Recent Advances in WS2 and Its Based Heterostructures for Water-Splitting Applications
Abstract
:1. Introduction
2. Fundamental Features of WS2 Nanostructures
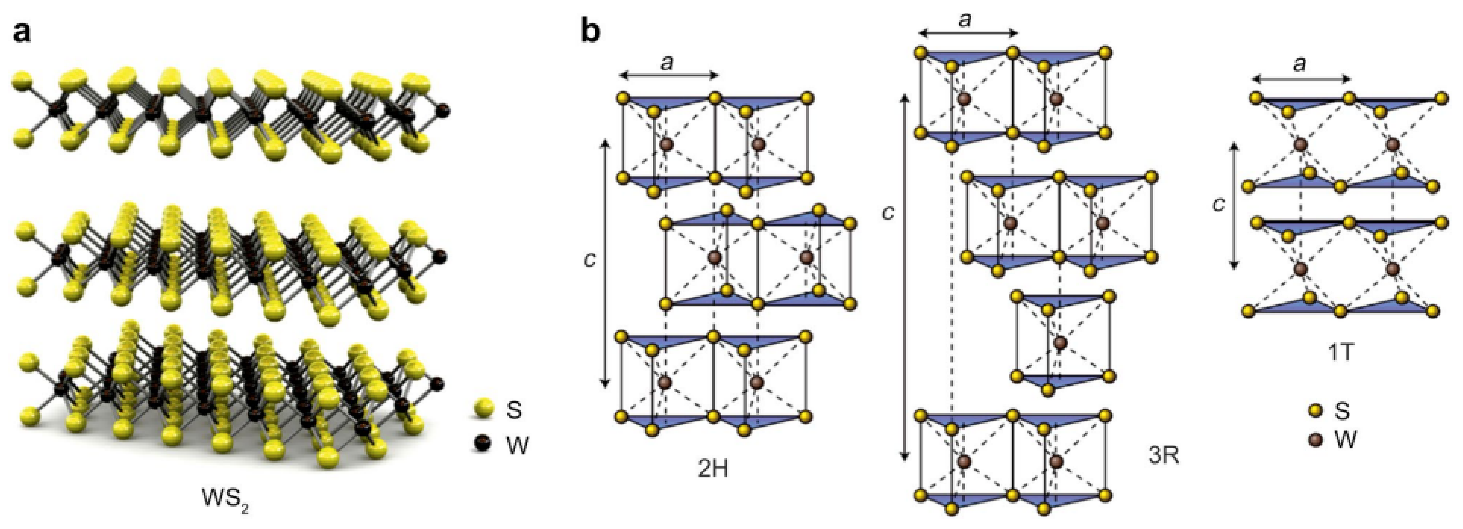
2.1. WS2 Crystallographic Structures
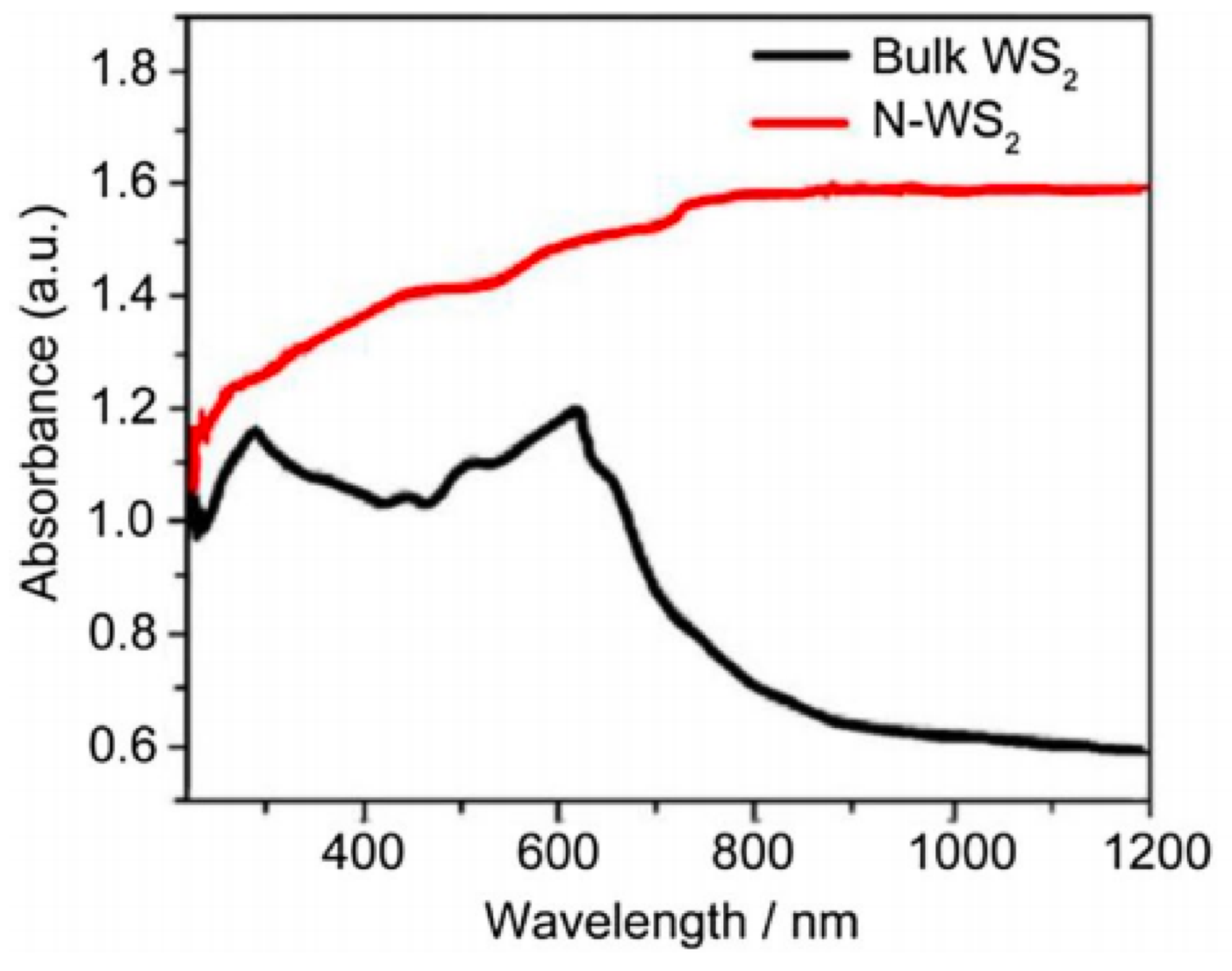
2.2. WS2 Electrical Properties
2.3. WS2 Electronic Band Structure
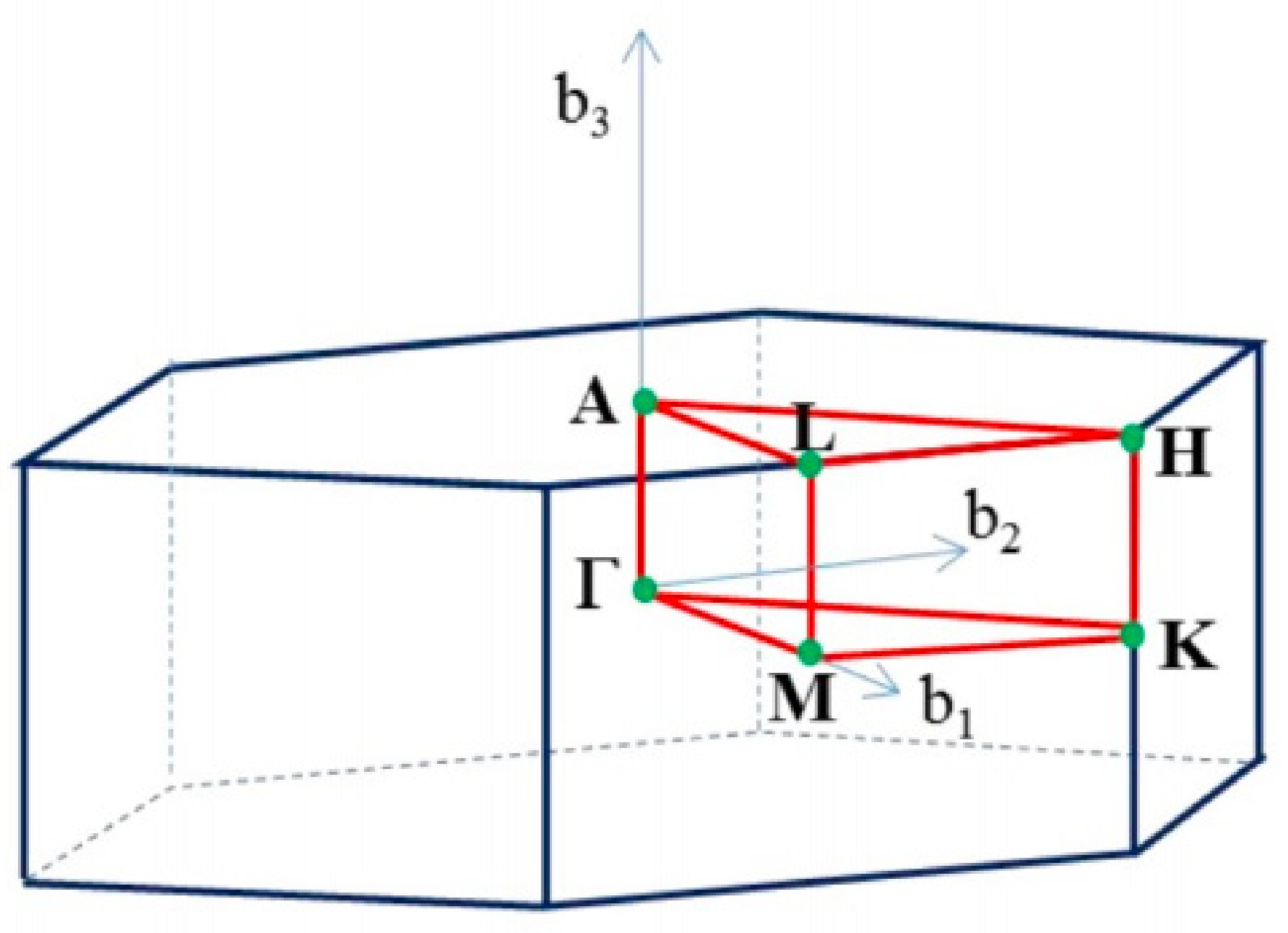
2.3.1. Brillouin Zone
2.3.2. Band Structure
3. Basic Principles of Water Splitting
3.1. Electrocatalytic Water Splitting
3.1.1. Electrocatalytic Mechanism of HER
Acid Electrolyte Mechanism
Alkaline Electrolyte Mechanism
3.1.2. Electrocatalytic Mechanism of OER
3.1.3. Parameters to Evaluate the Electrocatalytic Activity
Overpotential
Tafel Slope and Exchange Current Density
3.1.4. WS2 as an Electrocatalyst
3.2. Photocatalytic Water Splitting
3.2.1. Theory and Mechanism
3.2.2. Criteria for Efficient Photocatalyst
4. WS2 Synthesis Techniques
4.1. Mechanical Exfoliation
4.2. Chemical/Solvent Exfoliation
4.3. Chemical Vapor Deposition
4.4. Magnetron Sputtering
4.5. Hydrothermal Method
5. WS2-Based Heterostructures for Electrocatalytic Water Splitting
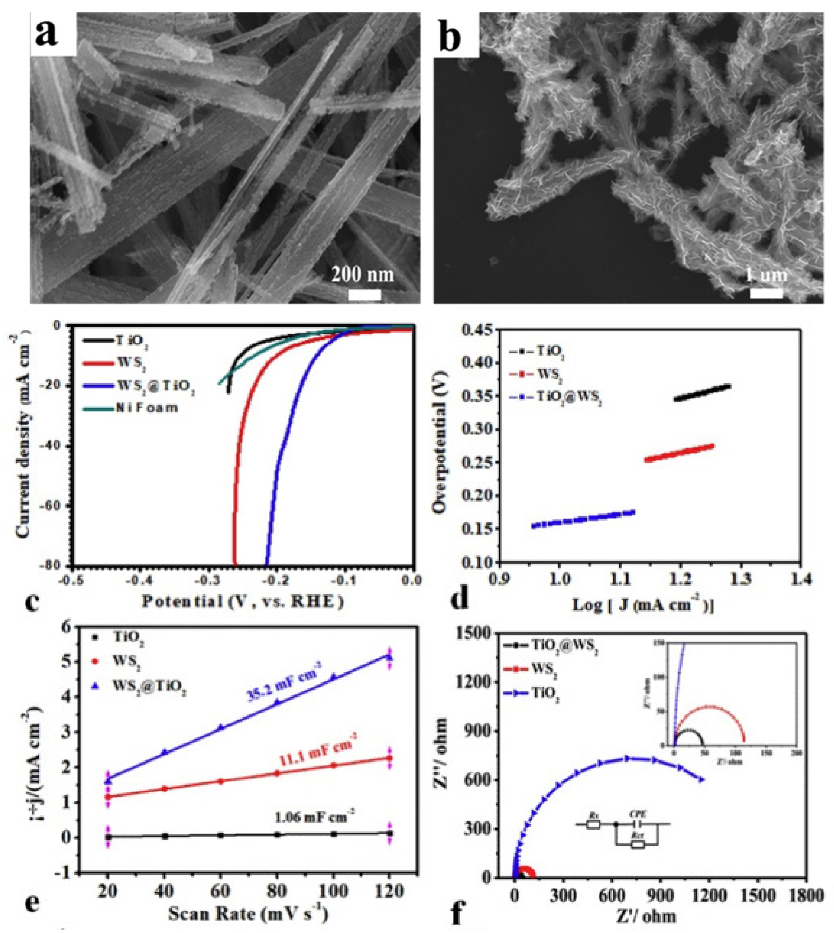
5.1. WS2-Metal Oxide Heterostructure
5.2. WS2-TMDs Heterostructures
5.3. WS2-Carbon-Based Material Heterostructures
6. WS2-Based Heterostructures for Photocatalytic Water Splitting
6.1. WS2-Metal Oxide Heterostructures
6.2. WS2-TMCs Heterostructures
6.3. WS2 Carbon-Based Material Heterostructures
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shanker, G.S.; Biswas, A.; Ogale, S. 2D materials and their heterostructures for photocatalytic water splitting and conversion of CO2 to value chemicals and fuels. J. Phys. Energy 2021, 3, 022003. [Google Scholar] [CrossRef]
- Rafiee, A.; Khalilpour, K.R.; Milani, D.; Panahi, M. Trends in CO2 conversion and utilization: A review from process systems perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K.M.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N. Review of fossil fuels and future energy technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3. [Google Scholar] [CrossRef]
- Konieczny, A.; Mondal, K.; Wiltowski, T.; Dydo, P. Catalyst development for thermocatalytic decomposition of methane to hydrogen. Int. J. Hydrog. Energy 2008, 1, 264–272. [Google Scholar] [CrossRef]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.-D.; Liu, S.; Teng, C.P.; Han, M.-Y. Recent Progress in Energy-Driven Water Splitting. Adv. Sci. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Tewary, V.K.; Zhang, Y. Modeling, Characterization and Production of Nanomaterials: Electronics, Photonics and Energy Applications; Woohead Publishing: Cambridge, UK, 2015; pp. 1–536. [Google Scholar] [CrossRef]
- Siahrostami, S.; Tsai, C.; Karamad, M.; Koitz, R.; García-Melchor, M.; Bajdich, M.; Vojvodic, A.; Abild-Pedersen, F.; Nørskov, J.K.; Studt, F. Two-Dimensional Materials as Catalysts for Energy Conversion. Catal. Lett. 2016, 146, 1917–1921. [Google Scholar] [CrossRef]
- Nam Trung, T.; Kamand, F.Z.; Al tahtamouni, T.M. Elucidating the mechanism for the chemical vapor deposition growth of vertical MoO2/MoS2 flakes toward photoelectrochemical applications. Appl. Surf. Sci. 2020, 505, 144551. [Google Scholar] [CrossRef]
- Shurbaji, S.; Huong, P.T.; Altahtamouni, T.M. Review on the Visible Light Photocatalysis for the Decomposition of Ciprofloxacin, Norfloxacin, Tetracyclines, and Sulfonamides Antibiotics in Wastewater. Catalysts 2021, 11, 437. [Google Scholar] [CrossRef]
- Li, H.; Jia, X.; Zhang, Q.; Wang, X. Metallic Transition-Metal Dichalcogenide Nanocatalysts for Energy Conversion. Chem 2018, 4, 1510–1537. [Google Scholar] [CrossRef] [Green Version]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef]
- Chia, X.; Eng, A.Y.S.; Ambrosi, A.; Tan, S.M.; Pumera, M. Electrochemistry of Nanostructured Layered Transition-Metal Dichalcogenides. Chem. Rev. 2015, 115, 11941–11966. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.-B.; Zhong, Y.-W.; Fu, W.-J.; Zhao, Z.-Q.; Liu, J.; Ding, J.; Han, X.-P.; Deng, Y.-D.; Hu, W.-B.; Zhong, C. Tungsten disulfide-based nanomaterials for energy conversion and storage. Tungsten 2020, 2, 109–133. [Google Scholar] [CrossRef]
- Xia, D.; Gong, F.; Pei, X.; Wang, W.; Li, H.; Zeng, W.; Wu, M.; Papavassiliou, D.V. Molybdenum and tungsten disulfides-based nanocomposite films for energy storage and conversion: A review. Chem. Eng. J. 2018, 348, 908–928. [Google Scholar] [CrossRef]
- Sang, Y.; Zhao, Z.; Zhao, M.; Hao, P.; Leng, Y.; Liu, H. From UV to Near-Infrared, WS2 Nanosheet: A Novel Photocatalyst for Full Solar Light Spectrum Photodegradation. Adv. Mater. 2015, 27, 363–369. [Google Scholar] [CrossRef]
- Wen, Y.; Xia, Y.; Zhang, S. Tungsten disulphide nanorattle: A new type of high performance electrocatalyst for hydrogen evolution reaction. J. Power Sources 2016, 307, 593–598. [Google Scholar] [CrossRef]
- Randall, J.N.; Luscombe, J.H.; Bate, R.T. Heterostructures and Quantum Devices; Elsevier: London, UK, 1994; Volume 24, ISBN 9780122341243. [Google Scholar]
- Nikam, R.D.; Lu, A.-Y.; Sonawane, P.A.; Kumar, U.R.; Yadav, K.; Li, L.-J.; Chen, Y.-T. Three-Dimensional Heterostructures of MoS2 Nanosheets on Conducting MoO2 as an Efficient Electrocatalyst To Enhance Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2015, 7, 23328–23335. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, W.; Hou, D.; Zhou, K.; Li, G.; Tang, Z.; Li, L.; Chen, S. Porous metallic MoO2-supported MoS2 nanosheets for enhanced electrocatalytic activity in the hydrogen evolution reaction. Nanoscale 2015, 7, 5203–5208. [Google Scholar] [CrossRef]
