Fe-TiO2/AC and Co-TiO2/AC Composites: Novel Photocatalysts Prepared from Waste Streams for the Efficient Removal and Photocatalytic Degradation of Cibacron Yellow F-4G Dye
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Adsorbents
2.1.1. TGA
2.1.2. Elemental Analysis
2.1.3. pHPZC
2.1.4. Characterization of Crystalline Phase
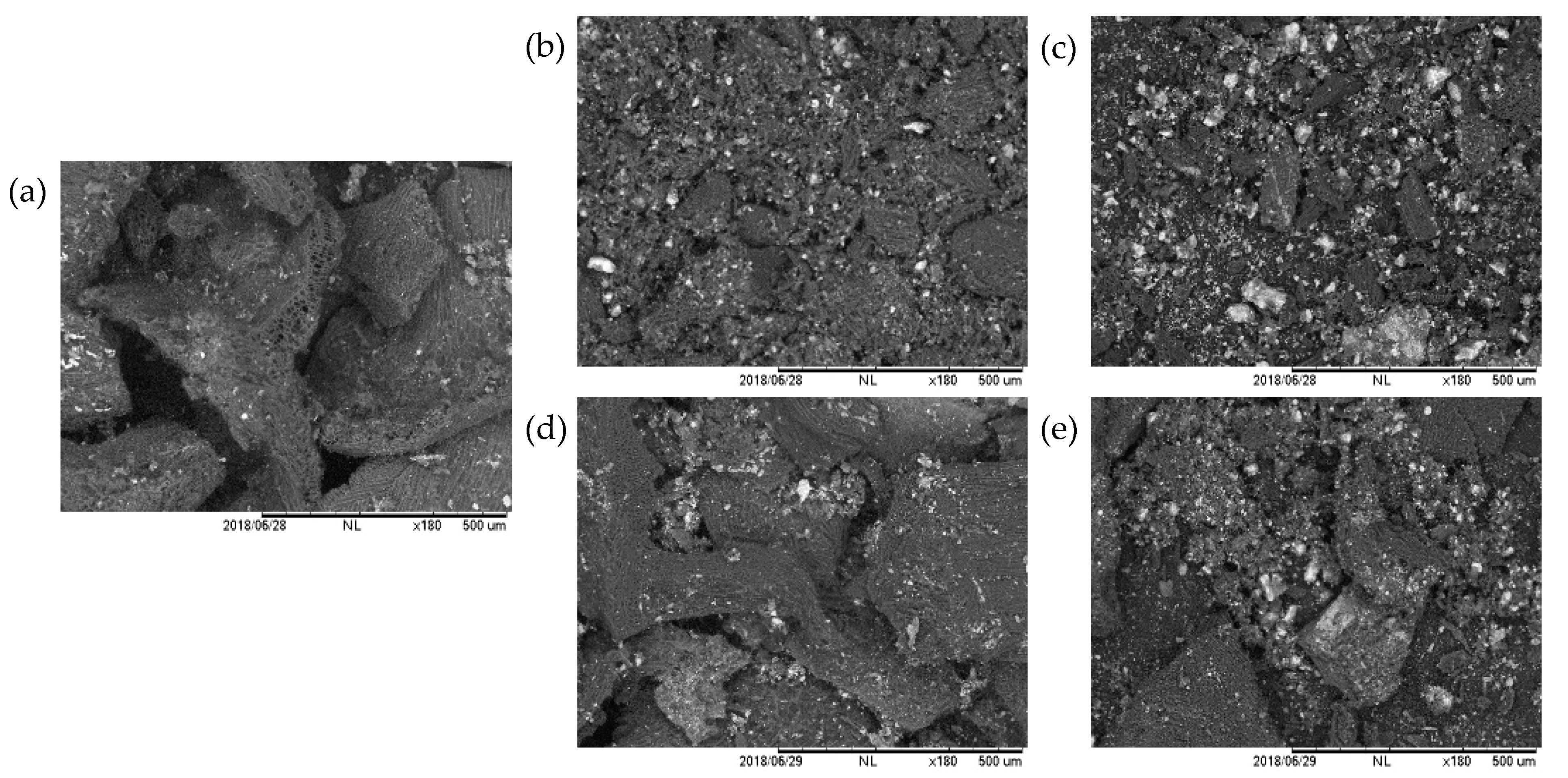
2.1.5. SEM-EDS and Binarization of SEM Images
2.1.6. X-ray Absorption (XRA)
2.2. Adsorption Studies
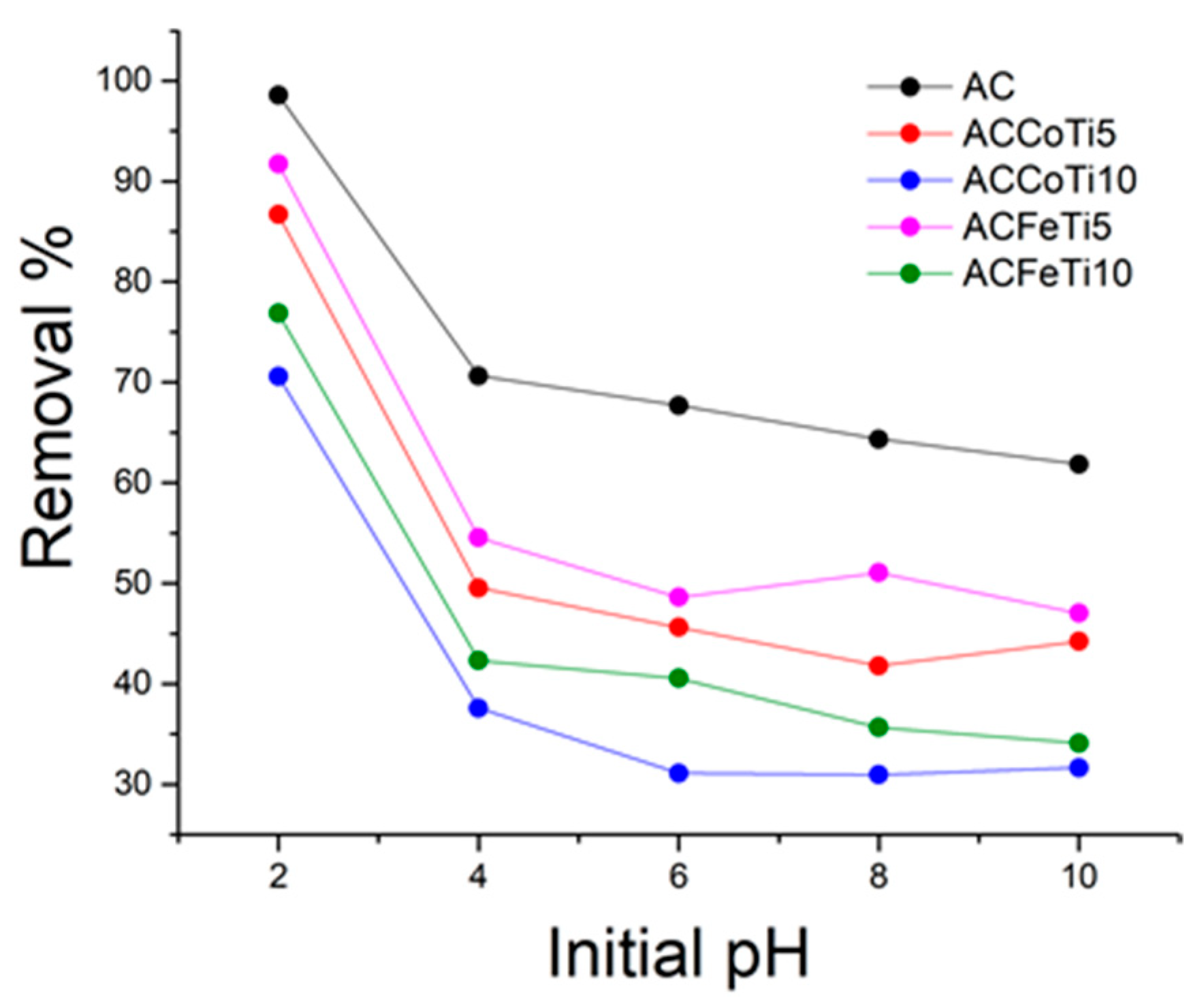
2.2.1. Effect of Initial pH
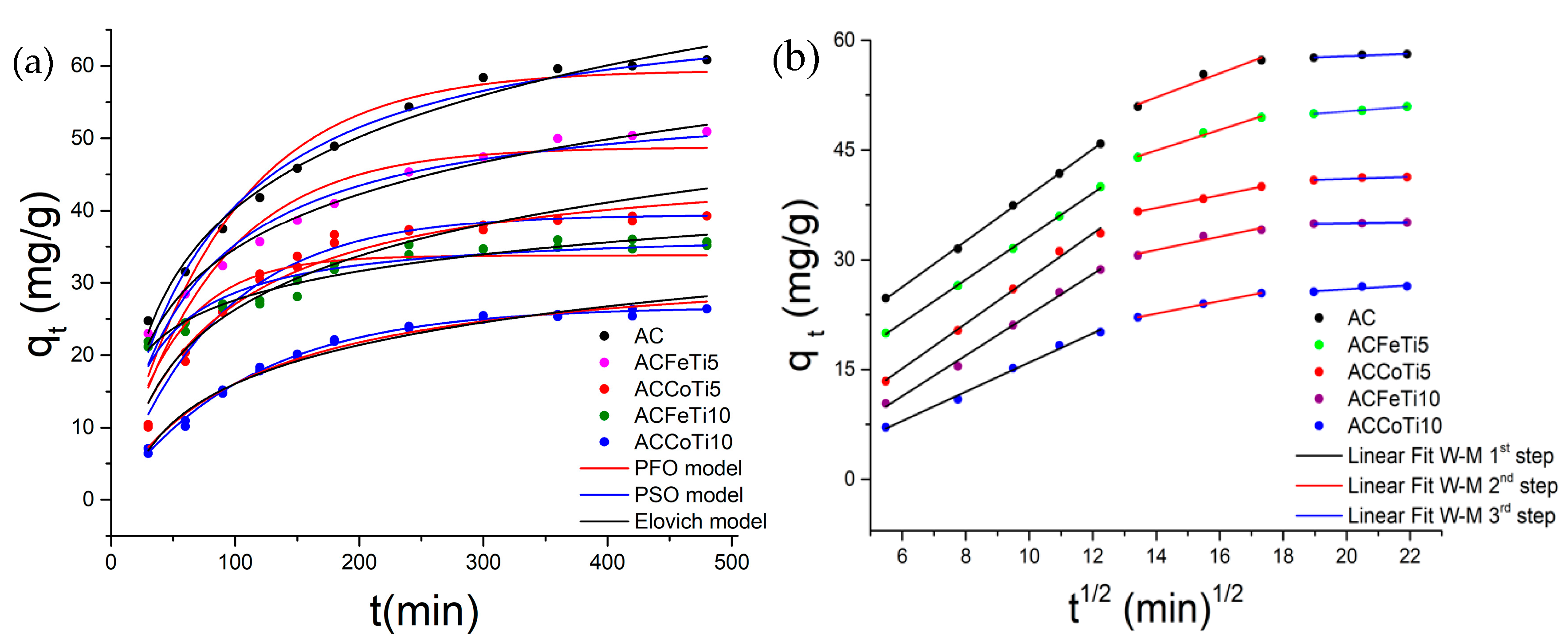
2.2.2. Adsorption Kinetics Studies
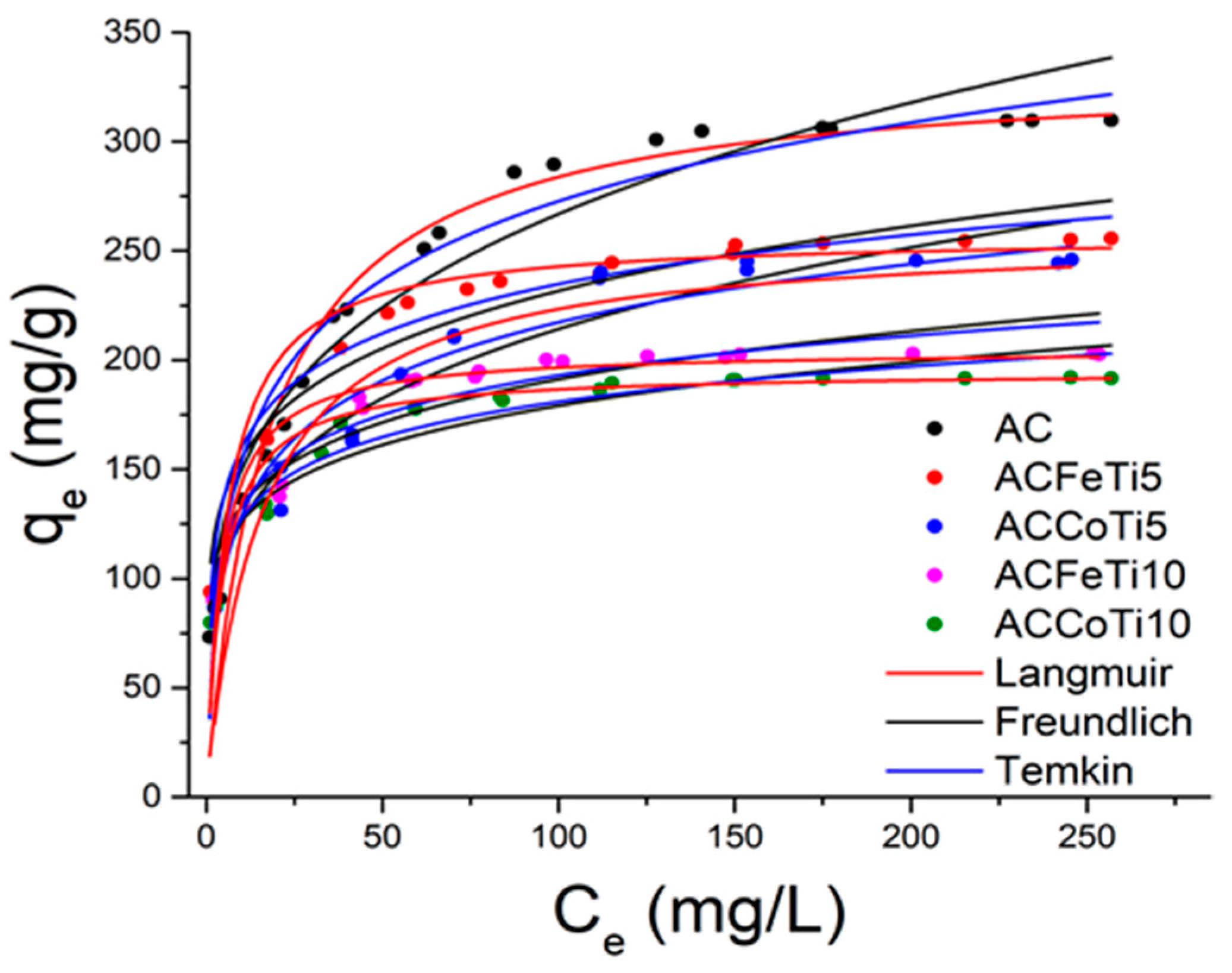
2.2.3. Adsorption Isotherms-Models
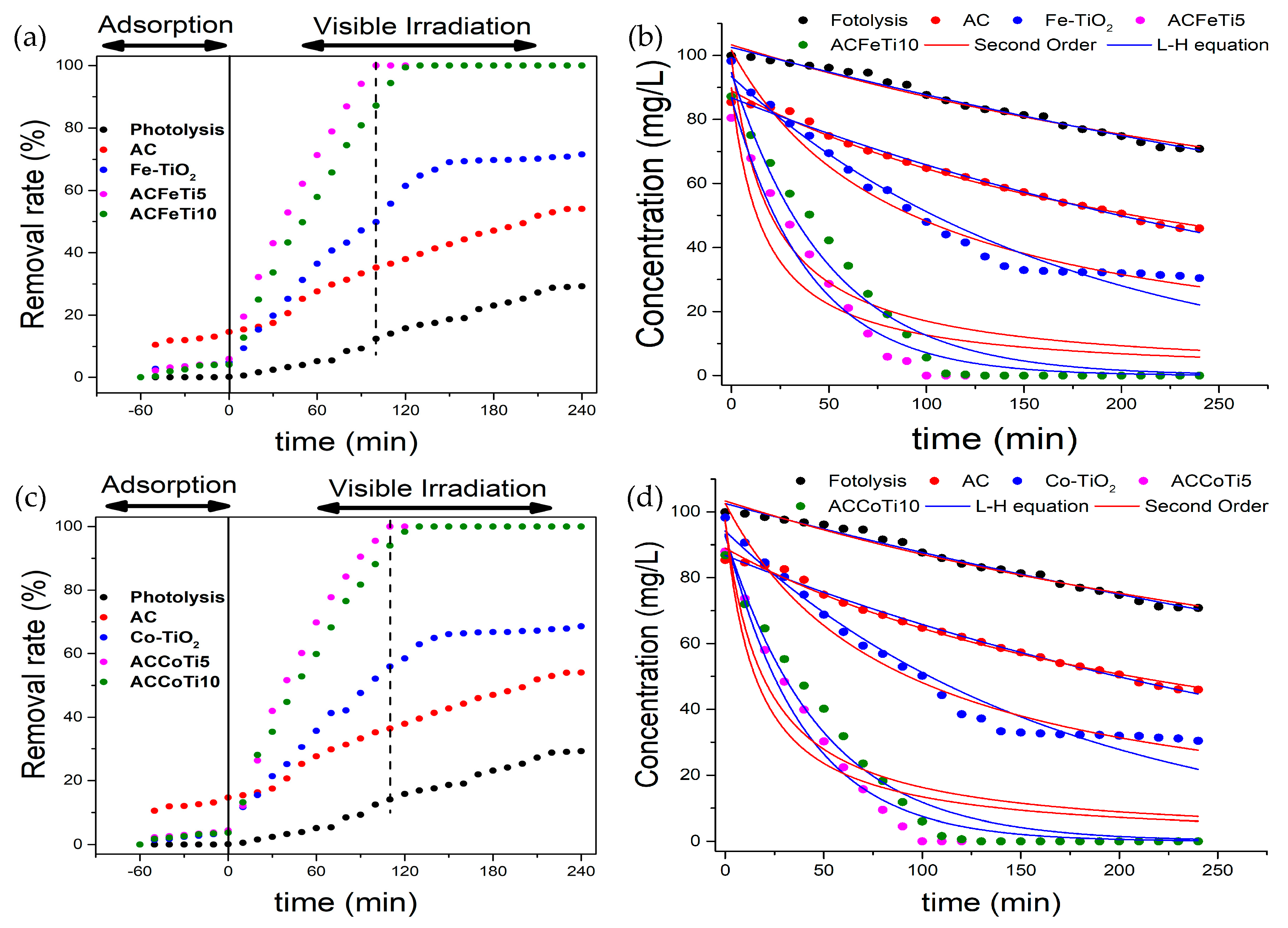
2.3. Photocatalytic Degradation of CYF-4G
3. Materials and Methods
3.1. Materials
3.2. Electrochemical Preparation of Nanosized TiO2
3.3. Preparation of Fe-TiO2/AC or Co-TiO2/AC Composites
3.4. Characterisation Techniques
3.4.1. Proximate Analysis
3.4.2. Elemental Analysis (EA)
3.4.3. pH at the Point of Zero Charge (pHPZC)
3.4.4. X-ray Diffraction (XRD)
3.4.5. Scanning Electron Microscopy (SEM)—Energy Dispersive X-ray Spectroscopy (EDS)
3.4.6. X-ray Absorption (XRA)
3.5. Batch Equilibrium Studies
3.6. Effect of Solution pH
3.7. Adsorption Equilibrium Isotherms
3.8. Adsorption Kinetic Models
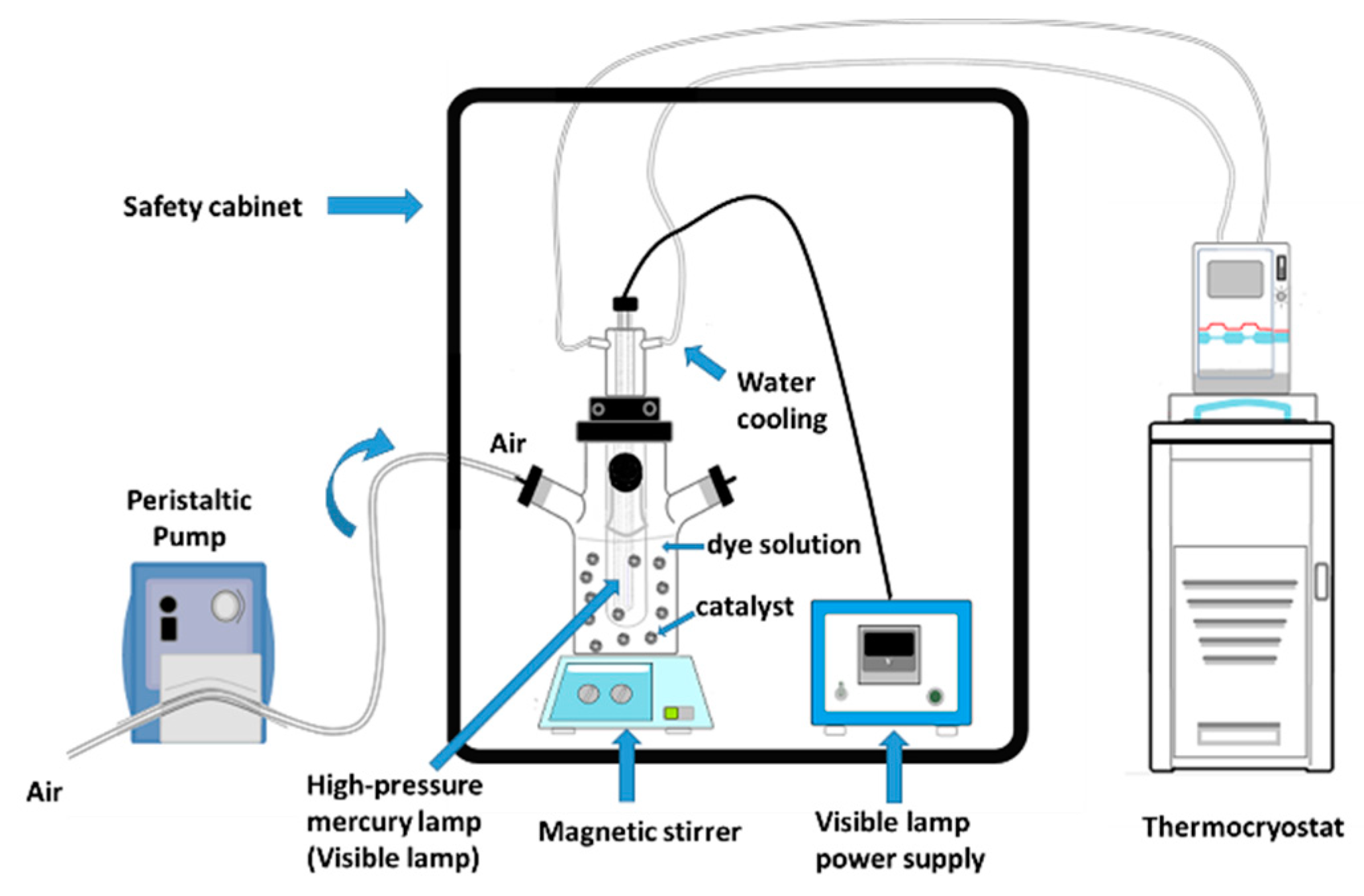
3.9. Photocatalytic Degradation Experiment
3.10. Kinetic Equations of CYF-4G Dye Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, C.H.; Tran, H.N.; Fu, C.-C.; Lu, Y.-T.; Juang, R.-S. Roles of adsorption and photocatalysis in removing organic pollutants from water by activated carbon–supported titania composites: Kinetic aspects. J. Taiwan Inst. Chem. Eng. 2020, 109, 51–61. [Google Scholar] [CrossRef]
- Galán, J.; Rodríguez, A.; Gómez, J.; Allen, S.; Walker, G. Reactive dye adsorption onto a novel mesoporous carbon. Chem. Eng. J. 2013, 219, 62–68. [Google Scholar] [CrossRef]
- Machado Garcia, R.; Carleer, R.; Arada Pérez, M.; Gryglewicz, G.; Maggen, J.; Haeldermans, T.; Yperman, J. Adsorption of Cibacron Yellow F-4G dye onto activated carbons obtained from peanut hull and rice husk: Kinetics and equilibrium studies. Biomass Convers. Biorefin. 2020, 1–17. [Google Scholar] [CrossRef]
- Gu, Y.; Yperman, J.; Carleer, R.; D’Haen, J.; Maggen, J.; Vanderheyden, S.; Vanreppelen, K.; Garcia, R.M. Adsorption and photocatalytic removal of Ibuprofen by activated carbon impregnated with TiO2 by UV–Vis monitoring. Chemosphere 2019, 217, 724–731. [Google Scholar] [CrossRef]
- GA, U. Transforming our World: The 2030 Agenda for Sustainable Development; Division for Sustainable Development Goals: New York, NY, USA, 2015. [Google Scholar]
- Murillo-Acevedo, Y.; Giraldo, L.; Moreno-Piraján, J.C. Nanoparticles size distribution and phenol photodegradation with TiO2/C support obtained by phosphoric acid activation of palm kernel shell. Microporous Mesoporous Mater. 2020, 304, 109325. [Google Scholar] [CrossRef]
- Asiltürk, M.; Şener, Ş. TiO2-activated carbon photocatalysts: Preparation, characterization and photocatalytic activities. Chem. Eng. J. 2012, 180, 354–363. [Google Scholar] [CrossRef]
- Velasco, L.F.; Parra, J.B.; Ania, C.O. Phenol adsorption and photo-oxidation on porous carbon/titania composites. Adsorpt. Sci. Technol. 2010, 28, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, F.; Gao, J.; Chen, Y.; Hao, X. Ordered mesoporous TiO2/activated carbon for adsorption and photocatalysis of acid red 18 solution. Bioresources 2017, 12, 9086–9102. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, S.; Zhang, J.; Zhong, W.; Huang, X. Cu2−xSe/CdS composite photocatalyst with enhanced visible light photocatalysis activity. Appl. Surf. Sci. 2019, 478, 762–769. [Google Scholar] [CrossRef]
