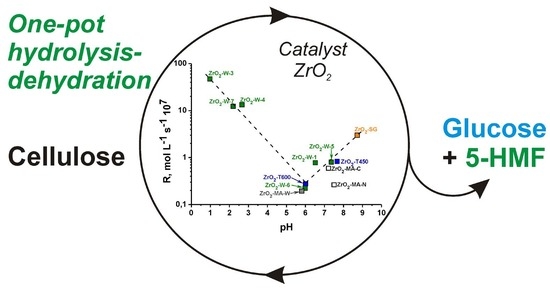
Impact of Design on the Activity of ZrO2 Catalysts in Cellulose Hydrolysis-Dehydration to Glucose and 5-Hydroxymethylfurfural
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.2.1. Thermodegradation (TD)
2.2.2. Microwave Treatment (MW)
2.2.3. Mechanical Activation (MA)
2.2.4. Microwave Heating Combined with Mechanical Pre-Activation (MW + MA)
2.2.5. Sol–Gel Method (SG)
2.3. Catalyst Characterization
2.4. Mechanical Activation and Characterization of Cellulose
2.5. Catalytic Tests
3. Results and Discussion
3.1. Catalyst Characterization
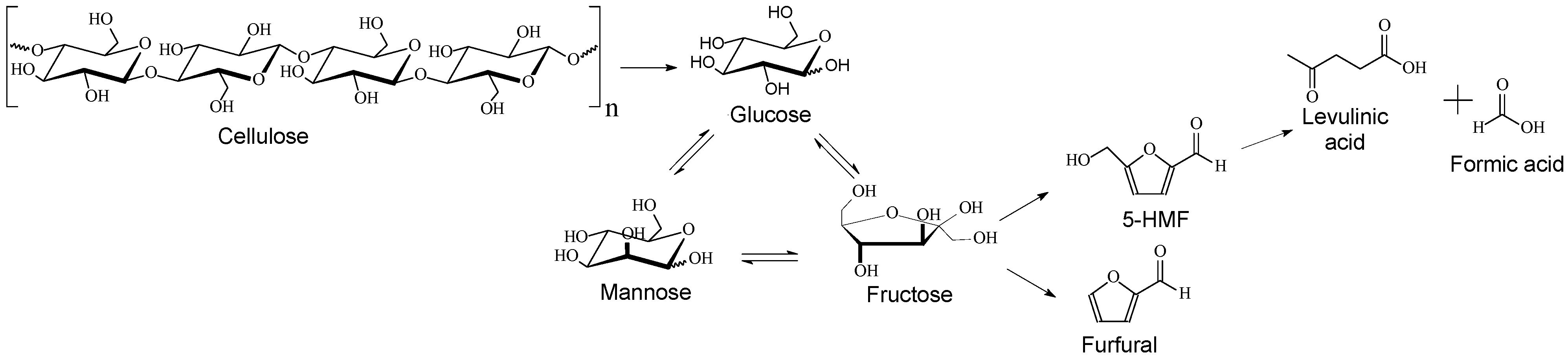
3.2. Cellulose Hydrolysis-Dehydration in the Presence of ZrO2 Catalysts
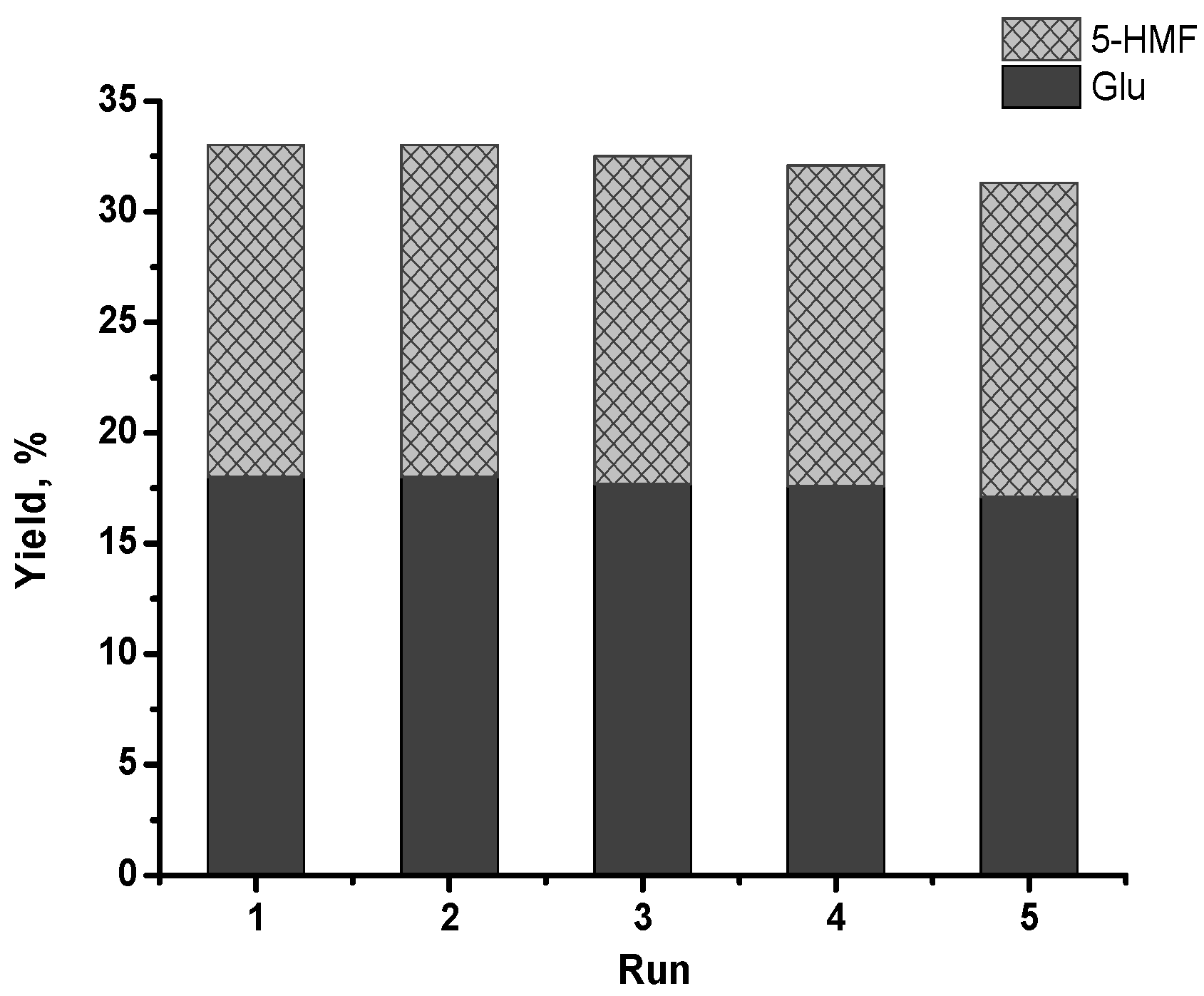
3.3. Perspectives of ZrO2 Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Agarwal, B.; Ahluwalia, V.; Pandey, A.; Sangwan, R.S.; Elumalai, S. Sustainable Production of Chemicals and Energy Fuel Precursors from Lignocellulosic Fractions. In Biofuels, Green Energy and Technology; Springer: Singapore, 2017; pp. 7–33. [Google Scholar] [CrossRef]
- Gromov, N.V.; Taran, O.P.; Parmon, V.N. CHAPTER 3 Catalysts for Depolymerization of Biomass. In Sustainable Catalysis for Biorefineries; The Royal Society of Chemistry: Croydon, UK, 2018; pp. 65–97. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Luque, R.; Sepúlveda-Escribano, A. Transformations of biomass-derived platform molecules: From high added-value chemicals to fuelsvia aqueous-phase processing. Chem. Soc. Rev. 2011, 11, 5266–5281. [Google Scholar] [CrossRef] [PubMed]
- Delidovich, I.; Leonhard, K.; Palkovits, R. Cellulose and hemicellulose valorisation: An integrated challenge of catalysis and reaction engineering. Energy Environ. Sci. 2014, 7, 2803–2830. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Kravchenko, O.A.; Ananikov, V.P. Conversion of plant biomass to furan derivatives and sustainable access to the new generation of polymers, functional materials and fuels. Russ. Chem. Rev. 2017, 86, 357–387. [Google Scholar] [CrossRef]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Centi, G.; Lanzafame, P.; Perathoner, S. Analysis of the alternative routes in the catalytic transformation of lignocellulosic materials. Catal. Today 2011, 167, 14–30. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, T.; Liu, Q.; Zhang, X.; Ma, W.; Zhang, Q. A review of thermal–chemical conversion of lignocellulosic biomass in China. Biotechnol. Adv. 2012, 30, 859–873. [Google Scholar] [CrossRef]
