Catalytic Activity of High-Surface-Area Amorphous MgO Obtained from Upsalite
Abstract
:1. Introduction
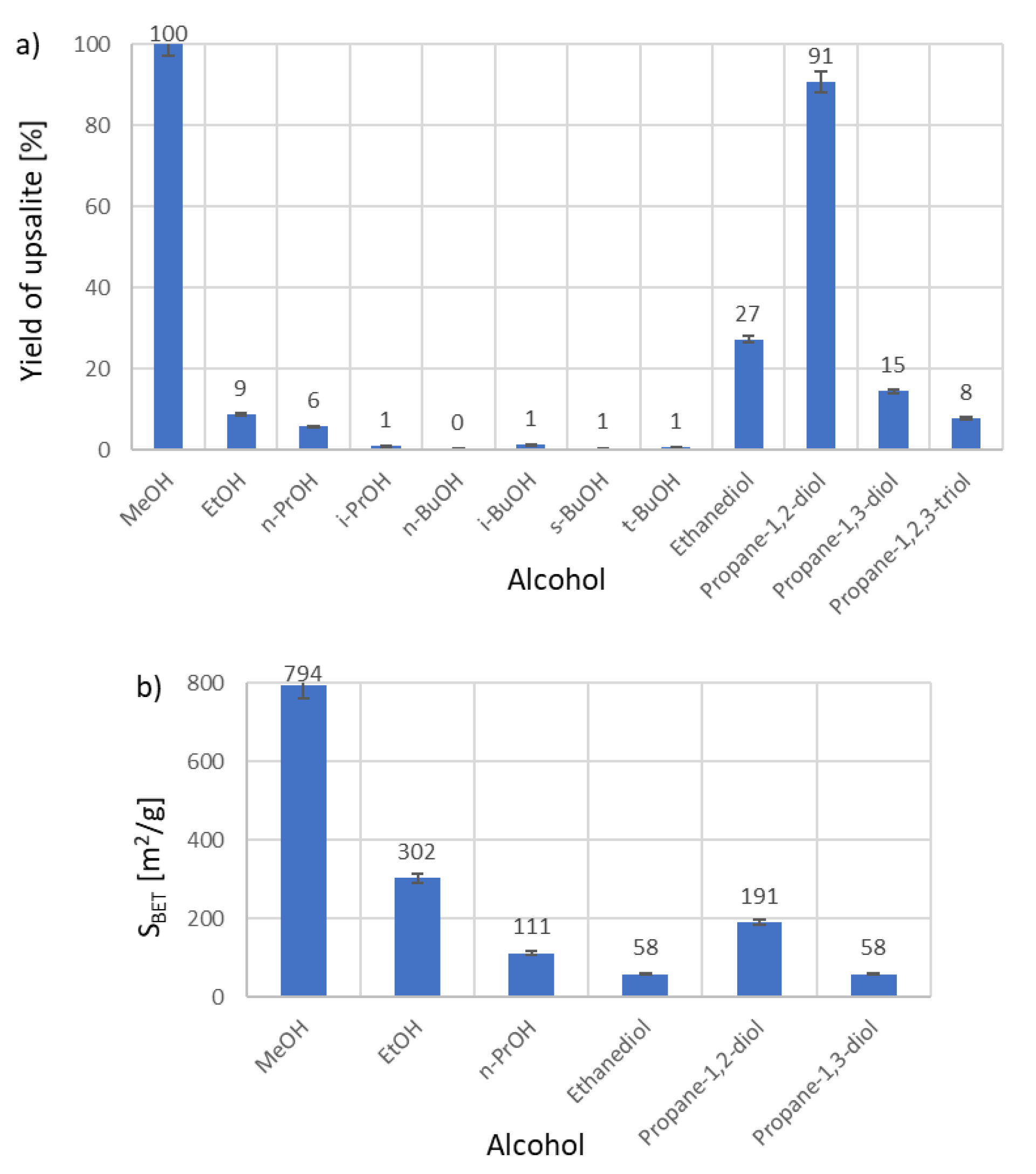
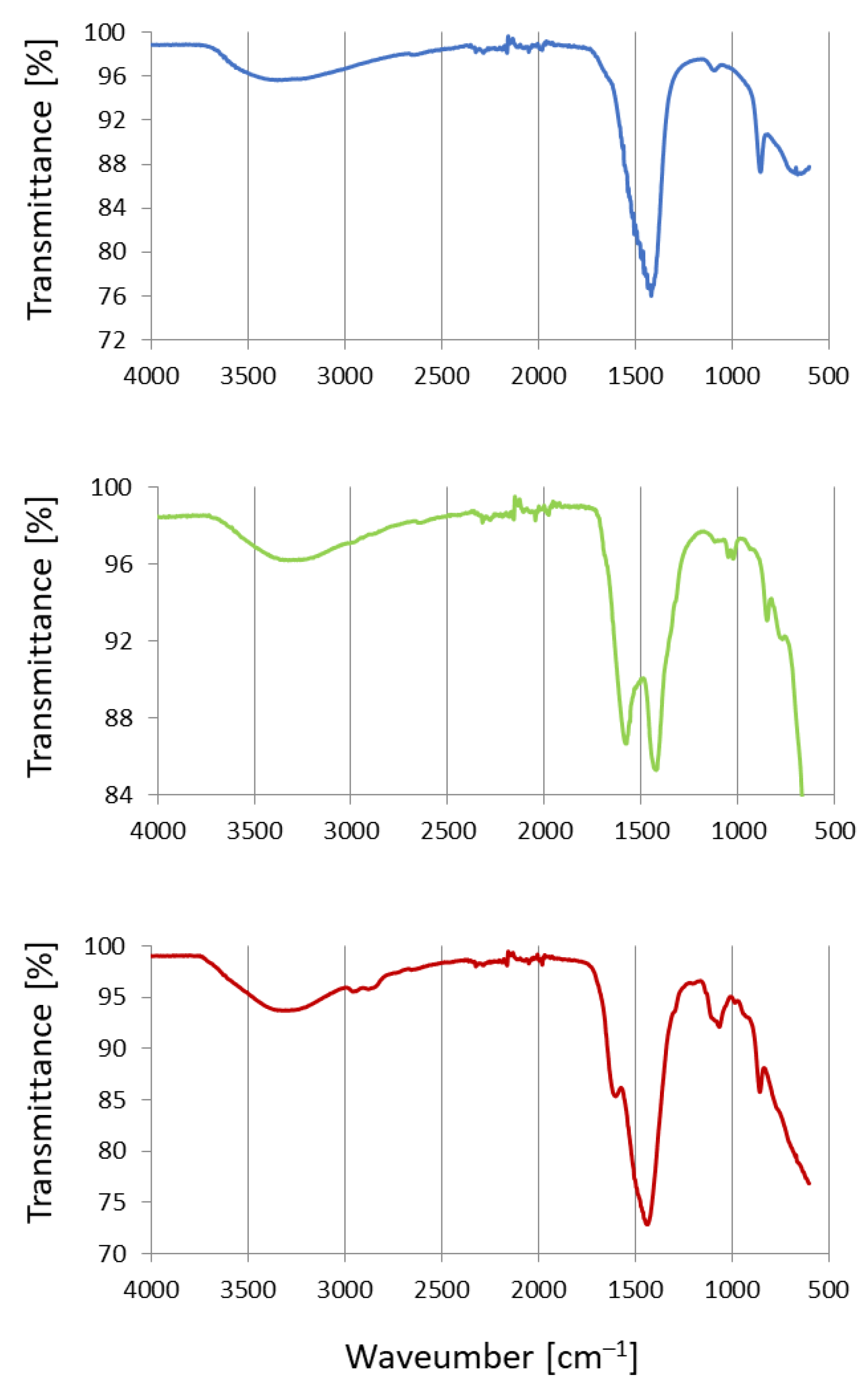
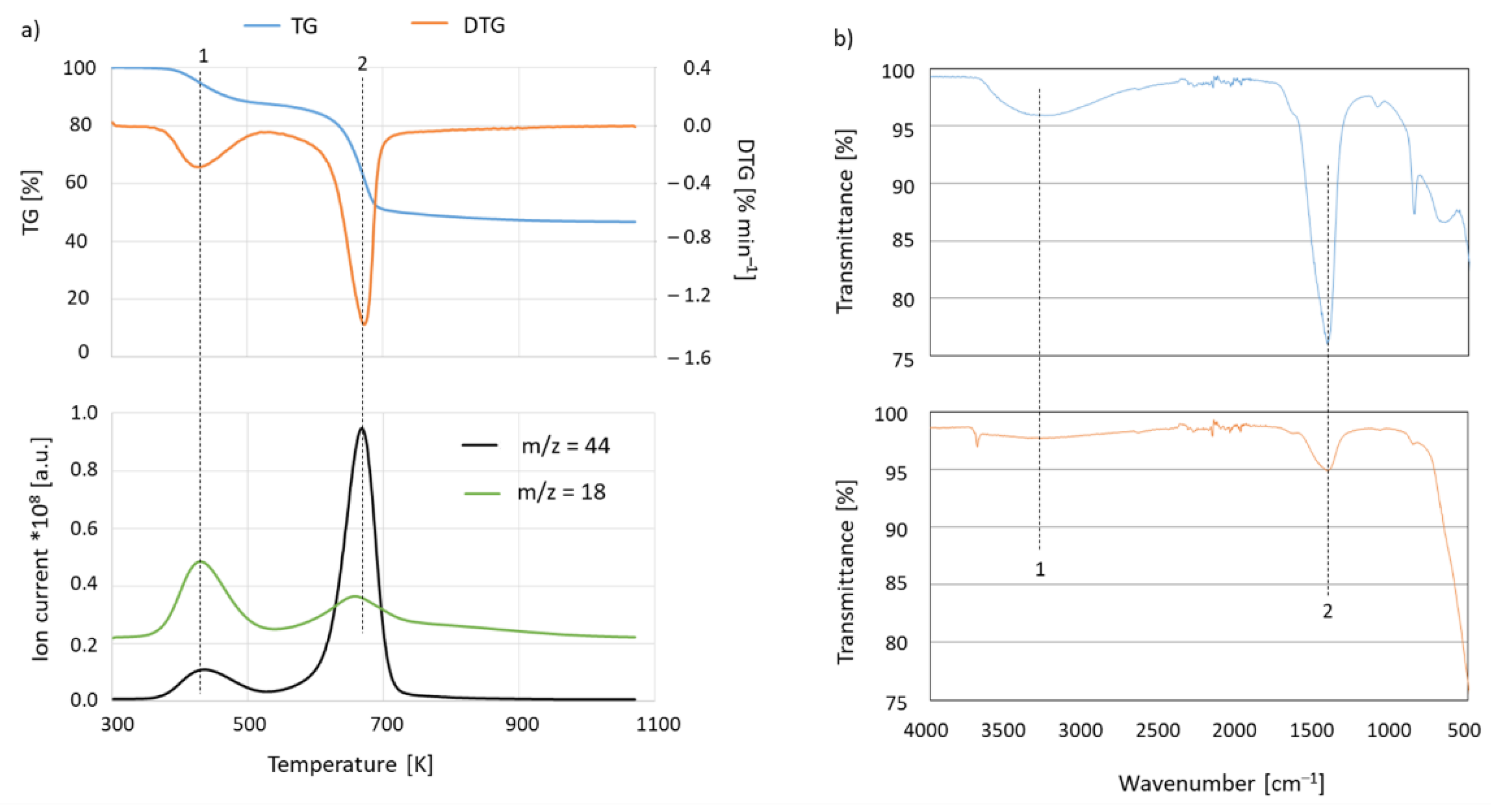
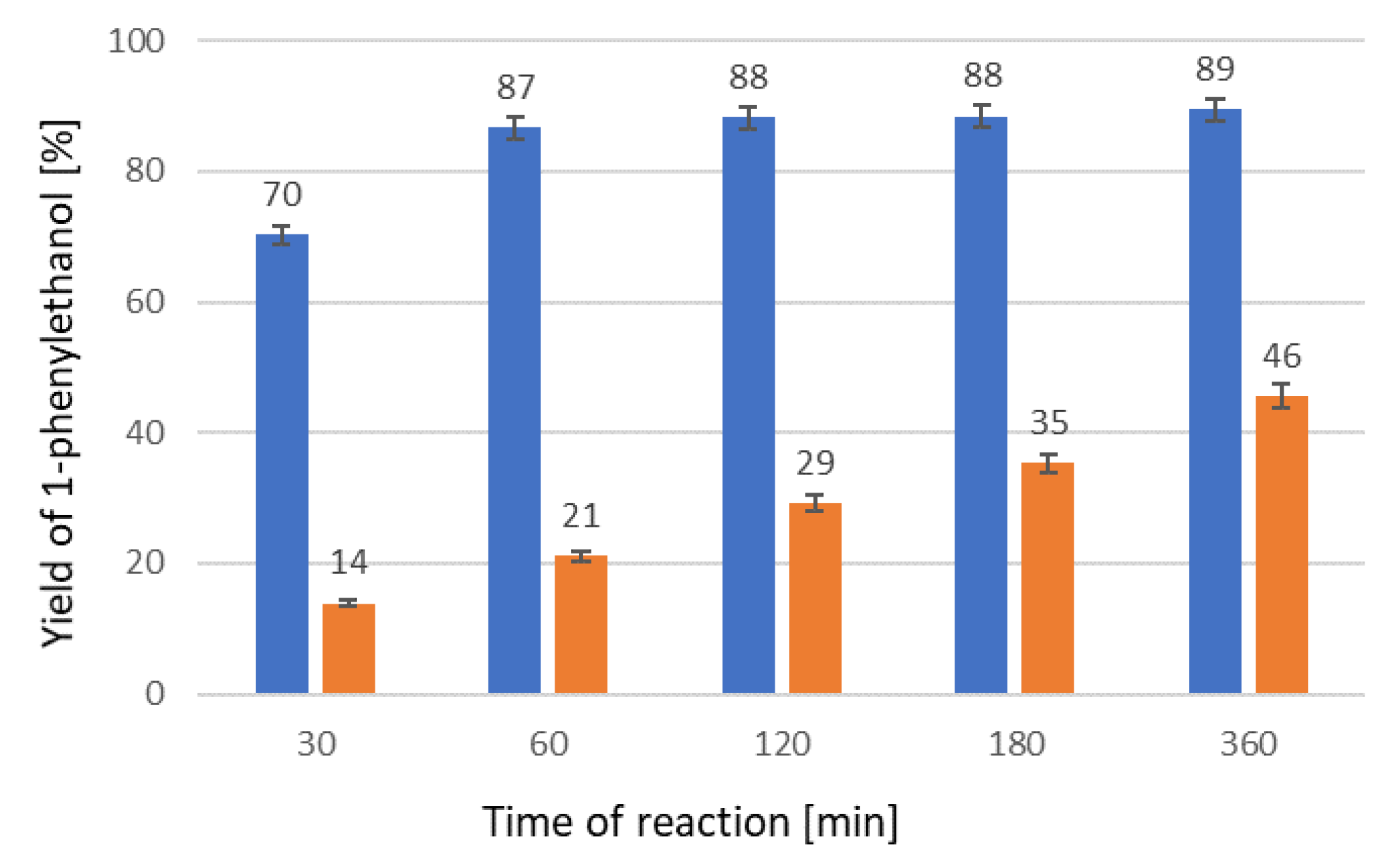
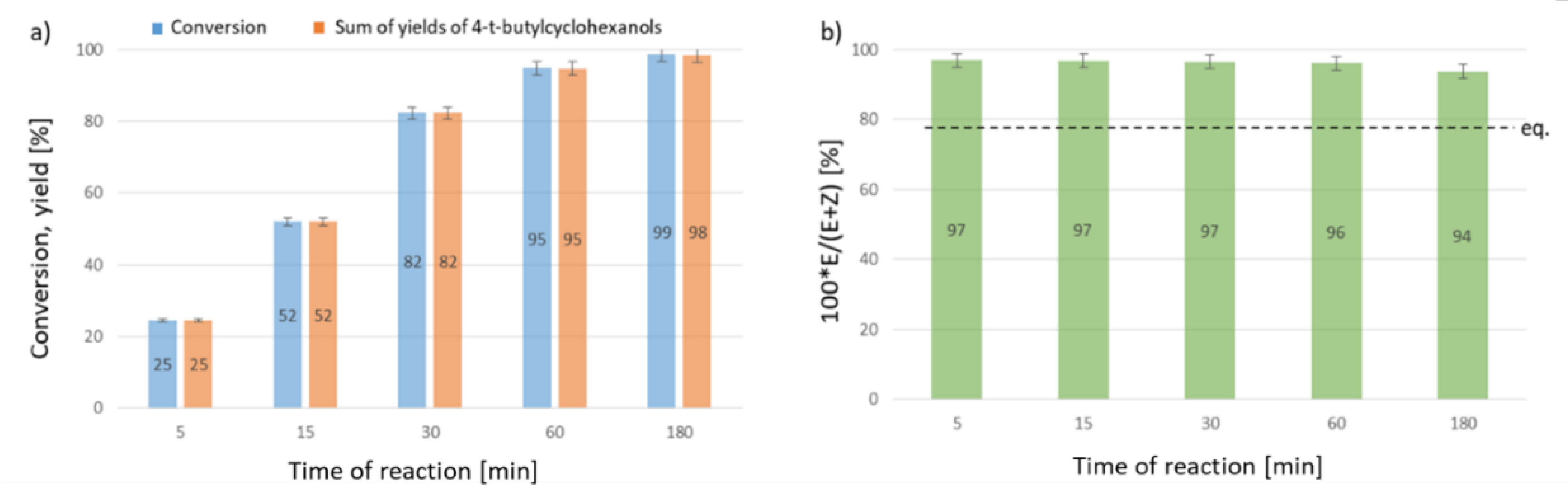
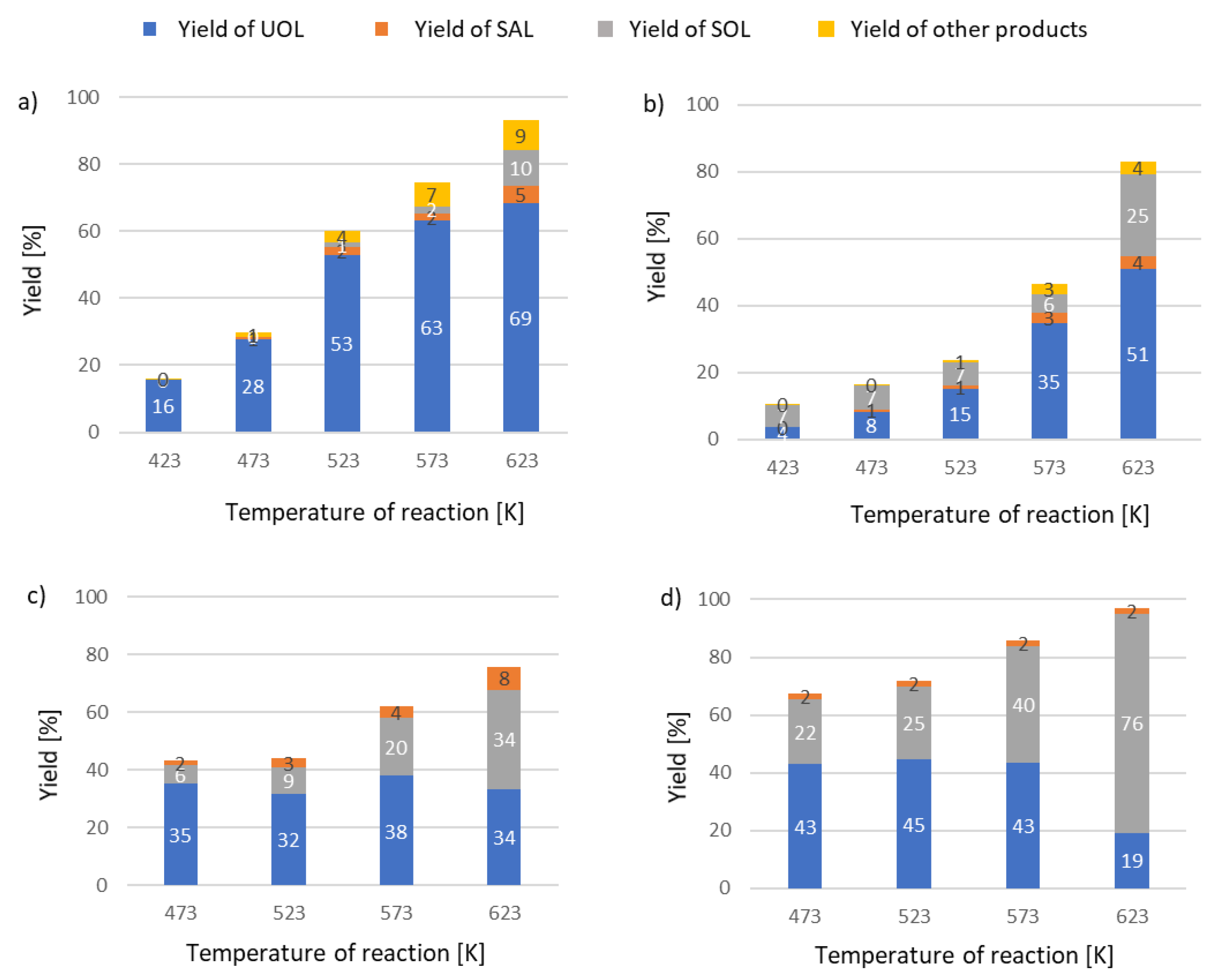
2. Results
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalytic Activity Measurements
3.3. Catalyst Characterization
3.3.1. Nitrogen Physisorption
3.3.2. X-ray Diffraction
3.3.3. Secondary Electron Microscopy-Energy Dispersive X-ray Spectroscopy (SEM-EDX)
3.3.4. X-ray Photoelectron Spectroscopy (XPS)
3.3.5. Attenuated Total Reflectance-Fourier Transform InfraRed Spectroscopy (ATR-FTIR)
3.3.6. Titration Measurements
3.4. Reagents and Solvents
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldsmith, B.R.; Peters, B.; Johnson, J.K.; Gates, B.C.; Scott, L.S. Beyond Ordered Materials: Understanding Catalytic Sites on Amorphous Solids. ACS Catal. 2017, 7, 7543–7557. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, H.J. Progress and Challenge of Amorphous Catalysts for Electrochemical Water Splitting. ACS Mater. Lett. 2021, 3, 136–147. [Google Scholar] [CrossRef]
- Liu, J.; Nai, J.; You, T.; An, P.; Zhang, J.; Ma, G.; Niu, X.; Liang, C.; Yang, S.; Guo, L. The Flexibility of an Amorphous Cobalt Hydroxide Nanomaterial Promotes the Electrocatalysis of Oxygen Evolution Reaction. Small 2018, 14, 1703514. [Google Scholar] [CrossRef]
