Halide-Doping Effect of Strontium Cobalt Oxide Electrocatalyst and the Induced Activity for Oxygen Evolution in an Alkaline Solution
Abstract
:1. Introduction
2. Results and Discussion
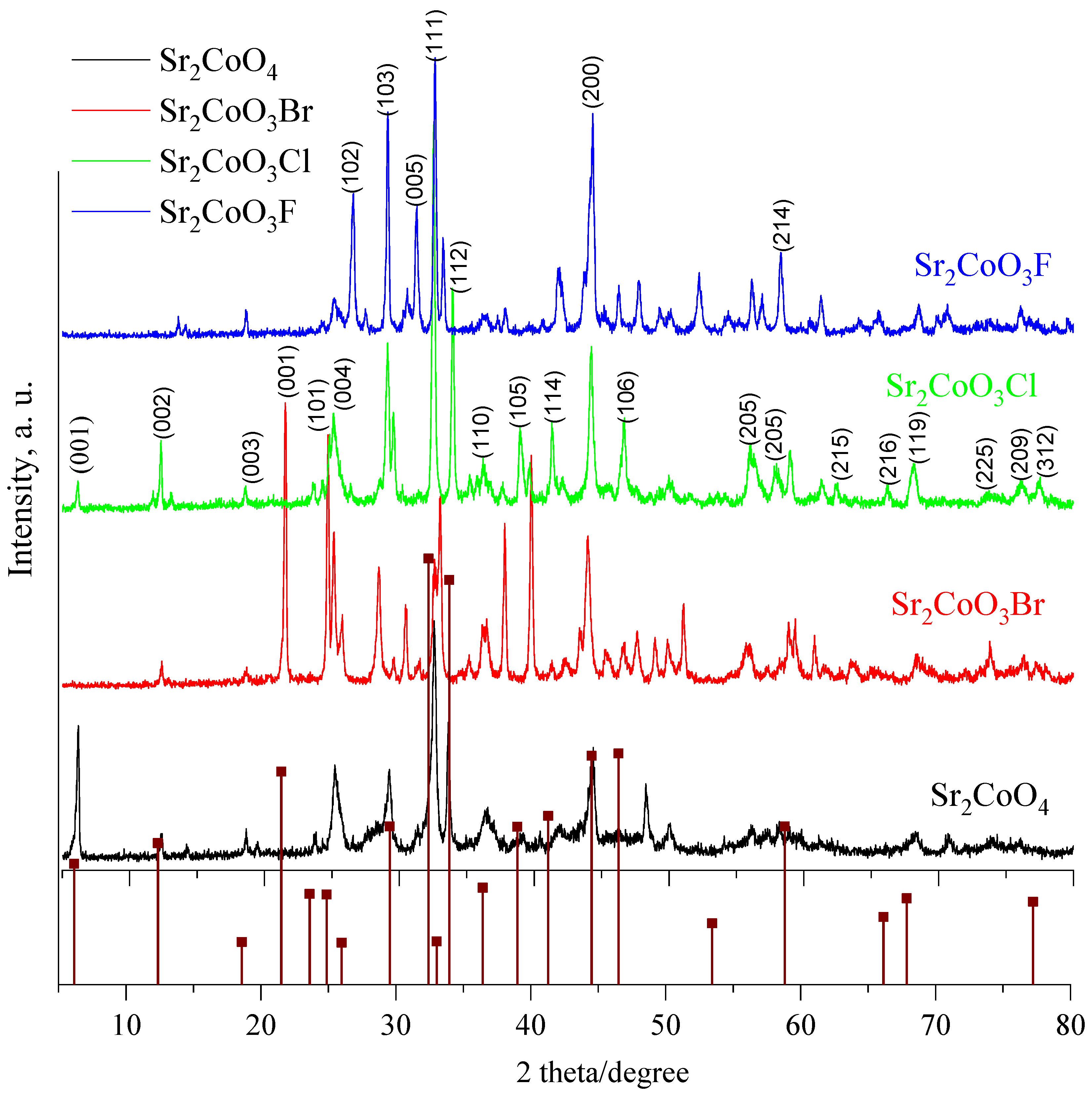
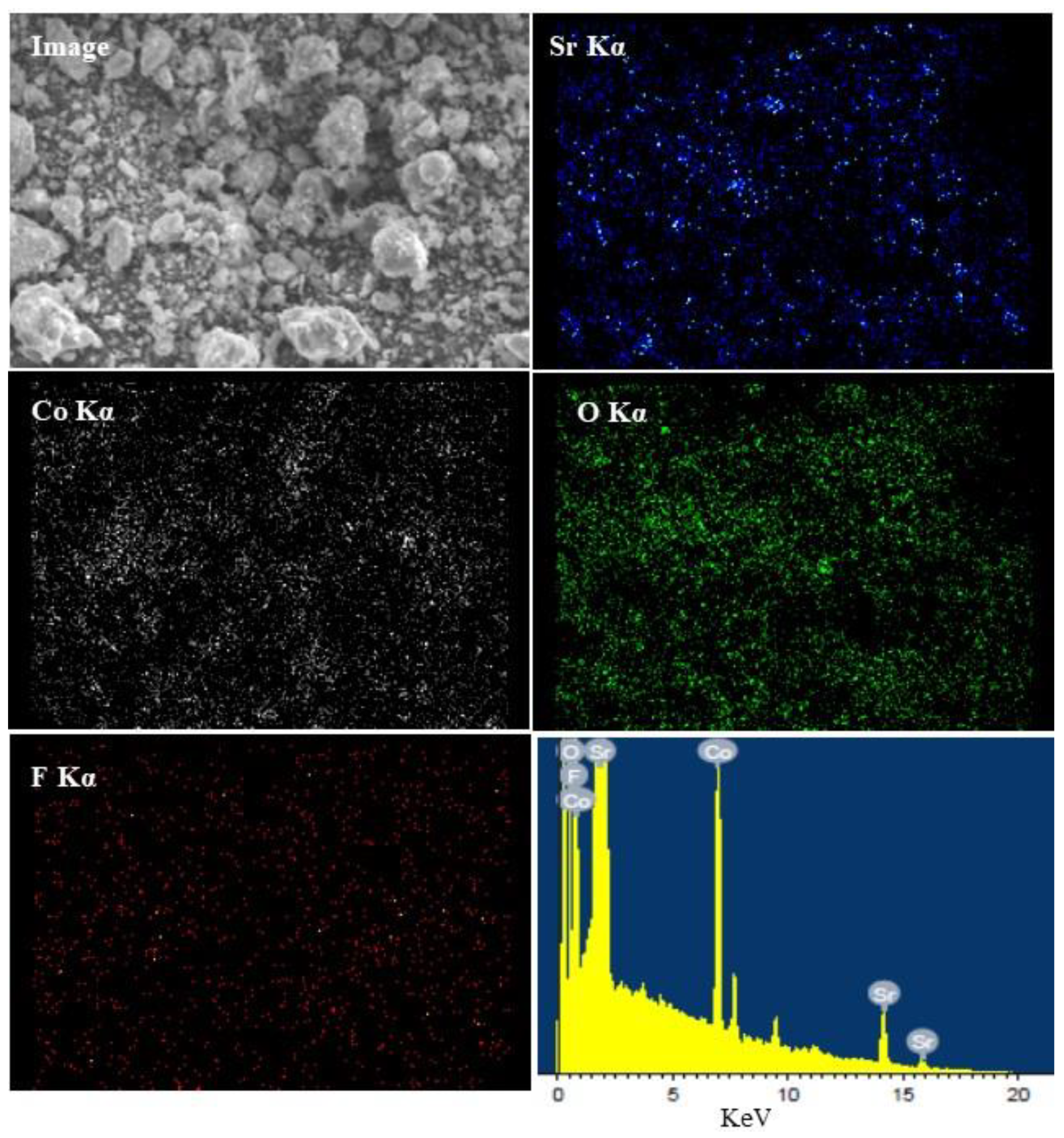
2.1. Structure and Morphology of the Sr2CoO3-xHx Catalysts
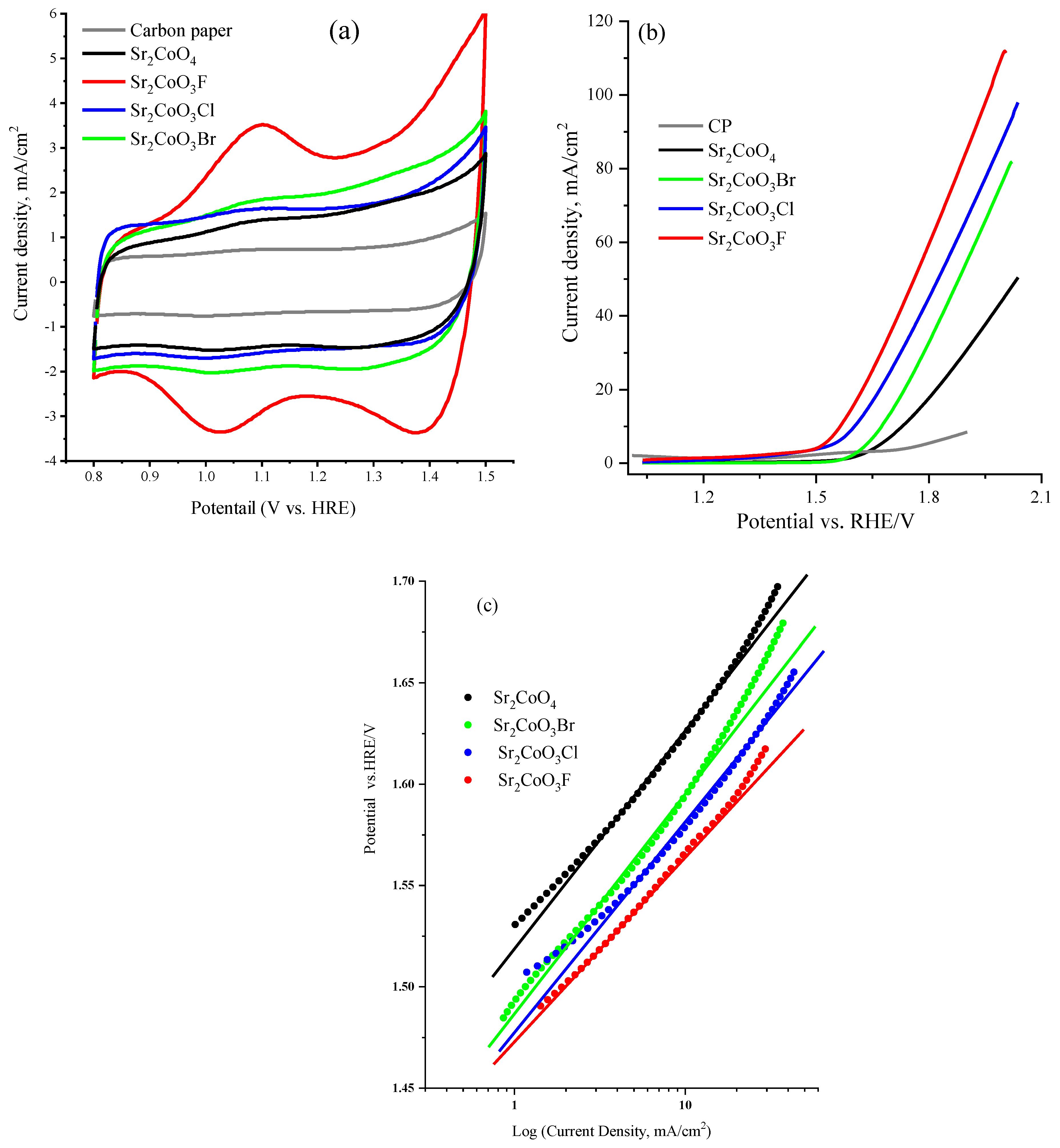
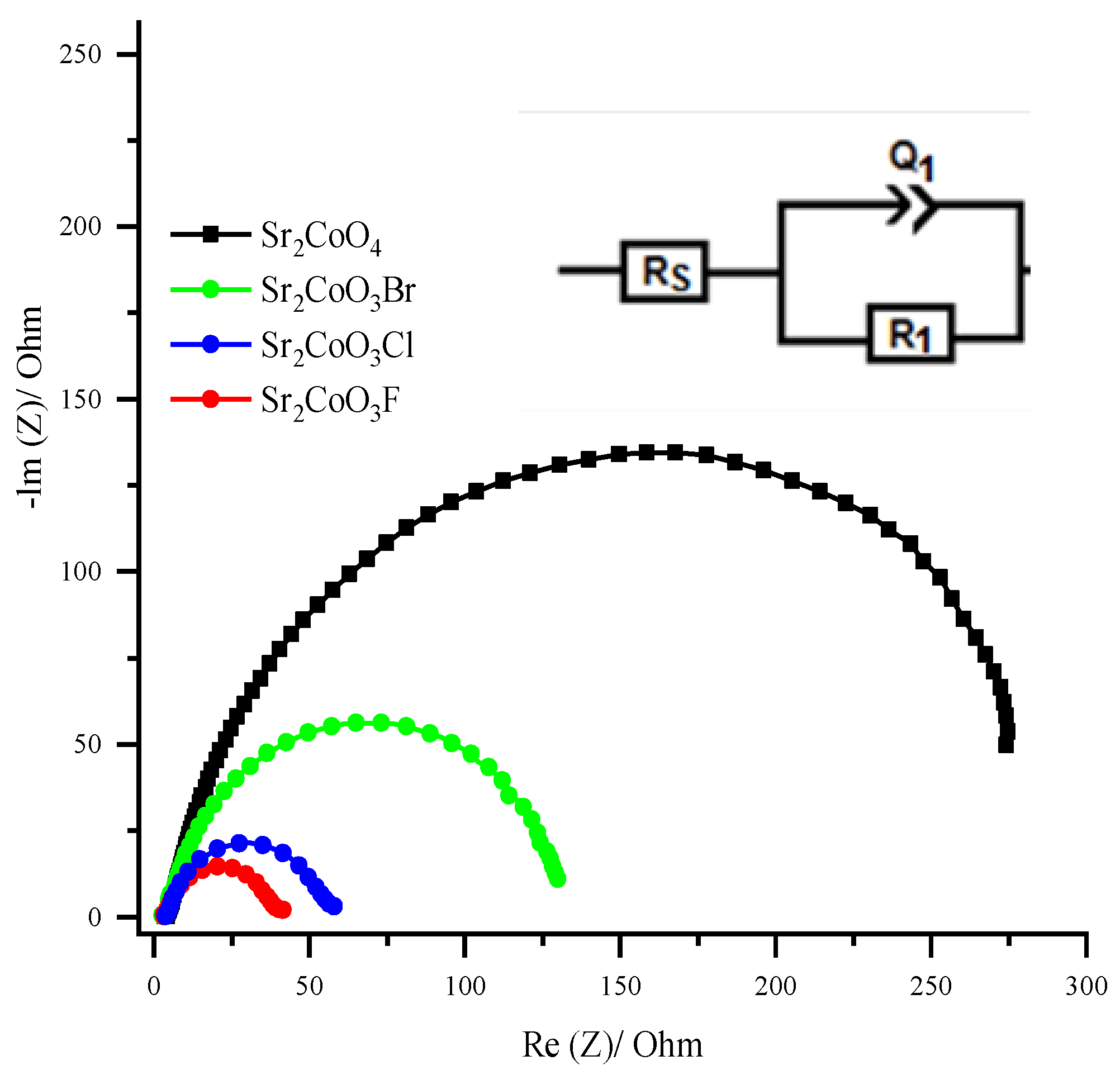
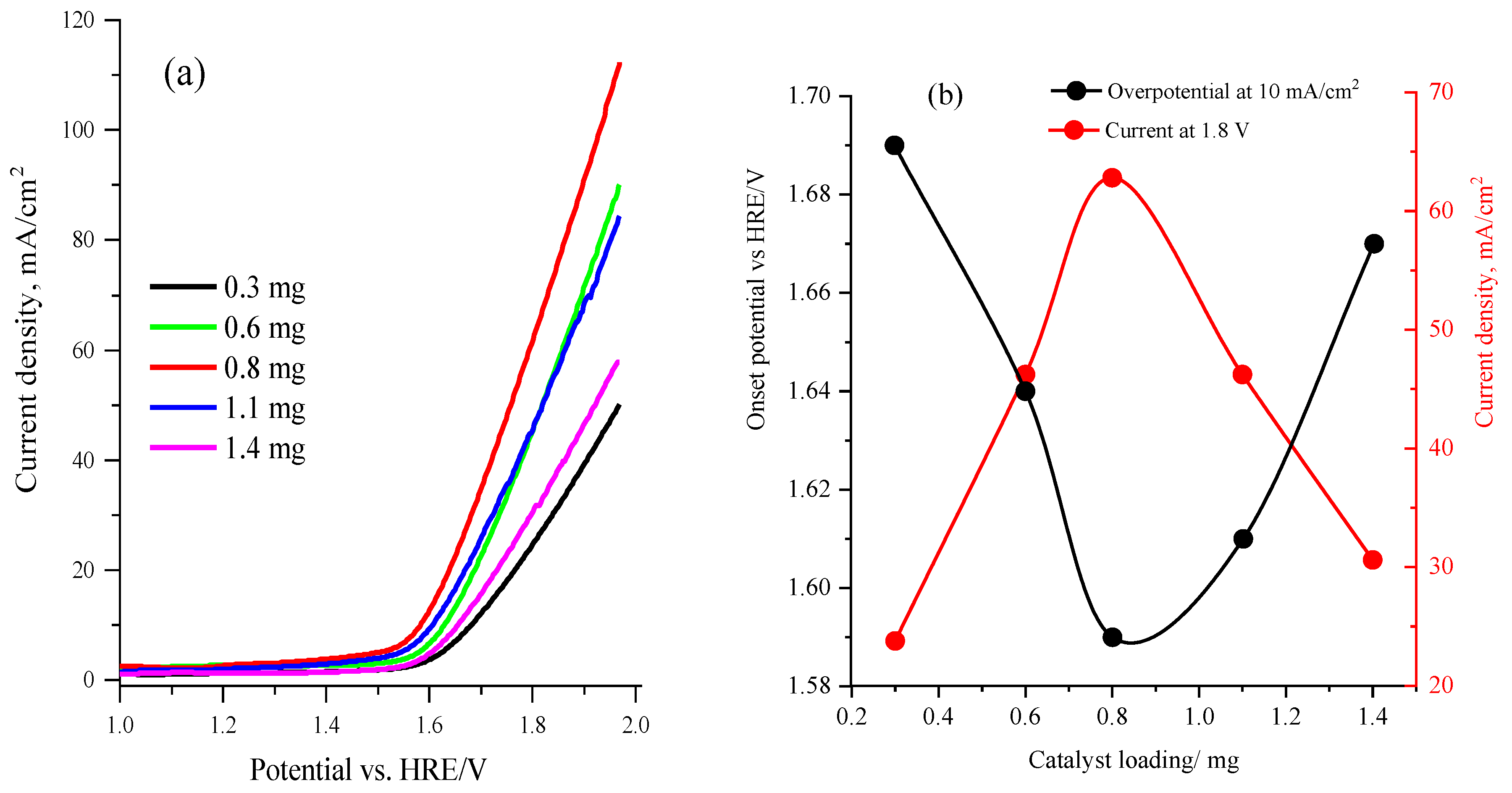
2.2. Electrocatalytic Activity of Cobalt Oxyhalide Catalysts in OER
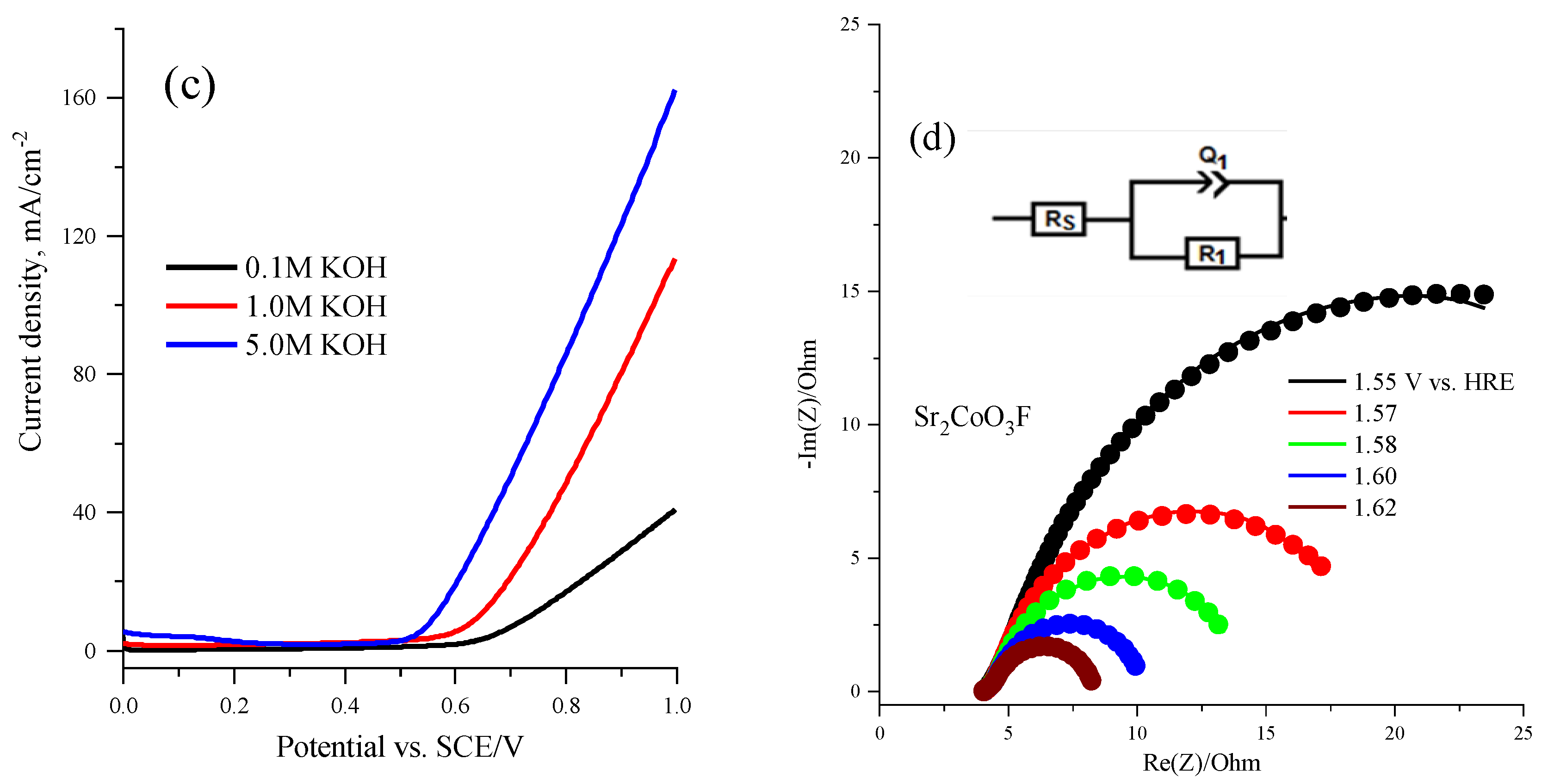
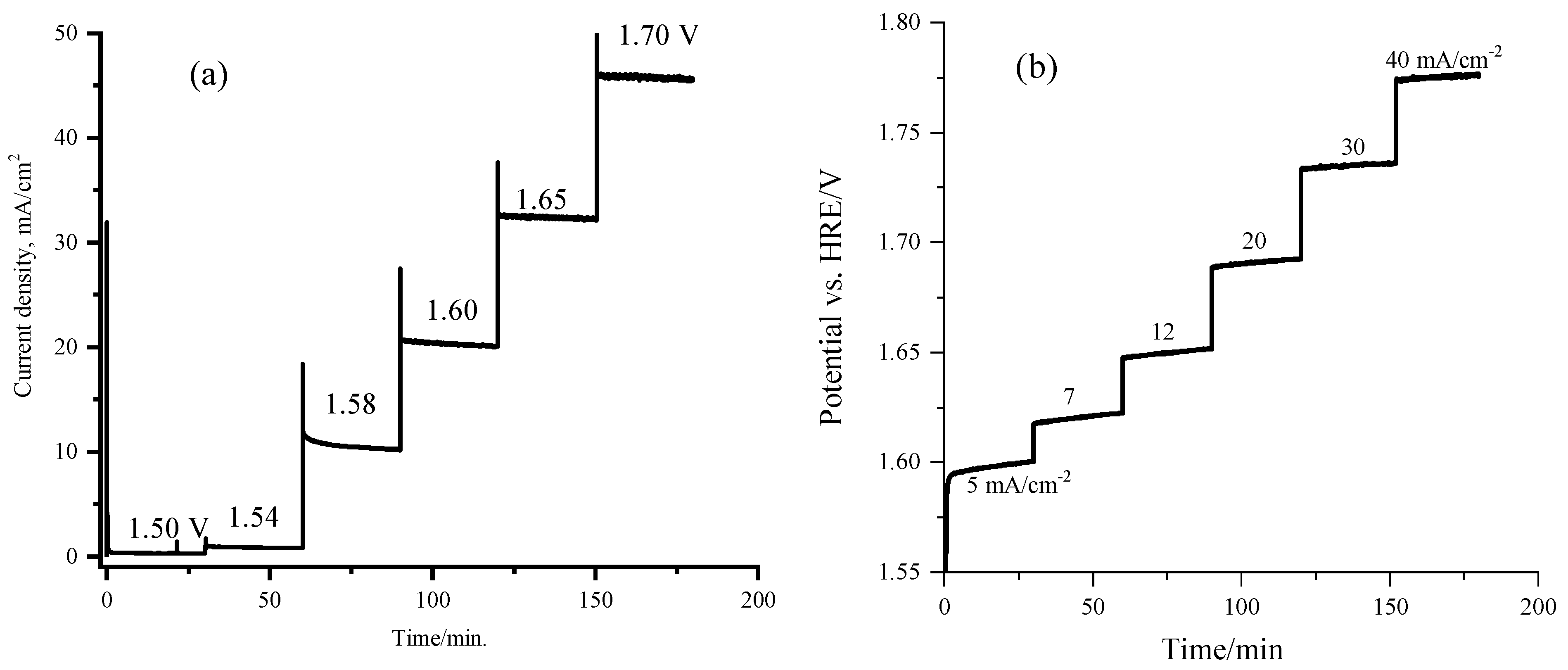
2.3. Electrochemical Performance of the Sr2CoO3F Catalyst
3. Materials and Methods
3.1. Catalyst Preparation Characterizations
3.2. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xia, W.; Mahmood, A.; Liang, Z.; Zou, R.; Guo, S. Earth-Abundant Nanomaterials for Oxygen Reduction. Angew. Chemie-Int. Ed. 2016, 55, 2650–2676. [Google Scholar] [CrossRef]
- Wang, Z.L.; Xu, D.; Xu, J.J.; Zhang, X.B. Oxygen Electrocatalysts in Metal-Air Batteries: From Aqueous to Nonaqueous Electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Burke, M.S.; Enman, L.J.; Batchellor, A.S.; Zou, S.; Boettcher, S.W. Oxygen Evolution Reaction Electrocatalysis on Transition Metal Oxides and (Oxy)Hydroxides: Activity Trends and Design Principles. Chem. Mater. 2015, 27, 7549–7558. [Google Scholar] [CrossRef]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.J.; Wang, Z.L. Electrocatalytic Oxygen Evolution Reaction for Energy Conversion and Storage: A Comprehensive Review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar Energy Supply and Storage for the Legacy and Nonlegacy Worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.F.; Wang, J.; Peng, Y.; Jung, C.Y.; Fisher, A.; Wang, X. Design of Efficient Bifunctional Oxygen Reduction/Evolution Electrocatalyst: Recent Advances and Perspectives. Adv. Energy Mater. 