A Thermal Study on Peat Oxidation Behavior in the Presence of an Iron-Based Catalyst
Abstract
:1. Introduction
2. Results
2.1. Experimental
2.1.1. Materials
2.1.2. Iron Tallate Preparation
2.1.3. Thermal Analysis
2.1.4. Kinetic Analysis
2.2. Discussion
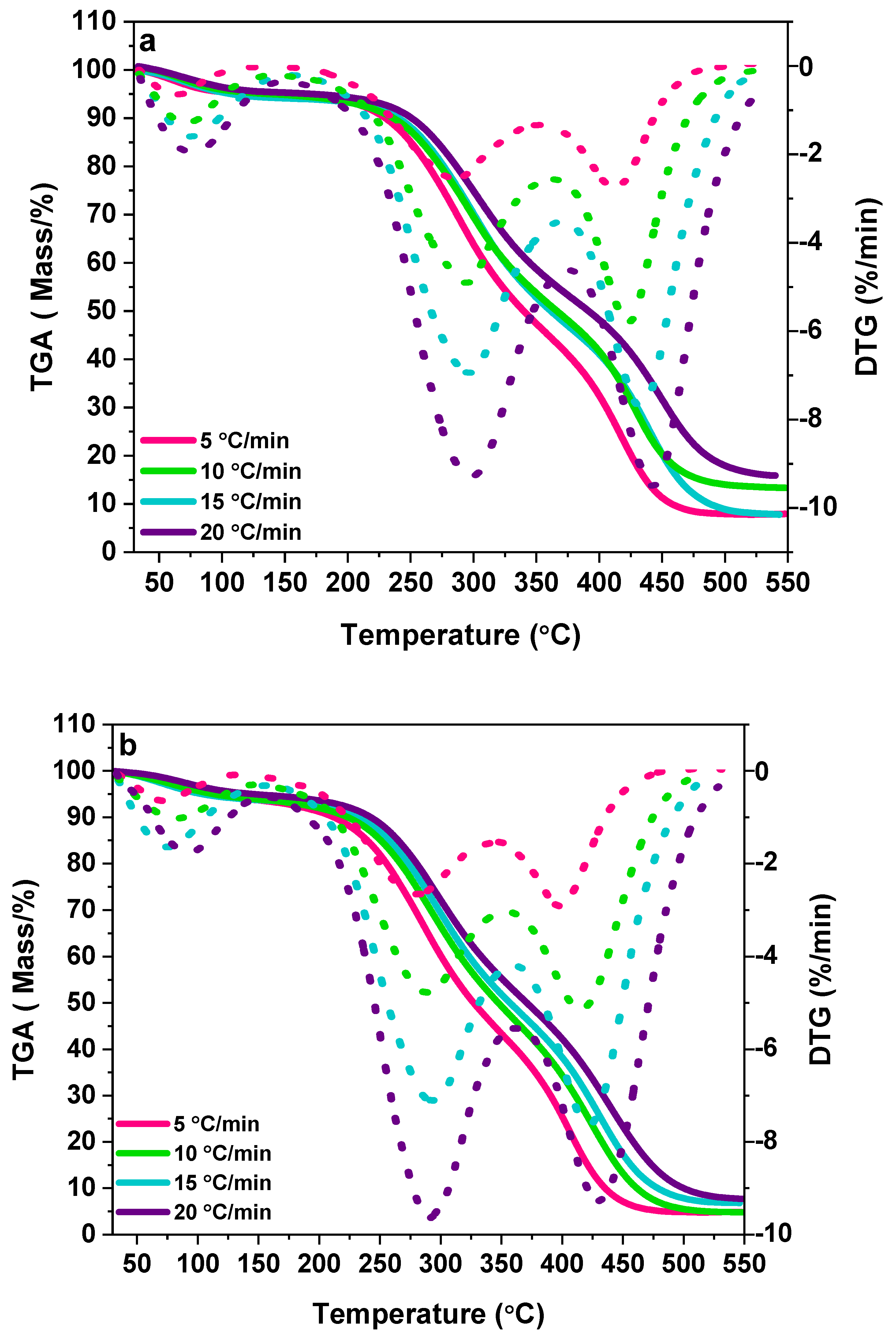
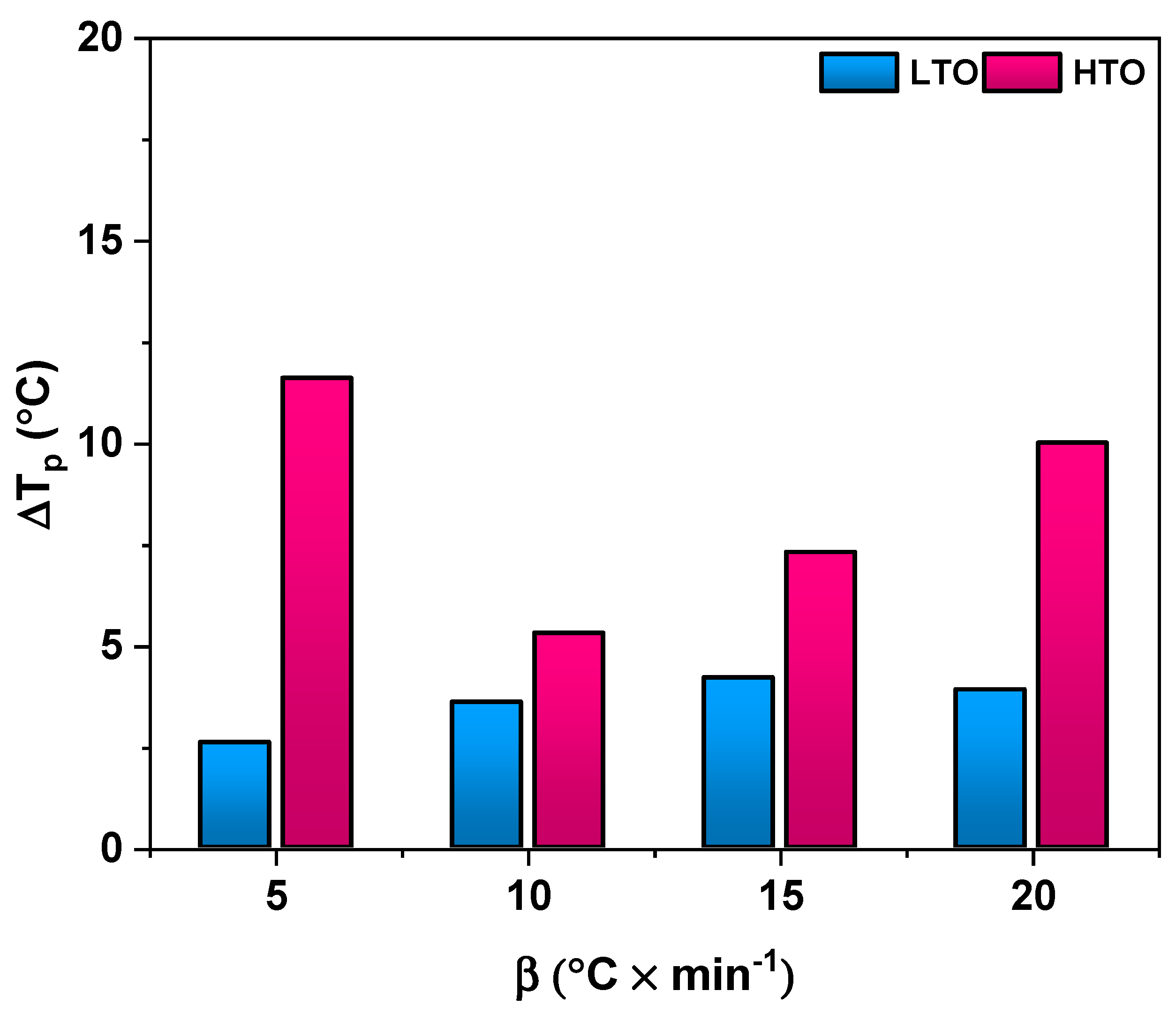
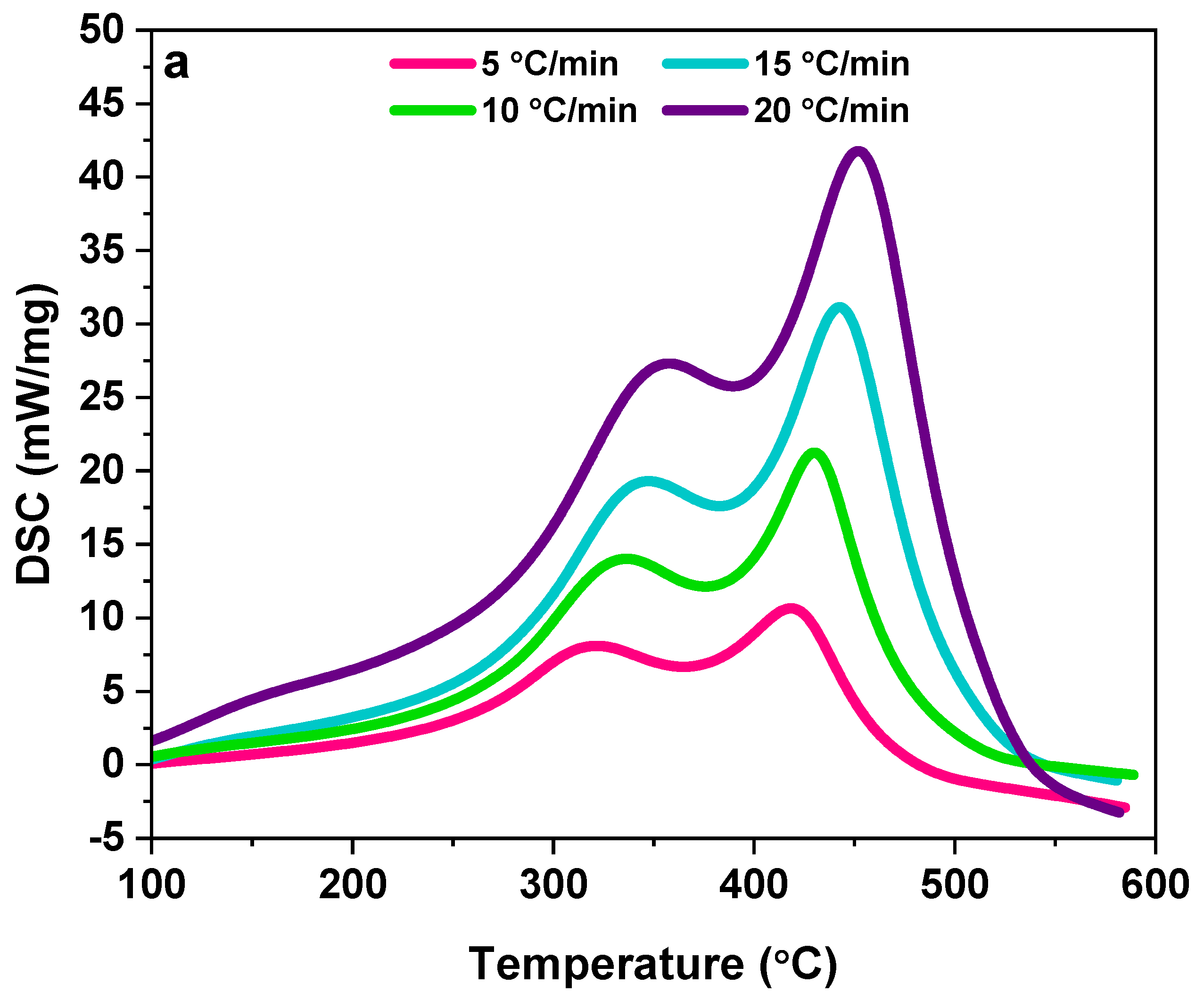
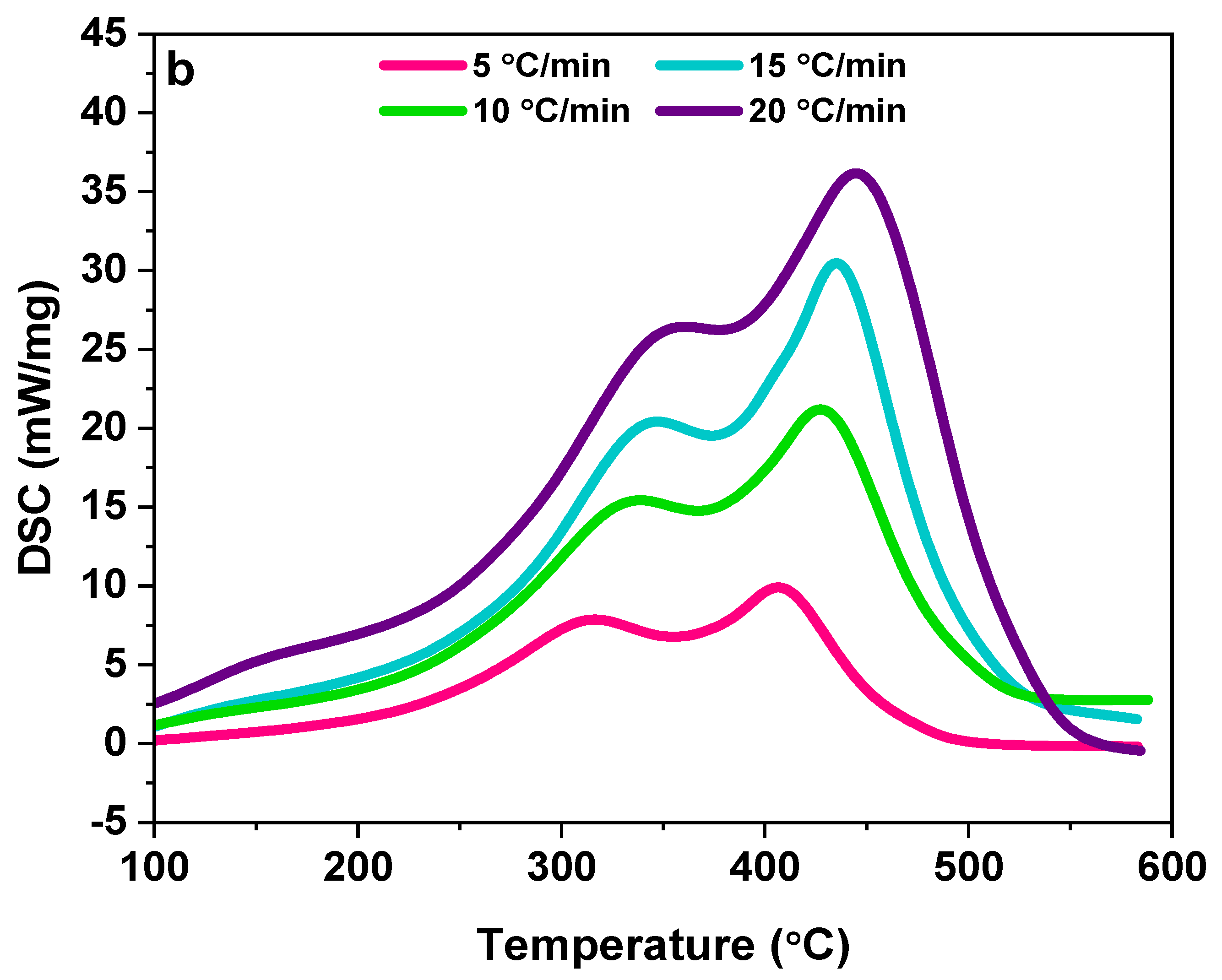
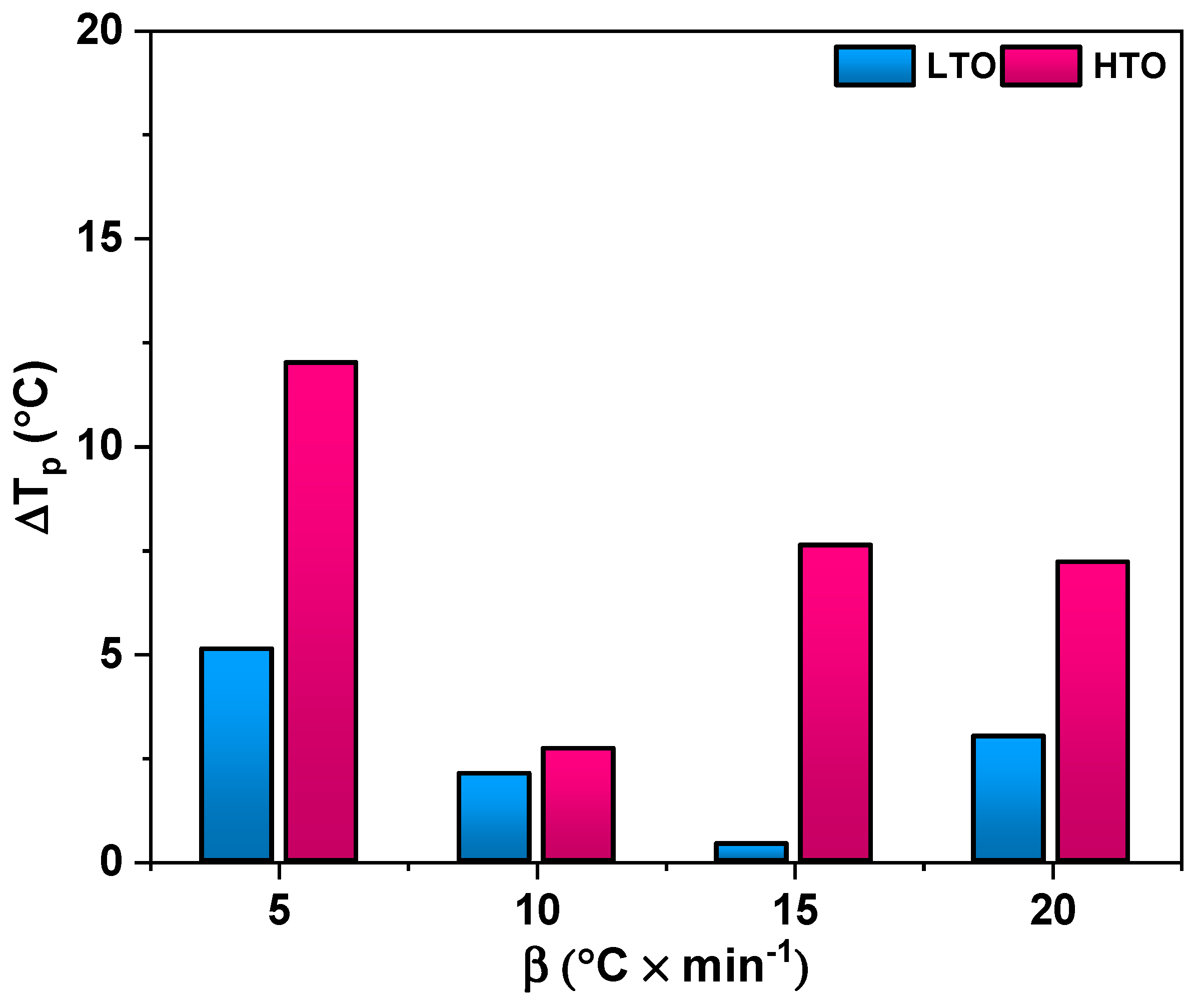
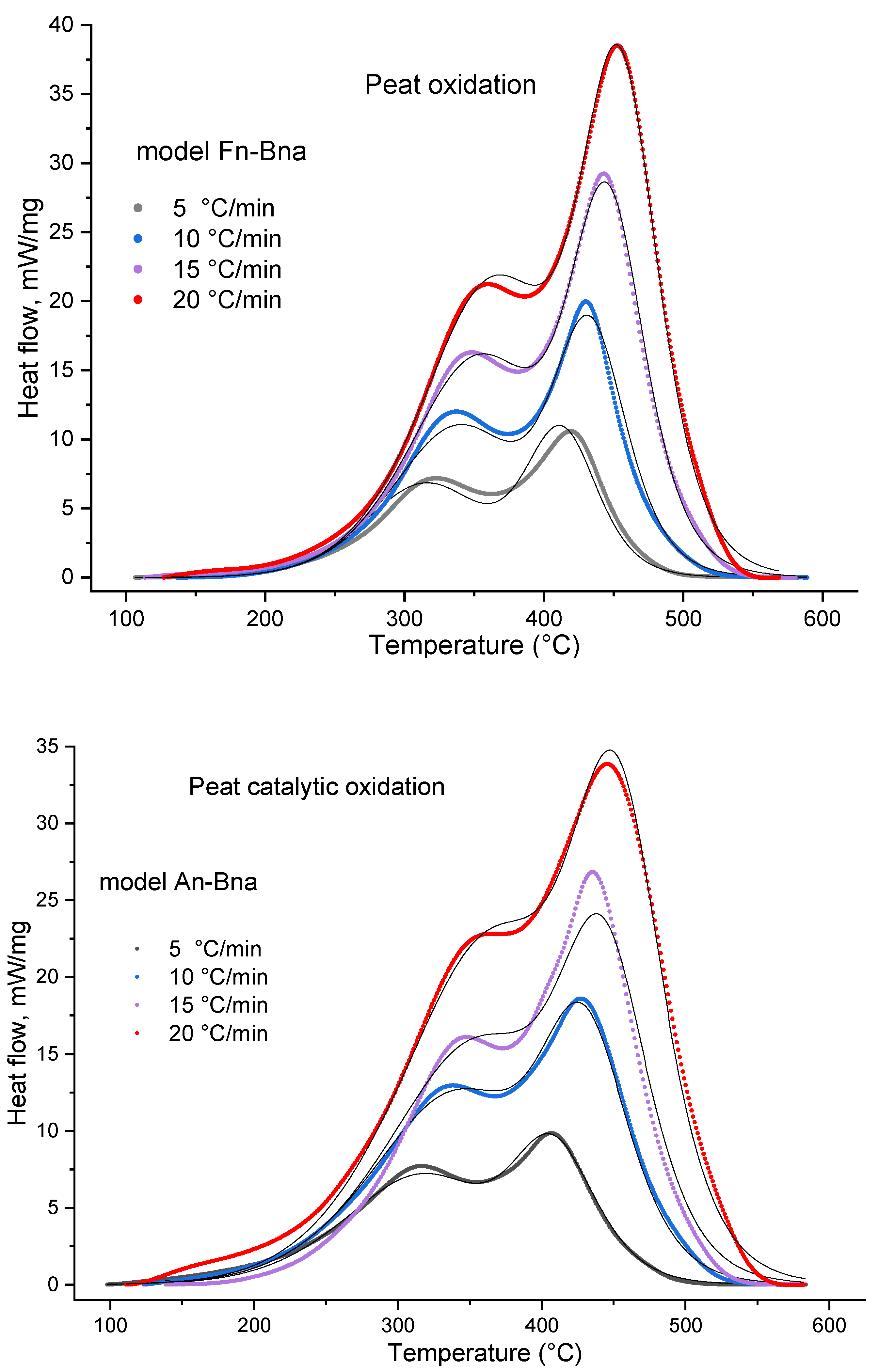
2.2.1. Thermal Analysis
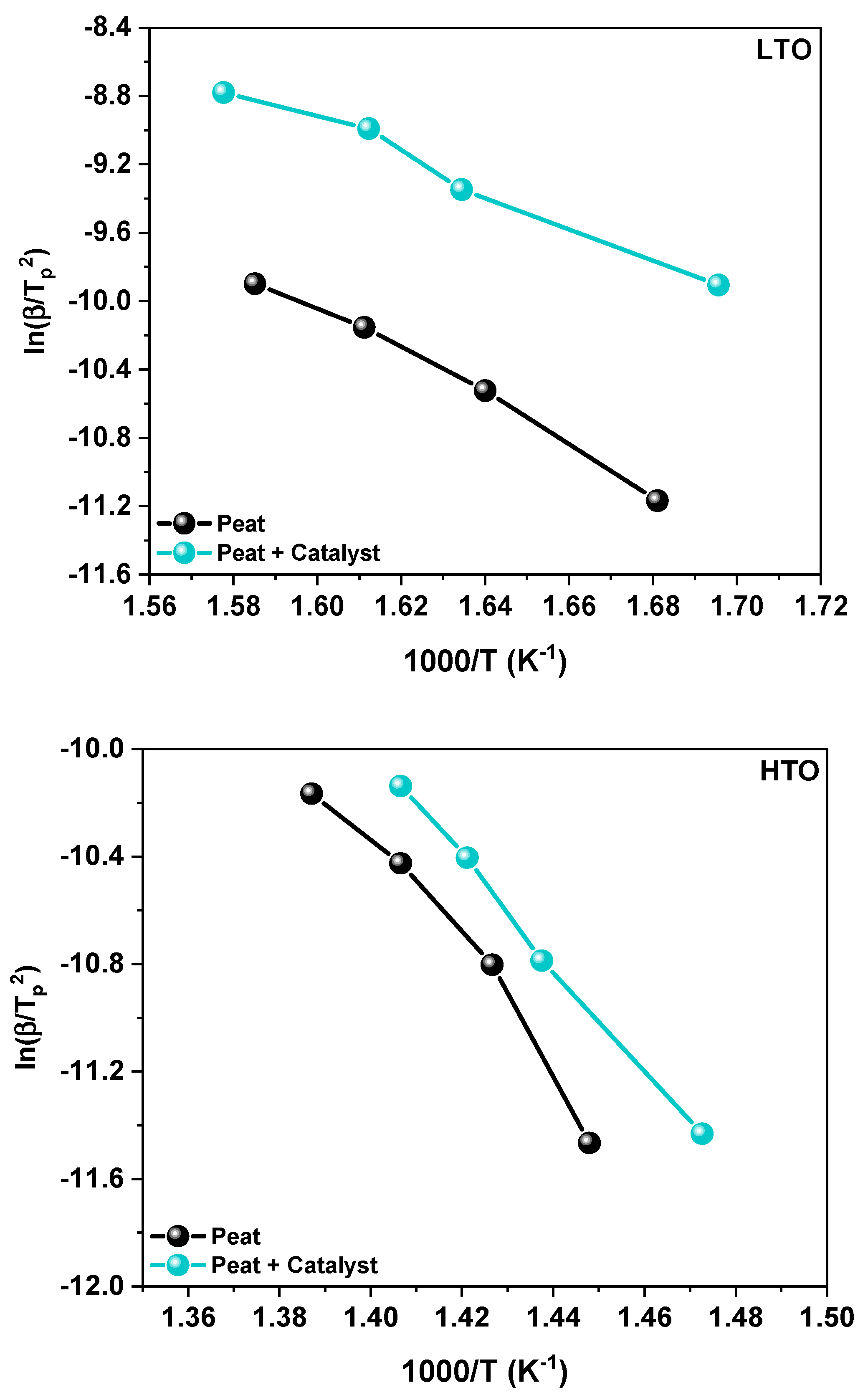
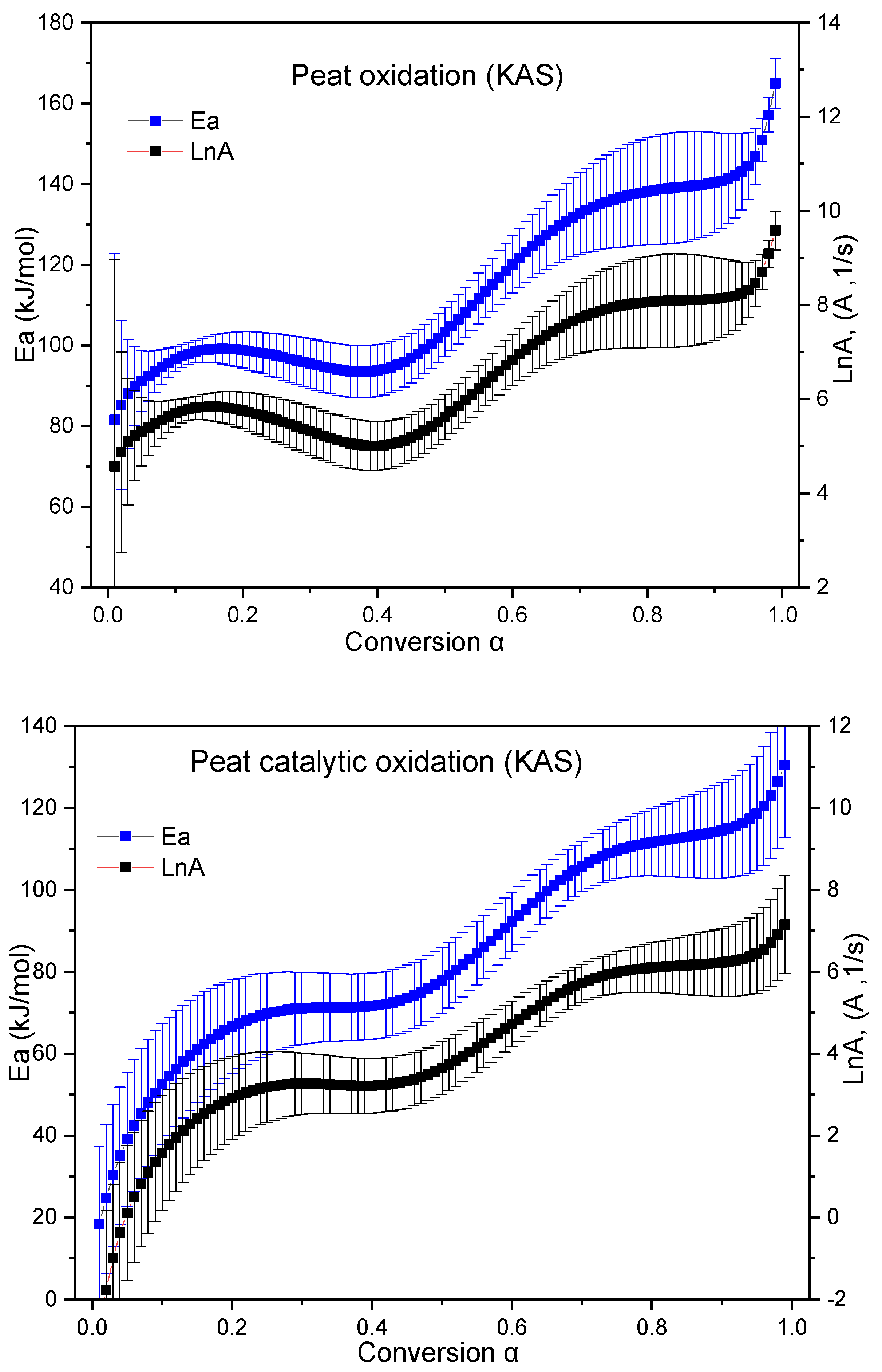
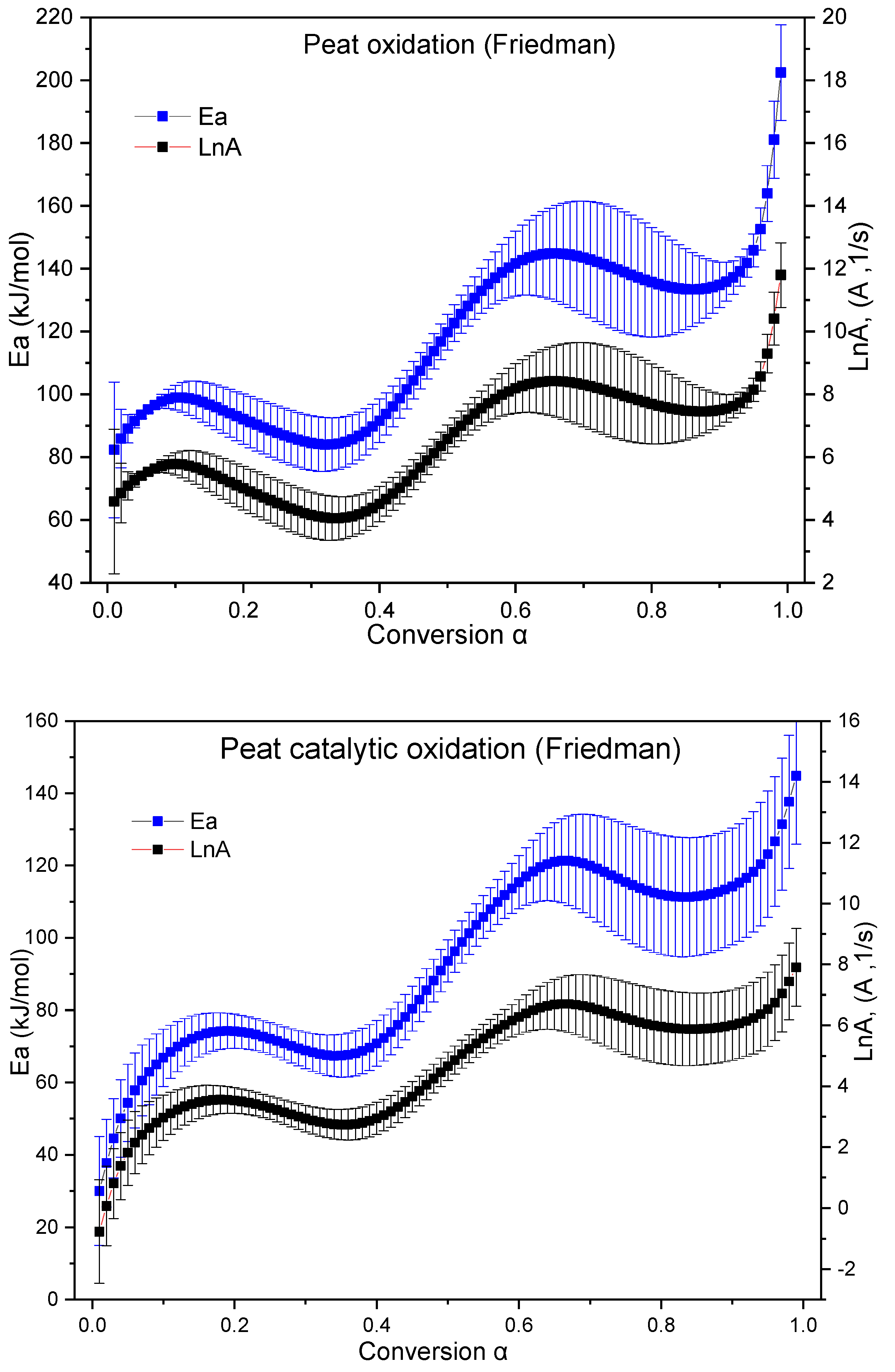
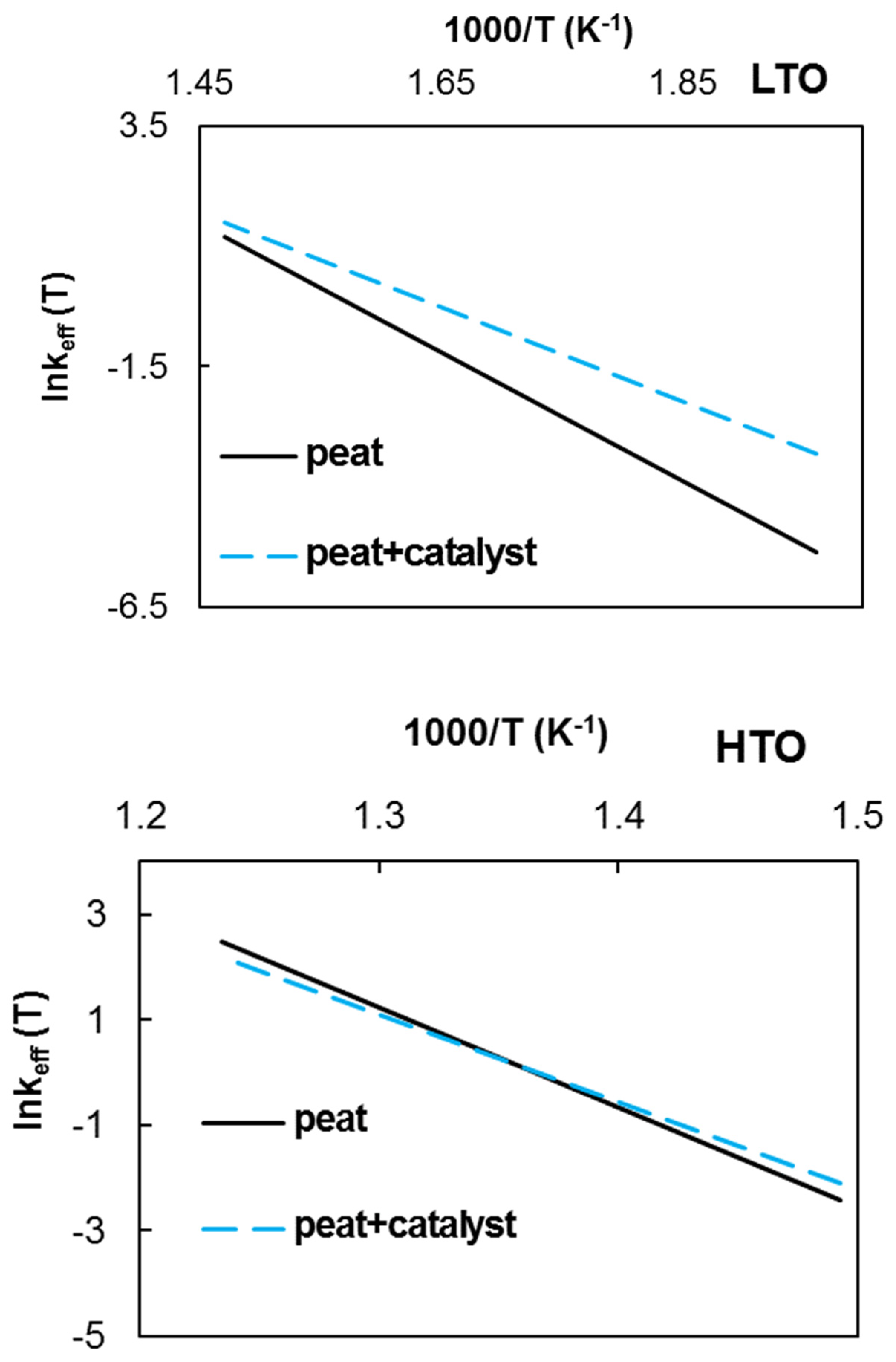
2.2.2. Kinetic Study
3. Peat Oxidation Rate Constant Calculation
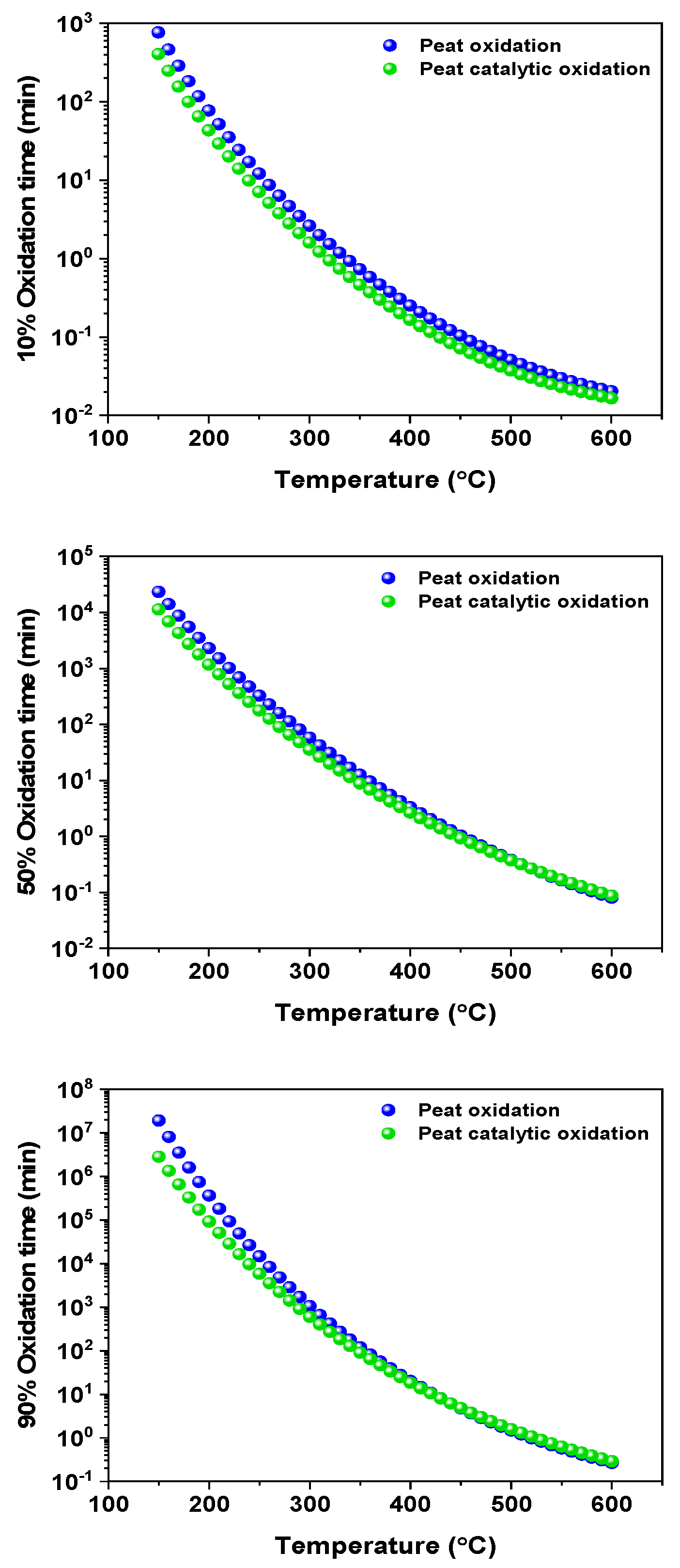
4. Kinetic Predictions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Kong, F.; Zheng, M.; Wang, D. Present Situation and Suggestions on the Exploitation and Utilization of Peat Resources in China. Conserv. Util. Miner. Resour. 2019, 39, 142–147. [Google Scholar]
- Waller, M.; Kirby, J. Coastal peat-beds and peatlands of the southern North Sea: Their past, present and future. Biol. Rev. 2021, 96, 408–432. [Google Scholar] [CrossRef]
- Lee, T.; Jung, S.; Hong, J.; Wang, C.-H.; Alessi, D.S.; Lee, S.S.; Park, Y.-K.; Kwon, E.E. Using CO2 as an oxidant in the catalytic pyrolysis of peat moss from the north polar region. Environ. Sci. Technol. 2020, 54, 6329–6343. