Cationic Dye Degradation and Real Textile Wastewater Treatment by Heterogeneous Photo-Fenton, Using a Novel Natural Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
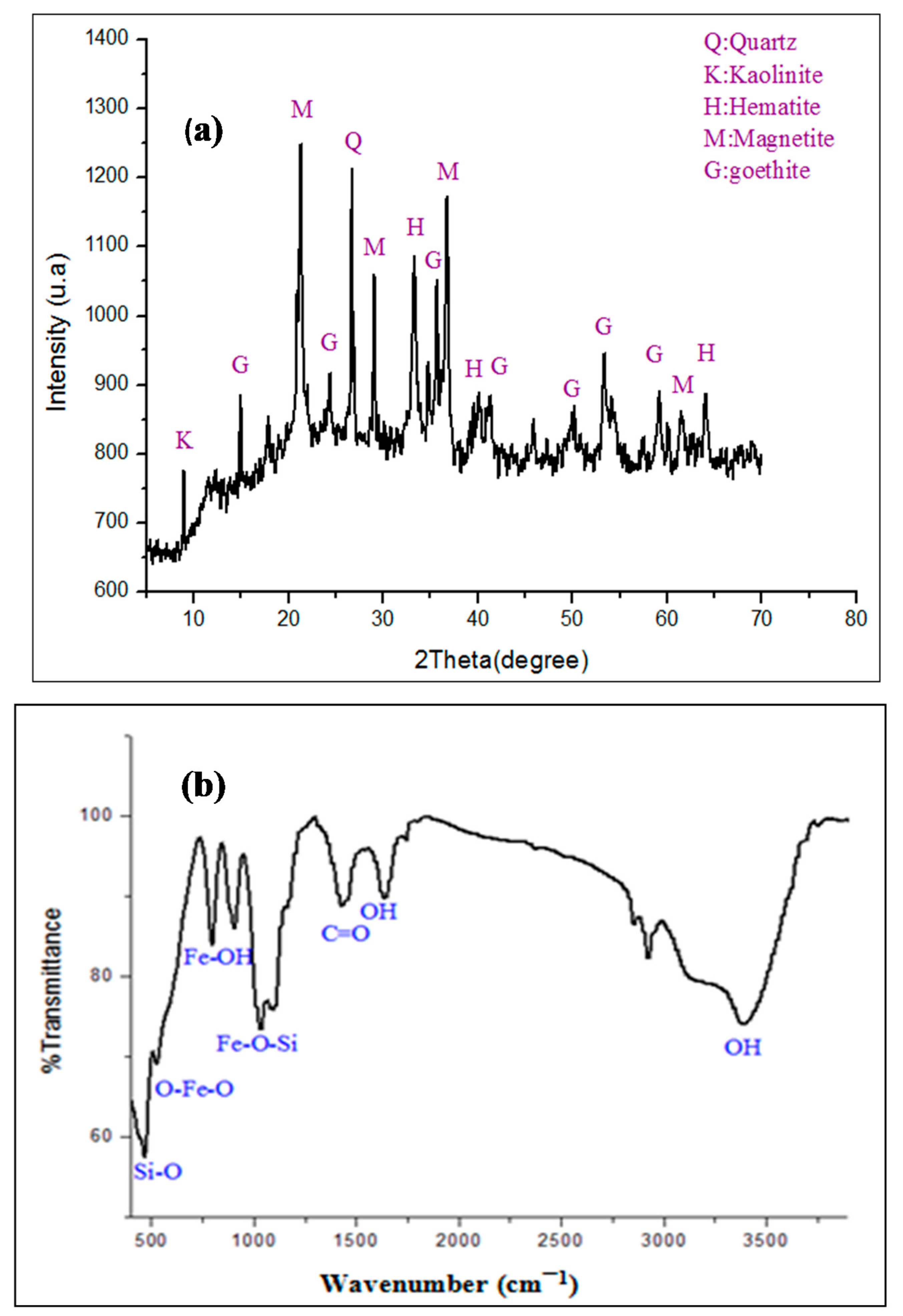
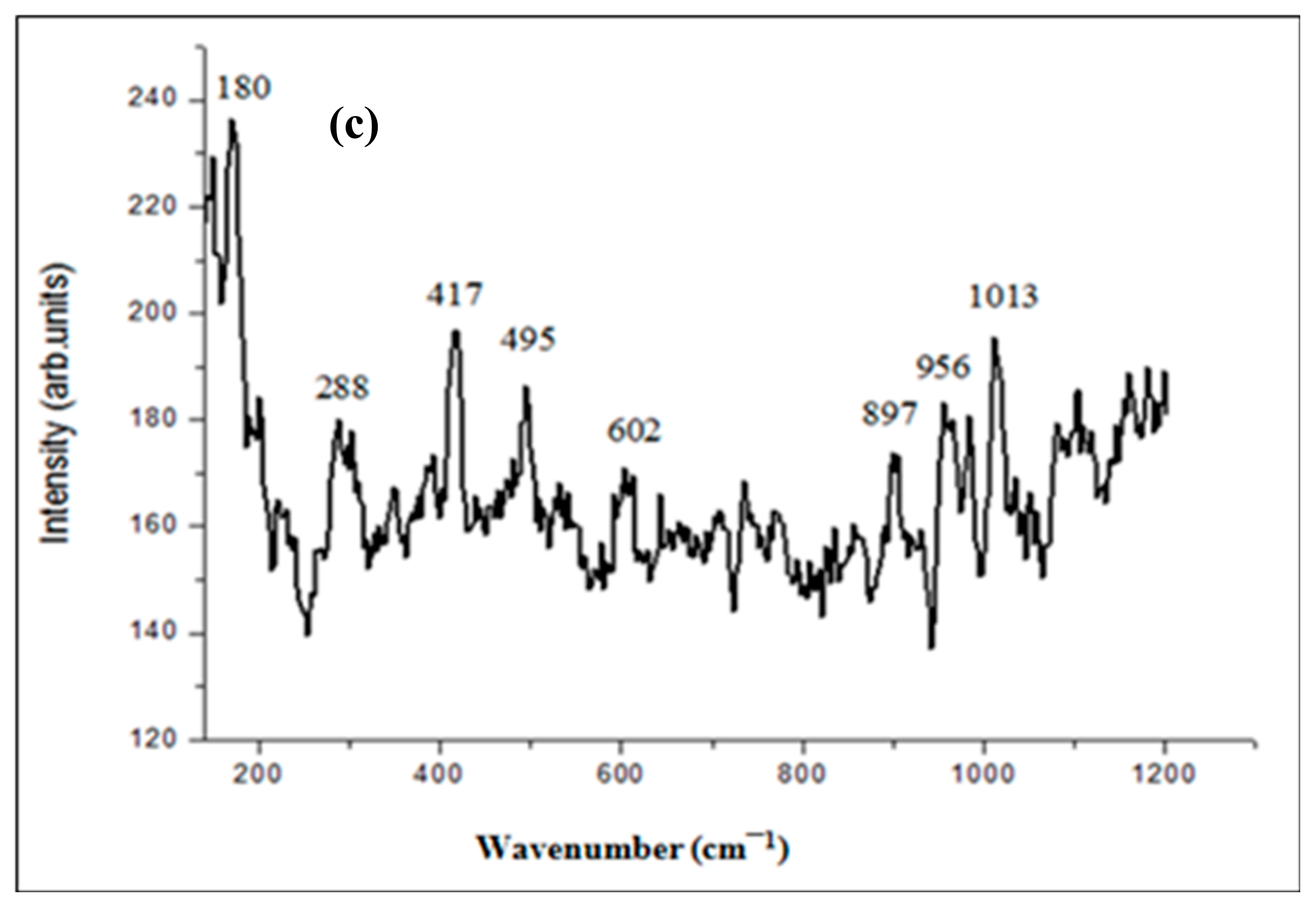
2.1.1. XRD, Raman, and FT-IR Analysis
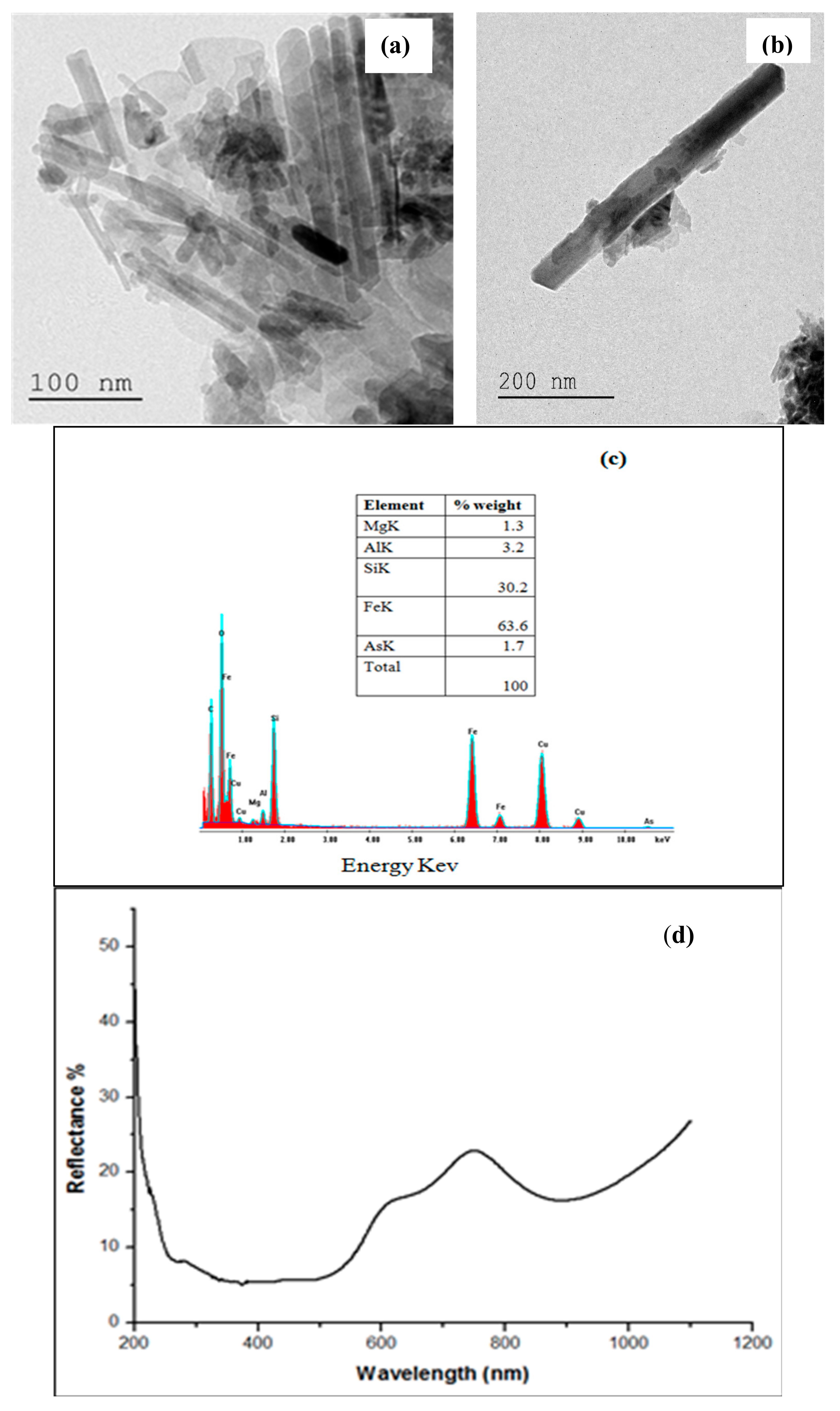
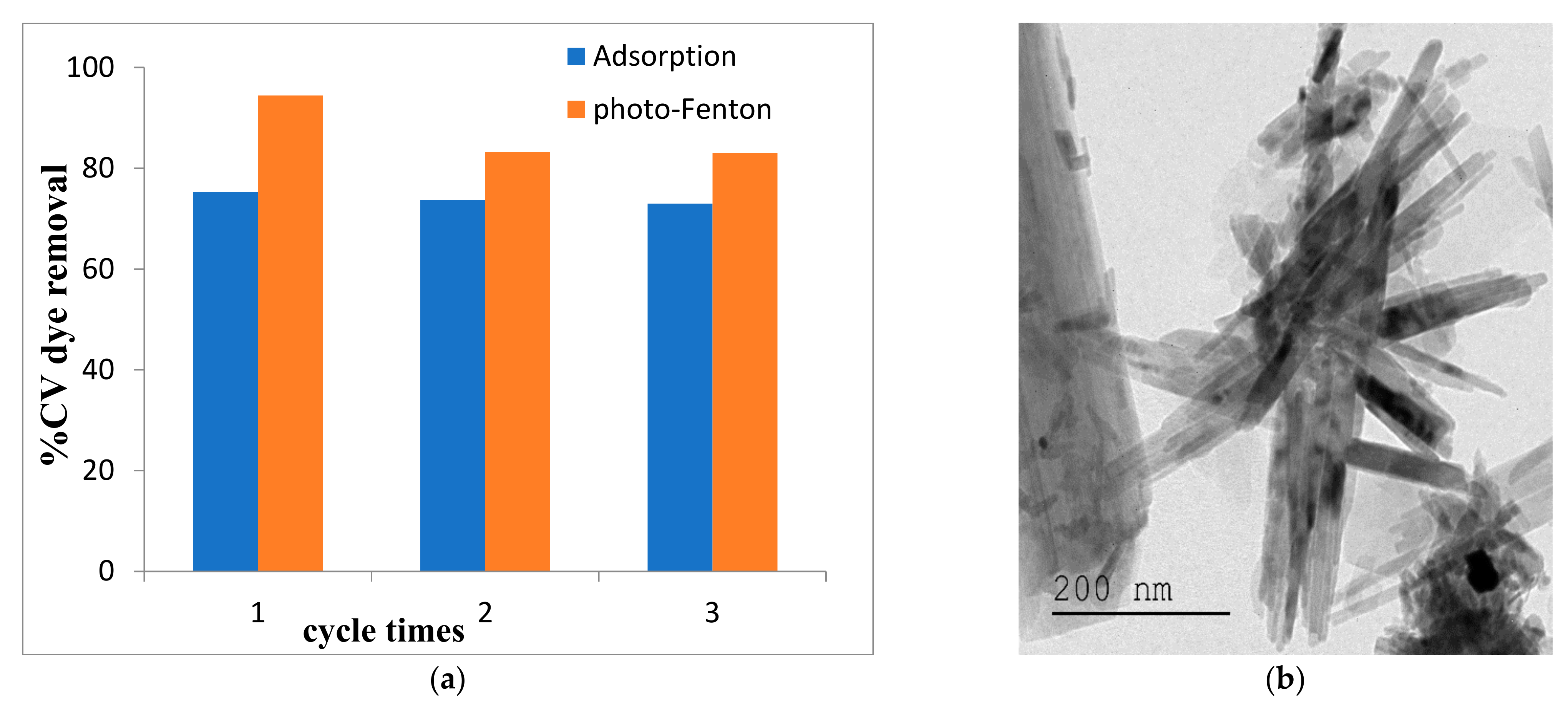
2.1.2. TEM–EDX and DRS Analyses
2.1.3. BET and BJH Analyses
2.2. Degradation of CV Dye in Aqueous Solution by AOPs
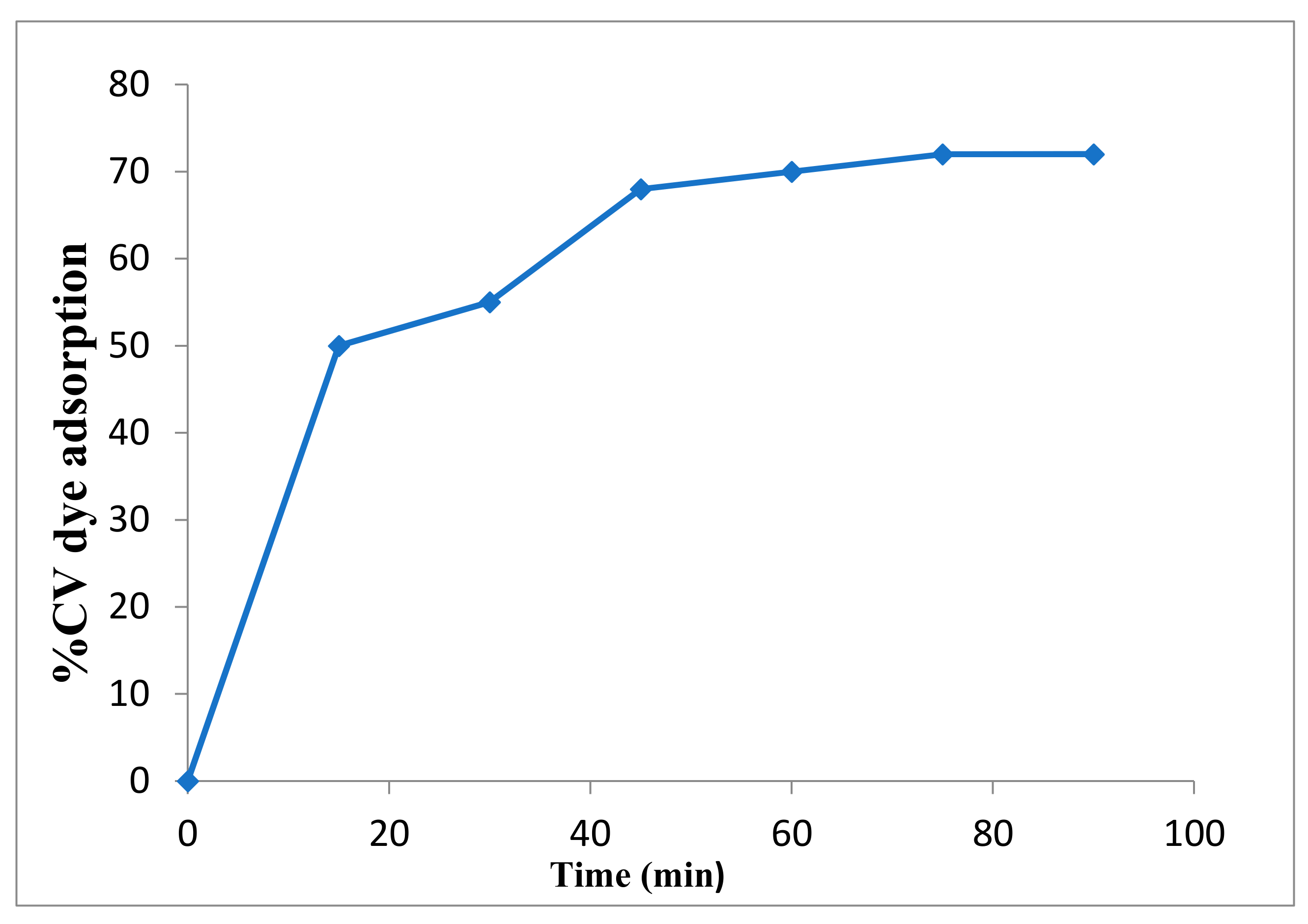
2.2.1. Dark Adsorption Test
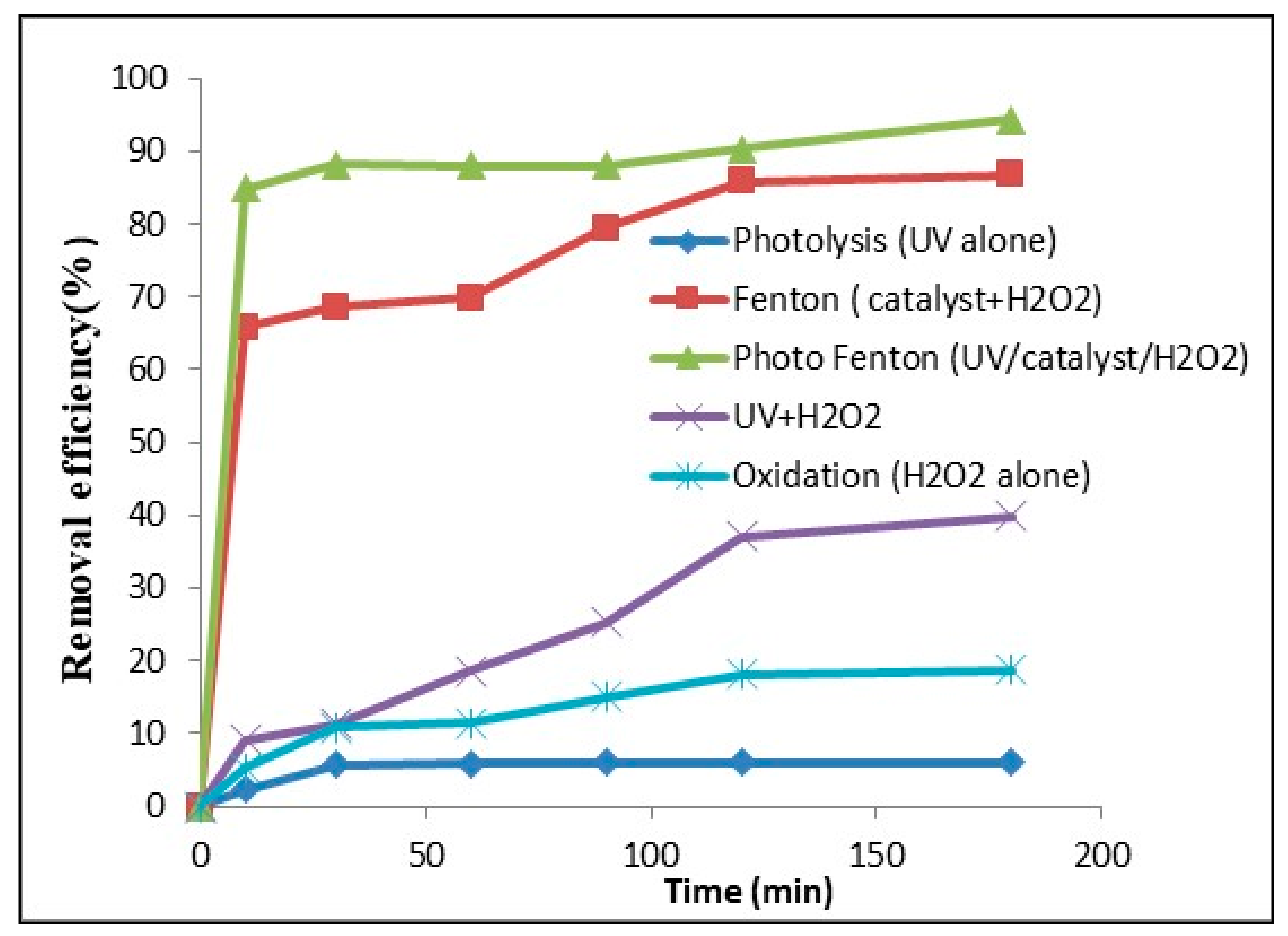
2.2.2. Photo-Fenton Experiments
2.2.3. CV Dye Degradation by Photo-Fenton Process: Effect of Different Parameters
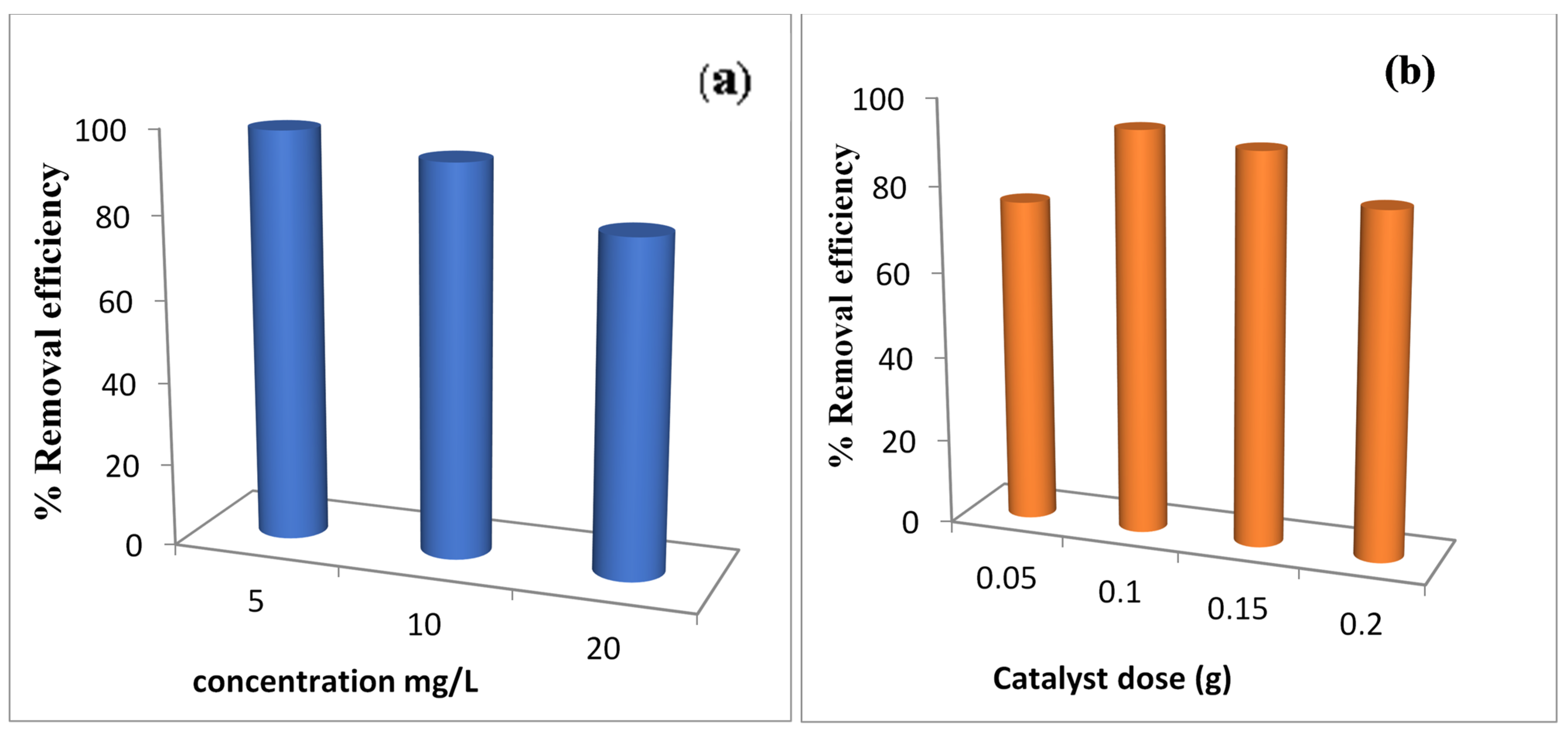
Effect of the Initial Pollutant Concentration
Effect of Catalyst Dose
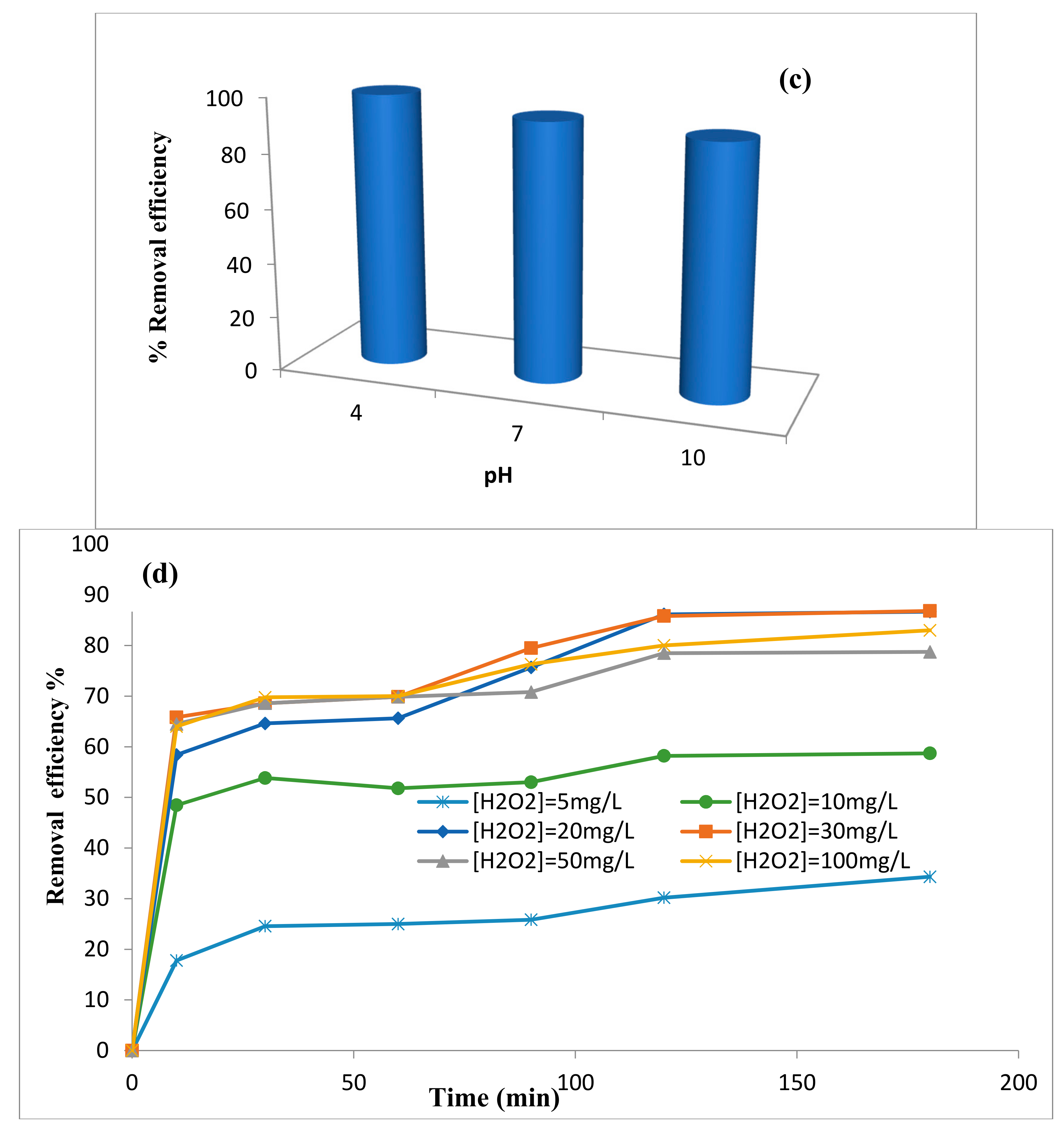
Effect of pH
Effect of H2O2 Concentration
2.3. Kinetic Study and Oxidation Intermediates Characterization
2.3.1. Kinetic Study
2.3.2. Characterization of Oxidation Intermediates
2.4. Catalyst Regeneration and Stability
2.5. Real Textile Wastewater Treatment
3. Methods and Materials
3.1. Reagents and Chemicals
3.2. Characterization of Catalyst
3.3. Advanced Oxidation and Control Tests
3.4. Analytical Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abukhadra, M.R.; Abukhadra, M.; Rabia, M.; Shaban, M.; Verpoort, F. Heulandite/polyaniline hybrid composite for efficient removal of acidic dye from water; kinetic, equilibrium studies and statistical optimization, optimization. Adv. Powder Tech. 2018, 10, 2501–2511. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Walker, G.M.; Weatherley, L.R. COD removal from textile industry effluent: Pilot plant studies. Chem. Eng. J. 2001, 84, 125–131. [Google Scholar] [CrossRef]
- Patel, T.M.; Chheda, H.; Baheti, A.; Patel, P.; Nath, K. Comparative performance of flat sheet and spiral wound modules in the nanofiltration of reactive dye solution. Environ. Sci. Pollut. 2012, 19, 2994–3004. [Google Scholar] [CrossRef]
- Zhang, X.; Geng, Z.; Jian, J.; Yiqiang, He.; Zipeng, Lv.; Xinxin, L.; Hongming, Y. Potassium ferrite as heterogeneous photo-fenton catalyst for highly efficient dye degradation. Catalysts 2020, 10, 293. [Google Scholar] [CrossRef] [Green Version]
