Recent Advances in the Brønsted/Lewis Acid Catalyzed Conversion of Glucose to HMF and Lactic Acid: Pathways toward Bio-Based Plastics
Abstract
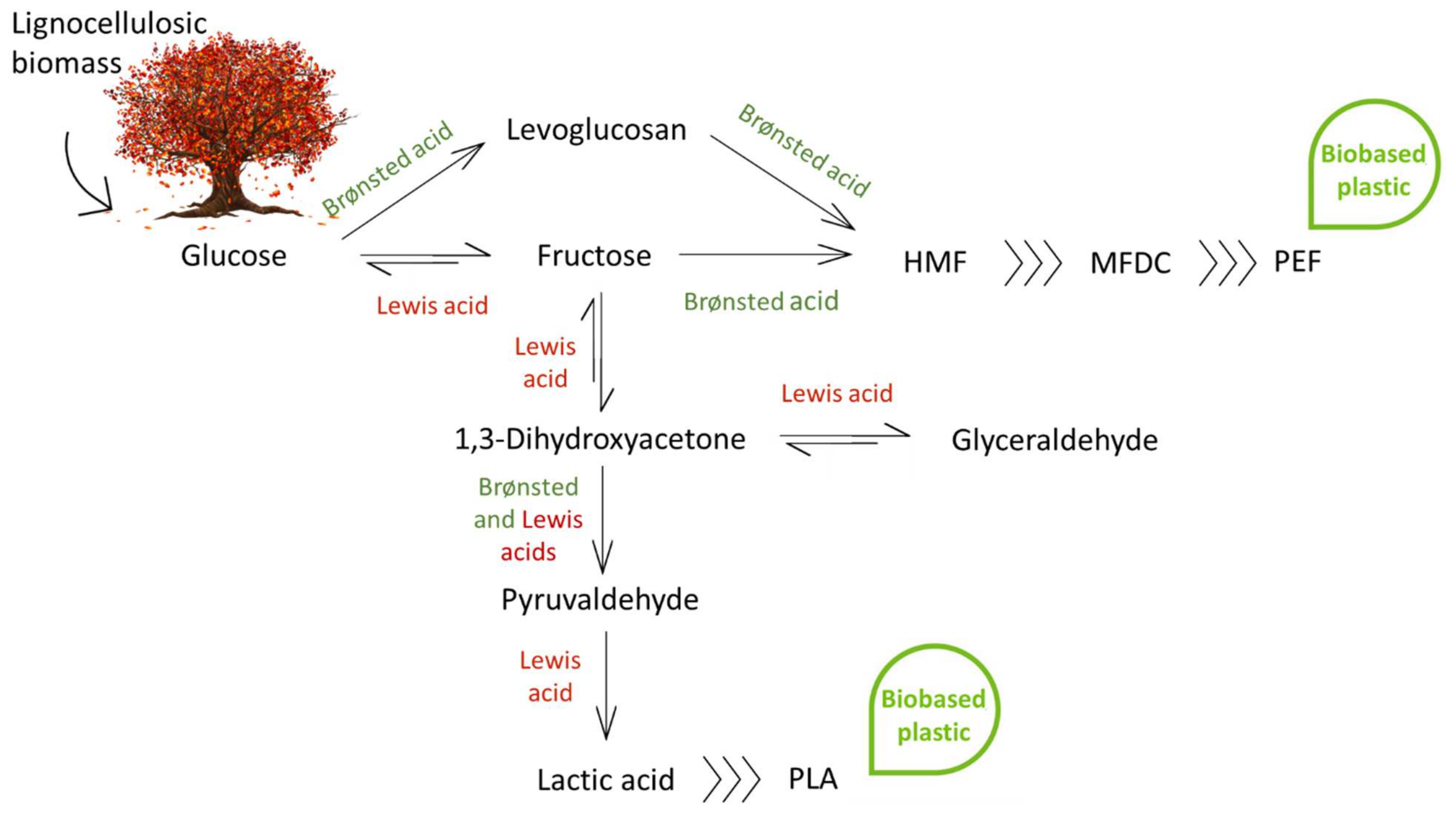
:1. Introduction
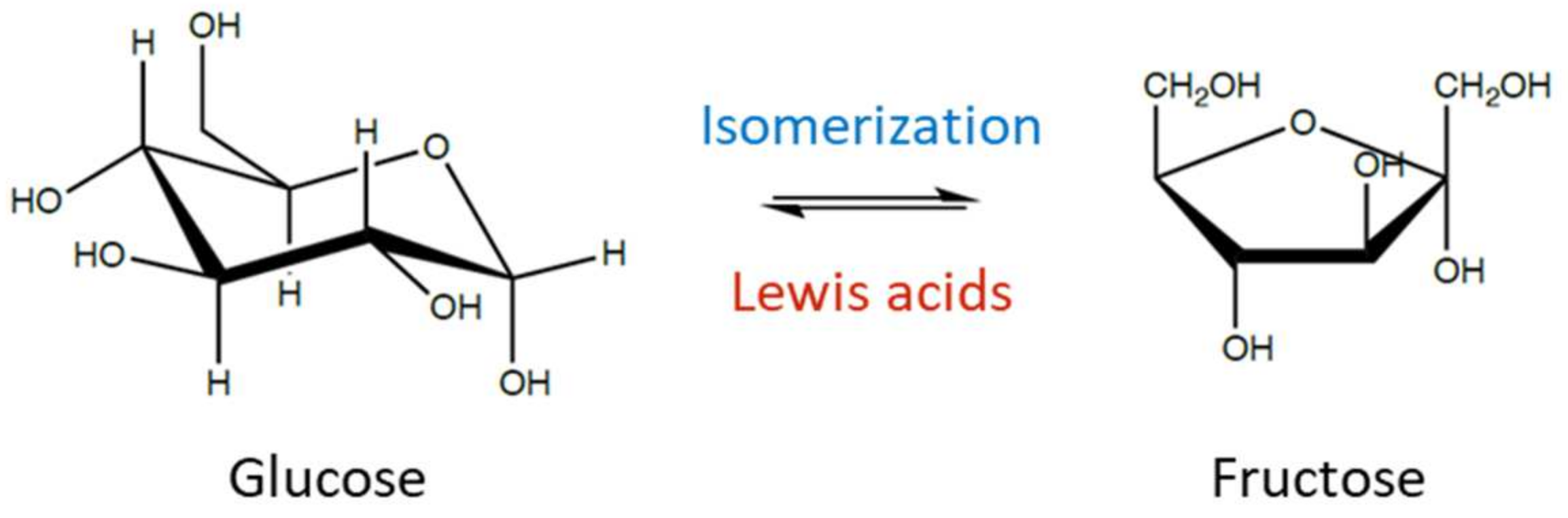
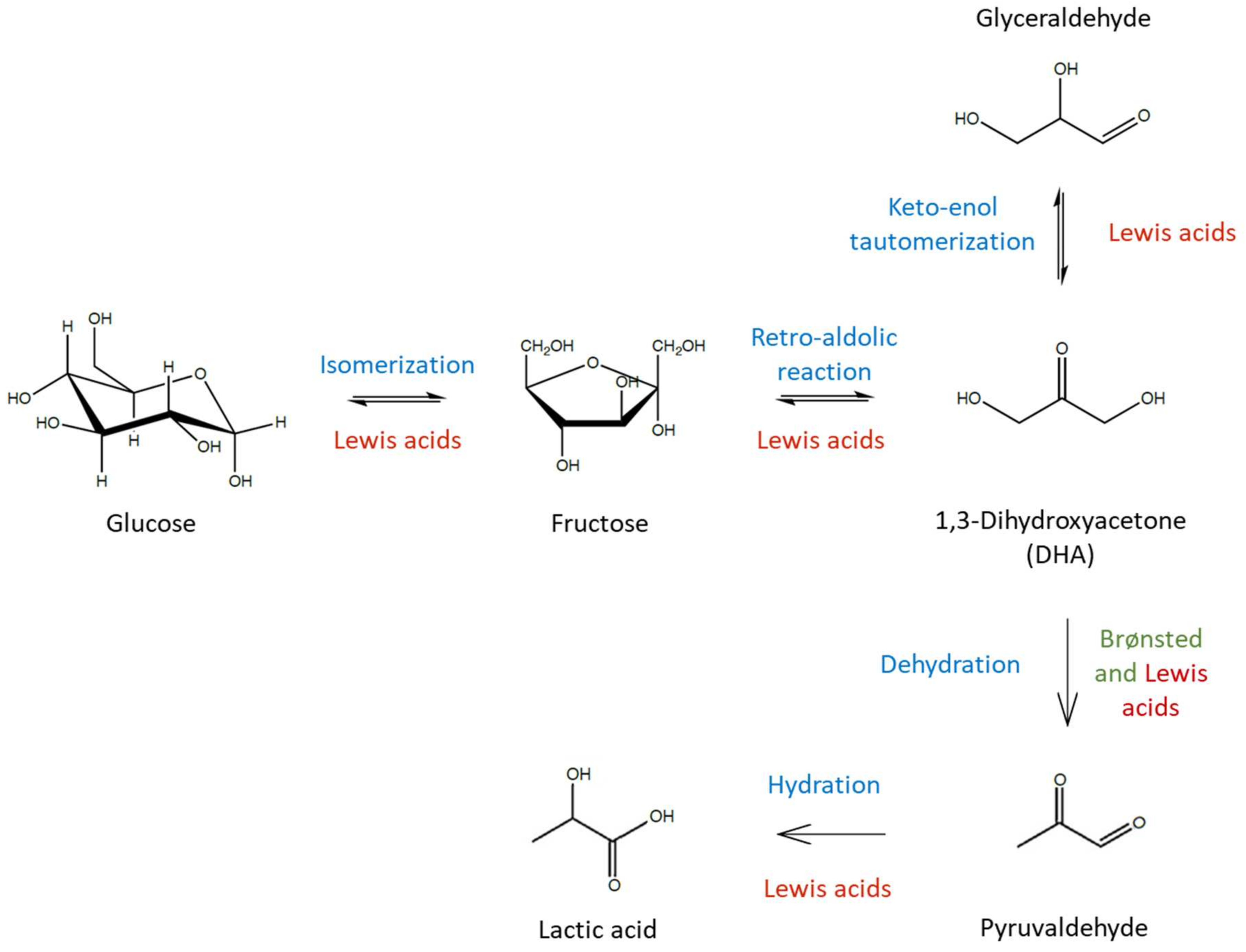
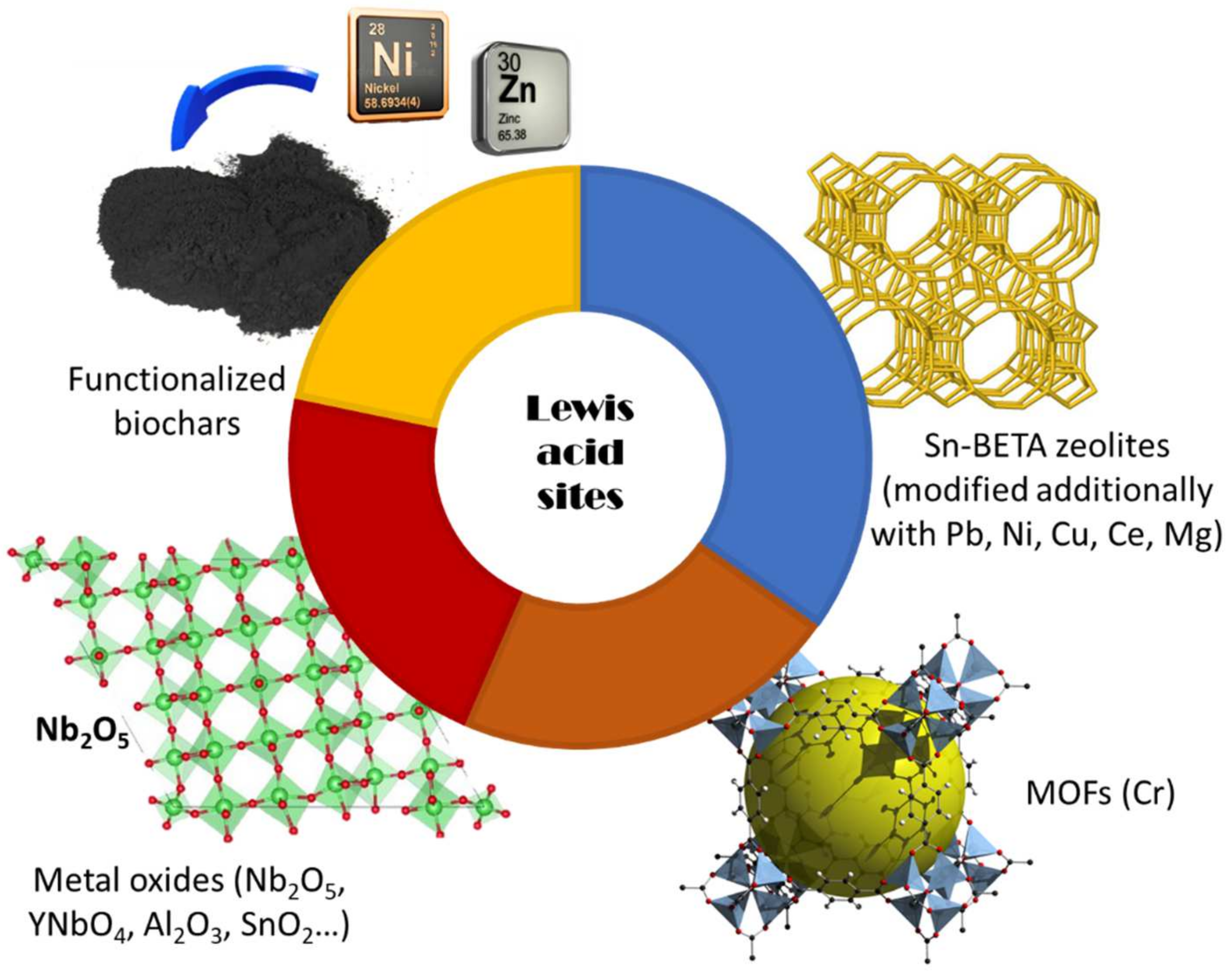
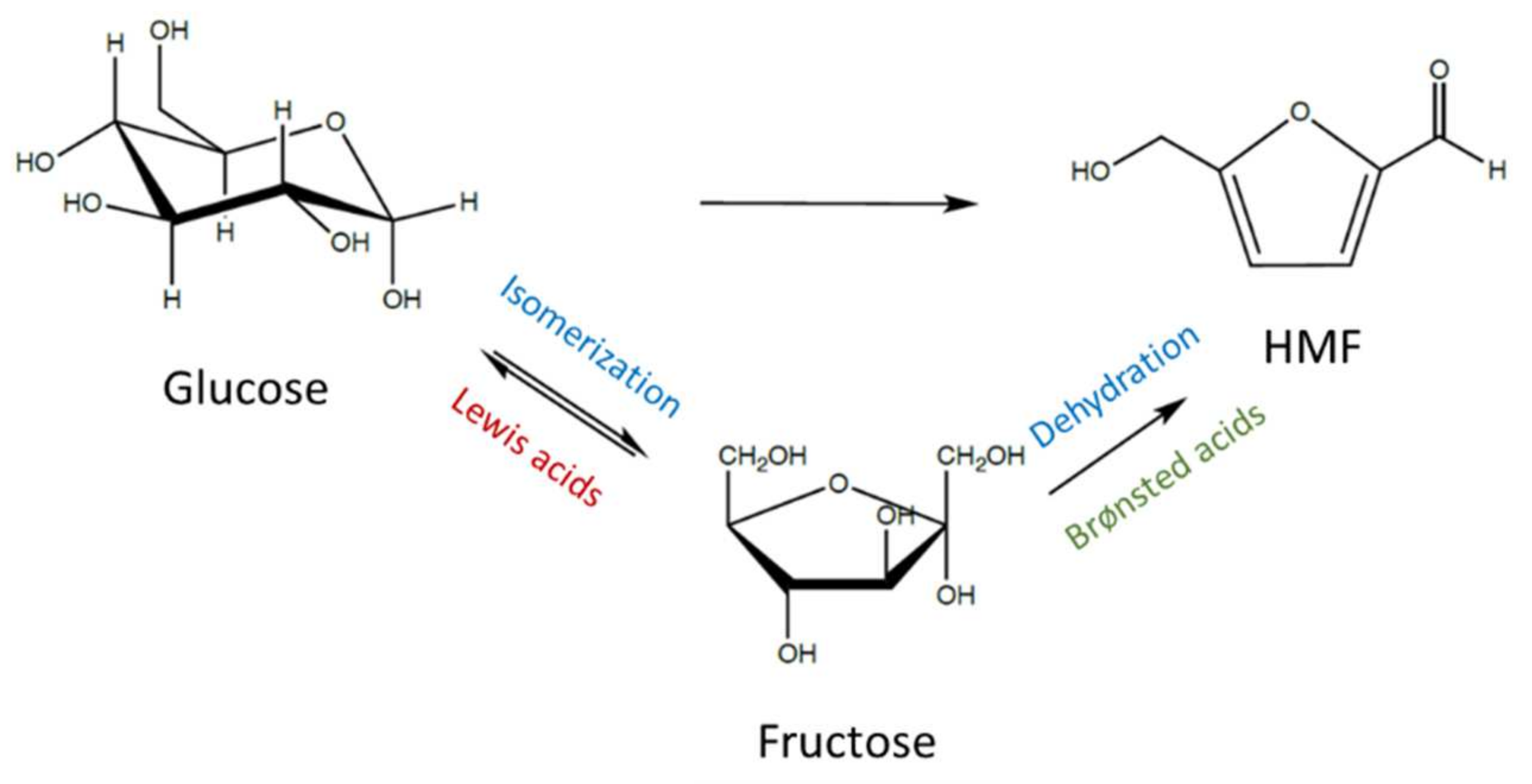
2. Glucose Isomerization to Fructose
3. Lactic Acid Synthesis
3.1. Triose Saccharide Conversion to Lactic Acid
