Ti2O3/TiO2-Assisted Solar Photocatalytic Degradation of 4-tert-Butylphenol in Water
Abstract
:1. Introduction
2. Results and Discussion
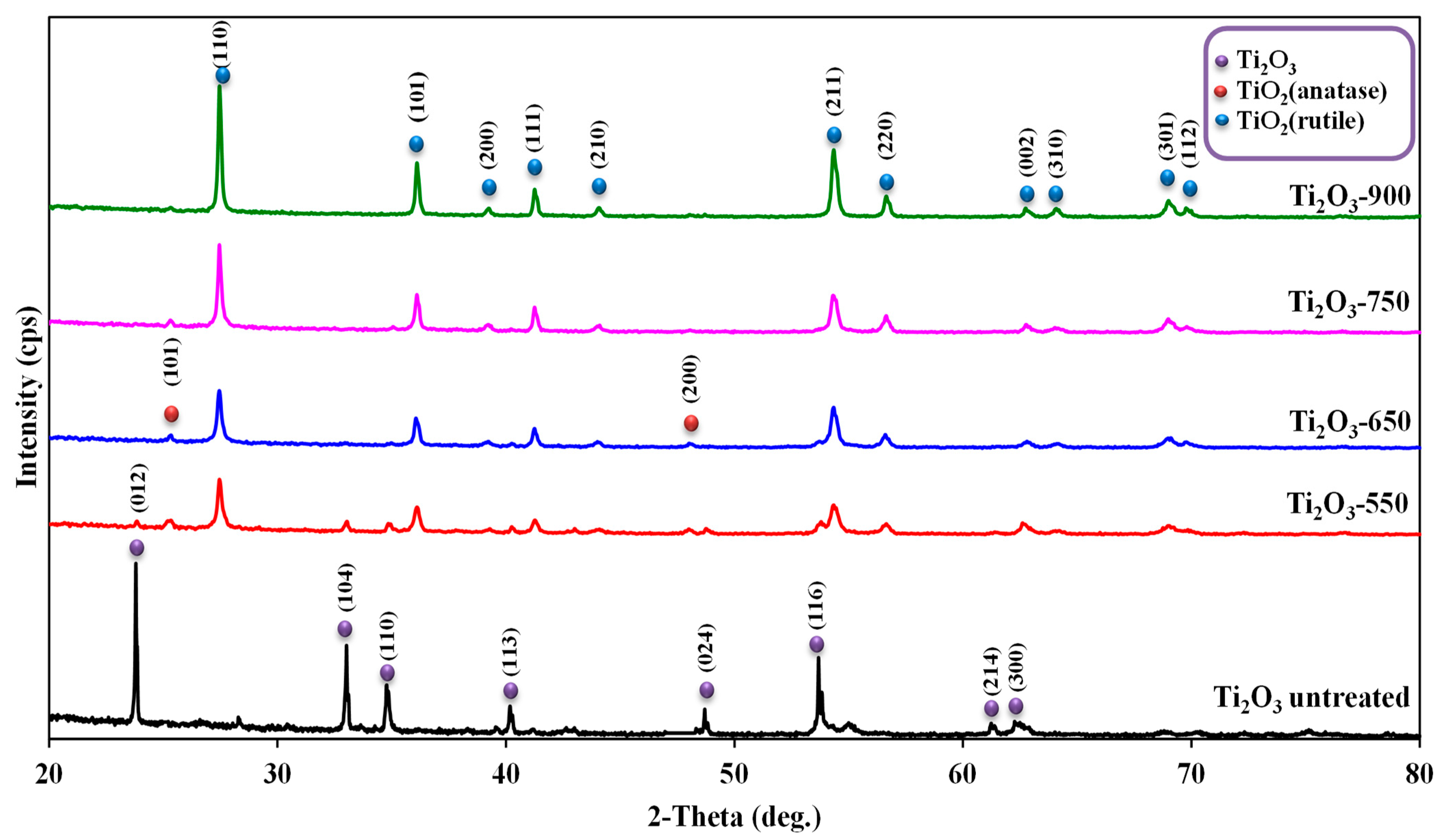
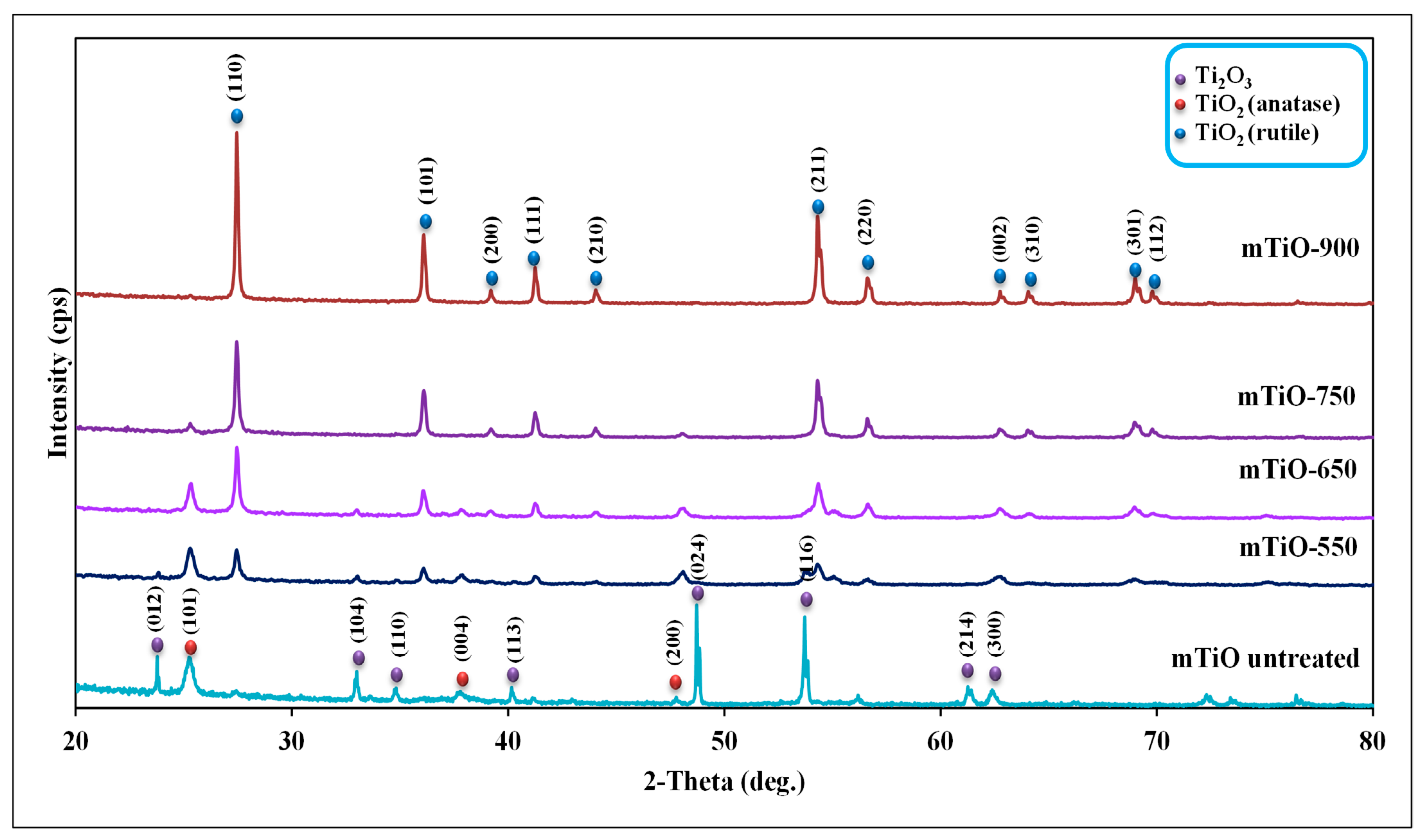
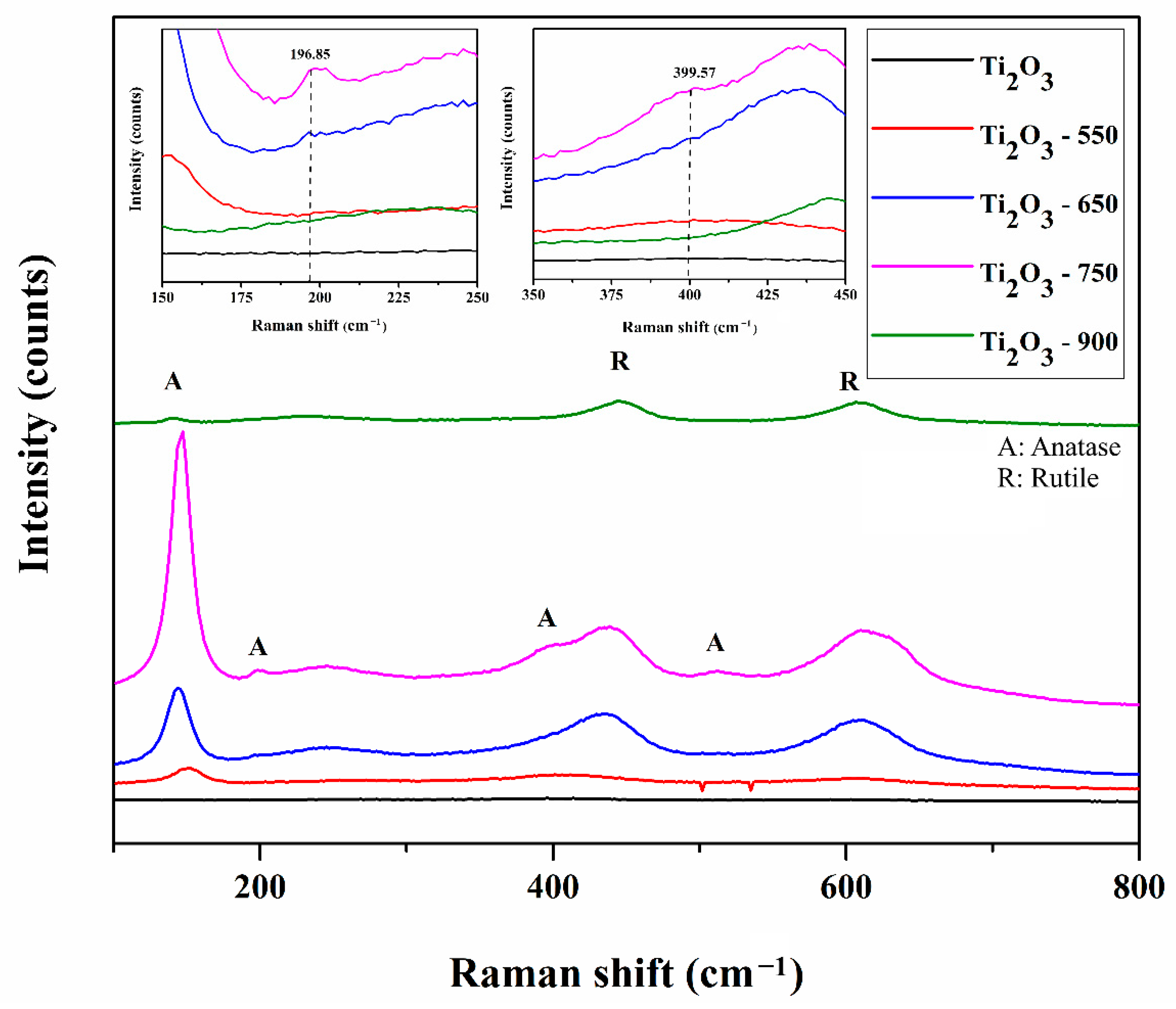
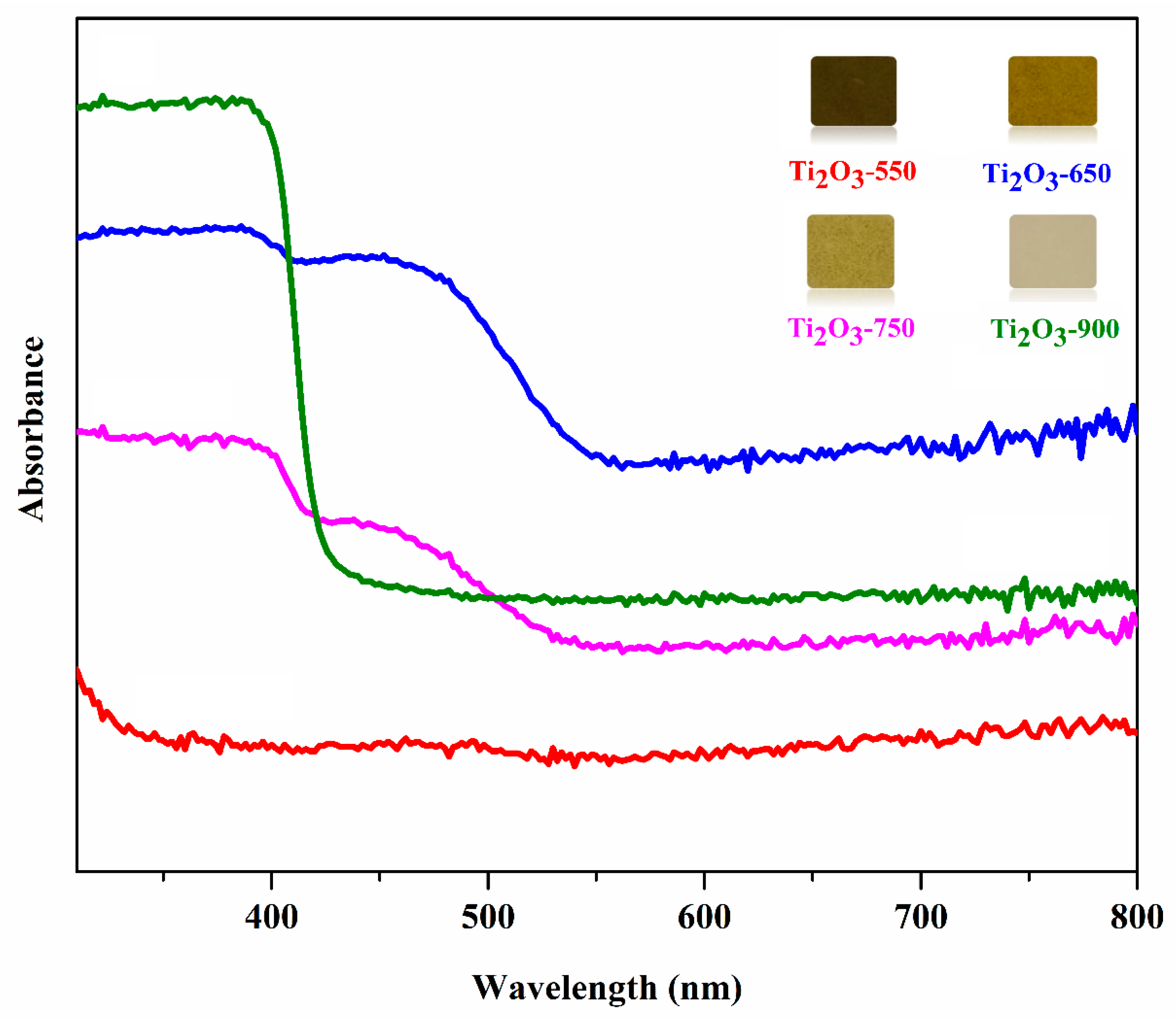
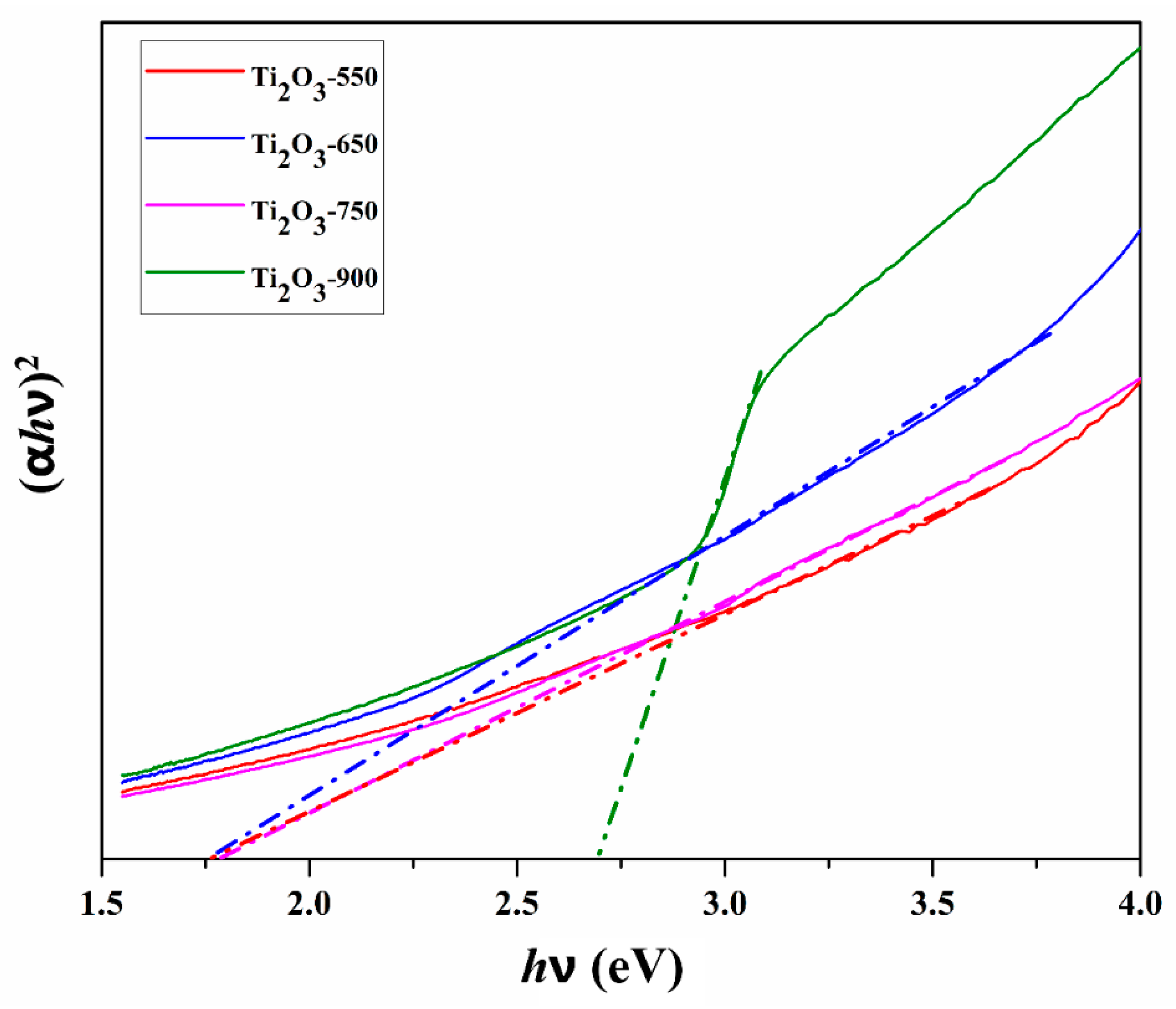
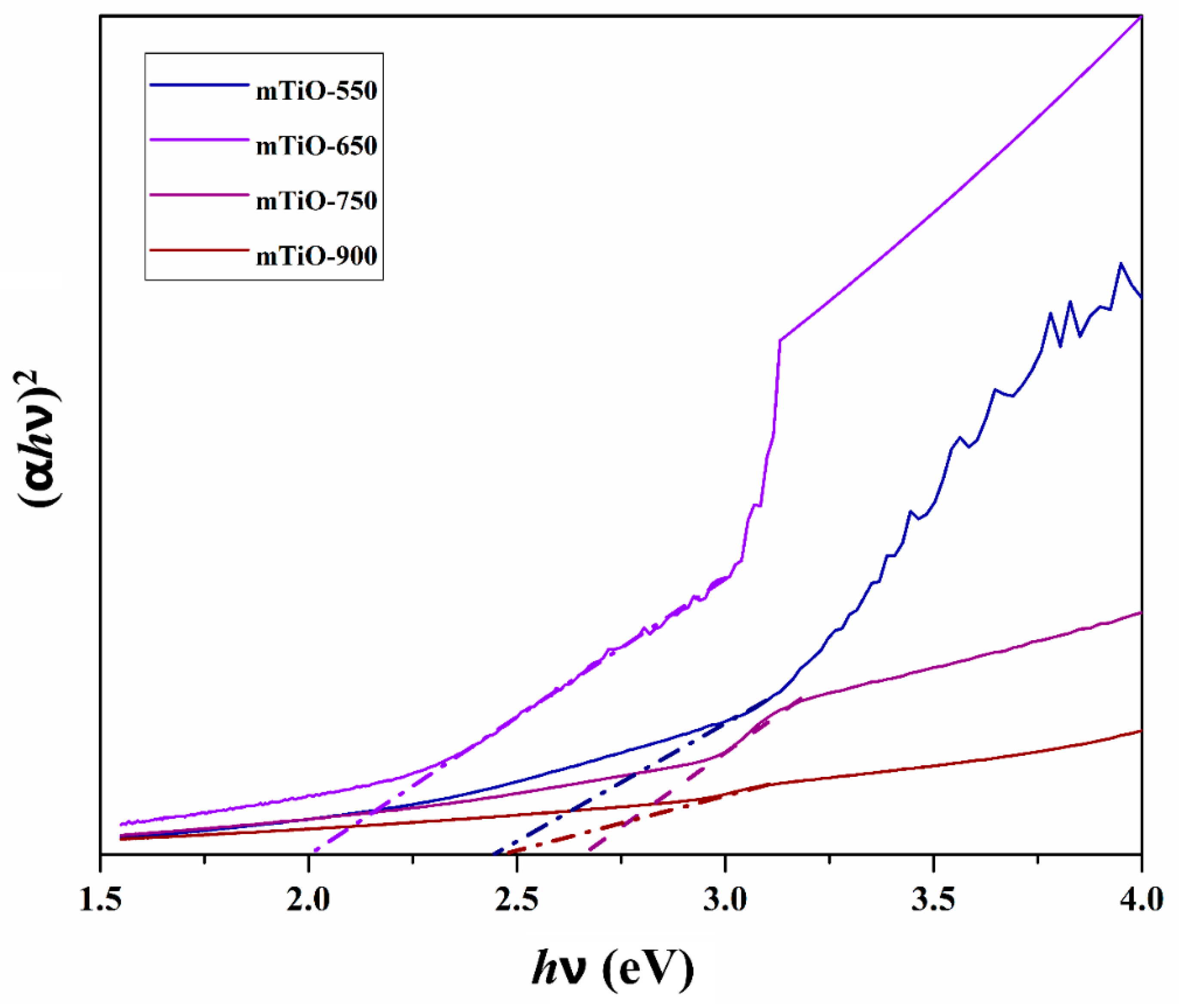
2.1. Characterization of Photocatalysts
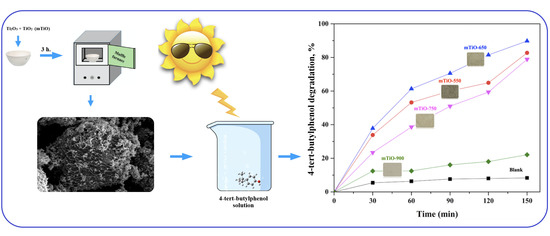
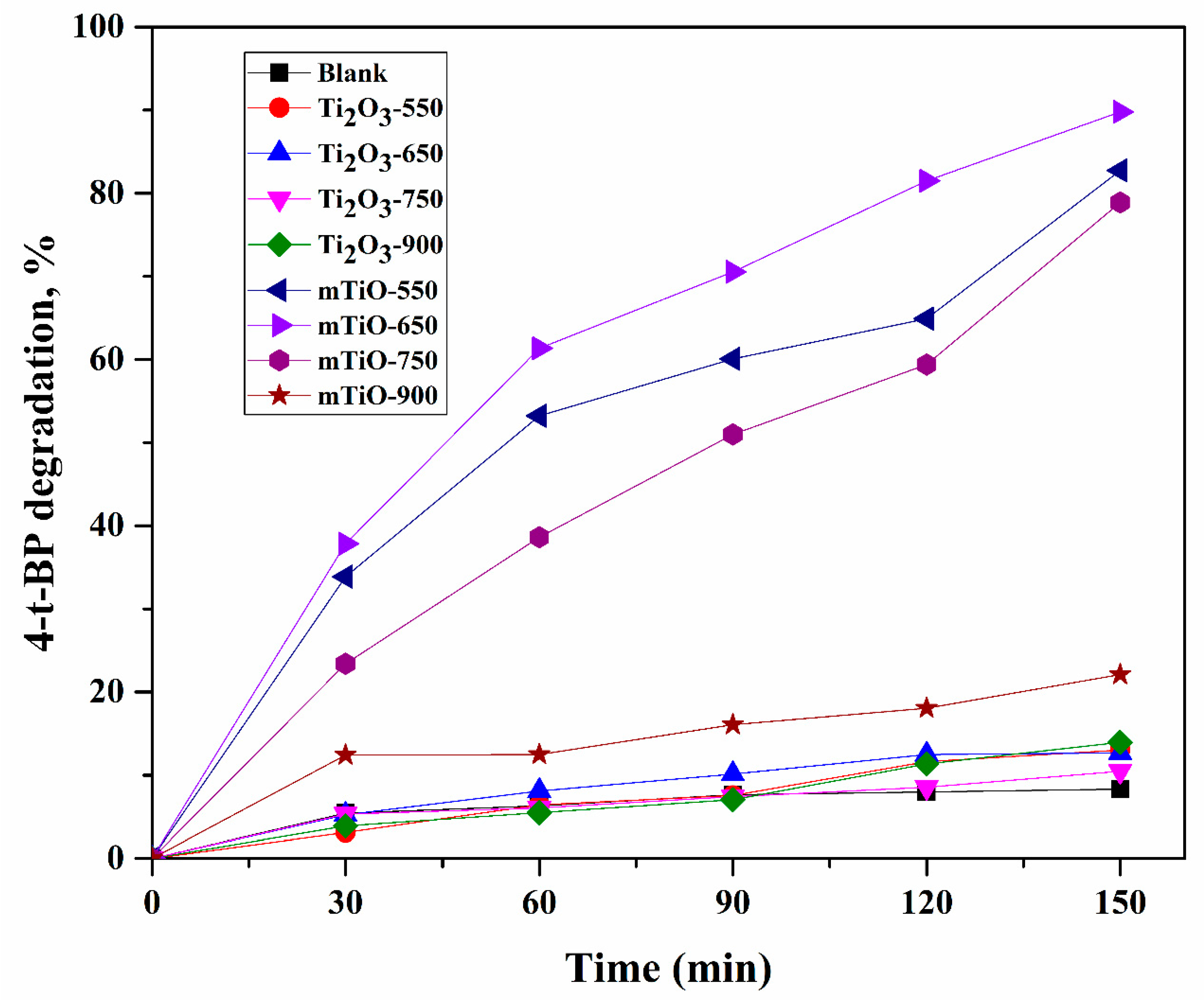
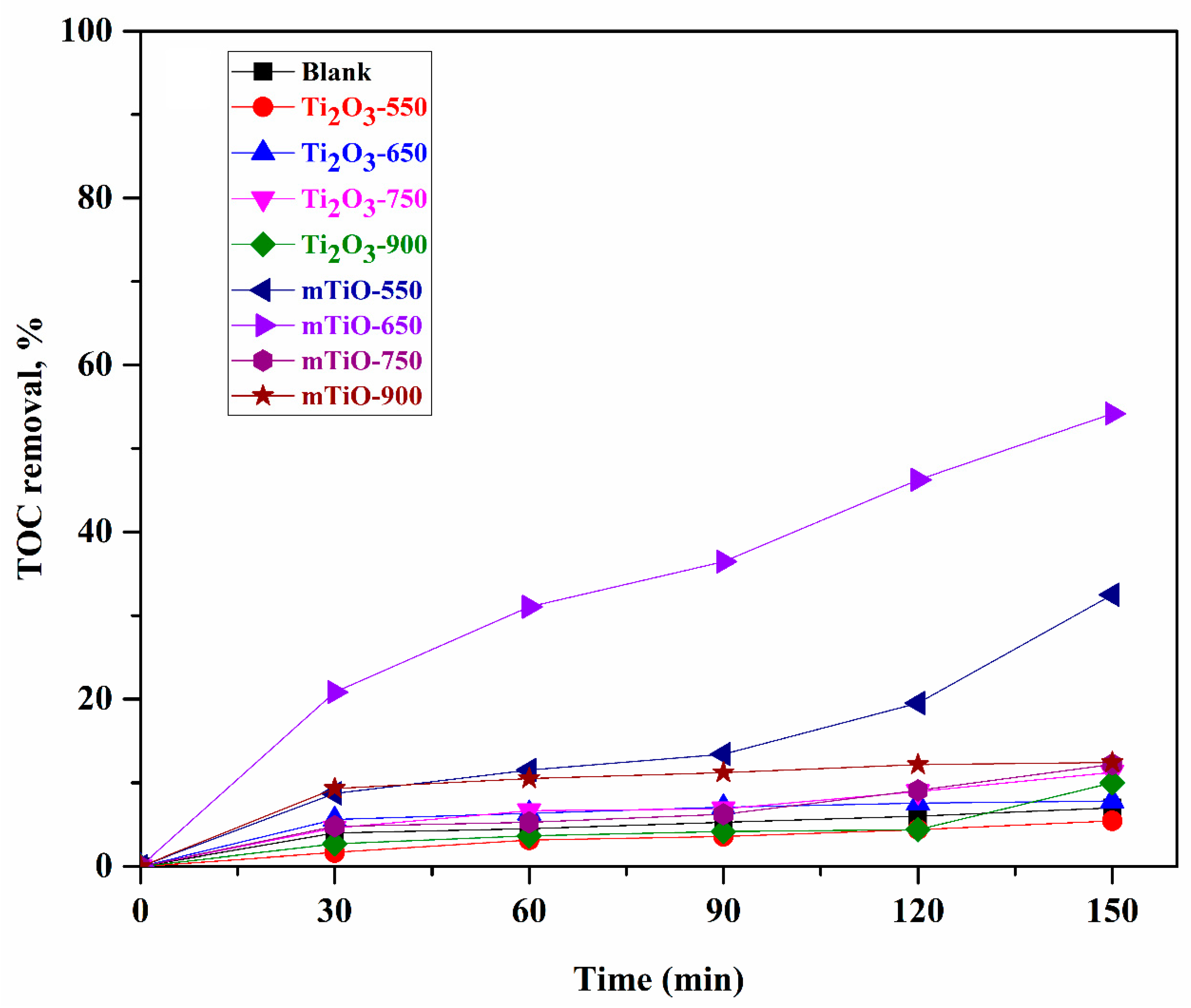
2.2. Photocatalytic Degradation of 4-t-BP in Aqueous Solution
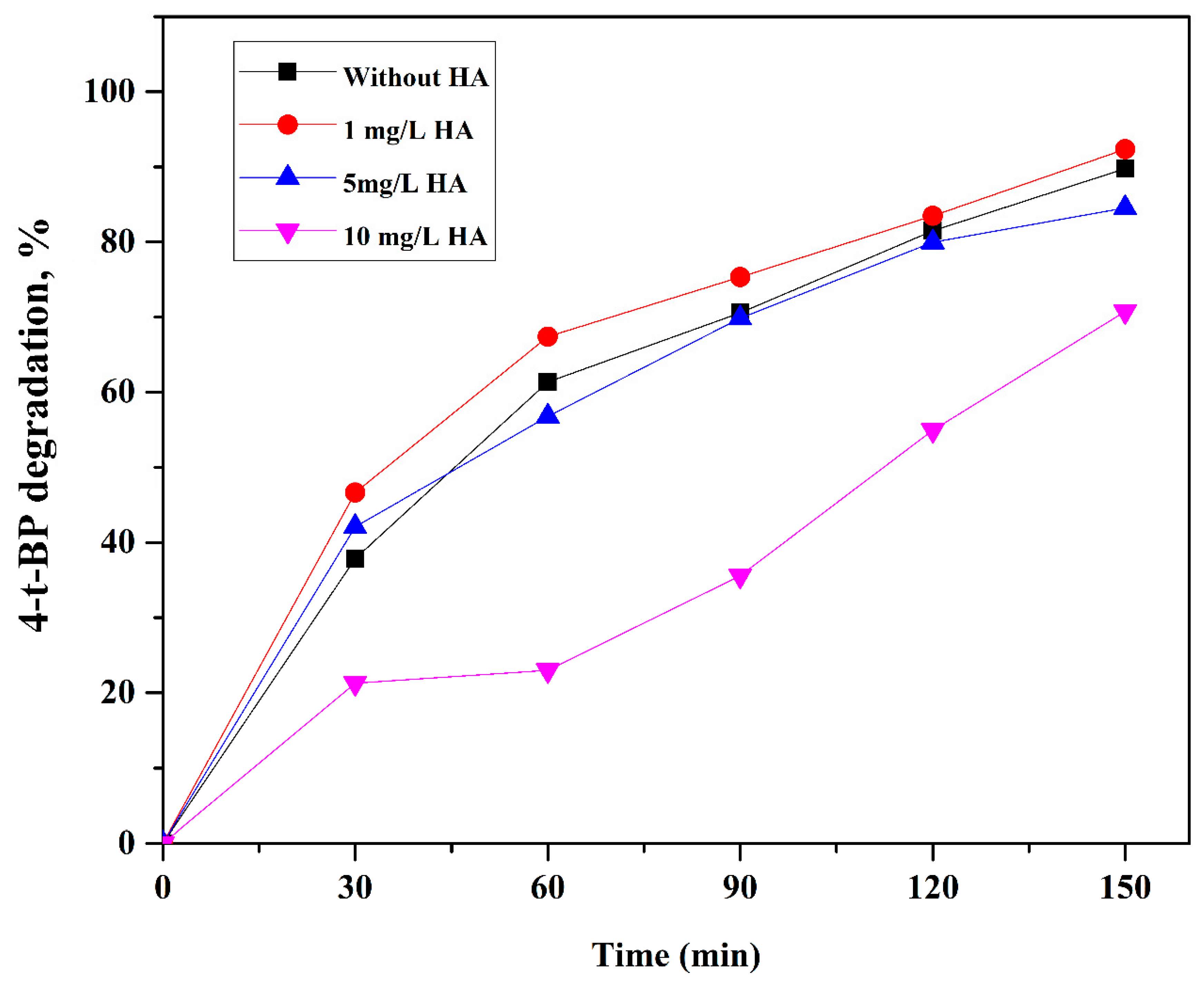
2.3. Effect of HA and Coexisting Ions (, , and ) on the Degradation of 4-t-BP
3. Materials and Methods
3.1. Materials
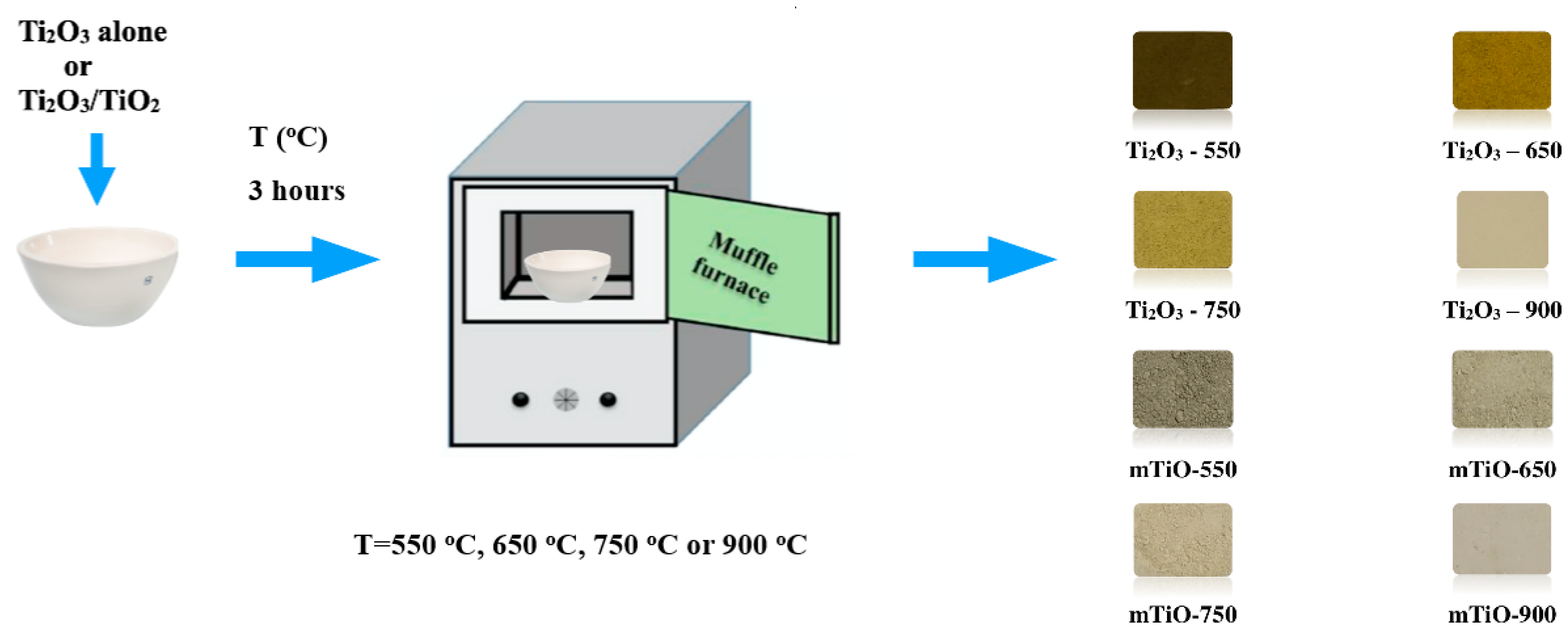
3.2. Preparation of the Photocatalysts
3.3. Characterization of the Photocatalysts
3.4. Photodegradation Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A Review on Fenton Process for Organic Wastewater Treatment Based on Optimization Perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sui, Q.; Lyu, S.; Zhao, W.; Liu, J.; Cai, Z.; Yu, G.; Barcelo, D. Municipal Solid Waste Landfills: An Underestimated Source of PPCPs in the Water Environment. Environ. Sci. Technol. 2020, 54, 9757–9768. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Rangabhashiyam, S.; Verma, P.; Singh, P.; Devi, P.; Kumar, P.; Kumar, K.S. Environmental and Health Impacts of Contaminants of Emerging Concerns: Recent Treatment Challenges and Approaches. Chemosphere 2021, 272, 129492. [Google Scholar] [CrossRef]
- Noguera-Oviedo, K.; Aga, D. Lessons Learned from More than Two Decades of Research on Emerging Contaminants in the Environment. J. Hazard. Mater. 2016, 316, 242–251. [Google Scholar] [CrossRef]
- Ferronato, N.; Torretta, V. Waste Mismanagement in Developing Countries: A Review of Global Issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fobbe, R.; Kuhlmann, B.; Nolte, J.; Preuß, G.; Skark, C.; Zullei-Seibert, N. Organic Pollutants in the Water Cycle: Properties, Occurrence, Analysis and Environmental Relevance of Polar Compounds. In Organic Pollutants in the Water Cycle: Properties, Occurrence, Analysis and Environmental Relevance of Polar Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 121–153. [Google Scholar] [CrossRef]
- Peck, A.M. Analytical Methods for the Determination of Persistent Ingredients of Personal Care Products in Environmental Matrices. Anal. Bioanal. Chem. 2006, 386, 907–939. [Google Scholar] [CrossRef] [PubMed]
