Chronicle of Nanocelluloses (NCs) for Catalytic Applications: Key Advances
Abstract
1. Introduction
2. Bacterial Nanocellulose
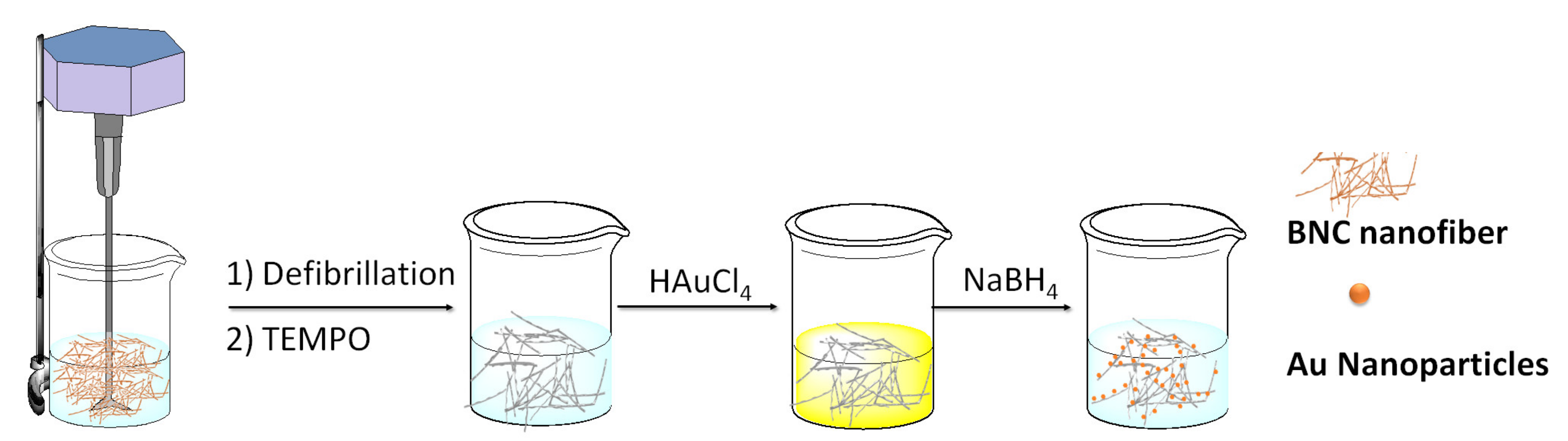
2.1. Inorganic Functionalization of Bacterial Nanocellulose and Catalytic Applications
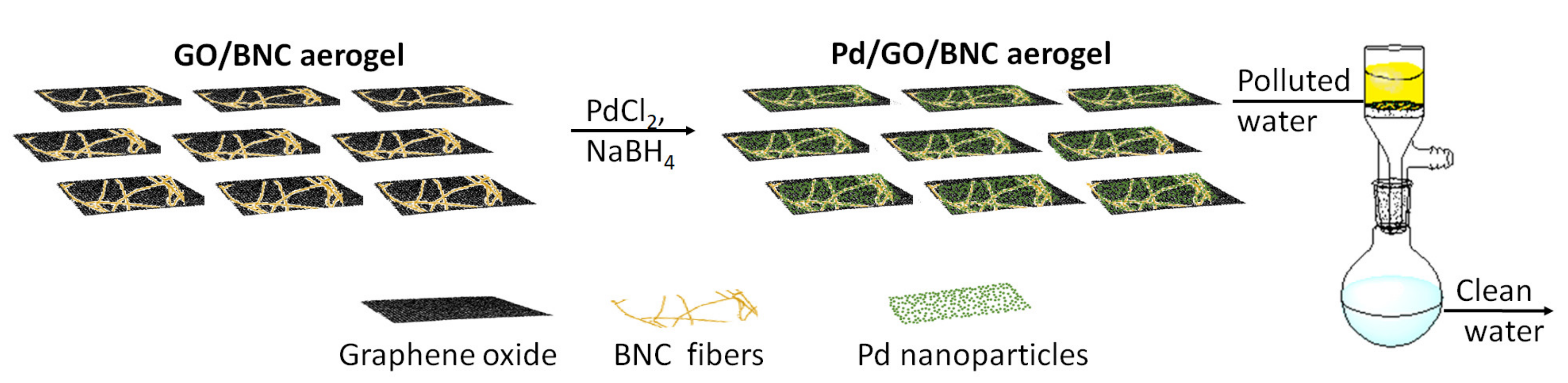
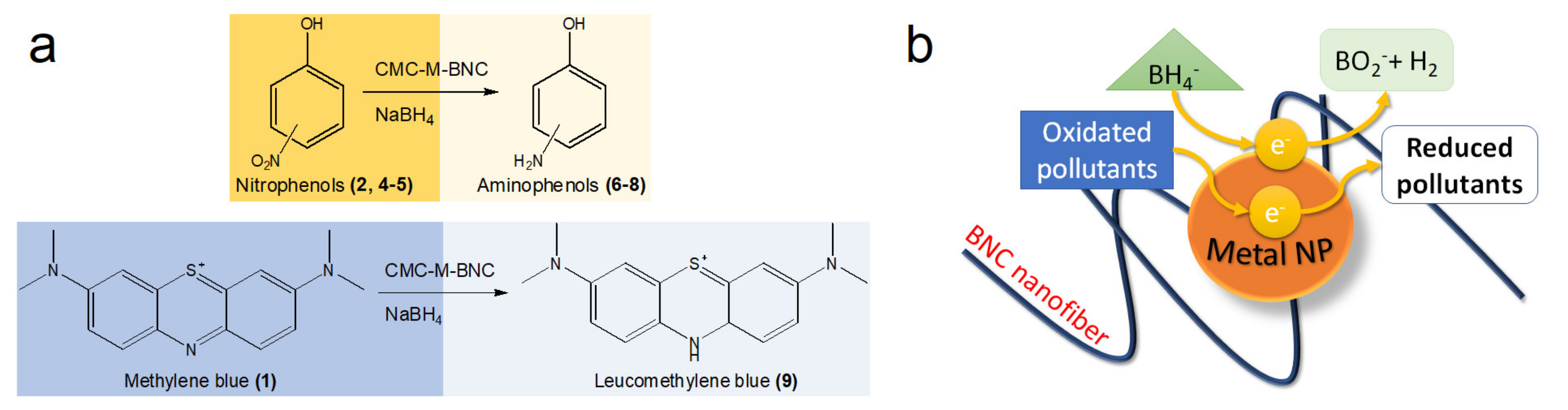
2.1.1. Catalytic Transformation of Organic Compounds for Environmental Purposes
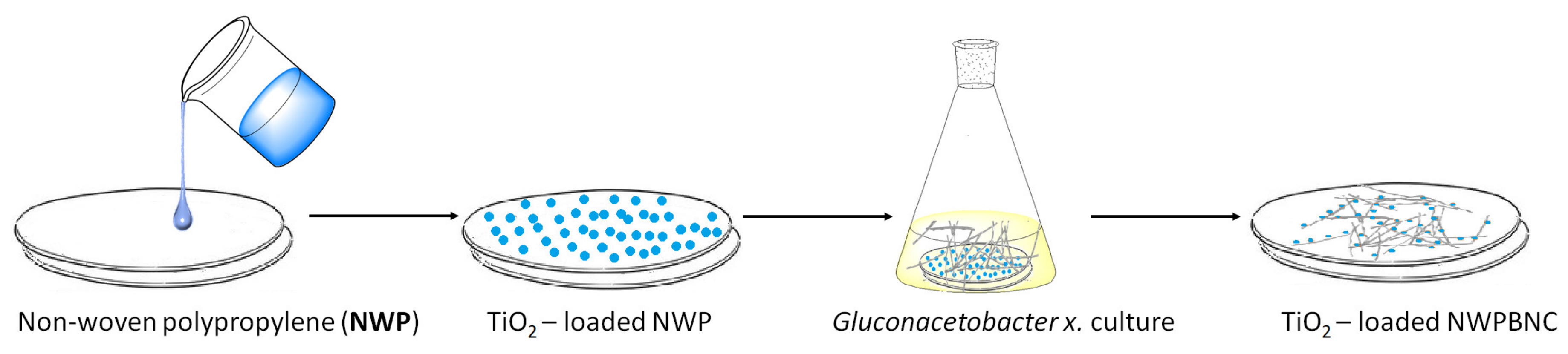
2.1.2. Photocatalytic Applications of BNC-Inorganic Composites for Environmental Remediation
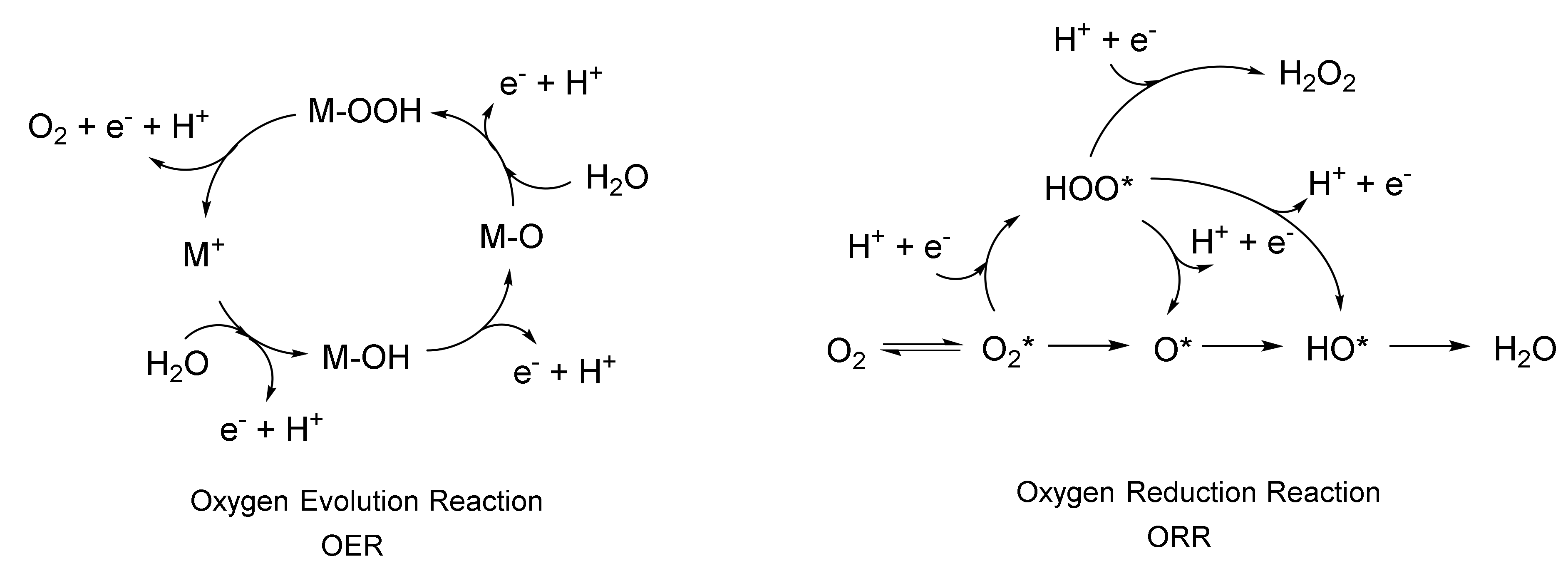
2.1.3. Electro-Catalytic Applications of Inorganic-Functionalized Bacterial Nanocellulose
2.1.4. Synthetic Applications of BNCs Grafted with Inorganic Catalysts
2.2. Surface Chemical Functionalization of Bacterial Nanocellulose and Catalytic Applications
3. Cellulose Nanocrystals
3.1. Inorganic Functionalization of Cellulose Nanocrystals and Catalytic Applications
3.2. Catalytic Trasformation of Inorganic Compounds for Environmental Purposes
3.3. Synthetic Applications of CNCs Grafted with Inorganic Catalysts
4. Cellulose Nanofibers
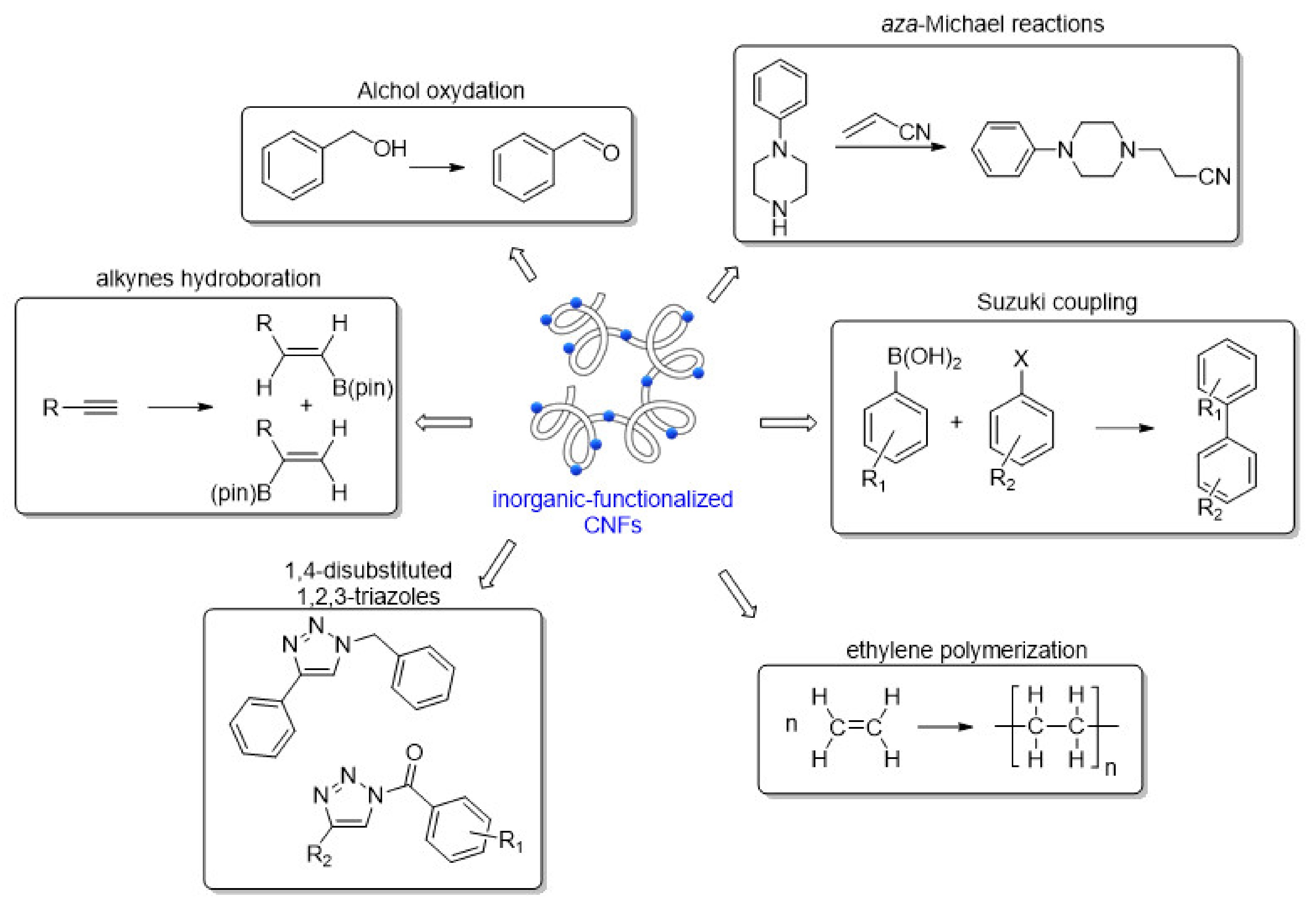
4.1. Inorganic-Functionalized CNFs Employed in Catalysis
4.1.1. Catalytic Transformation of Organic Compounds for Environmental Purposes
4.1.2. Electrocatalytic Applications of Inorganic-Functionalized CNFs
4.1.3. Photocalytic Applications of CNFs-Inorganic Composites for Environmental Remediation
4.1.4. Synthetic Applications of CNFs Grafted with Inorganic Catalysts
4.2. Organic-Functionalized CNF Employed in Catalysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- French, A.D.; Pérez, S.; Bulone, V.; Rosenau, T.; Gray, D. Cellulose; John Wiley & Sons: New York, NY, USA, 2018; Volume 1838, ISBN 0471440264. [Google Scholar]
- Kirk, O. Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; John Wiley & Sons: New York, NY, USA, 2004. [Google Scholar]
- Bellesia, G.; Asztalos, A.; Shen, T.; Langan, P.; Redondo, A.; Gnanakaran, S. In silico studies of crystalline cellulose and its degradation by enzymes. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 1184–1188. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Romeo, I.; Olivito, F.; Tursi, A.; Algieri, V.; Beneduci, A.; Chidichimo, G.; Maiuolo, L.; Sicilia, E.; De Nino, A. Totally green cellulose conversion into bio-oil and cellulose citrate using molten citric acid in an open system: Synthesis, characterization and computational investigation of reaction mechanisms. RSC Adv. 2020, 10, 34738–34751. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, B.K.; Rubiyah, H.M.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Wu, W.; Xiao, H. Methods and applications of nanocellulose loaded with inorganic nanomaterials: A review. Carbohydr. Polym. 2020, 229, 115454. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef]
- Brown, A.J. On an acetic ferment which forms cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439. [Google Scholar] [CrossRef]
- Foresti, M.L.; Vázquez, A.; Boury, B. Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: A review of recent advances. Carbohydr. Polym. 2017, 157, 447–467. [Google Scholar] [CrossRef]
- Qiu, K.; Netravali, A.N. A review of fabrication and applications of bacterial cellulose based nanocomposites. Polym. Rev. 2014, 54, 598–626. [Google Scholar] [CrossRef]
- Soriano, M.L.; Dueñas-Mas, M.J. Promising Sensing Platforms Based on Nanocellulose. In Carbon-Based Nanosensor Technology; Springer Series on Chemical Sensors and Biosensors (Methods and Applications); Kranz, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 273–301. [Google Scholar]
- Hu, W.; Chen, S.; Yang, J.; Li, Z.; Wang, H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial cellulose nanocomposites: An all-nano type of material. Mater. Sci. Eng. C 2019, 98, 1277–1293. [Google Scholar] [CrossRef] [PubMed]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Long, L.Y.; Weng, Y.X.; Wang, Y.Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers (Basel) 2018, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, L.; Calandra, P.; Amenitsch, H.; Proverbio, E.; Lombardo, D. Growth of fractal aggregates during template directed SAPO-34 zeolite formation. Microporous Mesoporous Mater. 2013, 167, 3–9. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Liang, H.W.; Chen, L.F.; Hu, B.C.; Yu, S.H. Bacterial Cellulose: A Robust Platform for Design of Three Dimensional Carbon-Based Functional Nanomaterials. Acc. Chem. Res. 2016, 49, 96–105. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Abd Mutalib, M.; Mohd Hir, Z.A.; Zain, M.F.M.; Mohamad, A.B.; Jeffery Minggu, L.; Awang, N.A.; Salleh, W.N.W. An overview on cellulose-based material in tailoring bio-hybrid nanostructured photocatalysts for water treatment and renewable energy applications. Int. J. Biol. Macromol. 2017, 103, 1232–1256. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, B.; Ma, M.G.; Wang, B. Review of Recent Development on Preparation, Properties, and Applications of Cellulose-Based Functional Materials. Int. J. Polym. Sci. 2018, 2018. [Google Scholar] [CrossRef]