- Torres-Mayanga, P.C.; Lachos-Perez, D.; Mudhoo, A.; Kumar, S.; Brown, A.B.; Tyufekchiev, M.; Dragone, G.; Mussatto, S.I.; Rostagno, M.A.; Timko, M.; et al. Production of biofuel precursors and value-added chemicals from hydrolysates resulting from hydrothermal processing of biomass: A review. Biomass Bioenergy 2019, 130, 105397. [Google Scholar] [CrossRef]
- van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, S.G.; Alonso, D.M.; Gürbüz, E.I.; Dumesic, J.A. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Siankevich, S.; Savoglidis, G.; Fei, Z.; Laurenczy, G.; Alexander, D.T.L.; Yan, N.; Dyson, P.J. A novel platinum nanocatalyst for the oxidation of 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic acid under mild conditions. J. Catal. 2014, 315, 67–74. [Google Scholar] [CrossRef]
- Lewkowski, J. Synthesis, Chemistry and Applications of 5-Hydroxymethyl-furfural And Its Derivatives. ARKIVOC 2001, 2001, 17–54. [Google Scholar] [CrossRef] [Green Version]
- Rout, P.K.; Nannaware, A.D.; Prakash, O.; Kalra, A.; Rajasekharan, R. Synthesis of hydroxymethylfurfural from cellulose using green processes: A promising biochemical and biofuel feedstock. Chem. Eng. Sci. 2016, 142, 318–346. [Google Scholar] [CrossRef]
- Krawielitzki, S.; Kläusli, T.M. Modified Hydrothermal Carbonization Process for Producing Biobased 5-HMF Platform Chemical. Ind. Biotechnol. 2015, 11, 6–8. [Google Scholar] [CrossRef]
- Hii, S.L.; Tan, J.S.; Ling, T.C.; Ariff, A.B. Pullulanase: Role in Starch Hydrolysis and Potential Industrial Applications. Enzym. Res. 2012, 2012, 921362. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Hoff, T.C.; Emdadi, L.; Wu, Y.; Bouraima, J.; Liu, D. Catalytic consequences of micropore topology, mesoporosity, and acidity on the hydrolysis of sucrose over zeolite catalysts. Catal. Sci. Technol. 2014, 4, 3064–3073. [Google Scholar] [CrossRef]
- Scholz, M.J.; Riley, M.R.; Cuello, J.L. Acid hydrolysis and fermentation of microalgal starches to ethanol by the yeast Saccharomyces cerevisiae. Biomass Bioenergy 2013, 48, 59–65. [Google Scholar] [CrossRef]
- Razumovskii, S.D.; Podmaster’ev, V.V.; Zelenetskii, A.N. Mechanochemical methods of activating processes of biomass pretreatment. Catal. Ind. 2011, 3, 23–27. [Google Scholar] [CrossRef]
