Synthesis of Methyl Mercaptan on Mesoporous Alumina Prepared with Hydroxysafflor Yellow A as Template: The Synergistic Effect of Potassium and Molybdenum
Abstract
:1. Introduction
2. Results and Discussion
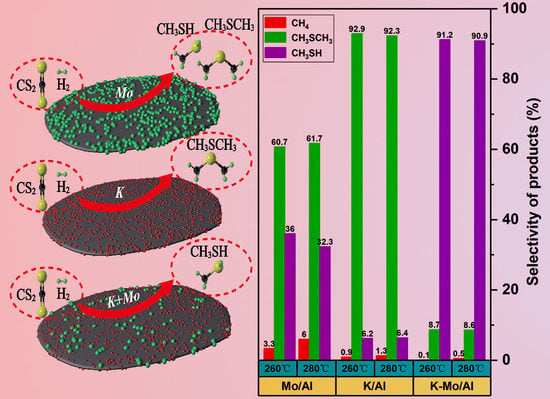
2.1. Catalytic Performances of the Catalysts
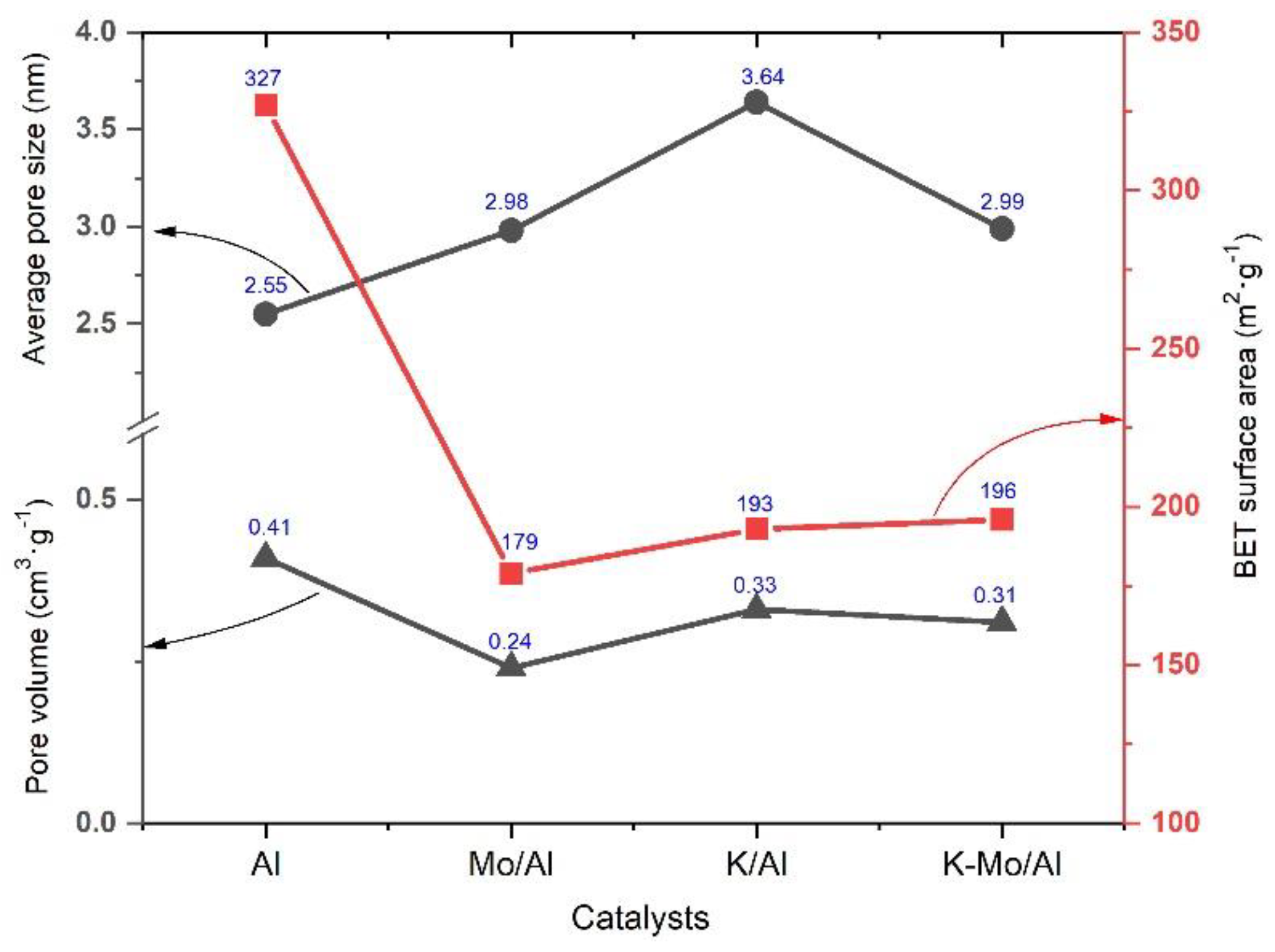
2.2. Textural Properties
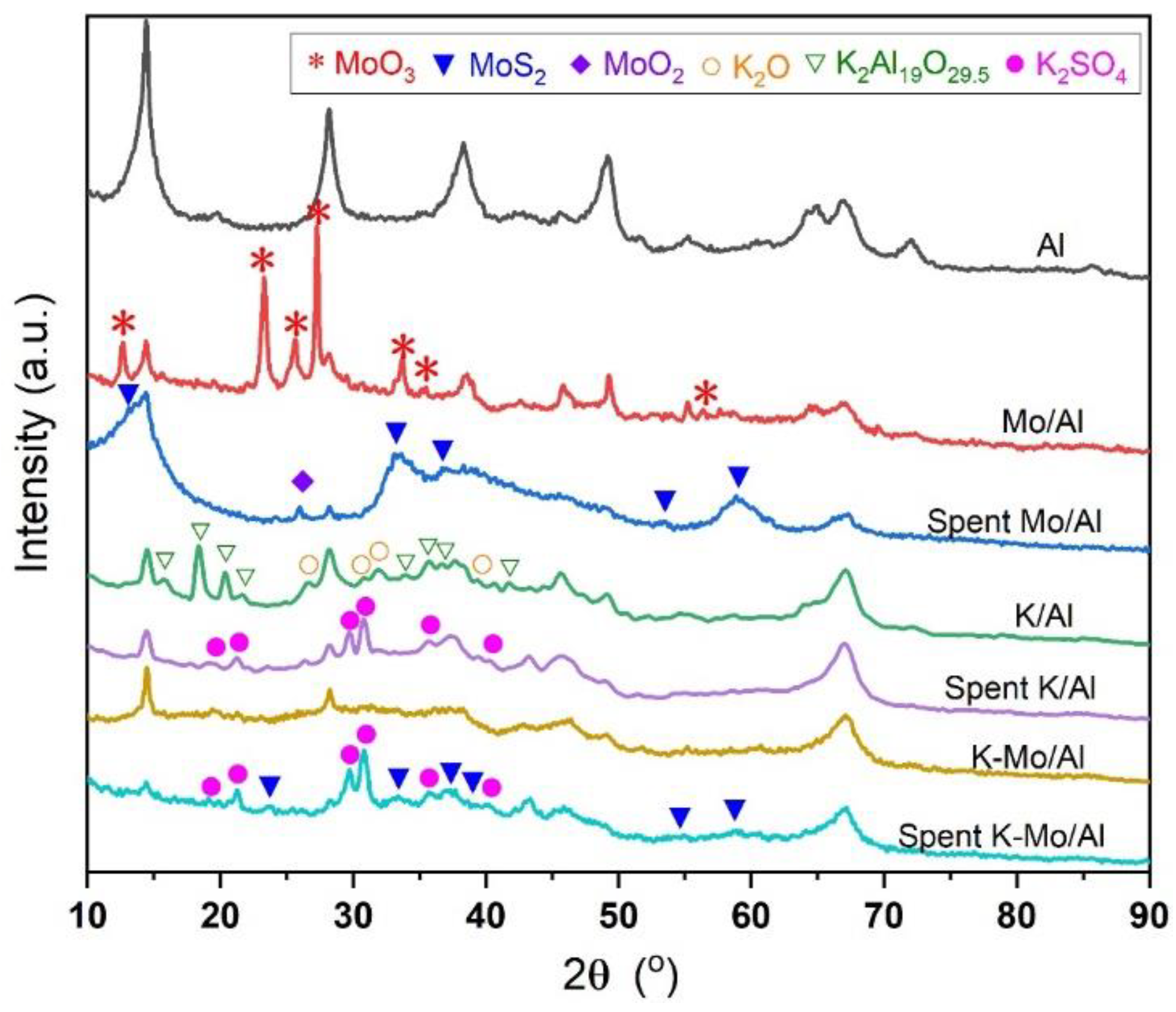
2.3. Crystalline Phase and Morphology
2.4. H2-TPR Studies
2.5. Surface Acid-Base Properties
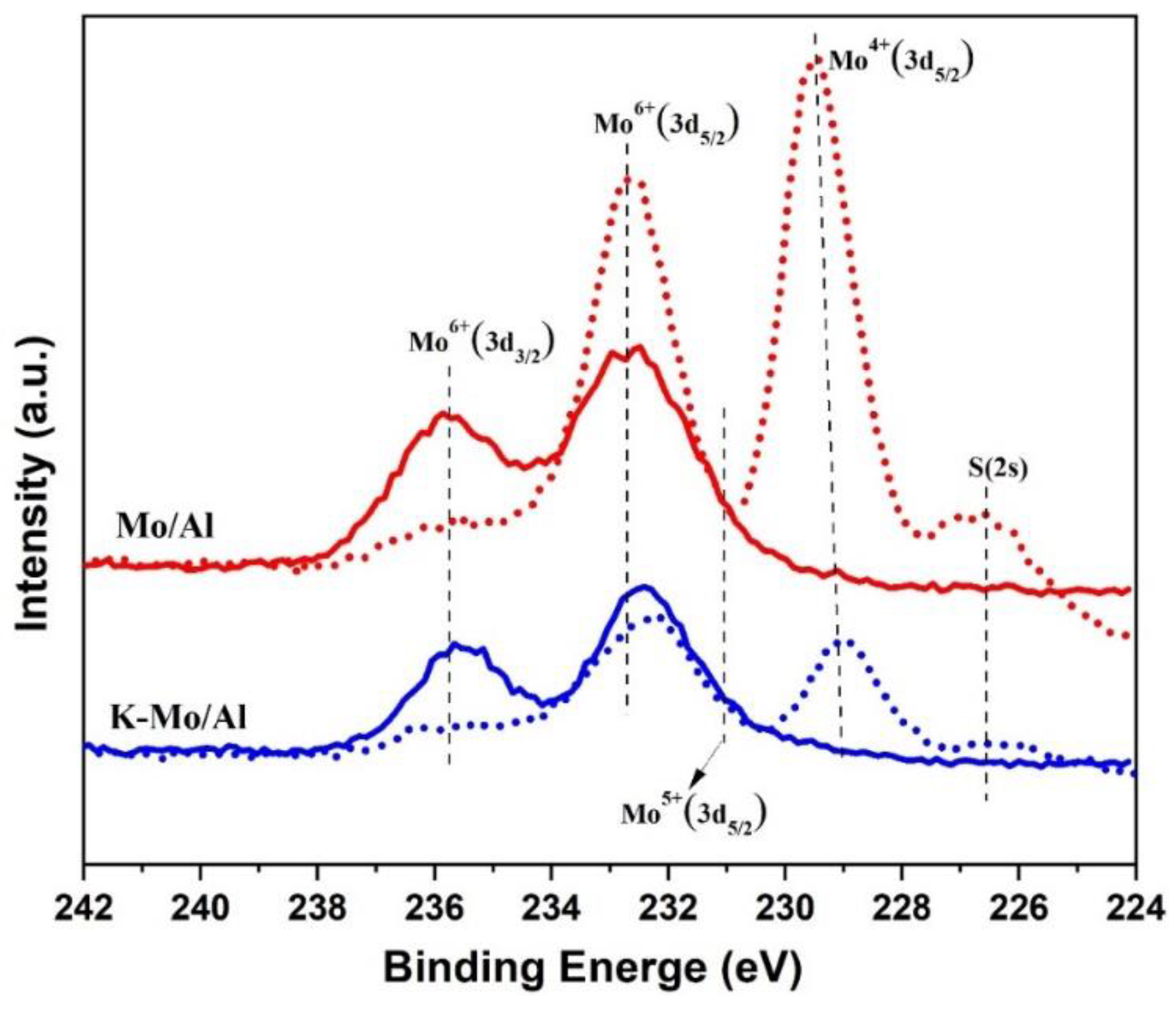
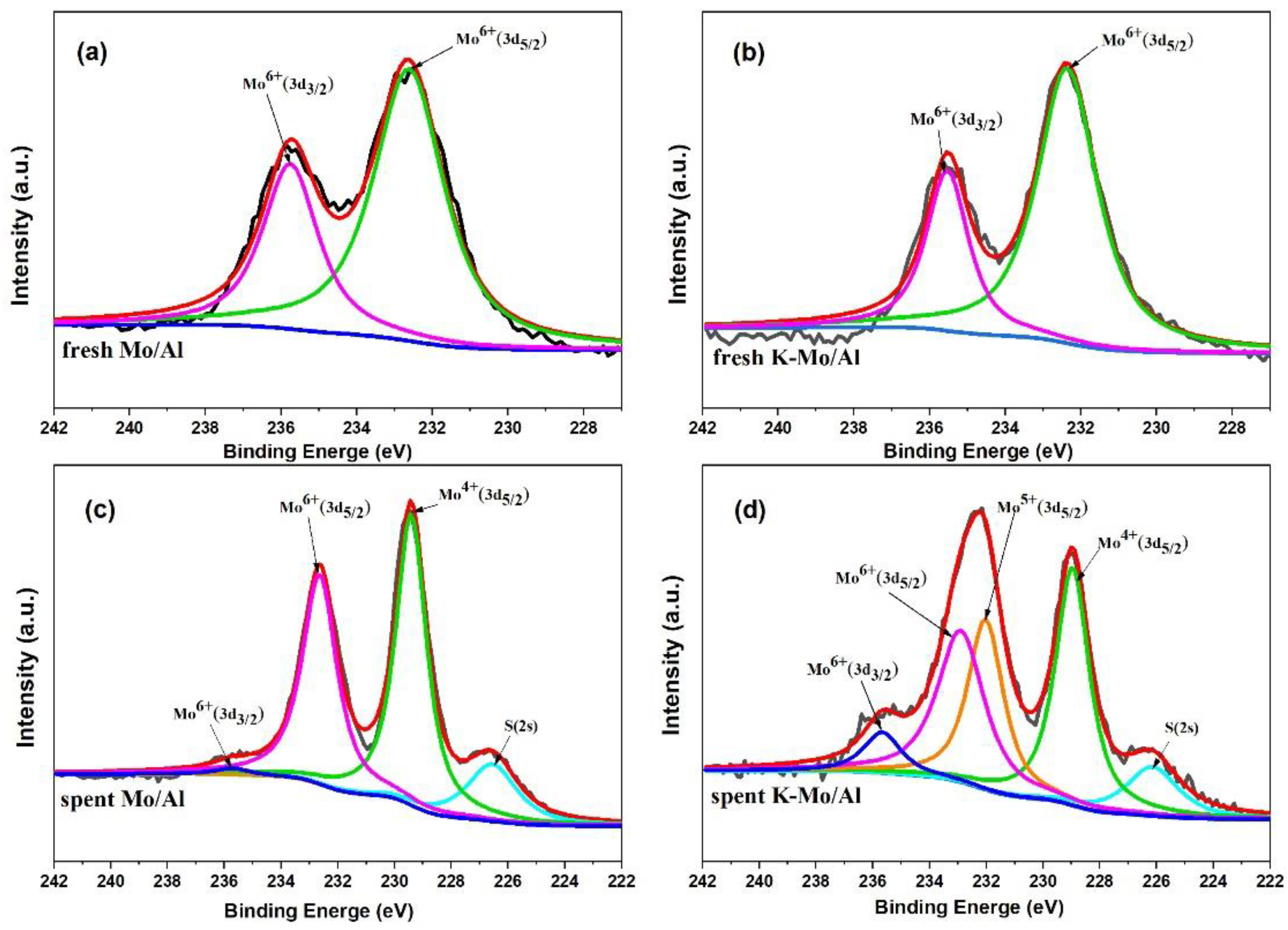
2.6. XPS Study of Catalysts
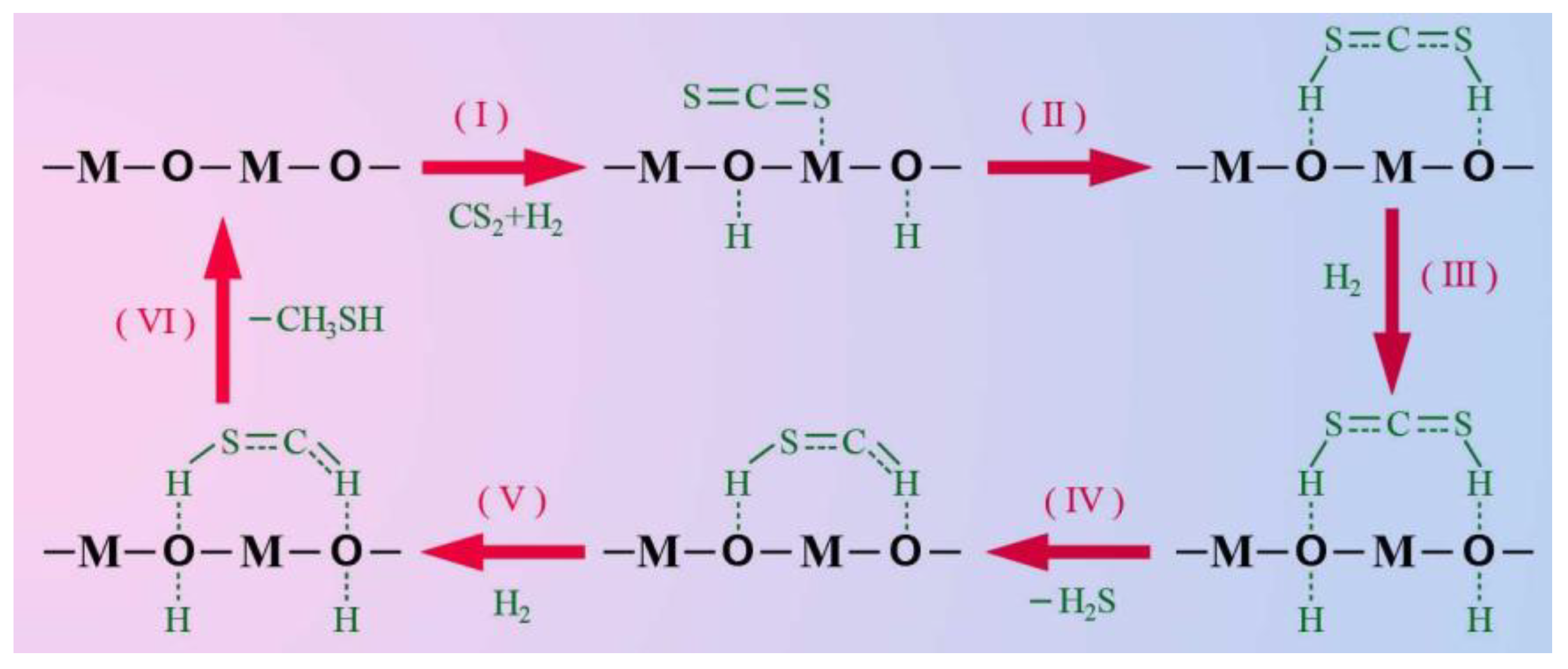
2.7. Proposed Mechanism on the Acid-Base Site
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.H.; Chen, S.P.; Wu, M.; Fang, W.P.; Yang, Y.Q. Promoting effect of SiO2 on the K2WO4/Al2O3 Catalysts for Methanethiol Synthesis from Methanol and H2S. Catal. Commun. 2012, 22, 48–51. [Google Scholar] [CrossRef]
- Bermejo-Deval, R.; Walter, R.M.H.; Gutiérrez, O.Y.; Lercher, J.A. On the Role of the Alkali Cations on Methanol Thiolation. Catal. Sci. Technol. 2017, 7, 4437–4443. [Google Scholar] [CrossRef]
- Pashigreva, A.V.; Kondratieva, E.; Bermejo-Deval, R.; Gutiérrez, O.Y.; Lercher, J.A. Methanol thiolation over Al2O3 and WS2 catalysts modified with cesium. J. Catal. 2017, 345, 308–318. [Google Scholar] [CrossRef]
- Weber-Stockbauer, M.; Gutiérrez, O.Y.; Bermejo-Deval, R.; Lercher, J.A. The Role of Weak Lewis Acid Sites for Methanol Thiolation. Catal. Sci. Technol. 2019, 9, 509–516. [Google Scholar] [CrossRef]
- Weber-Stockbauer, M.; Gutiérrez, O.Y.; Bermejo-Deval, R.; Lercher, J.A. Cesium Induced Changes in the Acid-Base Properties of Metal Oxides and the Consequences for Methanol Thiolation. ACS Catal. 2019, 9, 9245–9252. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.L.; Liu, F.; Zhao, T.X.; Wang, X.D.; Cao, J.X. High selectivity in methanethiol synthesis over a coated composite comprising ZSM-5 with t-ZrO2. Microporous Mesoporous Mater. 2020, 305, 110358–110365. [Google Scholar] [CrossRef]