3.2. Glucose/Fructose Conversion to Lactic Acid
3.3. Other Biomass Feedstocks to Lactic Acid
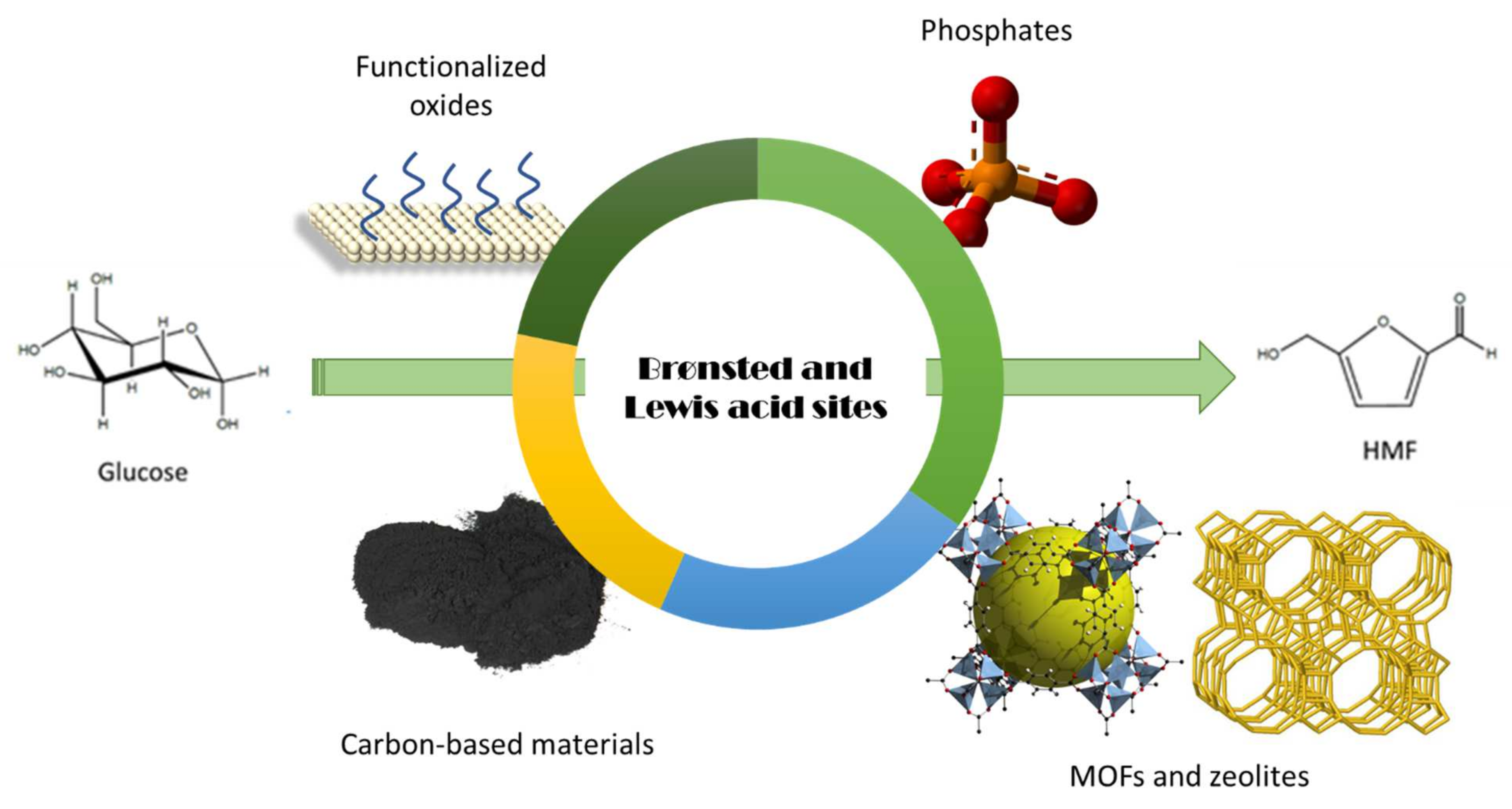
4. Glucose/Fructose Conversion to HMF
4.1. Fructose Dehydration to HMF
4.2. Glucose Conversion to HMF
4.3. Other Biomass Feedstocks to HMF
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Celik, G.; Kennedy, R.M.; Hackler, R.A.; Ferrandon, M.; Tennakoon, A.; Patnaik, S.; Lapointe, A.M.; Ammal, S.C.; Heyden, A.; Perras, F.A.; et al. Upcycling Single-Use Polyethylene into High-Quality Liquid Products. ACS Cent. Sci. 2019, 5, 1795–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.H.; Bhoi, P.R. An overview of non-biodegradable bioplastics. J. Clean. Prod. 2021, 294, 126218. [Google Scholar] [CrossRef]
- World Economic Forum, Ellen MacArthur Foundation and McKinsey & Company. The New Plastic Economy. Rethinking the Future of Plastics. 2016. Available online: http://www.ellenmacarthurfoundation.org/publications (accessed on 30 October 2021).