- Zallen, R. The Physics of Amorphous Solids; Wiley: Mörlenbach, Germany, 2008; 304p. [Google Scholar]
- Liu, W.-J.; Chang, Y.-H.; Chen, Y.-T.; Chiang, Y.-C.; Liu, Y.-C.; Wu, T.-H.; Chi, P.-W. Effect of Annealing on the Structural, Magnetic and Surface Energy of CoFeBY Films on Si (100) Substrate. Materials 2021, 14, 987. [Google Scholar] [CrossRef] [PubMed]
- Mahadeva, S.K.; Fan, J.; Biswas, A.; Sreelatha, K.S.; Belova, L.; Rao, K.V. Magnetism of amorphous and nano-crystallized Dc-sputter-deposited MgO thin films. Nanomaterials 2013, 3, 486–497. [Google Scholar] [CrossRef] [Green Version]
- Harnchana, V.; Hindmarch, A.T.; Brown, A.P.; Brydson, R.M.; Marrows, C.H. TEM investigation of MgO thin films for magnetic tunnel junction application. J. Phys. Conf. Ser. 2010, 241, 012039–0120342. [Google Scholar] [CrossRef]
- Durandurdu, M. Ferromagnetism in amorphous MgO. Phil. Mag. 2017, 97, 2129–2141. [Google Scholar] [CrossRef]
- Macêdo, M.I.F.; Bertran, C.A.; Osawa, C.C. Kinetics of the γ→α-alumina phase transformation by quantitative X-ray diffraction. J. Mater. Sci. 2007, 42, 2830–2836. [Google Scholar] [CrossRef]
- Stelzer, B.; Pingen, K.; Hans, M.; Holzapfel, D.M.; Richter, S.; Mayer, J.; Gokuldoss Pradeep, K.; Schneider, J.M. Phase Formation and Thermal Stability of Reactively Sputtered YTaO4–ZrO2 Coatings. Materials 2021, 14, 692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, T.; Wang, Y. Insights into TiO2 polymorphs: Highly selective synthesis, phase transition, and their polymorph-dependent properties. RSC Adv. 2017, 7, 52755–52761. [Google Scholar] [CrossRef] [Green Version]
