Performance Study of Zn-Co-Ni/AC Catalyst in Acetylene Acetylation
Abstract
:1. Introduction
2. Catalysts Characterization
3. Catalyst Evaluation
4. Results and Discussions
4.1. Catalytic Performance Evaluation
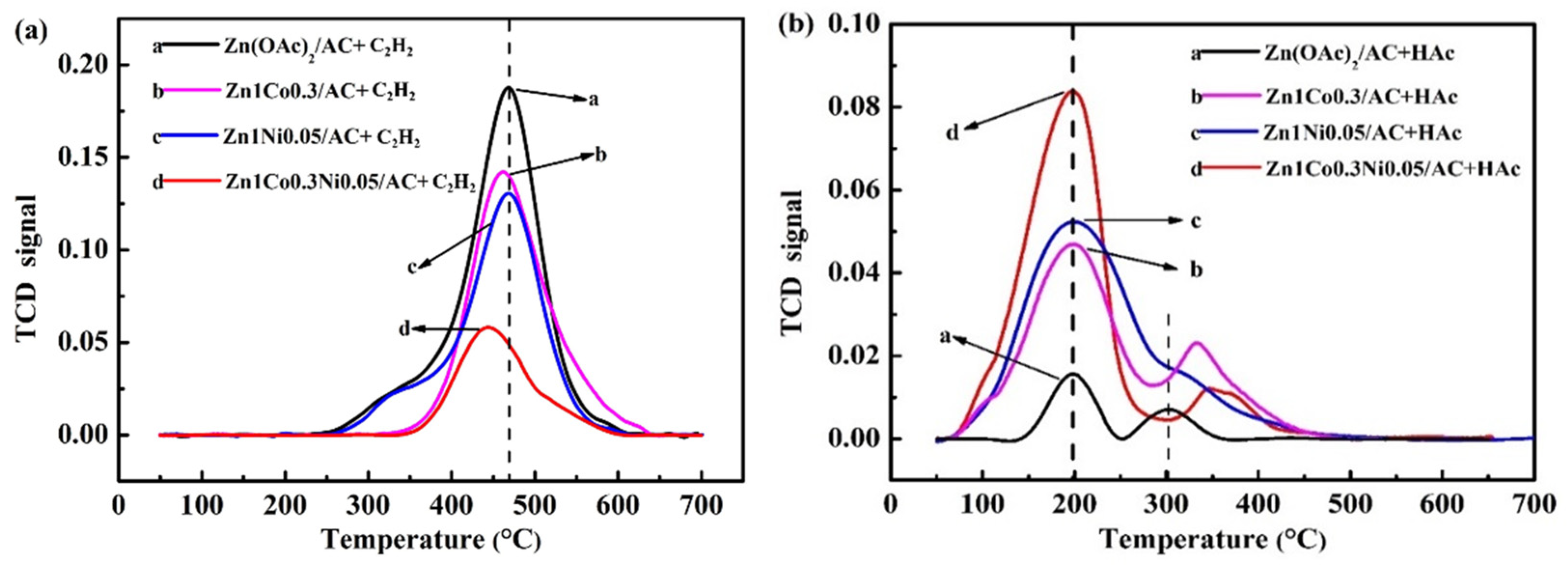
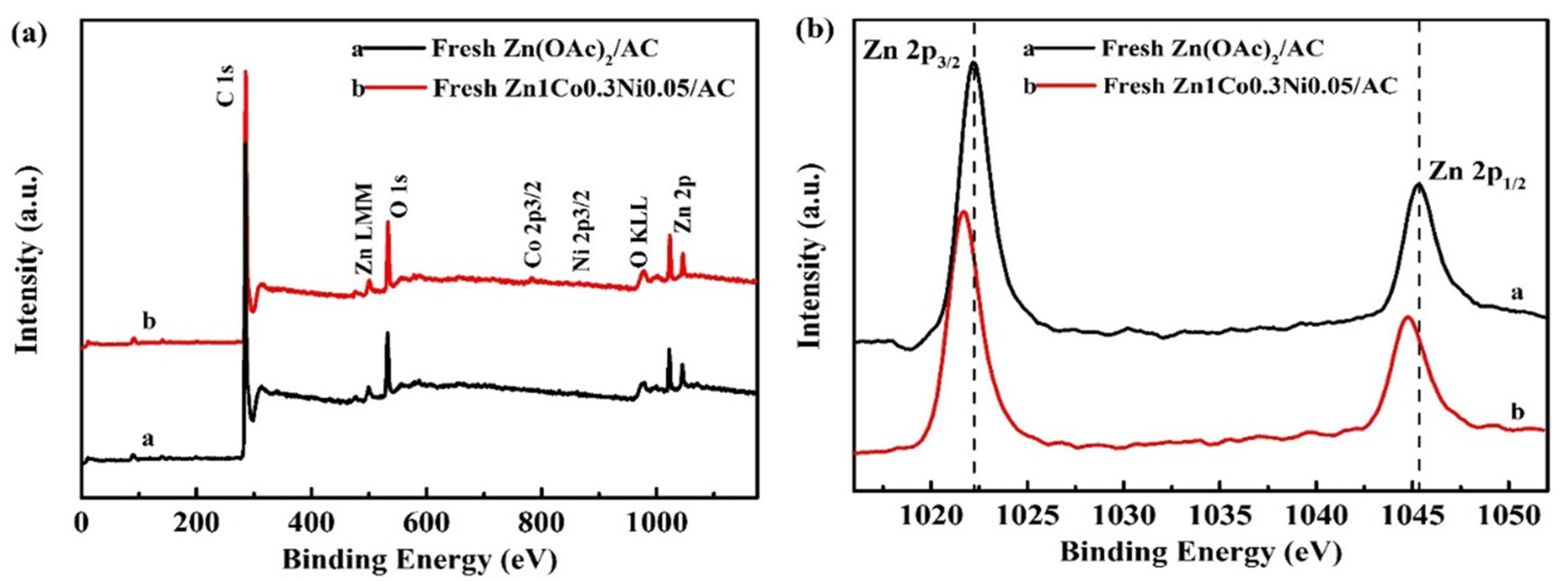
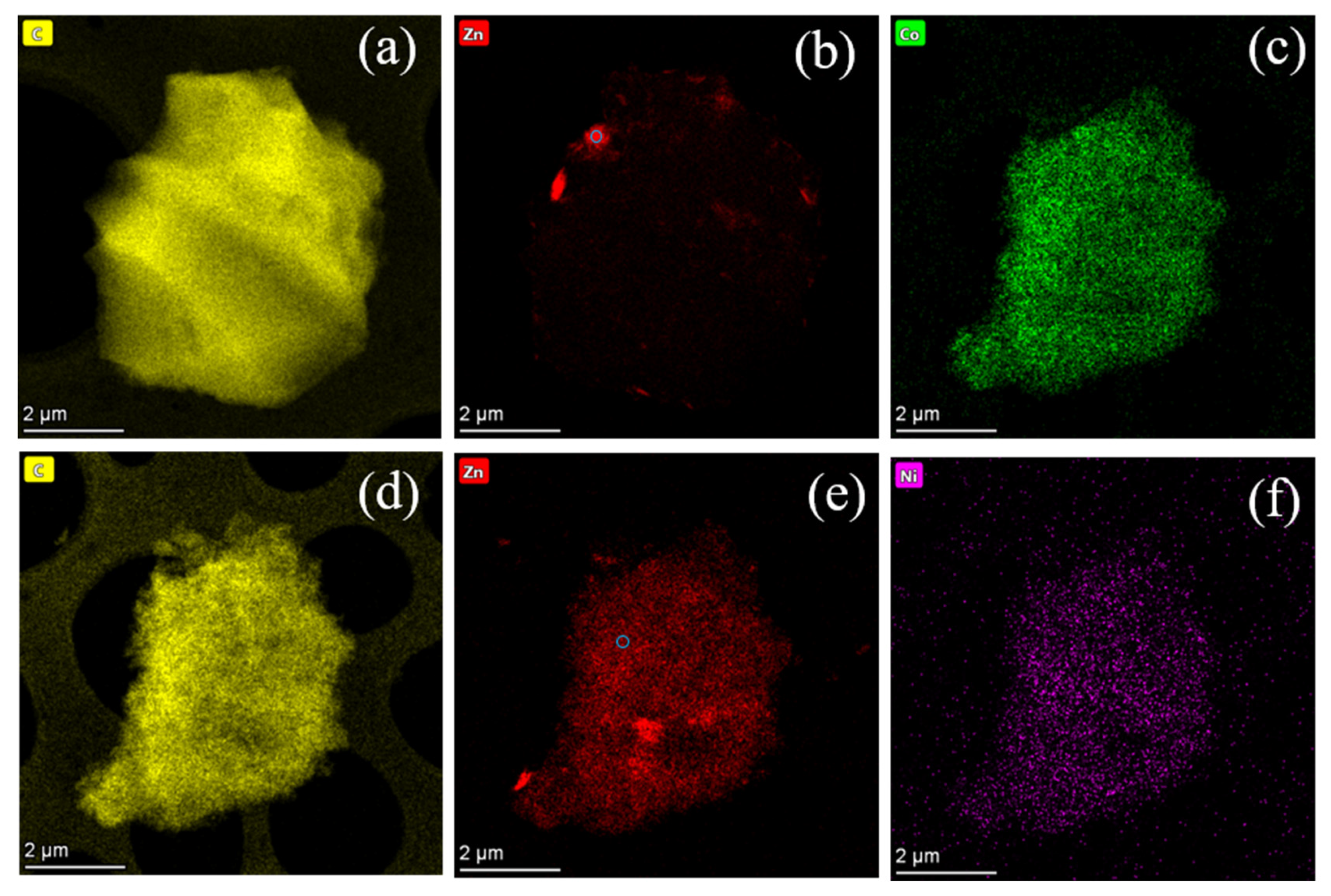
4.2. Structure Characterizations of the As-Prepared Catalysts
4.3. Effect of Co and Ni on the Zinc Catalyst
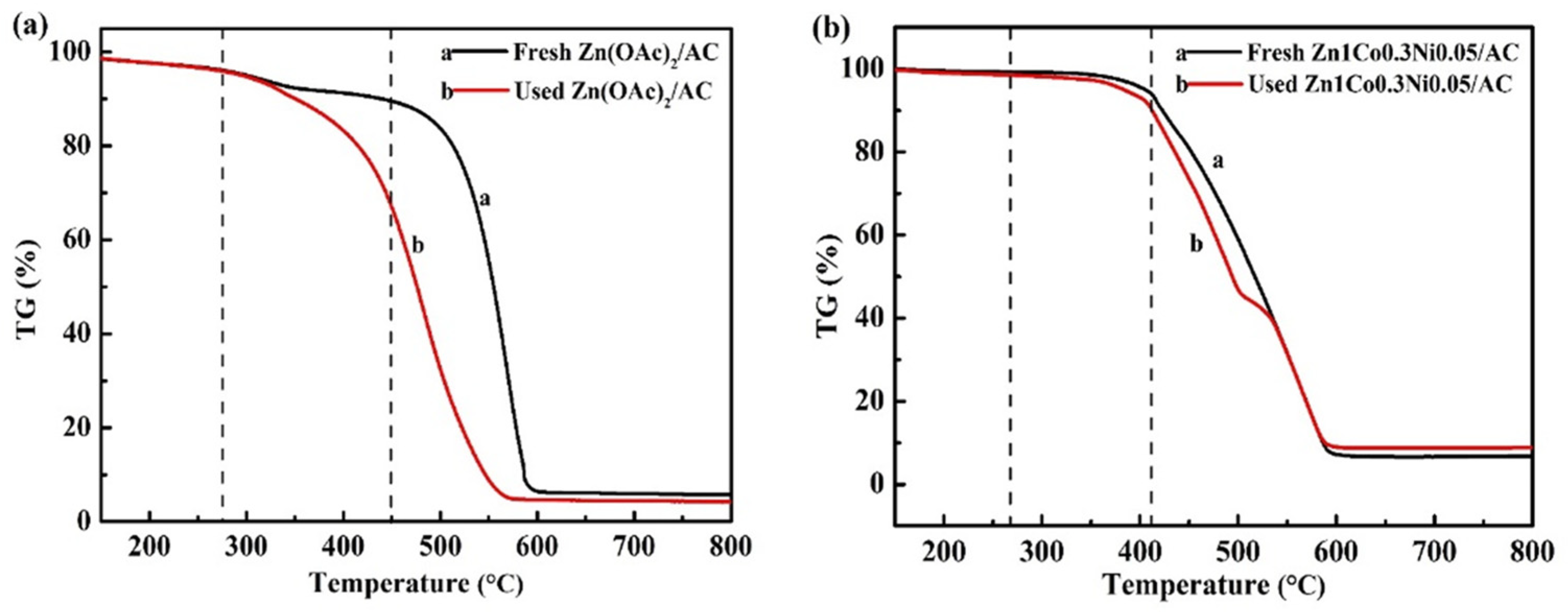
4.4. Catalytic Stability of the Zn-Co-Ni/AC Catalysts
5. Materials and Methods
5.1. Materials
5.2. Catalysts Preparation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez Caranton, A.R.; da Silva Pinto, J.C.C.; Stavale, F.; Barreto, J.; Schmal, M. Statistical analysis of the catalytic synthesis of Vinyl acetate over Pd-Cu/ZrO2 nanostructured based catalysts. Catal. Today 2020, 344, 108–117. [Google Scholar] [CrossRef]
- Han, Y.F.; Kumar, D.; Goodman, D.W. Particle size effects in vinyl acetate synthesis over Pd/SiO2. J. Catal. 2005, 230, 353–358. [Google Scholar] [CrossRef]
- Li, Z.J.; Thuening, T.; Tysoe, W.T. The adsorption of ethylene on Au/Pd (100) alloy surfaces. Surf. Sci. 2016, 646, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Schobert, H. Production of acetylene and acetylene-based chemicals from coal. Chem. Rev. 2014, 114, 1743–1760. [Google Scholar] [CrossRef]
