Efficiency of Microencapsulation of Proteolytic Enzymes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dependence of the Thickness of the Maltodextrin Layer on the Duration of Application to Pepsin
2.2. Influence of the Thickness of the Pepsin Coating on Its Activity
2.3. Dependence of Pepsin Activity on pH at a Coating Thickness of 4 μm with Maltodextrin
2.4. Dynamics of Pork pH Depending on the Duration of Fermentation
2.5. Dynamics of the Moisture-Binding Ability of Pork Samples Depending on the Duration of Fermentation
2.6. Dynamics of Solubility of Pork Proteins Depending on the Duration of Fermentation
2.7. Dynamics of Solubility of Sarcoplasmic of Myofibrillic Pork Proteins Depending on the Duration of Fermentation
2.8. Dynamics of Accumulation of Non-Protein Nitrogen Depending on the Duration of Fermentation
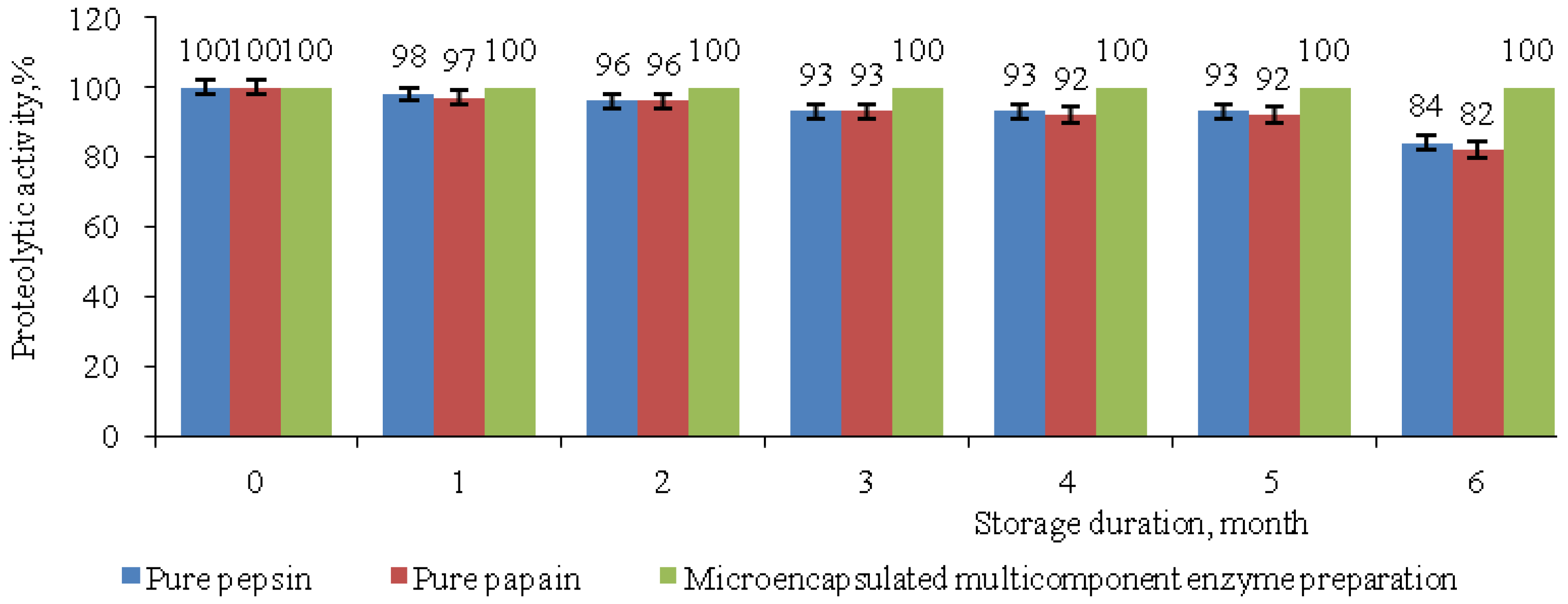
2.9. Dependence of the Proteolytic Activity of Pure Enzymes and Microencapsulated Complex Enzyme Preparation on the Duration of Storage
2.10. Influence of the Duration of Storage of Pure Pepsin, Pure Papain and Microencapsulated Complex Enzyme Preparation on the Structural and Mechanical Properties of Ham
3. Materials and Methods
3.1. The Objects of Research
3.2. Research Materials
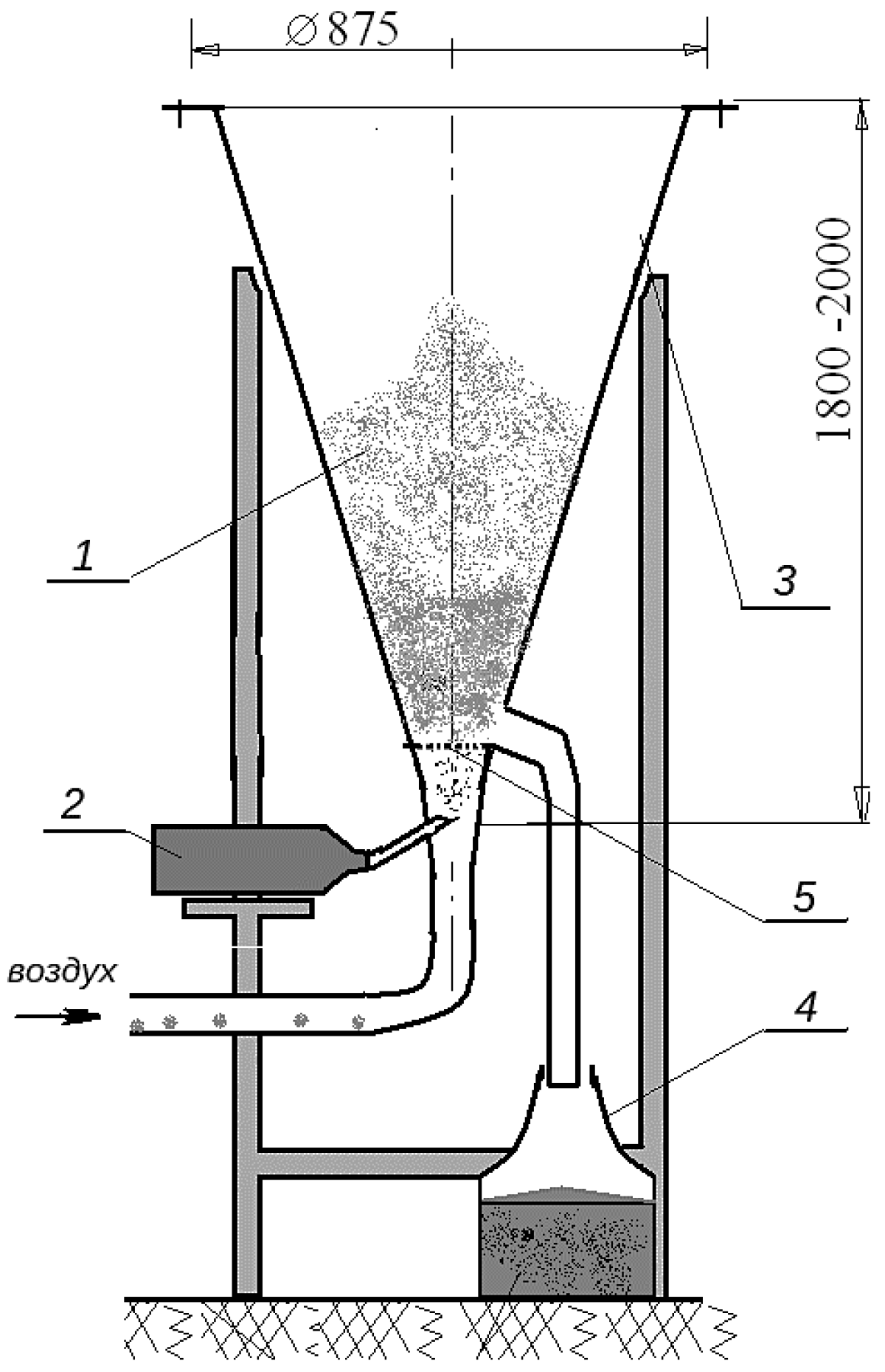
3.3. Microencapsulation Apparatus
3.4. The Composition of the Protective Layer
3.5. Storage of Enzymes and Enzyme Preparation
3.6. Methods for Determining the Thickness of a Protective Layer
3.7. Methods for Determining the Proteolytic Activity of Enzymes
3.8. Methods for the Determination of Protein Hydrolysis
3.9. Determination of Non-Protein Nitrogen
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Charmpi, C.; Vervaet, T.; Reckem, E.V.; Geeraerts, W.; Veken, D.V.D.; Ryckbosch, W.; Leroy, F.; Brengman, M. Assessing levels of traditionality and naturalness depicted on labels of fermented meat products in the retail: Exploring relations with price, quality and branding strategy. Meat Sci. 2021, 181, 108607. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, H.; Yazdani, P.; Ebrahimi, V.; Soofiyani, S.R.; Azargun, R.; Tarhriz, V.; Eyvazi, S. An Updated review on production of food derived bioactive peptides; focus on the psychrotrophic bacterial proteases. Biocatal. Agric. Biotechnol. 2021, 35, 102051. [Google Scholar] [CrossRef]
