Energetic Characterization of Faujasite Zeolites Using a Sensor Gas Calorimeter
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorption on NaX and NaY Zeolites
2.2. Adsorption on NaX and CaNaX Zeolites
2.3. Estimation of Energetic Contributions
3. Materials and Methods
3.1. Materials
3.2. Experimental Approach
3.2.1. Apparatus and Methods
3.2.2. Experimental Error
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Thermodynamic Evaluation of the Sensor Gas Calorimeter
References
- Schwab, G.-M.; Beebe, R.A.; Fricke, R.; Griffith, R.H.; Hunsmann, W.; Kohlschütter, H.W.; Straumanis, M.; Zimens, K.-E. (Eds.) Handbuch der Katalyse. Vierter Band: Heterogene Katalyse I; Springer: Vienna, Austria, 1943; ISBN 978-3-7091-7992-5. [Google Scholar]
- Schwab, G.-M.; Block, J.; Brovetto, P.; Constable, F.H.; Nasini, A.G.; Natta, G.; Noller, H.; Rigamonti, R.; Saini, G. (Eds.) Handbuch der Katalyse. Fünfter Band: Heterogene Katalyse II; Springer: Vienna, Austria, 1957; ISBN 978-3-7091-8040-2. [Google Scholar]
- Reschetilowski, W. Einführung in die heterogene Katalyse; Springer Spektrum: Berlin, Germany, 2015; ISBN 978-3-662-46983-5. [Google Scholar]
- Gregg, S.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982; ISBN 0-12-300956-1. [Google Scholar]
- Himeno, S.; Komatsu, T.; Fujita, S. High-Pressure Adsorption Equilibria of Methane and Carbon Dioxide on Several Activated Carbons. J. Chem. Eng. Data 2005, 50, 369–376. [Google Scholar] [CrossRef]
- Manca, C.; Martin, C.; Roubin, P. Comparative Study of Gas Adsorption on Amorphous Ice: Thermodynamic and Spectroscopic Features of the Adlayer and the Surface. J. Phys. Chem. B 2003, 107, 8929–8934. [Google Scholar] [CrossRef]
- Rouquerol, F. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2014; ISBN 978-0-08-097035-6. [Google Scholar]