- Mul, G.; Wachs, I.E.; Hirschon, A.S. Catalytic Synthesis of Methanethiol from Hydrogen Sulfide and Carbon Monoxide over Vanadium-based Catalysts. Catal. Today 2003, 78, 327–337. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Dai, S.J.; Yuan, Y.Z.; Lin, R.C.; Tang, D.L.; Zhang, H.B. The promoting effects of La2O3 and CeO2 on K2MoS4/SiO2 catalyst for methanethiol synthesis from synthesis gas blending with H2S. Appl. Catal. A 2000, 192, 175–180. [Google Scholar] [CrossRef]
- Chen, A.P.; Wang, Q.; Li, Q.L.; Hao, Y.J.; Fang, W.P.; Yang, Y.Q. Direct synthesis of methanethiol from H2S-rich syngas over sulfided Mo-based catalysts. J. Mol. Catal. A Chem. 2008, 283, 69–76. [Google Scholar] [CrossRef]
- Lu, J.C.; Luo, Y.M.; He, D.D.; Xu, Z.Z.; He, S.F.; Xie, D.L.; Mei, Y. An exploration into potassium (K) containing MoS2 active phases and its transformation process over MoS2 based materials for producing methanethiol. Catal. Today 2020, 339, 93–104. [Google Scholar] [CrossRef]
- Yu, M.; Kosinov, N.; Haandel, L.V.; Kooyman, P.J.; Hensen, E.J.M. Investigation of the Active Phase in K-Promoted MoS2 Catalysts for Methanethiol Synthesis. ACS Catal. 2020, 10, 1838–1846. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.C.; Liu, P.; Xu, Z.Z.; He, S.F.; Luo, Y.M. Investigation of the reaction pathway for synthesizing methyl mercaptan (CH3SH) from H2S-containing syngas over K–Mo-type materials. RSC Adv. 2018, 8, 21340–21353. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Lu, J.C.; Xu, Z.Z.; Liu, F.; Chen, D.K.; Yu, J.; Liu, J.P.; He, S.F.; Wan, G.P.; Luo, Y.M. The effect of alkali metals on the synthesis of methanethiol from CO/H2/H2S mixtures on the SBA-15 supported Mo-based catalysts. Mol. Catal. 2017, 442, 39–48. [Google Scholar] [CrossRef]
- Cordova, A.; Blanchard, P.; Lancelot, C.; Frémy, G.; Lamonier, C. Probing the Nature of the Active Phase of Molybdenum-Supported Catalysts for the Direct Synthesis of Methylmercaptan from Syngas and H2S. ACS Catal. 2015, 5, 2966–2981. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Zhong, L.S.; Zhu, Y.Z.; Lercher, J.A. Synthesis of Methanethiol from CS2 on Ni-, Co-, and K-Doped MoS2/SiO2 Catalysts. ChemCatChem 2013, 5, 3249–3259. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Kaufmann, C.; Lercher, J.A. Synthesis of Methanethiol from Carbonyl Sulfide and Carbon Disulfide on (Co)K-Promoted Sulfide Mo/SiO2 Catalysts. ACS Catal. 2011, 1, 1595–1603. [Google Scholar] [CrossRef]
- Wang, W.M.; Zhang, X.; Xia, Z.Q.; Yang, Y.Q. Catalytic synthesis of methanethiol from carbon disulfide and hydrogen over sulfided KMo/Al2O3 catalysts. Catal. Lett. 2015, 145, 1521–1528. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Kaufmann, C.; Hrabar, A.; Zhu, Y.Z.; Lercher, J.A. Synthesis of methyl mercaptan from carbonyl sulfide over sulfide K2MoO4/SiO2. J. Catal. 2011, 280, 264–273. [Google Scholar] [CrossRef]
- Wang, W.M.; Li, Y.; Zhang, X.; Fang, W.P.; Yang, Y.Q. Catalytic synthesis of methanethiol from methanol and carbon disulfide over KW/Al2O3 catalysts. Catal. Commun. 2015, 69, 104–108. [Google Scholar] [CrossRef]
- Wang, W.M.; Li, J.J.; He, Q.J.; Peng, S.; Li, M. Synthesis of methanethiol from methanol and carbon disulfide over CoKW/Al2O3 catalysts: The possible reaction network and reaction mechanism. ChemistrySelect 2018, 3, 9663–9671. [Google Scholar] [CrossRef]
- Lund, C.R.F. Microkinetics of Water-Gas Shift over Sulfided Mo/Al2O3 Catalysts. Ind. Eng. Chem. Res. 1996, 35, 2531–2538. [Google Scholar] [CrossRef]
- Liu, B.; Zong, Q.Y.; Edwards, P.P.; Zou, F.; Du, X.; Jiang, Z.; Xiao, T.C.; AlMegren, H. Effect of Titania Addition on the Performance of CoMo/Al2O3 Sour Water Gas Shift Catalysts under Lean Steam to Gas Ratio Conditions. Ind. Eng. Chem. Res. 2012, 51, 11674–11680. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, C.; Tan, X.Y.; Huang, B.; Bian, G.Q.; Shao, Q.; Bai, S.X.; Qian, Y.; Li, Y.Y.; Huang, X.Q. Se-Incorporation Stabilizes and Activates Metastable MoS2 for Efficient and Cost-Effective Water Gas Shift Reaction. ACS Nano 2019, 13, 11303–11309. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.U.; Guliants, V.V. Support Effects on Water Gas Shift Activity and Sulfur Dependence of Mo Sulfide Catalysts. Energy Fuels 2019, 33, 11655–11662. [Google Scholar] [CrossRef]
