Ru-gC3N4 Catalyzed Hydrodebenzylation of Benzyl Protected Alcohol and Acid Groups Using Sodium Hypophosphite as a Hydrogen Source
Abstract
1. Introduction
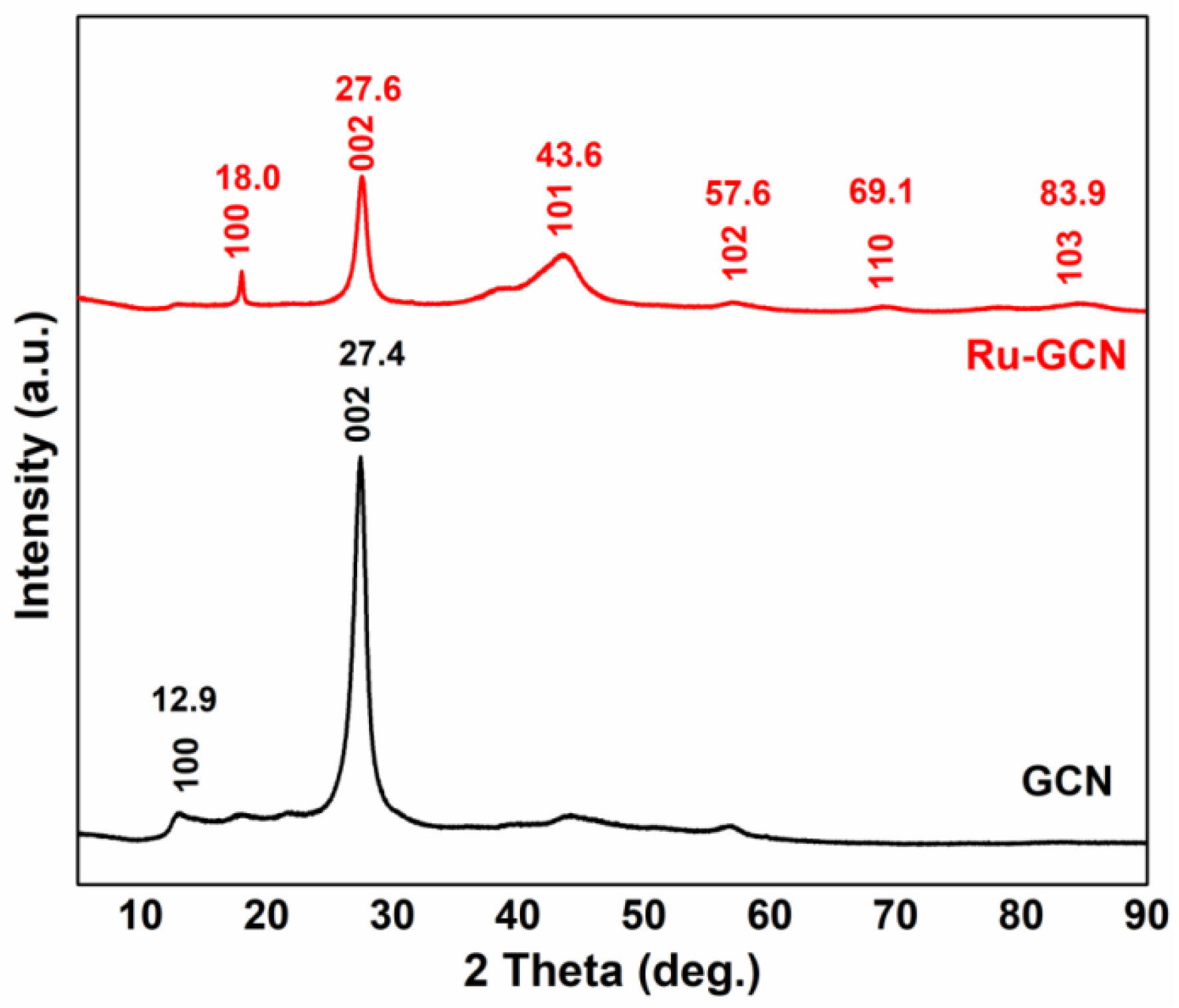
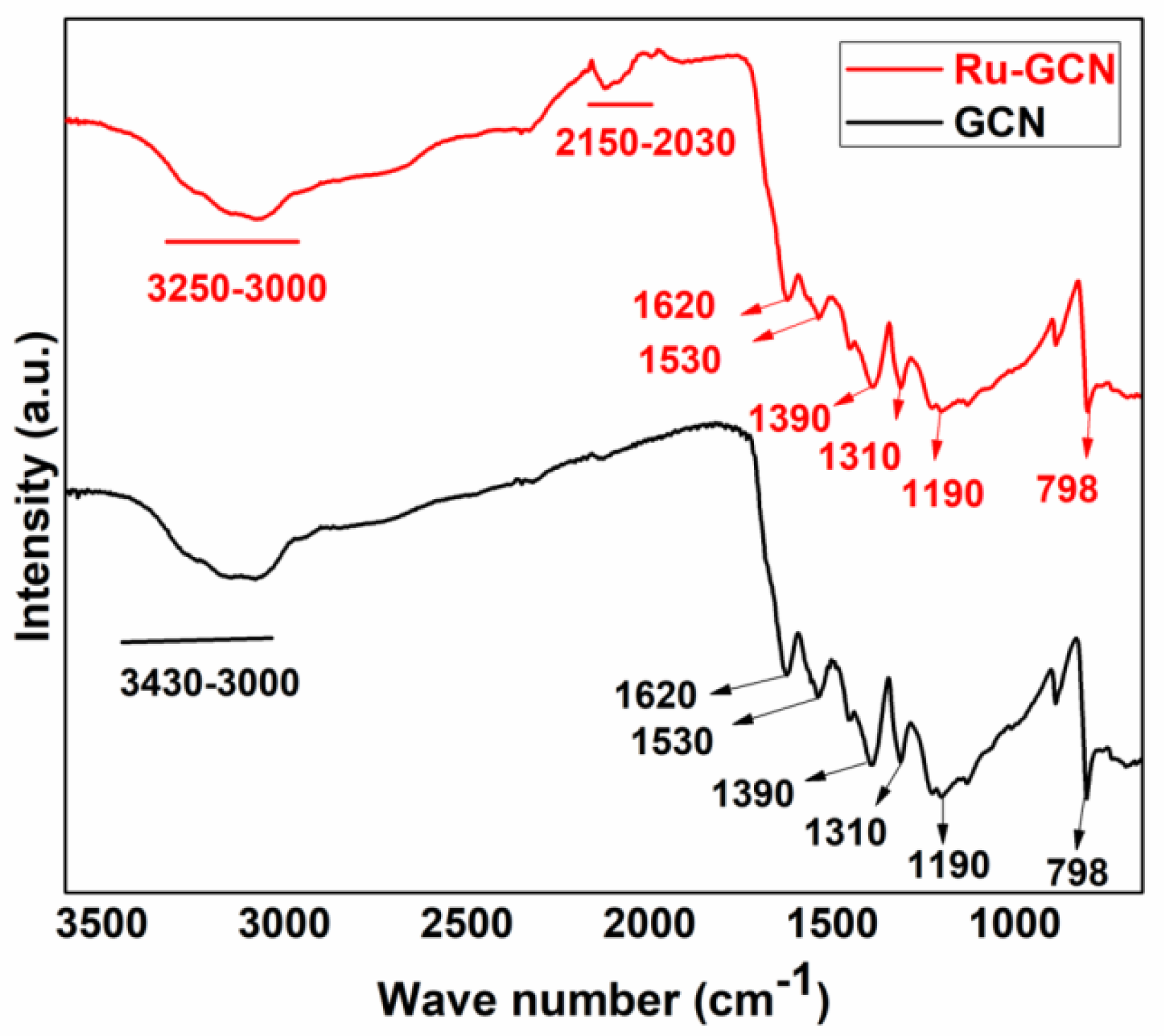
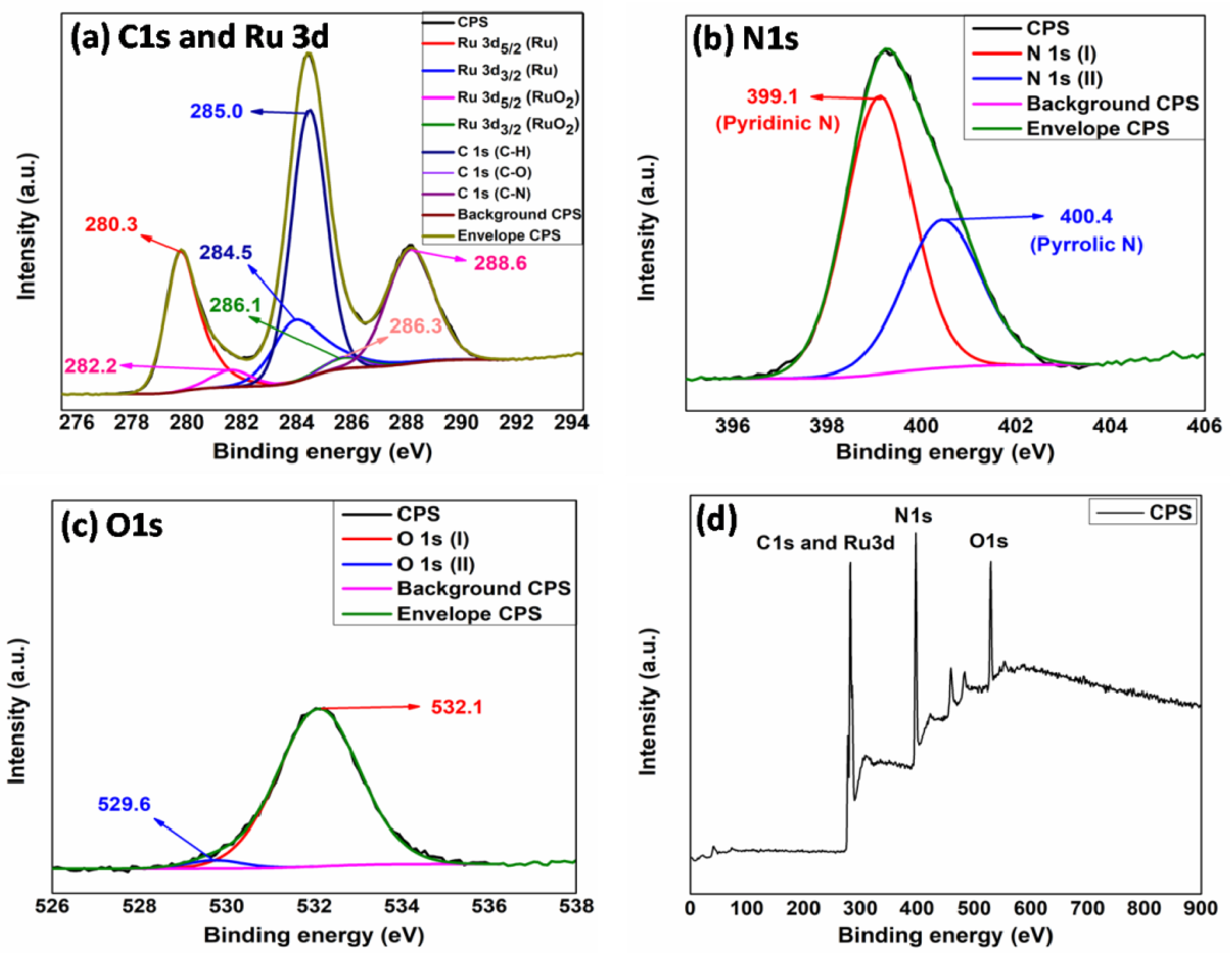
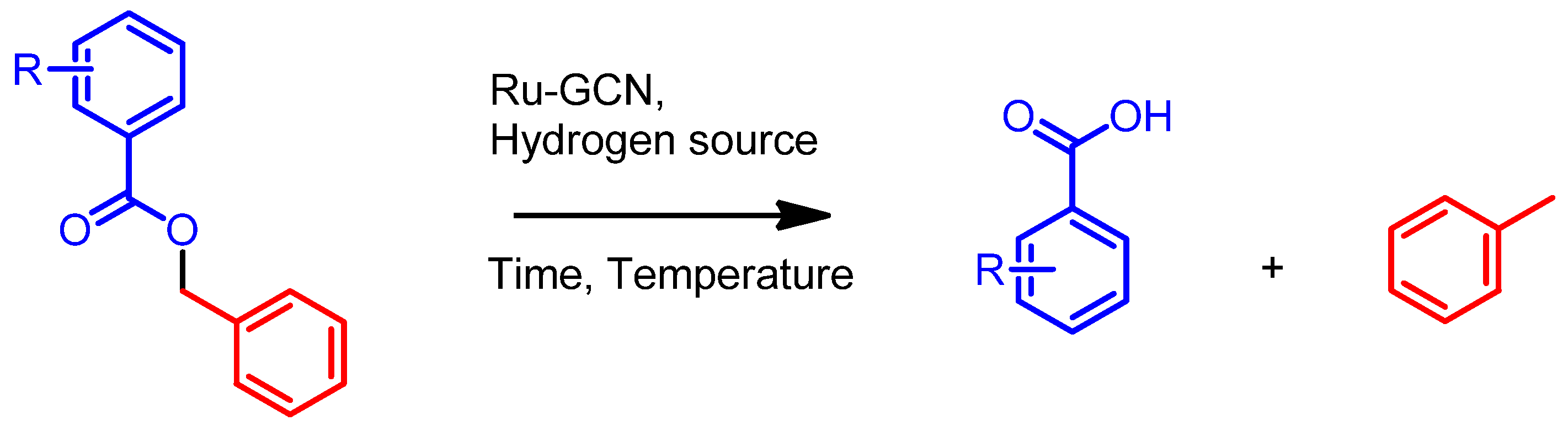
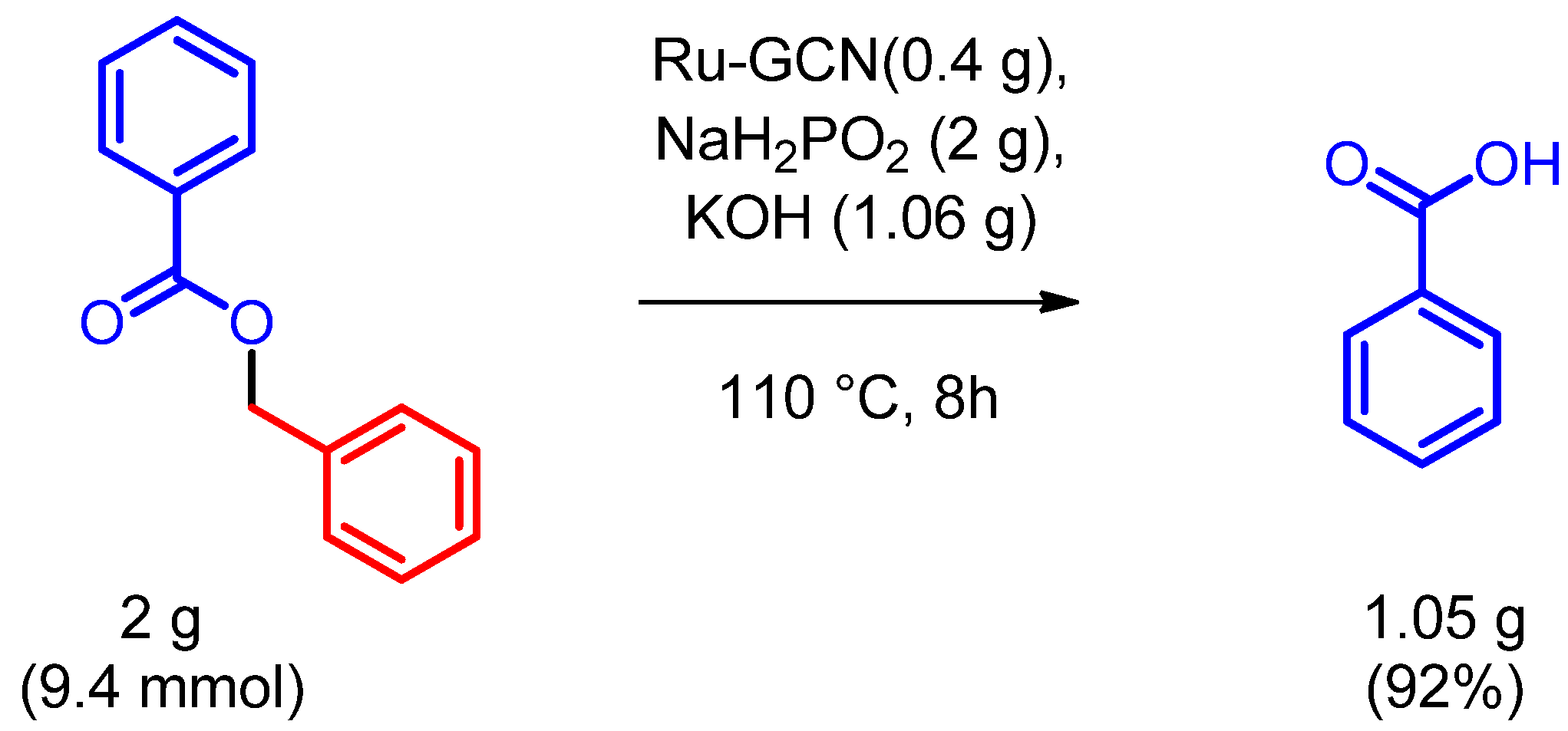
2. Results
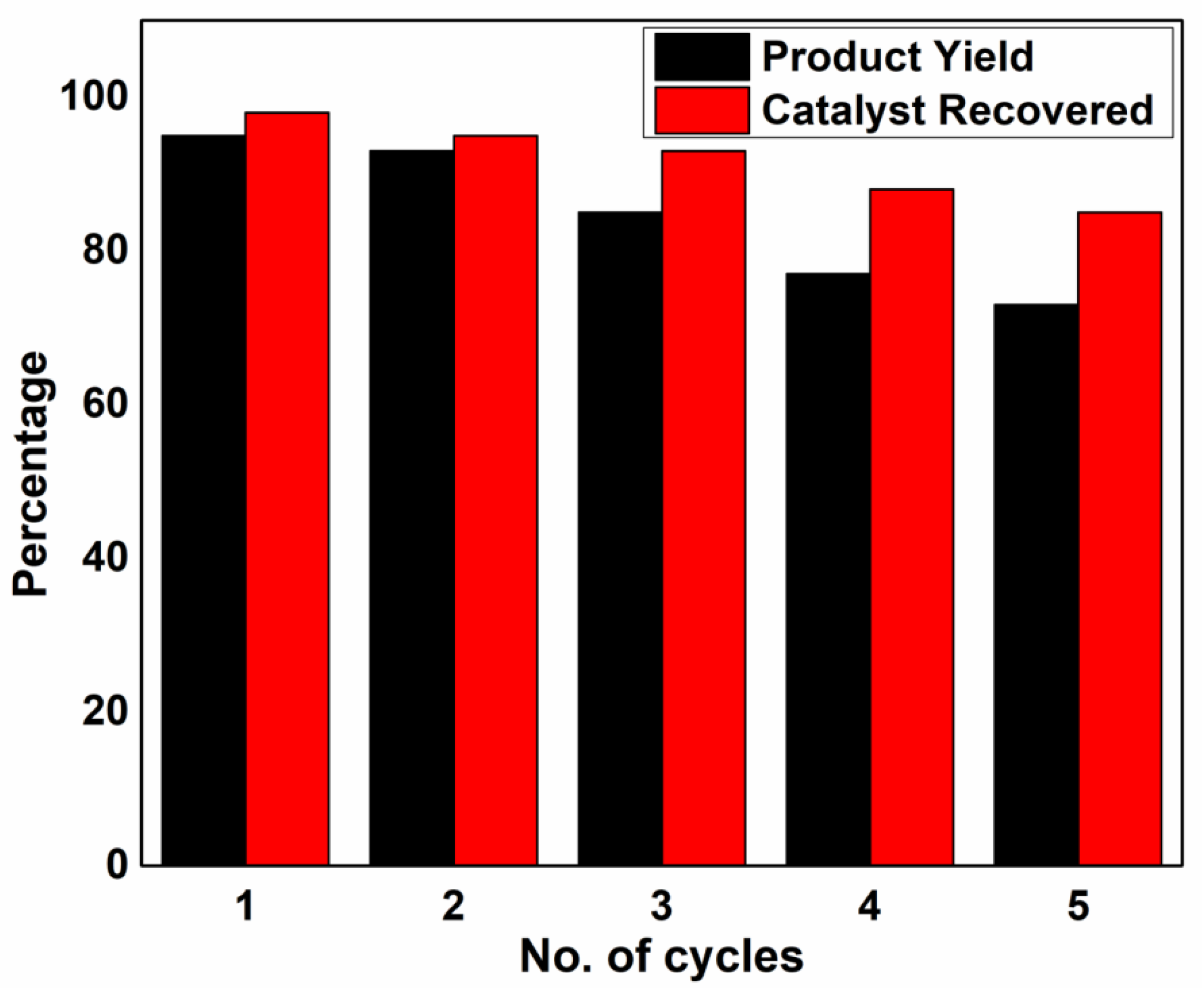
Catalytic Activity Studies
3. Material and Methods
3.1. Fabrication of Graphitic Carbon Nitride (GCN)
3.2. Synthesis of Ru-gC3N4 (Ru-GCN)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Experimental Section
References
- Greene, T.W.; Wuts, P.G. Protective Groups in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Yakukhnov, S.A.; Ananikov, V.P. Catalytic transfer hydrodebenzylation with low palladium loading. Adv. Synth. Catal. 2019, 361, 4781–4789. [Google Scholar] [CrossRef]
- Weissman, S.A.; Zewge, D. Recent advances in ether dealkylation. Tetrahedron 2005, 61, 7833–7863. [Google Scholar] [CrossRef]
- Wuts, P.G.M. Greene’s Protective Groups in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Zhou, L.; Wang, W.; Zuo, L.; Yao, S.; Wang, W.; Duan, W. Selective debenzylation of aromatic benzyl ethers by silica-supported sodium hydrogen sulfate. Tetrahedron Lett. 2008, 49, 4876. [Google Scholar] [CrossRef]
- Jensen, R.K.; Thykier, N.; Enevoldsen, M.V.; Lindhardt, A.T. A high mobility reactor unit for r&d continuous flow transfer hydrogenations. Org. Process Res. Dev. 2017, 21, 370–376. [Google Scholar]