- Montero, J.M.; Brown, D.R.; Gai, P.L.; Lee, A.F.; Wilson, K. In situ studies of structure–reactivity relations in biodiesel synthesis over nanocrystalline MgO. Chem. Eng. J. 2010, 161, 332–339. [Google Scholar] [CrossRef]
- Verziu, M.; Cojocaru, B.; Hu, J.; Richards, R.; Ciuculescu, C.; Filip, P.; Parvulescu, V.I. Sunflower and rapeseed oil transesterification to biodiesel over different nanocrystalline MgO catalysts. Green Chem. 2008, 10, 373–381. [Google Scholar] [CrossRef]
- Ferretti, C.A.; Olcese, R.N.; Apesteguia, C.R.; Di Cosimo, J.I. Heterogeneously-Catalyzed Glycerolysis of Fatty Acid Methyl Esters: Reaction Parameter Optimization. Ind. Eng. Chem. Res. 2009, 48, 10387–10394. [Google Scholar] [CrossRef]
- Cavani, F.; Maselli, L.; Passeri, S.; Lercher, J.A. Catalytic methylation of phenol on MgO—Surface chemistry and mechanism. J. Catal. 2010, 269, 340–350. [Google Scholar] [CrossRef]
- Tsai, T.-F.; Wang, F.-L. Ortho-alkylation of phenol derivatives with methanol over magnesium oxide catalysts. 1. Characterization of promoted magnesium oxide catalysts. Catal. Lett. 2001, 73, 167–173. [Google Scholar] [CrossRef]
- Baird, M.J.; Lunsford, J.H. Catalytic sites for the isomerization of 1-butene over magnesium oxide. J. Catal. 1972, 26, 440–450. [Google Scholar] [CrossRef]
- Jiang, W.; Moa, X.; Feng, S.; Xu, F.; Zhou, G.; Zhou, H.; Xua, C.; Chen, B. Effect of MgO on WO3/SiO2-catalyzed light olefin metathesis using different feedstocks. Mol. Catal. 2017, 442, 49–56. [Google Scholar] [CrossRef]
