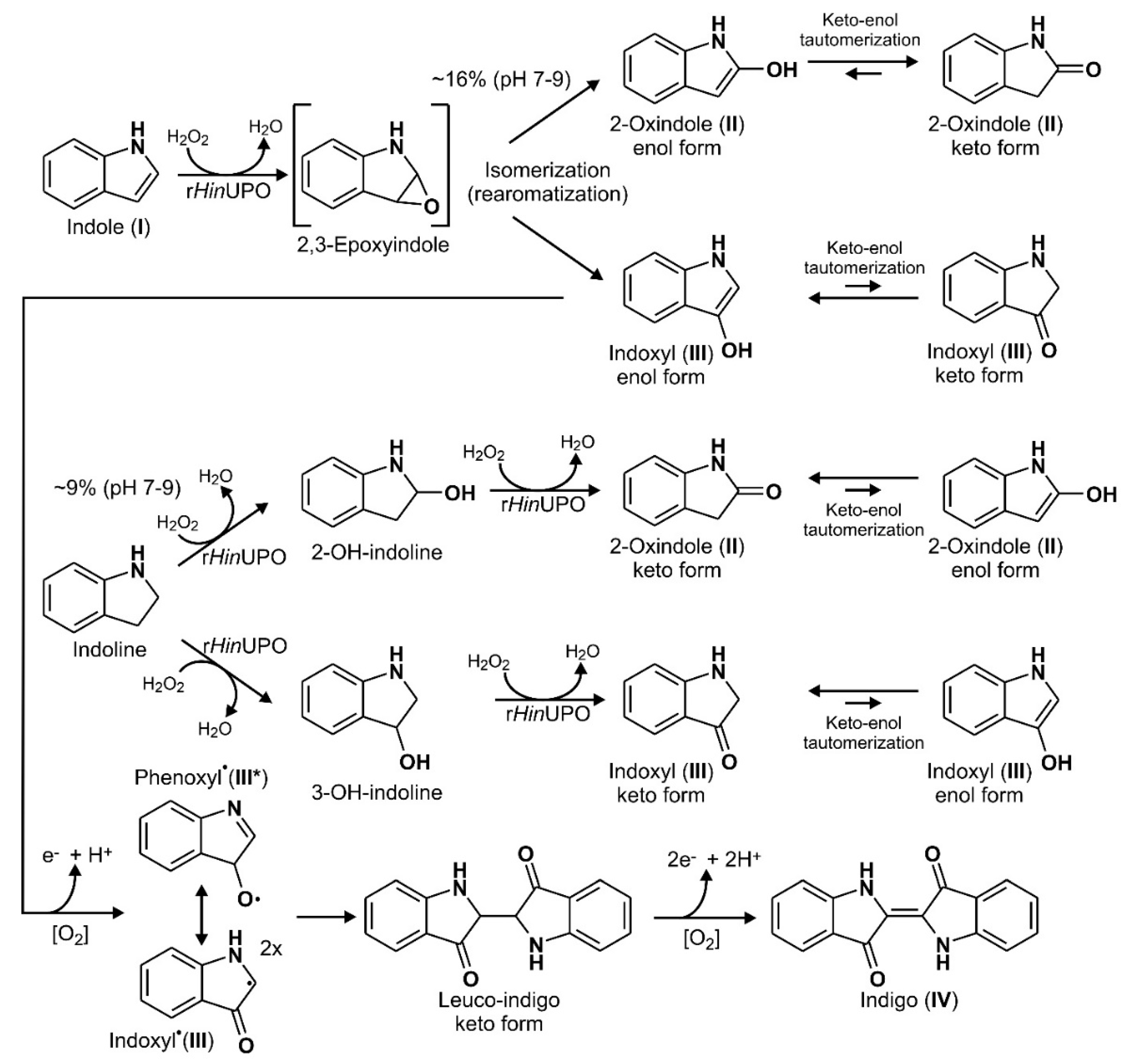
3.1. Reaction Sequence and Products
3.2. Considerations for the Commercialization of Peroxygenase-Catalyzed Indigoid Synthesis
3.3. Enzymatic Dyeing Process, of Interest for Textile Industry?
3.4. Products for Medicine and Other Applications
4. Materials and Methods
4.1. Chemicals
4.2. Enzymes
4.3. Enzyme Screening of Wild Types and Recombinantly Expressed Wild Types
4.4. Quantification of Indigo Using Sulfonation
4.5. Determination of pH Dependence of rHinUPO Catalyzed Indigo Formation
4.6. Variant Generation for Rational Design Enzyme Library of rHinUPO
4.7. Variant Screening in 96-Well Plate Assay
4.8. Verification of Screening Hits under Different Atmospheres
4.9. Proof of Indoxyl Formation
4.10. Comparison of UPO-Catalyzed Conversion of 5-Bromoindol and 5-Bromoindoline
4.11. In-Situ Dyeing Pre-Study
4.12. LC-MS Analysis
4.13. HPLC-IT-MS Analysis
4.14. Determination of the Reaction Time Course
4.15. Product Purification
4.16. Synthesis of 6,6′-Dibromoindigo
4.17. Quantification of Tyrian Purple
4.18. Common Chemical Dyeing by Application of Enzymatically Synthesized Dyes
4.19. Scaled up In-Situ Dyeing
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UPO | unspecific peroxygenase |
| PPB | potassium phosphate buffer |
| Hin | Humicola insolens |
| Aae | Agrocybe aegerita |
| Cci | Coprinopsis cinerea |
| ACN | acetonitrile |
| HPLC-IT-MS | high performance liquid chromatography/ion trap mass spectrometry |
| LRET | long range electron transfer. |
References
- Stieglitz, R.R. The Minoan origin of Tyrian purple. Biblic. Archaeol. 1994, 57, 46–54. [Google Scholar] [CrossRef]
- Wolk, J.L.; Frimer, A.A. Preparation of Tyrian purple (6,6′-Dibromoindigo): Past and present. Molecules 2010, 15, 5473. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, P. Über den Farbstoff des antiken Purpurs aus Murex brandaris. Ber. Dtsch. Chem. Ges. 1909, 42, 765–770. [Google Scholar] [CrossRef] [Green Version]
- Cooksey, C. Tyrian purple: 6,6′-dibromoindigo and related compounds. Molecules 2001, 6, 736–769. [Google Scholar] [CrossRef]
- Gillam, E.M.J.; Notley, L.M.; Cai, H.; De Voss, J.J.; Guengerich, F.P. Oxidation of indole by cytochrome P450 enzymes. Biochemistry 2000, 39, 13817–13824. [Google Scholar] [CrossRef]
- Hsu, T.M.; Welner, D.H.; Russ, Z.N.; Cervantes, B.; Prathuri, R.L.; Adams, P.D.; Dueber, J.E. Employing a biochemical protecting group for a sustainable indigo dyeing strategy. Nat. Chem. Biol. 2018, 14, 256–261. [Google Scholar] [CrossRef]
- Huisgen, R. Adolf von Baeyer’s scientific achievements—A legacy. Angew. Chem. Int. Ed. Engl. 1986, 25, 297–311. [Google Scholar] [CrossRef]