- Lednicer, D. The Organic Chemistry of Drug Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2007; Volume 8. [Google Scholar]
- McNair, R. Catalytic deprotection depro catalyst kit/catalytic deprotection. Aldrich ChemFiles 2009, 9, 21. [Google Scholar]
- Yin, J.; Weisel, M.; Ji, Y.; Liu, Z.; Liu, J.; Wallace, D.J.; Xu, F.; Sherry, B.D.; Yasuda, N. Improved preparation of a key hydroxylamine intermediate for relebactam: Rate enhancement of benzyl ether hydrogenolysis with dabco. Org. Process Res. Dev. 2018, 22, 273–277. [Google Scholar] [CrossRef]
- Oba, M.; Kojima, K.; Endo, M.; Sano, H.; Nishiyama, K. Palladium-catalyzed transfer hydrogenation of organic substrates by hypophosphite in water containing a nonionic surfactant. Green Chem. Lett. Rev. 2013, 6, 233–236. [Google Scholar] [CrossRef][Green Version]
- Mauriello, F.; Ariga-Miwa, H.; Paone, E.; Pietropaolo, R.; Takakusagi, S.; Asakura, K. Transfer hydrogenolysis of aromatic ethers promoted by the bimetallic pd/co catalyst. Catal. Today 2020, 357, 511–517. [Google Scholar] [CrossRef]
- Pandarus, V.; Béland, F.; Ciriminna, R.; Pagliaro, M. Selective debenzylation of benzyl protected groups with siliacat pd (0) under mild conditions. ChemCatChem 2011, 3, 1146–1150. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shimizu, E.; Ban, K.; Wada, Y.; Mizusaki, T.; Yoshimura, M.; Takagi, Y.; Sawama, Y.; Sajiki, H. Facile hydrogenative deprotection of n-benzyl groups using a mixed catalyst of palladium and niobic acid-on-carbon. ACS Omega 2020, 5, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, J.K.; Kang, K.H.; Song, J.C.; Song, I.K. Selective cleavage of co bond in benzyl phenyl ether to aromatics over pd–fe bimetallic catalyst supported on ordered mesoporous carbon. Appl. Catal. A Gen. 2015, 498, 142–149. [Google Scholar] [CrossRef]
- Li, L.; Dong, L.; Li, D.; Guo, Y.; Liu, X.; Wang, Y. Hydrogen-free production of 4-alkylphenols from lignin via self-reforming-driven depolymerization and hydrogenolysis. ACS Catal. 2020, 10, 15197–15206. [Google Scholar] [CrossRef]
- Zhang, J.; Ibrahim, M.; Collière, V.; Asakura, H.; Tanaka, T.; Teramura, K.; Philippot, K.; Yan, N. Rh nanoparticles with niox surface decoration for selective hydrogenolysis of co bond over arene hydrogenation. J. Mol. Catal. A Chem. 2016, 422, 188–197. [Google Scholar] [CrossRef]
- Ji, J.; Guo, H.; Li, C.; Qi, Z.; Zhang, B.; Dai, T.; Jiang, M.; Ren, C.; Wang, A.; Zhang, T. Tungsten-based bimetallic catalysts for selective cleavage of lignin c− o bonds. ChemCatChem 2018, 10, 415–421. [Google Scholar] [CrossRef]
- Wang, H.; Duan, Y.; Zhang, Q.; Yang, B. Effects of sugars, furans, and their derivatives on hydrodeoxygenation of biorefinery lignin-rich wastes to hydrocarbons. ChemSusChem 2018, 11, 2562–2568. [Google Scholar] [CrossRef]
- Gómez-Monedero, B.; Ruiz, M.; Bimbela, F.; Faria, J. Selective hydrogenolysis of αo4, βo4, 4o5 co bonds of lignin-model compounds and lignin-containing stillage derived from cellulosic bioethanol processing. Appl. Catal. A Gen. 2017, 541, 60–76. [Google Scholar] [CrossRef]