- Srivastava, V.C.; Mall, I.D.; Mishra, I.M. Adsorption thermodynamics and isosteric heat of adsorption of toxic metal ions onto bagasse fly ash (BFA) and rice husk ash (RHA). Chem. Eng. J. 2007, 132, 267–278. [Google Scholar] [CrossRef]
- Horn, J.R.; Russell, D.; Lewis, E.A.; Murphy, K.P. Van’t Hoff and calorimetric enthalpies from isothermal titration calorimetry: Are there significant discrepancies? Biochemistry 2001, 40, 1774–1778. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-C.; Chiang, P.-C.; Huang, C.-P. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Askalany, A.A.; Saha, B.B. Towards an accurate estimation of the isosteric heat of adsorption—A correlation with the potential theory. J. Colloid Interface Sci. 2017, 490, 59–63. [Google Scholar] [CrossRef]
- Dubinin, M.M. The Potential Theory of Adsorption of Gases and Vapors for Adsorbents with Energetically Nonuniform Surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Makowski, W. Quasi-equilibrated temperature programmed desorption and adsorption: A new method for determination of the isosteric adsorption heat. Thermochim. Acta 2007, 454, 26–32. [Google Scholar] [CrossRef]
- King, D.A. Thermal desorption from metal surfaces: A review. Surf. Sci. 1975, 47, 384–402. [Google Scholar] [CrossRef]
- Auroux, A. (Ed.) Calorimetry and Thermal Methods in Catalysis; Springer: Heidelberg/Berlin, Germany, 2013; ISBN 3642119530. [Google Scholar]
- Calvet, E.; Prat, H.; Skinner, H.A. Recent Progress in Microcalorimetry; Elsevier Science: Burlington, NY, USA, 1963; ISBN 9781483280134. [Google Scholar]
- Handy, B.E.; Sharma, S.B.; Spiewak, B.E.; Dumesic, J.A. A Tian-Calvet heat-flux microcalorimeter for measurement of differential heats of adsorption. Meas. Sci. Technol. 1993, 4, 1350–1356. [Google Scholar] [CrossRef]