- Lau, W.J.; Ismail, A.F. Polymeric nanofiltration membranes for textile dye wastewater treatment: Preparation, performance evaluation, transport modelling and fouling control. Desalination 2009, 245, 321–348. [Google Scholar] [CrossRef]
- Xiong, W.; Zeng, G.; Yang, Z.; Zhou, Y.; Zhang, C.; Cheng, M.; Liu, Y.; Hu, L.; Wan, J.; Zhou, C.; et al. Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53 (Fe) as new adsorbent. Sci. Total Environ. 2018, 627, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Rasalingam, S.; Peng, R.; Koodali, R.T. An insight into the adsorption and photocatalytic degradation of rhodamine B in periodic mesoporous materials. Appl. Catal. B Environ. 2015, 174, 49–59. [Google Scholar] [CrossRef]
- Achille, G.N.; Yi-lian, L. Mineralization of organic compounds in wastewater contaminated with petroleum hydrocarbon using Fenton’s reagent: A kinetic study. Am. J. Sci. 2010, 6, 58–66. [Google Scholar]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. Sci. 2019, 376, 120597. [Google Scholar] [CrossRef]
- De Carluccio, M.; Fiorentino, A.; Rizzo, L. Multi-barrier treatment of mature landfill leachate: Effect of Fenton oxidation and air stripping on activated sludge process and cost analysis. J. Environ. Chem. Eng. 2020, 8, 104444. [Google Scholar] [CrossRef]
- Fiorentino, A.; Cucciniello, R.; Di Cesare, A.; Fontaneto, D.; Prete, P.; Rizzo, L.; Corno, G.; Proto, A. Disinfection of urban wastewater by a new photo-Fenton like process using Cu-iminodisuccinic acid complex as catalyst at neutral pH. Water Res. 2018, 146, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Arzate, S.; Campos-Mañas, M.C.; Miralles-Cuevas, S.; Agüera, A.; García Sánchez, J.L.; Sánchez Pérez, J.A. Removal of contaminants of emerging concern by continuous flow solar photo-Fenton process at neutral pH in open reactors. J. Environ. Manag. 2020, 261, 110265. [Google Scholar] [CrossRef] [PubMed]
- Papoutsakis, S.; Brites-Nóbrega, F.F.; Pulgarin, C.; Malato, S. Benefits and limitations of using Fe(III)-EDDS for the treatment ofhighly contaminated water at near-neutral pH. J Photochem. Photobiol. A Chem. 2015, 303, 1–7. [Google Scholar] [CrossRef]
