Mechanochemically Prepared Co3O4-CeO2 Catalysts for Complete Benzene Oxidation
Abstract
:1. Introduction
2. Results and Discussion
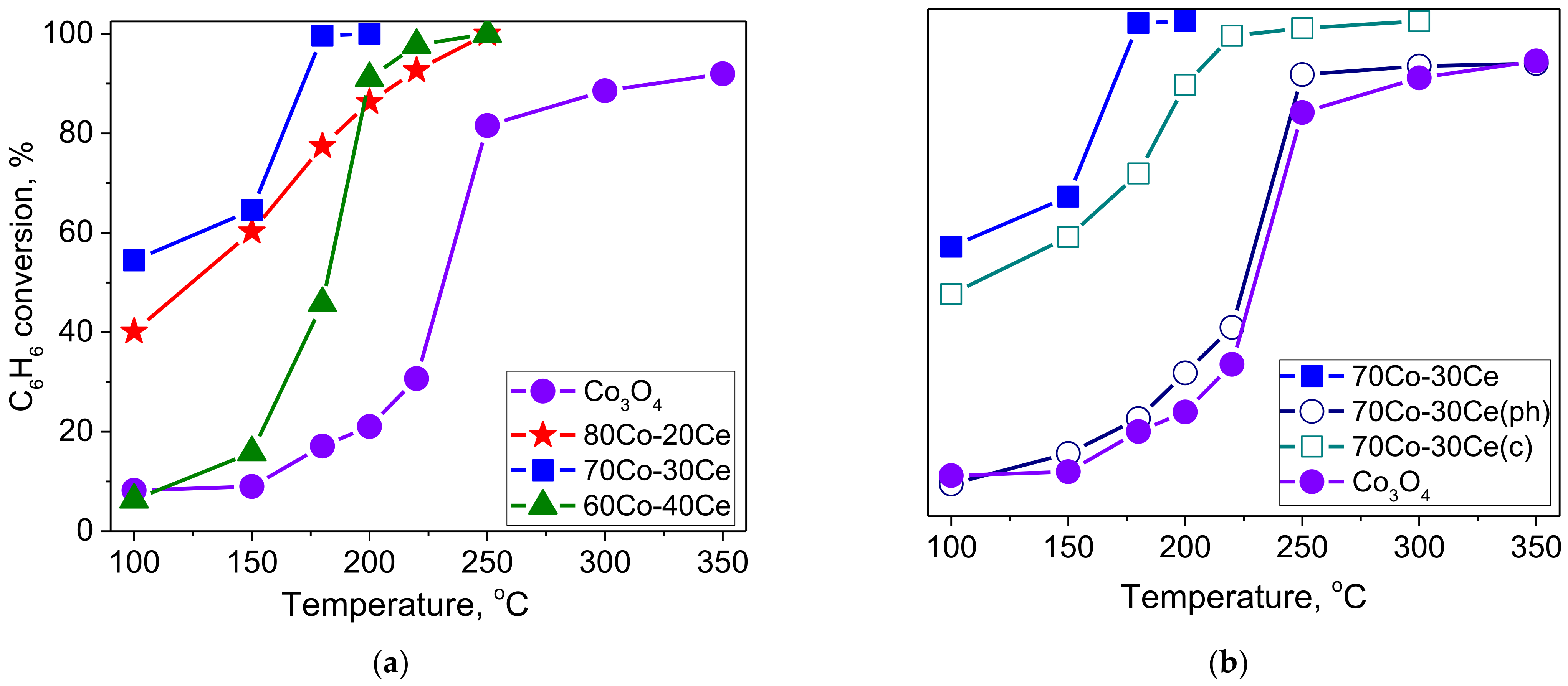
2.1. Catalytic Activity Measurements
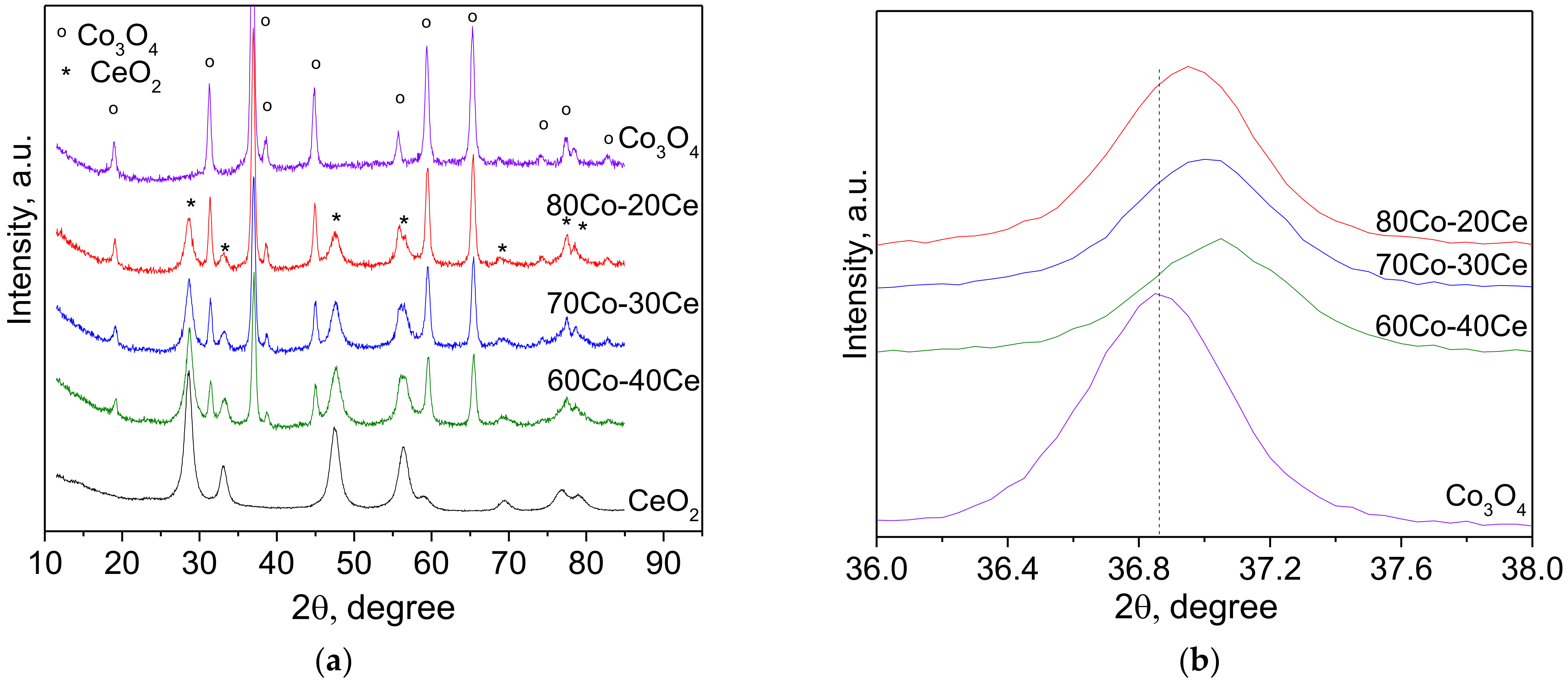
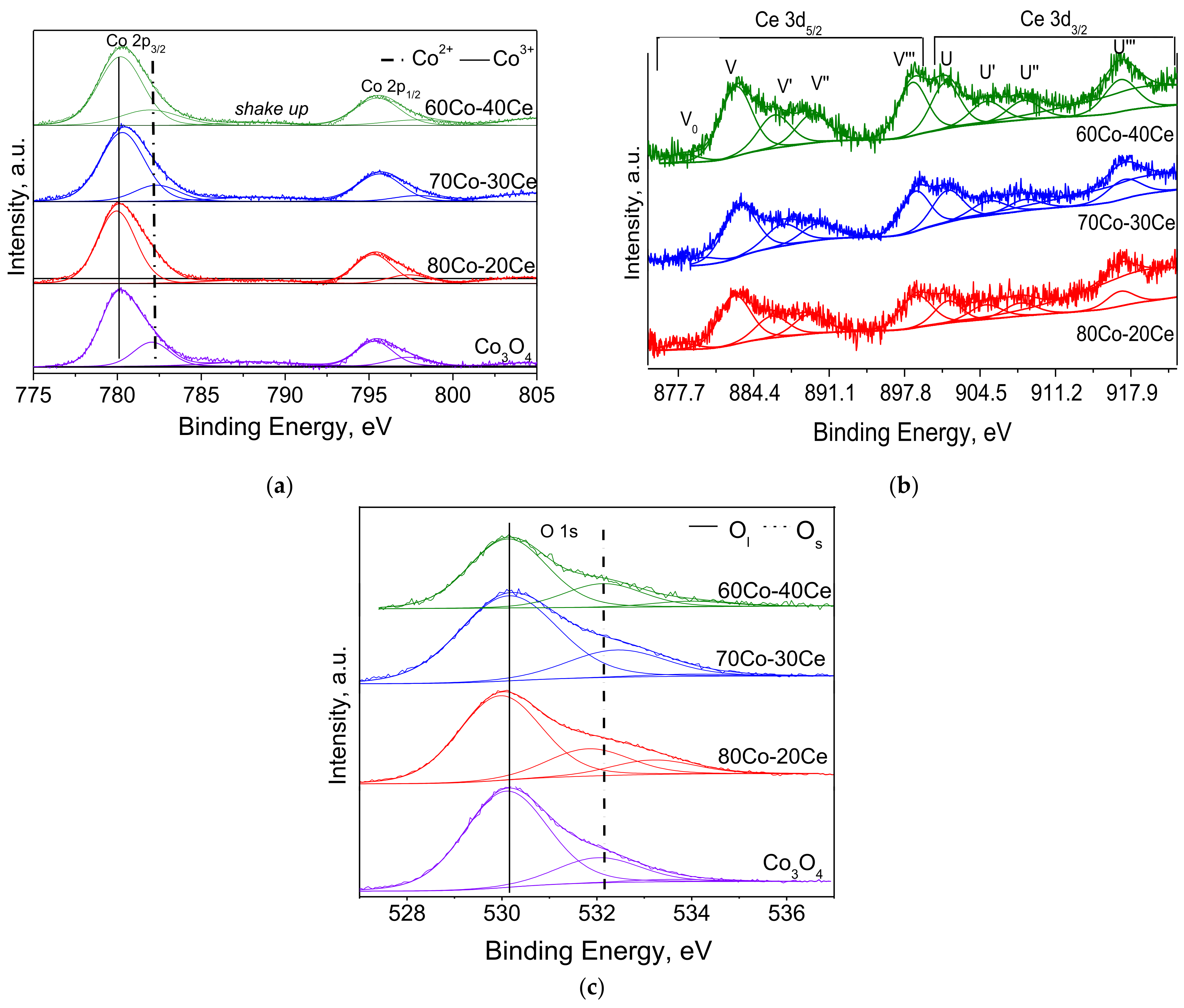
2.2. Sample Characterization
| CeO2 | 60Co-40Ce | 70Co-30Ce | 80Co-20Ce | Co3O4 | Band Assignments | Refs. |
|---|---|---|---|---|---|---|
| 399/113 | 355/143 | |||||
| 389/146 | 385/139 | 404/132 | - | Transversal stretching of topmost O-Ce | [61,64] | |
| 410/69 | ||||||
| 443/71 | 446/55 | 444/60 | 445/56 | Symmetric stretching of the of Ce4+-O8 units with F2g symmetry in CeO2 | [59,64] | |
| 465/77 | Eg modes of Co3O4 (470 cm−1) | [65,66] | ||||
| 493/43 | 490/38 | 491/47 | 494/43 | Subsurface oxygen vacancy in CeO2-x (111) | [60,61] | |
| 496/27 | Defect induced in CoO and F2g modes of nanosized Co3O4 | [67] | ||||
| 534/52 | 527/72 | 541/74 | 541/52 | Oxygen vacancies, VO∙∙, asymmetric Ce-O stretch of the surface peroxide O22−/Ce3+O7VO∙∙ (D1 at 550 cm−1) and O22−/Ce4+O7VO∙∙ (D1 at 525 cm−1) | [59,60,61] | |
| 595/73 | 600/80 | 602/71 | 598/85 | Defect induced (D2) mode with Oh symmetry | [59,60,61] | |
| 625/54 | F2g modes in Co3O4 | [67] | ||||
| 669/38 | 658/31 | A1g of CoO6 (675 cm−1) | [65,66,67] | |||
| 809/62 | 792/36 | 789/43 | 777/17 | 786/27 | O-O stretching of adsorbed O22− | [64] |
| 813/63 | 817/66 | |||||
| 852/10 | 828/30 | 827/29 | O22− adsorbed on isolated and clustered two-electron defect sites (830 and 860 cm−1) | [61,64] | ||
| 1064/52 | Two-phonon mode in CoO | [66] | ||||
| 1184/233 | 1181/210 | 1173/222 | 1178/225 | Second-order longitudinal optical (2LO) mode in CeO2 | [59,64] | |
| 1185/39 | ||||||
| 1228/32 | ||||||
| 0.0724 | 0.1052 | 0.2131 | 0.1113 | - | AD2/AF2g (CeO2) | [63] |
| 0.8675 | 1.4118 | 0.4454 | 0.8087 | - | ID1/ID2 | [59] |
| 0.9915 | 0.9962 | 0.9867 | 0.9913 | 0.9931 | R2 |
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalytic Activity Measurements
3.3. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Busca, G.; Daturi, M.; Finocchio, E.; Lorenzelli, V.; Ramis, G.; Willey, R. Transition metal mixed oxides as combustion catalysts: Preparation, characterization and activity mechanisms. Catal. Today 1997, 33, 239–249. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Kim, S.C. The catalytic oxidation of aromatic hydrocarbons over supported metal oxide. J. Hazard. Mater. 2002, 91, 285–299. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A Review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Ma, Z. Cobalt Oxide Catalysts for Environmental Remediation. Curr. Catal. 2014, 3, 15–26. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.-C.; Chen, M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Dry citrate-precursor synthesized nanocrystalline cobalt oxide as highly active catalyst for total oxidation of propane. J. Catal. 2009, 263, 104–113. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, L.; Wu, F.; Li, J. Decreasing Co3O4 particle sizes by ammonia-etching and catalytic oxidation of propane. Catal. Lett. 2017, 147, 407–415. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Bai, B.; Zhao, S.; Hu, F.; Li, J. Bridging the reaction route of toluene total oxidation and the structure of ordered mesoporous Co3O4: The roles of surface sodium and adsorbed oxygen. Catal. Today 2017, 297, 173–181. [Google Scholar] [CrossRef]
- Xie, S.; Liu, Y.; Deng, J.; Yang, J.; Zhao, X.; Han, Z.; Zhang, K.; Dai, H. Insights into the active sites of ordered mesoporous cobalt oxide catalysts for the total oxidation of o-xylene. J. Catal. 2017, 352, 282–292. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Wang, Z.; Huang, H.; Peng, H.; Li, X. Fabrication of mesoporous Co3O4 oxides by acid treatment and their catalytic performances for toluene oxidation. Appl. Catal. A Gen. 2018, 550, 67–76. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Y.; Hu, H.; Wang, S.; Ying, L.; Huang, Y. Achieving low temperature formaldehyde oxidation: A case study of NaBH4 reduced cobalt oxide nanowires. Inorg. Chem. Commun. 2017, 82, 20–23. [Google Scholar] [CrossRef]
- Bai, G.; Dai, H.; Deng, J.; Liu, Y.; Wang, F.; Zhao, Z.; Qiu, W.; Au, C.T. Porous Co3O4 nanowires and nanorods: Highly active catalysts for the combustion of toluene. Appl. Catal. A Gen. 2013, 450, 42–49. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, Z.; Dong, F.; Zhang, J. Controlled porous hollow Co3O4 polyhedral nanocages derived from metal-organic frameworks (MOFs) for toluene catalytic oxidation. Mol. Catal. 2019, 463, 77–86. [Google Scholar] [CrossRef]
- Szegedi, Á.; Popova, M.; Minchev, C. Catalytic activity of Co/MCM-41 and Co/SBA-15 materials in toluene oxidation. J. Mater. Sci. 2009, 44, 6710–6716. [Google Scholar] [CrossRef]
- Szegedi, Á.; Popova, M.; Dimitrova, A.; Cherkezova-Zheleva, Z.; Mitov, I. Effect of the pretreatment conditions on the physico-chemical and catalytic properties of cobalt- and iron-containing Ti-MCM-41 materials. Microporous Mesoporous Mater. 2010, 136, 106–114. [Google Scholar] [CrossRef]
