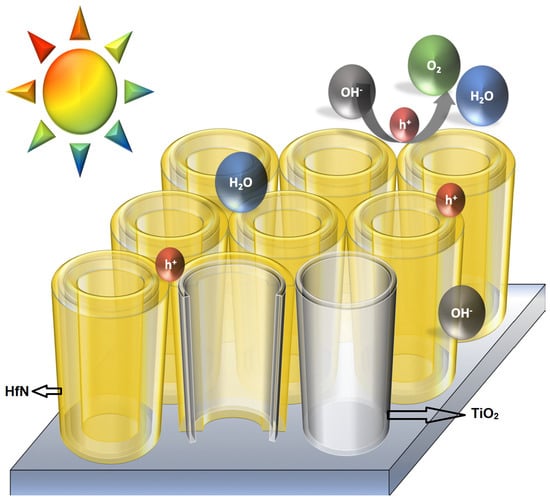
TiO2-HfN Radial Nano-Heterojunction: A Hot Carrier Photoanode for Sunlight-Driven Water-Splitting
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photoelectrochemical Performance
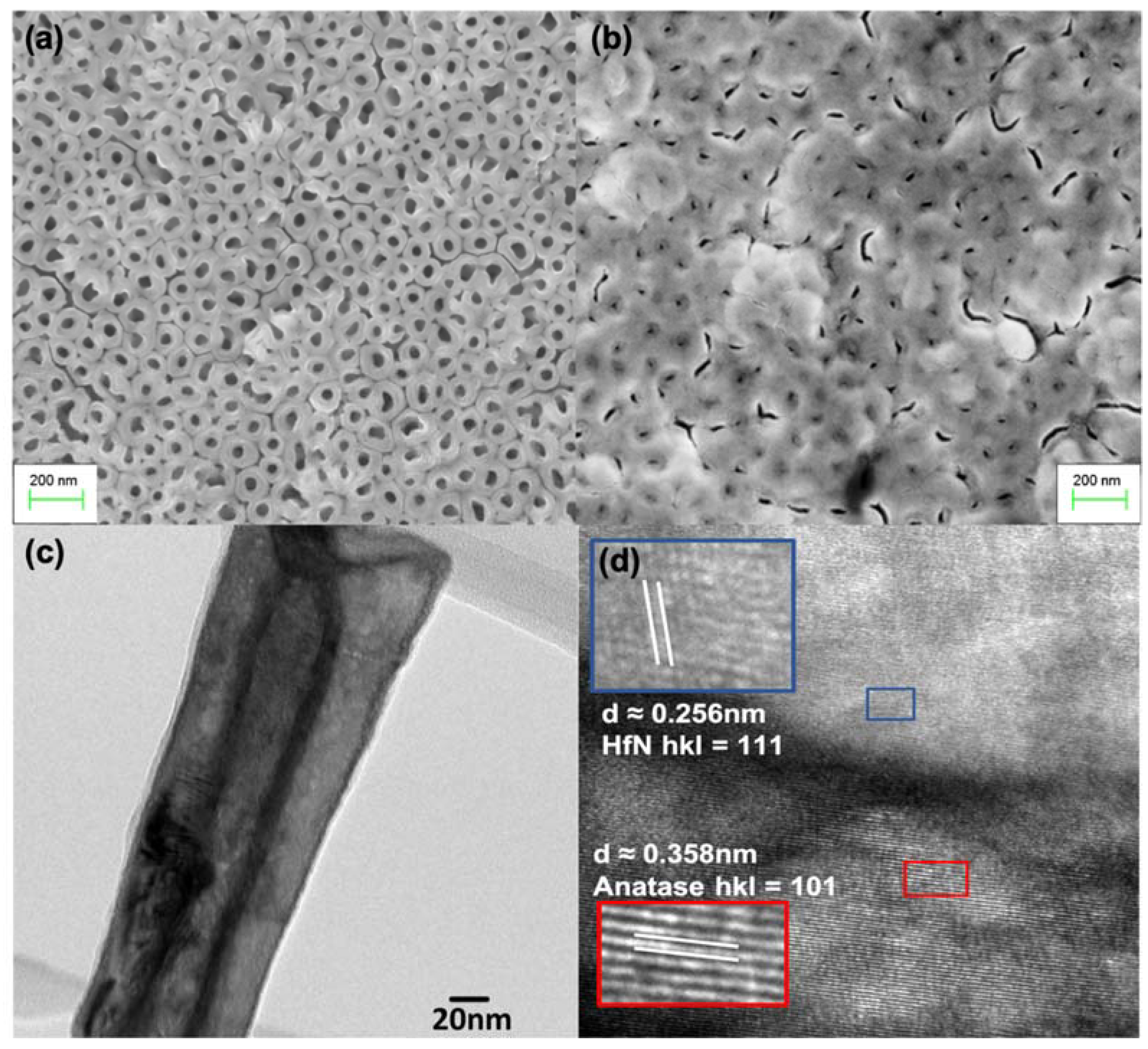
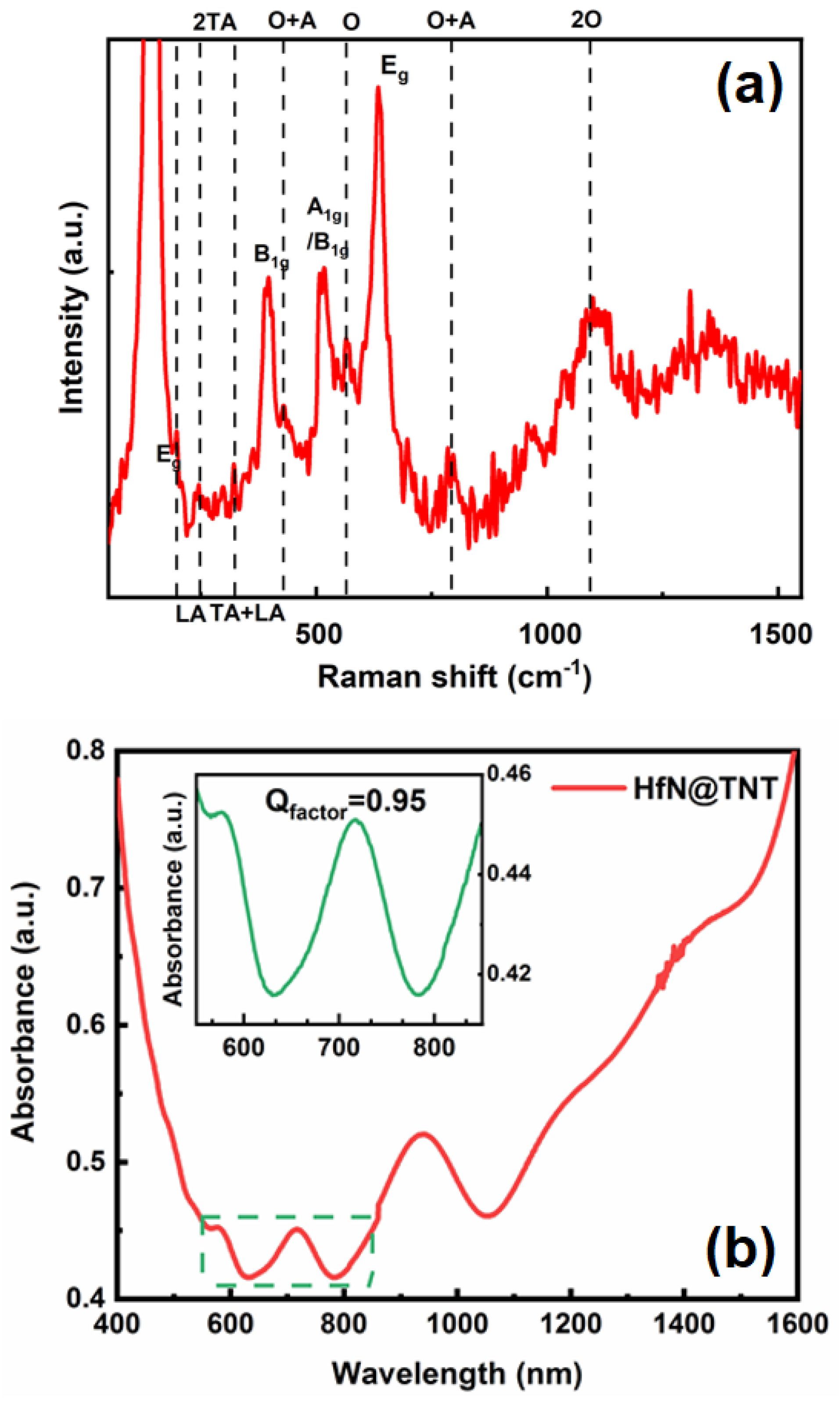
2.2. Physicochemical Characterization
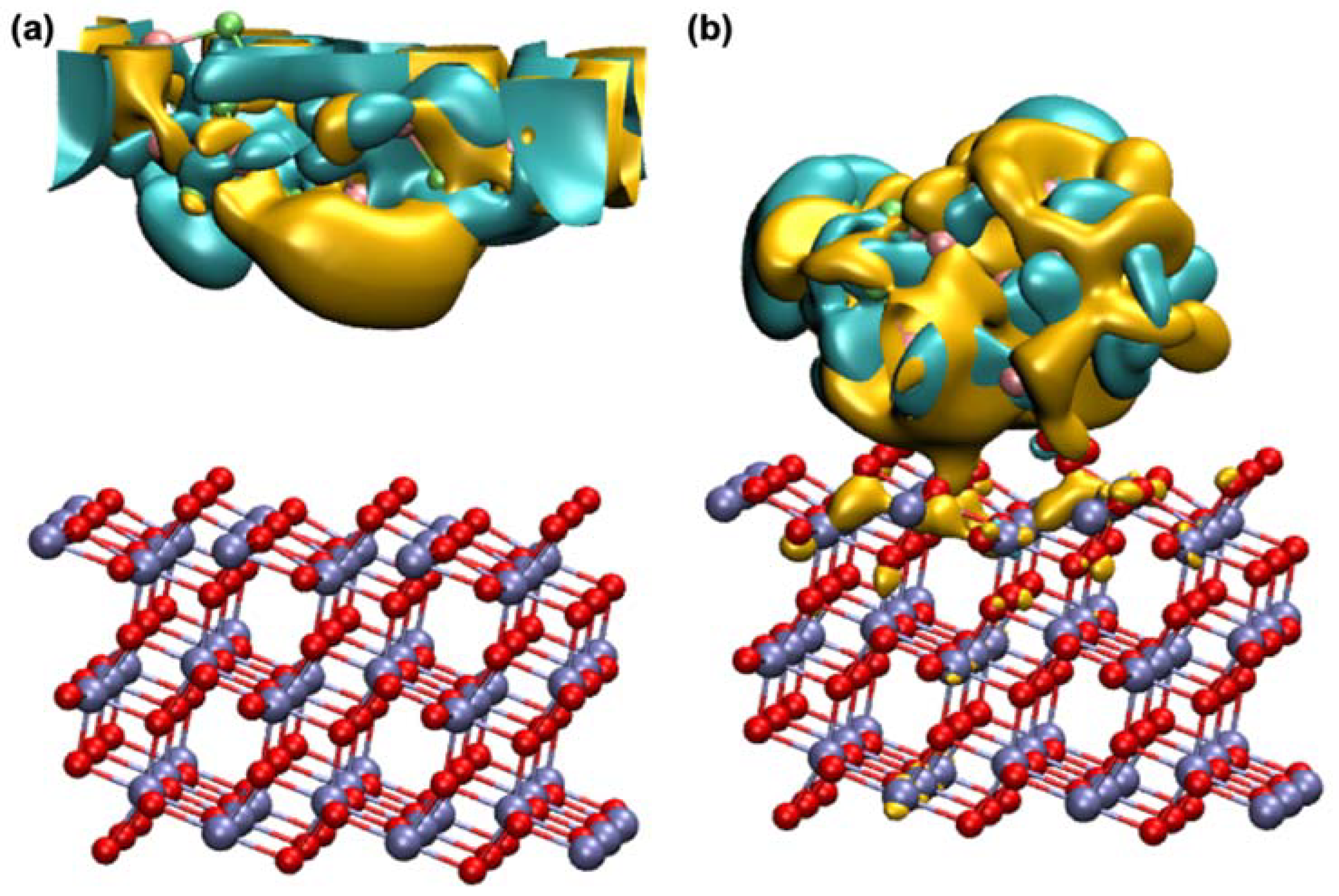
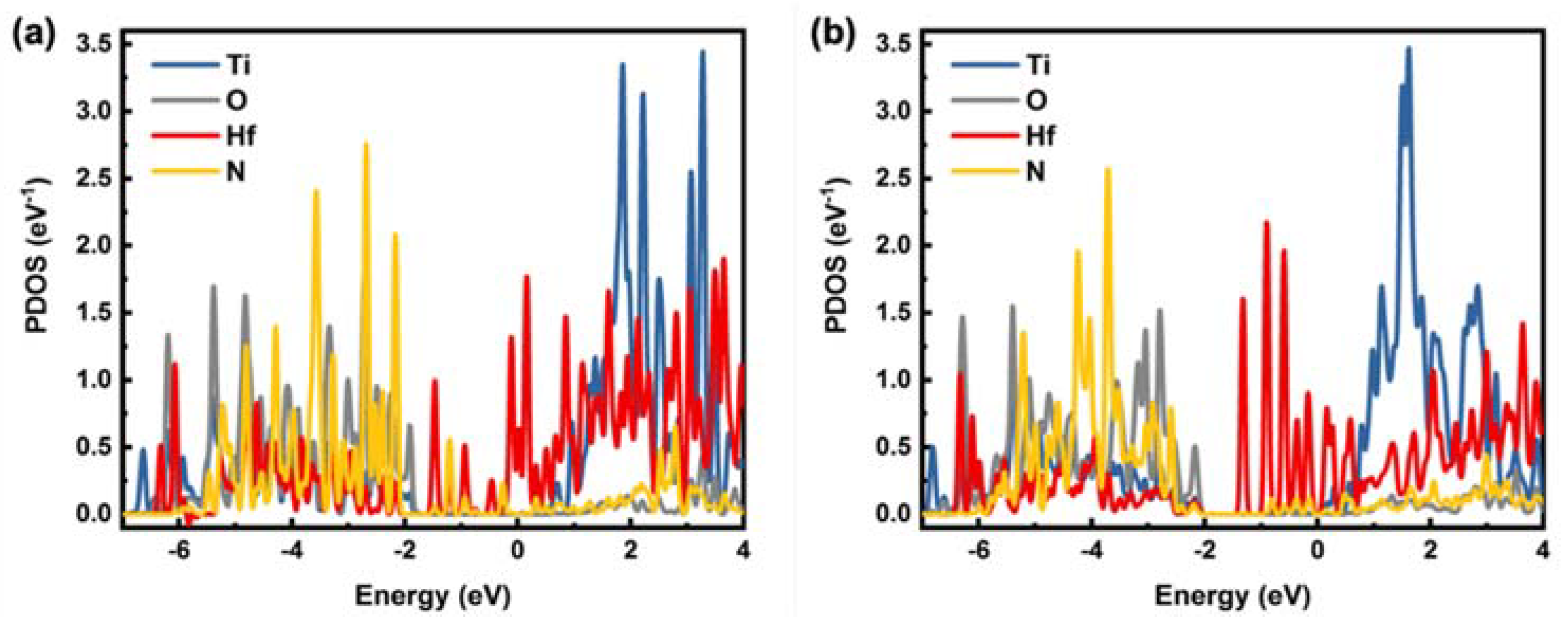
2.3. Quantum Computation Results
3. Materials and Methods
3.1. TiO2 Nanotube Array Synthesis
3.2. ALD Deposition of Hafnium Nitride
3.3. Materials Characterization
3.4. Photoelectrochemical Measurements
3.5. DFT Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oyama, S.T. Introduction to the chemistry of transition metal carbides and nitrides. In The Chemistry of Transition Metal Carbides and Nitrides; Oyama, S.T., Ed.; Springer: Dordrecht, The Netherlands, 1996; pp. 1–27. [Google Scholar] [CrossRef]
- Guler, U.; Shalaev, V.M.; Boltasseva, A. Nanoparticle plasmonics: Going practical with transition metal nitrides. Mater. Today 2014, 18, 227–237. [Google Scholar] [CrossRef]
- Sproul, W.D. Hafnium nitride coatings prepared by very high rate reactive sputtering. Thin Solid Films 1984, 118, 279–284. [Google Scholar] [CrossRef]
- Karaballi, R.A.; Humagain, G.; Fleischman, B.R.A.; Dasog, M. Synthesis of Plasmonic Group-4 Nitride Nanocrystals by Solid-State Metathesis. Angew. Chem. Int. Ed. 2019, 58, 3147–3150. [Google Scholar] [CrossRef] [PubMed]
- Patsalas, P.; Kalfagiannis, N.; Kassavetis, S. Optical Properties and Plasmonic Performance of Titanium Nitride. Materials 2015, 8, 3128–3154. [Google Scholar] [CrossRef] [Green Version]
- Naik, G.V.; Schroeder, J.L.; Ni, X.; Kildishev, A.V.; Sands, T.D.; Boltasseva, A. Titanium nitride as a plasmonic material for visible and near-infrared wavelengths. Opt. Mater. Express 2012, 2, 478–489. [Google Scholar] [CrossRef] [Green Version]
- Farsinezhad, S.; Shanavas, T.; Mahdi, N.; Askar, A.M.; Kar, P.; Sharma, H.; Shankar, K. Core–shell titanium dioxide–titanium nitride nanotube arrays with near-infrared plasmon resonances. Nanotechnology 2018, 29, 154006. [Google Scholar] [CrossRef]