- Gromov, N.V.; Taran, O.P.; Sorokina, K.N.; Mishchenko, T.I.; Uthandi, S.; Parmon, V.N. New methods for the one-pot processing of polysaccharide components (cellulose and hemicelluloses) of lignocellulose biomass into valuable products. Part 1: Methods for biomass activation. Catal. Ind. 2016, 8, 176–186. [Google Scholar] [CrossRef]
- Aymonier, C.; Gromov, N.V.; Taran, O.P.; Parmon, V.N. Hydrolysis–dehydration of cellulose to glucose and 5-hydroxymethylfurfural over Sibunit solid acid carbon catalysts under semi-flow conditions. Wood Sci. Technol. 2021, 55, 607–624. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Taran, O.P.; Bukhtiyarov, A.V.; Aymonier, C.; Prosvirin, I.P.; Parmon, V.N. Hydrothermal Solubilization–Hydrolysis–Dehydration of Cellulose to Glucose and 5-Hydroxymethylfurfural Over Solid Acid Carbon Catalysts. Top. Catal. 2018, 61, 1912–1927. [Google Scholar] [CrossRef]
- Gromov, N.V.; Taran, O.P.; Aymonier, C.; Parmon, V.N. Kinetic modeling of the multistep hydrolysis-dehydration of cellulose to platform molecules over a solid carbon acid catalyst in pure water. React. Kinet. Mech. Catal. 2020, 130, 669–684. [Google Scholar] [CrossRef]
- Pang, J.; Wang, A.; Zheng, M.; Zhang, T. Hydrolysis of cellulose into glucose over carbons sulfonated at elevated temperatures. Chem. Commun. 2010, 46, 6935–6937. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Iris, K.M.; Chen, S.S.; Tsang, D.C.; Wang, L.; Xiong, X.; Poon, C.S. Production of 5-hydroxymethylfurfural from starch-rich food waste catalyzed by sulfonated biochar. Bioresour. Technol. 2018, 252, 76–82. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon Materials as Catalyst Supports and Catalysts in the Transformation of Biomass to Fuels and Chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Kobayashi, H.; Komanoya , T.; Hara, K.; Fukuoka, A. Water-Tolerant Mesoporous-Carbon-Supported Ruthenium Catalysts for the Hydrolysis of Cellulose to Glucose. ChemSusChem 2010, 3, 440–443. [Google Scholar] [CrossRef]
- Onda, A.; Ochi, T.; Yanagisawa, K. Selective hydrolysis of cellulose into glucose over solid acid catalysts. Green Chem. 2008, 10, 1033–1037. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; He, H.; Wang, L. Glucose production from hydrolysis of cellulose over a novel silica catalyst under hydrothermal conditions. J. Environ. Sci. 2012, 24, 473–478. [Google Scholar] [CrossRef]