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, S.; Lu, T.; Wu, Y.; Yuan, H.; Chen, Y. Synthesis and evaluation of pyrolysis waste peat char supported catalyst for steam reforming of toluene. Renew. Energy 2020, 160, 964–973. [Google Scholar] [CrossRef]
- Fuchsman, C. Peat: Industrial Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0323157114. [Google Scholar]
- Elliott, D.C.; Baker, E.G.; Piskorz, J.; Scott, D.S.; Solantausta, Y. Production of liquid hydrocarbon fuels from peat. Energy Fuels 1988, 2, 234–235. [Google Scholar] [CrossRef]
- Weldemichael, Y.; Assefa, G. Assessing the energy production and GHG (greenhouse gas) emissions mitigation potential of biomass resources for Alberta. J. Clean. Prod. 2016, 112, 4257–4264. [Google Scholar] [CrossRef]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Marsh, G. Biofuels: Aviation alternative? Renew. Energy Focus 2008, 9, 48–51. [Google Scholar] [CrossRef]
- Yang, Q.Z.; Song, B. Sustainability assessment of biofuels as alternative energy resources. In Proceedings of the 2008 IEEE International Conference on Sustainable Energy Technologies, Singapore, 24–27 November 2008; pp. 1001–1006. [Google Scholar]
- Sternik, D.; Wiśniewska, M.; Nowicki, P. Thermal degradation of peat-based activated carbons covered with mixed adsorption layers of PAA polymer and SDS surfactant. Thermochim. Acta 2019, 676, 71–83. [Google Scholar] [CrossRef]