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial nanocellulose—a biobased polymer for active and intelligent food packaging applications: Recent advances and developments. Polymers (Basel) 2020, 12, 2209. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lawan, I.; Zhou, W.; Zhang, M.; Fernando, G.F.; Wang, L.; Yuan, Z. Synthesis, properties and photocatalytic activity of a semiconductor/cellulose composite for dye degradation-a review. Cellulose 2020, 27, 595–609. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, V.; Vitta, S. Bacterial cellulose based flexible multifunctional nanocomposite sheets. Cellulose 2017, 24, 3341–3351. [Google Scholar] [CrossRef]
- Xu, T.; Jiang, Q.; Ghim, D.; Liu, K.K.; Sun, H.; Derami, H.G.; Wang, Z.; Tadepalli, S.; Jun, Y.S.; Zhang, Q.; et al. Catalytically Active Bacterial Nanocellulose-Based Ultrafiltration Membrane. Small 2018, 14, 1–8. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Bacterial cellulose as support for biopolymer stabilized catalytic cobalt nanoparticles. Int. J. Biol. Macromol. 2019, 135, 1162–1170. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Ul-Islam, M.; Asiri, A.M. Microwave Assisted Synthesis and Carboxymethyl Cellulose Stabilized Copper Nanoparticles on Bacterial Cellulose Nanofibers Support for Pollutants Degradation. J. Polym. Environ. 2019, 27, 2867–2877. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Anionic polysaccharide stabilized nickel nanoparticles-coated bacterial cellulose as a highly efficient dip-catalyst for pollutants reduction. React. Funct. Polym. 2019, 145, 104395. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.; Wang, B.; Yao, J.; Wang, H. TEMPO-oxidized bacterial cellulose nanofibers-supported gold nanoparticles with superior catalytic properties. Carbohydr. Polym. 2017, 160, 34–42. [Google Scholar] [CrossRef]
- Sen, I.K.; Maity, K.; Islam, S.S. Green synthesis of gold nanoparticles using a glucan of an edible mushroom and study of catalytic activity. Carbohydr. Polym. 2013, 91, 518–528. [Google Scholar] [CrossRef]
- Wibowo, A.; Indrawan, R.F.; Triadhi, U.; Aimon, H.A.; Iskandar, F.; Ardy, H. Simple preparation of Fenton catalyst@bacterial cellulose for waste water treatment. Mater. Res. Express 2018, 5, 024005. [Google Scholar] [CrossRef]
- Hu, J.; Wu, D.; Feng, Q.; Wei, A.; Song, B. Soft High-Loading TiO2 Composite Biomaterial Film as an Efficient and Recyclable Catalyst for Removing Methylene Blue. Fibers Polym. 2020, 21, 1760–1766. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, X.; Li, X.; Fu, J.; Liu, J.; Hong, F.; Qiao, J. In-situ growth of CuO/Cu nanocomposite electrode for efficient CO2 electroreduction to CO with bacterial cellulose as support. J. CO2 Util. 2020, 37, 188–194. [Google Scholar] [CrossRef]
- Jeremic, S.; Djokic, L.; Ajdačić, V.; Božinović, N.; Pavlovic, V.; Manojlović, D.D.; Babu, R.; Senthamaraikannan, R.; Rojas, O.; Opsenica, I.; et al. Production of bacterial nanocellulose (BNC) and its application as a solid support in transition metal catalysed cross-coupling reactions. Int. J. Biol. Macromol. 2019, 129, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Kataria, R.; Cerrone, F.; Woods, T.; Kenny, S.; O’Donovan, A.; Guzik, M.; Shaikh, H.; Duane, G.; Gupta, V.K.; et al. Conversion of grass biomass into fermentable sugars and its utilization for medium chain length polyhydroxyalkanoate (mcl-PHA) production by Pseudomonas strains. Bioresour. Technol. 2013, 150, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Nikoofar, K.; Heidari, H.; Shahedi, Y. Investigation the catalytic activity of nanofibrillated and nanobacterial cellulose sulfuric acid in synthesis of dihydropyrimidoquinolinetriones. Res. Chem. Intermed. 2018, 44, 4533–4546. [Google Scholar] [CrossRef]