2017, 7, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Xia, B.Y.; Zhao, B.; Wang, X. A Review on Noble-Metal-Free Bifunctional Heterogeneous Catalysts for Overall Electrochemical Water Splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen-and Hydrogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Salim, J.; Li, S.; Sun, C.; Chen, L.; Goodenough, J.B.; Kim, Y. Perovskite Sr0.95Ce0.05CoO3-δ Loaded with Copper NanoParticles as a Bifunctional Catalyst for Lithium-Air Batteries. J. Mater. Chem. 2012, 22, 18902–18907. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design Principles for Oxygen-Reduction Activity on Perovskite Oxide Catalysts for Fuel Cells and Metal-Air Batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Misono, M. Advances in Designing Perovskite Catalysts. Curr. Opin. Solid State Mater. Sci. 2001, 5, 381–387. [Google Scholar] [CrossRef]
- Harris, J. Low-Cost Oxygen Electrode Material. Nature 1970, 226, 848–849. [Google Scholar] [CrossRef]
- Sunarso, J.; Torriero, A.A.J.; Zhou, W.; Howlett, P.C.; Forsyth, M. Oxygen Reduction Reaction Activity of La-Based Perovskite Oxides in Alkaline Medium: A Thin-Film Rotating Ring-Disk Electrode Study. J. Phys. Chem. C 2012, 116, 5827–5834. [Google Scholar] [CrossRef]
- Hyodo, T.; Hayashi, M.; Miura, N.; Yamazoe, N. Catalytic Activities of Rare-Earth Manganites for Cathodic Reduction of Oxygen in Alkaline Solution. J. Electrochem. Soc. 1996, 143, L266–L267. [Google Scholar] [CrossRef]