- Dhepe, P.L.; Fukuoka, A. Cellulose Conversion under Heterogeneous Catalysis. ChemSusChem 2008, 1, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Dhepe, P.L.; Ohashi, M.; Inagaki, S.; Ichikawa, M.; Fukuoka, A. Hydrolysis of sugars catalyzed by water-tolerant sulfonated mesoporous silicas. Catal. Lett. 2005, 102, 163–169. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Galande, N.D.; Thakur, P.; Sawant, S.D.; Zambre, V.P.; Bokade, V.V. One-Pot Synthesis of 5-Hydroxymethylfurfural by Cellulose Hydrolysis over Highly Active Bimodal Micro/Mesoporous H-ZSM-5 Catalyst. ACS Sustain. Chem. Eng. 2014, 2, 1928–1932. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Taran, O.P.; Timofeeva, M.N.; Parmon, V.N. Hydrolysis of Cellulose in the Presence of Catalysts Based on Cesium Salts of Heteropoly Acids. Catal. Ind. 2021, 13, 73–80. [Google Scholar] [CrossRef]
- Tian, J.; Fang, C.; Cheng, M.; Wang, X. Hydrolysis of Cellulose over CsxH3–xPW12O40 (X = 1–3) Heteropoly Acid Catalysts. Chem. Eng. Technol. 2011, 34, 482–486. [Google Scholar] [CrossRef]
- Pattnaik, F.; Nanda, S.; Kumar, V.; Naik, S.; Dalai, A.K. Subcritical water hydrolysis of Phragmites for sugar extraction and catalytic conversion to platform chemicals. Biomass Bioenergy 2021, 145, 105965. [Google Scholar] [CrossRef]
- Antonetti, C.; Melloni, M.; Licursi, D.; Fulignati, S.; Ribechini, E.; Rivas, S.; Parajó, J.C.; Cavani, F.; Raspolli Galletti, A.M. Microwave-assisted dehydration of fructose and inulin to HMF catalyzed by niobium and zirconium phosphate catalysts. Appl. Catal. B Environ. 2017, 206, 364–377. [Google Scholar] [CrossRef]
- Chareonlimkun, A.; Champreda, V.; Shotipruk, A.; Laosiripojana, N. Catalytic conversion of sugarcane bagasse, rice husk and corncob in the presence of TiO2, ZrO2 and mixed-oxide TiO2–ZrO2 under hot compressed water (HCW) condition. Bioresour. Technol. 2010, 101, 4179–4186. [Google Scholar] [CrossRef]
- Gavilà, L.; Güell, E.J.; Maru, B.T.; Medina, F.; Constantí, M. Combining catalytical and biological processes to transform cellulose into high value-added products. Phys. Sci. Rev. 2017, 2, 26. [Google Scholar] [CrossRef]