- Helali, S.; Polo-López, M.I.; Fernández-Ibáñez, P.; Ohtani, B.; Amano, F.; Malato, S.; Guillard, C. Solar photocatalysis: A green technology for E. coli contaminated water disinfection. Effect of concentration and different types of suspended catalyst. J. Photochem. Photobiol. A Chem. 2014, 276, 31–40. [Google Scholar] [CrossRef]
- Ribeiro, E.; Plantard, G.; Teyssandier, F.; Maury, F.; Sadiki, N.; Chaumont, D.; Goetz, V. Activated-carbon/TiO2 composites preparation: An original grafting by milling approach for solar water treatment applications. J. Environ. Chem. Eng. 2020, 8, 104115. [Google Scholar] [CrossRef]
- Tsang, C.H.A.; Li, K.; Zeng, Y.; Zhao, W.; Zhang, T.; Zhan, Y.; Xie, R.; Leung, D.Y.C.; Huang, H. Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: Overview and forecast. Environ. Int. 2019, 125, 200–228. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, N.; Sayadi, M.H.; Sobhani, S.; Hajiani, M. A potential natural solar light active photocatalyst using magnetic ZnFe2O4 TiO2/Cu nanocomposite as a high performance and recyclable platform for degradation of naproxen from aqueous solution. J. Clean. Prod. 2020, 268, 122023. [Google Scholar] [CrossRef]
- Vernardou, D.; Vlachou, K.; Spanakis, E.; Stratakis, E.; Katsarakis, N.; Kymakis, E.; Koudoumas, E. Influence of solution chemistry on the properties of hydrothermally grown TiO2 for advanced applications. Catal. Today 2009, 144, 172–176. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, B.; Wang, L.; Li, Y.; Qiao, Q. Enhanced photocatalytic activity of Fe-doped TiO2 coated on N-doped activated carbon composites for photocatalytic degradation of dyeing wastewater. AIP Conf. Proc. 2017, 1890, 020009. [Google Scholar] [CrossRef] [Green Version]
- Turkten, N.; Cinar, Z. Photocatalytic decolorization of azo dyes on TiO2: Prediction of mechanism via conceptual DFT. Catal. Today 2017, 287, 169–175. [Google Scholar] [CrossRef]
- Bezares, I.; del Campo, A.; Herrasti, P.; Muñoz-Bonilla, A. A simple aqueous electrochemical method to synthesize TiO2 nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 29319–29326. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Díaz, D.; Fernández, D.; Sepúlveda, S.; Lindeke, R.; Pérez-Bueno, J.; Peláez-Abellán, E.; Manríquez, J. Preparation of nanoparticulate TiO2 containing nanocrystalline phases of anatase and brookite by electrochemical dissolution of remelted titanium components. Arab. J. Chem. 2018, 13, 2858–2864. [Google Scholar] [CrossRef]
- Almaguer, M.; Aira, M.-J.; Rodríguez-Rajo, F.J.; Rojas, T.I. Temporal dynamics of airborne fungi in Havana (Cuba) during dry and rainy seasons: Influence of meteorological parameters. Int. J. Biometeorol. 2014, 58, 1459–1470. [Google Scholar] [CrossRef]
- Rosu, M.-C.; Coros, M.; Pogacean, F.; Magerusan, L.; Socaci, C.; Turza, A.; Pruneanu, S. Azo dyes degradation using TiO2-Pt/graphene oxide and TiO2-Pt/reduced graphene oxide photocatalysts under UV and natural sunlight irradiation. Solid State Sci. 2017, 70, 13–20. [Google Scholar] [CrossRef]
- Koh, P.W.; Yuliati, L.; Lee, S.L. Effect of transition metal oxide doping (Cr, Co, V) in the photocatalytic activity of TiO2 for congo red degradation under visible light. J. Teknol. 2014, 69, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Jo, W.-K.; Kumar, S.; Isaacs, M.A.; Lee, A.F.; Karthikeyan, S. Cobalt promoted TiO2/GO for the photocatalytic degradation of oxytetracycline and Congo Red. Appl. Catal. B Environ. 2017, 201, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, J.; Ma, M.; Ouyang, Y.; Yan, W. Preparation of TiO2/activated carbon with Fe ions doping photocatalyst and its application to photocatalytic degradation of reactive brilliant red K2G. Sci. China Ser. B Chem. 2009, 52, 1113–1119. [Google Scholar] [CrossRef]