- ELKhouly, S.M.; Fathy, N.A. Multi-walled carbon nanotubes supported amorphous Fe2O3 and Ag2O–Fe2O3 as Fenton catalysts for degradation of maxilon red dye. Asia-Pacific. Chem. Eng. Technol. 2018, 13, e2184. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and prospects for wastewater treatment by UV and visible-light-active heterogeneous photocatalysis: A critical review. Top. Curr. Chem. 2020, 378, 1–40. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; De Marco, I.; Vaiano, V. Zinc oxide nanoparticles obtained by supercritical antisolvent precipitation for the photocatalytic degradation of crystal violet dye. Catalysts 2019, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Mani, S.; Bharagava, R.N. Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety. Rev. Environ. Contam. Toxicol. 2016, 237, 71–104. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L. Bioassays as a tool for evaluating advanced oxidation processes in water and wastewater treatment. Water Res. 2011, 45, 4311–4340. [Google Scholar] [CrossRef] [PubMed]
- Kermani, M.; Mohammadi, F.; Kakavandi, B.; Esrafili, A.; Rostamifasih, Z. Simultaneous catalytic degradation of 2, 4-D and MCPA herbicides using sulfate radical-based heterogeneous oxidation over persulfate activated by natural hematite (α-Fe2O3/PS). J. Phys. Chem. Solids 2018, 117, 49–59. [Google Scholar] [CrossRef]
- Fahlepy, M.R.; Tiwow, V.A. Characterization of magnetite (Fe3O4) minerals from natural iron sand of Bonto Kanang Village Takalar for ink powder (toner) application. J. Phys. Conf. Ser. 2018, 997, 012036. [Google Scholar] [CrossRef]
- Jin, W.H.; Cao, G.T.; Sun, J.Y. Hybrid supercapacitor based on MnO2 and columned FeOOH using Li2SO4 electrolyte solution. J. Power Sources 2008, 175, 686–691. [Google Scholar] [CrossRef]
- Cai, J.; Chen, S.; Ji, M.; Hu, J.; Ma, Y.; Qi, L. Organic additive free synthesis of mesocrystalline hematite nanoplates via two-dimensional oriented attachment. CrystEngComm 2014, 16, 1553–1559. [Google Scholar] [CrossRef]