- Zhang, Q.; Mo, S.; Chen, B.; Zhang, W.; Huang, C.; Ye, D. Hierarchical Co3O4 nanostructures in-situ grown on 3D nickel foam towards toluene oxidation. Molec. Catal. 2018, 454, 12–20. [Google Scholar] [CrossRef]
- Solsona, B.; Davies, T.E.; Garcia, T.; Vazquez, I.; Dejoz, A.; Taylor, S.H. Total oxidation of propane using nanocrystalline cobalt oxide and supported cobalt oxide catalysts. Appl. Catal. B Environ. 2008, 84, 176–184. [Google Scholar] [CrossRef]
- Kouotou, P.M.; Pan, G.F.; Wenga, J.J.; Fan, S.B.; Tian, Z.Y. Stainless steel grid mesh-supported CVD made Co3O4 thin films for catalytic oxidation of VOCs of olefins type at low temperature. J. Ind. Eng. Chem. 2016, 35, 253–261. [Google Scholar] [CrossRef]
- Topka, P.; Dvořáková, M.; Kšírová, P.; Perekrestov, R.; Čada, M.; Balabánová, J.; Koštejn, J.; Jirátová, K.; Kovanda, F. Structured cobalt oxide catalysts for VOC abatement: The effect of preparation method. Environ. Sci. Pollut. Res. 2019, 27, 7608–7617. [Google Scholar] [CrossRef]
- Wyrwalski, F.; Giraudon, J.-M.; Lamonier, J.-F. Synergistic coupling of the redox properties of supports and cobalt oxide Co3O4 for the complete oxidation of volatile organic compounds. Catal. Lett. 2010, 137, 141–149. [Google Scholar] [CrossRef]
- Liotta, L.F.; Wu, H.; Pantaleo, P.; Venezia, A.M. Co3O4 nanocrystals and Co3O4–MOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: A review. Catal. Sci. Technol. 2013, 3, 3085–3102. [Google Scholar] [CrossRef]
- Chen, X.; Carabineiro, S.A.C.; Bastos, S.S.T.; Tavares, P.B.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic oxidation of ethyl acetate on cerium-containing mixed oxides. Appl. Catal. A Gen. 2014, 472, 101–112. [Google Scholar] [CrossRef]
- Konsolakis, M.; Carabineiro, S.A.C.; Marnellos, G.E.; Asad, M.F.; Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Effect of cobalt loading on the solid state properties and ethyl acetate oxidation performance of cobalt-cerium mixed oxides. J. Colloid Interface Sci. 2017, 496, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Carabineiro, S.A.C.; Chen, X.; Konsolakis, M.; Psarras, A.C.; Tavares, P.B.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic oxidation of toluene on Ce-Co and La-Co mixed oxides synthesized by exotemplating and evaporation methods. Catal. Today 2015, 244, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, X.; Zeng, X.; Zhu, T. Correlation between the physicochemical properties and catalytic performances of micro/mesoporous CoCeOx mixed oxides for propane combustion. Appl. Catal. A Gen. 2019, 572, 61–70. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Ma, L.; Bhat, A.; Li, Y.; Schwank, J.W. Effect of Ce and La dopants in Co3O4 nanorods on catalytic activity of CO and C3H6 oxidation. Catal. Sci. Technol. 2019, 9, 1165–1177. [Google Scholar] [CrossRef]