- Bartley, W.J.; Jobson, S.; Harkreader, G.G.; Kitson, M.; Lemanski, M.F. Catalysts and Processes for the Manufacture of Vinyl Acetate. U.S. Patent 5,185,308, 2 September 1993. [Google Scholar]
- Motahari, K.; Rempel, G.; Lashkarara, S.; Ghaseminezhad, K.; Borumandnejad, A.; Hatami, B. Modelling of deactivation of Pd-Au catalyst in vinyl acetate synthesis from oxidation of ethylene and acetic acid in the gaseous phase. Can. J. Chem. Eng. 2016, 94, 506–511. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, X.; Yu, Y.; Zhang, M. A comprehensive comparative DFT study on adsorption and reactions involved in vinyl acetate synthesis from acetoxylation of ethylene on pure Pd (100) and Pd-Au (100): Elucidating the role of Au. Appl. Surf. Sci. 2016, 387, 1021–1028. [Google Scholar] [CrossRef]
- Xu, H.; Yu, T.; Li, M. Zinc Acetate Immobilized on Mesoporous Materials by Acetate Ionic Liquids as Catalysts for Vinyl Acetate Synthesis. J. Chem. 2015, 2015, 238287. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, S. The Prevention of the Activity Decrease of Metallic Oxide Catalyst in Synthetic Process of Vinyl Acetate. J. Soc. Chem. Ind. Jpn. 1963, 66, 39–44. [Google Scholar]
- Miyazawa, S. The Change of the Activity and Composition of Metallic Oxide Catalyst in Synthetic Process of Vinyl Acetate. J. Soc. Chem. Ind. Jpn. 1961, 64, 1460–1463. [Google Scholar]