- Hartung, W.H.; Simonoff, R. Hydrogenolysis of benzyl groups attached to oxygen, nitrogen, or sulfur. Org. React. 2004, 7, 263–326. [Google Scholar]
- Oikawa, Y.; Tanaka, T.; Horita, K.; Yonemitsu, O. Selective hydrogenolysis of the benzyl protecting group for hydroxy function with raney nickel in the presence of the mpm (4-methoxybenzyl) and dmpm (3, 4-dimethoxybenzyl) protecting groups. Tetrahedron Lett. 1984, 25, 5397–5400. [Google Scholar] [CrossRef]
- Perosa, A.; Tundo, P.; Zinovyev, S. Mild catalytic multiphase hydrogenolysis of benzyl ethers. Green Chem. 2002, 4, 492–494. [Google Scholar] [CrossRef]
- Lange, S.; Formenti, D.; Lund, H.; Kreyenschulte, C.; Agostini, G.; Bartling, S.; Bachmann, S.; Scalone, M.; Junge, K.; Beller, M. Additive-free nickel-catalyzed debenzylation reactions via hydrogenative c–o and c–n bond cleavage. ACS Sustain. Chem. Eng. 2019, 7, 17107–17113. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Kang, T.-C.; Wang, Z.-Y.; Yoshikawa, T.; Nakasaka, Y.; Masuda, T.; Chuang, L.-C.; Wu, K.C.-W. Efficient liquid-phase hydrogenolysis of a lignin model compound (benzyl phenyl ether) using a ni/carbon catalyst. React. Chem. Eng. 2019, 4, 618–626. [Google Scholar] [CrossRef]
- Bernt, C.M.; Manesewan, H.; Chui, M.; Boscolo, M.; Ford, P.C. Temperature tuning the catalytic reactivity of cu-doped porous metal oxides with lignin models. ACS Sustain. Chem. Eng. 2018, 6, 2510–2516. [Google Scholar] [CrossRef]