- Ismail, A.; Li, M.; Zahid, M.; Fan, L.; Zhang, C.; Li, Z.; Zhu, Y. Effect of strong interaction between Co and Ce oxides in CoxCe1-xO2-δ oxides on its catalytic oxidation of toluene. Molec. Catal. 2021, 502, 111356. [Google Scholar] [CrossRef]
- Balzer, R.; Probst, L.F.D.; Drago, V.; Schreiner, W.H.; Fajardo, H.V. Catalytic oxidation of volatile organic compounds (n-hexane, benzene, toluene, o-xylene) promoted by cobalt catalysts supported on γ-Al2O3-CeO2. Braz. J. Chem. Eng. 2014, 31, 757–769. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yoshida, A.; Wang, P.; Yu, T.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Catalytic oxidation of volatile organic compound over cerium modified cobalt-based mixed oxide catalysts synthesized by electrodeposition method. Appl. Catal. B Environ. 2020, 271, 118941. [Google Scholar] [CrossRef]
- Blin, J.-L.; Michelin, L.; Lebeau, B.; Naydenov, A.; Velinova, R.; Kolev, H.; Gaudin, P.; Vidal, L.; Dotzeva, A.; Tenchev, K.; et al. Co–Ce oxides supported on SBA-15 for VOCs oxidation. Catalysts 2021, 11, 366. [Google Scholar] [CrossRef]
- Armor, J.N. New catalytic technology commercialized in the USA during the 1980’s. Appl. Catal. 1991, 78, 141–173. [Google Scholar] [CrossRef]
- Ataloglou, T.; Vakros, J.; Bourikas, K.; Fountzoula, C.; Kordulis, C.; Lycourghiotis, A. Influence of the preparation method on the structure–activity of cobalt oxide catalysts supported on alumina for complete benzene oxidation. Appl. Catal. B Environ. 2005, 57, 299–312. [Google Scholar] [CrossRef]
- Ataloglou, T.; Fountzoula, C.; Bourikas, K.; Vakros, J.; Lycourghiotis, A.; Kordulis, C. Cobalt oxide/γ-alumina catalysts prepared by equilibrium deposition filtration: The influence of the initial cobalt concentration on the structure of the oxide phase and the activity for complete benzene oxidation. Appl. Catal. A Gen. 2005, 288, 1–9. [Google Scholar] [CrossRef]
- Ding, Y.; Fan, Y.; Wie, X.; Li, D.; Xiao, Y.; Jiang, L. Total oxidation of benzene over cobalt-aluminum mixed oxides prepared from layered double hydroxides: Influence of preparation methods. Reac. Kinet. Mech. Cat. 2016, 118, 593–604. [Google Scholar] [CrossRef]