- Saha, B.; Acharya, J.; Sands, T.D.; Waghmare, U.V. Electronic structure, phonons, and thermal properties of ScN, ZrN, and HfN: A first-principles study. J. Appl. Phys. 2010, 107, 033715. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.; Shrestha, S.; Wen, X.; Feng, Y.; Gupta, N.; Xia, H.; Yu, P.; Tang, J.; Conibeer, G. Hafnium nitride for hot carrier solar cells. Sol. Energy Mater. Sol. Cells 2016, 144, 781–786. [Google Scholar] [CrossRef]
- Defilippi, C.; Shinde, D.V.; Dang, Z.; Manna, L.; Hardacre, C.; Greer, A.J.; D’Agostino, C.; Giordano, C. HfN Nanoparticles: An Unexplored Catalyst for the Electrocatalytic Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2019, 58, 15464–15470. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, F.; Yeh, Y.-W.; Selinsky, R.S.; Chen, Z.; Yao, N.; Tully, C.G.; Ju, Y.; Koel, B.E. Nitrogen-plasma treated hafnium oxyhydroxide as an efficient acid-stable electrocatalyst for hydrogen evolution and oxidation reactions. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Muneshwar, T.; Cadien, K. Comparing XPS on bare and capped ZrN films grown by plasma enhanced ALD: Effect of ambient oxidation. Appl. Surf. Sci. 2018, 435, 367–376. [Google Scholar] [CrossRef]
- Muneshwar, T.; Cadien, K. Low temperature plasma enhanced atomic layer deposition of conducting zirconium nitride films using tetrakis (dimethylamido) zirconium and forming gas (5% H2 + 95% N2) plasma. J. Vac. Sci. Technol. A 2015, 33, 31502. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, S.; Han, D.; Liu, T.; Wang, G.; Li, Y. Progress in Developing Metal Oxide Nanomaterials for Photoelectrochemical Water Splitting. Adv. Energy Mater. 2017, 7, 1700555. [Google Scholar] [CrossRef]
- Kumar, P.; Thakur, U.K.; Alam, K.; Kar, P.; Kisslinger, R.; Zeng, S.; Patel, S.; Shankar, K. Arrays of TiO2 nanorods embedded with fluorine doped carbon nitride quantum dots (CNFQDs) for visible light driven water splitting. Carbon 2018, 137, 174–187. [Google Scholar] [CrossRef]
- Zeng, S.; Karthik, S.; Thakur, U.K.; Shankar, K. A review on photocatalytic CO2 reduction using perovskite oxide nanomaterials. Nanotechnology 2017, 29, 052001. [Google Scholar] [CrossRef]