- Poux, T.; Napolskiy, F.S.; Dintzer, T.; Kéranguéven, G.; Istomin, S.Y.; Tsirlina, G.A.; Antipov, E.V.; Savinova, E.R. Dual Role of Carbon in the Catalytic Layers of Perovskite/Carbon Composites for the Electrocatalytic Oxygen Reduction Reaction. Catal. Today 2012, 189, 83–92. [Google Scholar] [CrossRef]
- Malkhandi, S.; Trinh, P.; Manohar, A.K.; Manivannan, A.; Balasubramanian, M.; Prakash, G.K.S.; Narayanan, S.R. Design Insights for Tuning the Electrocatalytic Activity of Perovskite Oxides for the Oxygen Evolution Reaction. J. Phys. Chem. C 2015, 119, 8004–8013. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, H.W.; Park, M.G.; Ismayilov, V.; Chen, Z. Synergistic Bifunctional Catalyst Design Based on Perovskite Oxide Nanoparticles and Intertwined Carbon Nanotubes for Rechargeable Zinc-Air Battery Applications. ACS Appl. Mater. Interfaces 2015, 7, 902–910. [Google Scholar] [CrossRef]
- Hardin, W.G.; Slanac, D.A.; Wang, X.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Highly Active, Nonprecious Metal Perovskite Electrocatalysts for Bifunctional Metal-Air Battery Electrodes. J. Phys. Chem. Lett. 2013, 4, 1254–1259. [Google Scholar] [CrossRef]
- Petrie, J.R.; Cooper, V.R.; Freeland, J.W.; Meyer, T.L.; Zhang, Z.; Lutterman, D.A.; Lee, H.N. Enhanced Bifunctional Oxygen Catalysis in Strained LaNiO3 Perovskites. J. Am. Chem. Soc. 2016, 138, 2488–2491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.; Bjørgum, E.; Mihai, O.; Yang, J.; Lein, H.L.; Grande, T.; Raaen, S.; Zhu, Y.-A.; Holmen, A.; Chen, D. Effects of Oxygen Mobility in La–Fe-Based Perovskites on the Catalytic Activity and Selectivity of Methane Oxidation. ACS Catal. 2020, 10, 3707–3719. [Google Scholar] [CrossRef]