- Li, D.; Ding, Y.; Wei, X.; Xiao, Y.; Jiang, L. Cobalt-aluminum mixed oxides prepared from layered double hydroxides for the total oxidation of benzene. Appl. Catal. A Gen. 2015, 507, 130–138. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Hao, Z.; Zhao, W. Mesoporous silica supported cobalt oxide catalysts for catalytic removal of benzene. J. Porous Mater. 2008, 15, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Mu, Z.; Li, J.J.; Duan, M.H.; Hao, Z.P.; Qiao, S.Z. Catalytic combustion of benzene on Co/CeO2/SBA-15 and Co/SBA-15 catalysts. Catal. Commun. 2008, 9, 1874–1877. [Google Scholar] [CrossRef]
- Ma, C.; Mu, Z.; He, C.; Li, P.; Li, J.; Hao, Z. Catalytic oxidation of benzene over nanostructured porous Co3O4-CeO2 composite catalysts. J. Environ. Sci. 2011, 23, 2078–2086. [Google Scholar] [CrossRef]
- Zuo, S.; Liu, F.; Tong, J.; Qi, C. Complete oxidation of benzene with cobalt oxide and ceria using the mesoporous support SBA-16. Appl. Catal. A Gen. 2013, 467, 1–6. [Google Scholar] [CrossRef]
- Yu, Y.; Takei, T.; Ohashi, H.; He, H.; Zhang, X.; Haruta, M. Pretreatments of Co3O4 at moderate temperature for CO oxidation at −80 °C. J. Catal. 2009, 267, 121–128. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Tavares, P.B.; Figueiredo, J.L. Gold on oxide-doped alumina supports as catalysts for CO oxidation. Appl. Nanosci. 2012, 2, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Ilieva, L.; Petrova, P.; Tabakova, T.; Zanella, R.; Abrashev, M.V.; Sobczak, J.W.; Lisowski, W.; Kaszkur, Z.; Andreeva, D. Relationship between structural properties and activity in complete benzene oxidation over Au/CeO2-CoOx catalysts. Catal. Today 2012, 187, 30–38. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Catalytic oxidation of vinyl chloride emissions over Co-Ce composite oxide catalysts. Chem. Eng. J. 2017, 315, 392–402. [Google Scholar] [CrossRef]
- Bishop, S.R.; Marrocchelli, D.; Chatzichristodoulou, C.; Perry, N.H.; Mogensen, M.B.; Tuller, H.L.; Wachsman, E.D. Chemical expansion: Implications for electrochemical energy storage and conversion devices. Annu. Rev. Mater. Res. 2014, 44, 205–239. [Google Scholar] [CrossRef]
- Kang, M.; Song, M.W.; Lee, C.H. Catalytic carbon monoxide oxidation over CoOx/CeO2 composite catalysts. Appl. Catal. A Gen. 2003, 251, 143–156. [Google Scholar] [CrossRef]
- Murgida, G.E.; Vildosola, V.; Ferrari, V.; Llois, A.M. Charge localization in Co-doped ceria with oxygen vacancies. Solid State Commun. 2012, 152, 368–371. [Google Scholar] [CrossRef]
- Zou, G.; Xu, Y.; Wang, S.; Chen, M.; Shangguan, W. The synergistic effect in Co–Ce oxides for catalytic oxidation of diesel soot. Catal. Sci. Technol. 2015, 5, 1084–1092. [Google Scholar] [CrossRef]