- Dunne, J.A.; Mariwala, R.; Rao, M.; Sircar, S.; Gorte, R.J.; Myers, A.L. Calorimetric Heats of Adsorption and Adsorption Isotherms. 1. O2, N2, Ar, CO2, CH4, C2H6, and SF6 on Silicalite. Langmuir 1996, 12, 5888–5895. [Google Scholar] [CrossRef]
- Llewellyn, P.L.; Maurin, G. Gas adsorption microcalorimetry and modelling to characterise zeolites and related materials. C. R. Chim. 2005, 8, 283–302. [Google Scholar] [CrossRef]
- Garcia-Cuello, V.; Moreno-Piraján, J.C.; Giraldo-Gutiérrez, L.; Sapag, K.; Zgrablich, G. Determination of Differential Enthalpy and Isotherm by Adsorption Calorimetry. Res. Lett. Phys. Chem. 2008, 2008, 1–4. [Google Scholar] [CrossRef][Green Version]
- García-Cuello, V.; Moreno-Piraján, J.C.; Giraldo-Gutiérrez, L.; Sapag, K.; Zgrablich, G. Design, Calibration, and Testing of a New Tian-Calvet Heat-Flow Microcalorimeter for Measurement of Differential Heats of Adsorption. Instrum. Sci. Technol. 2008, 36, 455–475. [Google Scholar] [CrossRef]
- Zimmermann, W.; Keller, J. A new calorimeter for simultaneous measurement of isotherms and heats of adsorption. Thermochim. Acta 2003, 405, 31–41. [Google Scholar] [CrossRef]
- Bläker, C.; Luckas, M.; Pasel, C.; Dreisbach, F.; Bathen, D. Entwicklung eines Messgeräts zur Kopplung von kalorimetrischen und volumetrischen Sorptionsmessungen. Chem. Ing. Tech. 2016, 88, 282–290. [Google Scholar] [CrossRef]
- Dunne, J.A.; Rao, M.; Sircar, S.; Gorte, R.J.; Myers, A.L. Calorimetric Heats of Adsorption and Adsorption Isotherms. 2. O2, N2, Ar, CO2, C4, C2H6, and S6 on NaX, H-ZSM-5, and Na-ZSM-5 Zeolites. Langmuir 1996, 12, 5896–5904. [Google Scholar] [CrossRef]
- Loughlin, K.F.; Hasanain, M.A.; Abdul-Rehman, H.B. Quaternary, ternary, binary, and pure component sorption on zeolites. 2. Light alkanes on Linde 5A and 13X zeolites at moderate to high pressures. Ind. Eng. Chem. Res. 1990, 29, 1535–1546. [Google Scholar] [CrossRef]