- Yang, W.; Hong, T.; Li, S.; Ma, Z.; Sun, C.; Xia, C.; Chen, L. Perovskite Sr1-XCexCoO3-δ (0.05 ≤ x ≤ 0.15) as Superior Cathodes for Intermediate Temperature Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2013, 5, 1143–1148. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Z.; He, B.; Wang, S.; Wu, X.; Xia, C. Effect of Co Doping on the Electrochemical Properties of Sr2Fe1.5Mo0.5O6 Electrode for Solid Oxide Fuel Cell. Int. J. Hydrog. Energy 2013, 38, 4108–4115. [Google Scholar] [CrossRef]
- Dai, N.; Feng, J.; Wang, Z.; Jiang, T.; Sun, W.; Qiao, J.; Sun, K. Synthesis and Characterization of B-Site Ni-Doped Perovskites Sr2Fe1.5-XNixMo0.5O6-δ (x = 0, 0.05, 0.1, 0.2, 0.4) as Cathodes for SOFCs. J. Mater. Chem. A 2013, 1, 14147–14153. [Google Scholar] [CrossRef]
- Dai, H.X.; Ng, C.F.; Au, C.T. Perovskite-Type Halo-Oxide La1-XSrxFeO3-ΔXσ (X=F, Cl) Catalysts Selective for the Oxidation of Ethane to Ethene. J. Catal. 2000, 189, 52–62. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Liu, T.; Chen, F.; Xia, C. Improving the Chemical Stability of BaCe0.8Sm0.2O3-δ Electrolyte by Cl Doping for Proton-Conducting Solid Oxide Fuel Cell. Electrochem. Commun. 2013, 28, 87–90. [Google Scholar] [CrossRef]
- Yajima, T.; Takeiri, F.; Aidzu, K.; Akamatsu, H.; Fujita, K.; Yoshimune, W.; Ohkura, M.; Lei, S.; Gopalan, V.; Tanaka, K.; et al. A Labile Hydride Strategy for the Synthesis of Heavily Nitridized BaTiO. Nat. Chem. 2015, 7, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Wan, Y.; Xie, Y.; Zhu, J.; Pan, H.; Zheng, X.; Xia, C. Perovskite Oxyfluoride Electrode Enabling Direct Electrolyzing Carbon Dioxide with Excellent Electrochemical Performances. Adv. Energy Mater. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Xu, X.; Marcel Veder, J.P.; Shao, Z. Recent Advances in Anion-Doped Metal Oxides for Catalytic Applications. J. Mater. Chem. A 2019, 7, 7280–7300. [Google Scholar] [CrossRef]