- Ren, Y.-L.; Tian, M.; Tian, X.-Z.; Wang, Q.; Shang, H.; Wang, J.; Zhang, Z.C. Highly selective reductive cleavage of aromatic carbon–oxygen bonds catalyzed by a cobalt compound. Catal. Commun. 2014, 52, 36–39. [Google Scholar] [CrossRef]
- Malara, A.; Paone, E.; Bonaccorsi, L.; Mauriello, F.; Macario, A.; Frontera, P. Pd/fe3o4 nanofibers for the catalytic conversion of lignin-derived benzyl phenyl ether under transfer hydrogenolysis conditions. Catalysts 2020, 10, 20. [Google Scholar] [CrossRef]
- Sun, K.-k.; Lu, G.-p.; Zhang, J.-w.; Cai, C. The selective hydrogenolysis of c–o bonds in lignin model compounds by pd–ni bimetallic nanoparticles in ionic liquids. Dalton Trans. 2017, 46, 11884–11889. [Google Scholar] [CrossRef]
- Qi, L.; Chamas, A.; Jones, Z.R.; Walter, E.D.; Hoyt, D.W.; Washton, N.M.; Scott, S.L. Unraveling the dynamic network in the reactions of an alkyl aryl ether catalyzed by Ni/γ-Al2O3 in 2-propanol. J. Am. Chem. Soc. 2019, 141, 17370–17381. [Google Scholar] [CrossRef]
- Bahuguna, A.; Sasson, Y. Formate-bicarbonate cycle as a vehicle for hydrogen and energy storage. ChemSusChem 2021, 14, 1258–1283. [Google Scholar] [CrossRef]
- Yang, S.; Jeong, S.; Ban, C.; Kim, H.; Kim, D.H. Catalytic cleavage of ether bond in a lignin model compound over carbon-supported noble metal catalysts in supercritical ethanol. Catalysts 2019, 9, 158. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bahuguna, A.; Sasson, Y. Advantage of using NaH2PO2 over alkali metal formates as a hydrogen source for pd-gc3n4 catalyzed hydro-dehalogenation of aryl halides. ChemistrySelect 2021, 6, 9477–9488. [Google Scholar] [CrossRef]