- Bong, H.K.; Binh, H.H.; Kurlyandskaya, I.I.; Nyrkova, A.N.; Yamandii, D.I.; Temkin, O.N. Regularities of adsorption of zinc acetate from aqueous solutions onto the surface of modified activated carbons. Russ. J. Appl. Chem. 2014, 86, 1691–1701. [Google Scholar] [CrossRef]
- Abanto-Chavez, H.J.; Kozhemyakina, I.A.; Bong, H.K.; Temkin, O.N. Adsorption of Zn (OAc) 2 from Aqueous Solutions on the Surface of Activated Carbons Modified with Acetic Acid. Russ. J. Appl. Chem. 2003, 76, 1418–1422. [Google Scholar]
- Yan, F.-W.; Guo, C.-Y.; Yan, F.; Li, F.-B.; Qian, Q.-L.; Yuan, G.-Q. Vinyl acetate formation in the reaction of acetylene with acetic acid catalyzed by zinc acetate supported on porous carbon spheres. Russ. J. Phys. Chem. A 2010, 84, 796–801. [Google Scholar] [CrossRef]
- Temkin, O.N.; Abanto-Chavez, H.I.; Hoang, K.B. Kinetic models of vinyl acetate synthesis on new-generation zinc acetate catalysts. React. Kinet. Catal. Lett. 2000, 41, 638–654. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Y.; Yu, Y.; Zhang, M. Density Functional Theory Investigation on the Synthesis Mechanism of Vinyl Acetate from Acetylene and Acetic Acid Catalyzed by Ordered Mesoporous Carbon-Supported Zinc Acetate. Ind. Eng. Chem. Res. 2018, 57, 7363–7373. [Google Scholar] [CrossRef]
- El-Sawi, M.; Emig, G.; Hofmann, H. A study of the kinetics of vinyl acetate synthesis. Chem. Eng. J. 1977, 13, 201–211. [Google Scholar] [CrossRef]