- Polyakov, A.Y.; Zak, A.; Tenne, R.; Goodilin, E.A.; Solntsev, K.A. Nanocomposites based on tubular and onion nanostructures of molybdenum and tungsten disulfides: Inorganic design, functional properties and applications. Russ. Chem. Rev. 2018, 87, 251–271. [Google Scholar] [CrossRef]
- Matte, H.S.; Gomathi, A.; Manna, A.K.; Late, D.J.; Datta, R.; Pati, S.K.; Rao, C.N.R. MoS2 and WS2 analogues of graphene. Angew. Chem. Int. Ed. 2010, 49, 4059–4062. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Chia, X.; Ambrosi, A.; Lazar, P.; Sofer, Z.; Pumera, M. Electrocatalysis of layered Group 5 metallic transition metal dichalcogenides (MX2, M = V, Nb, and Ta; X = S, Se, and Te). J. Mater. Chem. A 2016, 4, 14241–14253. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Z.; Yu, D.; Zhao, J.Z.; Su, Y.; Liu, Y.; Lin, Y.; Liu, W.; Xu, H.; Zhang, Z. Few-layer transition metal dichalcogenides (MoS2, WS2, and WSe2) for water splitting and degradation of organic pollutants: Understanding the piezocatalytic effect. Nano Energy 2019, 66, 104083. [Google Scholar] [CrossRef]
- Sokolikova, M.S.; Mattevi, C. Direct synthesis of metastable phases of 2D transition metal dichalcogenides. Chem. Soc. Rev. 2020, 49, 3952–3980. [Google Scholar] [CrossRef]
- Suzuki, R.; Sakano, M.; Zhang, Y.J.; Akashi, R.; Morikawa, D.; Harasawa, A.; Yaji, K.; Kuroda, K.; Miyamoto, K.; Okuda, T.; et al. Valley-dependent spin polarization in bulk MoS2 with broken inversion symmetry. Nat. Nanotechnol. 2014, 9, 611–617. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, D.; Allain, A.; Huang, Y.-S.; Dumcenco, D.; Kis, A. Electrical Transport Properties of Single-Layer WS2. ACS Nano 2014, 8, 8174–8181. [Google Scholar] [CrossRef] [PubMed]
- Jäger-Waldau, A.; Lux-Steiner, M.C.; Jäger-Waldau, G.; Bucher, E. WS2 thin films prepared by sulphurization. Appl. Surf. Sci. 1993, 70–71, 731–736. [Google Scholar] [CrossRef]
- Jo, S.; Ubrig, N.; Berger, H.; Kuzmenko, A.B.; Morpurgo, A.F. Mono- and Bilayer WS2 Light-Emitting Transistors. Nano Lett. 2014, 14, 2019–2025. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Ning, Z.; Liu, Y.; Xu, T.; Guo, Y.; Zak, A.; Zhang, Z.; Wang, S.; Tenne, R.; Chen, Q. Electrical transport properties of individual WS2 nanotubes and their dependence on water and oxygen absorption. Appl. Phys. Lett. 2012, 101, 113112. [Google Scholar] [CrossRef]
- Winslow, M.; Zhang, D.; Pandey, D.; Yap, R.K. Recent advancement on the optical properties of two-dimensional molybdenum disulfide (MoS2) thin films. Photonics 2015, 2, 288–307. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Bermel, P. Electronic and optical properties of ultra-thin 2D tungsten disulfide for photovoltaic applications. Sol. Energy Mater. Sol. Cells 2018, 174, 370–379. [Google Scholar] [CrossRef]
- Jiang, H. Electronic Band Structures of Molybdenum and Tungsten Dichalcogenides by the GW Approach. J. Phys. Chem. C 2012, 116, 7664–7671. [Google Scholar] [CrossRef]
- Kuc, A.; Zibouche, N.; Heine, T. Influence of quantum confinement on the electronic structure of the transition metal sulfide T S 2. Phys. Rev. B 2011, 83, 245213. [Google Scholar] [CrossRef] [Green Version]
- Johari, P.; Shenoy, V.B. Tuning the electronic properties of semiconducting transition metal dichalcogenides by applying mechanical strains. ACS Nano 2012, 6, 5449–5456. [Google Scholar] [CrossRef]
- Cao, S.; Liu, T.; Zeng, W.; Hussain, S.; Peng, X.; Pan, F. Synthesis and characterization of flower-like WS2 nanospheres via a facile hydrothermal route. J. Mater. Sci. Mater. Electron. 2014, 25, 4300–4305. [Google Scholar] [CrossRef]
- Seifert, G.; Terrones, H.; Terrones, M.; Jungnickel, G.; Frauenheim, T. On the electronic structure of WS2 nanotubes. Solid State Commun. 2000, 114, 245–248. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundmanentals and Applications; Wiley: New York, NY, USA, 2001; pp. 1–833. [Google Scholar]
- Danilovic, N.; Subbaraman, R.; Strmcnik, D.; Stamenkovic, V.R.; Markovic, N.M. Electrocatalysis of the HER in acid and alkaline media. J. Serbian Chem. Soc. 2013, 78, 2007–2015. [Google Scholar] [CrossRef]
- Quaino, P.; Juarez, F.; Santos, E.; Schmickler, W. Volcano plots in hydrogen electrocatalysis–Uses and abuses. Beilstein J. Nanotechnol. 2014, 5, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef] [Green Version]
- Takagi, Y.; Wang, H.; Uemura, Y.; Ikenaga, E.; Sekizawa, O.; Uruga, T.; Ohashi, H.; Senba, Y.; Yumoto, H.; Yamazaki, H.; et al. In situ study of an oxidation reaction on a Pt/C electrode by ambient pressure hard X-ray photoelectron spectroscopy. Appl. Phys. Lett. 2014, 105, 131602. [Google Scholar] [CrossRef]
- Casalongue, H.S.; Kaya, S.; Viswanathan, V.; Miller, D.J.; Friebel, D.; Hansen, H.A.; Nørskov, J.K.; Nilsson, A.; Ogasawara, H. Direct observation of the oxygenated species during oxygen reduction on a platinum fuel cell cathode. Nat. Commun. 2013, 4, 2817. [Google Scholar] [CrossRef]
- Guo, Y.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J.; et al. Nanoarchitectonics for Transition-Metal-Sulfide-Based Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, 1807134. [Google Scholar] [CrossRef]
- Loayza, J.M.; Bard, A.J.; Faulkner, L.R. Available online: https://www.academia.edu/15060550/Allen_J_Bard_Larry_R_Faulkner (accessed on 12 August 2021).