- Giokas, D.L.; Salvador, A.; Chisvert, A. UV Filters: From Sunscreens to Human Body and the Environment. TrAC Trends Anal. Chem. 2007, 26, 360–374. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Adams, C.D.; Gamagedara, S.; Stayton, I.; Timmons, T.; Ma, Y. Investigation of Pharmaceuticals in Missouri Natural and Drinking Water Using High Performance Liquid Chromatography-Tandem Mass Spectrometry. Water Res. 2011, 45, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.W.-P.; Lin, J.; Tung, H.-H.; Lo, S.-L.; Lin, A. Occurrence of Pharmaceuticals and Perfluorinated Compounds and Evaluation of the Availability of Reclaimed Water in Kinmen. Emerg. Contam. 2016, 2, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Wanda, E.M.M.; Nyoni, H.; Mamba, B.B.; Msagati, T.A.M. Occurrence of Emerging Micropollutants in Water Systems in Gauteng, Mpumalanga, and North West Provinces, South Africa. Int. J. Environ. Res. Public Health 2017, 14, E79. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Jaimes, J.A.; Postigo, C.; Melgoza-Alemán, R.M.; Aceña, J.; Barceló, D.; López de Alda, M. Study of Pharmaceuticals in Surface and Wastewater from Cuernavaca, Morelos, Mexico: Occurrence and Environmental Risk Assessment. Sci. Total Environ. 2018, 613–614, 1263–1274. [Google Scholar] [CrossRef]
- Nikolaou, A. Pharmaceuticals and Related Compounds as Emerging Pollutants in Water: Analytical Aspects. Glob. Nest J. 2013, 15, 1–12. [Google Scholar]
- Seibert, D.; Quesada, H.; Bergamasco, R.; Borba, F.; Pellenz, L. Presence of Endocrine Disrupting Chemicals in Sanitary Landfill Leachate, Its Treatment and Degradation by Fenton Based Processes: A Review. Process Saf. Environ. Prot. 2019, 131, 255–267. [Google Scholar] [CrossRef]
- Reis, B.; Silveira, A.; Tostes, L.; Okuma, A.; Lange, L.; Amaral, M. Organic Compounds Removal and Toxicity Reduction of Landfill Leachate by Commercial Bakers’ Yeast and Conventional Bacteria Based Membrane Bioreactor Integrated with Nanofiltration. Waste Manag. 2017, 70, 170–180. [Google Scholar] [CrossRef]
- Dan, A.; Fujii, D.; Soda, S.; Machimura, T.; Ike, M. Removal of Phenol, Bisphenol A, and 4-Tert-Butylphenol from Synthetic Landfill Leachate by Vertical Flow Constructed Wetlands. Sci. Total Environ. 2016, 578, 566–576. [Google Scholar] [CrossRef]