- Mentasty, L.; Woestyn, A.M.; Basaldella, E.; Kikot, A.; Zgrablich, G. High-Pressure Methane Adsorption on NaX and NaY Zeolites with Different Si/Al Ratios. Adsorpt. Sci. Technol. 1994, 11, 209–216. [Google Scholar] [CrossRef]
- Zhang, J.; Burke, N.; Zhang, S.; Liu, K.; Pervukhina, M. Thermodynamic analysis of molecular simulations of CO2 and CH4 adsorption in FAU zeolites. Chem. Eng. Sci. 2014, 113, 54–61. [Google Scholar] [CrossRef]
- Talu, O.; Zhang, S.Y.; Hayhurst, D.T. Effect of cations on methane adsorption by NaY, MgY, CaY, SrY, and BaY zeolites. J. Phys. Chem. 1993, 97, 12894–12898. [Google Scholar] [CrossRef]
- Triebe, R.W.; Tezel, F.H.; Khulbe, K.C. Adsorption of methane, ethane and ethylene on molecular sieve zeolites. Gas. Sep. Purif. 1996, 10, 81–84. [Google Scholar] [CrossRef]
- Habgood, H.W. Adsorptive and Gas Chromatographic Properties of Various Cationic Forms of Zeolite X. Can. J. Chem. 1964, 42, 2340–2350. [Google Scholar] [CrossRef]
- Sethia, G.; Somani, R.S.; Chand Bajaj, H. Adsorption of carbon monoxide, methane and nitrogen on alkaline earth metal ion exchanged zeolite-X: Structure, cation position and adsorption relationship. RSC Adv. 2015, 5, 12773–12781. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Talu, O.; Hayhurst, D.T. High-pressure adsorption of methane in zeolites NaX, MgX, CaX, SrX and BaX. J. Phys. Chem. 1991, 95, 1722–1726. [Google Scholar] [CrossRef]
- Bezus, A.G.; Kiselev, A.V.; Sedlaček, Z.; Du, P.Q. Adsorption of ethane and ethylene on X-zeolites containing Li+, Na+, K+, Rb+ and Cs+ cations. Trans. Faraday Soc. 1971, 67, 468–482. [Google Scholar] [CrossRef]
- Maurin, G.; Llewellyn, P.; Poyet, T.; Kuchta, B. Influence of extra-framework cations on the adsorption properties of X-faujasite systems: Microcalorimetry and molecular simulations. J. Phys. Chem. B 2005, 109, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Burke, N.; Yang, Y. Molecular Simulation of Propane Adsorption in FAU Zeolites. J. Phys. Chem. C 2012, 116, 9666–9674. [Google Scholar] [CrossRef]