- Degli Esposti, M.; Morselli, D.; Fava, F.; Bertin, L.; Cavani, F.; Viaggi, D.; Fabbri, P. The role of biotechnology in the transition from plastics to bioplastics: An opportunity to reconnect global growth with sustainability. FEBS Open Bio 2021, 11, 967–983. [Google Scholar] [CrossRef]
- De Vargas Mores, G.; Finocchio, C.P.S.; Barichello, R.; Pedrozo, E.A. Sustainability and innovation in the Brazilian supply chain of green plastic. J. Clean. Prod. 2018, 177, 12–18. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Németh, A. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef] [PubMed]
- Megías-Sayago, C.; Navarro-Jaén, S.; Castillo, R.; Ivanova, S. Recent advances in selective oxidation of biomass-derived platform chemicals over gold catalysts. Curr. Opin. Green Sustain. Chem. 2020, 21, 50–55. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Su, Y.; Aoshima, T.; Fukuoka, A.; Hensen, E.J.M.; Nakajima, K. Effective Strategy for High-Yield Furan Dicarboxylate Production for Biobased Polyester Applications. ACS Catal. 2019, 9, 4277–4285. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain Mobility, Thermal, and Mechanical Properties of Poly(ethylene furanoate) Compared to Poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Delidovich, I.; Hausoul, P.J.C.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative Monomers Based on Lignocellulose and Their Use for Polymer Production. Chem. Rev. 2016, 116, 1540–1599. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Xu, S.; Wu, Y.; Li, J.; He, T.; Xiao, Y.; Zhou, C.; Hu, C. Directing the Simultaneous Conversion of Hemicellulose and Cellulose in Raw Biomass to Lactic Acid. ACS Sustain. Chem. Eng. 2020, 8, 4244–4255. [Google Scholar] [CrossRef]
- Havstad, M.R. Biodegradable plastics. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; pp. 97–129. [Google Scholar]
- Son, P.A.; Nishimura, S.; Ebitani, K. Preparation of zirconium carbonate as water-tolerant solid base catalyst for glucose isomerization and one-pot synthesis of levulinic acid with solid acid catalyst. React. Kinet. Mech. Catal. 2014, 111, 183–197. [Google Scholar] [CrossRef]
- Bhosale, S.H.; Rao, M.B.; Deshpande, V.V. Molecular and industrial aspects of glucose isomerase. Microbiol. Rev. 1996, 60, 280–300. [Google Scholar] [CrossRef] [PubMed]
- Gounder, R.; Davis, M.E. Monosaccharide and disaccharide isomerization over Lewis acid sites in hydrophobic and hydrophilic molecular sieves. J. Catal. 2013, 308, 176–188. [Google Scholar] [CrossRef]
- Moliner, M.; Román-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Natl. Acad. Sci. USA 2010, 107, 6164–6168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román-Leshkov, Y.; Moliner, M.; Labinger, J.A.; Davis, M.E. Mechanism of Glucose Isomerization Using a Solid Lewis Acid Catalyst in Water. Angew. Chem. Int. Ed. 2010, 49, 8954–8957. [Google Scholar] [CrossRef] [Green Version]
- Cordon, M.J.; Hall, J.N.; Harris, J.W.; Bates, J.S.; Hwang, S.J.; Gounder, R. Deactivation of Sn-Beta zeolites caused by structural transformation of hydrophobic to hydrophilic micropores during aqueous-phase glucose isomerization. Catal. Sci. Technol. 2019, 9, 1654–1668. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Guo, Q.; Yang, P.; Liu, X.; Wang, Y. Synthesis of hierarchical Sn-Beta zeolite and its catalytic performance in glucose conversion. Catal. Today 2021, 367, 117–123. [Google Scholar] [CrossRef]
- Leus, K.; Bogaerts, T.; De Decker, J.; Depauw, H.; Hendrickx, K.; Vrielinck, H.; Van Speybroeck, V.; Van Der Voort, P. Systematic study of the chemical and hydrothermal stability of selected “stable” Metal Organic Frameworks. Microporous Mesoporous Mater. 2016, 226, 110–116. [Google Scholar] [CrossRef]
- Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Walton, R.I.; Degirmenci, V. Exceptionally Efficient and Recyclable Heterogeneous Metal–Organic Framework Catalyst for Glucose Isomerization in Water. ChemCatChem 2018, 10, 706–709. [Google Scholar] [CrossRef] [PubMed]
- De Mello, M.D.; Tsapatsis, M. Selective Glucose-to-Fructose Isomerization over Modified Zirconium UiO-66 in Alcohol Media. ChemCatChem 2018, 10, 2417–2423. [Google Scholar] [CrossRef]
- Rojas-Buzo, S.; Corma, A.; Boronat, M.; Moliner, M. Unraveling the reaction mechanism and active sites of metal-organic frameworks for glucose transformations in water: Experimental and theoretical studies. ACS Sustain. Chem. Eng. 2020, 8, 16143–16155. [Google Scholar] [CrossRef]
- Luo, Q.X.; Zhang, Y.B.; Qi, L.; Scott, S.L. Glucose Isomerization and Epimerization over Metal-Organic Frameworks with Single-Site Active Centers. ChemCatChem 2019, 11, 1903–1909. [Google Scholar] [CrossRef]
- Guo, Q.; Ren, L.; Kumar, P.; Cybulskis, V.J.; Mkhoyan, K.A.; Davis, M.E.; Tsapatsis, M. A Chromium Hydroxide/MIL-101(Cr) MOF Composite Catalyst and Its Use for the Selective Isomerization of Glucose to Fructose. Angew. Chem. 2018, 130, 5020–5024. [Google Scholar] [CrossRef]
- Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martin, J.M.; Abdelkader-Fernández, V.K.; Cunha-Silva, L.; Balula, S.S. Isomerization of glucose to fructose catalyzed by metal-organic framework. Sustain. Energy Fuels in press. 2021. [Google Scholar] [CrossRef]