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the Hydrogen Evolution Reaction (HER) with Molybdenum Sulfide Nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Luo, J.; Im, J.-H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.-G.; Tilley, S.D.; Fan, H.J.; Grätzel, M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef]
- Yu, P.; Wang, F.; Shifa, T.A.; Zhan, X.; Lou, X.; Xia, F.; He, J. Earth abundant materials beyond transition metal dichalcogenides: A focus on electrocatalyzing hydrogen evolution reaction. Nano Energy 2019, 58, 244–276. [Google Scholar] [CrossRef]
- Hamann, C.H.; Hamnett, A.; Vielstich, W. Electrochemistry; Wiley-VCH: Hoboken, NJ, USA, 2007; ISBN 978-3527310692. Available online: https://www.wiley.com/en-us/9783527310692 (accessed on 12 August 2016).
- Yu, Q.; Luo, Y.; Mahmood, A.; Liu, B.; Cheng, H.-M. Engineering Two-Dimensional Materials and Their Heterostructures as High-Performance Electrocatalysts. Electrochem. Energy Rev. 2019, 2, 373–394. [Google Scholar] [CrossRef]
- Parsons, R. The rate of electrolytic hydrogen evolution and the heat of adsorption of hydrogen. Trans. Faraday Soc. 1958, 54, 1053–1063. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem. Int. Ed. 2015, 54, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2021, 152, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Shi, Y.; Chiu, M.H.; Li, L.J. Emerging energy applications of two-dimensional layered transition metal dichalcogenides. Nano Energy 2015, 18, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.S.; Sai, N.; Lu, P.; Coker, E.N.; Liu, S.; Artyushkova, K.; Luk, T.S.; Kaehr, B.; Brinker, C.J. Understanding catalysis in a multiphasic two-dimensional transition metal dichalcogenide. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Qu, J.; Li, J. Two-dimensional layered MoS2: Rational design, properties and electrochemical applications. Energy Environ. Sci. 2016, 9, 1190–1209. [Google Scholar] [CrossRef]
- Li, H.; Tsai, C.; Koh, A.L.; Cai, L.; Contryman, A.W.; Fragapane, A.H.; Zhao, J.; Han, H.S.; Manoharan, H.C.; Abild-Pedersen, F.; et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 2016, 15, 48–53. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Chen, Z.; Reinecke, B.N.; Jaramillo, T. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Rossmeisl, J.; Christensen, C.H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46. [Google Scholar] [CrossRef]
- Tsai, C.; Chan, K.; Abild-Pedersen, F.; Nørskov, J.K. Active edge sites in MoSe2 and WSe2 catalysts for the hydrogen evolution reaction: A density functional study. Phys. Chem. Chem. Phys. 2014, 16, 13156–13164. [Google Scholar] [CrossRef]
- Woods, J.M.; Jung, Y.; Xie, Y.; Liu, W.; Liu, Y.; Wang, H.; Cha, J.J. One-Step Synthesis of MoS2/WS2 Layered Heterostructures and Catalytic Activity of Defective Transition Metal Dichalcogenide Films. ACS Nano 2016, 10, 2004–2009. [Google Scholar] [CrossRef]
- Wu, L.; van Hoof, A.J.F.; Dzade, N.Y.; Gao, L.; Richard, M.-I.; Friedrich, H.; De Leeuw, N.H.; Hensen, E.J.M.; Hofmann, J.P. Enhancing the electrocatalytic activity of 2H-WS2 for hydrogen evolution via defect engineering. Phys. Chem. Chem. Phys. 2019, 21, 6071–6079. [Google Scholar] [CrossRef] [Green Version]
- Alsabban, M.M.; Min, S.; Hedhili, M.N.; Ming, J.; Li, L.-J.; Huang, K.-W. Editors’ Choice—Growth of Layered WS2 Electrocatalysts for Highly Efficient Hydrogen Production Reaction. ECS J. Solid State Sci. Technol. 2016, 5, Q3067–Q3071. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, S.; Liang, H.; Dong, R.; Feng, X. Hierarchical Transition-Metal Dichalcogenide Nanosheets for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2015, 27, 7426–7431. [Google Scholar] [CrossRef]
- Yang, Y.; Fei, H.; Ruan, G.; Li, Y.; Tour, J.M. Vertically Aligned WS2 Nanosheets for Water Splitting. Adv. Funct. Mater. 2015, 25, 6199–6204. [Google Scholar] [CrossRef]
- Balasubramanyam, S.; Shirazi, M.; Bloodgood, M.A.; Wu, L.; Verheijen, M.A.; Vandalon, V.; Kessels, W.M.M.; Hofmann, J.P.; Bol, A.A. Edge-Site Nanoengineering of WS2 by Low-Temperature Plasma-Enhanced Atomic Layer Deposition for Electrocatalytic Hydrogen Evolution. Chem. Mater. 2019, 31, 5104–5115. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Yang, Z.; Zhang, Q.; Zhang, Y.; Cai, W.; Cheng, H. A self-template synthesis of defect-rich WS2 as a highly efficient electrocatalyst for the hydrogen evolution reaction. Chem. Commun. 2018, 54, 2631–2634. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Wang, G.; Zakhidov, D.; Larios, E.; Jose Yacaman, M.; Tour, J.M.; Lin, J.; Tour, J.M.; Wang, G.; et al. Enhanced Electrocatalysis for Hydrogen Evolution Reactions from WS2 Nanoribbons. Adv. Energy Mater. 2014. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, G.; Luo, H.; Zhang, Q.; Wang, J.; Zhao, C.; Rao, A.M.; Xu, B.; Lu, B. Enhancing catalytic activity of tungsten disulfide through topology. Appl. Catal. B Environ. 2019, 256, 117802. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, W.; Cheng, H.; Lu, Z.; Pan, H. WS2 Nanosheets with Highly-Enhanced Electrochemical Activity by Facile Control of Sulfur Vacancies. ChemCatChem 2019, 11, 2667–2675. [Google Scholar] [CrossRef]
