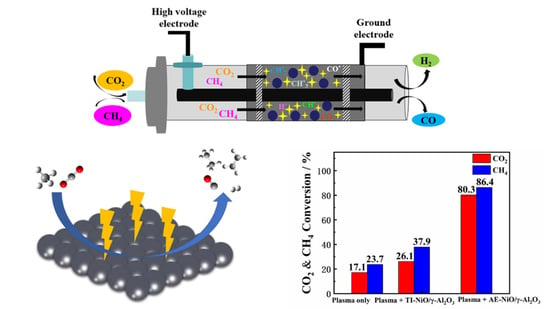
Plasma Promotes Dry Reforming Reaction of CH4 and CO2 at Room Temperature with Highly Dispersed NiO/γ-Al2O3 Catalyst
Abstract
:1. Introduction
2. Results and Discussion
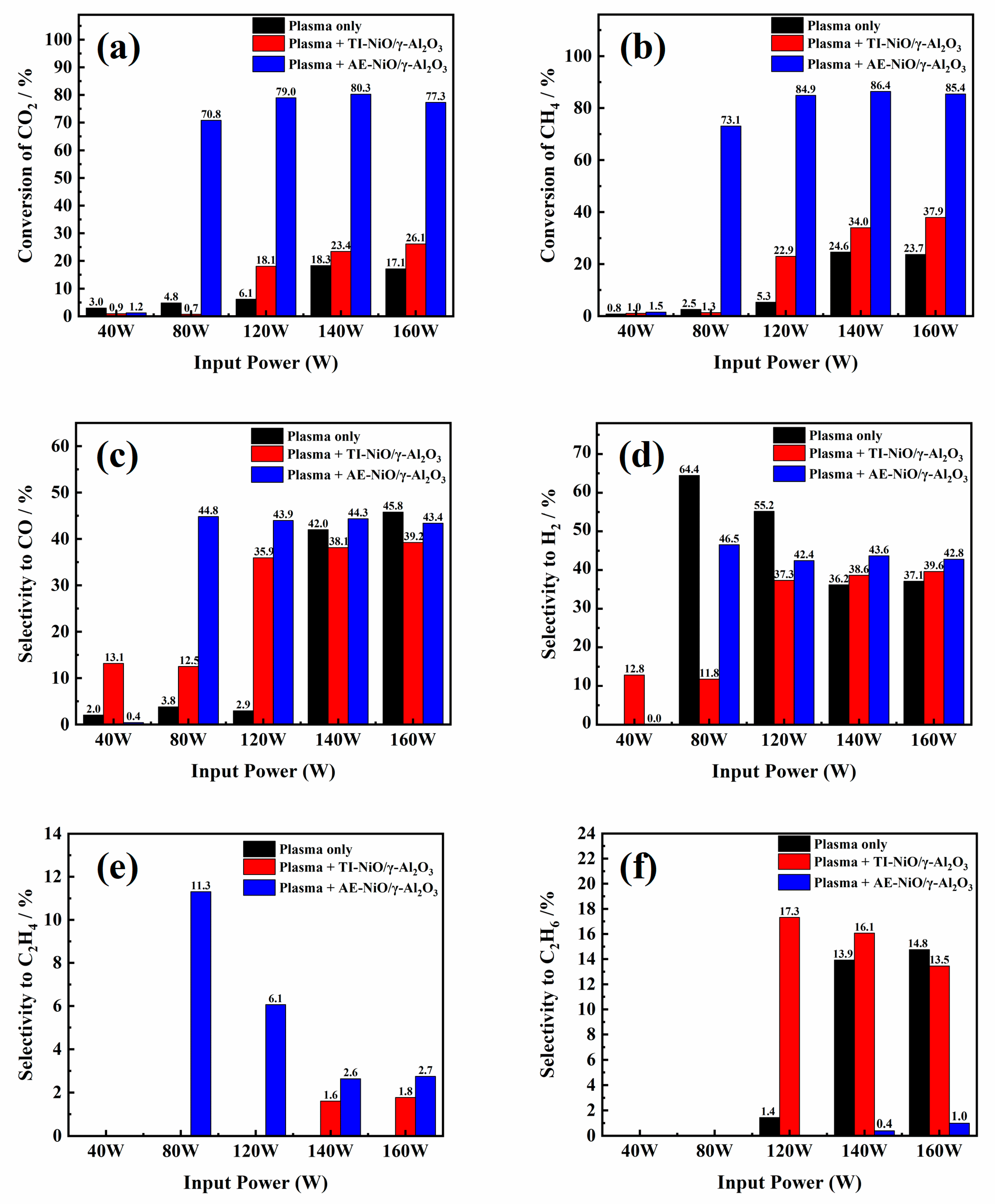
2.1. Effect of Preparation Methods on Catalyst Performance
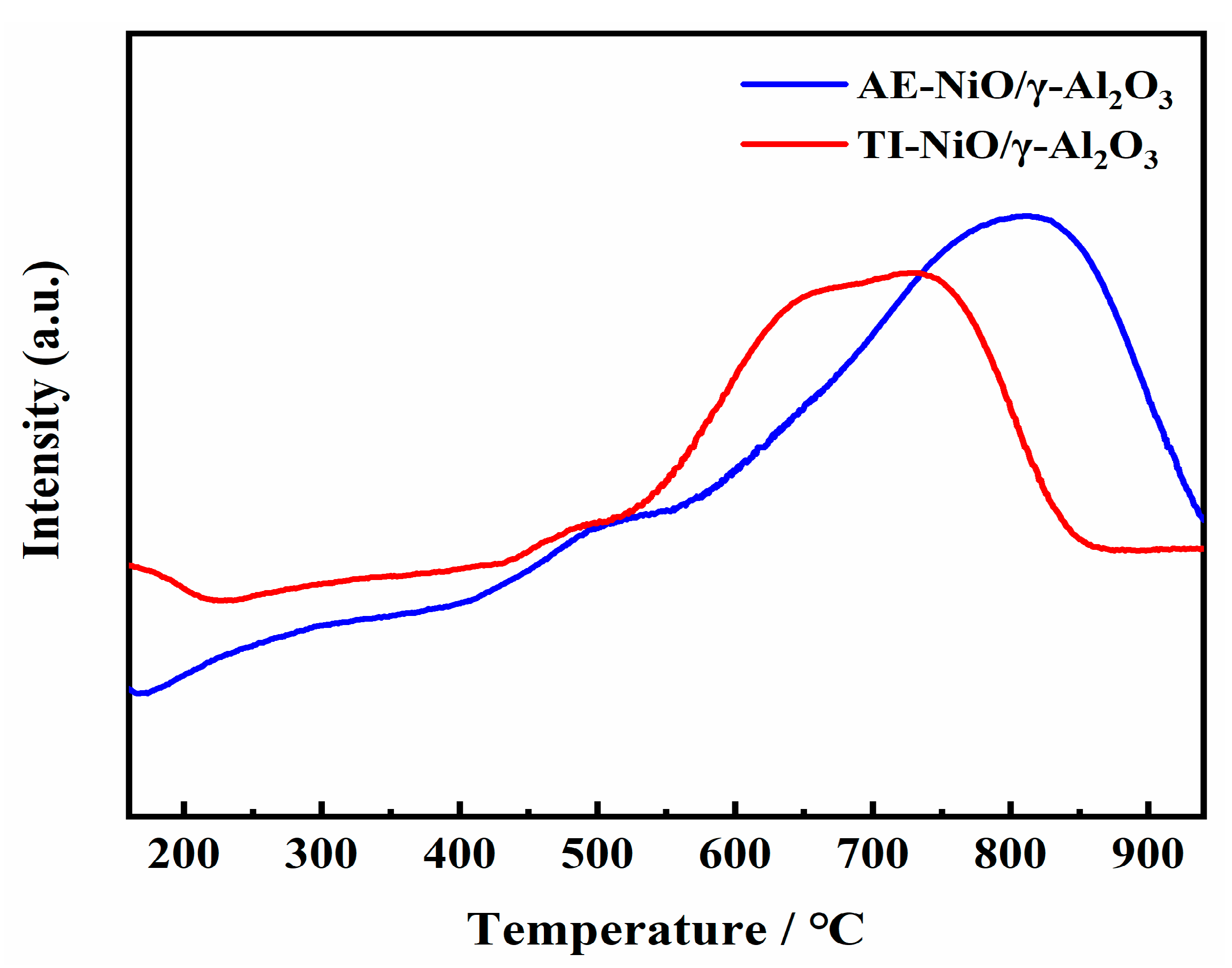
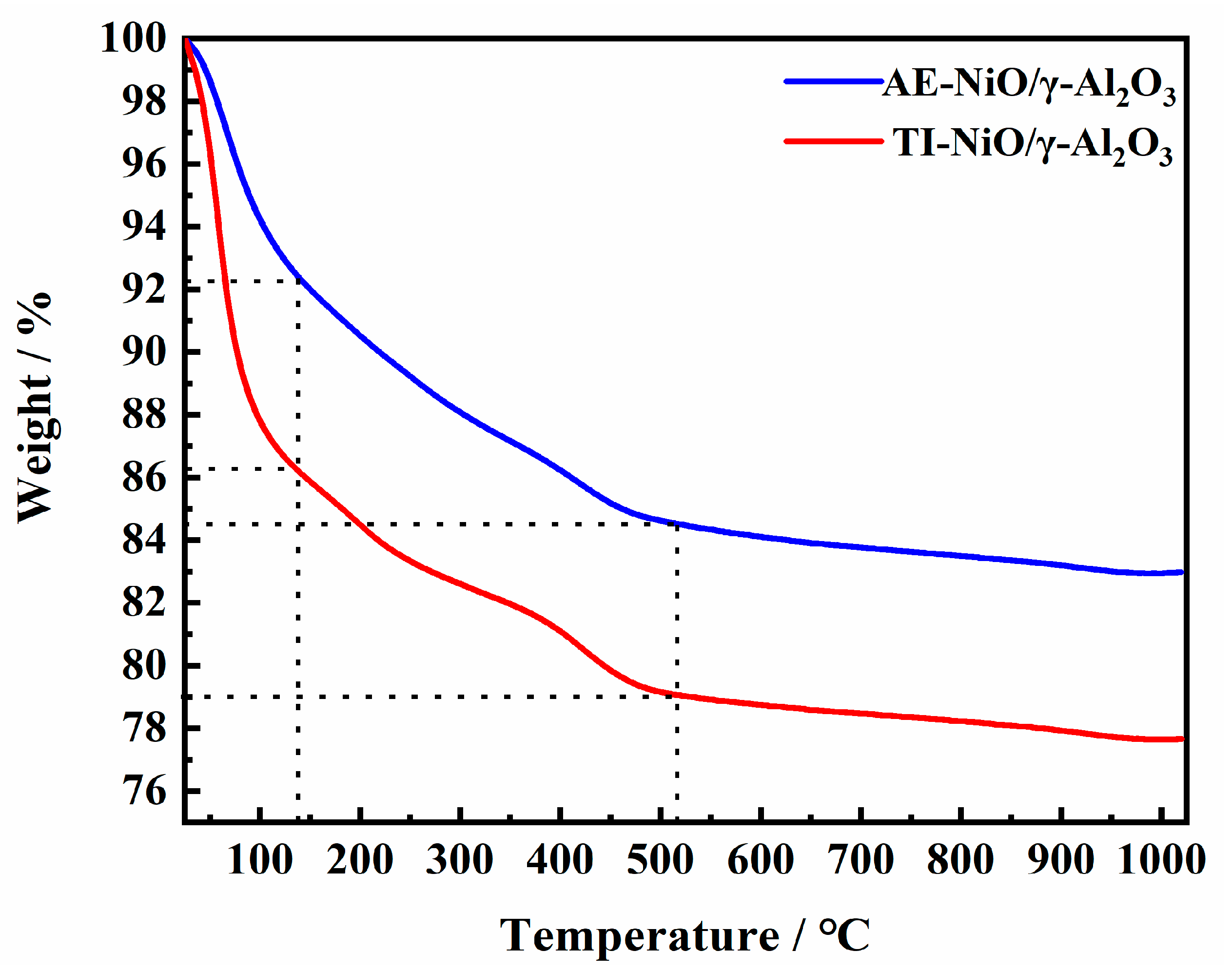
2.2. Catalyst Characterization
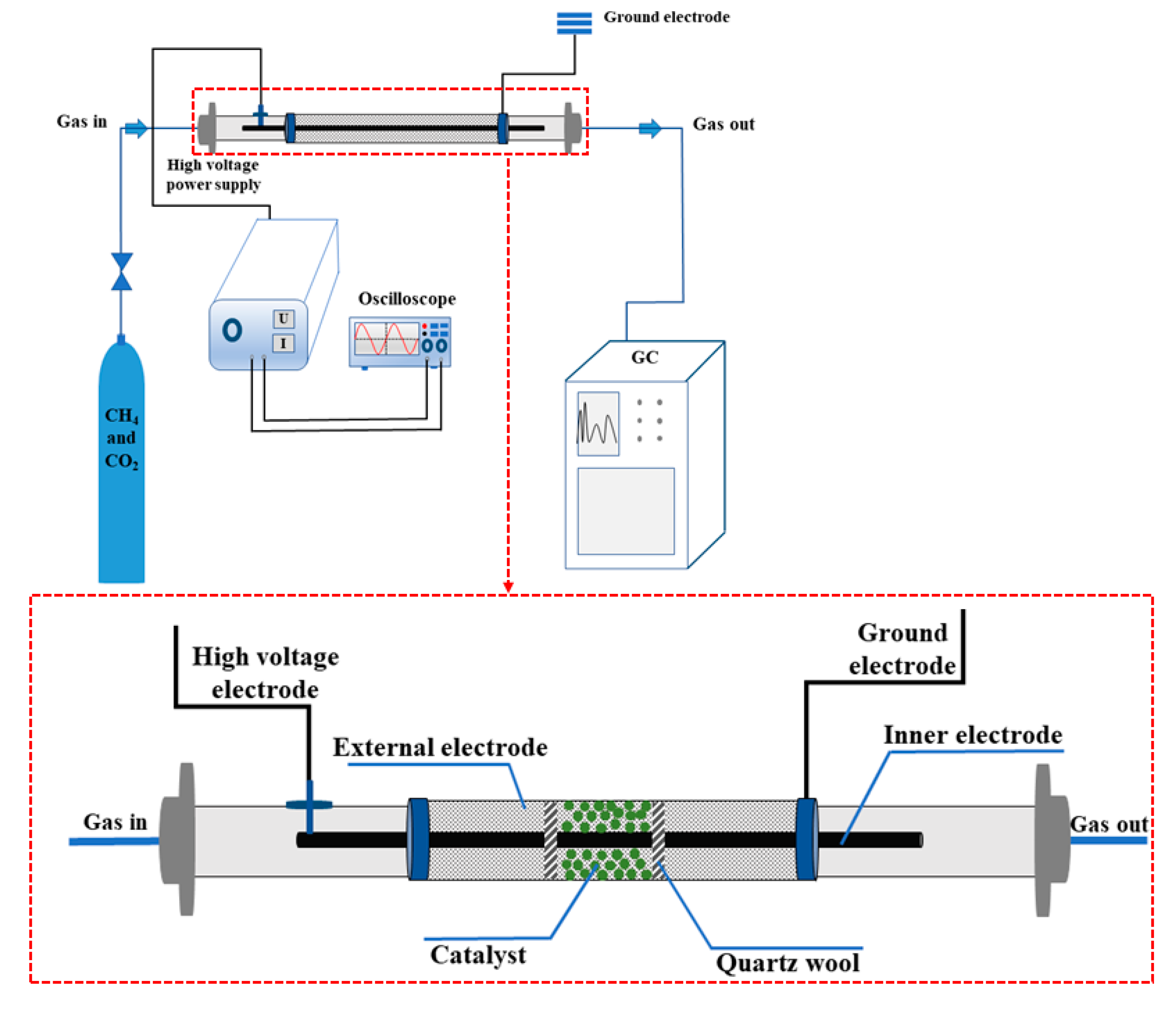
3. Experiment Section
3.1. Preparation of Catalysts
3.2. Characterization of Catalysts
3.3. Evaluation of Activity
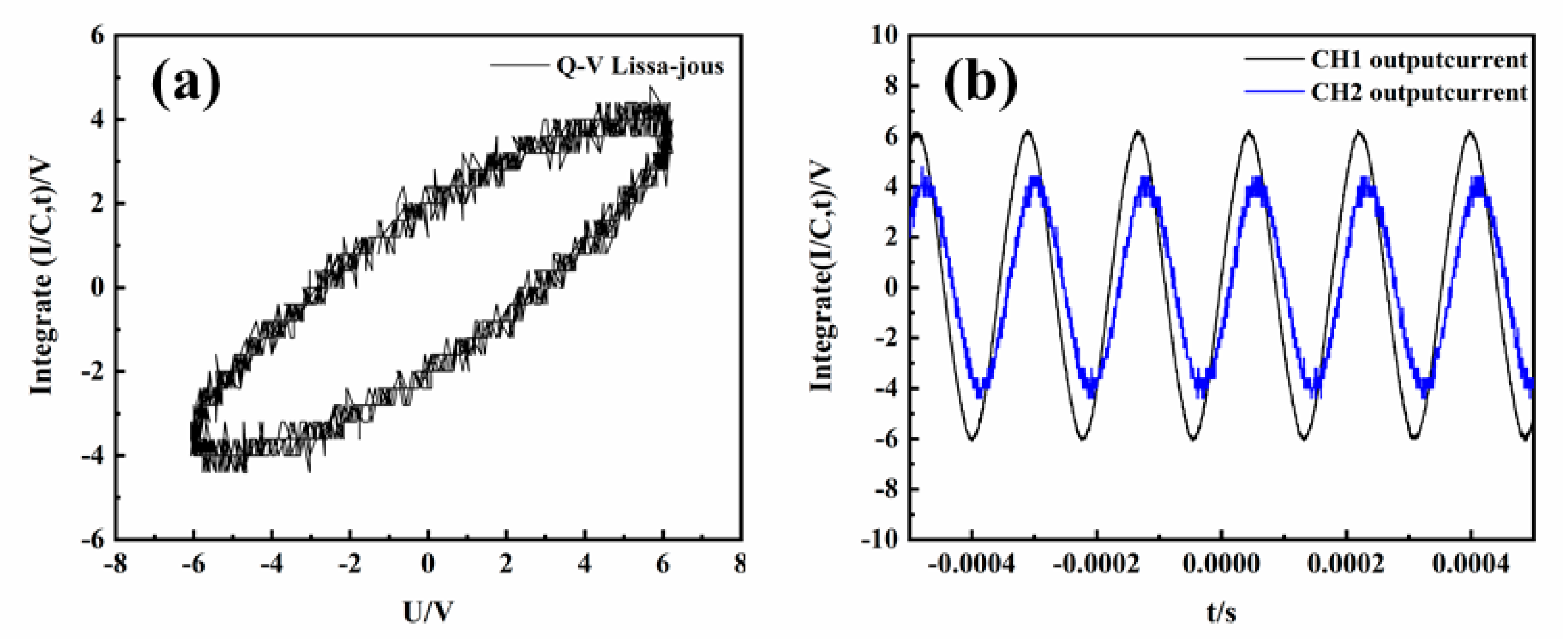
3.4. Calculation of Parameters
4. Conclusions
Reference:
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Lin, Z.; Li, T.; Huang, L.; Zhang, J.; Askari, S.; Dewangan, N.; Jangam, A.; Kawi, S. Recent Developments in Dielectric Barrier Discharge Plasma-Assisted Catalytic Dry Reforming of Methane over Ni-Based Catalysts. Catalysts 2021, 11, 455. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The future of energy supply: Challenges and opportunities. Angew. Chem. Int. Ed. Engl. 2007, 46, 52–66. [Google Scholar] [CrossRef]