- Kar, P.; Zeng, S.; Zhang, Y.; Vahidzadeh, E.; Manuel, A.; Kisslinger, R.; Alam, K.M.; Thakur, U.K.; Mahdi, N.; Kumar, P.; et al. High rate CO2 photoreduction using flame annealed TiO2 nanotubes. Appl. Catal. B Environ. 2018, 243, 522–536. [Google Scholar] [CrossRef]
- Kar, P.; Zhang, Y.; Farsinezhad, S.; Mohammadpour, A.; Wiltshire, B.D.; Sharma, H.; Shankar, K. Rutile phase n- and p-type anodic titania nanotube arrays with square-shaped pore morphologies. Chem. Commun. 2015, 51, 7816–7819. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, L.; Liu, Y.; Shao, C.; Gao, Y.; Fan, F.; Wang, J.; Li, J.; Yan, J.; Li, R.; et al. Surface-Polarity-Induced Spatial Charge Separation Boosts Photocatalytic Overall Water Splitting on GaN Nanorod Arrays. Angew. Chem. Int. Ed. 2019, 59, 935–942. [Google Scholar] [CrossRef]
- Alam, K.M.; Jensen, C.E.; Kumar, P.; Hooper, R.W.; Bernard, G.M.; Patidar, A.; Manuel, A.P.; Amer, N.; Palmgren, A.; Purschke, D.N.; et al. Photocatalytic mechanism control and study of carrier dynamics in CdS@C3N5 core-shell nanowires. ACS Appl. Mater. Inter. 2021, 13, 47418–47439. [Google Scholar] [CrossRef]
- You, D.; Xu, C.; Wang, X.; Wang, J.; Su, W.; Wang, R.; Chen, T.; Wang, R.; Shi, Z. A core@dual-shell nanorod array with a cascading band configuration for enhanced photocatalytic properties and anti-photocorrosion. J. Mater. Chem. A 2020, 8, 3726–3734. [Google Scholar] [CrossRef]
- Heab, S.; Yanc, C.; Chena, X.Z.; Wanga, Z.; Ouyanga, T.; Guob, M.L.; Liua, Z.Q. Construction of core-shell heterojunction regulating α-Fe2O3 layer on CeO2 nanotube arrays enables highly efficient Z-scheme photoelectrocatalysis. Appl. Catal. B Environ. 2020, 276, 119138. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, Y.; Li, L.; Liu, H.; Ren, C.; Liu, X.; Tian, G. Hierarchical ZnO nanorod/ZnFe2O4 nanosheet core/shell nanoarray decorated with PbS quantum dots for efficient photoelectrochemical water splitting. J. Alloy. Compd. 2020, 828, 154449. [Google Scholar] [CrossRef]
- Kumar, P.; Kar, P.; Manuel, A.P.; Zeng, S.; Thakur, U.K.; Alam, K.M.; Zhang, Y.; Kisslinger, R.; Cui, K.; Bernard, G.M.; et al. Noble Metal Free, Visible Light Driven Photocatalysis Using TiO2 Nanotube Arrays Sensitized by P-Doped C3N4 Quantum Dots. Adv. Opt. Mater. 2019, 8, 1901275. [Google Scholar] [CrossRef]