- Zhang, M.H.; Zhuang, J.Y.; Wu, X.Y.; Yu, Y.Z. Experimental and theoretical insights into the cyclotrimerization of acetylene during vinyl acetate synthesis. Chem. Eng. J. 2019, 378, 122183. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, X.; Huang, X.; Yang, B.; Yu, Y. DFT investigations on the conversion of acetylene to undesired vinyl acetylene during vinyl acetate synthesis. Comput. Theor. Chem. 2017, 1115, 253–260. [Google Scholar] [CrossRef]
- Morrow, B.A. The initial mechanism of vinyl acetate synthesis from acetic acid and acetylene catalyzed by active carbon-zinc acetate. J. Catal. 1984, 86, 328–332. [Google Scholar] [CrossRef]
- Hu, L.B.; Xu, Z.; He, P.J.; Wang, X.G.; Tian, Z.Q.; Yuan, H.F.; Yu, F.; Dai, B. Zinc and Nitrogen-Doped Carbon In-Situ Wrapped ZnO Nanoparticles as a High-Activity Catalyst for Acetylene Acetoxylation. Catal. Lett. 2020, 150, 1155–1162. [Google Scholar] [CrossRef]
- He, P.; Huang, L.; Wu, X.; Xu, Z.; Zhu, M.; Wang, X.; Dai, B. A Novel High-Activity Zn-Co Catalyst for Acetylene Acetoxylation. Catalysts 2018, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- He, P.; Wu, X.; Huang, L.; Zhu, M.; Wang, X.; Dai, B. Acetoxylation of acetylene to vinyl acetate monomer over bimetallic Zn-Ni/AC catalysts. Catal. Commun. 2018, 112, 5–9. [Google Scholar] [CrossRef]