- Heumann, K. Neue Synthesen des Indigos und verwandter Farbstoffe. Ber. Dtsch. Chem. Ges. 1890, 23, 3431–3435. [Google Scholar] [CrossRef]
- Steingruber, E. Indigo and indigo colorants. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012; pp. 55–63. [Google Scholar]
- Schmidt, H. Indigo—100 Jahre industrielle Synthese. Chem. Unserer Zeit 1997, 31, 121–128. [Google Scholar] [CrossRef]
- Russell, G.A.; Kaupp, G. Reactions of resonance stabilized carbanions. XXXI. Oxidation of carbanions. 4. Oxidation of indoxyl to indigo in basic solution. J. Am. Chem. Soc. 1969, 91, 3851–3859. [Google Scholar] [CrossRef]
- Rebelo, S.L.H.; Linhares, M.; Simões, M.M.Q.; Silva, A.M.S.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Freire, C. Indigo dye production by enzymatic mimicking based on an iron(III)porphyrin. J. Catal. 2014, 315, 33–40. [Google Scholar] [CrossRef]
- Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2003; p. 637. [Google Scholar]
- Sachs, F.; Kempf, R. Ueber p-Halogen-o-Nitrobenzaldehyde. Ber. Dtsch. Chem. Ges. 1903, 36, 3299–3303. [Google Scholar] [CrossRef] [Green Version]
- Baeyer, A.; Drewsen, V. Darstellung von Indigblau aus Orthonitrobenzaldehyd. Ber. Dtsch. Chem. Ges. 1882, 15, 2856–2864. [Google Scholar] [CrossRef] [Green Version]
- Sachs, F.; Sichel, E. Ueber p-substituirte o-Nitrobenzaldehyde. Ber. Dtsch. Chem. Ges. 1904, 37, 1861–1874. [Google Scholar] [CrossRef] [Green Version]
- Wolk, J.L.; Frimer, A.A. A simple, safe and efficient synthesis of Tyrian purple (6,6′-dibromoindigo). Molecules 2010, 15, 5561. [Google Scholar] [CrossRef] [PubMed]
- Parette, R.; McCrindle, R.; McMahon, K.S.; Pena-Abaurrea, M.; Reiner, E.; Chittim, B.; Riddell, N.; Voss, G.; Dorman, F.L.; Pearson, W.N. Halogenated indigo dyes: A likely source of 1,3,6,8-tetrabromocarbazole and some other halogenated carbazoles in the environment. Chemosphere 2015, 127, 18–26. [Google Scholar] [CrossRef]
- Kohlhaupt, R.; Bergmann, U. Purification of Indigo. U.S. Patent 5424453A, 14 June 1993. [Google Scholar]
- Ensley, B.; Ratzkin, B.; Osslund, T.; Simon, M.; Wackett, L.; Gibson, D. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science 1983, 222, 167–169. [Google Scholar] [CrossRef]
- Mouri, T.; Michizoe, J.; Ichinose, H.; Kamiya, N.; Goto, M. A recombinant Escherichia coli whole cell biocatalyst harboring a cytochrome P450cam monooxygenase system coupled with enzymatic cofactor regeneration. Appl. Microbiol. Biotechnol. 2006, 72, 514–520. [Google Scholar] [CrossRef]