- Smith, W.L.; Hobson, A.D. The structure of cobalt oxide, Co3O4. Acta Crystallogr. Sect. B 1973, 29, 362–363. [Google Scholar] [CrossRef]
- Yao, Y.F.Y. The oxidation of hydrocarbons and CO over metal oxides: III. Co3O4. J. Catal. 1974, 33, 108–122. [Google Scholar] [CrossRef]
- Yao, H.C.; Yao, Y.F.Y. Ceria in automotive exhaust catalysts: I. Oxygen storage. J. Catal. 1984, 86, 254–265. [Google Scholar] [CrossRef]
- Sanchez, M.G.; Gazquez, J.L. Oxygen vacancy model in strong metal–support interaction. J. Catal. 1987, 104, 120–135. [Google Scholar] [CrossRef]
- Laachir, A.; Perrichon, V.; Bardi, A.; Lamotte, J.; Catherine, E.; Lavalley, J.C.; El Fallah, J.; Hilaire, L.; Le Normand, F.; Quéméré, E.; et al. Reduction of CeO2 by hydrogen. J. Chem. Soc. Faraday Trans. 1991, 7, 1601–1609. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, C.; He, H.; Teraoka, Y. Catalytic decomposition of N2O over CeO2 promoted Co3O4 spinel catalyst. Appl. Catal. B Environ. 2007, 75, 167–174. [Google Scholar] [CrossRef]
- Li, J.; Lu, G.; Wu, G.; Mao, D.; Wang, Y.; Guo, Y. Promotional role of ceria on cobaltosic oxide catalyst for low-temperature CO oxidation. Catal. Sci. Technol. 2012, 2, 1865–1871. [Google Scholar] [CrossRef]
- Huan, W.; Li, J.; Ji, J.; Xing, M. In situ studies on ceria promoted cobalt oxide for CO oxidation. Chin. J. Catal. 2019, 40, 656–663. [Google Scholar] [CrossRef]
- Lu, S.; Wang, F.; Chen, C.; Huang, F.; Li, K. Catalytic oxidation of formaldehyde over CeO2–Co3O4 catalysts. J. Rare Earth 2017, 35, 867–874. [Google Scholar] [CrossRef]
- Luo, J.Y.; Meng, M.; Zha, Y.Q.; Guo, L.H. Identification of the active sites for CO and C3H8 total oxidation over nanostructured CuO-CeO2 and Co3O4-CeO2 catalysts. J. Phys. Chem. C 2008, 112, 8694–8701. [Google Scholar] [CrossRef]
- Taniguchi, T.; Watanabe, T.; Sugiyama, N.; Subramani, A.K.; Wagata, H.; Matsushita, N.; Yoshimura, M. Identifying defects in ceria-based nanocrystals by UV resonance Raman spectroscopy. J. Phys. Chem. C 2009, 113, 19789–19793. [Google Scholar] [CrossRef]
- Loridant, S. Raman spectroscopy as a powerful tool to characterize ceria-based catalysts. Catal. Today 2021, 373, 98–111. [Google Scholar] [CrossRef]
- Schilling, C.; Hofmann, A.; Hess, C.; Ganduglia-Pirovano, M.V. Raman spectra of polycrystalline CeO2: A Density Functional Theory study. J. Phys. Chem. C 2017, 121, 20834–20849. [Google Scholar] [CrossRef]