- Gliozzi, G.; Innorta, A.; Mancini, A.; Bortolo, R.; Perego, C.; Ricci, M.; Cavani, F. Zr/P/O catalyst for the direct acid chemo-hydrolysis of non-pretreated microcrystalline cellulose and softwood sawdust. Appl. Catal. B Environ. 2014, 145, 24–33. [Google Scholar] [CrossRef]
- Gromov, N.V.; Taran, O.P.; Semeykina, V.S.; Danilova, I.G.; Pestunov, A.V.; Parkhomchuk, E.V.; Parmon, V.N. Solid Acidic NbOx/ZrO2 Catalysts for Transformation of Cellulose to Glucose and 5-Hydroxymethylfurfural in Pure Hot Water. Catal. Lett. 2017, 147, 1485–1495. [Google Scholar] [CrossRef]
- Watanabe, M.; Aizawa, Y.; Iida, T.; Nishimura, R.; Inomata, H. Catalytic glucose and fructose conversions with TiO2 and ZrO2 in water at 473K: Relationship between reactivity and acid–base property determined by TPD measurement. Appl. Catal. A Gen. 2005, 295, 150–156. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; Gao, P.; Lv, X.-N.; Sun, X.; Liu, Z.-H.; Fan, H. Mild Hydrothermal Degradation of Cotton Cellulose by using a Mixed-Metal-Oxide ZnO–ZrO2 Catalyst. Energy Technol. 2013, 1, 581–586. [Google Scholar] [CrossRef]
- Qiao, Y.; Feng, L.; Li, Z.; Zhang, Z.; Chen, J.; Na, H.; Zhu, J.; Chen, L. Effect of Adsorption of ZrO2 in Catalysts on the Efficiency of Hydrolysisof Cellulose to Sugar in Aqueous System under Microwave Radiation. Chin. J. Chem. 2020, 38, 399–405. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhai, C.; Liu, F.; Chen, L.; Na, H.; Chen, J.; Zhu, J. Highly efficient microwave driven assisted hydrolysis of cellulose to sugar with the utilization of ZrO2 to inhibit recrystallization of cellulose. Carbohydr. Polym. 2020, 228, 115358. [Google Scholar] [CrossRef] [PubMed]
- Bolotov, V.A.; Chernousov, Y.D.; Udalov, E.I.; Tanashev, Y.Y.; Parmon, V.N. Features of high-temperature chemical reactions under the action of a microwave field. Vestnik NGU. Ser. Fiz. 2009, 4, 78–83. [Google Scholar]
- Avvakumov, E.G. Mechanical Methods of Activation of Chemical Processes; Nauka: Novosibirsk, Russia, 1986. [Google Scholar]
- Tsybulya, S.V.; Cherepanova, S.V.; Soloviyova, L.P. Polycrystal software package for IBM/PC. J. Struct. Chem. 1996, 37, 332–334. [Google Scholar] [CrossRef]
- Yatsenko, D.A.; Medvedeva, T.B. Estimating Crystality Index of Microcrystalline Cellulose Using Diffraction Methods. J. Struct. Chem. 2019, 60, 1430–1436. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Rodikova, Y.A.; Timofeeva, M.N.; Panchenko, V.N.; Taran, O.P.; Kozhevnikov, I.V.; Parmon, V.N. One-pot synthesis of sorbitol via hydrolysis-hydrogenation of cellulose in the presence of Ru-containing composites. Bioresour. Technol. 2021, 319, 124122. [Google Scholar] [CrossRef] [PubMed]