- Antipova, L.V.; Gorbunkov, M.V. Physicochemical and biocatalytic properties of the proteolytic complex of the drug “Protepsin”. Bull. Voronezh State Univ. Eng. Technol. 2016, 1, 89–93. [Google Scholar]
- Santos, J.M.D.; Ignácio, E.O.; Bis-Souza, C.V.; Silva-Barretto, A.C.D. Performance of reduced fat-reduced salt fermented sausage with added microcrystalline cellulose, resistant starch and oat fiber using the simplex design. Meat Sci. 2021, 175, 108433. [Google Scholar] [CrossRef]
- Dwivedi, P.; Sharma, A.K.; Singh, S.P. Biochemical properties and repression studies of an alkaline serine protease from a haloalkaliphilic actinomycete, Nocardiopsisdassonvillei subsp. albirubida OK-14. Biocatal. Agric. Biotechnol. 2021, 35, 102059. [Google Scholar] [CrossRef]
- El-Ghonemy, D.H.; Ali, T.H. Effective bioconversion of feather-waste Keratin by Thermo-Surfactant Stable Alkaline Keratinase produced from Aspergillus sp. DHE7 with promising biotechnological application in detergent formulations. Biocatal. Agric. Biotechnol. 2021, 35, 102052. [Google Scholar] [CrossRef]
- Naqvi, Z.B.; Campbell, M.A.; Latif, S.; Thomson, P.C.; McGill, D.M.; Warner, R.D.; Friend, M.A. Improving tenderness and quality of M. biceps femoris from older cows through concentrate feeding, zingibain protease and sous vide cooking. Meat Sci. 2021, 180, 108563. [Google Scholar] [CrossRef]
- Chanalia, P.; Gandhi, D.; Attri, P.; Dhanda, S. Extraction, purification and characterization of low molecular weight Prolineimino-peptidase from probiotic L. plantarum for meat tenderization. Int. J. Biol. Macromol. 2018, 109, 651–663. [Google Scholar] [CrossRef]