- Bahuguna, A.; Chakraborty, S.; Sasson, Y. Nio–ni/graphitic carbon nitride as a selective catalyst for transfer hydrogenation of carbonyl compounds using NaH2PO2 as a hydrogen source. Int. J. Hydrogen Energy 2021, 46, 28554–28564. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.W.; Shi, R.; Zhu, Y.F. Chemical Exfoliation of Graphitic Carbon Nitride for Efficient Heterogeneous Photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
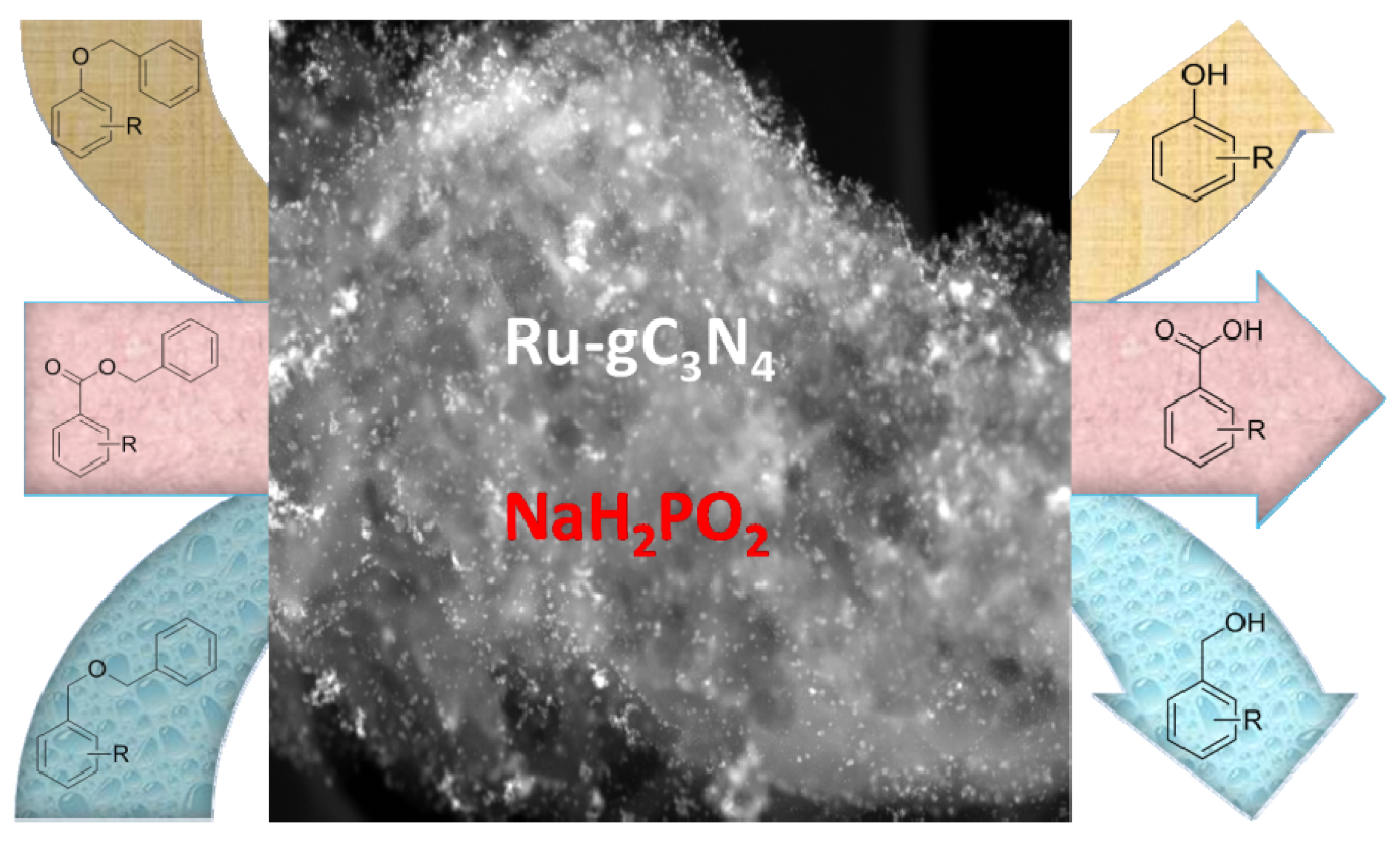


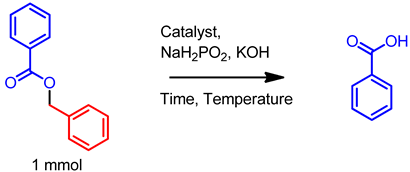 | |||||||
|---|---|---|---|---|---|---|---|
| Entry No. | Ru-GCN (mg) | NaH2PO2 (eq.) | KOH Additive (eq.) | Solvent | Temp. (°C) | Time (h) | Yield (%) |
| 1 | 42 | 2.0 | 2.0 | 1:1 EtOH/H2O | RT | 24 | 0 |
| 2 | 84 | 3.0 | 3.0 | 1:1 EtOH/H2O | RT | 24 | 0 |
| 3 | 42 | 2.0 | 2.0 | 1:1 EtOH/H2O | 50 | 24 | 0 |
| 4 | 42 | 2.0 | 2.0 | 1:1 EtOH/H2O | 80 | 24 | 40 |
| 5 | 42 | 2.0 | 2.0 | 1:1 EtOH/H2O | 110 | 8 | 95 |
| 6 | 21 | 2.0 | 2.0 | 1:1 EtOH/H2O | 110 | 8 | 70 |
| 7 | 00 | 2.0 | 2.0 | 1:1 EtOH/H2O | 110 | 24 | 0 |
| 8 | 42 | 1.0 | 2.0 | 1:1 EtOH/H2O | 110 | 8 | 60 |
| 9 | 42 | 3.0 | 2.0 | 1:1 EtOH/H2O | 110 | 8 | 95 |
| 10 | 42 | 2.0 | 1.0 | 1:1 EtOH/H2O | 110 | 8 | 65 |
| 11 | 42 | 2.0 | 00 | 1:1 EtOH/H2O | 110 | 8 | 40 |
| 12 | 42 | 2.0 | 2.0 | 1:1 EtOH/H2O | 110 | 6 | 82 |
| 13 | 42 | 2.0 | 2.0 | 1:1 EtOH/H2O | 110 | 4 | 65 |
| 14 | 42 | 2.0 | 2.0 | EtOH | 110 | 24 | 0 |
| 15 | 42 | 2.0 | 2.0 | 1:1 Dioxane/H2O | 110 | 8 | 20 |
| 16 | 42 | 2.0 | 0.0 | 1:1 AcOH/H2O | 110 | 8 | 0 |