- Vahidzadeh, E.; Zeng, S.; Alam, K.M.; Kumar, P.; Riddell, S.; Chaulagain, N.; Gusarov, S.; Kobryn, A.E.; Shankar, K. Harvesting Hot Holes in Plasmon-Coupled Ultrathin Photoanodes for High-Performance Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 42741–42752. [Google Scholar] [CrossRef]
- Manuel, A.; Shankar, K. Hot Electrons in TiO2–Noble Metal Nano-Heterojunctions: Fundamental Science and Applications in Photocatalysis. Nanomaterials 2021, 11, 1249. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Yu, Y.; Ma, Y.; Yang, W.; Wang, F.; Yin, X.; Wang, X. Surface-Plasmon-Resonance-Enhanced Photoelectrochemical Water Splitting from Au-Nanoparticle-Decorated 3D TiO2 Nanorod Architectures. J. Phys. Chem. C 2017, 121, 12071–12079. [Google Scholar] [CrossRef]
- Zeng, S.; Vahidzadeh, E.; VanEssen, C.G.; Kar, P.; Kisslinger, R.; Goswami, A.; Zhang, Y.; Mahdi, N.; Riddell, S.; Kobryn, A.E.; et al. Optical control of selectivity of high rate CO2 photoreduction via interband- or hot electron Z-scheme reaction pathways in Au-TiO2 plasmonic photonic crystal photocatalyst. Appl. Catal. B Environ. 2020, 267, 118644. [Google Scholar] [CrossRef]
- Farsinezhad, S.; Sharma, H.; Shankar, K. Interfacial band alignment for photocatalytic charge separation in TiO2 nanotube arrays coated with CuPt nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 29723–29733. [Google Scholar] [CrossRef] [Green Version]
- Wei, R.-B.; Kuang, P.-Y.; Cheng, H.; Chen, Y.-B.; Long, J.-Y.; Zhang, M.-Y.; Liu, Z.-Q. Plasmon-Enhanced Photoelectrochemical Water Splitting on Gold Nanoparticle Decorated ZnO/CdS Nanotube Arrays. ACS Sustain. Chem. Eng. 2017, 5, 4249–4257. [Google Scholar] [CrossRef]
- Zheng, B.-F.; Ouyang, T.; Wang, Z.; Long, J.; Chen, Y.; Liu, Z.-Q. Enhanced plasmon-driven photoelectrocatalytic methanol oxidation on Au decorated α-Fe2O3 nanotube arrays. Chem. Commun. 2018, 54, 9583–9586. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.S.T.; Carvalho, K.T.G.; Lopes, O.; Ribeiro, C. g-C3N4/Nb2O5 heterostructures tailored by sonochemical synthesis: Enhanced photocatalytic performance in oxidation of emerging pollutants driven by visible radiation. Appl. Catal. B Environ. 2017, 216, 70–79. [Google Scholar] [CrossRef]