- Makhova, A.A.; Minaev, M.Y.; Kulikovsky, A.V.; Vostrikova, N.L. Study of the enzymatic activity of recombinant metallopeptidase intended for use in the meat industry. Quest. Nutr. 2019, 88, 95–104. [Google Scholar] [CrossRef]
- Ribeiro, W.O.; Ozaki, M.M.; Santos, M.D.; Rodríguez, A.P.; Pflanzer, S.B.; Pollonio, M.A.R. Interaction between papain and transglutaminase enzymes on the textural softening of burgers. Meat Sci. 2021, 174, 108421. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, F.; Geng, F.; Luo, Y.; Gong, S.; Jiang, Z. A novel aspartic protease from Rhizomucormiehei expressed in Pichia pastoris and its application on meat tenderization and preparation of turtle peptides. Food Chem. 2017, 245, 570–577. [Google Scholar] [CrossRef]
- Brazzelli, M.; Cruickshank, M.; Tassie, E.; McNamee, P.; Robertson, C.; Elders, A.; Fraser, C.; Hernandez, R.; Lawrie, D.; Ramsay, C. Collagenase clostridium histolyticum for the treatment of Dupuytren’s contracture: Systematic review and economic evaluation. Health Technol. Assess. 2015, 19, 201–202. [Google Scholar] [CrossRef] [Green Version]
- Kudryashov, L.S.; Uzakov, Y.M.; Tikhonov, S.L.; Tikhonova, N.V.; Diachkova, A.V. Microencapsulation of proteolytic en-zymes for industrial application. News Natl. Acad. Sci. Repub. Kazakhstan. Ser. Geol. Tech. Sci. 2020, 3, 161–169. [Google Scholar]
- Smaoui, S.; Hlima, H.B.; Braiek, O.B.; Ennouri, K.; Mellouli, L.; Khaneghah, A.M. Recent advancements in encapsulation of bioactive compounds as a promising technique for meat preservation. Meat Sci. 2021, 181, 108585. [Google Scholar] [CrossRef] [PubMed]
- Beck, P.H.B.; Matiucci, M.A.; Neto, A.A.M.; Feihrmann, A.C. Sodium chloride reduction in fresh sausages using salt encapsulated in carnauba wax. Meat Sci. 2021, 175, 108462. [Google Scholar] [CrossRef] [PubMed]
- Graily-Moradi, F.; Hejazi, M.J.; Enayati, A.A.; Hamishehkar, H. Evaluation of co-nanoencapsulation process on the toxicity and biochemical metabolism of imidacloprid and lambda-cyhalothrin in Myzuspersicae (Sulzer). Biocatal. Agric. Biotechnol. 2021, 33, 101974. [Google Scholar] [CrossRef]