- Yang, X.; Yu, I.K.M.; Cho, D.W.; Chen, S.S.; Tsang, D.C.W.; Shang, J.; Yip, A.C.K.; Wang, L.; Ok, Y.S. Tin-Functionalized Wood Biochar as a Sustainable Solid Catalyst for Glucose Isomerization in Biorefinery. ACS Sustain. Chem. Eng. 2019, 7, 4851–4860. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Xiong, X.; Tsang, D.C.W.; Wang, L.; Hunt, A.J.; Song, H.; Shang, J.; Ok, Y.S.; Poon, C.S. Aluminium-biochar composites as sustainable heterogeneous catalysts for glucose isomerisation in a biorefinery. Green Chem. 2019, 21, 1267–1281. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Xiong, X.; Tsang, D.C.W.; Ng, Y.H.; Clark, J.H.; Fan, J.; Zhang, S.; Hu, C.; Ok, Y.S. Graphite oxide- and graphene oxide-supported catalysts for microwave-assisted glucose isomerisation in water. Green Chem. 2019, 21, 4341–4353. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Dutta, S.; Mašek, O.; Tsang, D.C.W. Valorization of humins from food waste biorefinery for synthesis of biochar-supported Lewis acid catalysts. Sci. Total Environ. 2021, 775, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.K.M.; Hanif, A.; Tsang, D.C.W.; Yip, A.C.K.; Lin, K.Y.A.; Gao, B.; Ok, Y.S.; Poon, C.S.; Shang, J. Tailoring acidity and porosity of alumina catalysts via transition metal doping for glucose conversion in biorefinery. Sci. Total Environ. 2020, 704, 135414. [Google Scholar] [CrossRef] [PubMed]
- Palai, Y.N.; Shrotri, A.; Asakawa, M.; Fukuoka, A. Silica supported Sn catalysts with tetrahedral Sn sites for selective isomerization of glucose to fructose. Catal. Today 2021, 365, 241–248. [Google Scholar] [CrossRef]
- Kumar, S.; Nepak, D.; Kansal, S.K.; Elumalai, S. Expeditious isomerization of glucose to fructose in aqueous media over sodium titanate nanotubes. RSC Adv. 2018, 8, 30106–30114. [Google Scholar] [CrossRef] [Green Version]
- Orazov, M.; Davis, M.E. Tandem catalysis for the production of alkyl lactates from ketohexoses at moderate temperatures. Proc. Natl. Acad. Sci. USA 2015, 112, 11777–11782. [Google Scholar] [CrossRef] [Green Version]
- Rasrendra, C.B.; Fachri, B.A.; Makertihartha, I.G.; Adisasmito, S.; Heeres, H.J. Catalytic conversion of dihydroxyacetone to lactic acid using metal salts in water. ChemSusChem 2011, 4, 768–777. [Google Scholar] [CrossRef]
- Taarning, E.; Saravanamurugan, S.; Holm, M.S.; Xiong, J.; West, R.M.; Christensen, C.H. Zeolite-catalyzed isomerization of triose sugars. ChemSusChem 2009, 2, 625–627. [Google Scholar] [CrossRef]
- Koito, Y.; Nakajima, K.; Kitano, M.; Hara, M. Efficient conversion of pyruvic aldehyde into lactic acid by Lewis acid catalyst in water. Chem. Lett. 2013, 42, 873–875. [Google Scholar] [CrossRef]
- Li, L.; Stroobants, C.; Lin, K.; Jacobs, P.A.; Sels, B.F.; Pescarmona, P.P. Selective conversion of trioses to lactates over Lewis acid heterogeneous catalysts. Green Chem. 2011, 13, 1175–1181. [Google Scholar] [CrossRef]
- Hayashi, Y.; Sasaki, Y. Tin-catalyzed conversion of trioses to alkyl lactates in alcohol solution. Chem. Commun. 2005, 36, 2716–2718. [Google Scholar] [CrossRef]
- Chambon, F.; Rataboul, F.; Pinel, C.; Cabiac, A.; Guillon, E.; Essayem, N. Cellulose hydrothermal conversion promoted by heterogeneous Brønsted and Lewis acids: Remarkable efficiency of solid Lewis acids to produce lactic acid. Appl. Catal. B Environ. 2011, 105, 171–181. [Google Scholar] [CrossRef]
- Ennaert, T.; Van Aelst, J.; Dijkmans, J.; De Clercq, R.; Schutyser, W.; Dusselier, M.; Verboekend, D.; Sels, B.F. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 2016, 47, 584–611. [Google Scholar] [CrossRef] [Green Version]
- Jeon, W.; Ban, C.; Park, G.; Woo, H.C.; Kim, D.H. Hydrothermal conversion of macroalgae-derived alginate to lactic acid catalyzed by metal oxides. Catal. Sci. Technol. 2015, 6, 1146–1156. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, F.; Hu, J.; Huo, Z. Improvement of lactic acid production from cellulose with the addition of Zn/Ni/C under alkaline hydrothermal conditions. Biores. Technol. 2011, 102, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Feliczak-Guzik, A.; Sprynskyy, M.; Nowak, I.; Buszewski, B. Catalytic isomerization of dihydroxyacetone to lactic acid and alkyl lactates over hierarchical zeolites containing tin. Catalysts 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Hirata, J.; Kim, M.; Gupta, N.K.; Murayama, T.; Yoshida, A.; Hiyoshi, N.; Fukuoka, A.; Ueda, W. Facile Formation of Lactic Acid from a Triose Sugar in Water over Niobium Oxide with a Deformed Orthorhombic Phase. ACS Catal. 2018, 8, 283–290. [Google Scholar] [CrossRef]
- Kim, M.; Ronchetti, S.; Onida, B.; Ichikuni, N.; Fukuoka, A.; Kato, H.; Nakajima, K. Lewis Acid and Base Catalysis of YNbO4 Toward Aqueous-Phase Conversion of Hexose and Triose Sugars to Lactic Acid in Water. ChemCatChem 2020, 12, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Song, Y.; Huang, C.; Wang, B. Crystalline niobium phosphates with water-tolerant and adjustable Lewis acid sites for the production of lactic acid from triose sugars. Sustain. Energy Fuels 2018, 2, 1530–1541. [Google Scholar] [CrossRef]
- Takagaki, A.; Goto, H.; Kikuchi, R.; Oyama, S.T. Silica-supported chromia-titania catalysts for selective formation of lactic acid from a triose in water. Appl. Catal. A Gen. 2019, 570, 200–208. [Google Scholar] [CrossRef]
- Xia, M.; Dong, W.; Gu, M.; Chang, C.; Shen, Z.; Zhang, Y. Synergetic effects of bimetals in modified beta zeolite for lactic acid synthesis from biomass-derived carbohydrates. RSC Adv. 2018, 8, 8965–8975. [Google Scholar] [CrossRef]
- Wang, S.; Chen, K.; Wang, Q. Ytterbium triflate immobilized on sulfo-functionalized SBA-15 catalyzed conversion of cellulose to lactic acid. J. Porous Mater. 2018, 25, 1531–1539. [Google Scholar] [CrossRef]