- Taran, O.P.; Polyanskaya, E.M.; Ogorodnikova, O.L.; Descorme, C.; Besson, M.; Parmon, V.N. Sibunit-based catalytic materials for the deep oxidation of organic ecotoxicants in aqueous solution: I. Surface properties of the oxidized sibunit samples. Catal. Ind. 2010, 2, 381–386. [Google Scholar] [CrossRef]


| Catalyst | Substrate | Reaction Conditions | Glucose Yield/Selectivity, (%) | 5-HMF Yield/Selectivity, (%) | Ref. |
|---|---|---|---|---|---|
| Si/Zr/O | Softwood | 423 K, autogenic pressure | - | 8.2 a/- | [42] |
| ZnO-ZrO2 | Cotton Cellulose | 463 K, 1.4 MPa | 6.01/- | 3.76/- | [45] |
| ZrO2 | Microcrystalline Cellulose | 453 K, 3 MPa | -/~2.3 | -/~20.6 | [41] |
| m/c-ZrO2 | Glucose | 473 K, 2.5 MPa | - | ~5.4/- b | [44] |
| Fructose | ~2.3/- | ~15/- | |||
| Zr-P-773 c | Sugarcane bagasse | 523 K, 34.5 MPa | ~1.8/- | ~4.2/- | [40] |
| Zr-P-873 | ~1.8/- | ~3.4/- | |||
| Zr-P-973 | ~1.2/- | ~2.5/- | |||
| Zr-S-773 | ~1.2/- | ~3.4/- | |||
| Zr-S-873 | ~0.9/- | ~3.3/- | |||
| Zr-S-973 | ~0.7/- | ~3.2/- | |||
| ZrO2 | Cellulose | 433 K [H2SO4] = 0.04 mol·L−1 | 65.2/- d | n/d | [47] |
| ZrO2-1 e | Cellulose | 433 K [H2SO4] = 0.04 mol L−1 | 62.3/- d | n/d | [46] |
| ZrO2-2 | 93.6/- d | n/d | |||
| ZrO2-3 | ~64.9/- d | n/d | |||
| ZrO2-4 | ~80.3/- d | n/d | |||
| ZrO2-5 | ~91.0/- d | n/d | |||
| ZrO2 | Cellulose | 453 K, 1 MPa | 12.7/- | 13.3/- | [43] |
| Synthesis Method a | Catalyst Sample | Precursor of ZrO2 | Synthesis Conditions |
|---|---|---|---|
| TD | ZrO2-T-723 | ZrO(NO3)2 | Thermodegradation at 723 K for 4 h |
| ZrO2-T-873 | ZrO(NO3)2 | Thermodegradation at 873 K for 4 h | |
| MW | ZrO2-W-1 | ZrO(NO3)2 | 9 min microwave treatment at 35 W (T = 523 K) |
| ZrO2-W-3 | ZrO(NO3)2 | Total treatment 18 min, microwave irradiation 9 min, 35–60 W (T = 523 K) | |
| ZrO2-W-4 | ZrCO3(OH)2 | Total treatment 11 min, microwave irradiation 3 min, 105 W (T = 603 K) | |
| ZrO2-W-5 | ZrO(NO3)2 | Total treatment 16 min, microwave irradiation 9 min, 80 W (T = 573 K) followed by 7 min, 150 W (T = 873 K) | |
| ZrO2-W-6 | ZrO(NO3)2 | Total treatment 48 min, microwave irradiation 24 min, 1000 W (T = 873 K) | |