- Homayouni-Rad, A.; Mortazavian, A.M.; Mashkani, M.G.; Hajipour, N.; Pourjafar, H. Effect of Alyssum homolocarpum mucilage and inulin microencapsulation on the survivability of Lactobacillus casei in simulated gastrointestinal and high-temperature conditions. Biocatal. Agric. Biotechnol. 2021, 35, 102075. [Google Scholar] [CrossRef]
- Stella, M.; Theeba, M.; Illani, Z.I. Organic fertilizer amended with immobilized bacterial cells for extended shelf-life. Biocatal. Agric. Biotechnol. 2019, 20, 101248. [Google Scholar] [CrossRef]
- Bahgat, F.; Hoda, S.E.; Amira, A.; Amal, M.H.; Nayra, S.H.M. The application of multi-particulate microcapsule containing probiotic bacteria and inulin nanoparticles in enhancing the probiotic survivability in yoghurt. Biocatal. Agric. Biotechnol. 2019, 22, 101391. [Google Scholar] [CrossRef]
- Shaymaa, A.I.; Mohamed, E.H.; Amal, M.H. Single step hydrolysis of chitin using thermophilic immobilized exochitinase on carrageenan-guar gum gel beads. Biocatal. Agric. Biotechnol. 2019, 21, 101281. [Google Scholar] [CrossRef]
- Wahba, M.I. Carrageenan stabilized calcium pectinate beads and their utilization as immobilization matrices. Biocatal. Agric. Biotechnol. 2021, 35, 102078. [Google Scholar] [CrossRef]
- Weber, D.; Nascimento, M.d.G.; Parize, A.L. Immobilization of Burkholderiacepacia lipase on cross-linked chitosan-based support for the synthesis of geranyl acetate. Biocatal. Agric. Biotechnol. 2019, 19, 101133. [Google Scholar] [CrossRef]
- Navya, A.; Mohanan, P.V. Template synthesized polypyrroles as a carrier for diastase alpha amylase immobilization. Biocatal. Agric. Biotechnol. 2019, 19, 101164. [Google Scholar] [CrossRef]
- Hala, R.W.; Mohamed, A.A.; Heba, M.E.; Hanan, F.Y. Nanoporous Zeolite-X as a new carrier for laccase immobilization and its application in dyes decolorization. Biocatal. Agric. Biotechnol. 2019, 19, 101135. [Google Scholar] [CrossRef]
- Bracco, L.F.; Levin, G.J.; Urtasun, N.; Cañizo, A.A.N.D.; Wolman, F.J.; Miranda, M.V.; Cascone, O. Covalent immobilization of soybean seed hull urease on chitosan mini-spheres and the impact on their properties. Biocatal. Agric. Biotechnol. 2019, 18, 101093. [Google Scholar] [CrossRef]