- Karnauskas, K.B.; Miller, S.L.; Schapiro, A.C. Fossil Fuel Combustion Is Driving Indoor CO2 Toward Levels Harmful to Human Cognition. Geohealth 2020, 4, e2019GH000237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, R.; Ranjith, P.; Haque, A.; Choi, X. A review of studies on CO2 sequestration and caprock integrity. Fuel 2010, 89, 2651–2664. [Google Scholar] [CrossRef]
- Zhang, Z. Energy and environment issues in carbon capture, utilization and storage. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–4. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Chung, W.-C.; Chang, M.-B. Review of catalysis and plasma performance on dry reforming of CH4 and possible synergistic effects. Renew. Sustain. Energy Rev. 2016, 62, 13–31. [Google Scholar] [CrossRef]
- Delikonstantis, E.; Scapinello, M.; Stefanidis, G. Investigating the Plasma-Assisted and Thermal Catalytic Dry Methane Reforming for Syngas Production: Process Design, Simulation and Evaluation. Energies 2017, 10, 1429. [Google Scholar] [CrossRef] [Green Version]
- Dybkjaer, I.; Aasberg-Petersen, K. Synthesis gas technology large-scale applications. Can. J. Chem. Eng. 2016, 94, 607–612. [Google Scholar] [CrossRef]
- Owgi, A.H.K.; Jalil, A.A.; Hussain, I.; Hassan, N.S.; Hambali, H.U.; Siang, T.J.; Vo, D.V.N. Catalytic systems for enhanced carbon dioxide reforming of methane: A review. Environ. Chem. Lett. 2021, 19, 2157–2183. [Google Scholar] [CrossRef]