| ZrO2-W-7 | ZrO(NO3)2 | Total treatment 30 min, microwave irradiation 15 min, 1000 W (T = 593 K) | |
| MA + MW | ZrO2-MA-W | ZrO(NO3)2 | Mechanical activation of ZrO2-T-873 2 min, followed by MW (total treatment 30 min, microwave irradiation 15 min, 1000 W (T = 593 K) |
| MA | ZrO2-MA-C | ZrCO3(OH)2 | Mechanical activation 2 min followed by calcination at 723 K during 4 h |
| ZrO2-MA-N | ZrO(NO3)2 | Mechanical activation 2 min followed by calcination at 723 K during 4 h | |
| SG | ZrO2-SG | Tetraisopropoxide Zr(IV) | Sol–gel method followed by calcination at 873 K during 8 h |
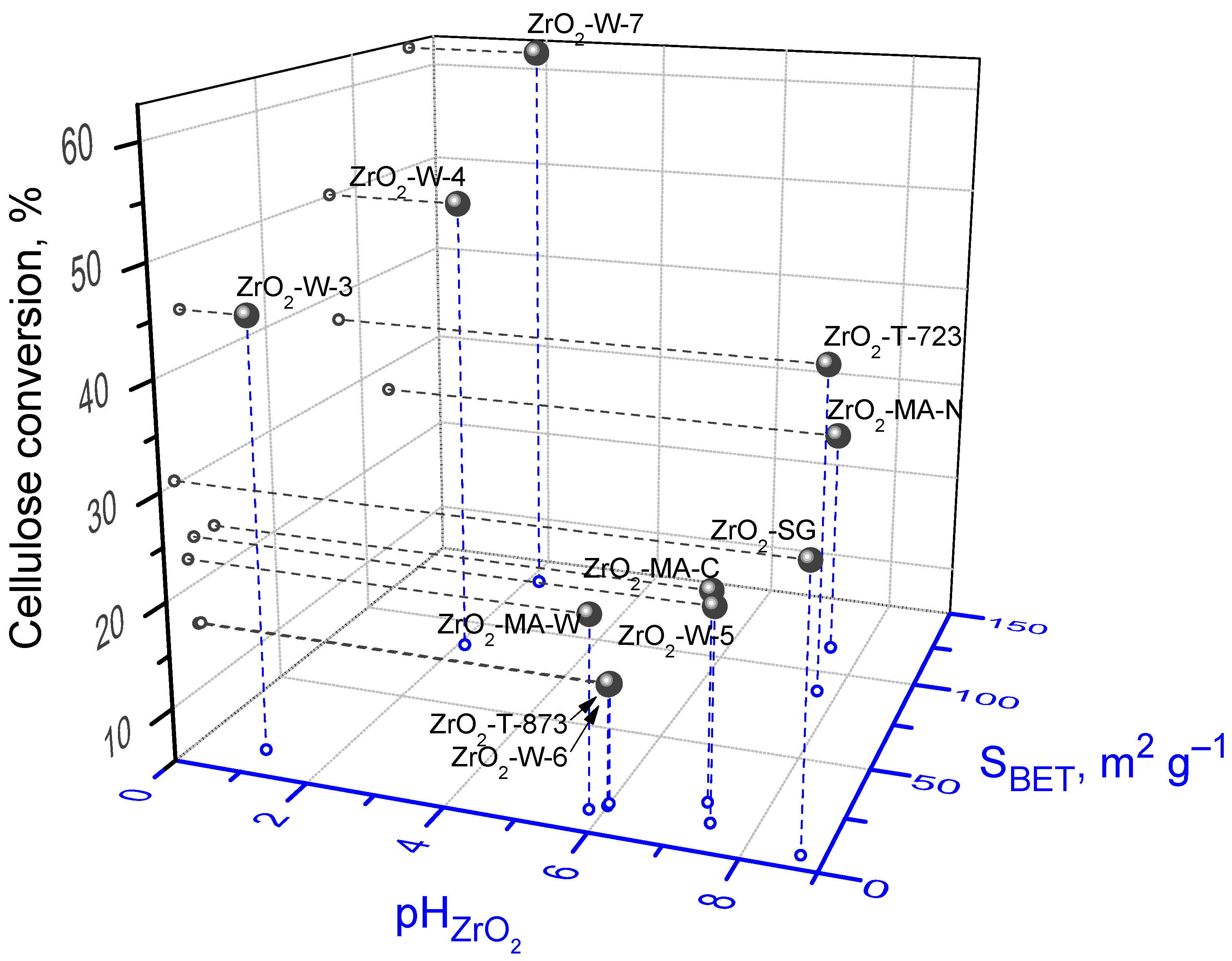
| No. | Catalyst Sample | Synthesis Method a | Texture Properties | Phase Composition b | pHZrO2 | ||
|---|---|---|---|---|---|---|---|
| SBET (m2·g−1) | VΣ (cm3 g−1) | Main | Admixture | ||||
| 1 | ZrO2-T-723 | TD | 88 | 0.24 | 50% M 50% T | - | 7.7 |
| 2 | ZrO2-T-873 | TD | 14 | 0.11 | n.d. | n.d. | 6.0 |
| 3 | ZrO2-W-1 | W | n.d. | n.d. | A | M | 6.5 |
| 4 | ZrO2-W-3 | W | 12 | 0.06 | A | - | 1.0 |
| 5 | ZrO2-W-4 | W | 85 | 0.18 | A | - | 2.1 |
| 6 | ZrO2-W-5 | W | 13 | 0.04 | A | - | 7.4 |
| 7 | ZrO2-W-6 | W | 13 | 0.08 | A | - | 6.0 |
| 8 | ZrO2-W-7 | W | 134 | 0.37 | A | - | 2.2 |
| 9 | ZrO2-MA-W | MA + MW | 10 | 0.39 | M | - | 5.8 |
| 10 | ZrO2-MA-C | MA | 22 | 0.06 | T | M | 7.2 |
| 11 | ZrO2-MA-N | MA | 116 | 0.24 | T | M | 7.5 |
| 12 | ZrO2-SG | SG | 6 | 0.02 | n.d. | n.d. | 8.7 |
| Entry No. | Catalyst Sample | X b, % | TOF c | Glucose Yield, % | 5-HMF Yield, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 5 h | 7 h | 0 h | 1 h | 2 h | 3 h | 5 h | 7 h | ||||
| 1 | ZrO2-T-723 | 37.4 | 0.12 | 0.0 | 0.5 | 0.6 | 1.3 | 1.4 | 1.4 | 0.0 | 0.3 | 1.0 | 2.4 | 3.0 | 3.8 |
| 2 | ZrO2-T-873 | 16.0 | 0.02 | 0.3 | 0.1 | 0.3 | 0.4 | 0.5 | 0.5 | 0.0 | 0.2 | 0.7 | 1.0 | 1.9 | 2.8 |
| 3 | ZrO2-W-1 | 37.8 | 0.09 | 0.0 | 0.5 | 0.2 | 1.4 | 1.9 | 0.3 | 0.0 | 0.5 | n/d | 2.6 | 3.9 | 0.9 |
| 4 | ZrO2-W-3 | 45.0 | 1.79 | 14.3 | 3.0 | 1.0 | 0.4 | 0.1 | 0.1 | 0.7 | 4.5 | 3.4 | 2.5 | 1.4 | 1.1 |
| 5 | ZrO2-W-4 | 50.1 | 0.62 | 8.1 | 9.1 | 8.8 | 7.9 | 4.5 | 2.8 | 2.2 | 4.0 | 6.2 | 7.0 | 7.5 | 7.4 |
| 6 | ZrO2-W-5 | 24.3 | 0.06 | 0.0 | 0.0 | 0.1 | 0.2 | 1.1 | 1.7 | 0.0 | 0.2 | 0.3 | 0.6 | 1.6 | 3.3 |
| 7 | ZrO2-W-6 | 16.1 | 0.02 | 0.0 | 0.1 | 0.2 | 0.4 | 0.6 | 0.7 | 0.0 | 0.2 | 0.5 | 0.7 | 1.6 | 2.4 |
| 8 | ZrO2-W-7 | 62.4 | 0.70 | 5.2 | n/d | 6.9 | 4.1 | 1.4 | 0.6 | 0.4 | 5.0 | 3.9 | 2.9 | 1.8 | 1.3 |