- Martín, M.C.; López, O.V.; Ciolino, A.E.; Morata, V.I.; Villar, M.A.; Ninago, M.D. Immobilization of enological pectinase in calcium alginate hydrogels: A potential biocatalyst for winemaking. Biocatal. Agric. Biotechnol. 2019, 18, 101091. [Google Scholar] [CrossRef] [Green Version]
- Balabushevitch, N.G.; Sukhorukov, G.B.; Moroz, N.A.; Volodkin, D.V.; Larionova, N.I.; Donath, E.; Mohwald, H. Encapsulation of proteins by layer-bylayer adsorption of polyelectrolytes onto protein aggregates: Factors regulating the protein release. Biotechnol. Bioeng. 2001, 76, 207–213. [Google Scholar] [CrossRef]
- Vergaro, V.; Baldassarre, F.; De Santis, F.; Ciccarella, G.; Giannelli, G.; Leporatti, S. TGF-Beta Inihibitor-loaded Polyelectrolyte Multilayers Capsules for Sustained Targetingof Hepatocarcinoma Cells. Curr. Pharm. Des. 2012, 18, 4155–4164. [Google Scholar] [CrossRef] [PubMed]
- Chandrawati, R.; Van Koeverden, M.P.; Lomas, H.; Caruso, F. Multicompartment particle assemblies for bioinspired encapsulated reactions. J. Phys. Chem. 2011, 2, 2639–2649. [Google Scholar] [CrossRef]
- Menshutina, N.V. Technologies of incapsulation. Pharm. Technol. 2014, 9, 30–33. [Google Scholar]
- Botinestean, C.; Hossain, M.; Mullen, A.M.; Kerry, J.P.; Hamill, R.M. The influence of the interaction of sous-vide cooking time and papain concentration on tenderness and technological characteristics of meat products. Meat Sci. 2021, 177, 108491. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.L.; Buckley, J.D. Protein hydrolysates and tissue repair. Nutr. Res. Rev. 2011, 24, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sviridenko, Y.Y.; Myagkonosov, D.S.; Abramov, D.V.; Ovchinnikova, E.G. Scientific and methodological approaches to the development of technology of protein hydrolysates for special nutrition. Part 2. Functional properties of protein hydrolysates depending on the specificity of proteolytic processes. Food Ind. 2017, 6, 50–53. [Google Scholar]