- Eshaghi, A.; Moradi, H. Optical and photocatalytic properties of the Fe-doped TiO2 nanoparticles loaded on the activated carbon. Adv. Powder Technol. 2018, 29, 1879–1885. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Hadidi, M.R.; Salehzadeh, D.; Nemati, A. Hydrophobic octadecylamine-functionalized graphene/TiO2 hybrid coating for corrosion protection of copper bipolar plates in simulated proton exchange membrane fuel cell environment. Int. J. Hydrogen Energy 2020, 45, 15380–15389. [Google Scholar] [CrossRef]
- Rouzafzay, F.; Shidpour, R.; Al-Abri, M.Z.; Qaderi, F.; Ahmadi, A.; Myint, M.T.Z. Graphene@ ZnO nanocompound for short-time water treatment under sun-simulated irradiation: Effect of shear exfoliation of graphene using kitchen blender. J. Alloys Compd. 2020, 829, 154614. [Google Scholar] [CrossRef]
- Cui, W.; Bai, H.; Shang, J.; Wang, F.; Xu, D.; Ding, J.; Fan, W.; Shi, W. Organic-inorganic hybrid-photoanode built from NiFe-MOF and TiO2 for efficient PEC water splitting. Electrochim. Acta 2020, 349, 136383. [Google Scholar] [CrossRef]
- Cui, W.; Kang, X.; Zhang, X.; Cui, X. Gel-like ZnO/Zr-MOF (bpy) nanocomposite for highly efficient adsorption of Rhodamine B dye from aqueous solution. J. Phys. Chem. Solids 2019, 134, 165–175. [Google Scholar] [CrossRef]
- El Bekkali, C.; Bouyarmane, H.; El Karbane, M.; Masse, S.; Saoiabi, A.; Coradin, T.; Laghzizil, A. Zinc oxide-hydroxyapatite nanocomposite photocatalysts for the degradation of ciprofloxacin and ofloxacin antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Chong, R.; Fan, Y.; Du, Y.; Liu, L.; Chang, Z.; Li, D. Hydroxyapatite decorated TiO2 as efficient photocatalyst for selective reduction of CO2 with H2O into CH4. Int. J. Hydrogen Energy 2018, 43, 22329–22339. [Google Scholar] [CrossRef]
- Ali, S.; Li, Z.; Chen, S.; Zada, A.; Khan, I.; Khan, I.; Ali, W.; Shaheen, S.; Qu, Y.; Jing, L. Synthesis of activated carbon-supported TiO2-based nano-photocatalysts with well recycling for efficiently degrading high-concentration pollutants. Catal. Today 2019, 335, 557–564. [Google Scholar] [CrossRef]
- Hernández-Barreto, D.F.; Rodriguez-Estupiñán, J.P.; Moreno-Piraján, J.C.; Sierra Ramírez, R.; Giraldo, L. Adsorption and Photocatalytic Study of Phenol Using Composites of Activated Carbon Prepared from Onion Leaves (Allium fistulosum) and Metallic Oxides (ZnO and TiO2). Catalysts 2020, 10, 574. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Open Access Libr. J. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Vanderheyden, S.R.H.; Vanreppelen, K.; Yperman, J.; Carleer, R.; Schreurs, S. Chromium(VI) removal using in-situ nitrogenized activated carbon prepared from Brewers’ spent grain. Adsorption 2018, 24, 147–156. [Google Scholar] [CrossRef]
- Rodiguez, M.H.; Yperman, J.; Carleer, R.; Maggen, J.; Dadi, D.; Gryglewicz, G.; Van der Bruggen, B.; Hernández, J.F.; Calvis, A.O. Adsorption of Ni (II) on spent coffee and coffee husk based activated carbon. J. Environ. Chem. Eng. 2018, 6, 1161–1170. [Google Scholar] [CrossRef]
- Smets, K.; De Jong, M.; Lupul, I.; Gryglewicz, G.; Schreurs, S.; Carleer, R.; Yperman, J. Rapeseed and raspberry seed cakes as inexpensive raw materials in the production of activated carbon by physical activation: Effect of activation conditions on textural and phenol adsorption characteristics. Materials 2016, 9, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupul, I.; Yperman, J.; Carleer, R.; Gryglewicz, G. Adsorption of atrazine on hemp stem-based activated carbons with different surface chemistry. Adsorption 2015, 21, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Hamm, W.; Hamilton, R.J.; Calliauw, G. (Eds.) Edible Oil Processing, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; p. 342. [Google Scholar]