- Ferrari, D.; Budroni, G.; Bisson, L.; Rane, N.J.; Dickie, B.D.; Kang, J.H.; Rozeveld, S.J. Effect of potassium addition method on MoS2 performance for the syngas to alcohol reaction. Appl. Catal. A-Gen. 2013, 462–463, 302–309. [Google Scholar] [CrossRef]
- Hensley, J.E.; Pylypenko, S.; Ruddy, D.A. Deactivation and stability of K-CoMoSx mixed alcohol synthesis catalysts. J. Catal. 2014, 309, 199–208. [Google Scholar] [CrossRef]
- Dorokhov, V.S.; Permyakov, E.A.; Nikulshin, P.A.; Maximov, V.V.; Kogan, V.M. Experimental and computational study of syngas and ethanol conversion mechanisms over K-modified transition metal sulfide catalysts. J. Catal. 2016, 344, 841–853. [Google Scholar] [CrossRef]
- Santos, V.P.; Linden, B.V.D.; Chojecki, A.; Budroni, G.; Corthals, S.; Shibata, H.; Meima, G.R.; Kapteijn, F.; Makkee, M.; Gascon, J. Mechanistic Insight into the Synthesis of Higher Alcohols from Syngas: The Role of K Promotion on MoS2 Catalysts. ACS Catal. 2013, 3, 1634–1637. [Google Scholar] [CrossRef]
- Zhang, L.F.; Ball, M.R.; Rivera-Dones, K.R.; Wang, S.C.; Kuech, T.F.; Huber, G.W.; Hermans, I.; Dumesic, J.A. Synthesis Gas Conversion over Molybdenum-Based Catalysts Promoted by Transition Metals. ACS Catal. 2020, 10, 365–374. [Google Scholar] [CrossRef]
- Maximov, V.V.; Permyakov, E.A.; Dorokhov, V.S.; Wang, A.J.; Kooyman, P.J.; Kogan, V.M. Effect of Promoter Nature on Synthesis Gas Conversion to Alcohols over (K)MeMoS2/Al2O3 Catalysts. ChemCatChem 2020, 12, 1443–1452. [Google Scholar] [CrossRef]
- Shi, G.J.; Zhao, H.Y.; Song, L.G.; Shen, J.Y. Effect of Solvents on the Hydrogenation and Isomerization of 1-Hexene over Sulfided Co-Mo/γ-Al2O3 Catalysts for Hydrodesulfurization. Energy Fuels 2008, 22, 2450–2454. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Singh, S.; Schachtl, E.; Kim, J.; Kondratieva, E.; Hein, J.; Lercher, J.A. Effects of the Support on the Performance and Promotion of (Ni)MoS2 Catalysts for Simultaneous Hydrodenitrogenation and Hydrodesulfurization. ACS Catal. 