- Ye, W.; Arif, M.; Fang, X.; Mushtaq, M.A.; Chen, X.; Yan, D. Efficient Photoelectrochemical Route for the Ambient Reduction of N2 to NH3 Based on Nanojunctions Assembled from MoS2 Nanosheets and TiO2. ACS Appl. Mater. Interfaces 2019, 11, 28809–28817. [Google Scholar] [CrossRef]
- Bhat, S.S.M.; Pawar, S.A.; Potphode, D.; Moon, C.-K.; Suh, J.M.; Kim, C.; Choi, S.; Patil, D.S.; Kim, J.-J.; Shin, J.C.; et al. Substantially enhanced photoelectrochemical performance of TiO2 nanorods/CdS nanocrystals heterojunction photoanode decorated with MoS2 nanosheets. Appl. Catal. B Environ. 2019, 259, 118102. [Google Scholar] [CrossRef]
- Sun, M.; Fang, Y.; Sun, S.; Wang, Y. Surface co-modification of TiO2 with N doping and Ag loading for enhanced visible-light photoactivity. RSC Adv. 2016, 6, 12272–12279. [Google Scholar] [CrossRef]
- Peng, C.; Wang, W.; Zhang, W.; Liang, Y.; Zhuo, L. Surface plasmon-driven photoelectrochemical water splitting of TiO2 nanowires decorated with Ag nanoparticles under visible light illumination. Appl. Surf. Sci. 2017, 420, 286–295. [Google Scholar] [CrossRef]
- Ge, M.-Z.; Cao, C.-Y.; Li, S.-H.; Tang, Y.-X.; Wang, L.-N.; Qi, N.; Huang, J.-Y.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y.-K. In situ plasmonic Ag nanoparticle anchored TiO2 nanotube arrays as visible-light-driven photocatalysts for enhanced water splitting. Nanoscale 2016, 8, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.B.; Linic, S. Water Splitting on Composite Plasmonic-Metal/Semiconductor Photoelectrodes: Evidence for Selective Plasmon-Induced Formation of Charge Carriers near the Semiconductor Surface. J. Am. Chem. Soc. 2011, 133, 5202–5205. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, D.; Yang, Y.; Liu, H.; Chen, J.; Wang, H. Preparation of Au/reduced graphene oxide/hydrogenated TiO2 nanotube arrays ternary composites for visible-light-driven photoelectrochemical water splitting. J. Alloy Compd. 2016, 661, 380–388. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Wang, H.; Liu, H. Ligand-exchange assisted preparation of plasmonic Au/TiO2 nanotube arrays photoanodes for visible-light-driven photoelectrochemical water splitting. J. Power Sources 2016, 303, 287–293. [Google Scholar] [CrossRef]