- Carredano, E.; Karlsson, A.; Kauppi, B.; Choudhury, D.; Parales, R.E.; Parales, J.V.; Lee, K.; Gibson, D.T.; Eklund, H.; Ramaswamy, S. Substrate binding site of naphthalene 1,2-dioxygenase: Functional implications of indole binding. J. Mol. Biol. 2000, 296, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Fabara, A.N.; Fraaije, M.W. An overview of microbial indigo-forming enzymes. Appl. Microbiol. Biotechnol. 2020, 104, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, R.; Nüske, J.; Scheibner, K.; Spantzel, J.; Hofrichter, M. Novel haloperoxidase from the agaric basidiomycete Agrocybe aegerita oxidizes aryl alcohols and aldehydes. Appl. Environ. Microbiol. 2004, 70, 4575–4581. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, R.; Hofrichter, M. The haloperoxidase of the agaric fungus Agrocybe aegerita hydroxylates toluene and naphthalene. FEBS Lett. 2005, 579, 6247–6250. [Google Scholar] [CrossRef] [Green Version]
- Gröbe, G.; Ullrich, R.; Pecyna, M.J.; Kapturska, D.; Friedrich, S.; Hofrichter, M.; Scheibner, K. High-yield production of aromatic peroxygenase by the agaric fungus Marasmius rotula. AMB Express 2011, 1, 31. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, R.; Poraj-Kobielska, M.; Scholze, S.; Halbout, C.; Sandvoss, M.; Pecyna, M.J.; Scheibner, K.; Hofrichter, M. Side chain removal from corticosteroids by unspecific peroxygenase. J. Inorg. Biochem. 2018, 183, 84–93. [Google Scholar] [CrossRef]
- Anh, D.H.; Ullrich, R.; Benndorf, D.; Svatoś, A.; Muck, A.; Hofrichter, M. The coprophilous mushroom Coprinus radians secretes a haloperoxidase that catalyzes aromatic peroxygenation. Appl. Environ. Microbiol. 2007, 73, 5477–5485. [Google Scholar] [CrossRef] [Green Version]
- Kiebist, J.; Schmidtke, K.-U.; Zimmermann, J.; Kellner, H.; Jehmlich, N.; Ullrich, R.; Zänder, D.; Hofrichter, M.; Scheibner, K. A peroxygenase from Chaetomium globosum catalyzes the selective oxygenation of testosterone. ChemBioChem 2017, 18, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Kluge, M.; Ullrich, R.; Dolge, C.; Scheibner, K.; Hofrichter, M. Hydroxylation of naphthalene by aromatic peroxygenase from Agrocybe aegerita proceeds via oxygen transfer from H2O2 and intermediary epoxidation. Appl. Microbiol. Biotechnol. 2009, 81, 1071–1076. [Google Scholar] [CrossRef]
- Bassanini, I.; Ferrandi, E.E.; Vanoni, M.; Ottolina, G.; Riva, S.; Crotti, M.; Brenna, E.; Monti, D. Peroxygenase-catalyzed enantioselective sulfoxidations. Eur. J. Org. Chem. 2017, 47, 7186–7189. [Google Scholar] [CrossRef]
- Aranda, E.; Kinne, M.; Kluge, M.; Ullrich, R.; Hofrichter, M. Conversion of dibenzothiophene by the mushrooms Agrocybe aegerita and Coprinellus radians and their extracellular peroxygenases. Appl. Microbiol. Biotechnol. 2008, 82, 1057–1066. [Google Scholar] [CrossRef]