2014, 4, 1487–1499. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, G.; Mei, J.L.; Xiao, C.K.; Hu, D.; Wang, A.C.; Song, Y.D.; Ni, Y.; Jiang, G.Y.; Duan, A.J. The Influence of Pore Structure and Acidity on the Hydrodesulfurization of Dibenzothiophene over NiMo-Supported Catalysts. ACS Omega 2020, 5, 15576–15585. [Google Scholar] [CrossRef]
- Mundotiya, S.; Singh, R.; Saha, S.; Kakkar, R.; Pal, S.; Kunzru, D.; Pala, R.G.S.; Sivakumar, S. Effect of Sodium on Ni-Promoted MoS2 Catalyst for Hydrodesulfurization Reaction: Combined Experimental and Simulation Study. Energy Fuels 2021, 35, 2368–2378. [Google Scholar] [CrossRef]
- Liu, C.; Virginie, M.; Griboval-Constant, A.; Khodakov, A.Y. Potassium promotion effects in carbon nanotube supported molybdenum sulfide catalysts for carbon monoxide hydrogenation. Catal. Today 2016, 261, 137–145. [Google Scholar] [CrossRef]
- Claure, M.T.; Morrill, M.R.; Goh, J.W.; Chai, S.H.; Dai, S.; Agrawal, P.K.; Jones, C.W. Insight into reaction pathways in CO hydrogenation reactions over K/MoS2 supported catalysts via alcohol/olefin co-feed experiments. Catal. Sci. Technol. 2016, 6, 1957–1966. [Google Scholar] [CrossRef]
- Zeng, F.; Xi, X.Y.; Cao, H.T.; Pei, Y.T.; Heeres, H.J.; Palkovits, R. Synthesis of mixed alcohols with enhanced C3+ alcohol production by CO hydrogenation over potassium promoted molybdenum sulfide. Appl. Catal. B-Environ. 2019, 246, 232–241. [Google Scholar] [CrossRef]
- Hu, J.; Yu, L.; Deng, J.; Wang, Y.; Cheng, K.; Ma, C.; Zhang, Q.; Wen, W.; Yu, S.; Pan, Y.; et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 2021, 4, 242–250. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Yuan, Y.Z.; Dai, S.J.; Wang, B.; Zhang, H.B. The catalytic properties of supported K2MoS4/SiO2 catalyst for methanethiol synthesis from high H2S-content synthesis gas. Catal. Lett. 1998, 54, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.J.; Yang, Y.Q.; Yuan, Y.Z.; Tang, D.L.; Lin, R.C.; Zhang, H.B. On methanethiol synthesis from H2S-containing synthesis gas over K2MoS4/SiO2 catalysts promoted with transition metal oxides. Catal. Lett. 1999, 61, 157–160. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.P.; Wang, Q.; Hao, Y.J.; Fang, W.P.; Yang, Y.Q. The Promoting Effect of Tellurium on K2MoO4/SiO2 Catalyst for Methanethiol Synthesis from High H2S-containing Syngas. Catal. Lett. 2007, 118, 295–299. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.P.; Wang, Q.; Hao, Y.J.; Fang, W.P.; Yang, Y.Q. Catalytic Synthesis of Methanethiol from H2S-rich Syngas Over Sulfided SiO2-supported Mo-based Catalysts. Catal. Lett. 2008, 121, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhu, H.; Ke, J.; Qin, R. Selection of a Taxon-Specific Reference Gene for Qualitative and Quantitative PCR Detection of Carthamus tinctorius. Food Anal. Methods 2017, 10, 2952–2963. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, H.; Zhan, W.; Yu, Z.; Qin, E.; Liu, S.; Yang, T.; Xiang, N.; Kudrna, D.; Chen, Y.; et al. The chromosome-scale reference genome of safflower (Carthamus tinctorius) provides insights into linoleic acid and flavonoid biosynthesis. Plant Biotechnol. J. 2021, 19, 1725–1742. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.L.; Laine, J. Reducibility of Ni-Mo/Al2O3 catalysts: A TPR study. J. Catal. 1993, 139, 540–550. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Dadyburjor, D.B.; Kugler, E.L. A temperature-programmed-reduction study on alkali-promoted, carbon-supported molybdenum catalysts. J. Catal. 2000, 191, 1–13. [Google Scholar] [CrossRef]
- Arnoldy, P.; de Jonge, J.C.M.; Moulijn, J.A. Temperature-programed reduction of molybdenum(VI) oxide and molybdenum(IV) oxide. J. Phys. Chem. 1985, 89, 4517–4526. [Google Scholar] [CrossRef]
- DeCanio, S.J.; Cataldo, M.C.; DeCanio, E.C.; Storm, D.A. Evidence from XPS for the stabilization of high-valent molybdenum by addition of potassium in Mo/Al2O3 catalysts. J. Catal. 1989, 119, 256–260. [Google Scholar] [CrossRef]
- Tatsumi, T.; Muramatsu, A.; Yokota, K.; Tominaga, H. Mechanistic study on the alcohol synthesis over molybdenum catalysts: Addition of probe molecules to CO-H2. J. Catal. 1989, 115, 388–398. [Google Scholar] [CrossRef]