- Costanzo, P.; Nardi, M.; Oliverio, M. Similarity and Competition between Biginelli and Hantzsch Reactions: An Opportunity for Modern Medicinal Chemistry. Eur. J. Org. Chem. 2020, 2020, 3954–3964. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Nardi, M.; Rivalta, I.; Procopio, A. Facile ecofriendly synthesis of monastrol and its structural isomers via biginelli reaction. ACS Sustain. Chem. Eng. 2014, 2, 1228–1233. [Google Scholar] [CrossRef]
- Costanzo, P.; Calandruccio, C.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Procopio, A. First multicomponent reaction exploiting glycerol carbonate synthesis. J. Clean. Prod. 2018, 202, 504–509. [Google Scholar] [CrossRef]
- Choudhury, P.; Ghosh, P.; Basu, B. Amine-functionalized graphene oxide nanosheets (AFGONs): An efficient bifunctional catalyst for selective formation of 1,4-dihydropyridines, acridinediones and polyhydroquinolines. Mol. Divers. 2020, 24, 283–294. [Google Scholar] [CrossRef]
- Said, M.S.; Khonde, N.S.; Thorat, M.N.; Atapalkar, R.S.; Kulkarni, A.A.; Gajbhiye, J.; Dastager, S.G. A New TBAF Complex, Highly Stable, Facile and Selective Source for Nucleophilic Fluorination: Applications in Batch and Flow Chemistry. Asian J. Org. Chem. 2020, 9, 1022–1026. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. Micro and nanocrystalline cellulose derivatives of lignocellulosic biomass: A review on synthesis, applications and advancements. Carbohydr. Polym. 2020, 250, 116937. [Google Scholar] [CrossRef] [PubMed]
- Carneiro de Oliveira, J.; Rigolet, S.; Marichal, C.; Roucoules, V.; Laborie, M.P. Grafting Diels-Alder moieties on cellulose nanocrystals through carbamation. Carbohydr. Polym. 2020, 250, 116966. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, T.; Naish, V.; O’Connor, B.; Blaise, C.; Gagné, F.; Hall, L.; Trudeau, V.; Martel, P. An ecotoxicological characterization of nanocrystalline cellulose (NCC). Nanotoxicology 2010, 4, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hsieh, Y. Lo Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Feng, K.; Gao, X.; Gu, Z.; Jin, Z. Improving Homogeneity of Iridescent Cellulose Nanocrystal Films by Surfactant-Assisted Spreading Self-Assembly. ACS Sustain. Chem. Eng. 2019, 7, 19062–19071. [Google Scholar] [CrossRef]
- Mendoza-Galván, A.; Tejeda-Galán, T.; Domínguez-Gómez, A.B.; Mauricio-Sánchez, R.A.; Järrendahl, K.; Arwin, H. Linear birefringent films of cellulose nanocrystals produced by dip-coating. Nanomaterials 2019, 9, 45. [Google Scholar] [CrossRef]
- Ali, S.D.; Imiete, I.E.; Orlandi, M.E.; Castellani, L.; Hanel, T.; Zoia, L. Novel CNC/silica hybrid as potential reinforcing filler for natural rubber compounds. J. Appl. Polym. Sci. 2020, 137, 1–11. [Google Scholar] [CrossRef]
- Wang, P.X.; Hamad, W.Y.; MacLachlan, M.J. Polymer and Mesoporous Silica Microspheres with Chiral Nematic Order from Cellulose Nanocrystals. Angew. Chem. Int. Ed. 2016, 55, 12460–12464. [Google Scholar] [CrossRef]
- Talantikite, M.; Beury, N.; Moreau, C.; Cathala, B. Arabinoxylan/Cellulose Nanocrystal Hydrogels with Tunable Mechanical Properties. Langmuir 2019, 35, 13427–13434. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, M.; Li, L.; Fan, B.; Liu, Y.; Li, R.; Ren, X.; Huang, T.S.; Kim, I.S. Construction of aerogels based on nanocrystalline cellulose and chitosan for high efficient oil/water separation and water disinfection. Carbohydr. Polym. 2020, 243, 116461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lou, C.; Hua, F.; Deng, H.; Tian, X. Cellulose nanocrystals-based flocculants for high-speed and high-efficiency decolorization of colored effluents. J. Clean. Prod. 2020, 251. [Google Scholar] [CrossRef]
- Wang, S.; Dong, L.; Li, Z.; Lin, N.; Xu, H.; Gao, S. Sustainable supercapacitors of nitrogen-doping porous carbon based on cellulose nanocrystals and urea. Int. J. Biol. Macromol. 2020, 164, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Friedman, H.M.; Bateman, M.; Moores, A. Cellulose nanocrystals as non-innocent supports for the synthesis of ruthenium nanoparticles and their application to arene hydrogenation. RSC Adv. 2015, 5, 53207–53210. [Google Scholar] [CrossRef]
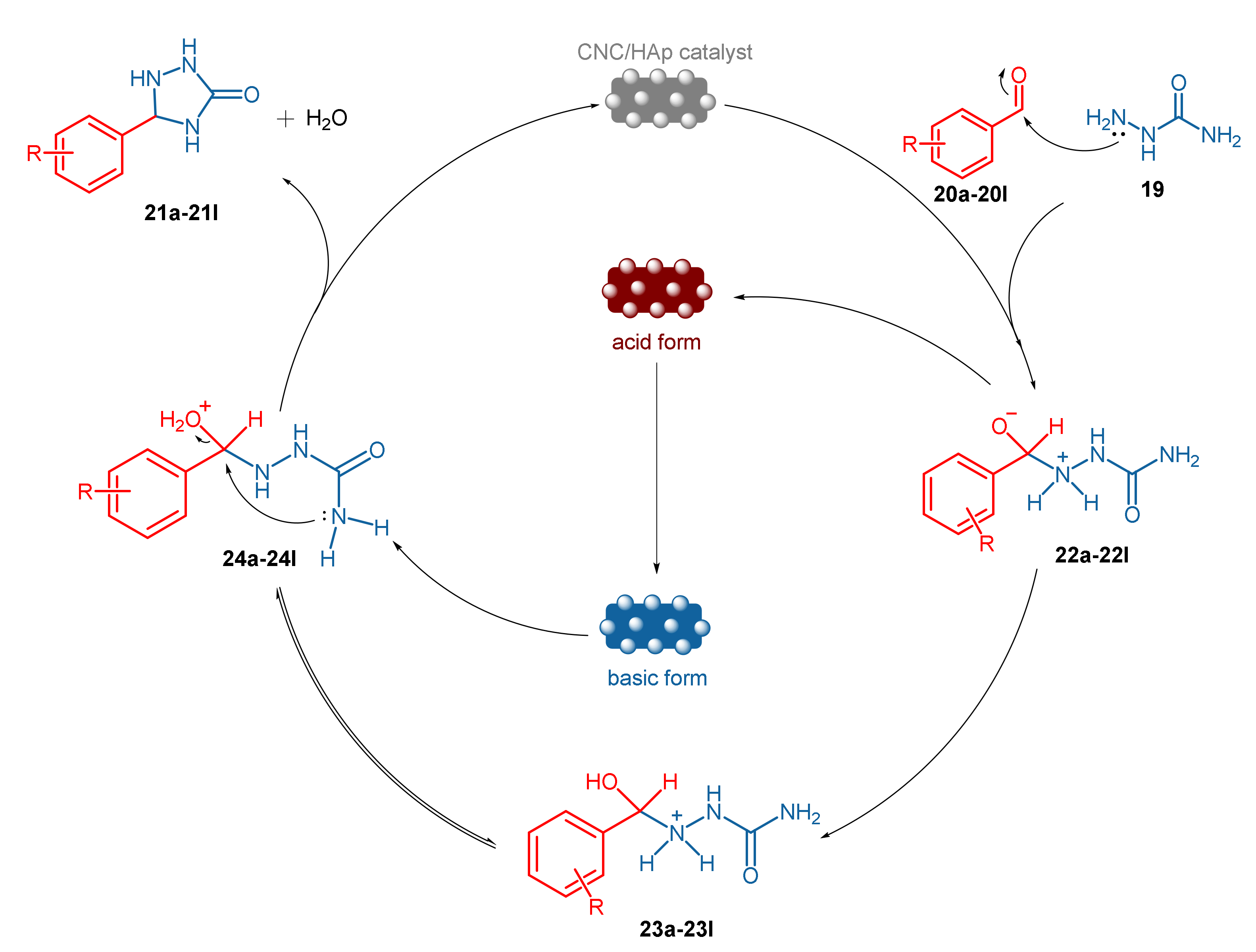
- Moodley, V.; Maddila, S.; Jonnalagadda, S.B.; Van Zyl, W.E. Synthesis of triazolidine-3-one derivatives through the nanocellulose/hydroxyapatite-catalyzed reaction of aldehydes and semicarbazide. New J. Chem. 2017, 41, 6455–6463. [Google Scholar] [CrossRef]
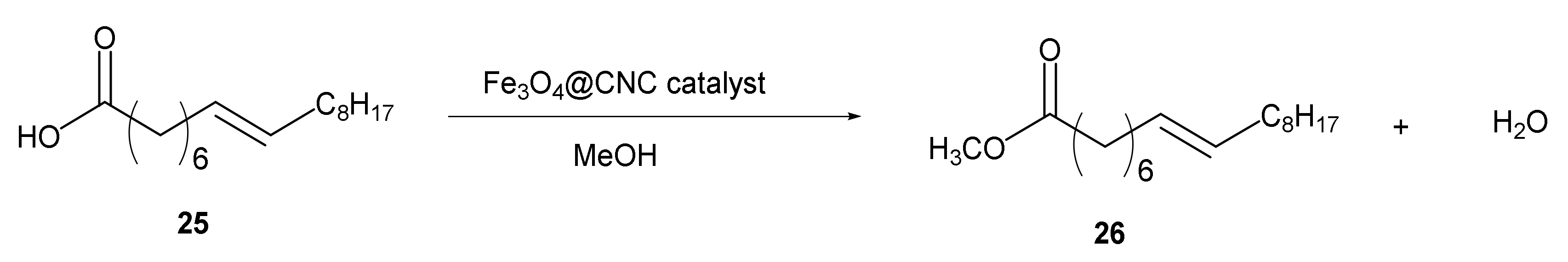