- Chen, J.; Bailey, C.S.; Hong, Y.; Wang, L.; Cai, Z.; Shen, L.; Hou, B.; Wang, Y.; Shi, H.; Sambur, J.; et al. Plasmon-Resonant Enhancement of Photocatalysis on Monolayer WSe2. ACS Photon. 2019, 6, 787–792. [Google Scholar] [CrossRef]
- Zhan, Z.; An, J.; Zhang, H.; Hansen, R.V.; Zheng, L. Three-Dimensional Plasmonic Photoanodes Based on Au-Embedded TiO2 Structures for Enhanced Visible-Light Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Wang, T.; Lv, R.; Zhang, J.; Zhang, P.; Lu, J.; Gong, J. Dendritic Au/TiO2 nanorod arrays for visible-light driven photoelectrochemical water splitting. Nanoscale 2013, 5, 9001–9009. [Google Scholar] [CrossRef]
- Pu, Y.-C.; Wang, G.; Chang, K.-D.; Ling, Y.; Lin, Y.-K.; Fitzmorris, B.C.; Liu, C.-M.; Lu, X.; Tong, Y.; Zhang, J.Z.; et al. Au Nanostructure-Decorated TiO2 Nanowires Exhibiting Photoactivity Across Entire UV-visible Region for Photoelectrochemical Water Splitting. Nano Lett. 2013, 13, 3817–3823. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Hedhili, M.N.; Zhang, H.; Wang, P. Plasmonic Gold Nanocrystals Coupled with Photonic Crystal Seamlessly on TiO2 Nanotube Photoelectrodes for Efficient Visible Light Photoelectrochemical Water Splitting. Nano Lett. 2012, 13, 14–20. [Google Scholar] [CrossRef]
- Shinkai, S.; Sasaki, K. Influence of Sputtering Parameters on the Formation Process of High-Quality and Low-Resistivity HfN Thin Film. Jpn. J. Appl. Phys. 1999, 38, 2097–2102. [Google Scholar] [CrossRef]
- Stoehr, M.; Seo, H.-S.; Petrov, I.; Greene, J.E. Effect of off stoichiometry on Raman scattering from epitaxial and polycrystalline HfNx (0.85 ≤ x ≤ 1.50) grown on MgO(001). J. Appl. Phys. 2008, 104, 033507. [Google Scholar] [CrossRef]
- Yuan, L.; Fang, G.; Li, C.; Wang, M.; Liu, N.; Ai, L.; Cheng, Y.; Gao, H.; Zhao, X. Influence of N2 flow ratio on the properties of hafnium nitride thin films prepared by DC magnetron sputtering. Appl. Surf. Sci. 2007, 253, 8538–8542. [Google Scholar] [CrossRef]
- Chung, S.; Shrestha, S.; Wen, X.; Feng, Y.; Gupta, N.; Xia, H.; Yu, P.; Tang, J.; Conibeer, G. Evidence for a large phononic band gap leading to slow hot carrier thermalisation. IOP Conf. Series Mater. Sci. Eng. 2014, 68, 012002. [Google Scholar] [CrossRef] [Green Version]