| Sl. No | Substrate | Product | Isolated Yield (%) |
|---|---|---|---|
| 1 | 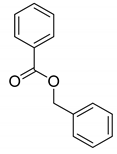 | 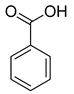 | 95 |
| 2 | 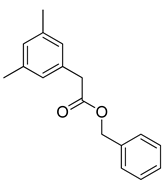 |  | 93 |
| 3 |  |  | 93 |
| 4 |  | 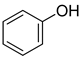 | 90 |
| 5 |  |  | 86 |
| 6 | 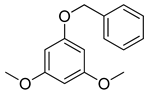 | 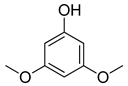 | 88 |
| 7 |  | 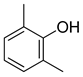 | 85 |
| 8 | 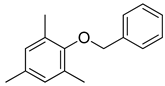 |  | 85 |
| 9 | 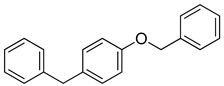 | 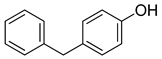 | 88 |
| 10 | 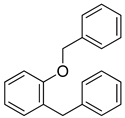 |  | 83 |
| 11 |  |  | 92 |
| 12 | 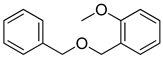 | 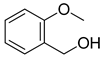 | 89 |
| Entry. No. | Catalyst | H2 Sources | Additive/Base | Nature of Catalyst | Temp. (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Pd(OAC)2 | Hydrogen (1 atm) | N/A | Het. | 25 °C | 14 | 99 | [2] |
| 2 | Pd/C | Hydrogen (50 pSi) | N,O-bis(trimethylsilyl)acetamide(BSA) and Dabco | Het. | RT | 8 | 88 | [9] |
| 3 | Pd/AC | Sodium hypophosphite monohydrate | Tween 20 as a surfactant | Het. | 50 °C | 1 | 94 | [10] |
| 4 | Pd/C | Sodium borohydride | N/A | Het. | 80 °C | 1 | 100 | [24] |
| 5 | Pd/Co | 2-isopropanol | N/A | Het. | 240 °C | 3 | 100 | [11] |
| 6 | Pd/C | Ammonium formate | N/A | Het. | 60 °C | 1/2 | 100 | [2] |
| 7 | Pd/C | Formic acid | KOH | Het. | 70 °C | 1 | 100 | [2] |