- López Sánchez, I. Biodegradación de Colorantes Textiles Industriales por las Enzimas Ligninolíticas de Trametes Maxima MUCL44155 Obtenidas en Medios con Bagazo de Caña de Azúcar. Ph.D. Thesis, Universidad de La Habana, La Habana, Cuba, 2011. [Google Scholar]
- Pérez, S.R.; Oduardo, N.G.; Savón, R.C.B.; Boizán, M.F.; Augur, C. Decolourisation of mushroom farm wastewater by Pleurotus ostreatus. Biodegradation 2008, 19, 519–526. [Google Scholar] [CrossRef]
- Puente Torres, J.; Crespo Sariol, H.; Yperman, J.; Brito Sauvanell, Á.; Carleer, R.; Navarro Campa, J. A novel X-ray radiography approach for the characterization of granular activated carbons used in the rum production. J. Anal. Sci. Technol. 2018, 9, 1. [Google Scholar] [CrossRef]
- Rizzo, L.; Meric, S.; Kassinos, D.; Guida, M.; Russo, F.; Belgiorno, V. Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Res. 2009, 43, 979–988. [Google Scholar] [CrossRef]
- Picard, M.; Thakur, S.; Misra, M.; Mielewski, D.F.; Mohanty, A.K. Biocarbon from peanut hulls and their green composites with biobased poly(trimethylene terephthalate) (PTT). Sci. Rep. 2020, 10, 3310. [Google Scholar] [CrossRef] [PubMed]
- Hilal, N.M.; Ahmed, I.A.; El-Sayed, R.E. Activated and Nonactivated Date Pits Adsorbents for the Removal of Copper(II) and Cadmium(II) from Aqueous Solutions. ISRN Phys. Chem. 2012, 2012, 985853. [Google Scholar] [CrossRef] [Green Version]
- Sayed, M.; Gul, M.; Shah, N.S.; Khan, J.A.; Khan, Z.U.H.; Rehman, F.; Khan, A.R.; Rauf, S.; Arandiyan, H.; Yang, C.P. In-situ dual applications of ionic liquid coated Co2+ and Fe3+ co-doped TiO2: Superior photocatalytic degradation of ofloxacin at pilot scale level and enhanced peroxidase like activity for calorimetric biosensing. J. Mol. Liq. 2019, 282, 275–285. [Google Scholar] [CrossRef]
- Xing, B.; Shi, C.; Zhang, C.; Yi, G.; Chen, L.; Guo, H.; Huang, G.; Cao, J. Preparation of TiO2/Activated Carbon Composites for Photocatalytic Degradation of RhB under UV Light Irradiation. J. Nanomater. 2016, 2016, 8393648. [Google Scholar] [CrossRef] [Green Version]
- Sayed, M.; Khan, J.A.; Shah, L.A.; Shah, N.S.; Shah, F.; Khan, H.M.; Zhang, P.; Arandiyan, H. Solar Light Responsive Poly(vinyl alcohol)-Assisted Hydrothermal Synthesis of Immobilized TiO2/Ti Film with the Addition of Peroxymonosulfate for Photocatalytic Degradation of Ciprofloxacin in Aqueous Media: A Mechanistic Approach. J. Phys. Chem. C 2018, 122, 406–421. [Google Scholar] [CrossRef]
- Isari, A.A.; Payan, A.; Fattahi, M.; Jorfi, S.; Kakavandi, B. Photocatalytic degradation of rhodamine B and real textile wastewater using Fe-doped TiO2 anchored on reduced graphene oxide (Fe-TiO2/rGO): Characterization and feasibility, mechanism and pathway studies. Appl. Surf. Sci. 2018, 462, 549–564. [Google Scholar] [CrossRef]
- Kapridaki, C.; Xynidis, N.; Vazgiouraki, E.; Kallithrakas-Kontos, N.; Maravelaki-Kalaitzaki, P. Characterization of Photoactive Fe-TiO2 Lime Coatings for Building Protection: The Role of Iron Content. Materials 2019, 12, 1847. [Google Scholar] [CrossRef] [Green Version]
- Ghorbanpour, M.; Feizi, A. Iron-doped TiO2 Catalysts with Photocatalytic Activity. J. Water Environ. Nanotechnol. 2019, 4, 60–66. [Google Scholar] [CrossRef]
- Ahadi, S.; Moalej, N.S.; Sheibani, S. Characteristics and photocatalytic behavior of Fe and Cu doped TiO2 prepared by combined sol-gel and mechanical alloying. Solid State Sci. 2019, 96, 105975. [Google Scholar] [CrossRef]
- El Mragui, A.; Logvina, Y.; Pinto da Silva, L.; Zegaoui, O.; Esteves da Silva, J.C.G. Synthesis of Fe- and Co-Doped TiO2 with Improved Photocatalytic Activity Under Visible Irradiation Toward Carbamazepine Degradation. Materials 2019, 12, 3874. [Google Scholar] [CrossRef] [Green Version]