- Kinne, M.; Poraj-Kobielska, M.; Ralph, S.A.; Ullrich, R.; Hofrichter, M.; Hammel, K.E. Oxidative cleavage of diverse ethers by an extracellular fungal peroxygenase. J. Biol. Chem. 2009, 284, 29343–29349. [Google Scholar] [CrossRef] [Green Version]
- Kiebist, J.; Holla, W.; Heidrich, J.; Poraj-Kobielska, M.; Sandvoss, M.; Simonis, R.; Gröbe, G.; Atzrodt, J.; Hofrichter, M.; Scheibner, K. One-pot synthesis of human metabolites of SAR548304 by fungal peroxygenases. Bioorganic Med. Chem. 2015, 23, 4324–4332. [Google Scholar] [CrossRef]
- Olmedo, A.; Río, J.C.d.; Kiebist, J.; Ullrich, R.; Hofrichter, M.; Scheibner, K.; Martínez, A.T.; Gutiérrez, A. Fatty acid chain shortening by a fungal peroxygenase. Chem. A Eur. J. 2017, 23, 16985–16989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinne, M.; Poraj-Kobielska, M.; Aranda, E.; Ullrich, R.; Hammel, K.E.; Scheibner, K.; Hofrichter, M. Regioselective preparation of 5-hydroxypropranolol and 4′-hydroxydiclofenac with a fungal peroxygenase. Bioorganic Med. Chem. Lett. 2009, 19, 3085–3087. [Google Scholar] [CrossRef] [PubMed]
- Poraj-Kobielska, M.; Kinne, M.; Ullrich, R.; Scheibner, K.; Kayser, G.; Hammel, K.E.; Hofrichter, M. Preparation of human drug metabolites using fungal peroxygenases. Biochem. Pharmacol. 2011, 82, 789–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiebist, J.; Hofrichter, M.; Zuhse, R.; Scheibner, K. Oxyfunctionalization of pharmaceuticals by fungal peroxygenases. In Pharmaceutical Biocatalysis: Chemoenzymatic Synthesis of Active Pharmaceutical Ingredients; Grunwald, P., Grunwald, P., Eds.; Pan Stanford Series on Biocatalysis; Jenny Stanford Publishing: Singapore, 2019; Volume 5. [Google Scholar]
- Hofrichter, M.; Ullrich, R. Oxidations catalyzed by fungal peroxygenases. Curr. Opin. Chem. Biol. 2014, 19, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, R.; Karich, A.; Hofrichter, M. Fungal peroxygenases—A versatile tool for biocatalysis. In Encyclopedia of Mycology; Zaragoza, Ó., Casadevall, A., Eds.; Elsevier: Oxford, UK, 2021; pp. 260–280. [Google Scholar]
- Kluge, M.; Ullrich, R.; Scheibner, K.; Hofrichter, M. Stereoselective benzylic hydroxylation of alkylbenzenes and epoxidation of styrene derivatives catalyzed by the peroxygenase of Agrocybe aegerita. Green Chem. 2012, 14, 440–446. [Google Scholar] [CrossRef]
- Karich, A.; Kluge, M.; Ullrich, R.; Hofrichter, M. Benzene oxygenation and oxidation by the peroxygenase of Agrocybe aegerita. AMB Express 2013, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- González-Benjumea, A.; Marques, G.; Herold-Majumdar, O.M.; Kiebist, J.; Scheibner, K.; del Río, J.C.; Martínez, A.T.; Gutiérrez, A. High epoxidation yields of vegetable oil hydrolyzates and methyl esters by selected fungal peroxygenases. Front. Bioeng. Biotechnol. 2021, 8, 1470. [Google Scholar] [CrossRef]