- Barse, A.V.; Chakrabarti, T.; Ghosh, T.; Pal, A.; Jadhao, S. One-Tenth Dose of LC50 of 4-Tert-Butylphenol Causes Endocrine Disruption and Metabolic Changes in Cyprinus Carpio. Pestic. Biochem. Physiol. 2006, 86, 172–179. [Google Scholar] [CrossRef]
- He, G.; Xing, C.; Xiao, X.; Hu, R.; Zuo, X.; Nan, J. Facile Synthesis of Flower-like Bi12O17Cl2/β-Bi2O3 Composites with Enhanced Visible Light Photocatalytic Performance for the Degradation of 4-Tert-Butylphenol. Appl. Catal. B Environ. 2015, 170, 1–9. [Google Scholar] [CrossRef]
- Xiao, X.; Xing, C.; He, G.; Zuo, X.; Nan, J.; Wang, L. Solvothermal Synthesis of Novel Hierarchical Bi4O5I2 Nanoflakes with Highly Visible Light Photocatalytic Performance for the Degradation of 4-Tert-Butylphe. Appl. Catal. B Environ. 2014, 148–149, 154–163. [Google Scholar] [CrossRef]
- Makhatova, A.; Ulykbanova, G.; Sadyk, S.; Sarsenbay, K.; Atabaev, T.; Inglezakis, V.; Poulopoulos, S. Degradation and Mineralization of 4-Tert-Butylphenol in Water Using Fe-Doped TiO2 Catalysts. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Black & Veatch Corporation. White’s Handbook of Chlorination and Alternative Disinfectants; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent Developments in Photocatalytic Water Treatment Technology: A Review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A Review of the Existing and Emerging Technologies in the Combination of AOPs and Biological Processes in Industrial Textile Wastewater Treatment. Chem. Eng. J. 2018, 376, 120597. [Google Scholar] [CrossRef]
- Wang, J.; XU, L. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Palmisano, L.; García-López, E.I.; Marcì, G. Inorganic Materials Acting as Heterogeneous Photocatalysts and Catalysts in the Same Reactions. Dalton Trans. 2016, 45, 11596–11605. [Google Scholar] [CrossRef]
- Novak Tusar, N.; Kaucic, V.; Zabukovec Logar, N. Functionalized Porous Silicates as Catalysts for Water and Air Purification. New Future Dev. Catal. Hybrid Mater. Compos. Organocatalysts 2013, 365–383. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Siti nor Hidayah, A.; Mohamed, R.; Al-Gheethi, A.; Lai, C.W.; Yashni, G. Heterogeneous Photocatalysis of Triclocarban and Triclosan in Greywater: A Systematic and Bibliometric Review Analysis. Int. J. Environ. Anal. Chem. 2021, 0, 1–19. [Google Scholar] [CrossRef]