- He, Q.; Wang, L.; Yin, K.; Luo, S. Vertically Aligned Ultrathin 1T-WS2 Nanosheets Enhanced the Electrocatalytic Hydrogen Evolution. Nanoscale Res. Lett. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Wu, H.; Tan, H.L.; Toe, C.Y.; Scott, J.; Wang, L.; Amal, R.; Ng, Y.H. Photocatalytic and Photoelectrochemical Systems: Similarities and Differences. Adv. Mater. 2020, 32, 1904717. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.-L.; Sa, B.; Ahuja, R. Review of two-dimensional materials for photocatalytic water splitting from a theoretical perspective. Catal. Sci. Technol. 2017, 7, 545–559. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.Y.; Jung, H.S.; Kang, Y.T. A review: Effect of nanostructures on photocatalytic CO2 conversion over metal oxides and compound semiconductors. J. CO2 Util. 2017, 20, 163–177. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Xiao, Z.; Zhou, Y.; Chen, H.; Khalil, A.; Xiang, T.; Xu, J.; Chu, W.; Wu, X.; et al. Stable Metallic 1T-WS2 Nanoribbons Intercalated with Ammonia Ions: The Correlation between Structure and Electrical/Optical Properties. Adv. Mater. 2015, 27, 4837–4844. [Google Scholar] [CrossRef]
- Cao, S.; Liu, T.; Hussain, S.; Zeng, W.; Pan, F.; Peng, X. Synthesis and characterization of novel chrysanthemum-like tungsten disulfide (WS2) nanostructure: Structure, growth and optical absorption property. J. Mater. Sci. Mater. Electron. 2015, 26, 809–814. [Google Scholar] [CrossRef]
- Notley, S.M. High yield production of photoluminescent tungsten disulphide nanoparticles. J. Colloid Interface Sci. 2013, 396, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Piskunov, S.; Lisovski, O.; Zhukovskii, Y.F.; D’yachkov, P.N.; Evarestov, R.A.; Kenmoe, S.; Spohr, E. First-Principles Evaluation of the Morphology of WS2 Nanotubes for Application as Visible-Light-Driven Water-Splitting Photocatalysts. ACS Omega 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adigilli, H.K.; Pandey, A.K.; Joardar, J. 2D-Nanolayered Tungsten and Molybdenum Disulfides: Structure, Properties, Synthesis, and Processing for Strategic Applications. In Handbook of Advanced Ceramics and Composites; Springer: Cham, Switzerland, 2020; pp. 1–47. [Google Scholar]
- Zhu, Y.Q.; Sekine, T.; Li, Y.H.; Wang, W.X.; Fay, M.W.; Edwards, H.; Brown, P.D.; Fleischer, N.; Tenne, R. WS2 and MoS2 Inorganic Fullerenes—Super Shock Absorbers at Very High Pressures. Adv. Mater. 2005, 17, 1500–1503. [Google Scholar] [CrossRef]
- Rapoport, L.; Fleischer, N.; Tenne, R. Fullerene-like WS2 Nanoparticles: Superior Lubricants for Harsh Conditions. Adv. Mater. 2003, 15, 651–655. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhang, J.; Wu, J.; Xu, H.; Wen, X.; Zhang, X.; Tiwary, C.S.; Yang, W.; Vajtai, R.; et al. Cryo-mediated exfoliation and fracturing of layered materials into 2D quantum dots. Sci. Adv. 2017, 3, e1701500. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Yoo, D.; Ahn, M.; Miró, P.; Heine, T.; Cheon, J. Tandem intercalation strategy for single-layer nanosheets as an effective alternative to conventional exfoliation processes. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ejigu, A.; Kinloch, I.A.; Prestat, E.; Dryfe, R.A.W. A simple electrochemical route to metallic phase trilayer MoS2: Evaluation as electrocatalysts and supercapacitors. J. Mater. Chem. A 2017, 5, 11316–11330. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-Layer Semiconducting Nanosheets: High-Yield Preparation and Device Fabrication. Angew. Chem. Int. Ed. 2011, 50, 11093–11097. [Google Scholar] [CrossRef]
- Paradisanos, I.; Pliatsikas, N.; Patsalas, P.; Fotakis, C.; Kymakis, E.; Kioseoglou, G.; Stratakis, E. Spatial non-uniformity in exfoliated WS2 single layers. Nanoscale 2016, 8, 16197–16203. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lo, T.W.; Sun, J.; Yip, C.T.; Lam, C.H.; Lei, D.Y. A comprehensive comparison study on the vibrational and optical properties of CVD-grown and mechanically exfoliated few-layered WS2. J. Mater. Chem. C 2017, 5, 11239–11245. [Google Scholar] [CrossRef]
- Dai, Y.; Wei, L.-L.; Chen, M.; Wang, J.-J.; Ren, J.; Wang, Q.; Wu, Y.-Z.; Wang, Y.-P.; Cheng, X.-N.; Yan, X.-H. Liquid Exfoliation and Electrochemical Properties of WS2 Nanosheets. J. Nanosci. Nanotechnol. 2017, 18, 3165–3170. [Google Scholar] [CrossRef]
- Lin, H.; Wang, J.; Luo, Q.; Peng, H.; Luo, C.; Qi, R.; Huang, R.; Travas-Sejdic, J.; Duan, C.G. Rapid and highly efficient chemical exfoliation of layered MoS2 and WS2. J. Alloys Compd. 2017, 699, 222–229. [Google Scholar] [CrossRef]