- Sankararamakrishman, N.; Gupta, A.; Viduarthi, S.B. Enhanced arsenic removal at neutral pH using functionalized multiwalled carbon nanotubes. J. Environ. Chem. Eng. 2014, 2, 802–810. [Google Scholar] [CrossRef]
- Ben Ayed, S.; Sbihi, H.M.; Azam, M.; Al-Resayes, S.I.; Ayadi, M.T.; Ayadi, F. Local iron ore identification: Comparison to synthesized Fe3O4 nanoparticles obtained by ultrasonic assisted reverse co-precipitation method for Auramine O dye adsorption. Desalin. Water Treat. 2021, 220, 446–458. [Google Scholar] [CrossRef]
- Shenoy, M.R.; Ayyasamy, S.; Reddy, M.V.V.; Kadarkarai, G.; Suryakanth, J.; Tamilarasan, A.; Jeyaramane, C. The effect of morphology-dependent surface charges of iron oxide on the visible light photocatalytic degradation of methylene blue dye. J. Mater. Sci. Mater. Electron. 2020, 31, 17703–17717. [Google Scholar] [CrossRef]
- Hanesch, M. Raman spectroscopy of iron oxides and oxy(hydroxides) at lower laser power and possible application in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Das, S.; Hendry, M.J. Application of Raman spectroscopy to identify iron minerals commonly found in mine wastes. Chem. Geol. 2011, 290, 101–108. [Google Scholar] [CrossRef]
- De Faria, D.L.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Lópes, F.N. Heated goethite and natural hematite: Can Raman spectroscopy be used to differentiate them? Vib. Spectrosc. 2007, 45, 117–121. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, C.C. Effect of nano-hematite morphology on photocatalytic activity. Phys. Chem. Miner. 2014, 41, 727–736. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the physisorption characterization of nanoporous gas storage materials. Eng. J. 2018, 4, 559566. [Google Scholar] [CrossRef]
- Vu, T.T.; Valdés-Solís, T.; Marbán, G. High surface area stainless steel wire mesh-supported TiO2 prepared by sacrificial template accelerated hydrolysis. A monolithic photocatalyst superior to P25 TiO2. J. Environ. Chem. Eng. 2014, 2, 2229–2235. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.Y.; Zhang, Y.Q.; Wang, J.W.; Zhou, W.; Zhang, C.; Liu, S. A novel approach to prepare MCM-41 supported CuO catalyst with high metal loading and dispersion. Microporous Mesoporous Mater. 2006, 88, 38–47. [Google Scholar] [CrossRef]