- Luisetto, I.; Sarno, C.; De Felicis, D.; Basoli, F.; Battocchio, C.; Tuti, S. Ni supported on γ-Al2O3 promoted by Ru for the dry reforming of methane in packed and monolithic reactors. Fuel Process. Technol. 2017, 158, 130–140. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serra, J.M. Exploring the Stability of Fe–Ni Alloy Nanoparticles Exsolved from Double-Layered Perovskites for Dry Reforming of Methane. Catalysts 2021, 11, 741. [Google Scholar] [CrossRef]
- Han, J.W.; Park, J.S.; Choi, M.S.; Lee, H. Uncoupling the size and support effects of Ni catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 203, 625–632. [Google Scholar] [CrossRef]
- Son, I.H.; Lee, S.J.; Song, I.Y.; Jeon, W.S.; Jung, I.; Yun, D.J.; Jeong, D.-W.; Shim, J.-O.; Jang, W.-J.; Roh, H.-S. Study on coke formation over Ni/γ-Al2O3, Co-Ni/γ-Al2O3, and Mg-Co-Ni/γ-Al2O3 catalysts for carbon dioxide reforming of methane. Fuel 2014, 136, 194–200. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Bogaerts, A.; Tu, X.; Whitehead, J.C.; Centi, G.; Lefferts, L.; Guaitella, O.; Azzolina-Jury, F.; Kim, H.-H.; Murphy, A.B.; Schneider, W.F.; et al. The 2020 plasma catalysis roadmap. J. Phys. D Appl. Phys. 2020, 53, 443001. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Recent developments in non-thermal catalytic DBD plasma reactor for dry reforming of methane. Energy Convers. Manag. 2019, 183, 529–560. [Google Scholar] [CrossRef]
- Ashford, B.; Tu, X. Non-thermal plasma technology for the conversion of CO2. Curr. Opin. Green Sustain. Chem. 2017, 3, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; Barboun, P.; Go, D.B.; Hicks, J.C.; Schneider, W.F. Catalysis Enabled by Plasma Activation of Strong Chemical Bonds: A Review. ACS Energy Lett. 2019, 4, 1115–1133. [Google Scholar] [CrossRef]
- Snoeckx, R.; Rabinovich, A.; Dobrynin, D.; Bogaerts, A.; Fridman, A. Plasma-based liquefaction of methane: The road from hydrogen production to direct methane liquefaction. Plasma Process. Polym. 2017, 14, e1600115. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.K.; Sunkara, M.K.; Bogaerts, A. Plasma Catalysis: Synergistic Effects at the Nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef]
- Tu, X.; Whitehead, J.C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: Understanding the synergistic effect at low temperature. Appl. Catal. B Environ. 2012, 125, 439–448. [Google Scholar] [CrossRef]
- Song, H.K.; Choi, J.-W.; Yue, S.H.; Lee, H.; Na, B.-K. Synthesis gas production via dielectric barrier discharge over Ni/γ-Al2O3 catalyst. Catal. Today 2004, 89, 27–33. [Google Scholar] [CrossRef]
- Mei, D.; Ashford, B.; He, Y.-L.; Tu, X. Plasma-catalytic reforming of biogas over supported Ni catalysts in a dielectric barrier discharge reactor: Effect of catalyst supports. Plasma Process. Polym. 2017, 14, e1600076. [Google Scholar] [CrossRef] [Green Version]
- Goujard, V.; Tatibouët, J.-M.; Batiot-Dupeyrat, C. Use of a non-thermal plasma for the production of synthesis gas from biogas. Appl. Catal. A Gen. 2009, 353, 228–235. [Google Scholar] [CrossRef]
- Wang, A.; Harrhy, J.H.; Meng, S.; He, P.; Liu, L.; Song, H. Nonthermal plasma-catalytic conversion of biogas to liquid chemicals with low coke formation. Energy Convers. Manag. 2019, 191, 93–101. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.; Wu, C.; Guo, H.; Tu, X. One-Step Reforming of CO2 and CH4 into High-Value Liquid Chemicals and Fuels at Room Temperature by Plasma-Driven Catalysis. Angew. Chem. Int. Ed. Engl. 2017, 56, 13679–13683. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Winter, L.R.; Chen, J.G. Review of Plasma-Assisted Catalysis for Selective Generation of Oxygenates from CO2 and CH4. ACS Catal. 