- Ritter, J.A.; Kapoor, A.; Yang, R.T. Localized Adsorption with Lateral Interaction on Random and Patchwise Heterogeneous Surfaces. J. Phys. Chem. 1990, 94, 6785–6791. [Google Scholar] [CrossRef]
- Yang, R.T. Adsorbents. Fundamentals and Applications; Wiley-Interscience: Hoboken, NJ, USA, 2003; ISBN 0-471-29741-0. [Google Scholar]
- Xu, X.; Zhao, X.; Sun, L.; Liu, X. Adsorption separation of carbon dioxide, methane, and nitrogen on Hβ and Na-exchanged β-zeolite. J. Nat. Gas. Chem. 2008, 17, 391–396. [Google Scholar] [CrossRef]
- Maurin, G.; Bourrelly, S.; Llewellyn, P.L.; Bell, R.G. Simulation of the adsorption properties of CH4 in faujasites up to high pressure: Comparison with microcalorimetry. Microporous Mesoporous Mater. 2006, 89, 96–102. [Google Scholar] [CrossRef]
- Pillai, R.S.; Sethia, G.; Jasra, R.V. Sorption of CO, CH4, and N2 in Alkali Metal Ion Exchanged Zeolite-X: Grand Canonical Monte Carlo Simulation and Volumetric Measurements. Ind. Eng. Chem. Res. 2010, 49, 5816–5825. [Google Scholar] [CrossRef]
- Wender, A.; Barreau, A.; Lefebvre, C.; Di Lella, A.; Boutin, A.; Ungerer, P.; Fuchs, A.H. Adsorption of n-alkanes in faujasite zeolites: Molecular simulation study and experimental measurements. Adsorption 2007, 13, 439–451. [Google Scholar] [CrossRef]
- Fuchs, A.H.; Cheetham, A.K. Adsorption of Guest Molecules in Zeolitic Materials: Computational Aspects. J. Phys. Chem. B 2001, 105, 7375–7383. [Google Scholar] [CrossRef]
- Zhu, L.; Seff, K. Reinvestigation of the Crystal Structure of Dehydrated Sodium Zeolite X. J. Phys. Chem. B 1999, 103, 9512–9518. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis. Equilibria and Kinetics; Imperial College Press: London, UK, 1998; ISBN 1-86094-130-3. [Google Scholar]