- Gliński, M. Catalytic hydrogen transfer over magnesia Vapour and liquid phase reduction of various aralkyl ketones. Appl. Catal. A Gen. 2008, 349, 133–139. [Google Scholar] [CrossRef]
- Gliński, M.; Ulkowska, U. Description of the structure-chemoselectivity relationship in the transfer hydrogenation of α,β-unsaturated aldehydes and ketones with alcohols in the presence of magnesium oxide. Appl. Catal. A Gen. 2018, 554, 117–124. [Google Scholar] [CrossRef]
- Hou, S.-F.; Chen, J.-Y.; Xue, M.; Jia, M.; Zhai, X.; Liao, R.-Z.; Tung, C.-H.; Wang, W. Cooperative Molybdenum-Thiolate Reactivity for Transfer Hydrogenation of Nitriles. ACS Catal. 2020, 10, 380–390. [Google Scholar] [CrossRef]
- Sloane, S.E.; Reyes, A.; Vang, Z.P.; Li, L.; Behlow, K.T.; Clark, J.R. Copper-Catalyzed Formal Transfer Hydrogenation/Deuteration of Aryl Alkynes. Org. Lett. 2020, 22, 9139–9144. [Google Scholar] [CrossRef]
- De Vrieze, J.E.; Urbina Blanco, C.A.; Thybaut, J.W.; Saeys, M. Autocatalytic Role of Molecular Hydrogen in Copper-Catalyzed Transfer Hydrogenation of Ketones. ACS Catal. 2019, 9, 8073–8082. [Google Scholar] [CrossRef]
- Nie, R.; Tao, Y.; Nie, Y.; Lu, T.; Wang, J.; Zhang, Y.; Lu, X.; Xu, C.C. Recent Advances in Catalytic Transfer Hydrogenation with Formic Acid over Heterogeneous Transition Metal Catalysts. ACS Catal. 2021, 11, 1071–1095. [Google Scholar] [CrossRef]