- Desai, J.A.; Adhikari, N.; Kaul, A.B. Chemical exfoliation efficacy of semiconducting WS2 and its use in an additively manufactured heterostructure graphene–WS2–graphene photodiode. RSC Adv. 2019, 9, 25805–25816. [Google Scholar] [CrossRef] [Green Version]
- Adilbekova, B.; Lin, Y.; Yengel, E.; Faber, H.; Harrison, G.; Firdaus, Y.; El-Labban, A.; Anjum, D.H.; Tung, V.; Anthopoulos, T.D. Liquid phase exfoliation of MoS2 and WS2 in aqueous ammonia and their application in highly efficient organic solar cells. J. Mater. Chem. C 2020, 8, 5259–5264. [Google Scholar] [CrossRef] [Green Version]
- Mayorga-Martinez, C.C.; Ambrosi, A.; Eng, A.Y.S.; Sofer, Z.; Pumera, M. Transition metal dichalcogenides (MoS2, MoSe2, WS2 and WSe2) exfoliation technique has strong influence upon their capacitance. Electrochem. Commun. 2015, 56, 24–28. [Google Scholar] [CrossRef]
- Xu, D.; Xu, P.; Zhu, Y.; Peng, W.; Li, Y.; Zhang, G.; Zhang, F.; Mallouk, T.E.; Fan, X. High Yield Exfoliation of WS2 Crystals into 1–2 Layer Semiconducting Nanosheets and Efficient Photocatalytic Hydrogen Evolution from WS2/CdS Nanorod Composites. ACS Appl. Mater. Interfaces 2018, 10, 2810–2818. [Google Scholar] [CrossRef]
- Tian, L.; Qiao, H.; Huang, Z.; Qi, X. Li-Ion Intercalated Exfoliated WS2 Nanosheets with Enhanced Electrocatalytic Hydrogen Evolution Performance. Cryst. Res. Technol. 2021, 56, 2000165. [Google Scholar] [CrossRef]
- Valappil, M.O.; Anil, A.; Shaijumon, M.; Pillai, V.K.; Alwarappan, S. A Single-Step Electrochemical Synthesis of Luminescent WS2 Quantum Dots. Chem. A Eur. J. 2017, 23, 9144–9148. [Google Scholar] [CrossRef]
- Subramanian, N.; Priya, K.M.; Valappil, M.O.; Nesakumar, N.; Kesavan, S.; Alwarappan, S. Electrochemically Exfoliated Porous WS2 Nanosheets: A Potential Electrochemical Sensing Platform for Chlorpromazine Detection. J. Electrochem. Soc. 2019, 166, B749. [Google Scholar] [CrossRef]
- Leong, S.X.; Mayorga-Martinez, C.C.; Chia, X.; Luxa, J.; Sofer, Z.; Pumera, M. 2H → 1T Phase Change in Direct Synthesis of WS2 Nanosheets via Solution-Based Electrochemical Exfoliation and Their Catalytic Properties. ACS Appl. Mater. Interfaces 2017, 9, 26350–26356. [Google Scholar] [CrossRef]
- Yu, J.; Hu, X.; Li, H.; Zhou, X.; Zhai, T. Large-scale synthesis of 2D metal dichalcogenides. J. Mater. Chem. C 2018, 6, 4627–4640. [Google Scholar] [CrossRef]
- Huang, L.; Yu, Y.; Li, C.; Cao, L. Substrate Mediation in Vapor Deposition Growth of Layered Chalcogenide Nanoplates: A Case Study of SnSe2. J. Phys. Chem. C 2013, 117, 6469–6475. [Google Scholar] [CrossRef]
- Cain, J.D.; Shi, F.; Wu, J.; Dravid, V.P. Growth Mechanism of Transition Metal Dichalcogenide Monolayers: The Role of Self-Seeding Fullerene Nuclei. ACS Nano 2016, 10, 5440–5445. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Jung, J.-W.; Baik, J.; Kang, J.-W.; Park, I.-K.; Bae, T.-S.; Chung, H.-S.; Cho, C.-H. Surface-diffusion-limited growth of atomically thin WS2 crystals from core–shell nuclei. Nanoscale 2019, 11, 8706–8714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Liu, Z.; Sun, D.-M.; Huang, L.; Ma, L.-P.; Yin, L.-C.; Ma, T.; Zhang, Z.; Ma, X.-L.; Peng, L.-M.; et al. Large-area synthesis of high-quality and uniform monolayer WS2 on reusable Au foils. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Aljarb, A.; Cao, Z.; Tang, H.-L.; Huang, J.-K.; Li, M.; Hu, W.; Cavallo, L.; Li, L.-J. Substrate Lattice-Guided Seed Formation Controls the Orientation of 2D Transition-Metal Dichalcogenides. ACS Nano 2017, 11, 9215–9222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.; Xie, S.; Huang, L.; Han, Y.; Huang, P.; Mak, K.F.; Kim, C.-J.; Muller, D.; Park, J. High-mobility three-atom-thick semiconducting films with wafer-scale homogeneity. Nature 2015, 520, 656–660. [Google Scholar] [CrossRef]
- Park, J.; Lee, W.; Choi, T.; Hwang, S.-H.; Myoung, J.M.; Jung, J.-H.; Kim, S.-H.; Kim, H. Layer-modulated synthesis of uniform tungsten disulfide nanosheet using gas-phase precursors. Nanoscale 2015, 7, 1308–1313. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Ji, Q.; Ju, J.; Yuan, H.; Shi, J.; Gao, T.; Ma, D.; Liu, M.; Liu, Z.; et al. Controlled growth of high-quality monolayer WS2 layers on sapphire and imaging its grain boundary. ACS Nano 2013, 7, 8963–8971. [Google Scholar] [CrossRef]
- Yun, S.J.; Chae, S.H.; Kim, H.; Park, J.C.; Park, J.H.; Han, G.H.; Lee, J.S.; Kim, S.M.; Oh, H.M.; Lee, Y.H.; et al. Synthesis of centimeter-scale monolayer tungsten disulfide film on gold foils. ACS Nano 2015, 9, 5510–5519. [Google Scholar] [CrossRef]
- Yang, P.; Zou, X.; Zhang, Z.; Hong, M.; Shi, J.; Chen, S.; Shu, J.; Zhao, L.; Jiang, S.; Zhou, X.; et al. Batch production of 6-inch uniform monolayer molybdenum disulfide catalyzed by sodium in glass. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Lan, C.; Li, C.; Ho, J.C.; Liu, Y. 2D WS2: From Vapor Phase Synthesis to Device Applications. Adv. Electron. Mater. 2021, 7, 2000688. [Google Scholar] [CrossRef]