- Klein, K.; Gross-Schmölders, M.; De la Rosa, J.M.; Alewell, C.; Leifeld, J. Investigating the influence of instrumental parameters and chemical composition on pyrolysis efficiency of peat. Commun. Soil Sci. Plant Anal. 2020, 51, 1572–1581. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 472–499. [Google Scholar]
- Taherzadeh, M.J.; Karimi, K. Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 707–738. [Google Scholar]
- Wen, Y. Study of the Performance of Peat Moss Pyrolysis; Kth Royal Institute of Technology: Stockholm, Sweden, 2019. [Google Scholar]
- Maftu’ah, E.; Fahmi, A.; Hayati, A. Changes in degraded peat land characteristic using FTIR-spectrocopy. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bandung, Indonesia, 2019; Volume 393, p. 12091. [Google Scholar]
- Kirkinen, J.; Minkkinen, K.; Penttilä, T.; Kojola, S.; Sievänen, R.; Alm, J.; Saarnio, S.; Silvan, N.; Laine, J.; Savolainen, I. Greenhouse Impact due to Different Peat Fuel Utilisation Chains in Finland—A Life-Cycle Approach; Boreal Environment Research Publishing Board: Helsinki, Finland, 2007. [Google Scholar]
- Lake, L.W. Enhanced Oil Recovery; Prentice Hall: Englewood Cliffs, NJ, USA, 1989. [Google Scholar]
- Sitnov, S.; Mukhamatdinov, I.; Aliev, F.; Khelkhal, M.A.; Slavkina, O.; Bugaev, K. Heavy oil aquathermolysis in the presence of rock-forming minerals and iron oxide (II, III) nanoparticles. Pet. Sci. Technol. 2020, 38, 574–579. [Google Scholar] [CrossRef]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Gulf Professional Publishing: Houston, TX, USA, 2010; ISBN 0080961630. [Google Scholar]