- Wu, X.Y.; He, P.J.; Wang, X.G.; Dai, B. Zinc acetate supported on N-doped activated carbon as catalysts for acetylene acetoxylation. Chem. Eng. J. 2017, 309, 172–177. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, M.; Kang, L. B-doped activated carbon as a support for a high-performance Zn-based catalyst in acetylene acetoxylation. Green Energy Environ. 2020. [Google Scholar] [CrossRef]
- Sakla, R.; Kaushik, R.; Kumar, V.; Jose, D.A.; Ghosh, A.; Mariappan, C.R. Light-induced water oxidation by polymorphs of the Zn-Co-Ni oxide spinel catalyst: A comparative study. Sustain. Energ Fuels 2019, 3, 786–792. [Google Scholar] [CrossRef]
- Zhu, L.L.; Hao, C.; Wang, X.H.; Guo, Y.N. Fluffy Cotton-Like GO/Zn-Co-Ni Layered Double Hydroxides Form from a Sacrificed Template GO/ZIF-8 for High Performance Asymmetric Supercapacitors. Acs Sustain. Chem. Eng. 2020, 8, 11618–11629. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Wang, Y.J.; Wang, S.F.; Yang, H.J.; Zhang, J.Y. Synthesis of MDI from dimethyl carbonate over solid catalysts. Ind. Eng. Chem. Res. 2002, 41, 5139–5144. [Google Scholar] [CrossRef]
- Li, F.; Li, W.B.; Li, J.; Xue, W.; Wang, Y.J.; Zhao, X.Q. Investigation of supported Zn (OAc)(2) catalyst and its stability in N-phenyl carbamate synthesis. Appl. Catal. A Gen. 2014, 475, 355–362. [Google Scholar] [CrossRef]
- Richardson, J.T. Principles of Catalyst Development; Springer Science & Business Media: New York, NY, USA, 1989; pp. 35–37. [Google Scholar]


| Samples | Composition of Samples | Acetic Acid Conversion (%) |