- Helmiyati; Anggraini, Y. Nanocomposites comprising cellulose and nanomagnetite as heterogeneous catalysts for the synthesis of biodiesel from oleic acid. Int. J. Technol. 2019, 10, 798–807. [Google Scholar] [CrossRef]
- Mirosanloo, A.; Zareyee, D.; Khalilzadeh, M.A. Recyclable cellulose nanocrystal supported Palladium nanoparticles as an efficient heterogeneous catalyst for the solvent-free synthesis of coumarin derivatives via von Pechmann condensation. Appl. Organomet. Chem. 2018, 32, 1–9. [Google Scholar] [CrossRef]
- Nikoofar, K.; Heidari, H.; Shahedi, Y. Nano crystalline cellulose sulfuric acid (s-NCC): A novel green nanocatalyst for the synthesis of polyhydroxy pyrimidine-fused heterocyclic compounds (PPFHs). Cellulose 2018, 25, 5697–5709. [Google Scholar] [CrossRef]
- An, X.; Long, Y.; Ni, Y. Cellulose nanocrystal/hexadecyltrimethylammonium bromide/silver nanoparticle composite as a catalyst for reduction of 4-nitrophenol. Carbohydr. Polym. 2017, 156, 253–258. [Google Scholar] [CrossRef]
- Turco Liveri, V.; Lombardo, D.; Pochylski, M.; Calandra, P. Molecular association of small amphiphiles: Origin of ionic liquid properties in dibutyl phosphate/propylamine binary mixtures. J. Mol. Liq. 2018, 263, 274–281. [Google Scholar] [CrossRef]
- Calandra, P.; Ruggirello, A.; Mele, A.; Turco Liveri, V. Self-assembly in surfactant-based liquid mixtures: Bis(2-ethylhexyl)phosphoric acid/bis(2-ethylhexyl)amine systems. J. Colloid Interface Sci. 2010, 348, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Calandra, P.; Turco Liveri, V.; Riello, P.; Freris, I.; Mandanici, A. Self-assembly in surfactant-based liquid mixtures: Octanoic acid/Bis(2-ethylhexyl)amine systems. J. Colloid Interface Sci. 2012, 367, 280–285. [Google Scholar] [CrossRef]
- Calandra, P.; Nicotera, I.; Oliviero Rossi, C.; Turco Liveri, V. Dynamical properties of self-assembled surfactant-based mixtures: Triggering of one-dimensional anomalous diffusion in Bis(2-ethylhexyl)phosphoric acid/ n -octylamine systems. Langmuir 2013, 29, 14848–14854. [Google Scholar] [CrossRef] [PubMed]
- Calandra, P.; Ruggirello, A.; Pistone, A.; Turco Liveri, V. Structural and Optical Properties of Novel Surfactant Coated TiO2-Ag Based Nanoparticles. J. Clust. Sci. 2010, 21, 767–778. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, D.; Hou, C.; Liang, C.; Li, H. Facile one-pot synthesis of cellulose nanocrystal-supported hollow CuFe2O4 nanoparticles as efficient catalyst for 4-nitrophenol reduction. J. Nanopart. Res. 2018, 20. [Google Scholar] [CrossRef]
- Zhan, Y.; Meng, Y.; Li, W.; Chen, Z.; Yan, N.; Li, Y.; Teng, M. Magnetic recoverable MnFe2O4/cellulose nanocrystal composites as an efficient catalyst for decomposition of methylene blue. Ind. Crops Prod. 2018, 122, 422–429. [Google Scholar] [CrossRef]
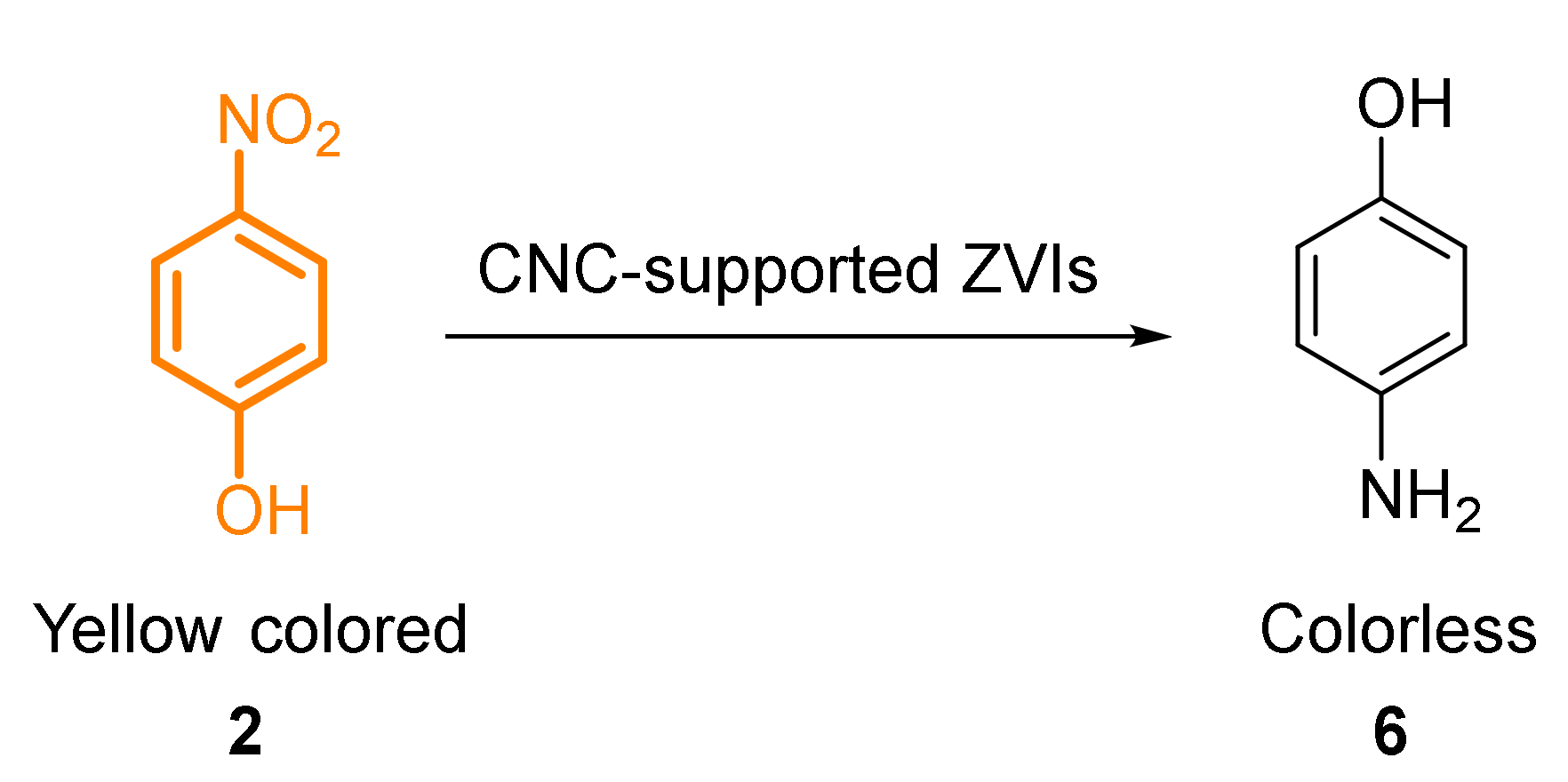
- Dhar, P.; Kumar, A.; Katiyar, V. Fabrication of cellulose nanocrystal supported stable Fe(0) nanoparticles: A sustainable catalyst for dye reduction, organic conversion and chemo-magnetic propulsion. Cellulose 2015, 22, 3755–3771. [Google Scholar] [CrossRef]
- Goswami, M.; Das, A.M. Synthesis of cellulose impregnated copper nanoparticles as an efficient heterogeneous catalyst for C–N coupling reactions under mild conditions. Carbohydr. Polym. 2018, 195, 189–198. [Google Scholar] [CrossRef]
- Chetia, M.; Ali, A.A.; Bordoloi, A.; Sarma, D. Facile route for the regioselective synthesis of 1,4-disubstituted 1,2,3-triazole using copper nanoparticles supported on nanocellulose as recyclable heterogeneous catalyst. J. Chem. Sci. 2017, 129, 1211–1217. [Google Scholar] [CrossRef]
- Maiuolo, L.; Algieri, V.; Olivito, F.; De Nino, A. Recent developments on 1,3-dipolar cycloaddition reactions by catalysis in green solvents. Catalysts 2020, 10, 65. [Google Scholar] [CrossRef]
- De Nino, A.; Algieri, V.; Tallarida, M.A.; Constanzo, P.; Pedrón, M.; Tejero, T.; Merino, P.; Maiuolo, L. Regioselective Synthesis of 1,4,5-Trisubstituted-1,2,3-Triazoles from Aryl Azides and Enaminones. Eur. J. Org. Chem. 2019, 2019, 5725–5731. [Google Scholar] [CrossRef]
- Maiuolo, L.; Russo, B.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Tallarida, M.A.; De Nino, A. Regioselective synthesis of 1,5-disubstituted 1,2,3-triazoles by 1,3-dipolar cycloaddition: Role of Er(OTf) 3, ionic liquid and water. Tetrahedron Lett. 2019, 60, 672–674. [Google Scholar] [CrossRef]