- Kosku Perkgöz, N. CVD growth and characterization of 2D transition metal dichalcogenides, MoS2 and WS2. ANADOLU Univ. J. Sci. Technol. A Appl. Sci. Eng. 2017, 18, 375–387. [Google Scholar] [CrossRef]
- Xu, Z.; Lv, Y.; Li, J.; Huang, F.; Nie, P.; Zhang, S.; Zhao, S.; Zhao, S.; Wei, G. CVD controlled growth of large-scale WS2 monolayers. RSC Adv. 2019, 9, 29628–29635. [Google Scholar] [CrossRef] [Green Version]
- Okada, M.; Okada, N.; Chang, W.-H.; Endo, T.; Ando, A.; Shimizu, T.; Kubo, T.; Miyata, Y.; Irisawa, T. Gas-Source CVD Growth of Atomic Layered WS2 from WF6 and H2S Precursors with High Grain Size Uniformity. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Ahmadi, A.; Shoushtari, M.Z.; Farbod, M. Photoelectrochemical application of WS2 nanosheets prepared via a low-temperature CVD method. J. Mater. Sci. Mater. Electron. 2019, 30, 6342–6349. [Google Scholar] [CrossRef]
- Shi, B.; Zhou, D.; Qiu, R.; Bahri, M.; Kong, X.; Zhao, H.; Tlili, C.; Wang, D. High-efficiency synthesis of large-area monolayer WS2 crystals on SiO2/Si substrate via NaCl-assisted atmospheric pressure chemical vapor deposition. Appl. Surf. Sci. 2020, 533, 147479. [Google Scholar] [CrossRef]
- Chen, Y. Growth of a Large, Single-Crystalline WS2 Monolayer for High-Performance Photodetectors by Chemical Vapor Deposition. Micromachines 2021, 12, 137. [Google Scholar] [CrossRef]
- Ji, H.G.; Solís-Fernández, P.; Erkılıç, U.; Ago, H. Stacking Orientation-Dependent Photoluminescence Pathways in Artificially Stacked Bilayer WS2 Nanosheets Grown by Chemical Vapor Deposition: Implications for Spintronics and Valleytronics. ACS Appl. Nano Mater. 2021, 4, 3717–3724. [Google Scholar] [CrossRef]
- Guo, H.; Lan, C.; Zhou, Z.; Sun, P.; Wei, D.; Li, C. Transparent, flexible, and stretchable WS2 based humidity sensors for electronic skin. Nanoscale 2017, 9, 6246–6253. [Google Scholar] [CrossRef]
- Jung, Y.; Shen, J.; Liu, Y.; Woods, J.M.; Sun, Y.; Cha, J.J. Metal seed layer thickness-induced transition from vertical to horizontal growth of MoS2 and WS2. Nano Lett. 2014, 14, 6842–6849. [Google Scholar] [CrossRef]
- Orofeo, C.M.; Suzuki, S.; Sekine, Y.; Hibino, H. Scalable synthesis of layer-controlled WS2 and MoS2 sheets by sulfurization of thin metal films. Appl. Phys. Lett. 2014, 105, 083112. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, M.F.; Shehzad, M.A.; Vikraman, D.; Iqbal, M.Z.; Choi, D.-C.; Song, W.; An, K.-S.; Seo, Y.; Eom, J.; et al. Layer-modulated, wafer scale and continuous ultra-thin WS2 films grown by RF sputtering via post-deposition annealing. J. Mater. Chem. C 2016, 4, 7846–7852. [Google Scholar] [CrossRef]
- Khan Sobayel Bin Rafiq, M.; Alharbi, H.; Luqman, M.; Ayob, A.; Alharthi, Y.S.; Alharthi, N.H.; Bais, B.; Akhtaruzzaman, M. WS2: A new Window Layer Material for Solar cell Application. Sci. Rep. 2020. [Google Scholar] [CrossRef]
- Morey, G.W.; Niggli, P. The Hydrothermal Formation of Silicates, A Review. J. Am. Chem. Soc. 2003, 35, 1086–1130. [Google Scholar] [CrossRef]
- Dubois, T.; Demazeau, G. Preparation of Fe3O4 fine particles through a solvothermal process. Mater. Lett. 1994, 19, 38–47. [Google Scholar] [CrossRef]
- Zhan, J.H.; Zhang, Z.D.; Qian, X.F.; Wang, C.; Xie, Y.; Qian, Y.T. Solvothermal Synthesis of Nanocrystalline MoS2 from MoO3 and Elemental Sulfur. J. Solid State Chem. 1998, 141, 270–273. [Google Scholar] [CrossRef]
- Ratha, S.; Rout, C.S. Supercapacitor electrodes based on layered tungsten disulfide-reduced graphene oxide hybrids synthesized by a facile hydrothermal method. ACS Appl. Mater. Interfaces 2013, 5, 11427–11433. [Google Scholar] [CrossRef]
- Cao, S.; Liu, T.; Hussain, S.; Zeng, W.; Peng, X.; Pan, F. Hydrothermal synthesis of variety low dimensional WS2 nanostructures. Mater. Lett. 2014, 129, 205–208. [Google Scholar] [CrossRef]
- Dang, H.; Chen, L.; Chen, L.; Yuan, M.; Yan, Z.; Li, M. Hydrothermal synthesis of 1T-WS2 nanosheets with excellent adsorption performance for dye removal from wastewater. Mater. Lett. 2019, 254, 42–45. [Google Scholar] [CrossRef]
- Ding, W.; Hu, L.; Dai, J.; Tang, X.; Wei, R.; Sheng, Z.; Liang, C.; Shao, D.; Song, W.; Liu, Q.; et al. Highly Ambient-Stable 1T-MoS2 and 1T-WS2 by Hydrothermal Synthesis under High Magnetic Fields. ACS Nano 2019, 13, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Mani, N.P.; Cyriac, J. Hydrothermal synthesis of WS2 quantum dots and their application as a fluorescence sensor for the selective detection of 2,4,6-trinitrophenol. New J. Chem. 2020, 44, 10840–10848. [Google Scholar] [CrossRef]
- Sengupta, S.; Kundu, M. Carbon Free Nanostructured Plate like WS2 with Excellent Lithium Storage Properties. ChemistrySelect 2020, 5, 14183–14189. [Google Scholar] [CrossRef]