- Iwanek, E.; Ulkowska, U.; Gliński, M. Magnesium oxide modified with various iodine-containing compounds–Surface studies. Surf. Interface Anal. 2017, 49, 945–952. [Google Scholar] [CrossRef]
- Feng, J.; Yang, C.; Zhang, D.; Wang, J.; Fu, H.; Chen, H.; Li, X. Catalytic transfer hydrogenolysis of α-methylbenzyl alcohol using palladium catalysts and formic acid. Appl. Catal. A 2009, 354, 38–43. [Google Scholar] [CrossRef]
- Gliński, M.; Markowska, A.; Wrońska, L.; Jerzak, A.; Tarkowska, M. Highly Selective Vapor and Liquid Phase Transfer Hydrogenation of Diaryl and Polycyclic Ketones with Secondary Alcohols in the Presence of Magnesium Oxide as Catalyst. Catalysts 2021, 11, 574. [Google Scholar] [CrossRef]
- Lan, X.; Wang, T. Highly Selective Catalysts for the Hydrogenation of Unsaturated Aldehydes: A Review. ACS Catal. 2020, 10, 2764–2790. [Google Scholar] [CrossRef]
- Chen, H.-J.; Chiu, C.-C.; Wang, T.; Lee, D.-S.; Lu, T.-J. Bis-NHC–Ag/Pd(OAc)2 Catalytic System Catalyzed Transfer Hydrogenation Reaction. Catalysts 2021, 11, 8. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, C.-A.; Gau, H.-M.; Bailey, J.A.; Akhadov, E.; Williams, D.; Wang, H.-L. Facile Synthesis of Polyaniline-Supported Pd Nanoparticles and Their Catalytic Properties toward Selective Hydrogenation of Alkynes and Cinnamaldehyde. Chem. Mater. 2008, 20, 2839–2844. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Acosta, A.; Apesteguia, C.R. Allylic alcohol synthesis by gas-phase hydrogen transfer reduction of unsaturated ketones. J. Mol. Catal. A Chem. 2005, 234, 111–120. [Google Scholar] [CrossRef]