- De Nino, A.; Merino, P.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Russo, B.; Tallarida, M.A.; Maiuolo, L. Synthesis of 1,5-functionalized 1,2,3-triazoles using ionic liquid/iron(III) chloride as an efficient and reusable homogeneous catalyst. Catalysts 2018, 8, 364. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- Algieri, V.; Algieri, C.; Maiuolo, L.; De Nino, A.; Pagliarani, A.; Tallarida, M.A.; Trombetti, F.; Nesci, S. 1,5-Disubstituted-1,2,3-triazoles as inhibitors of the mitochondrial Ca2+-activated F1FO-ATP(hydrol)ase and the permeability transition pore. Ann. N. Y. Acad. Sci. 2020, 1–13. [Google Scholar] [CrossRef]
- Maiuolo, L.; De Nino, A.; Algieri, V.; Nardi, M. Microwave-Assisted 1,3-Dipolar Cyclo-addition: Recent Advances In Synthesis of Isoxazolidines. Mini. Rev. Org. Chem. 2017, 14, 136–142. [Google Scholar] [CrossRef]
- Maiuolo, L.; Algieri, V.; Russo, B.; Tallarida, M.A.; Nardi, M.; Di Gioia, M.L.; Merchant, Z.; Merino, P.; Delso, I.; Nino, A. De Synthesis, biological and in silico evaluation of pure nucleobase-containing spiro (Indane-Isoxazolidine) derivatives as potential inhibitors of MDM2-p53 interaction. Molecules 2019, 24, 2909. [Google Scholar] [CrossRef]
- Maiuolo, L.; Feriotto, G.; Algieri, V.; Nardi, M.; Russo, B.; Di Gioia, M.L.; Furia, E.; Tallarida, M.A.; Mischiati, C.; De Nino, A. Antiproliferative activity of novel isatinyl/indanyl nitrones (INs) as potential spin trapping agents of free radical intermediates. Medchemcomm 2018, 9, 299–304. [Google Scholar] [CrossRef]
- Olivito, F.; Amodio, N.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Juli, G.; Tassone, P.; Procopio, A. Synthesis and preliminary evaluation of the anti-cancer activity on A549 lung cancer cells of a series of unsaturated disulfides. Medchemcomm 2019, 10, 116–119. [Google Scholar] [CrossRef]
- Olivito, F.; Costanzo, P.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Procopio, A. Efficient synthesis of organic thioacetates in water. Org. Biomol. Chem. 2018, 16, 7753–7759. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Chetia, M.; Ali, A.A.; Bordoloi, A.; Gehlot, P.S.; Kumar, A.; Sarma, D. Copper Nanoparticles Immobilized on Nanocellulose: A Novel and Efficient Heterogeneous Catalyst for Controlled and Selective Oxidation of Sulfides and Alcohols. Catal. Letters 2019, 149, 141–150. [Google Scholar] [CrossRef]
- Paonessa, R.; Nardi, M.; Di Gioia, M.L.; Olivito, F.; Oliverio, M.; Procopio, A. Eco-friendly synthesis of lipophilic EGCG derivatives and antitumor and antioxidant evaluation. Nat. Prod. Commun. 2018, 13, 1117–1122. [Google Scholar] [CrossRef]
- De Nino, A.; Tallarida, M.A.; Algieri, V.; Olivito, F.; Costanzo, P.; De Filpo, G.; Maiuolo, L. Sulfonated cellulose-based magnetic composite as useful media for water remediation from amine pollutants. Appl. Sci. 2020, 10, 8155. [Google Scholar] [CrossRef]
- Mirjalili, B.B.F.; Imani, M. Fe3O4@NCs/BF0.2: A magnetic bio-based nanocatalyst for the synthesis of 2,3-dihydro-1H-perimidines. J. Chinese Chem. Soc. 2019, 66, 1542–1549. [Google Scholar] [CrossRef]
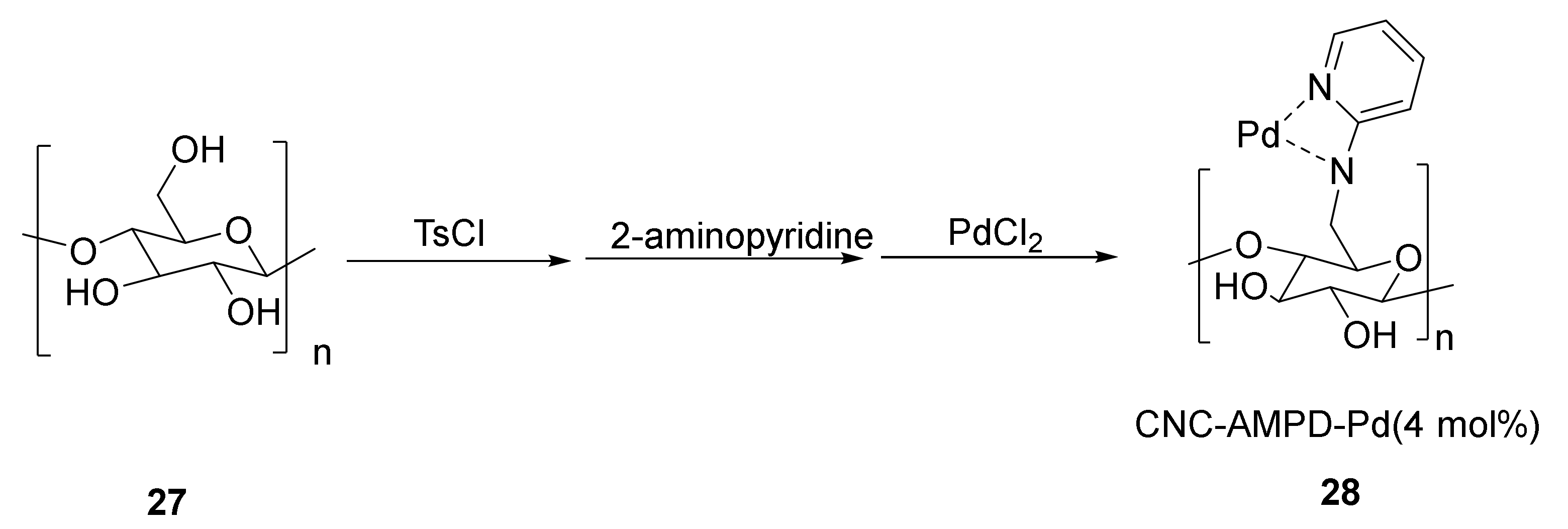
- Liu, J.; Plog, A.; Groszewicz, P.; Zhao, L.; Xu, Y.; Breitzke, H.; Stark, A.; Hoffmann, R.; Gutmann, T.; Zhang, K.; et al. Design of a Heterogeneous Catalyst Based on Cellulose Nanocrystals for Cyclopropanation: Synthesis and Solid-State NMR Characterization. Chem. A Eur. J. 2015, 21, 12414–12420. [Google Scholar] [CrossRef] [PubMed]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Herrick, F.W.; Casebier, R.L.; Hamilton, J.K.; Sandberg, K.R. Microfibrillated cellulose: Morphology and accessibility. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37, 797–813. [Google Scholar]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, B.S.; Kim, H.S.; Lee, Y.J.; Min, S.K.; Jang, D.; Abas, Z.; Kim, J. Review of nanocellulose for sustainable future materials. Int. J. Precis. Eng. Manuf. Green Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Pääkko, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, M.; Henriksson, G.; Berglund, L.A.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Wågberg, L.; Winter, L.; Ödberg, L.; Lindström, T. On the charge stoichiometry upon adsorption of a cationic polyelectrolyte on cellulosic materials. Colloids Surf. 1987, 27, 163–173. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chemie Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Hyuk Jang, K.; Ok Kang, Y.; Ho Park, W. International Journal of Biological Macromolecules Functional cellulose-based nanofibers with catalytic activity: Effect of Ag content and Ag phase. Int. J. Biol. Macromol. 2014, 67, 394–400. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhang, Y.; Van Bochove, B.; Mäkilä, E. Robust shape-retaining nanocellulose-based aerogels decorated with silver nanoparticles for fast continuous catalytic discoloration of organic dyes. Sep. Purif. Technol. 2020, 242, 116523. [Google Scholar] [CrossRef]