- Sánchez-Viesca, F.; Gómez, R. On the Baeyer-Emmerling synthesis of Indigo. World J. Org. Chem. 2018, 6, 6–12. [Google Scholar]
- Murdock, D.; Ensley, B.D.; Serdar, C.; Thalen, M. Construction of metabolic operons catalyzing the de novo biosynthesis of indigo in Escherichia coli. BioTechnology 1993, 11, 381–386. [Google Scholar] [CrossRef]
- McClay, K.; Boss, C.; Keresztes, I.; Steffan, R.J. Mutations of Toluene-4-monooxygenase that alter regiospecificity of indole oxidation and lead to production of novel indigoid pigments. Appl. Environ. Microbiol. 2005, 71, 5476–5483. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.-S.; Schwaneberg, U.; Fischer, P.; Schmid, R.D. Directed evolution of the fatty-acid hydroxylase P450 BM-3 into an indole-hydroxylating catalyst. Chem. Eur. J. 2000, 6, 1531–1536. [Google Scholar] [CrossRef]
- Churakova, E.; Kluge, M.; Ullrich, R.; Arends, I.; Hofrichter, M.; Hollmann, F. Specific photobiocatalytic oxyfunctionalization reactions. Angew. Chem. Int. Ed. 2011, 50, 10716–10719. [Google Scholar] [CrossRef]
- Horst, A.E.W.; Bormann, S.; Meyer, J.; Steinhagen, M.; Ludwig, R.; Drews, A.; Ansorge-Schumacher, M.; Holtmann, D. Electro-enzymatic hydroxylation of ethylbenzene by the evolved unspecific peroxygenase of Agrocybe aegerita. J. Mol. Catal. B Enzym. 2016, 133, S137–S142. [Google Scholar] [CrossRef]
- Aranda, C.; Municoy, M.; Guallar, V.; Kiebist, J.; Scheibner, K.; Ullrich, R.; del Río, J.C.; Hofrichter, M.; Martínez, A.T.; Gutiérrez, A. Selective synthesis of 4-hydroxyisophorone and 4-ketoisophorone by fungal peroxygenases. Catal. Sci. Technol. 2019, 9, 1398–1405. [Google Scholar] [CrossRef] [Green Version]
- Karich, A.; Scheibner, K.; Ullrich, R.; Hofrichter, M. Exploring the catalase activity of unspecific peroxygenases and the mechanism of peroxide-dependent heme destruction. J. Mol. Catal. B Enzym. 2016, 134, 238–246. [Google Scholar] [CrossRef]
- Ramig, K.; Islamova, A.; Scalise, J.; Karimi, S.; Lavinda, O.; Cooksey, C.; Vasileiadou, A.; Karapanagiotis, I. The effect of light and dye composition on the color of dyeings with indigo, 6-bromoindigo, and 6,6′-dibromoindigo, components of Tyrian purple. Struct. Chem. 2017, 28, 1553–1561. [Google Scholar] [CrossRef]
- Naegel, L.C.A.; Cooksey, C.J. Tyrian Purple from marine muricids, especially from Plicopurpura pansa (Gould, 1853). J. Shellfish Res. 2002, 21, 193–200. [Google Scholar]
- Fischer, E.; Jourdan, F. Ueber die Hydrazine der Brenztraubensäure. Ber. Dtsch. Chem. Ges. 1883, 16, 2241–2245. [Google Scholar] [CrossRef] [Green Version]