- Kulprathipanja, S. (Ed.) Zeolites in Industrial Separation and Catalysis; Wiley-VCH: Weinheim, Germany, 2010; ISBN 978-3-527-32505-4. [Google Scholar]
- Olson, D.H. The crystal structure of dehydrated NaX. Zeolites 1995, 15, 439–443. [Google Scholar] [CrossRef]
- Vitale, G.; Mellot, C.F.; Bull, L.M.; Cheetham, A.K. Neutron Diffraction and Computational Study of Zeolite NaX: Influence of SIII‘ Cations on Its Complex with Benzene. J. Phys. Chem. B 1997, 101, 4559–4564. [Google Scholar] [CrossRef]
- Buttefey, S.; Boutin, A.; Mellot-Draznieks, C.; Fuchs, A.H. A Simple Model for Predicting the Na+ Distribution in Anhydrous NaY and NaX Zeolites. J. Phys. Chem. B 2001, 105, 9569–9575. [Google Scholar] [CrossRef]
- Jaramillo, E.; Auerbach, S.M. New Force Field for Na Cations in Faujasite-Type Zeolites. J. Phys. Chem. B 1999, 103, 9589–9594. [Google Scholar] [CrossRef]
- Löwenstein, W. The Distribution of Aluminum in the Tetrahedra of Silicates and Aluminates. Am. Mineral. 1954, 39, 92–96. [Google Scholar]
- Xu, R. Chemistry of Zeolites and Related Porous Materials. Synthesis and Structure; Wiley: Chichester, UK, 2007; ISBN 978-0-470-82233-3. [Google Scholar]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-53064-6. [Google Scholar]
- Abrioux, C.; Coasne, B.; Maurin, G.; Henn, F.; Boutin, A.; Di Lella, A.; Nieto-Draghi, C.; Fuchs, A.H. A molecular simulation study of the distribution of cation in zeolites. Adsorption 2008, 14, 743–754. [Google Scholar] [CrossRef]
- Beauvais, C.; Guerrault, X.; Coudert, F.-X.; Boutin, A.; Fuchs, A.H. Distribution of Sodium Cations in Faujasite-Type Zeolite: A Canonical Parallel Tempering Simulation Study. J. Phys. Chem. B 2004, 108, 399–404. [Google Scholar] [CrossRef]
- Di Lella, A.; Desbiens, N.; Boutin, A.; Demachy, I.; Ungerer, P.; Bellat, J.-P.; Fuchs, A.H. Molecular simulation studies of water physisorption in zeolites. Phys. Chem. Chem. Phys. 2006, 8, 5396–5406. [Google Scholar] [CrossRef] [PubMed]