- Păcurariu, C.; Paşka, O.; Ianoş, R.; Muntean, S.G. Effective removal of methylene blue from aqueous solution using a new magnetic iron oxide nanosorbent prepared by combustion synthesis. Clean Technol. Environ. Policy 2016, 18, 705–715. [Google Scholar] [CrossRef]
- Alshamsi, F.A.; Albadwawi, A.S.; Alnuaimi, M.M.; Rauf, M.A.; Ashraf, S.S. Comparative efficiencies of the degradation of Crystal Violet using UV/hydrogen peroxide and Fenton’s reagent. Dyes Pigm. 2007, 74, 283–287. [Google Scholar] [CrossRef]
- Nawaz, S.; Shah, N.S.; Khan, J.A.; Sayed, M.; Al-Muhtaseb, A.; Andersen, H.R.; Muhammad, N.; Murtaza, B.; Khan, H.M. Removal efficiency and economic cost comparison of hydrated electron-mediated reductive pathways for treatment of bromated. Chem. Eng. Sci. 2017, 320, 523–531. [Google Scholar] [CrossRef]
- Rizzo, L.; Della Sala, A.; Fiorentino, A.G.; Puma, Li. Disinfection of urban wastewater by solar driven and UV lamp—TiO2 photocatalysis: Effect on a multi drug resistant Escherichia coli strain. Water Res. 2014, 53, 145–152. [Google Scholar] [CrossRef]
- Di Cesare, A.; De Carluccio, M.; Eckert, E.M.; Fontaneto, D.; Fiorentino, A.; Corno, G.; Rizzo, L. Combination of flow cytometry and molecular analysis to monitor the effect of UVC/H2O2 vs. UVC/H2O2/Cu-IDS processes on pathogens and antibiotic resistant genes in secondary wastewater effluents. Water Res. 2020, 184, 116194. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.; Hadi, F.; Khan, J.A.; Shah, N.S.; Shah, L.A.; Khan, H.M. Degradation of acetaminophen in aqueous media by H2O2 assisted gamma irradiation process. Z. Phys. Chem. 2018, 232, 545–558. [Google Scholar] [CrossRef]
- Zhao, L.; Lin, Z.R.; Ma, X.H.; Dong, Y.H. Catalytic activity of different iron oxides: Insight from pollutant degradation and hydroxyl radical formation in heterogene Fenton-like systems. Chem. Eng. J. 2018, 352, 343–351. [Google Scholar] [CrossRef]
- Valentine, R.L.; Wang, H.C.A. Iron oxide surface catalyzed oxidation of quinolone by hydrogen peroxide. J. Environ. Eng. 1998, 124, 31–38. [Google Scholar] [CrossRef]