- Younis, S.A.; Kim, K.-H. Heterogeneous Photocatalysis Scalability for Environmental Remediation: Opportunities and Challenges. Catalysts 2020, 10, 1109. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Luo, C.; Ren, X.; Dai, Z.; Zhang, Y.; Qi, X.; Pan, C. Present Perspectives of Advanced Characterization Techniques in TiO2-Based Photocatalysts. ACS Appl. Mater. Interfaces 2017, 9, 23265–23286. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent Progress in Enhancing Photocatalytic Efficiency of TiO2-Based Materials. Appl. Catal. Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 Photocatalyst (Degussa, P-25) Consisting of Anatase and Rutile Crystalline Phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Sangchay, W.; Sikong, L.; Kooptarnond, K. Comparison of Photocatalytic Reaction of Commercial P25 and Synthetic TiO2-AgCl Nanoparticles. Procedia Eng. 2012, 32, 590–596. [Google Scholar] [CrossRef] [Green Version]
- Obaid, D. Bulk TiO2 vs alternative Ti-based photocatalysts for the mild aerobic oxidation of alcohols. Ph.D. Thesis, Université Pierre et Marie Curie-Paris VI, Paris, France, 2017. [Google Scholar]
- Khan, H.; Swati, I.K. Fe3+-Doped Anatase TiO2 with d–d Transition, Oxygen Vacancies and Ti3+ Centers: Synthesis, Characterization, UV–Vis Photocatalytic and Mechanistic Studies. Ind. Eng. Chem. Res. 2016, 55, 6619–6633. [Google Scholar] [CrossRef]
- Karthik, P.M.; Vinesh, V.; Shaheer, A.R.M.; Neppolian, B. Self-Doping of Ti3+ in TiO2 through Incomplete Hydrolysis of Titanium (IV) Isopropoxide: An Efficient Visible Light Sonophotocatalyst for Organic Pollutants Degradation. Appl. Catal. Gen. 2019, 585, 117208. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zhu, X.; Liu, X.; Li, H. 1T and 2H Mixed Phase MoS2 Nanobelts Coupled with Ti3+ Self-Doped TiO2 Nanosheets for Enhanced Photocatalytic Degradation of RhB under Visible Light. Appl. Surf. Sci. 2021, 556, 149768. [Google Scholar] [CrossRef]
- Wu, C.; Gao, Z.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y. Ti3+ Self-Doped TiO2 Photoelectrodes for Photoelectrochemical Water Splitting and Photoelectrocatalytic Pollutant Degradation. J. Energy Chem. 2016, 25, 726–733. [Google Scholar] [CrossRef]
- Li, G.; Li, J.; Li, G.; Jiang, G. N and Ti3+ Co-Doped 3D Anatase TiO2 Superstructures Composed of Ultrathin Nanosheets with Enhanced Visible Light Photocatalytic Activity. J. Mater. Chem. A 2015, 3, 22073–22080. [Google Scholar] [CrossRef]
- Randorn, C.; Irvine, J.T.S. Synthesis and Visible Light Photoactivity of a High Temperature Stable Yellow TiO2 Photocatalyst. J. Mater. Chem. 2010, 20, 8700–8704. [Google Scholar] [CrossRef]