- Heidari, H. Ag Nanoparticle / Nanofibrillated Cellulose Composite as an Effective and Green Catalyst for Reduction of 4-Nitrophenol. J. Clust. Sci. 2018, 29, 475–481. [Google Scholar] [CrossRef]
- Gopiraman, M.; Deng, D.; Saravanamoorthy, S.; Chung, I.; Kim, I.S. Gold, silver and nickel nanoparticle anchored cellulose nanofiber composites as highly active catalysts for the rapid and selective reduction of nitrophenols in water. RSC Adv. 2018, 8, 3014–3023. [Google Scholar] [CrossRef]
- Gopiraman, M.; Saravanamoorthy, S. Green synthesis of Ag @ Au bimetallic regenerated cellulose nanofibers for catalytic applications †. New J. Chem. 2019, 43, 17090–17103. [Google Scholar] [CrossRef]
- Cunha Arantes, A.C.; das Gracas Almeida, C.; Leite Dauzacker, L.C.; Bianchi, M.L.; Wood, D.F.; Williams, T.G.; Orts, W.J.; Denzin Tonoli, G.H. Renewable hybrid nanocatalyst from magnetite and cellulose for treatment of textile effluents. Carbohydr. Polym. 2017, 163, 101–107. [Google Scholar] [CrossRef]
- Hou, C.; Chen, W.; Fu, L.; Zhang, S.; Liang, C.; Wang, Y. Facile synthesis of a Co/Fe bi-MOFs/CNF membrane nanocomposite and its application in the degradation of tetrabromobisphenol A. Carbohydr. Polym. 2020, 247, 116731. [Google Scholar] [CrossRef]
- Gu, J.; Hu, C.; Zhang, W.; Dichiara, A.B. Applied Catalysis B: Environmental Reagentless preparation of shape memory cellulose nano fi bril aerogels decorated with Pd nanoparticles and their application in dye discoloration. Appl. Catal. B Environ. 2018, 237, 482–490. [Google Scholar] [CrossRef]
- Ujihara, M.; Hsu, M.; Liou, J.; Imae, T. Journal of the Taiwan Institute of Chemical Engineers Hybridization of cellulose nanofiber with amine-polymers and its ability on sick house syndrome gas decomposition. J. Taiwan Inst. Chem. Eng. 2018, 92, 106–111. [Google Scholar] [CrossRef]
- Esquivel-Pena, V.; Guccini, V.; Kumar, S.; Salazar-Alvarez, G.; Rodriguez de San Miguel, E.; de Gyves, J. Hybrids based on borate-functionalized cellulose nanofibers and noble-metal nanoparticles as sustainable catalysts for environmental applications. RSC Adv. 2020, 10, 12460–12468. [Google Scholar] [CrossRef]
- Idrissi, N.E.L.; Mouden, A.E.L.; Kaddami, H. Novel biohybrid aerogel composites based on cellulosic and cobalt metallic nanoparticles: Efficient and recyclable catalysts for green reduction reactions Novel biohybrid aerogel composites based on cellulosic and cobalt metallic nanoparticles: Efficient. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Marrakech, Morocco, 13–15 November 2019; 2020; p. 827. [Google Scholar]
- Zhang, Y.; Zhou, K.; Zhang, L.; Wu, H.; Guo, J. Synthesis of mesoporous Γ-Al2O3 by using cellulose nanofiber as template for hydrodesulfurization of dibenzothiophene. Fuel 2019, 253, 431–440. [Google Scholar] [CrossRef]
- Tian, C.; Liu, Z.; Wu, Y.; Lu, X.; Yang, T.; Tao, X.; Qing, Y. Natural-Cellulose-Nanofibril-Tailored NiFe Nanoparticles for Efficient Oxygen Evolution Reaction. ChemElectroChem 2019, 6, 3303–3310. [Google Scholar] [CrossRef]
- Tao, X.; Luo, S.; Tian, C.; Qing, Y.; Lu, X.; Yan, N.; Wu, Y. Ni@Ni2P Encapsulation in Interconnected N-Doped Carbonized Cellulose Nanofibril Network for Efficient Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2020, 8, 1859–1867. [Google Scholar] [CrossRef]
- Tian, C.; Yang, T.; Liu, Z.; Qing, Y.; Zhang, B.; Zhou, J.; Wu, Y. Well-aligned arrangement CoFe nanoparticles assisted with cellulose nanofibrils for efficient oxygen evolution reaction. Appl. Surf. Sci. 2020, 510, 145484. [Google Scholar] [CrossRef]
- Lee, Y.R.; Yoo, H.; Choi, J.; Ahn, W.S. Electrocatalytic oxygen reduction over Co@Co3O4/N-doped porous carbon derived from pyrolysis of ZIF-8/67 on cellulose nanofibers. Cellulose 2020, 27, 2723–2735. [Google Scholar] [CrossRef]
- Meng, H.; Liu, Y.; Liu, H.; Pei, S.; Yuan, X.; Li, H.; Zhang, Y. ZIF67@MFC-Derived Co/N-C@CNFs Interconnected Frameworks with Graphitic Carbon-Encapsulated Co Nanoparticles as Highly Stable and Efficient Electrocatalysts for Oxygen Reduction Reactions. ACS Appl. Mater. Interfaces 2020, 12, 41580–41589. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Mohamed, A.; Aboamera, N.M.; Osman, T.A.; Khattab, A. Photocatalytic degradation of organic dyes using composite nanofibers under UV irradiation. Appl. Nanosci. 2018, 8, 155–161. [Google Scholar] [CrossRef]