- Li, F.F.; Liu, D.R.; Gao, G.M.; Xue, B.; Jiang, Y.S. Improved Visible-Light Photocatalytic Activity of NaTaO3 with Perovskite-like Structure via Sulfur Anion Doping. Appl. Catal. B Environ. 2015, 166–167, 104–111. [Google Scholar] [CrossRef]
- Ruddlesden, S.N.; Popper, P. New Compounds of the K 2 NIF 4 Type. Acta Crystallogr. 1957, 10, 538–539. [Google Scholar] [CrossRef]
- Popper, S.R.P. Mixed Bismuth Oxides with Layer Lattices I. The Structure Type of CaNb2Bi2O. Acta Crystallogr. 1957, 10, 538. [Google Scholar]
- Uma, S.; Raju, A.R.; Gopalakrishnan, J. Bridging the Ruddiesden-Popper and the Dion-Jacobson Series of Layered Perovskites: Synthesis of Layered Oxides, A2-XLa2Ti3-XNbxO10 (A = K, Rb), Exhibiting Ion Exchange. J. Mater. Chem. 1993, 3, 709–713. [Google Scholar] [CrossRef]
- Francis S., G. Structure, Properties and Preparation of Perovskite-Type Compounds; International Series of Monographs in Solid State Physics; Pergamon Press Ltd.: London, UK, 1969. [Google Scholar]
- Takeguchi, T.; Yamanaka, T.; Takahashi, H.; Watanabe, H.; Kuroki, T.; Nakanishi, H.; Orikasa, Y.; Uchimoto, Y.; Takano, H.; Ohguri, N.; et al. Layered Perovskite Oxide: A Reversible Air Electrode for Oxygen Evolution/Reduction in Rechargeable Metal-Air Batteries. J. Am. Chem. Soc. 2013, 135, 11125–11130. [Google Scholar] [CrossRef] [PubMed]
- Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.L.; Risch, M.; Hong, W.T.; Zhou, J.; Shao-Horn, Y. Double Perovskites as a Family of Highly Active Catalysts for Oxygen Evolution in Alkaline Solution. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.A.; Arunachalam, P.; Almayouf, A.; Weller, M.T. Efficient Bi-Functional Electrocatalysts of Strontium Iron Oxy-Halides for Oxygen Evolution and Reduction Reactions in Alkaline Media. J. Electrochem. Soc. 2016, 163, H450–H458. [Google Scholar] [CrossRef]