- Xiu, Z.; Guo, M.; Zhao, T.; Pan, K.; Xing, Z.; Li, Z.; Zhou, W. Recent Advances in Ti3+ Self-Doped Nanostructured TiO2 Visible Light Photocatalysts for Environmental and Energy Applications. Chem. Eng. J. 2019, 382, 123011. [Google Scholar] [CrossRef]
- Ghosh, N.G.; Sarkar, A.; Zade, S.S. The Type-II n-n Inorganic/Organic Nano-Heterojunction of Ti3+ Self-Doped TiO2 Nanorods and Conjugated Co-Polymers for Photoelectrochemical Water Splitting and Photocatalytic Dye Degradation. Chem. Eng. J. 2021, 407, 127227. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Meng, X.; Wang, J.; Wang, S.; Lou, Z.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y. Metallic Zinc- Assisted Synthesis of Ti3+ Self-Doped TiO2 with Tunable Phase Composition and Visible-Light Photocatalytic Activity. Chem. Commun. Camb. Engl. 2012, 49, 868–870. [Google Scholar] [CrossRef]
- Fang, W.; Xing, M.; Zhang, J. A New Approach to Prepare Ti3+ Self-Doped TiO2 via NaBH4 Reduction and Hydrochloric Acid Treatment. Appl. Catal. B Environ. 2014, 160–161, 240–246. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-Light Photocatalytic, Solar Thermal and Photoelectrochemical Properties of Aluminium-Reduced Black Titania. Energy Environ. Sci. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Jeon, J.-P.; Yu, J.-S. A New Approach to Prepare Highly Active and Stable Black Titania for Visible Light-Assisted Hydrogen Production. Energy Environ. Sci. 2015, 8, 3539–3544. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Lian, Z.; Li, X.; Xu, Y.; Wang, W.; Zhang, D.; Tian, F.; Li, H. Ionothermal Synthesis of Black Ti3+-Doped Single-Crystal TiO2 as an Active Photocatalyst for Pollutant Degradation and H2 Generation. J. Mater. Chem. A 2015, 3, 3748–3756. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, G.; Wang, G.; Yuan, X.; Lin, T.; Huang, F. Progress in Black Titania: A New Material for Advanced Photocatalysis. Adv. Energy Mater. 2016, 6, 1600452. [Google Scholar] [CrossRef]
- Kako, T.; Umezawa, N.; Xie, K.; Ye, J. Undoped Visible-Light-Sensitive Titania Photocatalyst. J. Mater. Sci. 2013, 48, 108–114. [Google Scholar] [CrossRef]
- Xu, M.; Zada, A.; Yan, R.; Li, H.; Sun, N.; Qu, Y. Ti2O3/TiO2 Heterophase Junctions with Enhanced Charge Separation and Spatially Separated Active Sites for Photocatalytic CO2 Reduction. Phys. Chem. Chem. Phys. 2020, 22, 4526–4532. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Shu, X.; Wan, D.; Wei, N.; Yu, X.; Breese, M.B.H.; Venkatesan, T.; Xue, J.M.; Liu, Y.; et al. From Titanium Sesquioxide to Titanium Dioxide: Oxidation-Induced Structural, Phase, and Property Evolution. Chem. Mater. 