- Leofanti, G.; Solari, M.; Tauszik, G.R.; Garbasi, F.; Galvagno, S.; Schwank, J. Magnesium oxide as a catalyst support: The influence of chlorine. Appl. Catal. 1982, 3, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Utamapanya, S.; Klabunde, K.J.; Schlup, J.R. Nanoscale Metal Oxide Particles/Clusters as Chemical Reagents. Synthesis and Properties of Ultrahigh Surface Area Magnesium Hydroxide and Magnesium Oxide. Chem. Mater. 1991, 3, 175–181. [Google Scholar] [CrossRef]
- Singh, J.P.; Singh, V.; Sharma, A.; Pandey, G.; Chae, K.H.; Lee, S. Approaches to synthesize MgO nanostructures for diverse applications. Heliyon 2020, 6, e04882. [Google Scholar] [CrossRef] [PubMed]
- Busca, G. Bases and Basic Materials in Chemical and Environmental Processes. Liquid versus Solid Basicity. Chem. Rev. 2010, 110, 2217–2249. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S. Optimization of Alkaline Earth Metal Oxide and Hydroxide Catalysts for Base-Catalyzed Reactions. Adv. Catal. 2006, 49, 239–302. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Chen, R.; Yang, C.; Xiang, L.; Yi, M. A vacuum calcination route to high-surface-area MgO nanoplates for superior arsenate adsorption and catalytic properties. Vacuum 2018, 158, 231–235. [Google Scholar] [CrossRef]
- Matsuda, T.; Sugimoto, M. High activity of MgO catalyst prepared from magnesium oxalate in hydrogenation of butadiene. React. Kinet. Catal. Lett. 1991, 44, 69–73. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, K.D.; Moon, D.J. Shape controlled synthesis of nanostructured magnesium oxide particles in supercritical carbon dioxide with ethanol cosolvent. Mater. Res. Bull. 2013, 48, 2817–2823. [Google Scholar] [CrossRef]