- Iglesias, J.; Moreno, J.; Morales, G.; Melero, J.A.; Juárez, P.; López-Granados, M.; Mariscal, R.; Martínez-Salazar, I. Sn-Al-USY for the valorization of glucose to methyl lactate: Switching from hydrolytic to retro-aldol activity by alkaline ion exchange. Green Chem. 2019, 21, 5876–5885. [Google Scholar] [CrossRef]
- Tang, B.; Li, S.; Song, W.C.; Yang, E.C.; Zhao, X.J.; Guan, N.; Li, L. Fabrication of Hierarchical Sn-Beta Zeolite as Efficient Catalyst for Conversion of Cellulosic Sugar to Methyl Lactate. ACS Sustain. Chem. Eng. 2020, 8, 3796–3808. [Google Scholar] [CrossRef]
- Yang, X.; Lv, B.; Lu, T.; Su, Y.; Zhou, L. Promotion effect of Mg on a post-synthesized Sn-Beta zeolite for the conversion of glucose to methyl lactate. Catal. Sci. Technol. 2020, 10, 700–709. [Google Scholar] [CrossRef]
- Xia, M.; Dong, W.; Shen, Z.; Xiao, S.; Chen, W.; Gu, M.; Zhang, Y. Efficient production of lactic acid from biomass-derived carbohydrates under synergistic effects of indium and tin in In-Sn-Beta zeolites. Sustain. Energy Fuels 2020, 4, 5327–5338. [Google Scholar] [CrossRef]
- Cai, Q.; Yue, X.; Dong, W.S. Hierarchical Fe–Sn/Beta catalyzes the conversion of glucose to methyl lactate. J. Porous Mater. 2021, 28, 1315–1324. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, L.; Wang, H.; Miao, G.; Kong, L.; Li, S.; Sun, Y. Efficient production of lactic acid from sugars over Sn-Beta zeolite in water: Catalytic performance and mechanistic insights. Sustain. Energy Fuels 2019, 3, 1163–1171. [Google Scholar] [CrossRef]
- Cao, D.; Cai, W.; Tao, W.; Zhang, S.; Wang, D.; Huang, D. Lactic Acid Production from Glucose Over a Novel Nb2O5 Nanorod Catalyst. Catal. Lett. 2017, 147, 926–933. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yabushita, M.; Kim, M.; Hirayama, J.; Motokura, K.; Fukuoka, A.; Nakajima, K. Catalytic Conversion of Biomass-Derived Carbohydrates to Methyl Lactate by Acid-Base Bifunctional γ-Al2O3. ACS Sustain. Chem. Eng. 2018, 6, 8113–8117. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, F.; Hu, J.; Zhang, W. Role of metallic Zn, Ni and activated carbon additives in improving the hydrothermal conversion of glucose into lactic acid. J. Chem. Technol. Biotechnol. 2017, 92, 1046–1052. [Google Scholar] [CrossRef]
- Zhao, B.; Yue, X.; Li, H.; Li, J.; Liu, C.L.; Xu, C.; Dong, W.S. Lanthanum-modified phosphomolybdic acid as an efficient catalyst for the conversion of fructose to lactic acid. React. Kinet. Mech. Catal. 2018, 125, 55–69. [Google Scholar] [CrossRef]
- Murillo, B.; Zornoza, B.; de la Iglesia, O.; Wang, S.; Serre, C.; Téllez, C.; Coronas, J. Tin-Carboxylate MOFs for Sugar Transformation into Methyl Lactate. Eur. J. Inorg. Chem. 2019, 2624–2629. [Google Scholar] [CrossRef]
- Swesi, Y.; Nguyen, C.; Ha Vu, T.T.; Rataboul, F.; Eternot, M.; Fongarland, P.; Essayem, N. Direct Solid Lewis Acid Catalyzed Wood Liquefaction into Lactic Acid: Kinetic Evidences that Wood Pretreatment Might Not be a Prerequisite. ChemCatChem 2017, 9, 2377–2382. [Google Scholar] [CrossRef] [Green Version]
- Wattanapaphawong, P.; Reubroycharoen, P.; Yamaguchi, A. Conversion of cellulose into lactic acid using zirconium oxide catalysts. RSC Adv. 2017, 7, 18561–18568. [Google Scholar] [CrossRef] [Green Version]
- Wattanapaphawong, P.; Sato, O.; Sato, K.; Mimura, N.; Reubroycharoen, P.; Yamaguchi, A. Conversion of cellulose to lactic acid by using ZrO2–Al2O3 catalysts. Catalysts 2017, 7, 221. [Google Scholar] [CrossRef] [Green Version]
- Candu, N.; Anita, F.; Podolean, I.; Cojocaru, B.; Parvulescu, V.I.; Coman, S.M. Direct conversion of cellulose to α-hydroxy acids (AHAs) over Nb2O5-SiO2-coated magnetic nanoparticles. Green Process. Synth. 2017, 6, 255–264. [Google Scholar] [CrossRef]
- Verziu, M.; Serano, M.; Jurca, B.; Parvulescu, V.I.; Coman, S.M.; Scholz, G.; Kemnitz, E. Catalytic features of Nb-based nanoscopic inorganic fluorides for an efficient one-pot conversion of cellulose to lactic acid. Catal. Today 2018, 306, 102–110. [Google Scholar] [CrossRef]
- Kong, L.; Shen, Z.; Zhang, W.; Xia, M.; Gu, M.; Zhou, X.; Zhang, Y. Conversion of Sucrose into Lactic Acid over Functionalized Sn-Beta Zeolite Catalyst by 3-Aminopropyltrimethoxysilane. ACS Omega 2018, 3, 17430–17438. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Wu, H.Z.; Ren, H.F.; Liu, C.L.; Xu, C.L.; Dong, W.S. Er/β-zeolite-catalyzed one-pot conversion of cellulose to lactic acid. J. Porous Mater. 2017, 24, 697–706. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass; National Renewable Energy Lab.: Golden, CO, USA, 2004; Volume I. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Davidson, M.G.; Elgie, S.; Parsons, S.; Young, T.J. Production of HMF, FDCA and their derived products: A review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies. Green Chem. 2021, 23, 3154–3171. [Google Scholar] [CrossRef]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, X.; Hu, Y.; Hu, C. Controlling the Reaction Networks for Efficient Conversion of Glucose into 5-Hydroxymethylfurfural. ChemSusChem 2020, 13, 4812–4832. [Google Scholar] [CrossRef] [PubMed]
- Körner, P.; Jung, D.; Kruse, A. The effect of different Brønsted acids on the hydrothermal conversion of fructose to HMF. Green Chem. 2018, 20, 2231–2241. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Hacking, J.; Heeres, H.J.; Yue, J. Selective fructose dehydration to 5-hydroxymethylfurfural from a fructose-glucose mixture over a sulfuric acid catalyst in a biphasic system: Experimental study and kinetic modelling. Chem. Eng. J. 2021, 409, 128182. [Google Scholar] [CrossRef]