2018, 30, 4383–4392. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Ishihara, H.; Sharma, R.; Biris, A.S. Photoelectroactivity and Raman Spectroscopy of Anodized Titania (TiO2) Photoactive Water-Splitting Catalysts as a Function of Oxygen- Annealing Temperature. J. Mater. Chem. 2011, 21, 6337–6345. [Google Scholar] [CrossRef]
- Yahya, N.; Aziz, F.; Jaafar, J.; Lau, W.J.; Yusof, N.; Norharyati, W.; Ismail, A.; Aziz, M. Impacts of Annealing Temperature on Morphological, Optical and Photocatalytic Properties of Gel-Combustion-Derived LaFeO3 Nanoparticles. Arab. J. Sci. Eng. 2020, 46, 6153–6165. [Google Scholar] [CrossRef]
- Ji, J.; Xu, Y.; Huang, H.; He, M.; Liu, S.; Liu, G.; Xie, R.; Feng, Q.; Shu, Y.; Zhan, Y.; et al. Mesoporous TiO2 under VUV Irradiation: Enhanced Photocatalytic Oxidation for VOCs Degradation at Room Temperature. Chem. Eng. J. 2017, 327, 490–499. [Google Scholar] [CrossRef]
- Hamdy, M.; Saputera, W.; Groenen, E.; Mul, G. A Novel TiO2 Composite for Photocatalytic Wastewater Treatment. J. Catal. 2014, 310, 75–83. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, H.; Xin, Y.; Cheng, X. TiO2 Nanobelts—Effect of Calcination Temperature on Optical, Photoelectrochemical and Photocatalytic Properties. Electrochim. Acta 2013, 111, 284–291. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ai, Z.; Jia, F.; Zhang, L. Oxygen Vacancy-Mediated Photocatalysis of BiOCl: Reactivity, Selectivity and Perspective. Angew. Chem. Int. Ed. Engl. 2017, 57, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ao, Z.; Sun, H.; Duan, X.; Wang, S. Activation of Peroxymonosulfate by Carbonaceous Oxygen Groups: Experimental and Density Functional Theory Calculations. Appl. Catal. B Environ. 2016, 198, 295–302. [Google Scholar] [CrossRef]
- Yang, W.; Bradford, S.; Wang, Y.; Sharma, P.; Shang, J.; Baoguo, L. Transport of Biochar Colloids in Saturated Porous Media in the Presence of Humic Substances or Proteins. Environ. Pollut. 2018, 246, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Liu, B.-M.; Lu, M.-F.; Li, Y.-P.; Jiang, Y.-Y.; Zhao, M.-X.; Huang, Z.-X.; Pan, Y.; Miao, H.-F.; Ruan, W.-Q. Characterization of Algal Organic Matter as Precursors for Carbonaceous and Nitrogenous Disinfection Byproducts Formation: Comparison with Natural Organic Matter. J. Environ. Manag. 2021, 282, 111951. [Google Scholar] [CrossRef]