- Ilieva, L.; Petrova, P.; Pantaleo, G.; Zanella, R.; Liotta, L.F.; Georgiev, V.; Boghosian, S.; Kaszkur, Z.; Sobczak, J.W.; Lisowski, W.; et al. Gold catalysts supported on Y-modified ceria for CO-free hydrogen production via PROX. Appl. Catal. B Environ. 2016, 188, 154–168. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; He, H.; Wu, Z.; Wu, J.; Chen, L.; Ye, D.; Fu, M. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Appl. Catal. B Environ. 2018, 223, 91–102. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M.; Overbury, S.H. Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, N.M.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, W.; Qin, F.; Fang, H.; Hadjiev, V.G.; Litvinov, D.; Bao, J. Identification of cobalt oxides with Raman scattering and Fourier Transform Infrared Spectroscopy. J. Phys. Chem. C 2016, 120, 4511–4516. [Google Scholar] [CrossRef]
- Hongyan, X.; Jiangtao, D.; Zhenyin, H.; Libo, G.; Qiang, Z.; Jun, T.; Binzhen, Z.; Chenyang, X. A study of the growth process of Co3O4 microcrystals synthesized via a hydrothermal method. Cryst. Res. Technol. 2016, 51, 123–128. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Chuang, T.J.; Brundle, C.R.; Rice, D.W. Interpretation of the x-ray photoemission spectra of cobalt oxides and cobalt oxide surfaces. Surf. Sci. 1976, 59, 413–429. [Google Scholar] [CrossRef]
- Liotta, L.F.; Pantaleo, G.; Puleo, F.; Venezia, A.M. Au/CeO2-SBA-15 catalysts for CO oxidation: Effect of ceria loading on physicochemical properties and catalytic performances. Catal. Today 2012, 187, 10–19. [Google Scholar] [CrossRef]
- Qu, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. 3D Gold-Modified Cerium and Cobalt Oxide Catalyst on a Graphene Aerogel for Highly Efficient Catalytic Formaldehyde Oxidation. Small 2018, 1804415. [Google Scholar] [CrossRef]
- Dang-Bao, T.; Anh, N.P.; Phuong, P.H.; Van, N.T.T.; Nghia, T.N.D.; Tien, H.V.; Tri, N. Fabrication of cobalt-doped ceria nanorods for p-xylene deep oxidation: Effects of cobalt precursor and loading. Mater. Trans. 2020, 61, 1294–1300. [Google Scholar] [CrossRef]
- Bortolotti, M.; Lutterotti, L.; Lonardelli, I. ReX: A computer program for structural analysis using powder diffraction data. J. Appl. Cryst. 2009, 42, 538–539. [Google Scholar] [CrossRef]
- Kotzev, N.; Shopov, D. A thermodesorption study of the system olefin—NiO. J. Catal. 1971, 22, 297–301. [Google Scholar] [CrossRef]
- Monti, D.A.M.; Baiker, A. Temperature-programmed reduction, parametric sensitivity and estimation of kinetic parameters. J. Catal. 1983, 83, 323–335. [Google Scholar] [CrossRef]