- Meng, F.; Zhu, T.; Yuan, Z.; Qin, W.; Gao, H.; Zhang, H. Investigation of Mixed-Phase WS2Nanomaterials for Ammonia Gas Sensing. IEEE Sens. J. 2021, 21, 7268–7274. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Han, C.; Xing, Z.; Yang, X. When NiO@Ni Meets WS2 Nanosheet Array: A Highly Efficient and Ultrastable Electrocatalyst for Overall Water Splitting. ACS Cent. Sci. 2017, 4, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Xu, Y.; Chanda, D.; Tan, L.; Xing, R.; Li, X.; Mao, L.; Kazuya, N.; Fujishima, A. Ultrathin WS2 nanosheets vertically aligned on TiO2 nanobelts as efficient alkaline hydrogen evolution electrocatalyst. Int. J. Hydrog. Energy 2020, 45, 1697–1705. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, X.; Xiong, S.; Wu, X.; Shan, Y.; Chu, P.K. Synergistic WO3·2H2O Nanoplates/WS2 Hybrid Catalysts for High-Efficiency Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 13966–13972. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Akbar, K.; Truong, L.; Kathalingam, A.; Chun, S.-H.; Jung, J.; Park, H.J.; Kim, H.-S. Improved Hydrogen Evolution Reaction Performance using MoS2–WS2 Heterostructures by Physicochemical Process. ACS Sustain. Chem. Eng. 2018, 6, 8400–8409. [Google Scholar] [CrossRef]
- Hussain, S.; Akbar, K.; Vikraman, D.; Liu, H.; Chun, S.H.; Jung, J. WS2/CoSe2 heterostructure: A designed structure as catalysts for enhanced hydrogen evolution performance. J. Ind. Eng. Chem. 2018, 65, 167–174. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Patil, S.A.; Truong, L.; Arbab, A.A.; Jeong, S.H.; Chun, S.-H.; Jung, J.; Kim, H.-S. Engineering MoSe2/WS2 Hybrids to Replace the Scarce Platinum Electrode for Hydrogen Evolution Reactions and Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 5061–5072. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, F.; Sun, J.; He, R.; Wang, Y.; Guo, C.F.; Wang, F.; Lan, Y.; Ren, Z.; Chen, S. Highly active and durable self-standing WS2/graphene hybrid catalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 9472–9476. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Kim, D.; Kim, Y.; Lee, H. Bifunctional Oxygen Electrocatalysis through Chemical Bonding of Transition Metal Chalcogenides on Conductive Carbons. Adv. Energy Mater. 2017, 7, 1602217. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, R.; Wu, Q.; Li, W.; Shen, C.; Ni, L.; Yan, H.; Diao, G.; Chen, M. Ultrathin WS2 nanosheets vertically embedded in a hollow mesoporous carbon framework – a triple-shell structure with enhanced lithium storage and electrocatalytic properties. J. Mater. Chem. A 2018, 6, 19004–19012. [Google Scholar] [CrossRef]
- Ma, S.; Zeng, L.; Tao, L.; Tang, C.Y.; Yuan, H.; Long, H.; Cheng, P.K.; Chai, Y.; Chen, C.; Fung, K.H.; et al. Enhanced Photocatalytic Activity of WS2 Film by Laser Drilling to Produce Porous WS2/WO3 Heterostructure. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayebi, M.; Masoumi, Z.; Lee, B.K. Ultrasonically prepared photocatalyst of W/WO3 nanoplates with WS2 nanosheets as 2D material for improving photoelectrochemical water splitting. Ultrason. Sonochem. 2021, 70, 105339. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Devarayapalli, K.C.; Nagajyothi, P.C.; Shim, J. Binder-free WS2/ZrO2 hybrid as a photocatalyst for organic pollutant degradation under UV/simulated sunlight and tests for H2 evolution. J. Alloys Compd. 2019, 809, 151805. [Google Scholar] [CrossRef]
- Reddy, D.A.; Park, H.; Ma, R.; Kumar, D.P.; Lim, M.; Kim, T.K. Heterostructured WS2-MoS2 Ultrathin Nanosheets Integrated on CdS Nanorods to Promote Charge Separation and Migration and Improve Solar-Driven Photocatalytic Hydrogen Evolution. ChemSusChem 2017, 10, 1563–1570. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, R.; Li, X.; Sun, X. Enhanced photocatalytic activity for hydrogen evolution from water by Zn0.5Cd0.5S/WS2 heterostructure. Mater. Sci. Semicond. Process. 2017, 59, 68–75. [Google Scholar] [CrossRef]
- Lai, G.J.; Lyu, L.M.; Huang, Y.S.; Lee, G.C.; Lu, M.P.; Perng, T.P.; Lu, M.Y.; Chen, L.J. Few-layer WS2–MoS2 in-plane heterostructures for efficient photocatalytic hydrogen evolution. Nano Energy 2021, 81, 105608. [Google Scholar] [CrossRef]
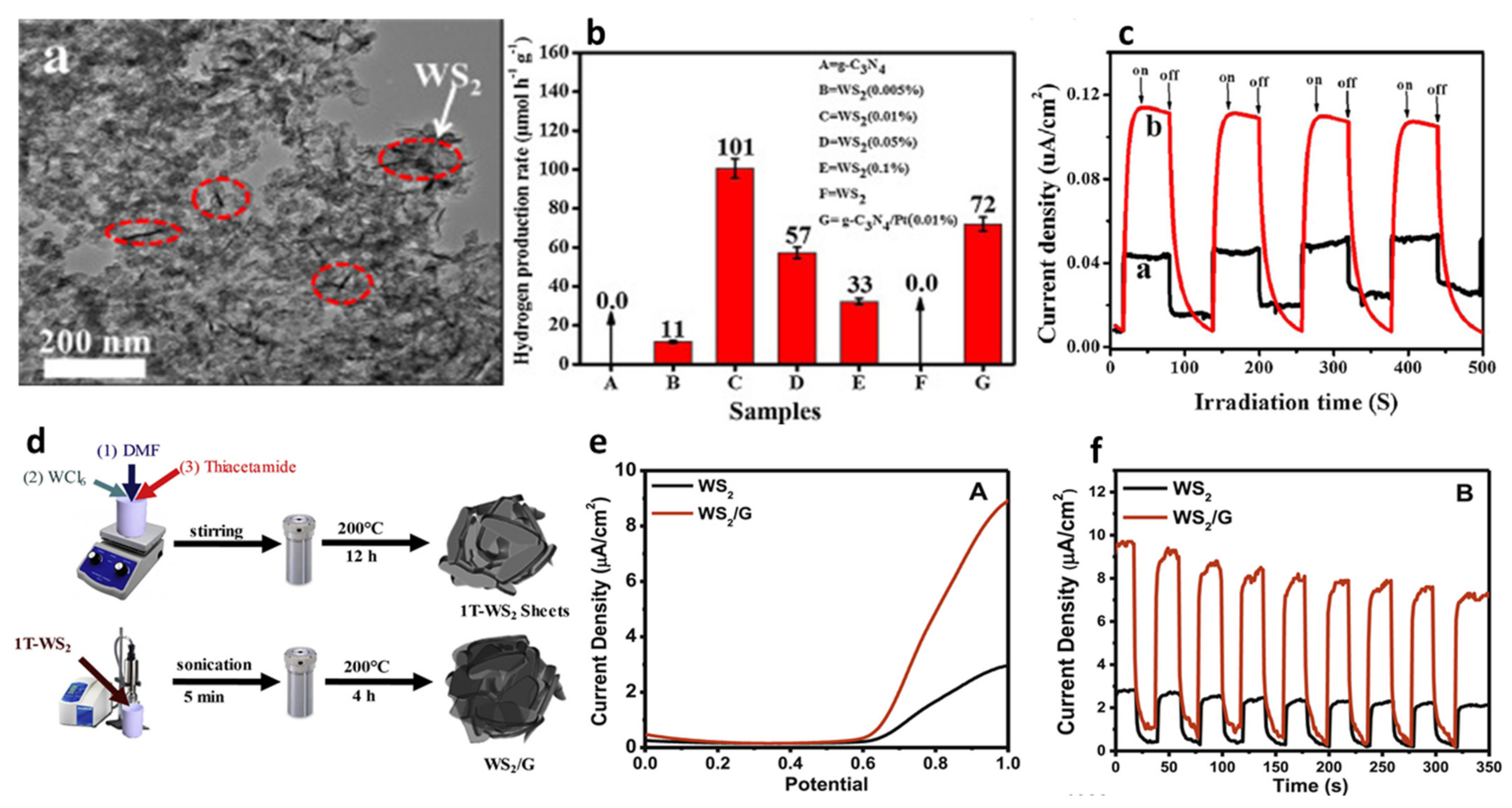
- Akple, M.S.; Low, J.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Zhang, J. Enhanced visible light photocatalytic H2-production of g-C3N4/WS2 composite heterostructures. Appl. Surf. Sci. 2015, 358, 196–203. [Google Scholar] [CrossRef]
- Khan, A.; Khan, I.; Khan, M.Y.; Dafallah, H.; Qurashi, A. Facile synthesis of 1T-WS2/graphite nanocomposite for efficient solar-driven oxygen evolution reaction. Int. J. Hydrog. Energy 2020, 45, 24045–24053. [Google Scholar] [CrossRef]


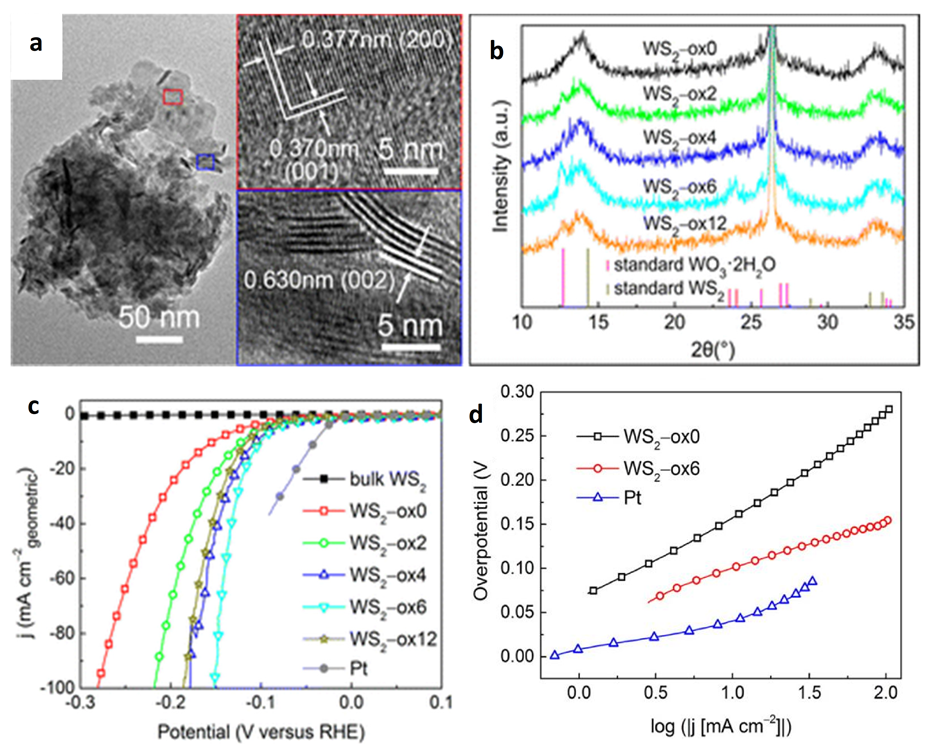
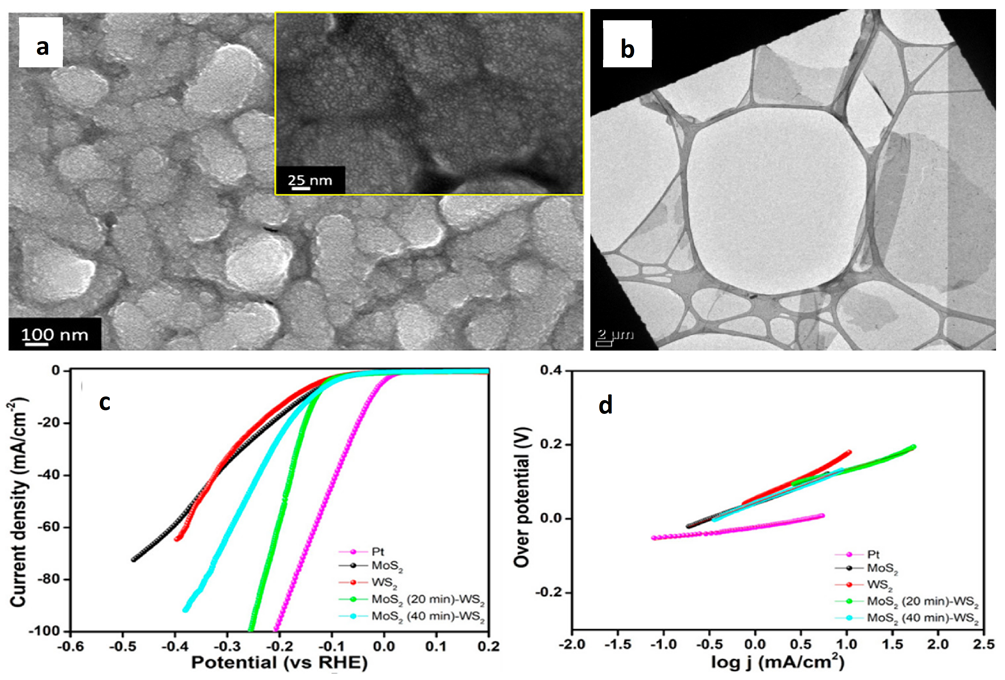
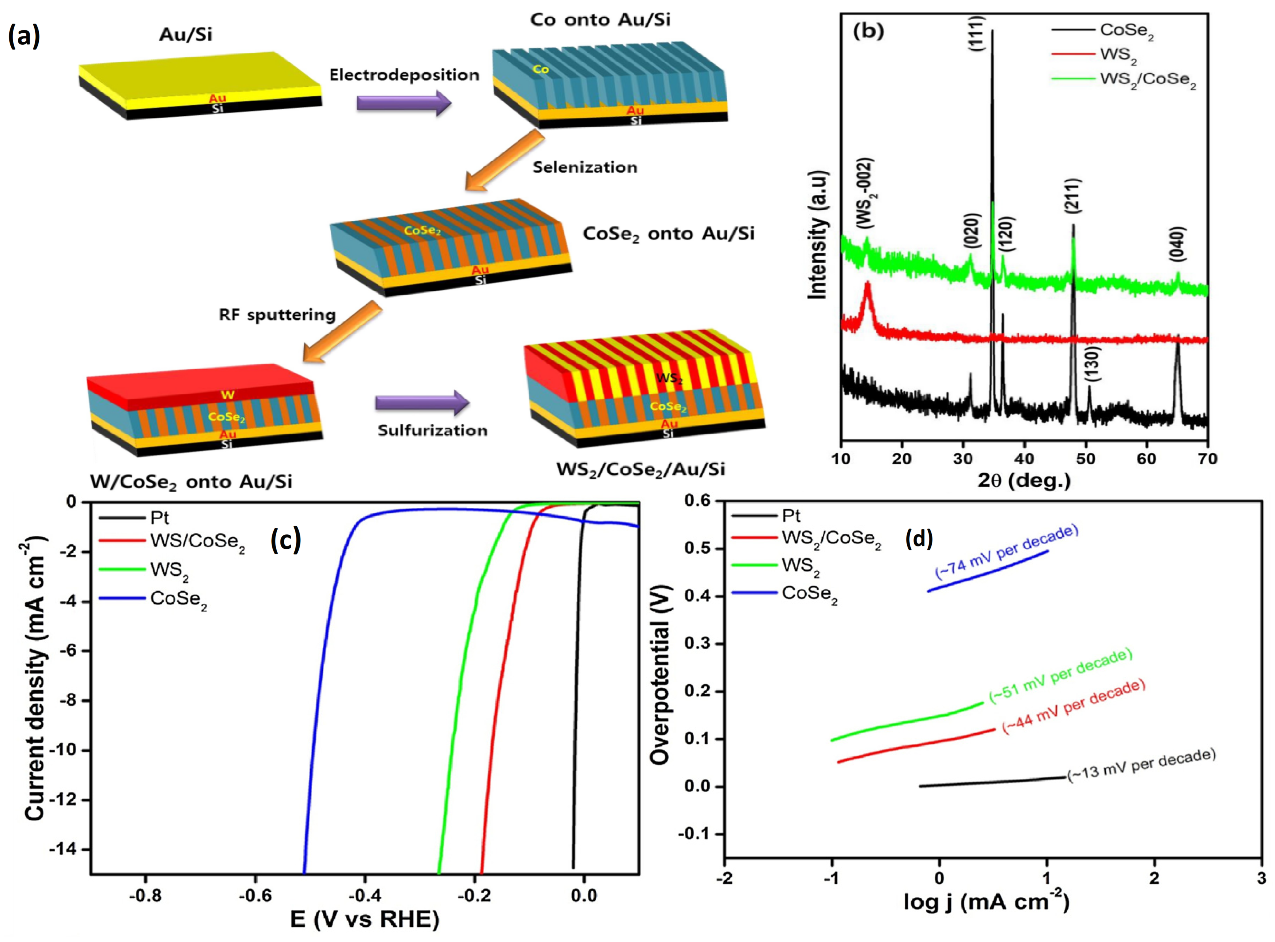

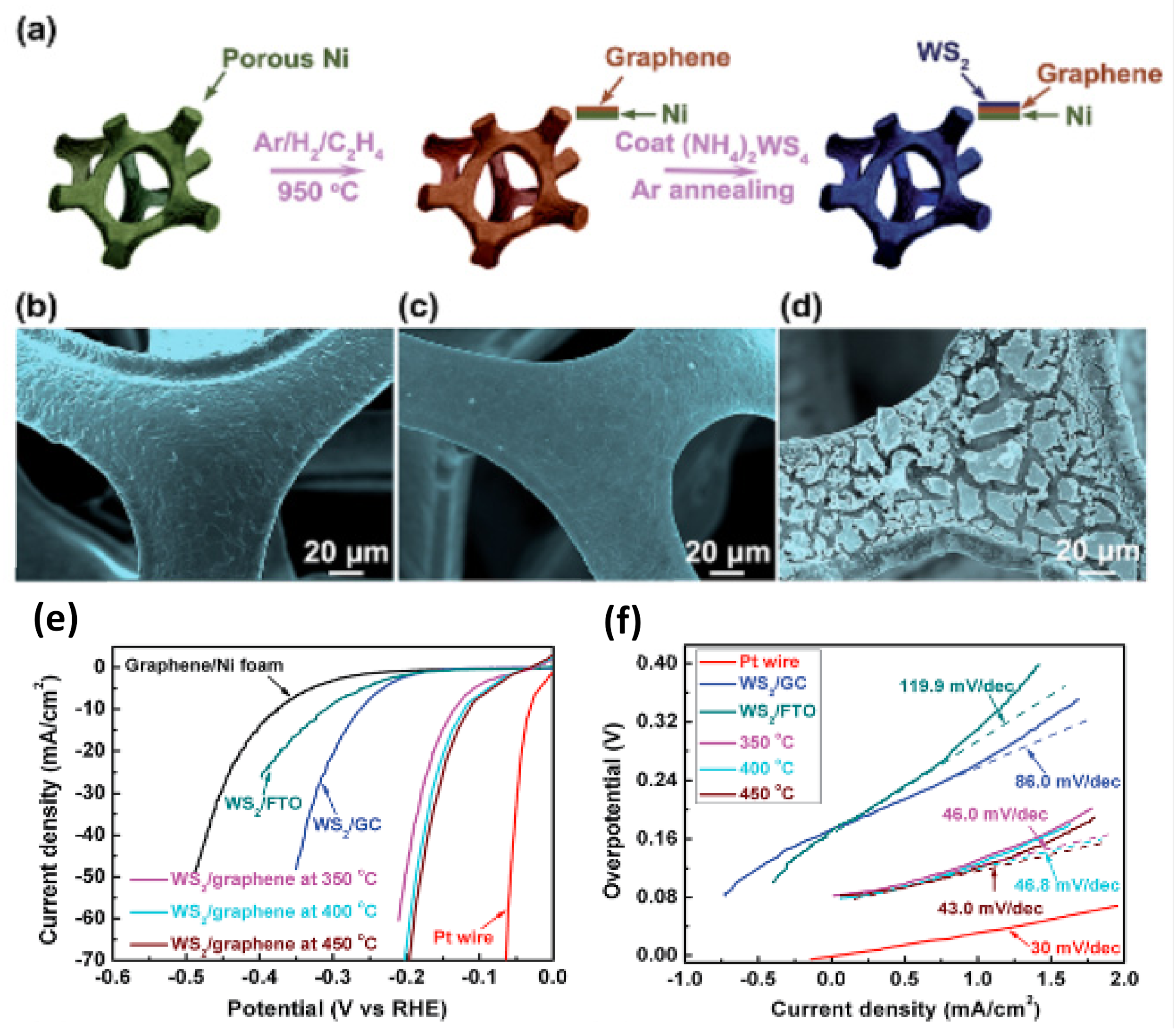

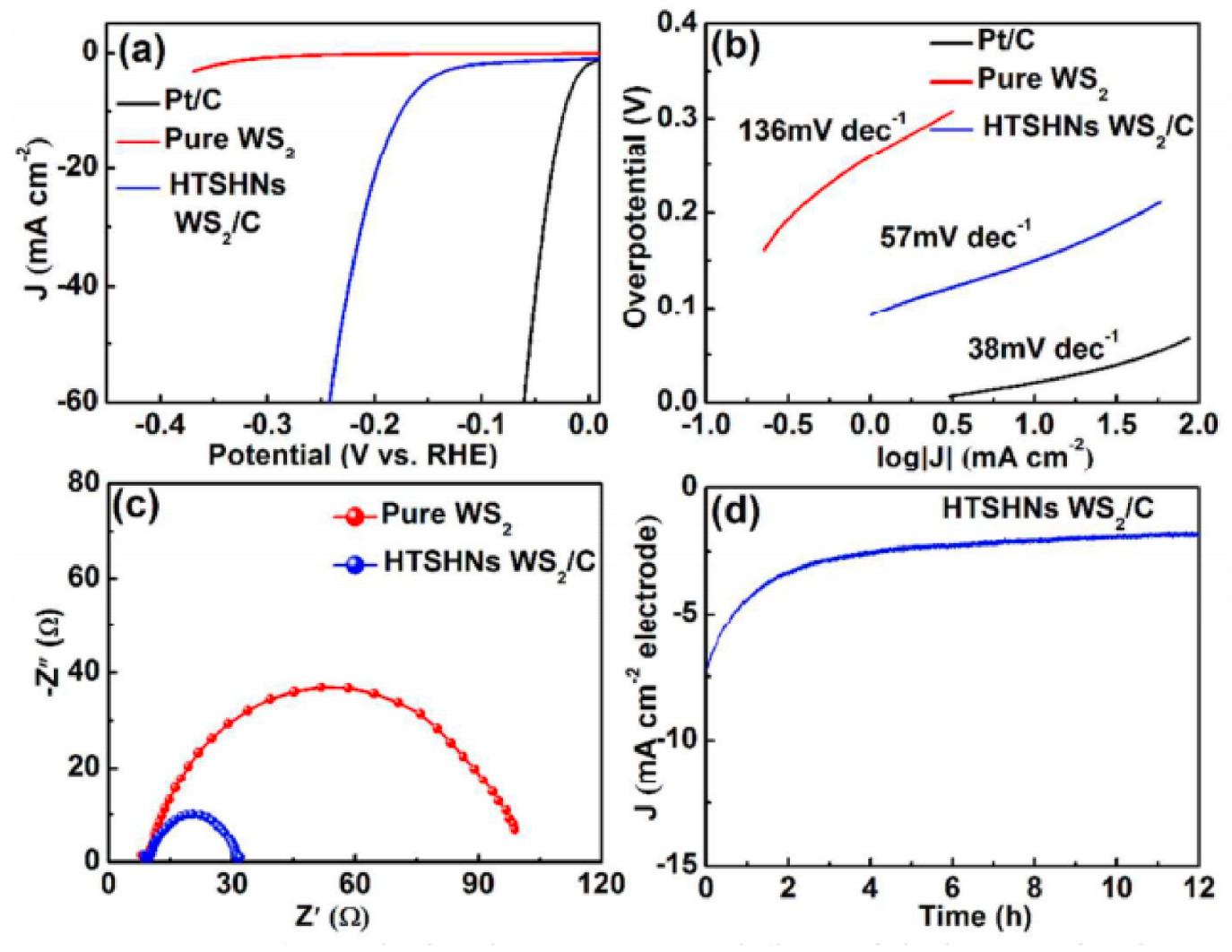


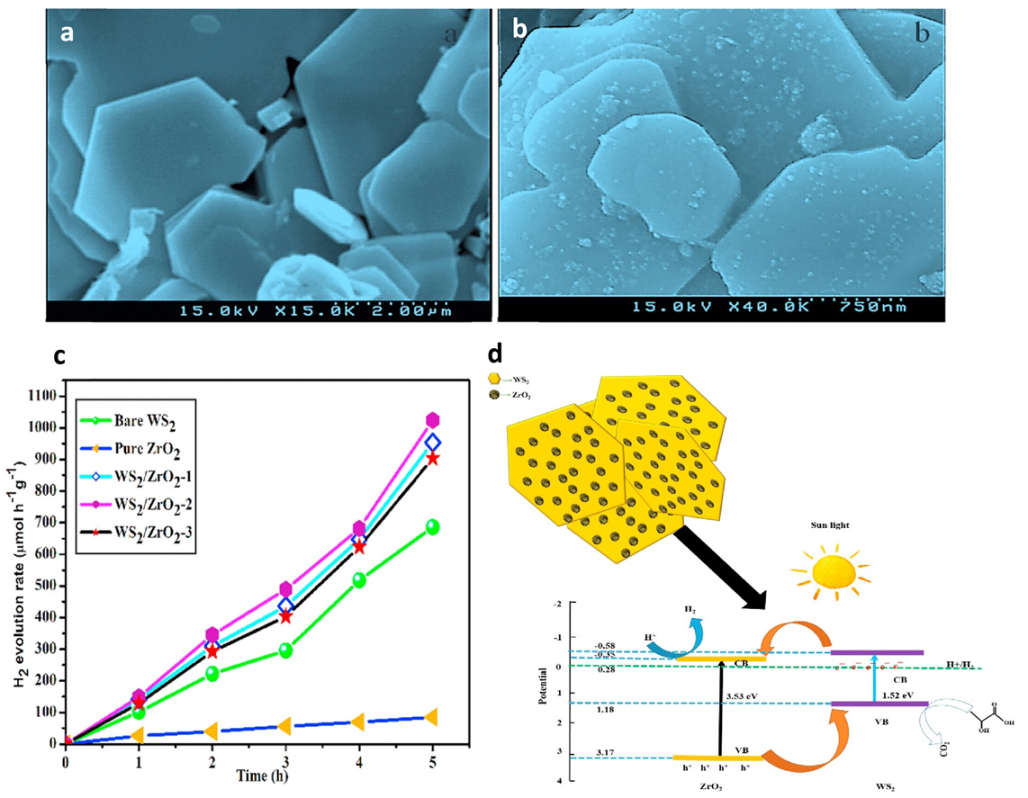