- Wu, W.; Guoqiang, S.; Wang, S.; Zhu, L.; Yue, L.; Xiang, Q.; Zhang, Y.; Li, Z. Environmentally Relevant Impacts of Nano-TiO2 on Abiotic Degradation of Bisphenol A under Sunlight Irradiation. Environ. Pollut. 2016, 216, 166–172. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Z.; Wang, D.; Gao, C.; Ji, R. Photocatalytic Mineralization of Dimethoate in Aqueous Solutions Using TiO2: Parameters and by-Products Analysis. Desalination 2010, 258, 28–33. [Google Scholar] [CrossRef]
- Cheng, R.; Kang, M.; Shen, Z.-P.; Shi, L.; Zheng, X. Visible-Light-Driven Photocatalytic Inactivation of Bacteriophage F2 by Cu-TiO2 Nanofibers in the Presence of Humic Acid. J. Environ. Sci. 2018, 77. [Google Scholar] [CrossRef]
- Li, L.; Zheng, X.; Chi, Y.; Wang, Y.; Sun, X.; Yue, Q.; Zhou, W.; Xu, S. Molecularly Imprinted Carbon Nanosheets Supported TiO2: Strong Selectivity and Synergic Adsorption-Photocatalysis for Antibiotics Removal. J. Hazard. Mater. 2019, 383, 121211. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Guilong, Z.; Pang, T.; Zhang, X.; Cai, D.; Wu, Z. In Situ Degradation of Antibiotic Residues in Medical Intravenous Infusion Bottles Using High Energy Electron Beam Irradiation. Sci. Rep. 2017, 7, 39928. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, S.; Zhao, Y.; Cao, H.; Liu, F. Radiolytic Decomposition of Ciprofloxacin Using γ Irradiation in Aqueous Solution. Environ. Sci. Pollut. Res. 2015, 22, 15772–15780. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Yu, N.; Xin, S.; Xin, Y.; Zhang, C.; Ma, X.; Gao, M. Photoelectrocatalytic Degradation of P-Chloronitrobenzene by g-C3N4/TiO2 Nanotube Arrays Photoelectrodes under Visible Light Irradiation. Chemosphere 2021, 267, 129242. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, Z.; Ma, D.; Liu, G.; Song, N.; Xiang, D.; Xin, Y.; Zhang, G.; Chen, Q. Improving Photocatalytic Activity by Construction of Immobilized Z-Scheme CdS/Au/TiO2 Nanobelt Photocatalyst for Eliminating Norfloxacin from Water. J. Colloid Interface Sci. 2021, 586, 243–256. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A.; Prakoso, S.P. Fabrication of Ag2O/TiO2 Composites on Nanographene Platelets for the Removal of Organic Pollutants: Influence of Oxidants and Inorganic Anions. Appl. Surf. Sci. 2019, 480, 697–708. [Google Scholar] [CrossRef]
- Chládková, B.; Evgenidou, E.; Kvitek, L.; Panacek, A.; Zboril, R.; Kovář, P.; Lambropoulou, D. Adsorption and Photocatalysis of Nanocrystalline TiO2 Particles for Reactive Red 195 Removal: Effect of Humic Acids, Anions and Scavengers. Environ. Sci. Pollut. Res. Int. 2015, 22, 16514–16524. [Google Scholar] [CrossRef] [PubMed]