- Venezia, A.M.; Murania, R.; Pantaleo, G.; Deganello, G. Pd and PdAu on mesoporous silica for methane oxidation: Effect of SO2. J. Catal. 2007, 251, 94–102. [Google Scholar] [CrossRef]


| Sample | SSA (m2 g−1) | DCeO2 (nm) | aCeO2 (Å) | DCo3O4 (nm) | aCo3O4 (Å) |
|---|---|---|---|---|---|
| Co3O4 | 44 | - | - | 18.0 (2) | 8.0868 (7) |
| CeO2 | 61 | 5.0 (4) | 5.4118 (2) | - | - |
| 80Co-20Ce | 54 | 5.1 (2) | 5.4093 (2) | 18.0 (2) | 8.0717 (4) |
| 70Co-30 Ce | 64 | 5.2 (3) | 5.4043 (5) | 17.0 (4) | 8.0630 (6) |
| 60Co-40Ce | 67 | 4.8 (2) | 5.3980 (4) | 16.0 (4) | 8.0557 (4) |
| Sample | Tmax (°C) | HC (µmol g−1) |
|---|---|---|
| Co3O4 | 157 | 184 |
| 80Co-20Ce | 157 | 154 |
| 70Co-30Ce | 165 | 195 |
| 60Co-40Ce | 176 | 97 |
| Sample | BE Co 2p3/2 a (eV) | BE Ce 3d5/2 (eV) | BE O 1s (eV) | Ce/Co b | Ce3+/Cetot | Co3+/Co2+ | Os/Ol c |
|---|---|---|---|---|---|---|---|
| Co3O4 | 780.1(63) (Co3+) | 530.1 (78) | 1.70 | 0.26 | |||
| 782.0(37) (Co2+) | 532.0 (20) | ||||||
| 533.5 (02) | |||||||
| 80Co-20Ce | 780.0(69) (Co3+) | 883.1 (V) | 530.0 (67) | 0.07 | 0.20 | 2.25 | 0.31 |
| 781.9(31) (Co2+) | 886.0 (V’) | 531.8 (21) 533.2 (12) | (0.07) | ||||
| 70Co-30Ce | 780.3(73) (Co3+) | 883.1 (V) | 530.1 (75) | 0.16 | 0.22 | 2.70 | 0.31 |
| 782.3(27) (Co2+) | 886.3 (V’) | 532.4 (23) | (0.10) | ||||
| 533.6 (02) | |||||||
| 60Co-40Ce | 780.2(72) (Co3+) | 882.9 (V) | 530.1 (70) | 0.22 | 0.19 | 2.57 | 0.33 |
| 782.1(28) (Co2+) | 886.1 (V’) | 532.1 (24) 533.9 (06) | (0.16) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilieva, L.; Petrova, P.; Venezia, A.M.; Anghel, E.M.; State, R.; Avdeev, G.; Tabakova, T. Mechanochemically Prepared Co3O4-CeO2 Catalysts for Complete Benzene Oxidation. Catalysts 2021, 11, 1316. https://doi.org/10.3390/catal11111316
Ilieva L, Petrova P, Venezia AM, Anghel EM, State R, Avdeev G, Tabakova T. Mechanochemically Prepared Co3O4-CeO2 Catalysts for Complete Benzene Oxidation. Catalysts. 2021; 11(11):1316. https://doi.org/10.3390/catal11111316
Chicago/Turabian StyleIlieva, Lyuba, Petya Petrova, Anna Maria Venezia, Elena Maria Anghel, Razvan State, Georgi Avdeev, and Tatyana Tabakova. 2021. "Mechanochemically Prepared Co3O4-CeO2 Catalysts for Complete Benzene Oxidation" Catalysts 11, no. 11: 1316. https://doi.org/10.3390/catal11111316
APA StyleIlieva, L., Petrova, P., Venezia, A. M., Anghel, E. M., State, R., Avdeev, G., & Tabakova, T. (2021). Mechanochemically Prepared Co3O4-CeO2 Catalysts for Complete Benzene Oxidation. Catalysts, 11(11), 1316. https://doi.org/10.3390/catal11111316