- Taber, D.F.; Tirunahari, P.K. Indole synthesis: A review and proposed classification. Tetrahedron 2011, 67, 7195–7210. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Arza, C.R.; Zhang, B. Indole as a new sustainable aromatic unit for high quality biopolyesters. Polym. Chem. 2018, 9, 4706–4710. [Google Scholar] [CrossRef] [Green Version]
- Buzzetti, F.; Longo, A.; Colombo, M. 2-Oxindole Compounds which Have Useful Tyrosine Kinase Activity. U.S. Patent 5488057A, 23 August 1994. [Google Scholar]
- Marte, W.; Rys, P. Dyeing and Printing of Cellulosic Fibre Materials with Vat Dyes with Mono- or Di-Hydroxyacetone as Reducing Agent. U.S. Patent 4950306-A, 27 July 1989. [Google Scholar]
- Bechtold, T.; Burtscher, E.; Turcanu, A.; Bobleter, O. Dyeing behavior of Indigo reduced by indirect electrolysis. Text. Res. J. 1997, 67, 635–642. [Google Scholar] [CrossRef]
- Meksi, N.; Ben Ticha, M.; Kechida, M.; Mhenni, M.F. Using of ecofriendly α-hydroxycarbonyls as reducing agents to replace sodium dithionite in indigo dyeing processes. J. Clean. Prod. 2012, 24, 149–158. [Google Scholar] [CrossRef]
- Božič, M.; Kokol, V.; Guebitz, G.M. Indigo dyeing of polyamide using enzymes for dye reduction. Text. Res. J. 2009, 79, 895–907. [Google Scholar] [CrossRef]
- Song, J.; Imanaka, H.; Imamura, K.; Kajitani, K.; Nakanishi, K. Development of a highly efficient indigo dyeing method using indican with an immobilized β-glucosidase from Aspergillus niger. J. Biosci. Bioeng. 2010, 110, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-K.; See, L.-C.; Huang, Y.-H.; Chang, Y.-C.; Tsou, T.-C.; Lin, T.-Y.; Lin, N.-L. Efficacy and safety of Indigo naturalis extract in oil (Lindioil) in treating nail psoriasis: A randomized, observer-blind, vehicle-controlled trial. Phytomedicine 2014, 21, 1015–1020. [Google Scholar] [CrossRef]
- Lee, C.-L.; Wang, C.-M.; Hu, H.-C.; Yen, H.-R.; Song, Y.-C.; Yu, S.-J.; Chen, C.-J.; Li, W.-C.; Wu, Y.-C. Indole alkaloids indigodoles A–C from aerial parts of Strobilanthes cusia in the traditional Chinese medicine Qing Dai have anti-IL-17 properties. Phytochemistry 2019, 162, 39–46. [Google Scholar] [CrossRef]
- Leclerc, S.; Garnier, M.; Hoessel, R.; Marko, D.; Bibb, J.A.; Snyder, G.L.; Greengard, P.; Biernat, J.; Wu, Y.-Z.; Mandelkow, E.-M.; et al. Indirubins inhibit glycogen synthase kinase-3β and CDK5/P25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease: A property common to most cyclin-dependent kinase inhibitors? J. Biol. Chem. 2001, 276, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Eisenbrand, G.; Hippe, F.; Jakobs, S.; Muehlbeyer, S. Molecular mechanisms of indirubin and its derivatives: Novel anticancer molecules with their origin in traditional Chinese phytomedicine. J. Cancer Res. Clin. Oncol. 2004, 130, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Kunikata, T.; Tatefuji, T.; Aga, H.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Indirubin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur. J. Pharm. 2000, 410, 93–100. [Google Scholar] [CrossRef]