- Barka, N.; Qourzal, S.; Assabbane, A.; Nounah, A.; Ait-Ichou, Y. Factors Influencing the Photocatalytic Degradation of Rhodamine B by TiO2-Coated Non-Woven Paper. J. Photochem. Photobiol. Chem. 2008, 195, 346–351. [Google Scholar] [CrossRef]
- Ziegmann, M.; Doll, T.; Frimmel, F.H. Matrix Effects on the Photocatalytical Degradation of Dichloroacetic Acid and Atrazine in Water. Acta Hydrochim. Hydrobiol. 2006, 34, 146–154. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Motti, C.; Glass, B.; Oelgemöller, M. TiO2 Photocatalysis of Naproxen: Effect of the Water Matrix, Anions and Diclofenac on Degradation Rates. Chemosphere 2015, 139, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Bekkouche, S.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Efficient Photocatalytic Degradation of Safranin O by Integrating Solar-UV/TiO2/Persulfate Treatment: Implication of Sulfate Radical in the Oxidation Process and Effect of Various Water Matrix Components. J. Photochem. Photobiol. Chem. 2017, 345, 80–91. [Google Scholar] [CrossRef]

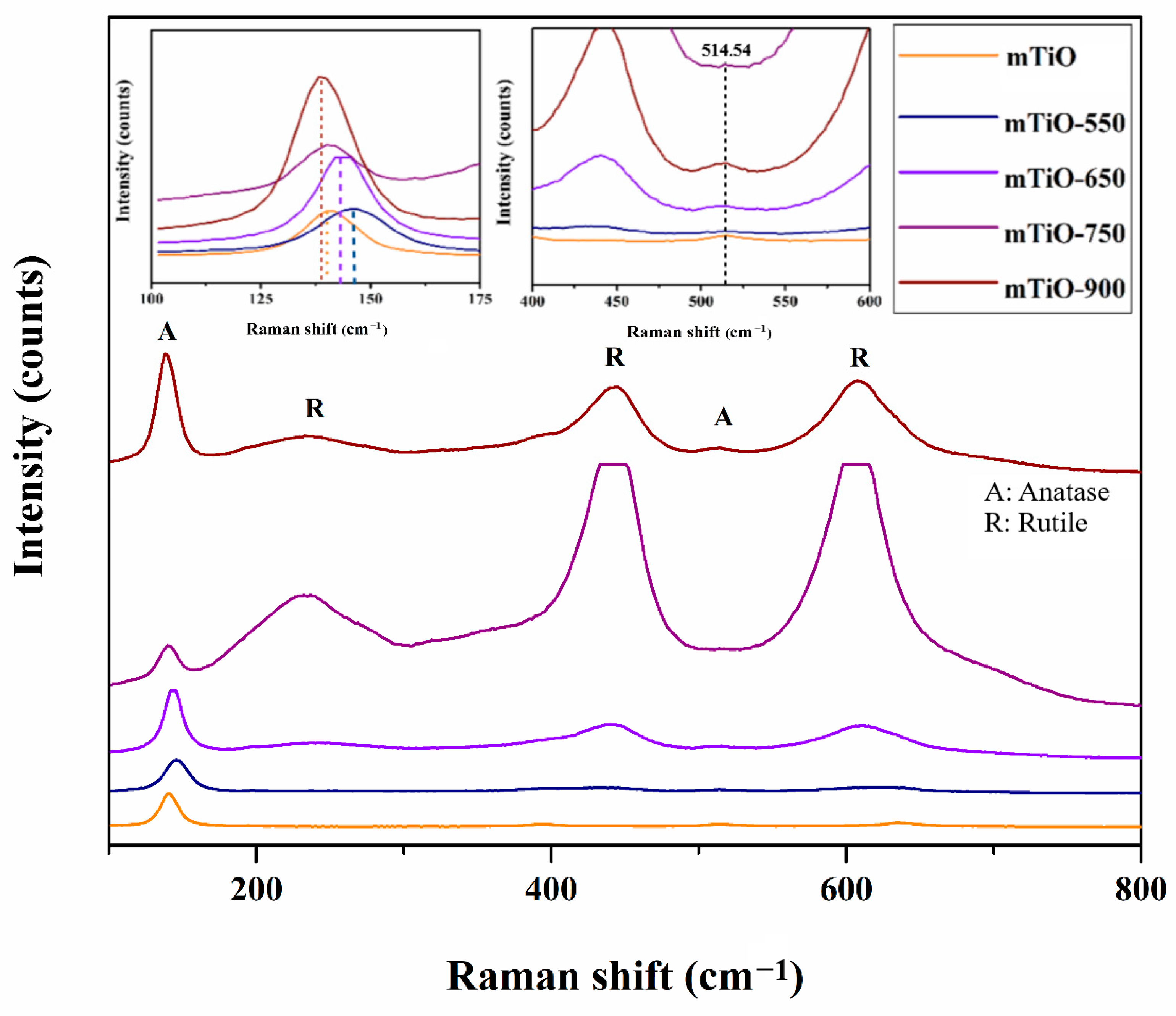
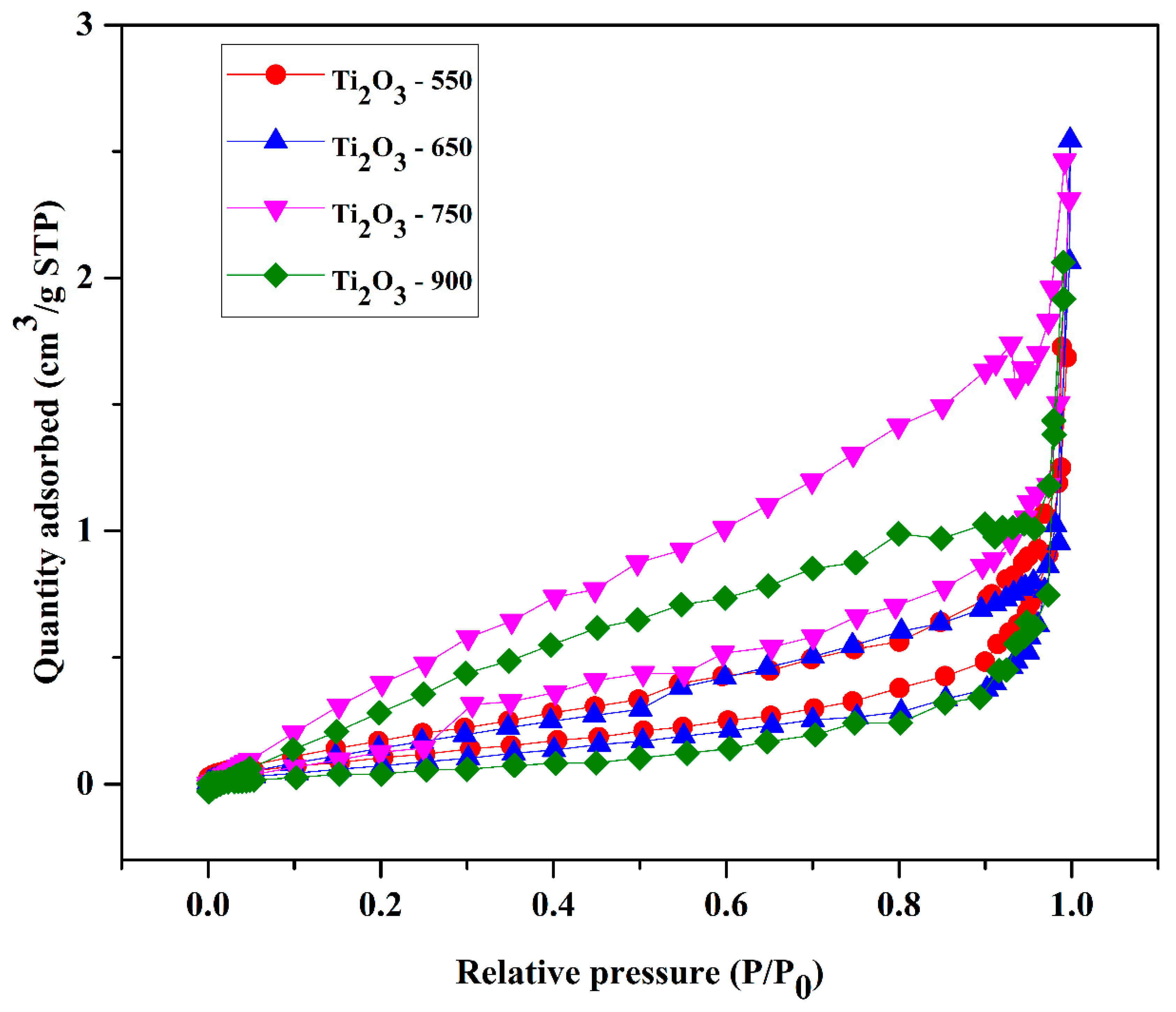
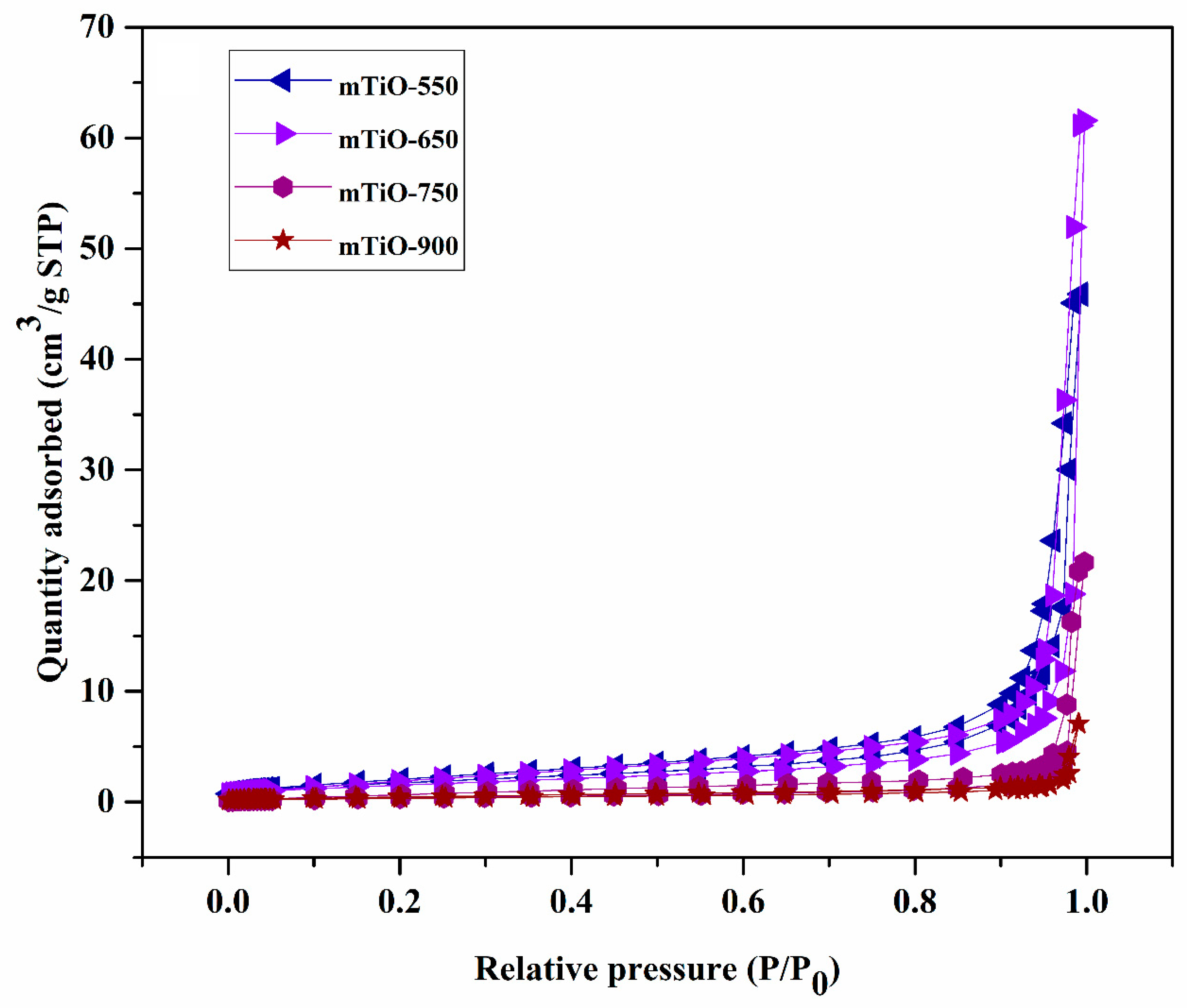
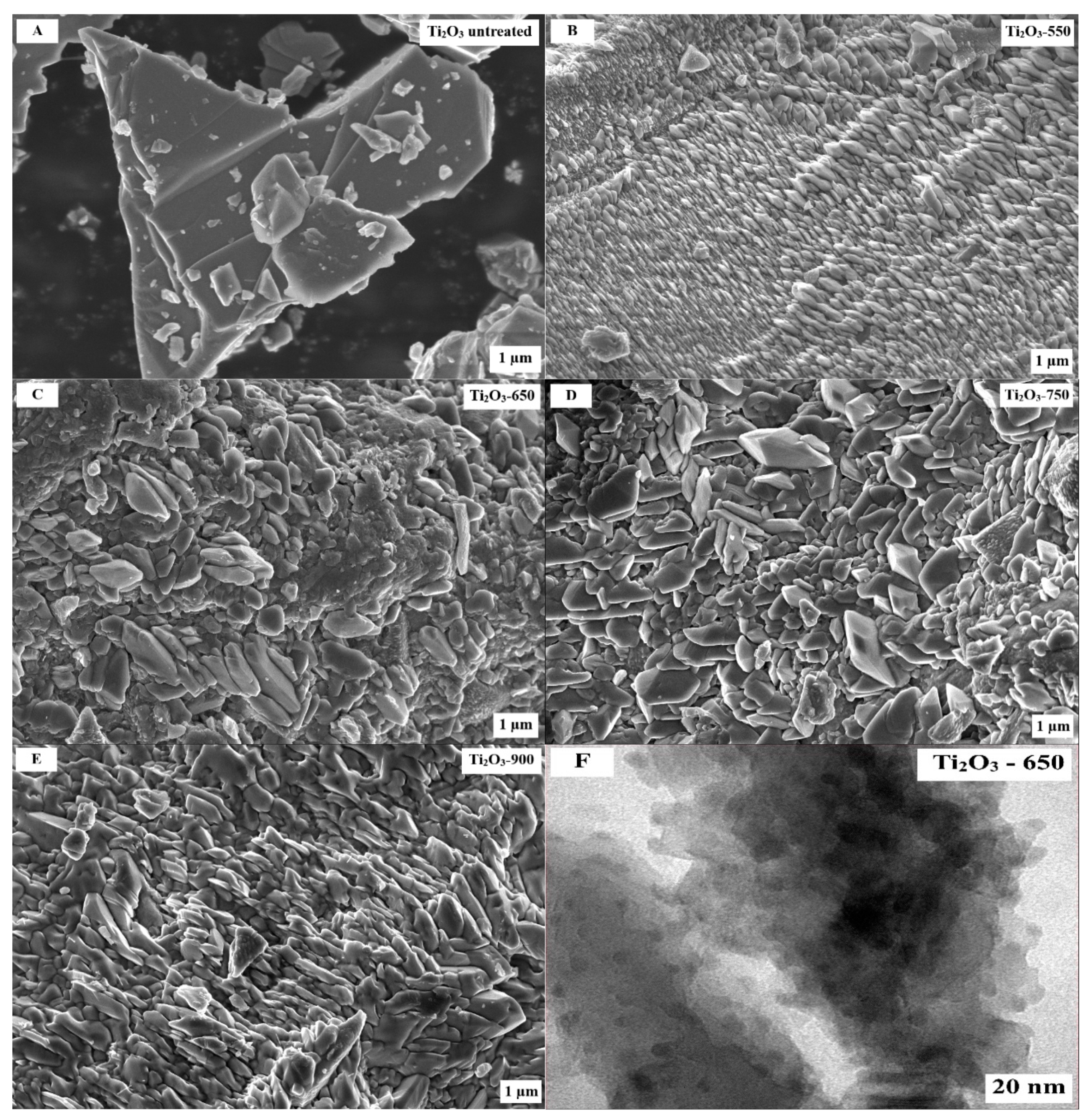



| Photocatalyst | SBET (m2/g) | Vp (cm3/g) |
|---|---|---|
| Ti2O3-550 | 1.629 | 0.009 |
| Ti2O3-650 | 1.985 | 0.017 |
| Ti2O3-750 | 2.733 | 0.014 |
| Ti2O3-900 | 0.974 | 0.012 |
| mTiO-550 | 23.012 | 0.255 |
| mTiO-650 | 20.894 | 0.347 |
| mTiO-750 | 5.593 | 0.134 |
| mTiO-900 | 3.443 | 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mergenbayeva, S.; Sh. Atabaev, T.; Poulopoulos, S.G. Ti2O3/TiO2-Assisted Solar Photocatalytic Degradation of 4-tert-Butylphenol in Water. Catalysts 2021, 11, 1379. https://doi.org/10.3390/catal11111379
Mergenbayeva S, Sh. Atabaev T, Poulopoulos SG. Ti2O3/TiO2-Assisted Solar Photocatalytic Degradation of 4-tert-Butylphenol in Water. Catalysts. 2021; 11(11):1379. https://doi.org/10.3390/catal11111379
Chicago/Turabian StyleMergenbayeva, Saule, Timur Sh. Atabaev, and Stavros G. Poulopoulos. 2021. "Ti2O3/TiO2-Assisted Solar Photocatalytic Degradation of 4-tert-Butylphenol in Water" Catalysts 11, no. 11: 1379. https://doi.org/10.3390/catal11111379
APA StyleMergenbayeva, S., Sh. Atabaev, T., & Poulopoulos, S. G. (2021). Ti2O3/TiO2-Assisted Solar Photocatalytic Degradation of 4-tert-Butylphenol in Water. Catalysts, 11(11), 1379. https://doi.org/10.3390/catal11111379