| 9 | ZrO2-MA-W | 22.6 | 0.03 | 0.0 | 0.1 | 0.2 | 0.3 | 0.5 | 0.7 | 0.0 | 0.2 | 0.6 | 1.0 | 2.2 | 3.5 |
| 10 | ZrO2-MA-C | 24.2 | 0.02 | 0.0 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 0.0 | 0.0 | 0.2 | 0.4 | 0.8 | 1.8 |
| 11 | ZrO2-MA-N | 27.3 | 0.01 | 0.0 | 0.1 | 0.1 | 0.1 | 0.4 | 0.8 | 0.1 | 0.2 | 0.4 | 1.0 | 1.8 | 2.8 |
| 12 | ZrO2-SG | 30.3 | 0.21 | 0.0 | 0.9 | 2.6 | 4.6 | 7.0 | 7.9 | 0.0 | 0.5 | 1.4 | 2.9 | 5.7 | 8.2 |
| Catalyst Sample | [Cat]/[Cell] | pHZrO2 | X c (%) | R, (mol·L−1·s−1·107) | Maximum Yields of the Products (%) | |||
|---|---|---|---|---|---|---|---|---|
| YGlu | τ, h | Y5-HMF | τ, h | |||||
| ZrO2-W-7 | 1:1 | 2.2 | 62.4 | 12.4 | 6.2 | 2 | 5.0 | 1 |
| 1:20 b | 3.4 | 53.1 | 18.6 | 18.0 | 7 | 15.0 | 7 | |
| Reaction Conditions | TOF, (mmol·g−1·h−1) | Ref. | |
|---|---|---|---|
| T, P | Cell:H2O:Cat a | ||
| 463 K, 1.4 MPa | 1:100:1 | 0.10 | [45] |
| 453 K, 3 MPa | 4:250:1 | 3.5 × 10−5 | [41] |
| 453 K, 1 MPa | 1:100:1 | 0.83 | [43] |
| 453 K, 1 MPa | 20:2000:1 | 15.1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medvedeva, T.B.; Ogorodnikova, O.L.; Yakovleva, I.S.; Isupova, L.A.; Taran, O.P.; Gromov, N.V.; Parmon, V.N. Impact of Design on the Activity of ZrO2 Catalysts in Cellulose Hydrolysis-Dehydration to Glucose and 5-Hydroxymethylfurfural. Catalysts 2021, 11, 1359. https://doi.org/10.3390/catal11111359
Medvedeva TB, Ogorodnikova OL, Yakovleva IS, Isupova LA, Taran OP, Gromov NV, Parmon VN. Impact of Design on the Activity of ZrO2 Catalysts in Cellulose Hydrolysis-Dehydration to Glucose and 5-Hydroxymethylfurfural. Catalysts. 2021; 11(11):1359. https://doi.org/10.3390/catal11111359
Chicago/Turabian StyleMedvedeva, Tatiana B., Olga L. Ogorodnikova, Irina S. Yakovleva, Lyubov A. Isupova, Oxana P. Taran, Nikolay V. Gromov, and Valentin N. Parmon. 2021. "Impact of Design on the Activity of ZrO2 Catalysts in Cellulose Hydrolysis-Dehydration to Glucose and 5-Hydroxymethylfurfural" Catalysts 11, no. 11: 1359. https://doi.org/10.3390/catal11111359
APA StyleMedvedeva, T. B., Ogorodnikova, O. L., Yakovleva, I. S., Isupova, L. A., Taran, O. P., Gromov, N. V., & Parmon, V. N. (2021). Impact of Design on the Activity of ZrO2 Catalysts in Cellulose Hydrolysis-Dehydration to Glucose and 5-Hydroxymethylfurfural. Catalysts, 11(11), 1359. https://doi.org/10.3390/catal11111359