- ZabihiSahebi, A.; Koushkbaghi, S.; Pishnamazi, M.; Askari, A.; Khosravi, R.; Irani, M. Synthesis of cellulose acetate/chitosan/SWCNT/Fe3O4/TiO2 composite nanofibers for the removal of Cr(VI), As(V), Methylene blue and Congo red from aqueous solutions. Int. J. Biol. Macromol. 2019, 140, 1296–1304. [Google Scholar] [CrossRef]
- Gan, L.; Geng, A.; Song, C.; Xu, L.; Wang, L.; Fang, X.; Han, S.; Cui, J.; Mei, C. Simultaneous removal of rhodamine B and Cr(VI) from water using cellulose carbon nanofiber incorporated with bismuth oxybromide: The effect of cellulose pyrolysis temperature on photocatalytic performance. Environ. Res. 2020, 185, 109414. [Google Scholar] [CrossRef]
- Tian, C.; Tao, X.; Luo, S.; Qing, Y.; Lu, X.; She, J.; Wu, Y. Cellulose nanofibrils anchored Ag on graphitic carbon nitride for efficient photocatalysis under visible light. Environ. Sci. Nano 2018, 5, 2129–2143. [Google Scholar] [CrossRef]
- Gupta, K.; Kaushik, A.; Tikoo, K.B.; Kumar, V.; Singhal, S. Enhanced catalytic activity of composites of NiFe2O4 and nano cellulose derived from waste biomass for the mitigation of organic pollutants. Arab. J. Chem. 2020, 13, 783–798. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Sun, J.J.; Fakhri, A.; Surendar, A.; Ibatova, A.Z.; Liu, J.B. Synthesis, photocatalytic, optical, electronic and biological properties of the CoS2–CuS on cellulose nanocomposites as novel nano catalyst by a sonochemical technology. J. Mater. Sci. Mater. Electron. 2018, 29, 18531–18539. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q.; Liu, L.; Wang, Y.; Leung, M.K.H. Activation of peroxymonosulfate and recycled effluent filtration over cathode membrane CNFs-CoFe2O4/PVDF in a photocatalytic fuel cell for water pollution control. Chem. Eng. J. 2020, 399, 125731. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, M.; Liu, S.; Wang, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Xu, B.; Sui, X. Copper-loaded nanocellulose sponge as a sustainable catalyst for regioselective hydroboration of alkynes. Carbohydr. Polym. 2018, 191, 17–24. [Google Scholar] [CrossRef]
- Gopiraman, M.; Bang, H.; Yuan, G.; Yin, C.; Song, K.H.; Lee, J.S.; Chung, I.M.; Karvembu, R.; Kim, I.S. Noble metal/functionalized cellulose nanofiber composites for catalytic applications. Carbohydr. Polym. 2015, 132, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Jebali, Z.; Granados, A.; Nabili, A.; Boufi, S.; do Rego, A.M.B.; Majdoub, H.; Vallribera, A. Cationic cellulose nanofibrils as a green support of palladium nanoparticles: Catalyst evaluation in Suzuki reactions. Cellulose 2018, 25, 6963–6975. [Google Scholar] [CrossRef]
- Hees, T.; Zhong, F.; Rudolph, T.; Walther, A.; Mülhaupt, R. Nanocellulose Aerogels for Supporting Iron Catalysts and In Situ Formation of Polyethylene Nanocomposites. Adv. Funct. Mater. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Koga, H.; Azetsu, A.; Tokunaga, E.; Saito, T.; Isogai, A.; Kitaoka, T. Topological loading of Cu(I) catalysts onto crystalline cellulose nanofibrils for the Huisgen click reaction. J. Mater. Chem. 2012, 22, 5538–5542. [Google Scholar] [CrossRef]
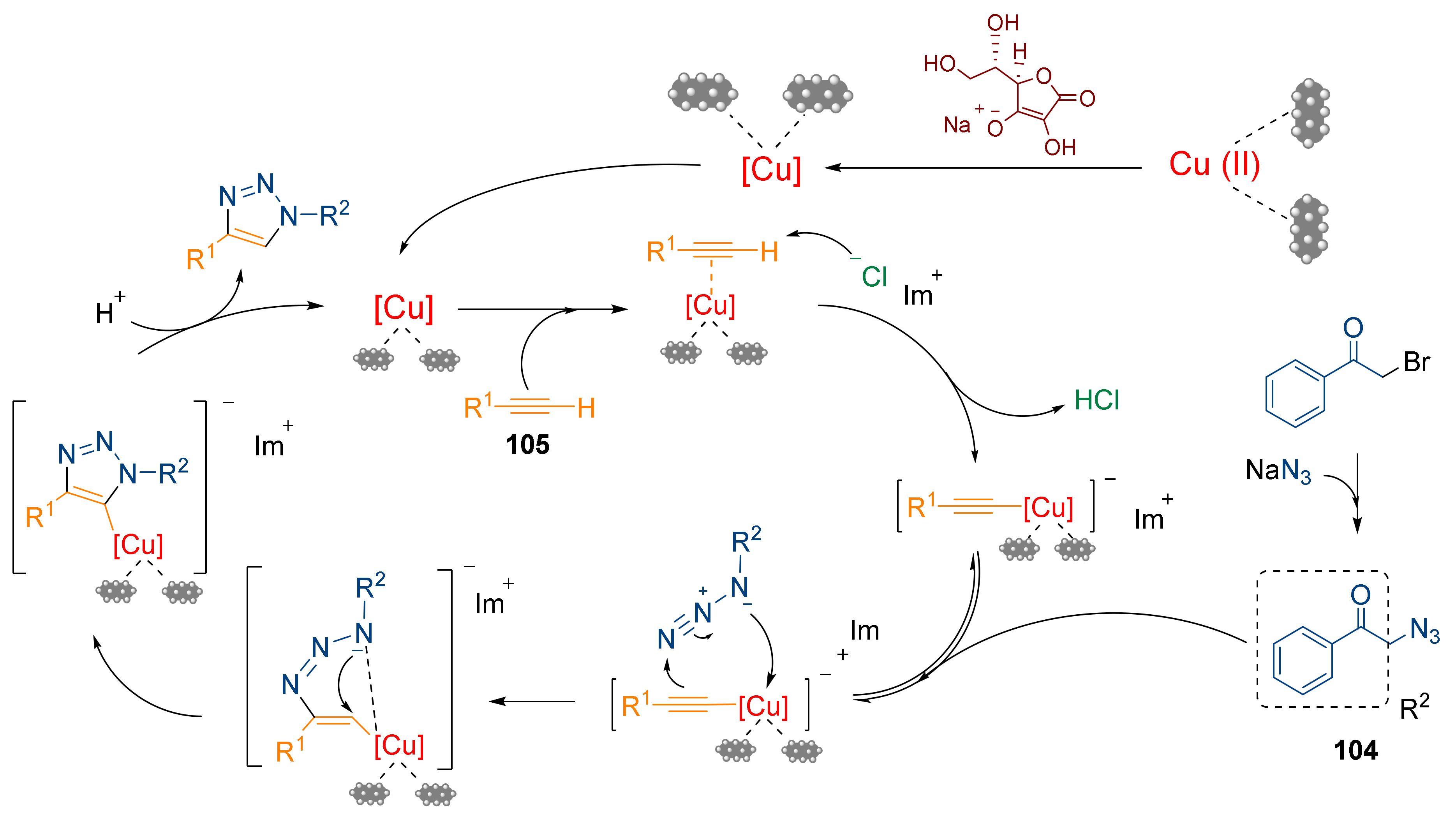
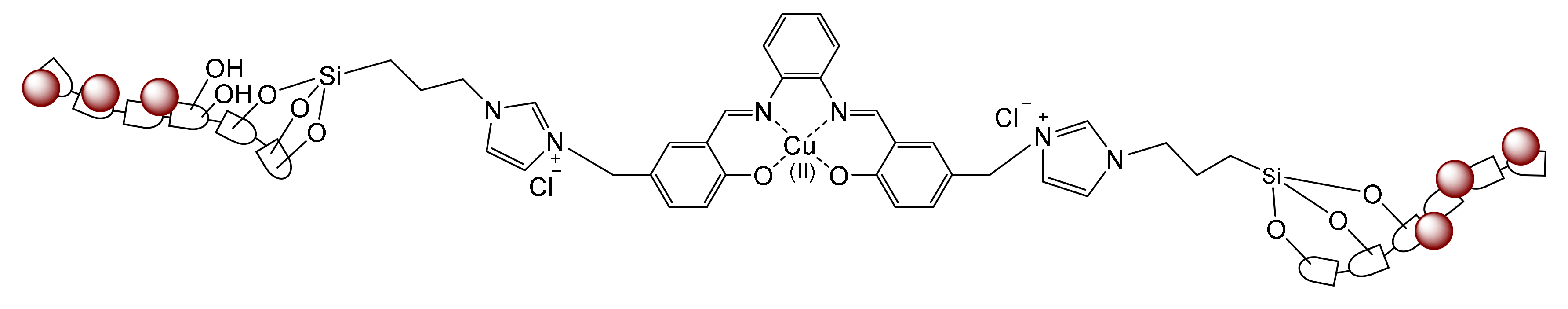
- Ghamari kargar, P.; Bagherzade, G.; Eshghi, H. Design and synthesis of magnetic Fe3O4@NFC-ImSalophCu nanocatalyst based on cellulose nanofibers as a new and highly efficient, reusable, stable and green catalyst for the synthesis of 1,2,3-triazoles. RSC Adv. 2020, 10, 32927–32937. [Google Scholar] [CrossRef]
- Tamura, Y.; Kanomata, K.; Kitaoka, T. Interfacial Hydrolysis of Acetals on Protonated TEMPO-oxidized Cellulose Nanofibers. Sci. Rep. 2018, 8, 6–10. [Google Scholar] [CrossRef]
- Kanomata, K.; Tatebayashi, N.; Habaki, X.; Kitaoka, T. Cooperative catalysis of cellulose nanofiber and organocatalyst in direct aldol reactions. Sci. Rep. 2018, 8, 6–10. [Google Scholar] [CrossRef]
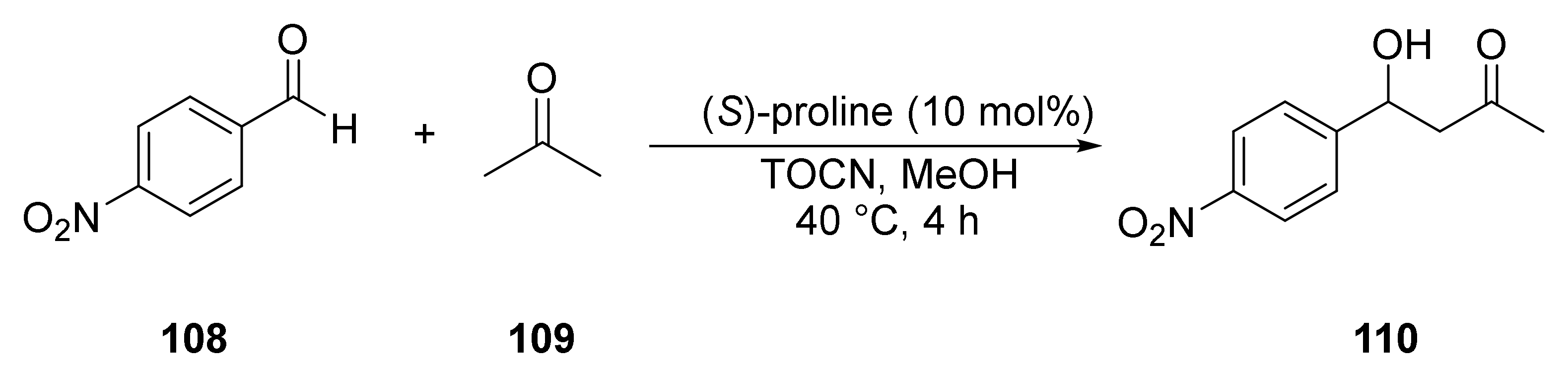
- Ranaivoarimanana, N.J.; Kanomata, K.; Kitaoka, T. Concerted catalysis by nanocellulose and proline in organocatalytic Michael additions. Molecules 2019, 24, 1231. [Google Scholar] [CrossRef] [PubMed]
- Ellebracht, N.C.; Jones, C.W. Functionalized cellulose nanofibril aerogels as cooperative acid–base organocatalysts for liquid flow reactions. Carbohydr. Polym. 2020, 233, 115825. [Google Scholar] [CrossRef] [PubMed]