- Watson, R.B.; Ozkan, U.S. Propane and propylene adsorption effects over MoOx-based catalysts induced by low levels of alkali doping. J. Mol. Catal. A 2003, 194, 115–135. [Google Scholar] [CrossRef]
- Chen, K.; Xie, S.; Bell, A.T.; Iglesia, E. Alkali Effects on Molybdenum Oxide Catalysts for the Oxidative Dehydrogenation of Propane. J. Catal. 2000, 195, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, S.; Marzari, J.A.; Miranda, R. Silica-alumina-supported Mo oxide catalysts: Genesis and demise of Brønsted-Lewis acidity. J. Catal. 1995, 151, 192–203. [Google Scholar] [CrossRef]
- Baker, M.A.; Gilmore, R.; Lenardi, C.; Gissler, W. XPS investigation of preferential sputtering of S from MoS2 and determination of MoSx stoichiometry from Mo and S peak positions. Appl. Surf. Sci. 1999, 150, 255–262. [Google Scholar] [CrossRef]
- Brown, N.M.D.; Cui, N.; McKinley, A. An XPS study of the surface modification of natural MoS2 following treatment in an RF-oxygen plasma. Appl. Surf. Sci. 1998, 134, 11–21. [Google Scholar] [CrossRef]
- Kantschewa, M.; Delannay, F.; Jeziorowski, H.; Delgado, E.; Eder, S. Nature and properties of a potassium-promoted NiMo/Al2O3 water gas shift catalyst. J. Catal. 1984, 87, 482–496. [Google Scholar] [CrossRef]
- Spevack, P.A.; McIntyre, S. Reactivity and stability of sulphided thin films of molybdenum to dry air. Appl. Catal. 1990, 64, 191–207. [Google Scholar] [CrossRef]
- Byskov, L.S.; Bollinger, M.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H. Molecular aspects of the H2 activation on MoS2 based catalysts—The role of dynamic surface arrangements. J. Mol. Catal. A 2000, 163, 117–122. [Google Scholar] [CrossRef]
- Startsev, A.N.; Zakharov, I.I.; Parmon, V.N. An unexpected phenomenon in heterogeneous catalysis: Oxidative addition of hydrogen to the sulfide catalysts. J. Mol. Catal. A 2003, 192, 113–127. [Google Scholar] [CrossRef]
- Bian, G.Z.; Fu, Y.L.; Yamada, M. Reaction stability and structure studies of sulfided K-MoO3/γ-Al2O3 catalyst for the synthesis of mixed alcohols. Appl. Catal. A 1996, 144, 79–91. [Google Scholar] [CrossRef]