- Cao, Z.; Li, M.; Chen, Y.; Shen, T.; Tang, C.; Zhu, C.; Ying, H. Dehydration of fructose into 5-hydroxymethylfurfural in a biphasic system using EDTA as a temperature-responsive catalyst. Appl. Catal. A Gen. 2019, 569, 93–100. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, J.; Luo, W.; Su, L.; Huang, Z. Catalytic conversion of carbohydrates into 5-hydroxymethyl furfural over sulfonated hyper-cross-linked polymer in DMSO. Chem. Eng. J. 2018, 334, 1055–1064. [Google Scholar] [CrossRef]
- Mayer, S.F.; Falcón, H.; Dipaola, R.; Ribota, P.; Moyano, L.; Morales-delaRosa, S.; Mariscal, R.; Campos-Martín, J.M.; Alonso, J.A.; Fierro, J.L.G. Dehydration of fructose to HMF in presence of (H3O)xSbxTe(2-x)O6 (x = 1, 1.1, 1.25) in H2O-MIBK. Mol. Catal. 2020, 481, 110276. [Google Scholar] [CrossRef] [Green Version]
- Pyo, S.H.; Sayed, M.; Hatti-Kaul, R. Batch and Continuous Flow Production of 5-Hydroxymethylfurfural from a High Concentration of Fructose Using an Acidic Ion Exchange Catalyst. Org. Process Res. Dev. 2019, 23, 952–960. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Itabaiana, I. Engineering the future: Perspectives in the 2,5-furandicarboxylic acid synthesis. Catal. Today 2020, 354, 211–217. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Z.; Qin, D.; Yang, G. Sulfonic acid-functionalized hierarchical SAPO-34 for fructose dehydration to 5-hydroxymethylfurfural. React. Kinet. Mech. Catal. 2019, 128, 523–538. [Google Scholar] [CrossRef]
- Bounoukta, C.E.; Megías-Sayago, C.; Ivanova, S.; Penkova, A.; Ammari, F.; Centeno, M.A.; Odriozola, J.A. Effect of the sulphonating agent on the catalytic behavior of activated carbons in the dehydration reaction of fructose in DMSO. Appl. Catal. A Gen. 2021, 617, 118108. [Google Scholar] [CrossRef]
- Lv, G.; Deng, L.; Lu, B.; Li, J.; Hou, X.; Yang, Y. Efficient dehydration of fructose into 5-hydroxymethylfurfural in aqueous medium over silica-included heteropolyacids. J. Clean. Prod. 2017, 142, 2244–2251. [Google Scholar] [CrossRef]
- Bounoukta, C.E.; Megías-Sayago, C.; Ammari, F.; Ivanova, S.; Monzon, A.; Centeno, M.A.; Odriozola, J.A. Dehydration of glucose to 5-Hydroxymethlyfurfural on bifunctional carbon catalysts. Appl. Catal. B Environ. 2021, 286, 119938. [Google Scholar] [CrossRef]
- Delgado Martín, G.; Bounoukta, C.E.; Ammari, F.; Domínguez, M.I.; Monzón, A.; Ivanova, S.; Centeno, M.A. Fructose dehydration reaction over functionalized nanographitic catalysts in MIBK/H2O biphasic system. Catal. Today 2021, 366, 68–76. [Google Scholar] [CrossRef]
- Galaverna, R.; Breitkreitz, M.C.; Pastre, J.C. Conversion of d-Fructose to 5-(Hydroxymethyl)furfural: Evaluating Batch and Continuous Flow Conditions by Design of Experiments and In-Line FTIR Monitoring. ACS Sustain. Chem. Eng. 2018, 6, 4220–4230. [Google Scholar] [CrossRef]
- Sayed, M.; Warlin, N.; Hulteberg, C.; Munslow, I.; Lundmark, S.; Pajalic, O.; Tunå, P.; Zhang, B.; Pyo, S.H.; Hatti-Kaul, R. 5-Hydroxymethylfurfural from fructose: An efficient continuous process in a water-dimethyl carbonate biphasic system with high yield product recovery. Green Chem. 2020, 22, 5402–5413. [Google Scholar] [CrossRef]
- Antonetti, C.; Melloni, M.; Licursi, D.; Fulignati, S.; Ribechini, E.; Rivas, S.; Parajó, J.C.; Cavani, F.; Raspolli Galletti, A.M. Microwave-assisted dehydration of fructose and inulin to HMF catalyzed by niobium and zirconium phosphate catalysts. Appl. Catal. B Environ. 2017, 206, 364–377. [Google Scholar] [CrossRef]
- Wang, C.; Gong, W.; Lu, X.; Xiang, Y.; Ji, P. Heparin Immobilized on Multiwalled Carbon Nanotubes for Catalytic Conversion of Fructose in Water with High Yield and Selectivity. ACS Omega 2019, 4, 16808–16815. [Google Scholar] [CrossRef] [Green Version]
- Yan, P.; Xia, M.; Chen, S.; Han, W.; Wang, H.; Zhu, W. Unlocking biomass energy: Continuous high-yield production of 5-hydroxymethylfurfural in water. Green Chem. 2020, 22, 5274–5284. [Google Scholar] [CrossRef]
- Testa, M.L.; Miroddi, G.; Russo, M.; Parola, V.L.; Marcì, G. Dehydration of fructose to 5-HMF over acidic TiO2 catalysts. Materials 2020, 13, 1178. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, M.; Singh, S.K.; Khilari, S.; Sahu, M.; Ranganath, K.V.S. Graphene oxide as a sustainable metal and solvent free catalyst for dehydration of fructose to 5-HMF: A new and green protocol. Catal. Commun. 2018, 106, 64–67. [Google Scholar] [CrossRef]
- Karimi, S.; Shekaari, H.; Halimehjani, A.Z.; Niakan, M. Solvent-Free Production of 5-Hydroxymethylfurfural from Deep Eutectic Substrate Reaction Mixtures over a Magnetically Recoverable Solid Acid Catalyst. ACS Sustain. Chem. Eng. 2021, 9, 326–336. [Google Scholar] [CrossRef]
- Tomer, R.; Biswas, P. Dehydration of glucose/fructose to 5-hydroxymethylfurfural (5-HMF) over an easily recyclable sulfated titania (SO42-/TiO2) catalyst. New J. Chem. 2020, 44, 20734–20750. [Google Scholar] [CrossRef]
- Zhang, T.; Fan, W.; Li, W.; Xu, Z.; Xin, H. One-Pot Conversion of Carbohydrates into 5-(Hydroxymethyl ) furfural using Heterogeneous Lewis-Acid and Brønsted-Acid Catalysts. Energy Technol. 2017, 5, 747–755. [Google Scholar] [CrossRef]
- Takagaki, A.; Ohara, M.; Nishimura, S.; Ebitani, K. A one-pot reaction for biorefinery : Combination of solid acid and base catalysts for direct production of 5-hydroxymethylfurfural from saccharides. Chem. Commun. 2009, 41, 6276–6278. [Google Scholar] [CrossRef]
- Nikolla, E.; Rom, Y.; Moliner, M.; Davis, M.E. “One-Pot” Synthesis of 5-(Hydroxymethyl)furfural from Carbohydrates using Tin-Beta Zeolite. Acs Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef] [Green Version]