- Kim, M.; Park, W.-T.; Park, S.A.; Park, C.W.; Ryu, S.U.; Lee, D.H.; Noh, Y.-Y.; Park, T. Controlling ambipolar charge transport in isoindigo-based conjugated polymers by altering fluorine substitution position for high-performance organic field-effect transistors. Adv. Funct. Mater. 2019, 29, 1805994. [Google Scholar] [CrossRef]
- Weiner, M.P.; Costa, G.L.; Schoettlin, W.; Cline, J.; Mathur, E.; Bauer, J.C. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene 1994, 151, 119–123. [Google Scholar] [CrossRef]
- Matsui, T.; Udagawa, H.; Kishishita, S.; Skovlund, D.; Jin, Q. Recombinase-Mediated Integration of a Polynucleotide Library. Patent WO 2016026938A1, 20 August 2015. [Google Scholar]



| Variant | Amino Acid | Proposed Effect |
|---|---|---|
| per27.0002 | A55L | flanks active site on one -helix |
| per27.0006 | N71Q | removes a potential N-glycosylation, which could affect access to the active site |
| per27.0032 | I154V | flanks active site on another -helix |
| per27.0043 | W183I | presumably affects the long-range electron transfer (LRET) |
| per27.0044 | W183V | presumably affects the LRET |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullrich, R.; Poraj-Kobielska, M.; Herold-Majumdar, O.M.; Vind, J.; Hofrichter, M. Synthesis of Indigo-Dyes from Indole Derivatives by Unspecific Peroxygenases and Their Application for In-Situ Dyeing. Catalysts 2021, 11, 1495. https://doi.org/10.3390/catal11121495
Ullrich R, Poraj-Kobielska M, Herold-Majumdar OM, Vind J, Hofrichter M. Synthesis of Indigo-Dyes from Indole Derivatives by Unspecific Peroxygenases and Their Application for In-Situ Dyeing. Catalysts. 2021; 11(12):1495. https://doi.org/10.3390/catal11121495
Chicago/Turabian StyleUllrich, René, Marzena Poraj-Kobielska, Owik M. Herold-Majumdar, Jesper Vind, and Martin Hofrichter. 2021. "Synthesis of Indigo-Dyes from Indole Derivatives by Unspecific Peroxygenases and Their Application for In-Situ Dyeing" Catalysts 11, no. 12: 1495. https://doi.org/10.3390/catal11121495
APA StyleUllrich, R., Poraj-Kobielska, M., Herold-Majumdar, O. M., Vind, J., & Hofrichter, M. (2021). Synthesis of Indigo-Dyes from Indole Derivatives by Unspecific Peroxygenases and Their Application for In-Situ Dyeing. Catalysts, 11(12), 1495. https://doi.org/10.3390/catal11121495