2020, 10, 2855–2871. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, B.-H.; Jin, Y.; Cheng, Y. Dry Reforming of Methane in a Dielectric Barrier Discharge Reactor with Ni/Al2O3 Catalyst: Interaction of Catalyst and Plasma. Energy Fuels 2009, 23, 4196–4201. [Google Scholar] [CrossRef]
- Ray, D.; Nepak, D.; Janampelli, S.; Goshal, P.; Subrahmanyam, C. Dry Reforming of Methane in DBD Plasma over Ni-Based Catalysts: Influence of Process Conditions and Support on Performance and Durability. Energy Technol. 2019, 7, 1801008. [Google Scholar] [CrossRef]
- Ray, D.; Reddy, P.M.K.; Subrahmanyam, C. Ni-Mn/γ-Al2O3 assisted plasma dry reforming of methane. Catal. Today 2018, 309, 212–218. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, Z.; Jin, J.; Zhou, H.; Cohron, M.; Zhao, H.; Liu, H.; Pan, W. Synthesis Gas Production with an Adjustable H2/CO Ratio through the Coal Gasification Process: Effects of Coal Ranks and Methane Addition. Energy Fuels 2008, 22, 1720–1730. [Google Scholar] [CrossRef]
- Dieuzeide, M.L.; Iannibelli, V.; Jobbagy, M.; Amadeoa, N. Steam reforming of glycerol over Ni/Mg/γ-Al2O3 catalysts. Effect of calcination temperatures. Int. J. Hydrog. Energy 2012, 37, 14926–14930. [Google Scholar] [CrossRef]