- Catrinck, M.N.; Ribeiro, E.S.; Monteiro, R.S.; Ribas, R.M.; Barbosa, M.H.P.; Teófilo, R.F. Direct conversion of glucose to 5-hydroxymethylfurfural using a mixture of niobic acid and niobium phosphate as a solid acid catalyst. Fuel 2017, 210, 67–74. [Google Scholar] [CrossRef]
- Li, K.; Du, M.; Ji, P. Multifunctional Tin-Based Heterogeneous Catalyst for Catalytic Conversion of Glucose to 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2018, 6, 5636–5644. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Zhang, Y.; Cui, H.; Yi, W.; Song, F.; Zhao, P.; Sun, X.; Xie, Y.; Wang, L.; et al. AlNb/SBA-15 Catalysts with Tunable Lewis and Bronsted Acidic Sites for Glucose Conversion to HMF. ChemistrySelect 2018, 3, 3555–3560. [Google Scholar] [CrossRef]
- Han, B.; Zhao, P.; He, R.; Wu, T.; Wu, Y. Catalytic Conversion of Glucose to 5-Hydroxymethyfurfural Over B2O3 Supported Solid Acids Catalysts. Waste Biomass Valorization 2018, 9, 2181–2190. [Google Scholar] [CrossRef]
- Rao, K.T.V.; Souzanchi, S.; Yuan, Z.; Xu, C. One-pot sol-gel synthesis of a phosphated TiO2 catalyst for conversion of monosaccharide, disaccharides, and polysaccharides to 5-hydroxymethylfurfural. New J. Chem. 2019, 43, 12483–12493. [Google Scholar] [CrossRef]
- He, R.; Huang, X.; Zhao, P.; Han, B.; Wu, T.; Wu, Y. The Synthesis of 5-Hydroxymethylfurfural from Glucose in Biphasic System by Phosphotungstic Acidified Titanium–Zirconium Dioxide. Waste Biomass Valorization 2018, 9, 657–668. [Google Scholar] [CrossRef]
- Vieira, J.L.; Paul, G.; Iga, G.D.; Cabral, N.M.; Bueno, J.M.C.; Bisio, C.; Gallo, J.M.R. Niobium phosphates as bifunctional catalysts for the conversion of biomass-derived monosaccharides. Appl. Catal. A Gen. 2021, 617, 118099. [Google Scholar] [CrossRef]
- Huang, F.; Su, Y.; Long, Z.; Chen, G.; Yao, Y. Enhanced Formation of 5-Hydroxymethylfurfural from Glucose Using a Silica-Supported Phosphate and Iron Phosphate Heterogeneous Catalyst. Ind. Eng. Chem. Res. 2018, 57, 10198–10205. [Google Scholar] [CrossRef]
- Song, X.; Yue, J.; Zhu, Y.; Wen, C.; Chen, L.; Liu, Q.; Ma, L.; Wang, C. Efficient Conversion of Glucose to 5-Hydroxymethylfurfural over a Sn-Modified SAPO-34 Zeolite Catalyst. Ind. Eng. Chem. Res. 2021, 60, 5838–5851. [Google Scholar] [CrossRef]
- Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Kashtiban, R.J.; Huband, S.; Walton, R.I.; Degirmenci, V. Systematic Modification of UiO-66 Metal-Organic Frameworks for Glucose Conversion into 5-Hydroxymethyl Furfural in Water. ChemCatChem 2021, 13, 2517–2529. [Google Scholar] [CrossRef]
- Tangsermvit, V.; Pila, T.; Boekfa, B.; Somjit, V.; Klysubun, W.; Limtrakul, J.; Horike, S.; Kongpatpanich, K. Incorporation of Al3+ Sites on Brønsted Acid Metal–Organic Frameworks for Glucose-to-Hydroxylmethylfurfural Transformation. Small 2021, 17, 2006541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Wang, J.; Wang, Y.; Wang, M.; Cui, H.; Song, F.; Sun, X.; Xie, Y.; Yi, W. Al2O3-TiO2 Modified Sulfonated Carbon with Hierarchically Ordered Pores for Glucose Conversion to 5-HMF. ChemistrySelect 2019, 4, 5724–5731. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Wang, S.; Wu, Y. Recent Advances in Aqueous-Phase Catalytic Conversions of Biomass Platform Chemicals Over Heterogeneous Catalysts. Front. Chem. 2020, 7, 948. [Google Scholar] [CrossRef]
- Gliozzi, G.; Innorta, A.; Mancini, A.; Bortolo, R.; Perego, C.; Ricci, M.; Cavani, F. Zr/P/O catalyst for the direct acid chemo-hydrolysis of non-pretreated microcrystalline cellulose and softwood sawdust. Appl. Catal. B Environ. 2014, 145, 24–33. [Google Scholar] [CrossRef]
- Li, Z.; Su, K.; Ren, J.; Yang, D.; Cheng, B.; Kim, C.K.; Yao, X. Direct catalytic conversion of glucose and cellulose. Green Chem. 2018, 20, 863–872. [Google Scholar] [CrossRef]
- Tang, Z.; Su, J. Direct conversion of cellulose to 5-hydroxymethylfurfural (HMF) using an efficient and inexpensive boehmite catalyst. Carbohydr. Res. 2019, 481, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Cao, X.; Zhong, L.; Peng, X.; Sun, R.; Liu, J. Effectively enhancing conversion of cellulose to HMF by combining in-situ carbonic acid from CO2 and metal oxides. Ind. Crop. Prod. 2018, 126, 151–157. [Google Scholar] [CrossRef]
- Sezgin, E.; Esen Keçeci, M.; Akmaz, S.; Koc, S.N. Heterogeneous Cr-zeolites (USY and Beta) for the conversion of glucose and cellulose to 5-hydroxymethylfurfural (HMF). Cellulose 2019, 26, 9035–9043. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megías-Sayago, C.; Navarro-Jaén, S.; Drault, F.; Ivanova, S. Recent Advances in the Brønsted/Lewis Acid Catalyzed Conversion of Glucose to HMF and Lactic Acid: Pathways toward Bio-Based Plastics. Catalysts 2021, 11, 1395. https://doi.org/10.3390/catal11111395
Megías-Sayago C, Navarro-Jaén S, Drault F, Ivanova S. Recent Advances in the Brønsted/Lewis Acid Catalyzed Conversion of Glucose to HMF and Lactic Acid: Pathways toward Bio-Based Plastics. Catalysts. 2021; 11(11):1395. https://doi.org/10.3390/catal11111395
Chicago/Turabian StyleMegías-Sayago, Cristina, Sara Navarro-Jaén, Fabien Drault, and Svetlana Ivanova. 2021. "Recent Advances in the Brønsted/Lewis Acid Catalyzed Conversion of Glucose to HMF and Lactic Acid: Pathways toward Bio-Based Plastics" Catalysts 11, no. 11: 1395. https://doi.org/10.3390/catal11111395
APA StyleMegías-Sayago, C., Navarro-Jaén, S., Drault, F., & Ivanova, S. (2021). Recent Advances in the Brønsted/Lewis Acid Catalyzed Conversion of Glucose to HMF and Lactic Acid: Pathways toward Bio-Based Plastics. Catalysts, 11(11), 1395. https://doi.org/10.3390/catal11111395