- El Mragui, A.; Zegaoui, O.; Daou, I.; Esteves da Silva, J.C.G. Preparation, characterization, and photocatalytic activity under UV and visible light of Co, Mn, and Ni mono-doped and (P,Mo) and (P,W) co-doped TiO2 nanoparticles: A comparative study. Environ. Sci. Pollut. Res. 2021, 28, 25130–25145. [Google Scholar] [CrossRef]
- Sreethawong, T.; Yamada, Y.; Kobayashi, T.; Yoshikawa, S. Catalysis of nanocrystalline mesoporous TiO2 on cyclohexene epoxidation with H2O2: Effects of mesoporosity and metal oxide additives. J. Mol. Catal. A Chem. 2005, 241, 23–32. [Google Scholar] [CrossRef]
- Majeed Khan, M.A.; Siwach, R.; Kumar, S.; Alhazaa, A.N. Role of Fe doping in tuning photocatalytic and photoelectrochemical properties of TiO2 for photodegradation of methylene blue. Opt. Laser Technol. 2019, 118, 170–178. [Google Scholar] [CrossRef]
- Kashale, A.A.; Rasal, A.S.; Kamble, G.P.; Ingole, V.H.; Dwivedi, P.K.; Rajoba, S.J.; Jadhav, L.D.; Ling, Y.-C.; Chang, J.-Y.; Ghule, A.V. Biosynthesized Co-doped TiO2 nanoparticles based anode for lithium-ion battery application and investigating the influence of dopant concentrations on its performance. Compos. Part B Eng. 2019, 167, 44–50. [Google Scholar] [CrossRef]
- Gaur, L.K.; Kumar, P.; Kushavah, D.; Khiangte, K.R.; Mathpal, M.C.; Agrahari, V.; Gairola, S.P.; Soler, M.A.G.; Swart, H.C.; Agarwal, A. Laser induced phase transformation influenced by Co doping in TiO2 nanoparticles. J. Alloys Compd. 2019, 780, 25–34. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, L.; Li, F.; Yu, R.; Jin, C. Structural evolution in the graphitization process of activated carbon by high-pressure sintering. Carbon 2009, 47, 744–751. [Google Scholar] [CrossRef]
- Marsh, H.; Reinoso, F.R. Activated Carbon; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Chekem, C.T.; Richardson, Y.; Plantard, G.; Blin, J.; Goetz, V. From Biomass Residues to Titania Coated Carbonaceous Photocatalysts: A Comparative Analysis of Different Preparation Routes for Water Treatment Application. Waste Biomass Valoriz. 2017, 8, 2721–2733. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Zhuang, Y.; Fu, X. New Insight for Enhanced Photocatalytic Activity of TiO2 by Doping Carbon Nanotubes: A Case Study on Degradation of Benzene and Methyl Orange. J. Phys. Chem. C 2010, 114, 2669–2676. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- El Mouchtari, E.M.; Daou, C.; Rafqah, S.; Najjar, F.; Anane, H.; Piram, A.; Hamade, A.; Briche, S.; Wong-Wah-Chung, P. TiO2 and activated carbon of Argania Spinosa tree nutshells composites for the adsorption photocatalysis removal of pharmaceuticals from aqueous solution. J. Photochem. Photobiol. A Chem. 2020, 388, 112183. [Google Scholar] [CrossRef]
- Puente Torres, J.; Crespo Sariol, H.; Yperman, J.; Adriaensens, P.; Carleer, R.; Mariño Peacok, T.; Brito Sauvanell, Á.; Thijssen, E.; Reggers, G.; Haeldermans, T.; et al. X-ray absorption as an alternative method to determine the exhausting degree of activated carbon layers in water treatment system for medical services. Talanta 2019, 205, 120058. [Google Scholar] [CrossRef] [PubMed]
- Puente Torres, J.; Crespo Sariol, H.; Mariño Peacok, T.; Yperman, J.; Adriaensens, P.; Carleer, R.; Brito Sauvanell, Á. X-ray Absorption (XRA): A New Technique for the Characterization of Granular Activated Carbons. Materials 2021, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.P.; Codorniu, R.T.; Baracaldo, R.L.; Sariol, H.C.; Peacok, T.M.; Yperman, J.; Adriaensens, P.; Carleer, R.; Sauvanell, Á.B. A convolutional neural networks approach using X-Ray absorption images for studying granular activated carbon. SN Appl. Sci. 2020, 2, 2088. [Google Scholar] [CrossRef]