- Forsgren, J.; Frykstrand, S.; Grandfield, K.; Mihranyan, A.; Strømme, M.A. Template-Free, Ultra-Adsorbing, High Surface Area Carbonate Nanostructure. PLoS ONE 2013, 8, e68486. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Forsgren, J.; Strømme, M. Stabilisation of amorphous ibuprofen in Upsalite, a mesoporous magnesium carbonate, as an approach to increasing the aqueous solubility of poorly soluble drugs. Int. J. Pharm. 2014, 472, 185–191. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef]
- Chuah, G.K.; Jaenicke, S.; Zhu, Y.Z.; Liu, S.H. Meerwein-Ponndorf-Verley Reduction over Heterogeneous Catalysts. Curr. Org. Chem. 2006, 10, 1639–1654. [Google Scholar] [CrossRef]
- Bartók, M. Unexpected Inversions in Asymmetric Reactions: Reactions with Chiral Metal Complexes, Chiral Organocatalysts, and Heterogeneous Chiral Catalysts. Chem. Rev. 2010, 110, 1663–1705. [Google Scholar] [CrossRef]
- Graf, D.L. Crystallographic tables for the rhombohedral carbonates. Am. Mineral. 1961, 46, 1283–1316. [Google Scholar]
- Acquista, N.; Schoen, L.J.; Lide, D.R., Jr. Infrared Spectrum of the Matrix-Isolated OR Radical. J. Chem. Phys. 1968, 48, 1534. [Google Scholar] [CrossRef]