- Jeewanthi, R.K.C.; Lee, N.K.; Paik, H.D. Improved functional performance of whey protein hydrolysates in the food industry. Korean J. Food Sci. Anim. Resour. 2015, 35, 350–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäumler, H.; Georgieva, R. Coupled Enzyme Reactions in Multicompartment Microparticles. Biomacromolecules 2010, 11, 1480–1487. [Google Scholar] [CrossRef]
- Kuiper, S.M.; Nallani, M.; Vriezema, D.M.; Cornelissen, J.J.L.M.; van Hest, J.C.M.; Nolte, R.J.M.; Rowan, A.E. Enzymes containing porous polymersomes as nano reaction vessels for cascade reactions. Org. Biomol. Chem. 2008, 6, 4315–4318. [Google Scholar] [CrossRef]
- Minaev, M.Y.; Makhova, A.A. Possibility of using recombinant metallopeptidase M9 for tenderizing meat. Food Ind. 2019, 4, 63–64. [Google Scholar]
- Zhuravskaya, N.K. Research and Quality Control of Meat and Meat Products; Agropromizdat: Moscow, Russia, 1985. [Google Scholar]


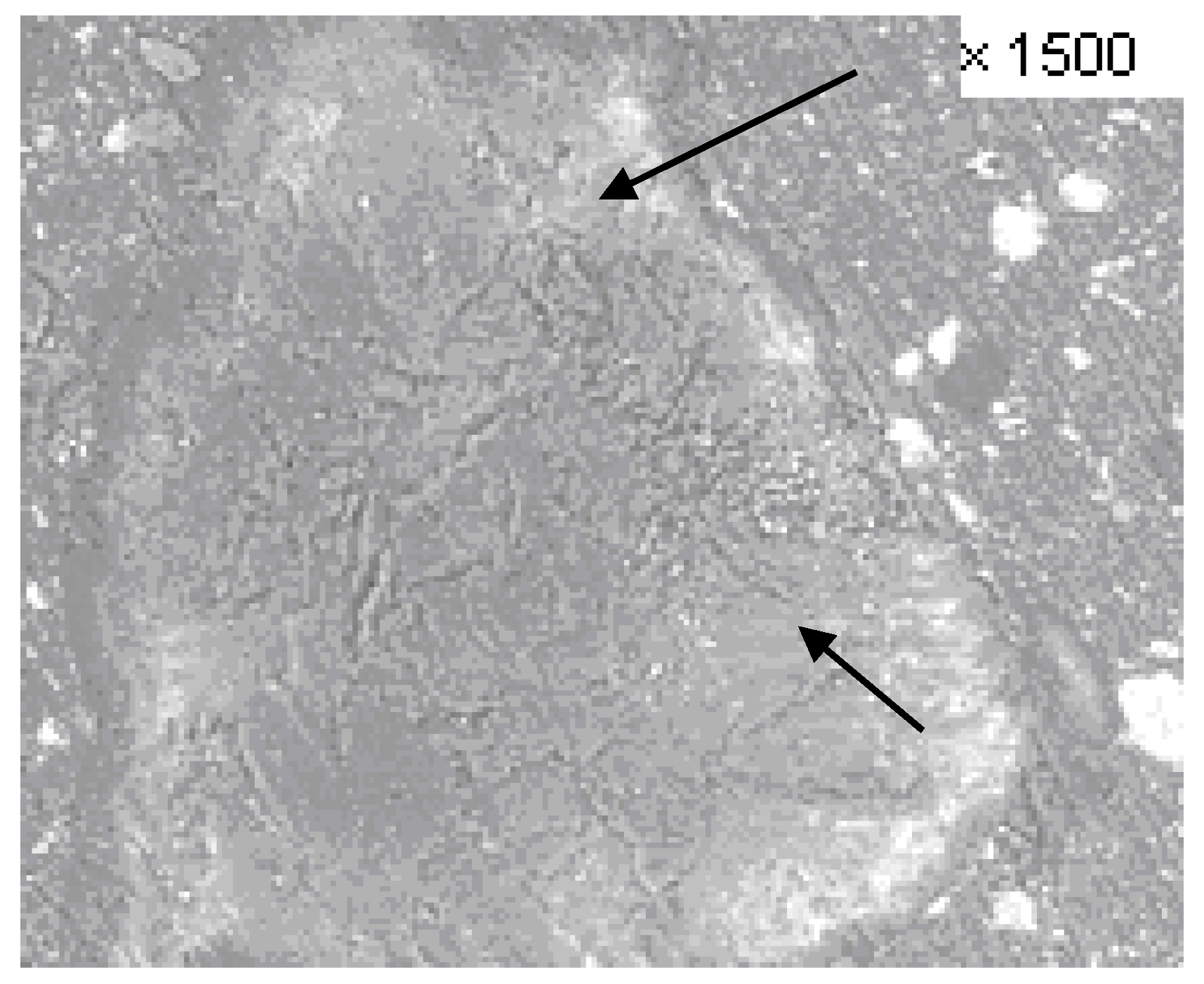



| Maltodextrin Layer Thickness, μm | Dispersion | Standard Deviation |
|---|---|---|
| Pure enzyme | 708.49 | 26.62 |
| Maltodextrin layer thickness 2 μm | 852.28 | 29.19 |
| Maltodextrin layer thickness 4 μm | 830.56 | 28.82 |
| Maltodextrin layer thickness 6 μm | 767.48 | 27.70 |
| Fermentation Duration, h | pH Value * | ||
|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | |
| Before fermentation | 5.72 ± 0,02 | 5.72 ± 0.02 | 5.72 ± 0.02 |
| 12 | 5.72 ± 0,01 | 5.72 ± 0.01 | 5.82 ± 0.01 |
| 24 | 5.74 ± 0,02 | 5.76 ± 0.02 | 5.91 ± 0.02 |
| 36 | 5.77 ± 0,02 | 5.79 ± 0.01 | 6.14 ± 0.02 |
| Fermentation Duration, h | The Value of the Moisture-Binding Ability, % * | ||
|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | |
| Before fermentation | 58.6 ± 0.20 | 58.5 ± 0.22 | 58.7 ± 0.23 |
| 12 | 59.8 ± 0.22 | 59.7 ± 0.20 | 63.4 ± 0.20 |
| 24 | 60.5 ± 0.23 | 60.4 ± 0.21 | 64.9 ± 0.21 |
| 36 | 61.3 ± 0.22 | 61.8 ± 0.25 | 67.2 ± 0.24 |
| Fermentation Duration, h | The Amount of Nitrogen of Sarcoplasmic Proteins to the Total Nitrogen, % * | ||