- Peck, M.A.; Langell, M.A. Comparison of Nanoscaled and Bulk NiO Structural and Environmental Characteristics by XRD, XAFS, and XPS. Chem. Mater. 2012, 24, 4483–4490. [Google Scholar] [CrossRef]
- Xu, Z.; Zhen, M.; Bi, Y.; Zhen, K. Catalytic properties of Ni modified hexaaluminates LaNiyAl12−yO19−δ for CO2 reforming of methane to synthesis gas. Appl. Catal. A Gen. 2000, 198, 267–273. [Google Scholar] [CrossRef]
- Huang, X.; Sun, N.; Xue, G.; Wang, C.; Zhan, H.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. Effect of pore geometries on the catalytic properties of NiO–Al2O3 catalysts in CO2 reforming of methane. RSC Adv. 2015, 5, 21090–21098. [Google Scholar] [CrossRef]
- Olszewska, D. Application of XPS method in the research into Ni ion-modified montmorillonite as a SO2 sorbent. Fuel Process. Technol. 2012, 95, 90–95. [Google Scholar] [CrossRef]
- Snoeckx, R.; Aerts, R.; Tu, X.; Bogaerts, A. Plasma-Based Dry Reforming: A Computational Study Ranging from the Nanoseconds to Seconds Time Scale. J. Phys. Chem. C 2013, 117, 4957–4970. [Google Scholar] [CrossRef]




| Sample | a Ni Loading | b SBET (m2g−1) | c Average Pore Diameter (nm) | d Pore Volume (cm3g−1) |
|---|---|---|---|---|