- Gliński, M.; Ulkowska, U. Reactivity of Alcohols in Chemoselective Transfer Hydrogenation of Acrolein over Magnesium Oxide as the Catalyst. Catal. Lett. 2011, 141, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Gliński, M. Catalytic Transfer Hydrogenation of Cycloalkanones on MgO. Vapour and Liquid Phase Modes of Reaction. Pol. J. Chem. 2009, 83, 187–194. [Google Scholar]
- Iwanek, E.M.; Liotta, L.F.; Williams, S.; Hu, L.; Calilung, K.; Pantaleo, G.; Kaszkur, Z.; Kirk, D.W.; Gliński, M. Application of Potassium Ion Deposition in Determining the Impact of Support Reducibility on Catalytic Activity of Au/Ceria-Zirconia Catalysts in CO Oxidation, NO Oxidation, and C3H8 Combustion. Catalysts 2020, 10, 688. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gliński, M.; Iwanek, E.M.; Ulkowska, U.; Czajka, A.; Kaszkur, Z. Catalytic Activity of High-Surface-Area Amorphous MgO Obtained from Upsalite. Catalysts 2021, 11, 1338. https://doi.org/10.3390/catal11111338
Gliński M, Iwanek EM, Ulkowska U, Czajka A, Kaszkur Z. Catalytic Activity of High-Surface-Area Amorphous MgO Obtained from Upsalite. Catalysts. 2021; 11(11):1338. https://doi.org/10.3390/catal11111338
Chicago/Turabian StyleGliński, Marek, Ewa M. Iwanek (nee Wilczkowska), Urszula Ulkowska, Agnieszka Czajka, and Zbigniew Kaszkur. 2021. "Catalytic Activity of High-Surface-Area Amorphous MgO Obtained from Upsalite" Catalysts 11, no. 11: 1338. https://doi.org/10.3390/catal11111338
APA StyleGliński, M., Iwanek, E. M., Ulkowska, U., Czajka, A., & Kaszkur, Z. (2021). Catalytic Activity of High-Surface-Area Amorphous MgO Obtained from Upsalite. Catalysts, 11(11), 1338. https://doi.org/10.3390/catal11111338