- Vakhin, A.V.; Khelkhal, M.A.; Tajik, A.; Gafurov, M.R.; Morozov, O.G.; Nasybullin, A.R.; Karandashov, S.A.; Ponomarev, A.A.; Krapivnitskaia, T.O.; Glyavin, M.Y. The Role of Nanodispersed Catalysts in Microwave Application during the Development of Unconventional Hydrocarbon Reserves: A Review of Potential Applications. Processes 2021, 9, 420. [Google Scholar] [CrossRef]
- Green, D.W.; Willhite, G.P. Enhanced oil Recovery; Henry L. Doherty Memorial Fund of AIME, Society of Petroleum Engineers: Richardson, TX, USA, 1998; Volume 6. [Google Scholar]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Khelkhal, M.A.; Vakhin, A.V. Application of Aromatic and Industrial Solvents for Enhancing Heavy Oil Recovery from the Ashalcha Field. Energy Fuels 2020, 35, 374–385. [Google Scholar] [CrossRef]
- Mokheimer, E.M.A.; Hamdy, M.; Abubakar, Z.; Shakeel, M.R.; Habib, M.A.; Mahmoud, M. A comprehensive review of thermal enhanced oil recovery: Techniques evaluation. J. Energy Resour. Technol. 2019, 141, 30801. [Google Scholar] [CrossRef]
- Mahinpey, N.; Ambalae, A.; Asghari, K. In situ combustion in enhanced oil recovery (EOR): A review. Chem. Eng. Commun. 2007, 194, 995–1021. [Google Scholar] [CrossRef]