| Catalysts | Basic Site Distribution from CO2-TPD [a] | Acid Site Distribution from NH3-TPD [a] | ||||||
|---|---|---|---|---|---|---|---|---|
| Weak | Medium | Strong | Total | Weak | Medium | Strong | Total | |
| Al | 1.0 | - | - | 1.0 | 0.41 | 0.34 | 0.25 | 1.0 |
| Mo/Al | 0.03 | - | - | 0.03 | 0.53 | 0.40 | 0.33 | 1.26 |
| K/Al | 3.67 | 1.84 | 2.35 | 7.86 | 0.26 | 0.11 | 0.03 | 0.4 |
| K-Mo/Al | 1.42 | - | - | 1.42 | 0.14 | 0.15 | 0.28 | 0.57 |
| Catalysts | Binding Energy of Mo(3d5/2) eV | Concentration (%) | ||||
|---|---|---|---|---|---|---|
| Mo4+ | Mo5+ | Mo6+ | Mo4+ | Mo5+ | Mo6+ | |
| Mo/Al | 229.4 | - | 232.6 | 55.1 | 0 | 44.9 |
| K-Mo/Al | 229.0 | 232.0 | 232.9 | 38.0 | 30.8 | 31.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Zeng, D.; Li, J.; Peng, S.; Xiong, J.; Wang, W.; Chen, Y.; Liu, H.; Liu, H.; Qin, R. Synthesis of Methyl Mercaptan on Mesoporous Alumina Prepared with Hydroxysafflor Yellow A as Template: The Synergistic Effect of Potassium and Molybdenum. Catalysts 2021, 11, 1365. https://doi.org/10.3390/catal11111365
Peng C, Zeng D, Li J, Peng S, Xiong J, Wang W, Chen Y, Liu H, Liu H, Qin R. Synthesis of Methyl Mercaptan on Mesoporous Alumina Prepared with Hydroxysafflor Yellow A as Template: The Synergistic Effect of Potassium and Molybdenum. Catalysts. 2021; 11(11):1365. https://doi.org/10.3390/catal11111365
Chicago/Turabian StylePeng, Chuang, Dong Zeng, Jianjun Li, Shuai Peng, Jun Xiong, Weiming Wang, Yingming Chen, Hong Liu, Hao Liu, and Rui Qin. 2021. "Synthesis of Methyl Mercaptan on Mesoporous Alumina Prepared with Hydroxysafflor Yellow A as Template: The Synergistic Effect of Potassium and Molybdenum" Catalysts 11, no. 11: 1365. https://doi.org/10.3390/catal11111365
APA StylePeng, C., Zeng, D., Li, J., Peng, S., Xiong, J., Wang, W., Chen, Y., Liu, H., Liu, H., & Qin, R. (2021). Synthesis of Methyl Mercaptan on Mesoporous Alumina Prepared with Hydroxysafflor Yellow A as Template: The Synergistic Effect of Potassium and Molybdenum. Catalysts, 11(11), 1365. https://doi.org/10.3390/catal11111365