- Acevedo, B.; Barriocanal, C. Simultaneous adsorption of Cd2+ and reactive dye on mesoporous nanocarbons. RSC Adv. 2015, 5, 95247–95255. [Google Scholar] [CrossRef] [Green Version]
- Din, A.T.M.; Hameed, B.; Ahmad, A.L. Batch adsorption of phenol onto physiochemical-activated coconut shell. J. Hazard. Mater. 2009, 161, 1522–1529. [Google Scholar] [CrossRef]
- Elovich, S.Y.; Larinov, O. Theory of adsorption from solutions of non electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form,(II) verification of the equation of adsorption isotherm from solutions. Izvestiya Akademii Nauk. SSSR Otd. Khimicheskikh Nauk. 1962, 2, 209–216. [Google Scholar]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Pei, Z.; Kaiqiang, Z.; Yu, D.; Bo, B.; Weisheng, G.; Yourui, S. Adsorption of Organic Dyes by TiO2 @Yeast-Carbon Composite Microspheres and Their In Situ Regeneration Evaluation. J. Nanomater. 2015, 2015, 498304. [Google Scholar] [CrossRef]
- Weber Walter, J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Doğan, M.; Alkan, M. Adsorption kinetics of methyl violet onto perlite. Chemosphere 2003, 50, 517–528. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K. Mass transfer, kinetics and equilibrium studies for the biosorption of methylene blue using Paspalum notatum. J. Hazard. Mater. 2007, 146, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Salman, J.; Njoku, V.; Hameed, B. Bentazon and carbofuran adsorption onto date seed activated carbon: Kinetics and equilibrium. Chem. Eng. J. 2011, 173, 361–368. [Google Scholar] [CrossRef]
- Cheung, W.; Szeto, Y.; McKay, G. Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Mahmoud, D.K.; Salleh, M.A.M.; Karim, W.A.W.A.; Idris, A.; Abidin, Z.Z. Batch adsorption of basic dye using acid treated kenaf fibre char: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2012, 181, 449–457. [Google Scholar] [CrossRef]
- Chen, L.; Bai, B. Equilibrium, Kinetic, Thermodynamic, and in Situ Regeneration Studies about Methylene Blue Adsorption by the Raspberry-like TiO2@yeast Microspheres. Ind. Eng. Chem. Res. 2013, 52, 15568–15577. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Temkin, M.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Phys.-Chim. Sin. 1940, 12, 217–222. [Google Scholar]
- Popa, N.; Visa, M. New hydrothermal charcoal TiO2 composite for sustainable treatment of wastewater with dyes and cadmium cations load. Mater. Chem. Phys. 2021, 258, 123927. [Google Scholar] [CrossRef]
- Andriantsiferana, C.; Mohamed, E.F.; Delmas, H. Photocatalytic degradation of an azo-dye on TiO2/activated carbon composite material. Environ. Technol. 2014, 35, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atout, H.; Bouguettoucha, A.; Chebli, D.; Gatica, J.M.; Vidal, H.; Yeste, M.P.; Amrane, A. Integration of Adsorption and Photocatalytic Degradation of Methylene Blue Using TiO2 Supported on Granular Activated Carbon. Arab. J. Sci. Eng. 2017, 42, 1475–1486. [Google Scholar] [CrossRef]
- Shah, J.A.; Ashfaq, T.; Khan, M.S.; Riaz, N.; Shah, K.H.; Arshad, M.; Shah, S.H.; Amin, B.A.Z.; Arfan, M.; Zhang, Y.; et al. Melia azedarach Activated Carbon and its novel TiO2 Nanocomposite for Chemisorption and Photodecoloration of Reactive Orange 16: Isotherm and Kinetic Modeling. Curr. Anal. Chem. 2021, 17, 107–119. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Gómez-Avilés, A.; Belver, C.; Rodriguez, J.J.; Bedia, J. Degradation pathways of emerging contaminants using TiO2-activated carbon heterostructures in aqueous solution under simulated solar light. Chem. Eng. J. 2020, 392, 124867. [Google Scholar] [CrossRef]
- Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Souza, J.R.B.; Zhang, T.; Pilau, E.J.; Asefa, T.; Almeida, V.C. Sol-gel synthesis of new TiO2/activated carbon photocatalyst and its application for degradation of tetracycline. Ceram. Int. 2017, 43, 4411–4418. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, W.; Xia, T.; Ao, C.; Zhao, J.; Huang, B.; Wang, Q.; Zhang, W.; Lu, C. A TiO2 Coated Carbon Aerogel Derived from Bamboo Pulp Fibers for Enhanced Visible Light Photo-Catalytic Degradation of Methylene Blue. Nanomaterials 2021, 11, 239. [Google Scholar] [CrossRef]
- Bansal, P.; Singh, D.; Sud, D. Photocatalytic degradation of azo dye in aqueous TiO2 suspension: Reaction pathway and identification of intermediates products by LC/MS. Sep. Purif. Technol. 2010, 72, 357–365. [Google Scholar] [CrossRef]
- Dong, X.; Ding, W.; Zhang, X.; Liang, X. Mechanism and kinetics model of degradation of synthetic dyes by UV–vis/H2O2/Ferrioxalate complexes. Dyes Pigment. 2007, 74, 470–476. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, O.; Gürkan, T. Monitoring the Decolorization of Acid Orange 8 and Acid Red 44 from Aqueous Solution Using Fenton’s Reagents by Online Spectrophotometric Method: Effect of Operation Parameters and Kinetic Study. Ind. Eng. Chem. Res. 2013, 52, 1414–1425. [Google Scholar] [CrossRef]
- Kudelko, A.; Olesiejuk, M.; Luczynski, M.; Swiatkowski, M.; Sieranski, T.; Kruszynski, R. 1,3,4-Thiadiazole-Containing Azo Dyes: Synthesis, Spectroscopic Properties and Molecular Structure. Molecules 2020, 25, 2822. [Google Scholar] [CrossRef]
- Sun, J.-H.; Sun, S.-P.; Wang, G.-L.; Qiao, L.-P. Degradation of azo dye Amido black 10B in aqueous solution by Fenton oxidation process. Dyes Pigment. 2007, 74, 647–652. [Google Scholar] [CrossRef]
- Herrmann, J.-M.; Matos, J.; Disdier, J.; Guillard, C.; Laine, J.; Malato, S.; Blanco, J. Solar photocatalytic degradation of 4-chlorophenol using the synergistic effect between titania and activated carbon in aqueous suspension. Catal. Today 1999, 54, 255–265. [Google Scholar] [CrossRef]
- Lim, T.-T.; Yap, P.-S.; Srinivasan, M.; Fane, A.G. TiO2/AC Composites for Synergistic Adsorption-Photocatalysis Processes: Present Challenges and Further Developments for Water Treatment and Reclamation. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1173–1230. [Google Scholar] [CrossRef]
- Matos, J.; Laine, J.; Herrmann, J.-M. Synergy effect in the photocatalytic degradation of phenol on a suspended mixture of titania and activated carbon. Appl. Catal. B Environ. 1998, 18, 281–291. [Google Scholar] [CrossRef]
- Kangwansupamonkon, W.; Jitbunpot, W.; Kiatkamjornwong, S. Photocatalytic efficiency of TiO2/poly[acrylamide-co-(acrylic acid)] composite for textile dye degradation. Polym. Degrad. Stab. 2010, 95, 1894–1902. [Google Scholar] [CrossRef]
- Parilti, N.B.; Demirel, C.S.U.; Bekbolet, M. Response surface methodological approach for the assessment of the photocatalytic degradation of NOM. J. Photochem. Photobiol. A Chem. 2011, 225, 26–35. [Google Scholar] [CrossRef]
- Cho, I.-H.; Zoh, K.-D. Photocatalytic degradation of azo dye (Reactive Red 120) in TiO2/UV system: Optimization and modeling using a response surface methodology (RSM) based on the central composite design. Dyes Pigment. 2007, 75, 533–543. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Vanreppelen, K.; Vanderheyden, S.; Kuppens, T.; Schreurs, S.; Yperman, J.; Carleer, R. Activated carbon from pyrolysis of brewer’s spent grain: Production and adsorption properties. Waste Manag. Res. 2014, 32, 634–645. [Google Scholar] [CrossRef]
- Belekbir, S.; El Azzouzi, M.; El Hamidi, A.; Rodríguez-Lorenzo, L.; Santaballa, J.A.; Canle, M. Improved Photocatalyzed Degradation of Phenol, as a Model Pollutant, over Metal-Impregnated Nanosized TiO2. Nanomaterials 2020, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.V.; Basumatary, A.K.; Ghoshal, A.K.; Pugazhenthi, G. Performance assessment of an analcime-C zeolite–ceramic composite membrane by removal of Cr (VI) from aqueous solution. RSC Adv. 2015, 5, 6246–6254. [Google Scholar] [CrossRef]