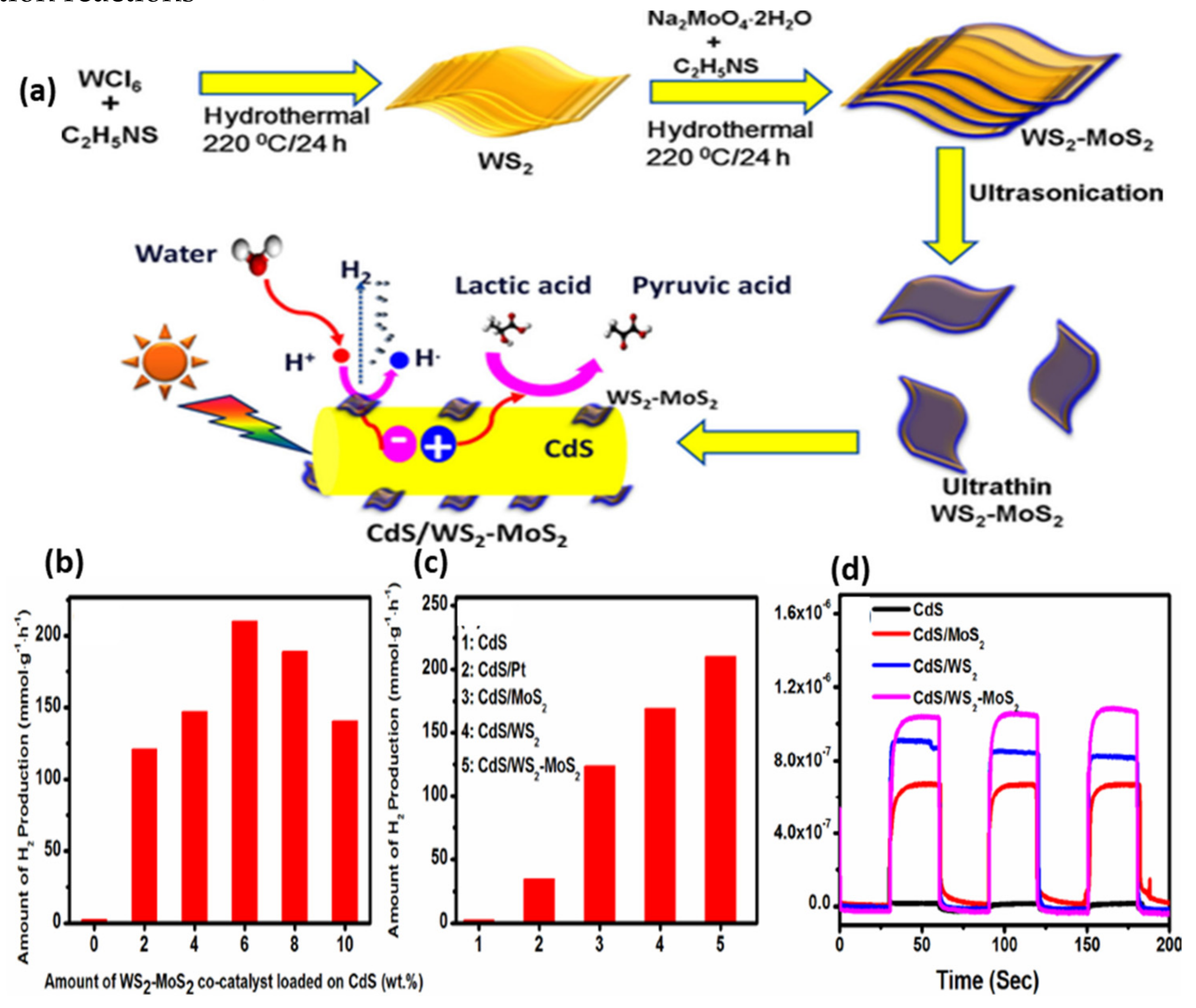

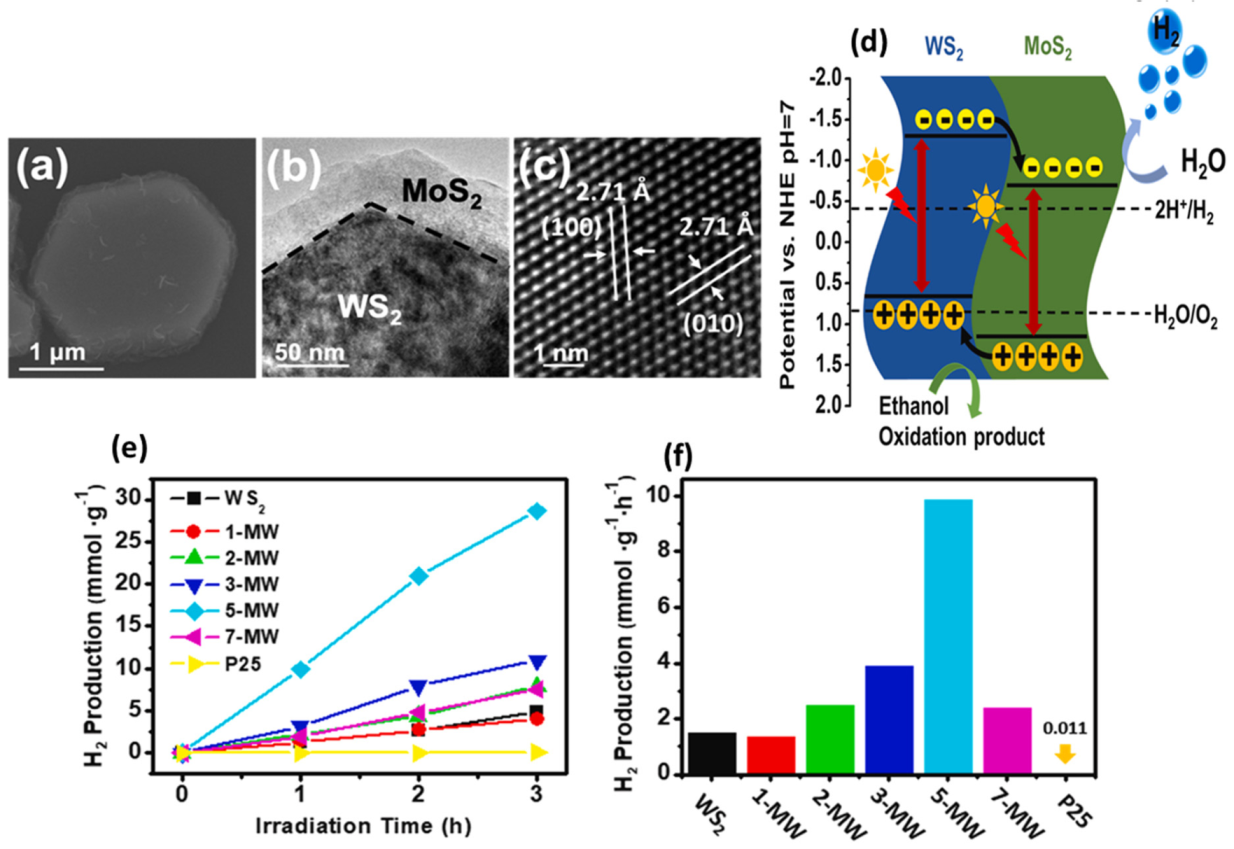
| Catalyst | Substrate | Synthesis Method | Current Density mA·cm−2 | Overpotential mV | Tafel Slope mV dec−1 | Ref. |
|---|---|---|---|---|---|---|
| Layered WS2 | CC | Thermolysis | 10 | 184 | 79 | [68] |
| WS2 nanosheets | GCE | Polarization/decomposition | 10 | 157 | 60 | [69] |
| WS2 nanosheets | W foil | Anodization/sulfurization | 10 | 136 | 61 | [70] |
| WS2 film | Si/glassy carbon | Atomic layer deposition | 10 | 137 | 54 | [71] |
| WS2 nanosheets | GCE | Self-template | 10 | 145 | 67 | [72] |
| WS2 nanoribbons | Ti/Au | Chemical unzipping | 10 | 240 | 68 | [73] |
| 1T-WS2 nanobelts | GC | Electrochemical activation | 10 | 170 | 40 | [74] |
| WS2 nanosheets | CC | Hydrothermal | 10 | 116 | 37.5 | [75] |
| WS2 nanoflakes | Si | Hydrothermal | 10 | 118 | 43 | [76] |
| Synthesis Method | Technique/Solvent/Electrolyte | Morphology | Ref. |
|---|---|---|---|
| Mechanical exfoliation | Scotch tape using Si/SiO2 wafer | Monolayers/flakes | [93] |
| Scotch tape using quartz wafer | Mono/few monolayer | [94] | |
| Chemical exfoliation | NaNO3/HCl solution | Nanosheets (4.3 nm) | [95] |
| Sodium dodecyl sulfate solution | Nanosheets | [96] | |
| Cyclohexanone/Ethyl cellulose by magnetic stirring (MS), shear mixing (SM), and horn-tip (HT) | Single/few nanosheets | [97] | |
| Aqueous ammonia | 2H nanosheets | [98] | |
| DI (deionized) water and IPA (isopropyl alcohol/vacuum filtration | Nano flakes (3.9) | ||
| Li-intercalation | Methyllithium(Me-Li), n-butyllithium (n-Bu-Li) and tert-butyllithium (t-Bu-Li) | Nano flakes | [99] |
| n-Butyllithium | 2H and 1 T nanosheets | [100] | |
| Ethylene glycol/Li hydroxide | Few layers nanosheets | [101] | |
| Electrochemical Li-intercalation | Propylene carbonate with 0.1 wt% LiClO4 | Quantum dots QD | [102] |
| A WS2 pellet, platinum mesh, and platinum wire are used as working, counter, and quasi-reference electrodes g 0.1 wt% of lithium perchlorate in deoxygenated propylene carbonate as electrolyte | Porous WS2 nanosheets | [103] | |
| Bipolar platinum electrodes, 0.5 M Na2SO4 | 1T and 2H nanosheets | [104] |
| Precursors | Substrate | Growth Conditions | Morphology | Ref. |
|---|---|---|---|---|
| WO3 and elemental S powders | Si/SiO2 wafer | WO3 at 700 °C, S at 150 °C for 3 min, 380 SCCM N2, 20 sccm H2 | Nano flakes | [117] |
| WS2 and Na2S2O3 powders | Si/SiO2 wafer | 700 °C, atmospheric P, for 1 h with 30 sccm Ar/H2 (5% H2) | Leaf-like film | [118] |
| H2S and WF6 gases | Si/SiO2 wafer | 650 °C, 1 kPa with 100 sccm Ar, 15–60 min | Atomic monolayers | [119] |
| WO3 and elemental S powders | FTO/ITO | 500 °C, atmospheric gas of Ar/H2 at a pressure of about 10−2 Torr, for 3 min with 40 sccm gas flow | Nanosheets | [120] |
| WO3, elemental S and NaCl powders | Si/SiO2 wafer | 700 °C, atmospheric P for 5 min with 200 sccm Ar | Large-area monolayers | [121] |
| Tungstic acid and elemental sulfur | Si/SiO2 wafer | 900 °C, for 30 min with 50 sccm Ar flow | Nano flakes | [122] |
| WO3 and elemental S powders | c-plane sapphire | 970–1080 °C for 30 min with 500 sccm Ar flow | Bilayer nanosheets | [123] |
| Precursors | Conditions | Morphology | Application | Ref. |
|---|---|---|---|---|
| Hexaammonium heptatungstate, thiourea powders | Autoclave, titanium substrate, 200 for 7 h. | Vertical and flat 1-T nanosheets | Electrocatalytic hydrogen revolution | [76] |
| WCl6, thioacetamide powders | Autoclave, 230 for 24 h. | 1-T nanosheets | Wastewater treatment | [134] |
| WCl6, thioacetamide powders | Autoclave, 240 for 24 h under different magnetic field | 1-T nanosheets | Anode for sodium ion batteries | [135] |
| WS2 powder, NaOH solution | Autoclave, 220 for 24 h | Quantum dots | Fluorescence sensor | [136] |
| WCl6, thiourea powders | Autoclave, 180 for 48 h | Nanoplate like structure | Lithium ion battery | [137] |
| Sodium tungstate, thioacetamide | Autoclave, 200 for 24 h | Mixed phase nanosheets | Ammonia gas sensing | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiehmed, Z.; Shakoor, A.; Altahtamouni, T. Recent Advances in WS2 and Its Based Heterostructures for Water-Splitting Applications. Catalysts 2021, 11, 1283. https://doi.org/10.3390/catal11111283
Thiehmed Z, Shakoor A, Altahtamouni T. Recent Advances in WS2 and Its Based Heterostructures for Water-Splitting Applications. Catalysts. 2021; 11(11):1283. https://doi.org/10.3390/catal11111283
Chicago/Turabian StyleThiehmed, Zeineb, Abdul Shakoor, and Talal Altahtamouni. 2021. "Recent Advances in WS2 and Its Based Heterostructures for Water-Splitting Applications" Catalysts 11, no. 11: 1283. https://doi.org/10.3390/catal11111283
APA StyleThiehmed, Z., Shakoor, A., & Altahtamouni, T. (2021). Recent Advances in WS2 and Its Based Heterostructures for Water-Splitting Applications. Catalysts, 11(11), 1283. https://doi.org/10.3390/catal11111283