|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | |
| Before fermentation | 8.22 ± 0.05 | 8.21 ± 0.04 | 8.11 ± 0.04 |
| 12 | 9.41 ± 0.04 | 9.63 ± 0.05 | 12.72 ± 0.06 |
| 24 | 10.70 ± 0.06 | 10.92 ± 0.05 | 15.91 ± 0.05 |
| 36 | 12.61 ± 0.04 | 12.82 ± 0.04 | 18.11 ± 0.04 |
| Fermentation Duration, h | The Amount of Nitrogen of Myofibrillary Proteins to the Total Nitrogen, % * | ||
|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | |
| Before fermentation | 15.11 ± 0.04 | 15.12 ± 0.03 | 15.21 ± 0.04 |
| 12 | 15.42 ± 0.05 | 15.64 ± 0.04 | 17.72 ± 0.03 |
| 24 | 16.30 ± 0.06 | 16.40 ± 0.05 | 18.54 ± 0.04 |
| 36 | 16.91 ± 0.04 | 17.11 ± 0.04 | 20.80 ± 0.05 |
| Fermentation Duration, h | Non-Protein Nitrogen to Total Nitrogen, % * | ||
|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | |
| Before fermentation | 5.72 ± 0.03 | 5.72 ± 0.02 | 5.72 ± 0.02 |
| 12 | 6.13 ± 0.04 | 6.31 ± 0.03 | 7.94 ± 0.03 |
| 24 | 6.45 ± 0.02 | 6.72 ± 0.01 | 8.93 ± 0.04 |
| 36 | 6.81 ± 0.02 | 7.11 ± 0.04 | 10.53 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhonov, S.L.; Tikhonova, N.V.; Kudryashov, L.S.; Kudryashova, O.A.; Moskovenko, N.V.; Tretyakova, I.N. Efficiency of Microencapsulation of Proteolytic Enzymes. Catalysts 2021, 11, 1270. https://doi.org/10.3390/catal11111270
Tikhonov SL, Tikhonova NV, Kudryashov LS, Kudryashova OA, Moskovenko NV, Tretyakova IN. Efficiency of Microencapsulation of Proteolytic Enzymes. Catalysts. 2021; 11(11):1270. https://doi.org/10.3390/catal11111270
Chicago/Turabian StyleTikhonov, Sergey L., Natalya V. Tikhonova, Leonid S. Kudryashov, Olga A. Kudryashova, Nadezhda V. Moskovenko, and Irina N. Tretyakova. 2021. "Efficiency of Microencapsulation of Proteolytic Enzymes" Catalysts 11, no. 11: 1270. https://doi.org/10.3390/catal11111270
APA StyleTikhonov, S. L., Tikhonova, N. V., Kudryashov, L. S., Kudryashova, O. A., Moskovenko, N. V., & Tretyakova, I. N. (2021). Efficiency of Microencapsulation of Proteolytic Enzymes. Catalysts, 11(11), 1270. https://doi.org/10.3390/catal11111270