- Miyahara, Y.; Miyazaki, K.; Fukutsuka, T.; Abe, T. Strontium Cobalt Oxychlorides: Enhanced Electrocatalysts for Oxygen Reduction and Evolution Reactions. Chem. Commun. 2017, 53, 2713–2716. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Sathish, C.I.; Matsushita, Y.; Yamaura, K.; Uchikoshi, T. New Members of Layered Oxychloride Perovskites with Square Planar Coordination: Sr2MO2Cl2 (M = Mn, Ni) and Ba2PdO2Cl. Chem. Commun. 2014, 50, 5915–5918. [Google Scholar] [CrossRef] [PubMed]
- Knee, C.; Price, D.; Lees, M.; Weller, M. Two-And Three-Dimensional Magnetic Order in the Layered Cobalt Oxychloride Sr2CoO3Cl. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 68, 1–8. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Yamaura, K.; Takayama-Muromachi, E. Oxyfluoride Chemistry of Layered Perovskite Compounds. Appl. Sci. 2012, 2, 206–219. [Google Scholar] [CrossRef]
- Wang, X.L.; Takayama-Muromachi, E. Magnetic and Transport Properties of the Layered Perovskite System Sr2-YYyCoO4 (0≤y≤1). Phys. Rev. B Condens. Matter Mater. Phys. 2005, 72, 1–7. [Google Scholar]
- Hector, A.L.; Knee, C.S.; MacDonald, A.I.; Price, D.J.; Weller, M.T. An unusual magnetic structure in Sr2FeO3F and magnetic structures of K2NiF4-type iron(III) oxides and oxide halides, including the cobalt substituted series Sr2Fe1−xCoxO3Cl. J. Mater. Chem. 2005, 15, 3093–3103. [Google Scholar] [CrossRef]
- Knee, C.S.; Price, D.J.; Lees, M.R.; Weller, M.T. Two- and three-dimensional magnetic order in the layered cobalt oxychloride Sr2CoO3Cl. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 68, 174407–174415. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Manna, L. What Defines a Halide Perovskite? ACS Energy Lett. 2020, 5, 604–610. [Google Scholar] [CrossRef] [Green Version]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319. [Google Scholar] [CrossRef]