| γ-Al2O3 | – | 234.1 | 7.6 | 0.56 |
| TI-Ni/γ-Al2O3 | 7.9% | 222.2 | 8.3 | 0.60 |
| AE-Ni/γ-Al2O3 | 6.8% | 219.4 | 8.3 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-S.; Li, P.-R.; Jiang, H.-B.; Diao, J.-F.; Xu, Z.-N.; Guo, G.-C. Plasma Promotes Dry Reforming Reaction of CH4 and CO2 at Room Temperature with Highly Dispersed NiO/γ-Al2O3 Catalyst. Catalysts 2021, 11, 1433. https://doi.org/10.3390/catal11121433
Lin S-S, Li P-R, Jiang H-B, Diao J-F, Xu Z-N, Guo G-C. Plasma Promotes Dry Reforming Reaction of CH4 and CO2 at Room Temperature with Highly Dispersed NiO/γ-Al2O3 Catalyst. Catalysts. 2021; 11(12):1433. https://doi.org/10.3390/catal11121433
Chicago/Turabian StyleLin, Shan-Shan, Peng-Rui Li, Hui-Bo Jiang, Jian-Feng Diao, Zhong-Ning Xu, and Guo-Cong Guo. 2021. "Plasma Promotes Dry Reforming Reaction of CH4 and CO2 at Room Temperature with Highly Dispersed NiO/γ-Al2O3 Catalyst" Catalysts 11, no. 12: 1433. https://doi.org/10.3390/catal11121433
APA StyleLin, S.-S., Li, P.-R., Jiang, H.-B., Diao, J.-F., Xu, Z.-N., & Guo, G.-C. (2021). Plasma Promotes Dry Reforming Reaction of CH4 and CO2 at Room Temperature with Highly Dispersed NiO/γ-Al2O3 Catalyst. Catalysts, 11(12), 1433. https://doi.org/10.3390/catal11121433