- Kroon, R.; Lenes, M.; Hummelen, J.C.; Blom, P.W.M.; De Boer, B. Small Bandgap Polymers for Organic Solar Cells (Polymer Material Development in the Last 5 Years). Polym. Rev. 2008, 48, 531–582. [Google Scholar] [CrossRef]
- Moreno, J.J.G.; Nolan, M. Ab Initio Study of the Atomic Level Structure of the Rutile TiO2(110)–Titanium Nitride (TiN) Interface. ACS Appl. Mater. Interfaces 2017, 9, 38089–38100. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dai, Y.; Yu, L.; Huang, B.-B. Energy transfer in plasmonic photocatalytic composites. Light. Sci. Appl. 2016, 5, e16017. [Google Scholar] [CrossRef] [Green Version]
- Kale, M.J.; Avanesian, T.; Christopher, P. Direct Photocatalysis by Plasmonic Nanostructures. ACS Catal. 2013, 4, 116–128. [Google Scholar] [CrossRef]
- Farsinezhad, S.; Mohammadpour, A.; Dalrymple, A.N.; Geisinger, J.; Kar, P.; Brett, M.J.; Shankar, K. Transparent Anodic TiO2 Nanotube Arrays on Plastic Substrates for Disposable Biosensors and Flexible Electronics. J. Nanosci. Nanotechnol. 2013, 13, 2885–2891. [Google Scholar] [CrossRef]
- Ozaki, T. Variationally optimized atomic orbitals for large-scale electronic structures. Phys. Rev. B 2003, 67, 155108. [Google Scholar] [CrossRef]
- Bachelet, G.B.; Hamann, D.R.; Schlüter, M. Pseudopotentials that work: From H to Pu. Phys. Rev. B 1982, 26, 4199–4228. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Dhifallah, M.; Iachella, M.; Dhouib, A.; Di Renzo, F.; Loffreda, D.; Guesmi, H. Support Effects Examined by a Comparative Theoretical Study of Au, Cu, and CuAu Nanoclusters on Rutile and Anatase Surfaces. J. Phys. Chem. C 2019, 123, 4892–4902. [Google Scholar] [CrossRef]
- Capdevila-Cortada, M.; Lodziana, Z.; López, N. Performance of DFT+U Approaches in the Study of Catalytic Materials. ACS Catal. 2016, 6, 8370–8379. [Google Scholar] [CrossRef] [Green Version]


| Sample | Photocurrent (µA/cm2) | Light Intensity (mW/cm2) | Light Spectrum | Applied Bias | Electrolyte | Reference |
|---|---|---|---|---|---|---|
| HfN-TNT | 1800 | 100 | visible light (>420 nm) | 0.6 V | 1 M KOH | This work |