- Farhadian, A.; Khelkhal, M.A.; Tajik, A.; Lapuk, S.E.; Rezaeisadat, M.; Eskin, A.A.; Rodionov, N.O.; Vakhin, A.V. Effect of Ligand Structure on the Kinetics of Heavy Oil Oxidation: Toward Biobased Oil-Soluble Catalytic Systems for Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2021, 60, 14713–14727. [Google Scholar] [CrossRef]
- Burger, J.G.; Sahuquet, B.C. Chemical Aspects of in-Situ Combustion—Heat of Combustion and Kinetics. Soc. Pet. Eng. J. 1972, 12, 410–422. [Google Scholar] [CrossRef]
- Sarathi, P. In-Situ Combustion Handbook–Principles and Practices; National Petroleum Technology Office: Tulsa, OK, USA, 1999. [Google Scholar]
- Lin, C.Y.; Chen, W.H.; Lee, S.T.; Culham, W.E. Numerical simulation of combustion tube experiments and the associated kinetics of in-situ combustion processes. Soc. Pet. Eng. J. 1984, 24, 657–666. [Google Scholar] [CrossRef]
- Abuhesa, M.B.; Hughes, R. Comparison of conventional and catalytic in situ combustion processes for ol recovery. Energy Fuels 2009, 23, 186–192. [Google Scholar] [CrossRef]
- Moore, R.G.; Laureshen, C.J.; Mehta, S.A.; Ursenbach, M.G.; Belgrave, J.D.M.; Weissman, J.G.; Kessler, R.V. A downhole catalytic upgrading process for heavy oil using in situ combustion. J. Can. Pet. Technol. 1999, 38. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. In situ combustion synthesis of structured Cu-Ce-O and Cu-Mn-O catalysts for the production and purification of hydrogen. Appl. Catal. B Environ. 2006, 66, 168–174. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Nurgaliev, D.K.; Vakhin, A.V. Thermal Study on Stabilizing the Combustion Front via Bimetallic Mn@Cu Tallates during Heavy Oil Oxidation. Energy Fuels 2020, 34, 5121–5127. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Vakhin, A.V. Kinetic Study on Heavy Oil Oxidation by Copper Tallates. Energy Fuels 2019, 33, 12690–12695. [Google Scholar] [CrossRef]
- Galukhin, A.; Nosov, R.; Eskin, A.; Khelkhal, M.; Osin, Y. Manganese Oxide Nanoparticles Immobilized on Silica Nanospheres as a Highly Efficient Catalyst for Heavy Oil Oxidation. Ind. Eng. Chem. Res. 2019, 58, 8990–8995. [Google Scholar] [CrossRef]
- Feoktistov, D.A.; Kayukova, G.P.; Vakhin, A.V.; Sitnov, S.A. Catalytic Aquathermolysis of High-Viscosity Oil Using Iron, Cobalt, and Copper Tallates. Chem. Technol. Fuels Oils 2018, 53, 905–912. [Google Scholar] [CrossRef]
- Fassihi, M.R.; Brigham, W.E.; Ramey, H.J. Reaction Kinetics of in-Situ Combustion: Part 1—Observations. Soc. Pet. Eng. J. 1984, 24, 399–407. [Google Scholar] [CrossRef]
- Bousaid, I.S.; Ramey, H.J. Oxidation of Crude Oil in Porous Media. Soc. Pet. Eng. J. 1968, 8, 137–148. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Lapuk, S.E.; Zubaidullina, L.S.; Ziganshin, M.A.; Mukhametzyanov, T.A.; Schick, C.; Gerasimov, A.V. Kinetic stability of amorphous solid dispersions with high content of the drug: A fast scanning calorimetry investigation. Int. J. Pharm. 2019, 562, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. (1934) 1956, 57, 217. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Lapuk, S.E.; Mukhametzyanov, T.A.; Schick, C.; Gerasimov, A.V. Crystallization kinetics and glass-forming ability of rapidly crystallizing drugs studied by Fast Scanning Calorimetry. Int. J. Pharm. 2021, 599, 120427. [Google Scholar] [CrossRef] [PubMed]
- Lapuk, S.E.; Mukhametzyanov, T.A.; Schick, C.; Gerasimov, A.V. Kinetic stability of amorphous dipyridamole: A fast scanning calorimetry investigation. Int. J. Pharm. 2020, 574, 118890. [Google Scholar] [CrossRef]
- Freund, J.E.; Perles, B.M. Modern Elementary Statistics; Pearson College Division: Upper Saddle River, NJ, USA, 2007. [Google Scholar]
| Component Composition of Peat Organic Matter | Mass Fraction in the Composition, % |
|---|---|
| Cellulose | 4–10 |
| Humic acids | 15–50 |
| Lignin | 5–20 |
| Easily hydrolyzable compounds | 20–40 |
| Water-soluble substances | 1–5 |
| Model. | Equation |
|---|---|
| Reaction of nth order (Fn) | f = (1 − α)n |
| Two-dimensional phase boundary (R2) | f = 2(1 − α)1/2 |
| Three-dimensional phase boundary (R3) | f = 3(1 − α)2/3 |
| N-dimensional nucleation according to Avrami-Erofeev (An) | f = n·(1 − α)·[−ln(1 − α)](n−1)/n |
| Expanded Prout–Tompkins equation (Bna) | f = (1 − α) n·αAutocatOrder |
| Reaction of nth order with m-Power autocatalysis by product (Cnm) | f = (1 − α)n·(1 + AutocatOrder ·αm) |
| Non-Catalytic | Catalytic | |||
|---|---|---|---|---|
| LTO | HTO | LTO | HTO | |
| Ea, kJ/mol | 110.8 ± 7.8 | 157.8 ± 19.1 | 81.8 ± 7.5 | 137.6 ± 9.3 |