| 8 | Ni/CB | Sodium borohydride | N/A | Het. | 80 °C | 2 | 90 | [24] |
| 9 | Ru/AC | Molecular Hydrogen (25 bar) | N/A | Het. | 150 °C | 2 | 100 | [19] |
| 10 | Ru/AC | Supercritical ethanol | N/A | Het. | 270 °C | 4 | 60 | [31] |
| 11 | Ru-W/AC | Molecular Hydrogen (0.7 MPa) | N/A | Het. | 260 °C | 10 | 93 | [17] |
| 12 | Indium triflate, Ru/Al2O3 | Molecular Hydrogen (580 pSi) | N/A | Het. | 250 °C | 2 | 80 | [18] |
| 13 | Ru-gC3N4 | Sodium hypophosphite | KOH | Het. | 110 °C | 8 | Upto 95 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, S.; Bahuguna, A.; Sasson, Y. Ru-gC3N4 Catalyzed Hydrodebenzylation of Benzyl Protected Alcohol and Acid Groups Using Sodium Hypophosphite as a Hydrogen Source. Catalysts 2021, 11, 1227. https://doi.org/10.3390/catal11101227
Chakraborty S, Bahuguna A, Sasson Y. Ru-gC3N4 Catalyzed Hydrodebenzylation of Benzyl Protected Alcohol and Acid Groups Using Sodium Hypophosphite as a Hydrogen Source. Catalysts. 2021; 11(10):1227. https://doi.org/10.3390/catal11101227
Chicago/Turabian StyleChakraborty, Sourav, Ashish Bahuguna, and Yoel Sasson. 2021. "Ru-gC3N4 Catalyzed Hydrodebenzylation of Benzyl Protected Alcohol and Acid Groups Using Sodium Hypophosphite as a Hydrogen Source" Catalysts 11, no. 10: 1227. https://doi.org/10.3390/catal11101227
APA StyleChakraborty, S., Bahuguna, A., & Sasson, Y. (2021). Ru-gC3N4 Catalyzed Hydrodebenzylation of Benzyl Protected Alcohol and Acid Groups Using Sodium Hypophosphite as a Hydrogen Source. Catalysts, 11(10), 1227. https://doi.org/10.3390/catal11101227