| Ag/N-TiO2 | 26 | 120 | visible light (>420 nm) | 0 V | 0.5 M Na2SO4 | [35] |
| AgNPs/TiO2 NWs | 47 | 100 | visible light (>420 nm) | 0.4 V | 0.1 M Na2SO4 | [36] |
| In situ AgNPs/TNTs | 40 | 80 | visible light (>420 nm) | 0.3 V | 0.1 M Na2SO4 | [37] |
| Ag/N-TiO2 | 0.5 | 500 | 400–900 nm | 0.3 V | 1 M KOH | [38] |
| Au/RGO/H-TNTs | 224 | 100 | visible light (>400 nm) | 0.2 V | 1 M KOH | [39] |
| LE-Au/TNTs | 202 | 100 | visible light (>400 nm) | 0.2 V | 1 M KOH | [40] |
| AuNPs/TiO2 | 23 | 7000 | 532 nm, 633 nm | 0 V | 1 M KOH | [41] |
| Au embedded TiO2 | 3 | visible light (>420 nm) | 0.2 V | 1 M KOH + 25% MeOH | [42] | |
| AuNPs/TiO2 BNRs | 125 | 100 | visible light (>420 nm) | 0.5 V | 1 M KOH | [43] |
| AuNPs/TiO2 NWs | 11 | 73.3 | visible light (>430 nm) | 0 V | 1 M KOH | [44] |
| AuNPs/TiO2 PhC | 150 | 100 | visible light (>420 nm) | 0.2 V | 1 M KOH | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, S.; Muneshwar, T.; Riddell, S.; Manuel, A.P.; Vahidzadeh, E.; Kisslinger, R.; Kumar, P.; Alam, K.M.M.; Kobryn, A.E.; Gusarov, S.; et al. TiO2-HfN Radial Nano-Heterojunction: A Hot Carrier Photoanode for Sunlight-Driven Water-Splitting. Catalysts 2021, 11, 1374. https://doi.org/10.3390/catal11111374
Zeng S, Muneshwar T, Riddell S, Manuel AP, Vahidzadeh E, Kisslinger R, Kumar P, Alam KMM, Kobryn AE, Gusarov S, et al. TiO2-HfN Radial Nano-Heterojunction: A Hot Carrier Photoanode for Sunlight-Driven Water-Splitting. Catalysts. 2021; 11(11):1374. https://doi.org/10.3390/catal11111374
Chicago/Turabian StyleZeng, Sheng, Triratna Muneshwar, Saralyn Riddell, Ajay Peter Manuel, Ehsan Vahidzadeh, Ryan Kisslinger, Pawan Kumar, Kazi Mohammad Monirul Alam, Alexander E. Kobryn, Sergey Gusarov, and et al. 2021. "TiO2-HfN Radial Nano-Heterojunction: A Hot Carrier Photoanode for Sunlight-Driven Water-Splitting" Catalysts 11, no. 11: 1374. https://doi.org/10.3390/catal11111374
APA StyleZeng, S., Muneshwar, T., Riddell, S., Manuel, A. P., Vahidzadeh, E., Kisslinger, R., Kumar, P., Alam, K. M. M., Kobryn, A. E., Gusarov, S., Cadien, K. C., & Shankar, K. (2021). TiO2-HfN Radial Nano-Heterojunction: A Hot Carrier Photoanode for Sunlight-Driven Water-Splitting. Catalysts, 11(11), 1374. https://doi.org/10.3390/catal11111374