- Lemoine, K.; Lhoste, J.; Hémon-Ribaud, A.; Heidary, N.; Maisonneuve, V.; Guiet, A.; Kornienko, N. Investigation of mixed-Metal (oxy)fluorides as a new class of water oxidation electrocatalysts. Chem. Sci. 2019, 10, 9209–9218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, W.; Xu, C.; Ren, R.; Yang, X.; Qiao, J.; Wang, Z.; Sun, K. Attenuating a metal–Oxygen bond of a double perovskite oxide via anion doping to enhance its catalytic activity for the oxygen reduction reaction, J. Mater. Chem. A 2020, 8, 14091–14098. [Google Scholar] [CrossRef]


| Catalyst | Sr wt. % ± 2.0% | Co wt. % ± 2.0% | O wt. % ± 2.0% | H wt. % ± 2.0% |
|---|---|---|---|---|
| Sr2CoO4 | 59.06 | 19.64 | 21.30 | -- |
| Sr2CoO3F | 58.18 | 19.56 | 15.93 | 6.33 |
| Sr2CoO3Cl | 55.15 | 18.55 | 15.13 | 11.17 |
| Sr2CoO3Br | 49.63 | 16.70 | 13.60 | 20.07 |
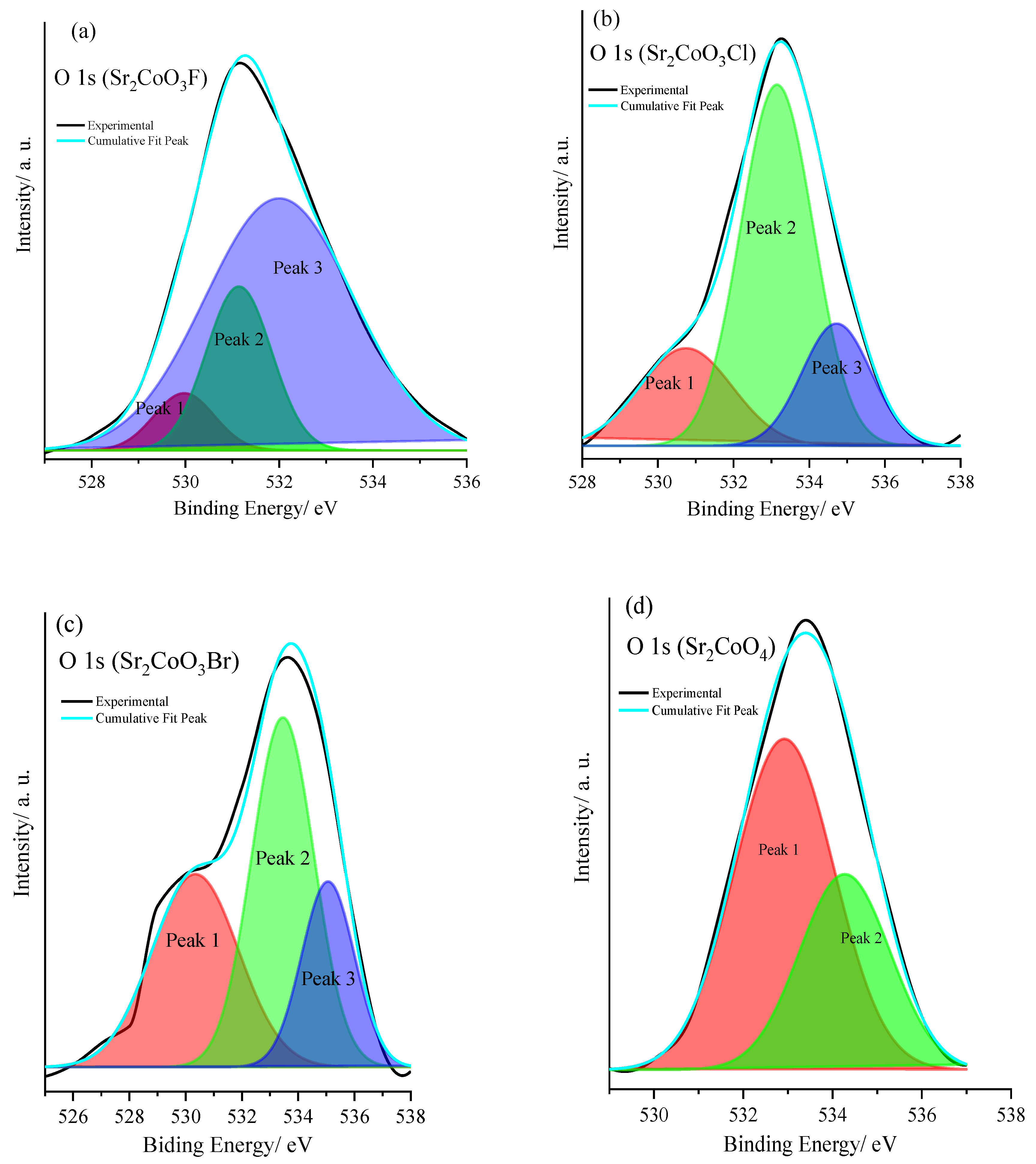
| Catalyst | Peak BE (O2−)/eV | Peak 1 Area % | Peak 2 (O1−)/eV | Peak Area % | Peak 3 (Ochem)/eV | Peak 3 Area % | [O1−/ O2−] | [O1− + Ochem/O2−] |
|---|---|---|---|---|---|---|---|---|
| Sr2CoO4 | 532.90 | 61.4 | 534.2 | 38.6 | -- | -- | 0.63 | 0.63 |
| Sr2CoO3Br | 530.34 | 34.3 | 533.45 | 43.9 | 535.06 | 21.8 | 1.28 | 1.91 |
| Sr2CoO3Cl | 530.74 | 20.7 | 533.14 | 60.1 | 534.72 | 19.2 | 2.90 | 3.83 |
| Sr2CoO3F | 529.96 | 6.7 | 531.14 | 20.8 | 532.01 | 72.5 | 3.10 | 13.20 |
| Catalyst | Onset Potential ± 0.005 vs. RHE/ V | Onset Potential ± 0.005 vs. RHE/ V (RDE = 1600 rpm) | Potential at 10 mA/cm2 | Potential at 10 mA/cm2 (RDE = 1600 rpm) | Tafel slope, mV/dec (at 1.0 mV s−1 Using GCE) |
|---|---|---|---|---|---|
| Sr2CoO3F | 1.50 | 1.45 | 1.56 | 1.56 | 88 |
| Sr2CoO3Cl | 1.53 | 1.49 | 1.60 | 1.57 | 98 |