- Huang, H.H.; Lu, M.C.; Chen, J.N. Catalytic decomposition of hydrogen peroxide and 2-chlorophenol with iron oxides. Water Res. 2001, 35, 2291–2299. [Google Scholar] [CrossRef]
- Garrido-Ramírez, E.G.; Theng, B.K.G.; Mora, M.L. Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions—A review. Appl. Clay Sci. 2010, 47, 182–192. [Google Scholar] [CrossRef]
- Lin, S.S.; Gurol, M.D. Catalytic decomposition of hydrogen peroxide on iron oxide: Kinetics, mechanism, and implications. Environ. Sci. Technol. 1998, 32, 1417–1423. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic perox-dase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Nicell, J.A.; Wright, H. A model of peroxidase activity with inhibition by hydrogen peroxide. Enzym. Microb. Technol. 1997, 21, 302–310. [Google Scholar] [CrossRef]
- Guz, L.; Curutchet, G.; Torres Sánchez, R.M.; Candal, R. Adsorption of crystal violet on montmorillonite (or iron modified montmorillonite) followed by degradation through Fenton or photo-Fenton type reactions. J. Environ. Chem. Eng. 2014, 2, 2344–2351. [Google Scholar] [CrossRef]
- Ain, Q.U.; Rasheed, U.; Yaseen, M.; Zhang, H.; Tong, Z. Superior dye degradation and adsorption capability of polydopamine modified Fe3O4-pillared bentonite composite. J. Hazard. Mater. 2020, 397, 122758. [Google Scholar] [CrossRef]
- Rizzo, L.; Meric, S.; Kassinos, D.; Guida, M.; Russo, F.; Belgiorno, V. Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Res. 2009, 43, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.; Sayed, M.; Khan, J.A.; Shah, L.A.; Shah, N.S.; Khan, H.M.; Khattak, R. Degradation of crystal violet dye by Fenton and photo-Fenton oxidation processes. Z. Phys. Chem. 2018, 232, 1771–1786. [Google Scholar] [CrossRef]
- Fan, H.J.; Huang, S.T.; Chung, W.H.; Jan, J.L.; Lin, W.Y.; Chen, C.C. Degradation pathways of crystal violet by Fenton and Fenton-like systems: Condition optimization and intermediate separation and identification. J. Hazard. Mater. 2009, 171, 1032–1104. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chen, W.C.; Chiou, M.R.; Chen, S.W.; Chen, Y.Y.; Fan, H.J. Degradation of crystal violet dye an FeGAC/H2O2 process. J. Hazard. Mater. 2011, 196, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.J.; Lu, C.S.; Lee, W.L.W.; Chiou, M.R.; Chen, C.C. Mechanistic pathways differences between P25-TiO2 and Pt-TiO2 mediated CV photodegradation. J. Hazard. Mater. 2011, 185, 227–235. [Google Scholar] [CrossRef]