|---|---|---|
| Zn/AC | 10 wt% Zn(OAc)2 | 19.9 |
| Co/AC | 10 wt% Co(OAc)2 | 4.8 |
| Ni/AC | 10 wt% Ni(OAc)2 | 4.4 |
| Samples | SBET (m2 g–1) | V (cm3 g–1) | D (nm) |
|---|---|---|---|
| AC | 1134.4 | 0.61 | 2.16 |
| Zn(OAc)2/AC | 929.9 | 0.50 | 2.17 |
| Zn1Co0.1Ni0.05/AC | 795.7 | 0.42 | 2.09 |
| Zn1Co0.3Ni0.05/AC | 718.9 | 0.39 | 2.12 |
| Zn1Co0.5Ni0.05/AC | 621.2 | 0.34 | 2.11 |
| Zn1Co0.3Ni0.01/AC | 730.5 | 0.33 | 2.10 |
| Zn1Co0.3Ni0.09/AC | 724.6 | 0.38 | 2.12 |
| Catalysts | Zn (OAc)2 Loading, wt% | Co (OAc)2 Loading, wt% | Ni (OAc)2 Loading, wt% |
|---|---|---|---|
| Zn (OAc)2/AC | 8.69 | - | - |
| Zn1Co0.3Ni0.05/AC | 8.75 | 2.61 | 0.38 |
| Zn (OAc)2/AC-U | 7.51 | - | - |
| Zn1Co0.3Ni0.05/AC-U | 7.71 | 0.92 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; He, P.; Chen, Y.; Zhu, M.; Wang, X.; Dai, B. Performance Study of Zn-Co-Ni/AC Catalyst in Acetylene Acetylation. Catalysts 2021, 11, 1271. https://doi.org/10.3390/catal11111271
Xu Z, He P, Chen Y, Zhu M, Wang X, Dai B. Performance Study of Zn-Co-Ni/AC Catalyst in Acetylene Acetylation. Catalysts. 2021; 11(11):1271. https://doi.org/10.3390/catal11111271
Chicago/Turabian StyleXu, Zhuang, Peijie He, Yuhao Chen, Mingyuan Zhu, Xugen Wang, and Bin Dai. 2021. "Performance Study of Zn-Co-Ni/AC Catalyst in Acetylene Acetylation" Catalysts 11, no. 11: 1271. https://doi.org/10.3390/catal11111271