| log10A, A in min−1 | 11.2 ± 1.53 | 16.0 ± 3.2 | 6.7 ± 1.47 | 12.9 ± 1.6 |
| Models Please indicate LTO and HTO | Peat Catalytic Oxidation | Peat Oxidation |
| An: Ea = 75.8 kJ·mole−1, LgA = 4.1 s−1, Dimension n = 0.78 | Fn: Ea = 76.3 kJ·mole−1, LgA = 4.1 s−1, ReactOrder n = 1.45 | |
| Bna: E = 116 kJ·mole−1, LgA = 6.4 s−1, ReactOrder n = 1.51, AutocatOrder 0.206 | Bna: E = 132.2 kJ·mole−1, LgA = 7.7 s−1, ReactOrder n = 1.48, AutocatOrder 0.254 | |
| R2 | 0.99715 | 0.99833 |
| F-test | 1.000 | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khelkhal, M.A.; Lapuk, S.E.; Ignashev, N.E.; Eskin, A.A.; Glyavin, M.Y.; Peskov, N.Y.; Krapivnitskaia, T.O.; Vakhin, A.V. A Thermal Study on Peat Oxidation Behavior in the Presence of an Iron-Based Catalyst. Catalysts 2021, 11, 1344. https://doi.org/10.3390/catal11111344
Khelkhal MA, Lapuk SE, Ignashev NE, Eskin AA, Glyavin MY, Peskov NY, Krapivnitskaia TO, Vakhin AV. A Thermal Study on Peat Oxidation Behavior in the Presence of an Iron-Based Catalyst. Catalysts. 2021; 11(11):1344. https://doi.org/10.3390/catal11111344
Chicago/Turabian StyleKhelkhal, Mohammed A., Semen E. Lapuk, Nikita E. Ignashev, Alexey A. Eskin, Mikhail Yu. Glyavin, Nikolay Yu. Peskov, Tatiana O. Krapivnitskaia, and Alexey V. Vakhin. 2021. "A Thermal Study on Peat Oxidation Behavior in the Presence of an Iron-Based Catalyst" Catalysts 11, no. 11: 1344. https://doi.org/10.3390/catal11111344