- APAT; IRSA-CNR. Metodi Analitici per le Acque; APAT Manuali e Linee Guida 29/2003; APAT: Rome, Italy, 2003. [Google Scholar]
- Rizzo, L.; Agovino, T.; Nahim-Granados, S.; Castro-Alférez, M.; Fernández-Ibáñe, P.; Polo-López, M.I. Tertiary treatment of urban wastewater by solar and UV-C driven advanced oxidation with peracetic acid: Effect on contaminants of emerging concern and antibiotic resistance. Water Res. 2019, 149, 272–281. [Google Scholar] [CrossRef] [PubMed]



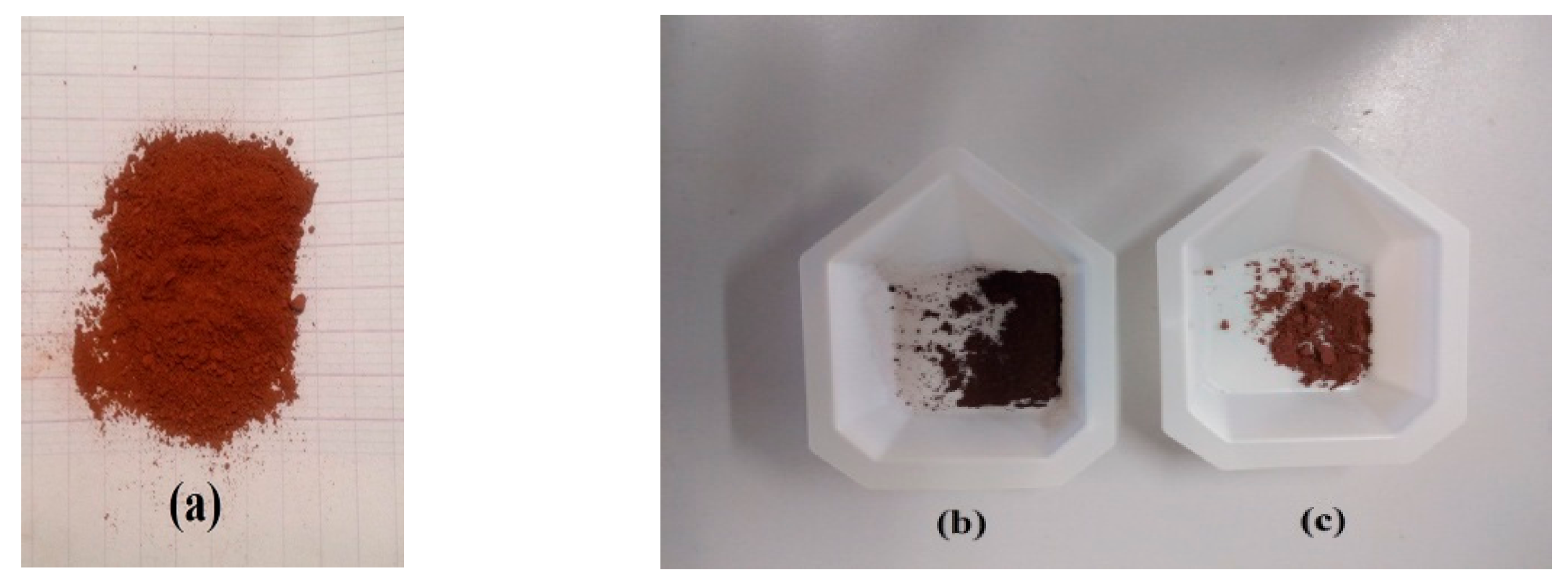

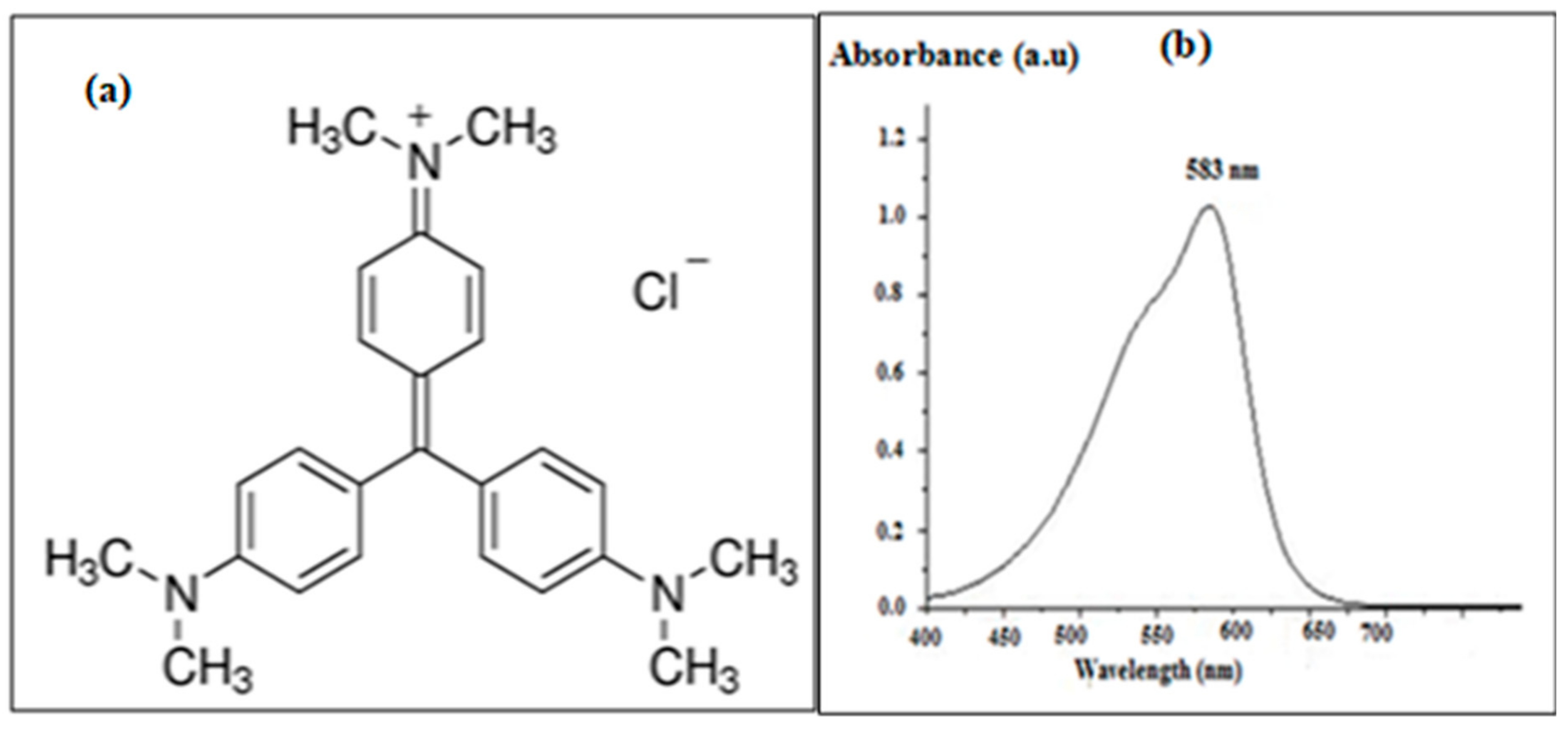
| SBET (m2/g) | SBJH/mesp (m2/g) | VBJH/mesp (cm2/g) | Vads (cm3/g) | D (A°) |
|---|---|---|---|---|
| 45.86 | 44.17 | 0.092 | 0.1 | 20 |
| Catalyst | Operating Conditions | Degradation Percentage | Reference |
|---|---|---|---|
| Fe-Montmorillonite | Dosage: 0.15 g/L; (H2O2) = 50 mM; (CV) = 0.06 mM, visible light irradiation | 90% | [48] |
| K2Fe4O7 | Dosage: 0.03g/L; (H2O2) = 5 mM; (CV) = 20 mg/L, visible light irradiation | 92% | [5] |
| Fe3O4- polydopamine–bentonite composite | Dosage: 0.03g/L; (H2O2) = 1 mM; (CV) = 20 mg/L; UV light irradiation | 93% | [49] |
| Natural iron oxide | Dosage: 1g /L; (H2O2) = 30 mg/L; (CV) = 10 mg/L, UVC light irradiation | 98% | This work |
| PFO Kinetic (Yi = ai⋅x + bi) | 6.8 × 10−2⋅x + 0.1 | 4.7 × 10−4 ⋅x + 2.107 | 8.0 × 10−3⋅x + 1.369 |
|---|---|---|---|
| Ri2 | 0.982 | 0.992 | 0.995 |
| ki (min−1) | 2.726 × 10−3 | 5.206 × 10−6 | 0.0076 |
| ai | 0.1498 | 2.10721 | 1.36934 |
| bi | 0.06818 | 4.69515 × 10−4 | 0.00836 |
| Molecular Structure | Chemical Name | m/z Value |
|---|---|---|
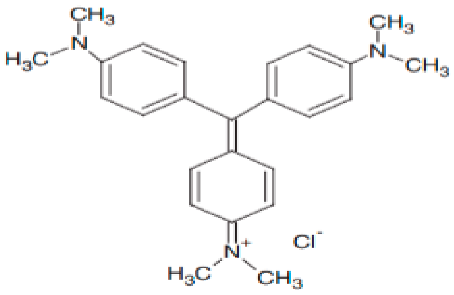 | N,N,N,N′,N″,N″-hexamethyl pararosaniline (Crystal violet) | 372.14 |
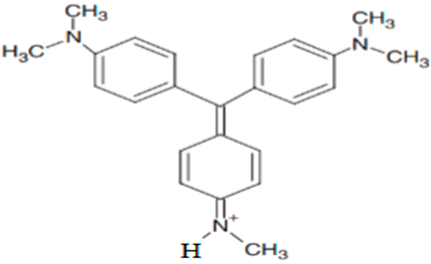 | N,N- dimethyl-N′,N′-dimethyl-N″-methyl pararosaniline | 358.18 |