- Buttefey, S.; Boutin, A.; Fuchs, A.H. Cation distribution in faujasite-type zeolites: A test of semi-empirical force fields for Na cations. Mol. Simul. 2002, 28, 1049–1062. [Google Scholar] [CrossRef]
- Bläker, C.; Pasel, C.; Luckas, M.; Dreisbach, F.; Bathen, D. Investigation of load-dependent heat of adsorption of alkanes and alkenes on zeolites and activated carbon. Microporous Mesoporous Mater. 2017, 241, 1–10. [Google Scholar] [CrossRef]
- Bläker, C.; Pasel, C.; Luckas, M.; Dreisbach, F.; Bathen, D. A study on the load-dependent enthalpy of adsorption and interactions in adsorption of C5 and C6 hydrocarbons on zeolites 13X and ZSM-5 and an activated carbon. Microporous Mesoporous Mater. 2020, 302, 110205. [Google Scholar] [CrossRef]
- Atkins, P.W.; De Paula, J. Physikalische Chemie, 4th ed.; Wiley-VCH: Weinheim, Germany, 2006; ISBN 3-527-31546-2. [Google Scholar]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics. A Ready-Reference Book of Chemical and Physical Data, 97th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-5429-3. [Google Scholar]
- Kast, W. Adsorption aus der Gasphase. In Ingenieurwissenschaftliche Grundlagen und Technische Verfahren; VCH: Weinheim, Germany, 1988; ISBN 3-527-26719-0. [Google Scholar]
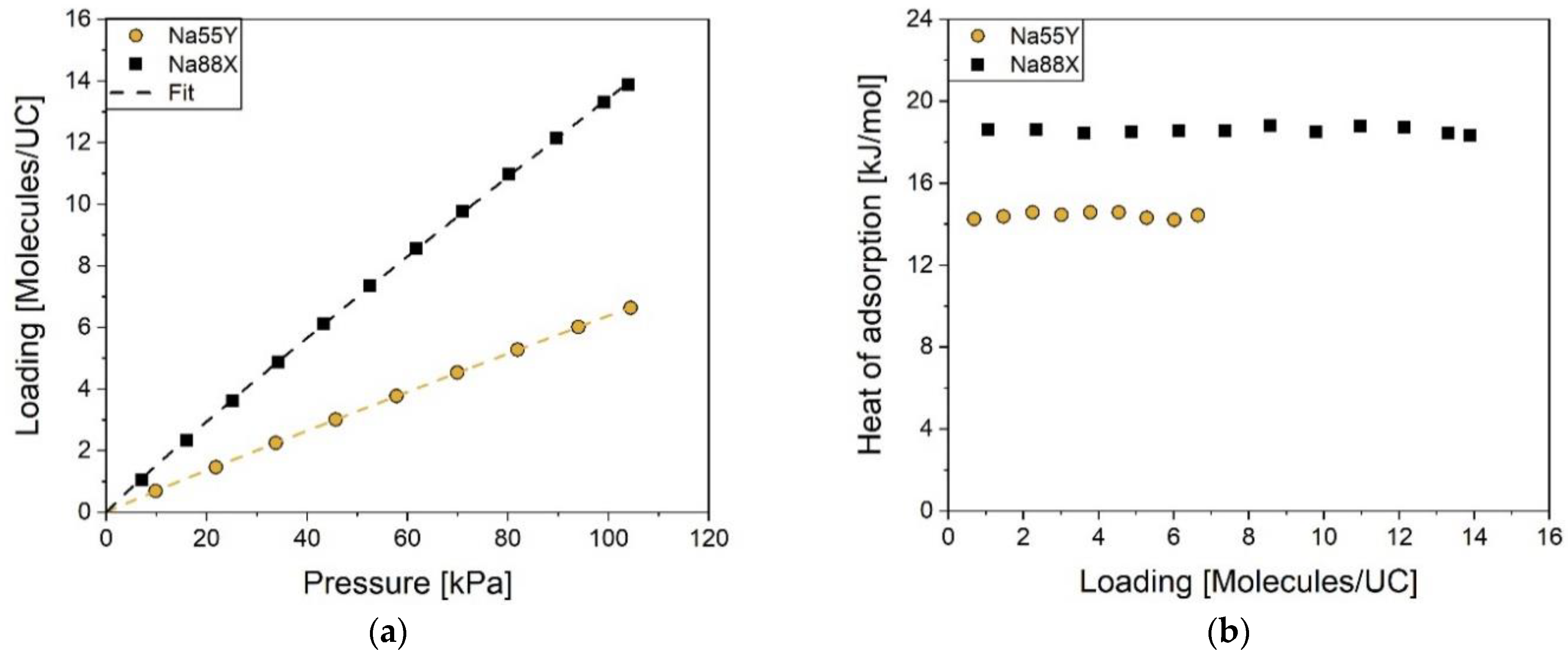

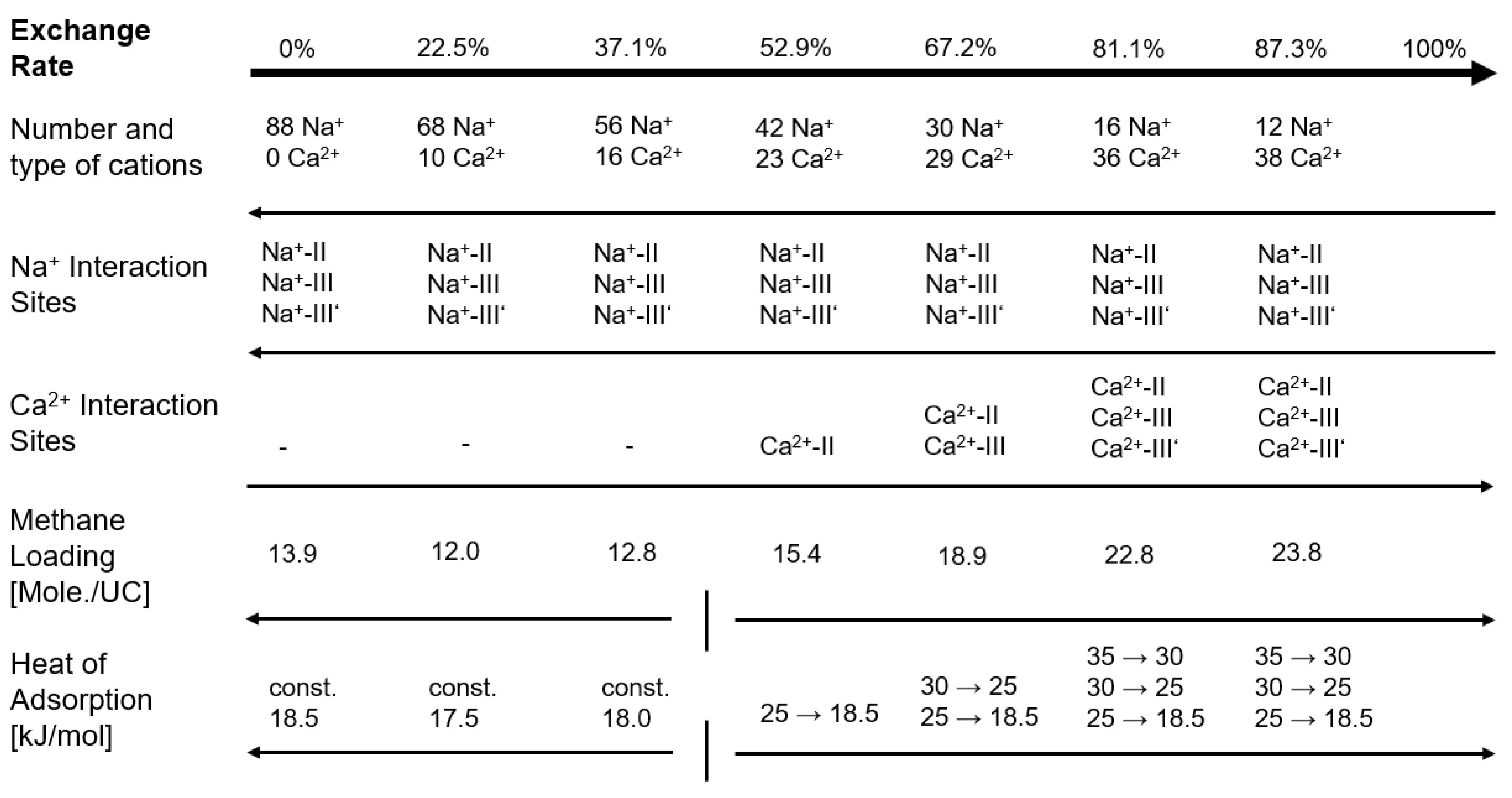
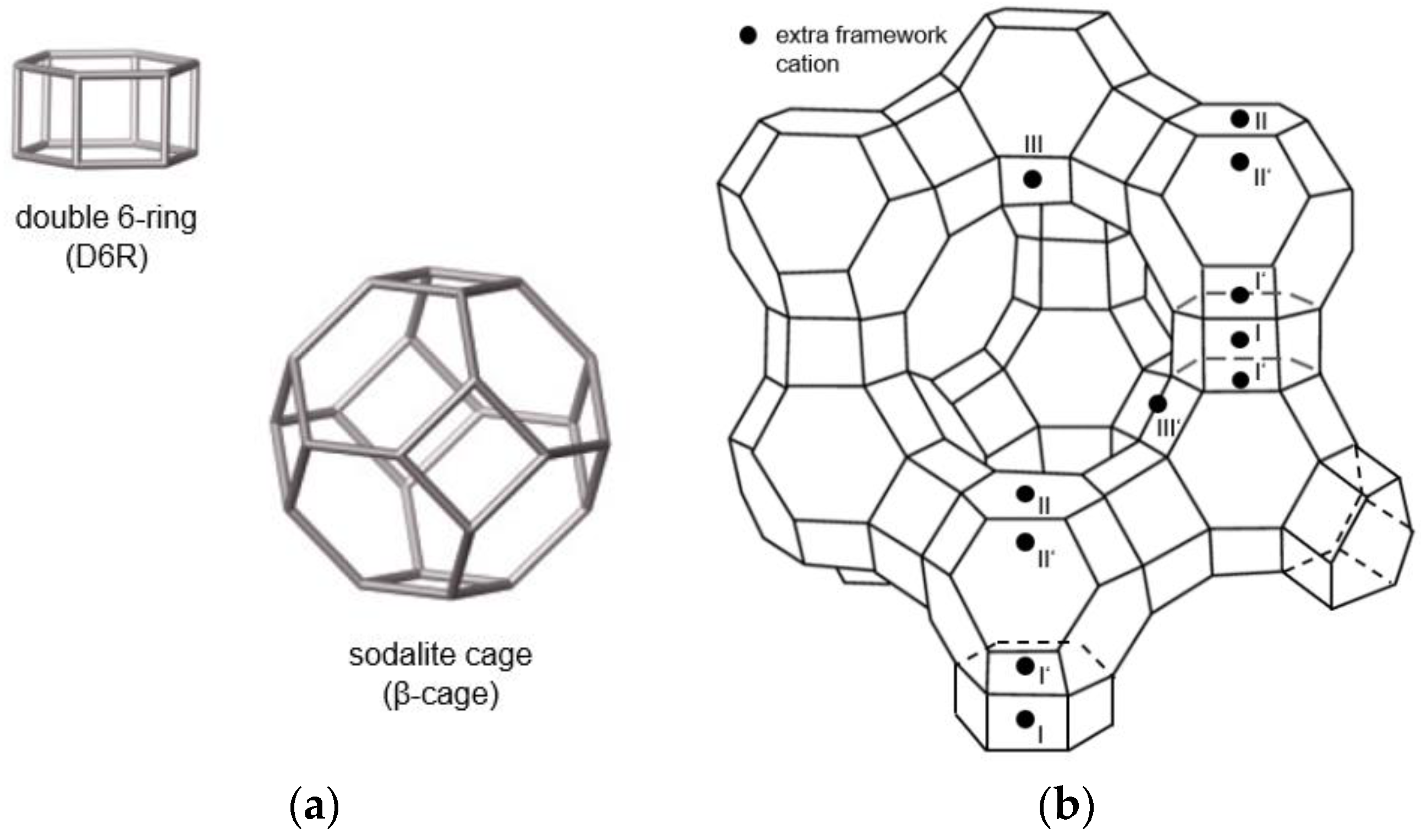
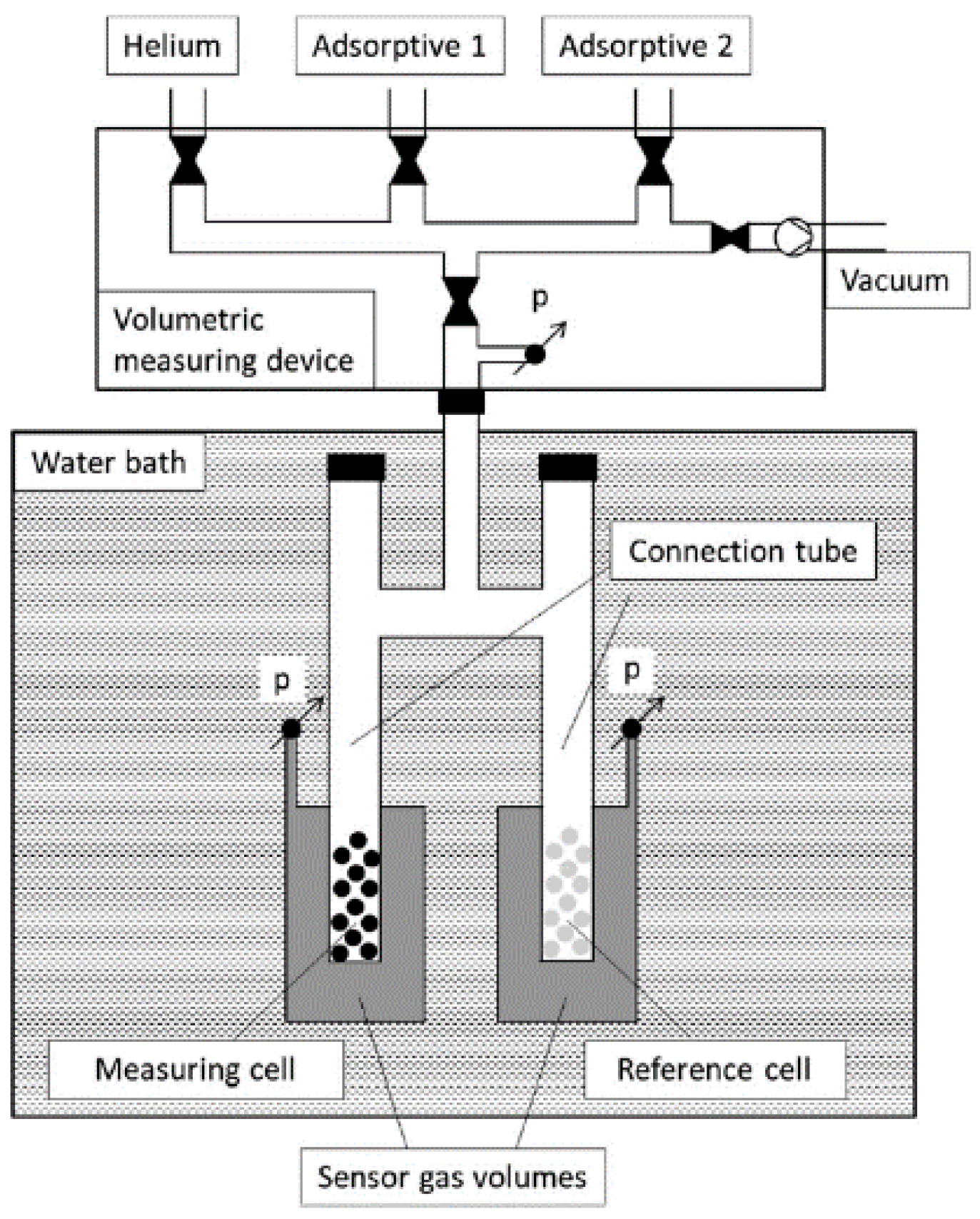
| Zeolite | |||
|---|---|---|---|
| Na88X | 0.2206 | 0.9426 | 0.9999 |
| Na55Y | 0.1013 | 0.9589 | 0.9999 |
| Ca10Na68X | 0.1817 | 0.9520 | 0.9999 |
| Ca16Na56X | 0.2017 | 0.9427 | 0.9999 |
| Ca23Na42X | 0.4060 | 0.8330 | 0.9996 |
| Ca29Na30X | 0.9654 | 0.6931 | 0.9986 |
| Ca36Na16X | 2.2700 | 0.5520 | 0.9950 |
| Ca38Na12X | 2.9913 | 0.4989 | 0.9927 |