- Chaki, N.; Shaikh, S.H.; Saeed, K. A comprehensive survey on image binarization techniques. Explor. Image Bin. Tech. 2014, 560, 5–15. [Google Scholar] [CrossRef]
- Temkin, M.I. Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and in the interaction between adsorbed molecules. Zhurnal Fiz. Khimii 1941, 15, 296–332. [Google Scholar]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Ballav, N.; Nyoni, H.; Maity, A.; Pillay, K. Optimization and mechanism elucidation of the catalytic photo-degradation of the dyes Eosin Yellow (EY) and Naphthol blue black (NBB) by a polyaniline-coated titanium dioxide nanocomposite. Appl. Catal. B Environ. 2015, 163, 330–342. [Google Scholar] [CrossRef]

| Adsorbent. | Azo-Dye | Isotherm | (mg/g) | Reference |
|---|---|---|---|---|
| AC-DSD-TiO2 | Reactive orange 16 | Langmuir | 92.84 | [88] |
| TiO2/AC | Yellow 5 | Langmuir | 107.1 | [86] |
| M-TiO2/AC | Acid Red 18 | Langmuir | 161.3 | [9] |
| TiO2/C-500 | Methylene blue | Langmuir | 49.64 | [87] |
| TiO2/AC | Acid Red 18 | Langmuir | 158.7 | [9] |
| ACFeTi5 | CYF-4G | Langmuir | 243.2 | This study |
| ACFeTi10 | CYF-4G | Langmuir | 189.2 | This study |
| ACCoTi5 | CYF-4G | Langmuir | 258.4 | This study |
| ACCoTi10 | CYF-4G | Langmuir | 201.1 | This study |
| Parameter | CYF-4G * | AC ** | Co-TiO2 | AC- CoTi5 | AC- CoTi10 | Fe-TiO2 | AC- FeTi5 | AC- FeTi10 |
|---|---|---|---|---|---|---|---|---|
| Second-order | ||||||||
| Co (mg/L) | 99.36 | 88.97 | 99.57 | 97.41 | 97.28 | 99.77 | 89.87 | 98.85 |
| k2 (mg/g.min)·10−5 | 1.81 | 4.24 | 10.56 | 48.89 | 41.21 | 11.44 | 53.31 | 39.35 |
| R2 | 0.976 | 0.991 | 0.976 | 0.832 | 0.833 | 0.969 | 0.832 | 0.819 |
| RMSE | 1.54 | 1.26 | 3.23 | 12.39 | 12.66 | 3.91 | 11.85 | 13.35 |
| Langmuir-Hinshelwood | ||||||||
| Co (mg/L) | 102.5 | 86.77 | 92.24 | 93.19 | 92.60 | 94.51 | 86.71 | 94.59 |
| kLH (1/min)·10−3 | 0.37 | 1.07 | 6.15 | 25.19 | 20.62 | 6.01 | 24.96 | 20.19 |
| R2 | 0.986 | 0.993 | 0.955 | 0.975 | 0.971 | 0.968 | 0.973 | 0.965 |
| RMSE | 1.18 | 1.02 | 4.40 | 4.78 | 5.26 | 3.98 | 4.80 | 5.96 |
| t1/2 (min) | 1868 | 636 | 113 | 28.5 | 33.6 | 115 | 27.8 | 34.3 |
| RC | - | - | - | 4.09 | 3.29 | - | 4.15 | 3.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado Garcia, R.; Carleer, R.; Arada Pérez, M.; Puente Torres, J.; Gu, Y.; Samyn, P.; Yperman, J. Fe-TiO2/AC and Co-TiO2/AC Composites: Novel Photocatalysts Prepared from Waste Streams for the Efficient Removal and Photocatalytic Degradation of Cibacron Yellow F-4G Dye. Catalysts 2021, 11, 1137. https://doi.org/10.3390/catal11101137
Machado Garcia R, Carleer R, Arada Pérez M, Puente Torres J, Gu Y, Samyn P, Yperman J. Fe-TiO2/AC and Co-TiO2/AC Composites: Novel Photocatalysts Prepared from Waste Streams for the Efficient Removal and Photocatalytic Degradation of Cibacron Yellow F-4G Dye. Catalysts. 2021; 11(10):1137. https://doi.org/10.3390/catal11101137
Chicago/Turabian StyleMachado Garcia, Roberto, Robert Carleer, Maria Arada Pérez, Jeamichel Puente Torres, Ying Gu, Pieter Samyn, and Jan Yperman. 2021. "Fe-TiO2/AC and Co-TiO2/AC Composites: Novel Photocatalysts Prepared from Waste Streams for the Efficient Removal and Photocatalytic Degradation of Cibacron Yellow F-4G Dye" Catalysts 11, no. 10: 1137. https://doi.org/10.3390/catal11101137
APA StyleMachado Garcia, R., Carleer, R., Arada Pérez, M., Puente Torres, J., Gu, Y., Samyn, P., & Yperman, J. (2021). Fe-TiO2/AC and Co-TiO2/AC Composites: Novel Photocatalysts Prepared from Waste Streams for the Efficient Removal and Photocatalytic Degradation of Cibacron Yellow F-4G Dye. Catalysts, 11(10), 1137. https://doi.org/10.3390/catal11101137