| Sr2CoO3Br | 1.61 | 1.51 | 1.65 | 1.60 | 103 |
| Sr2CoO4 | 1.64 | 1.55 | 1.73 | 1.65 | 110 |
| Catalyst | Rs (ohm) | Q1 (mF) | R1 (ohm) |
|---|---|---|---|
| Sr2CoO4 | 4.18 | 21.680 | 296.1 |
| Sr2CoO3Br | 4.37 | 21.890 | 128.5 |
| Sr2CoO3Cl | 4.17 | 22.310 | 52.9 |
| Sr2CoO3F | 4.23 | 38.300 | 36.8 |
| Potential (V) vs. HRE | Rs (ohm) | Q1 (mF) | R1 (ohm) |
|---|---|---|---|
| 1.55 | 4.23 | 38.300 | 36.8 |
| 1.57 | 4.22 | 38.600 | 15.9 |
| 1.58 | 4.13 | 41.750 | 10.40 |
| 1.60 | 4.19 | 41.760 | 6.30 |
| 1.62 | 4.16 | 41.510 | 4.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanem, M.A.; Amer, M.S.; Al-Mayouf, A.M.; Arunachalam, P.; Weller, M.T. Halide-Doping Effect of Strontium Cobalt Oxide Electrocatalyst and the Induced Activity for Oxygen Evolution in an Alkaline Solution. Catalysts 2021, 11, 1408. https://doi.org/10.3390/catal11111408
Ghanem MA, Amer MS, Al-Mayouf AM, Arunachalam P, Weller MT. Halide-Doping Effect of Strontium Cobalt Oxide Electrocatalyst and the Induced Activity for Oxygen Evolution in an Alkaline Solution. Catalysts. 2021; 11(11):1408. https://doi.org/10.3390/catal11111408
Chicago/Turabian StyleGhanem, Mohamed A., Mabrook S. Amer, Abdullah M. Al-Mayouf, Prabhakarn Arunachalam, and Mark T. Weller. 2021. "Halide-Doping Effect of Strontium Cobalt Oxide Electrocatalyst and the Induced Activity for Oxygen Evolution in an Alkaline Solution" Catalysts 11, no. 11: 1408. https://doi.org/10.3390/catal11111408
APA StyleGhanem, M. A., Amer, M. S., Al-Mayouf, A. M., Arunachalam, P., & Weller, M. T. (2021). Halide-Doping Effect of Strontium Cobalt Oxide Electrocatalyst and the Induced Activity for Oxygen Evolution in an Alkaline Solution. Catalysts, 11(11), 1408. https://doi.org/10.3390/catal11111408