| Energetic Contribution. | kJ/mol |
|---|---|
| Zeolite framework | ≈13.0 |
| Na+ on site III’ | ≈5.5 |
| Ca2+ on site II | ≈12.0 |
| Ca2+ on site III | ≈17.0 |
| Ca2+ on site III’ | ≈22.0 |
| Chemical Composition (wt%) | |||||||
|---|---|---|---|---|---|---|---|
| Zeolite | Si/Al Ratio | Al2O3 | SiO2 | Na2O | CaO | Sodium Exchange [%] | Abbreviation |
| Na88Al88Si104O384 | 1.175 | 28.00 | 36.90 | 17.70 | 0.03 | 0.0 | Na88X |
| Na55Al55Si137O384 | 2.500 | 18.90 | 56.50 | 11.80 | 0.06 | 0.0 | Na55Y |
| Ca10Na68Al88Si104O384 | 1.175 | 28.30 | 37.20 | 13.60 | 3.50 | 22.5 | Ca10Na68X |
| Ca16Na56Al88Si104O384 | 1.175 | 28.20 | 37.00 | 10.90 | 5.76 | 37.1 | Ca16Na56X |
| Ca23Na42Al88Si104O384 | 1.175 | 28.10 | 36.70 | 8.04 | 8.17 | 52.9 | Ca23Na42X |
| Ca29Na30Al88Si104O384 | 1.175 | 27.60 | 36.20 | 5.30 | 10.20 | 67.2 | Ca29Na30X |
| Ca36Na16Al88Si104O384 | 1.175 | 27.80 | 36.50 | 2.93 | 12.40 | 81.1 | Ca36Na16X |
| Ca38Na12Al88Si104O384 | 1.175 | 28.10 | 36.90 | 1.82 | 13.50 | 87.3 | Ca38Na12X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauer, V.; Bläker, C.; Pasel, C.; Bathen, D. Energetic Characterization of Faujasite Zeolites Using a Sensor Gas Calorimeter. Catalysts 2021, 11, 98. https://doi.org/10.3390/catal11010098
Mauer V, Bläker C, Pasel C, Bathen D. Energetic Characterization of Faujasite Zeolites Using a Sensor Gas Calorimeter. Catalysts. 2021; 11(1):98. https://doi.org/10.3390/catal11010098
Chicago/Turabian StyleMauer, Volker, Christian Bläker, Christoph Pasel, and Dieter Bathen. 2021. "Energetic Characterization of Faujasite Zeolites Using a Sensor Gas Calorimeter" Catalysts 11, no. 1: 98. https://doi.org/10.3390/catal11010098
APA StyleMauer, V., Bläker, C., Pasel, C., & Bathen, D. (2021). Energetic Characterization of Faujasite Zeolites Using a Sensor Gas Calorimeter. Catalysts, 11(1), 98. https://doi.org/10.3390/catal11010098