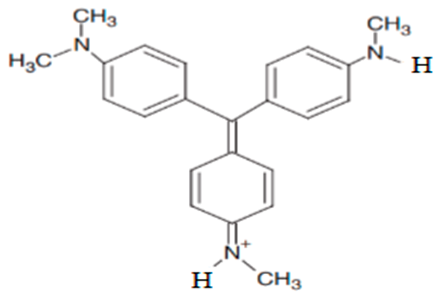 | N,N-dimethyl-N′-methyl-N″- methyl pararosaniline | 344.16 |
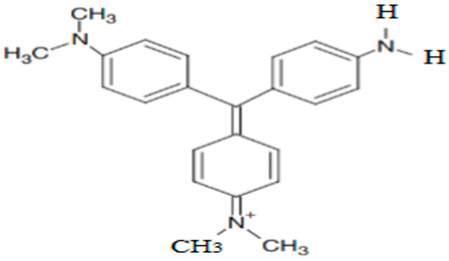 | N,N-dimethyl-N′,N′-dimethyl pararosaniline | 344.10 |
 |
N
-methyl-N′-methyl-N″- methylpararosaniline | 330.15 |
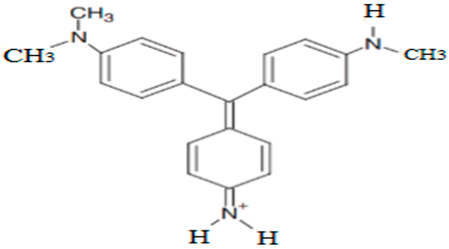 | N,N-dimethyl-N′- methylpararosaniline | 330.15 |
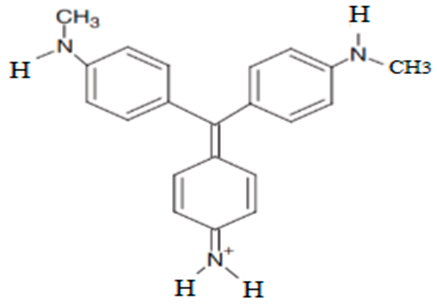 | N -methyl-N′-methylpararosaniline | 316.25 |
 | N,N-dimethylpararosaniline | 316.13 |
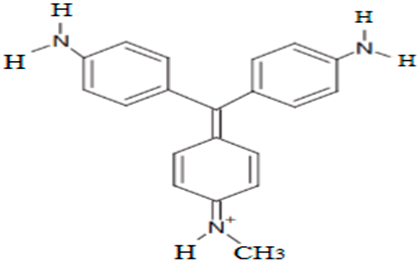 | N-methylpararosaniline | 302.10 |
 | Pararosaniline | 288.07 |
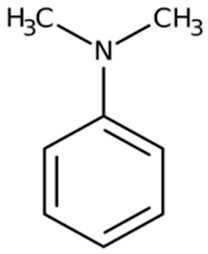 | N,N-dimethyl aniline | 121.15 |
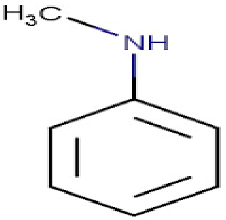 | N-methylaniline | 107.06 |
| Quality Indicator | Raw Effluent | Treated Effluent | Italian Standards for Disposal of Industrial Wastewater into Sewerage |
|---|---|---|---|
| COD (mg/L) | 803 | 290 | 500 |
| Turbidity (NTU) | 57 | 25 | - |
| pH | 9.18 | 8.31 | 5.5–9.5 |
| BOD5 (mg/L) | 352 | 149 | 250 |
| Conductivity (μS/cm) | 15.05 | 13.51 | - |
| TSS (mg/L) | 130 | Traces | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ayed, S.; Azam, M.; Al-Resayes, S.I.; Ayari, F.; Rizzo, L. Cationic Dye Degradation and Real Textile Wastewater Treatment by Heterogeneous Photo-Fenton, Using a Novel Natural Catalyst. Catalysts 2021, 11, 1358. https://doi.org/10.3390/catal11111358
Ben Ayed S, Azam M, Al-Resayes SI, Ayari F, Rizzo L. Cationic Dye Degradation and Real Textile Wastewater Treatment by Heterogeneous Photo-Fenton, Using a Novel Natural Catalyst. Catalysts. 2021; 11(11):1358. https://doi.org/10.3390/catal11111358
Chicago/Turabian StyleBen Ayed, Sirine, Mohammad Azam, Saud I. Al-Resayes, Fadhila Ayari, and Luigi Rizzo. 2021. "Cationic Dye Degradation and Real Textile Wastewater Treatment by Heterogeneous Photo-Fenton, Using a Novel Natural Catalyst" Catalysts 11, no. 11: 1358. https://doi.org/10.3390/catal11111358
APA StyleBen Ayed, S., Azam, M., Al-Resayes, S. I., Ayari, F., & Rizzo, L. (2021). Cationic Dye Degradation and Real Textile Wastewater Treatment by Heterogeneous Photo-Fenton, Using a Novel Natural Catalyst. Catalysts, 11(11), 1358. https://doi.org/10.3390/catal11111358