- Lalanne-Tisné, M.; Mees, M.A.; Eyley, S.; Zinck, P.; Thielemans, W. Organocatalyzed ring opening polymerization of lactide from the surface of cellulose nanofibrils. Carbohydr. Polym. 2020, 250. [Google Scholar] [CrossRef] [PubMed]
- Lasemi, Z.; Tajbakhsh, M.; Alinezhad, H.; Mehrparvar, F. 1,8-Diazabicyclo [5.4.0] undec-7-ene functionalized cellulose nanofibers as an efficient and reusable nanocatalyst for the synthesis of tetraketones in aqueous medium. Res. Chem. Intermed. 2020, 46, 3667–3682. [Google Scholar] [CrossRef]

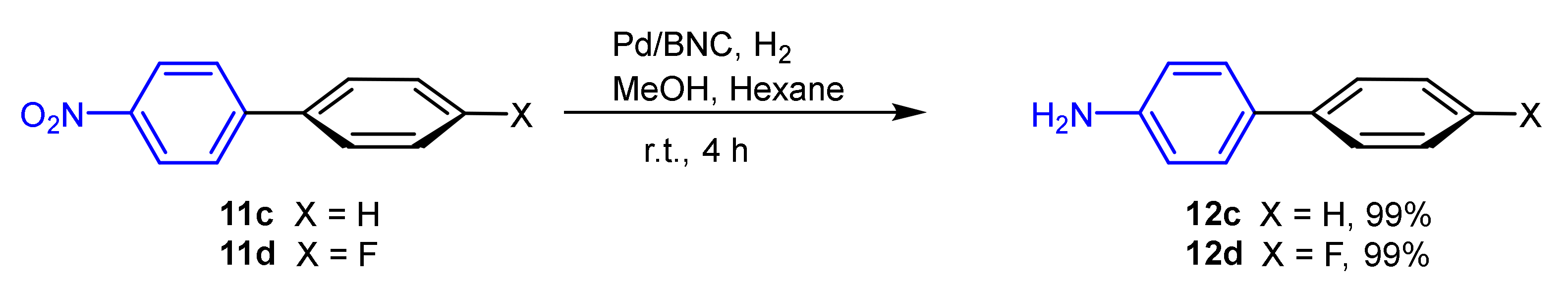
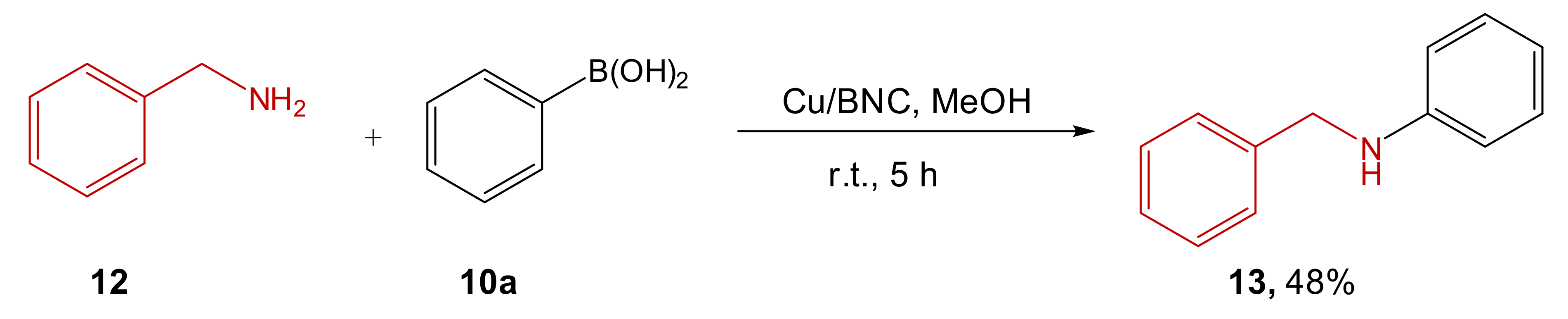
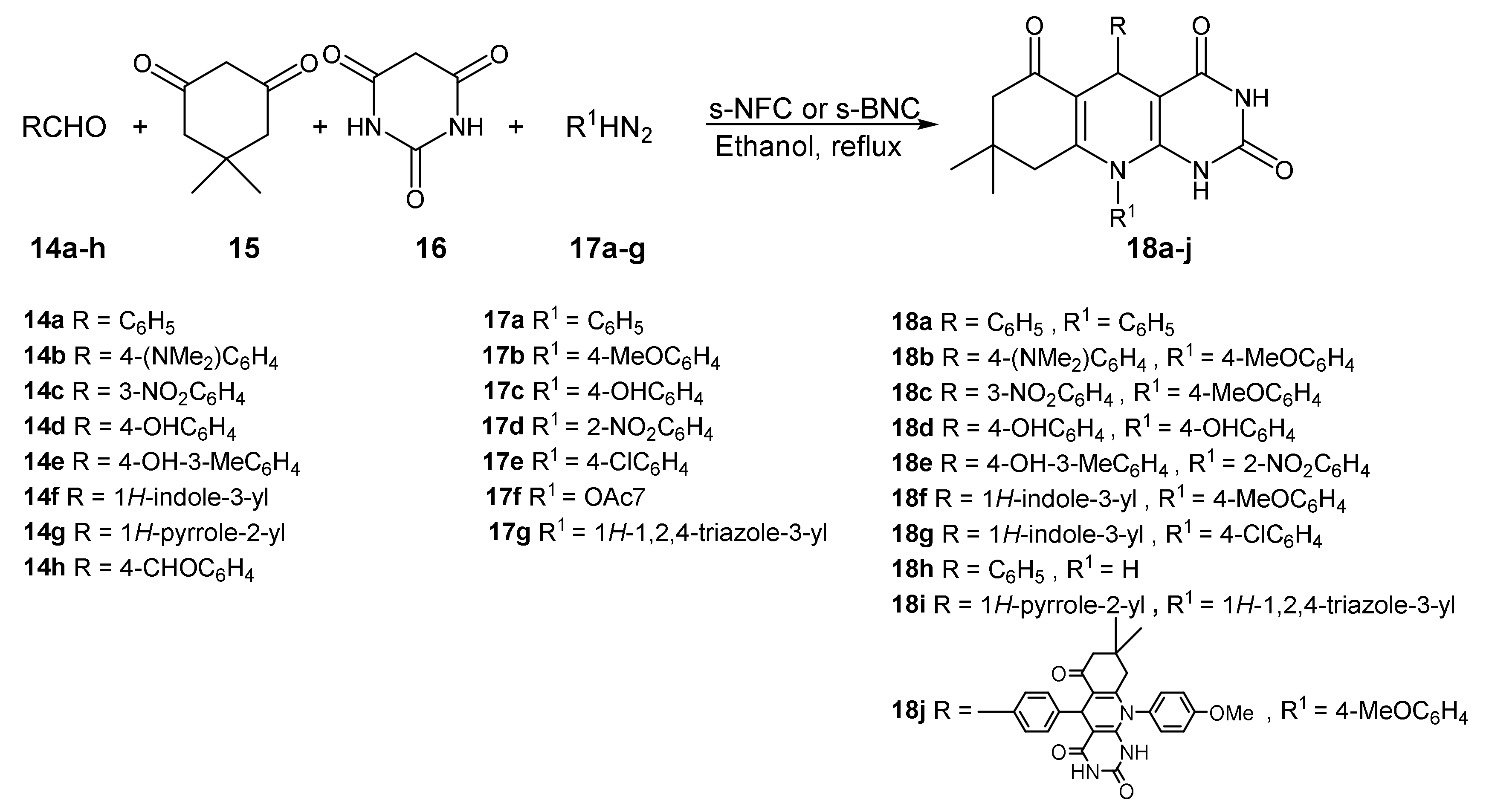
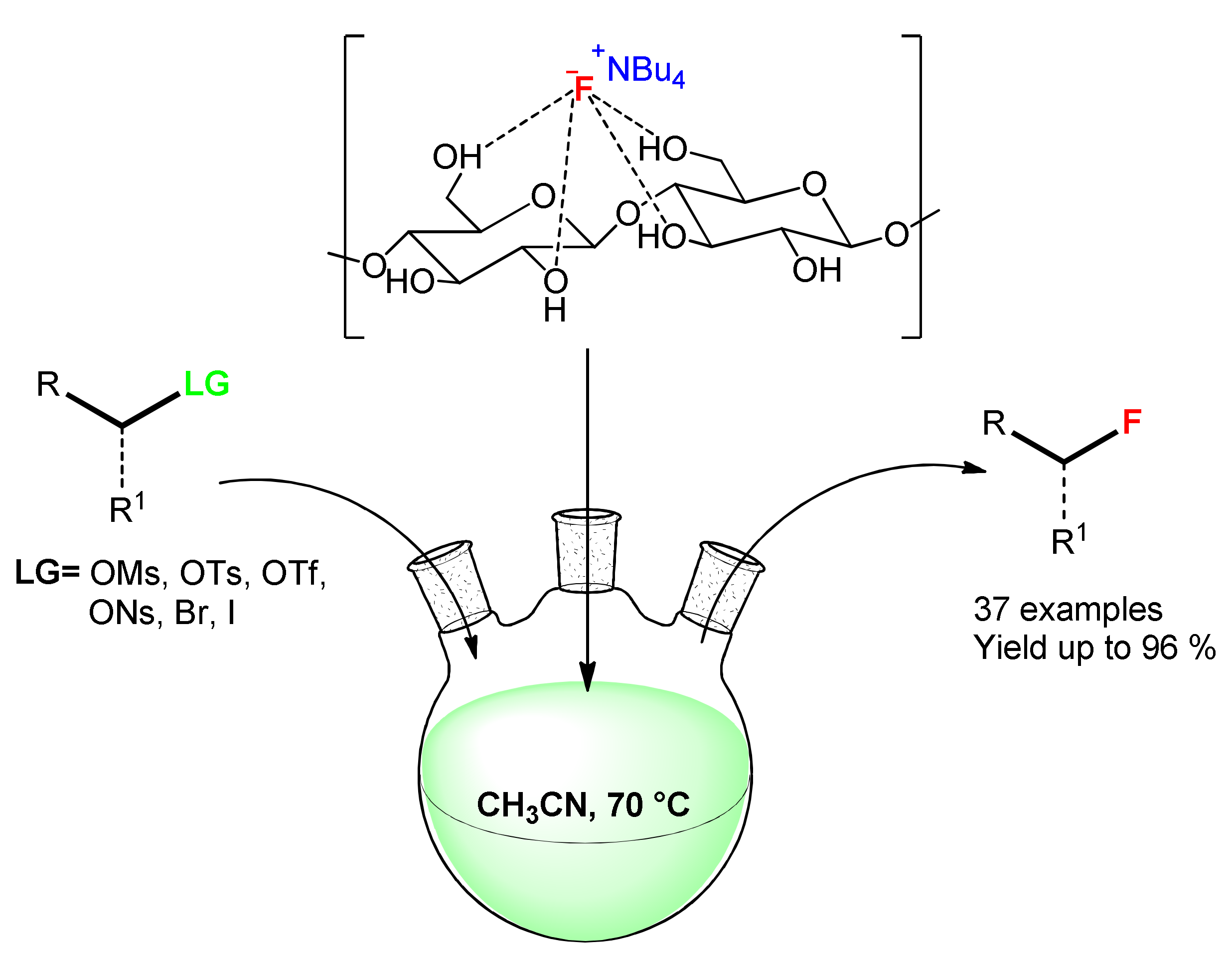



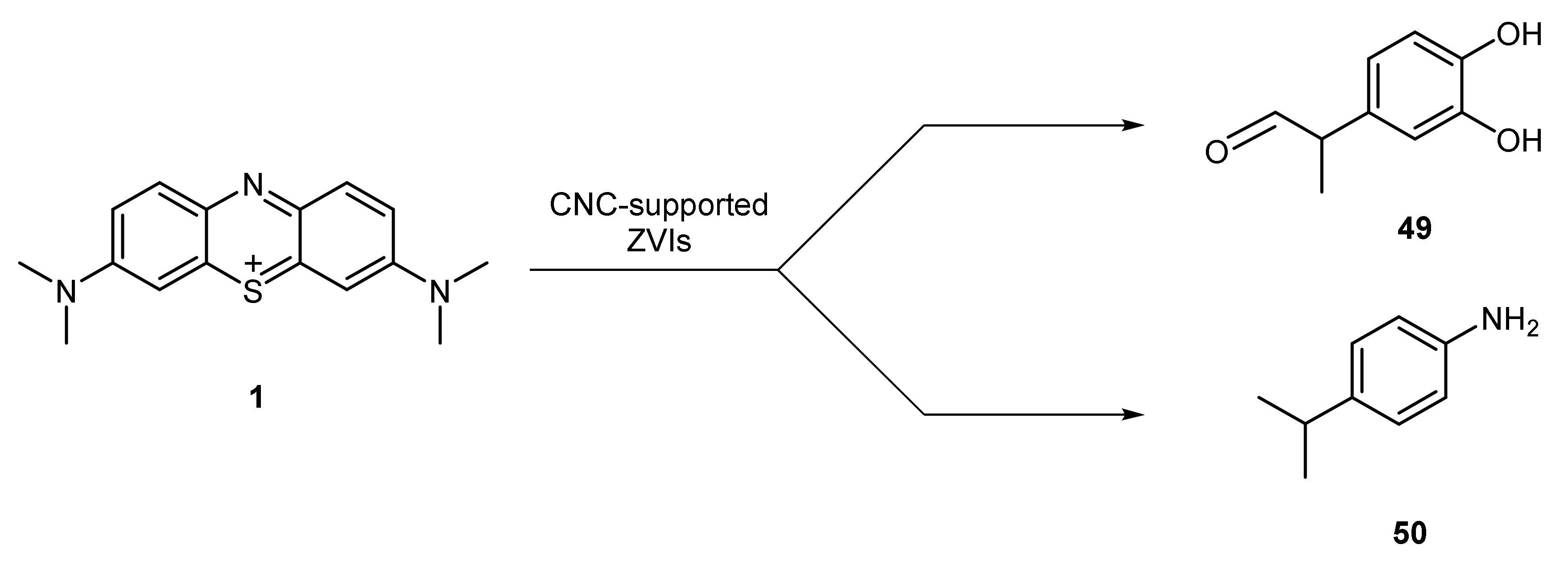



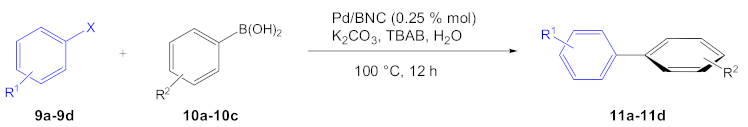
| Entry | Aryl Halide | Aryl Boronic Acid | Product | Yield (%) | TON a | TOF b |
|---|---|---|---|---|---|---|
| 1 |  9a |  10a |  11a | 14 | 56 | 4.5 |
| 2 | 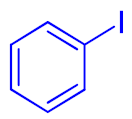 9b | 78 | 312 | 26 | ||
| 3 | 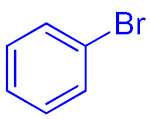 9c | 87 | 352 | 29 | ||
| 4 |  9c | 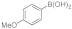 10b |  11b | 77 | 308 | 26 |
| 5 |  9d | 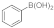 10a | 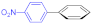 11c | 86 | 344 | 28.6 |
| 6 | 23 c | 92 | 7.6 | |||
| 7 | 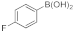 10c |  11d | 91 | 372 | 31 |
| Entry | Aldehyde | Amine | Product | s-NFC | s-BNC | ||
|---|---|---|---|---|---|---|---|
| Time (Min) | Yield (%) | Time (Min) | Yield (%) | ||||
| 1 | 14a | 15a | 18a | 45 | 92,90,90 a | 40 | 94 |
| 2 | 14b | 15b | 18b | 90 | 90 | 70 | 91 |
| 3 | 14c | 15b | 18c | 60 | 85 | 50 | 83 |
| 4 | 14d | 15c | 18d | 105 | 95 | 90 | 94 |
| 5 | 14e | 15d | 18e | 90 | 93 | 80 | 90 |
| 6 | 14f | 15b | 18f | 165 | 93 | 150 | 91 |
| 7 | 14f | 15e | 18g | 105 | 95 | 95 | 90 |
| 8 | 14a | 15f | 18h | 75 | 90 | 60 | 88 |
| 9 | 14g | 15g | 18i | 75 | 90 | 65 | 88 |
| 10 b | 14h | 15b | 18j | 90 | 55 | 80 | 54 |
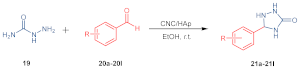
| Entry a | R | Product | Yield (%) b |
|---|---|---|---|
| 1 | 2-OMe | 21a | 96 |
| 2 | 4-OMe | 21b | 96 |
| 3 | 4-Et | 21c | 95 |
| 4 | 2-F | 21d | 90 |
| 5 | 2-Cl | 21e | 94 |
| 6 | 4-Cl | 21f | 91 |
| 7 | 4-Br | 21g | 90 |
| 8 | 2-NO2 | 21h | 92 |
| 9 | 3-OH | 21i | 93 |
| 10 | 2,3-(OMe)2 | 21j | 94 |
| 11 | 2,5-(OMe)2 | 21k | 94 |
| 12 | 3,4-(OMe)2 | 21l | 91 |

| Entry | Phenols 29a–29m | Reaction Time (h) | Products 31a–31m | Yield (%) a |
|---|---|---|---|---|
| 1 | 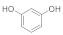 | 2 | 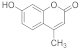 | 94 |
| 2 | 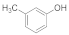 | 3 | 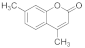 | 96 |
| 3 |  | 2 | 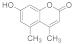 | 97 |
| 4 |  | 1.5 | 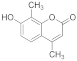 | 95 |
| 5 |  | 0.5 |  | 92 |
| 6 |  | 0.5 | 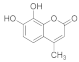 | 95 |
| 7 | 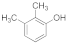 | 2 | 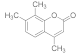 | 90 |
| 8 | 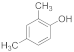 | 3 | 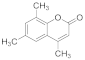 | 89 |
| 9 | 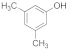 | 2 |  | 87 |
| 10 |  | 7 | 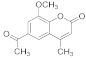 | 45 |
| 11 |  | 3 | 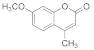 | 86 |
| 12 | 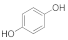 | 6 | 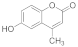 | 84 |
| 13 | 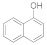 | 6 |  | 85 |

| Entry a | Amine 51a–51f | Vinylic Compound 52–53 | Product 54a–54f, 55a–55f | Timet (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 |  |  | 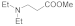 | 0.75 | 93 |
| 2 | 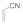 | 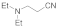 | 1 | 90 | |
| 3 | 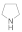 |  |  | 1.5 | 92 |
| 4 | 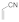 | 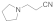 | 1.5 | 88 | |
| 5 |  |  | 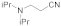 | 3.5 | 82 |
| 6 | 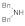 | 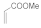 |  | 2 | 93 |
| 7 | 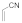 |  | 1.5 | 88 | |
| 8 |  | 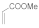 | 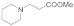 | 0.75 | 95 |
| 9 | 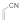 |  | 0.75 | 95 | |
| 10 | 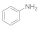 | 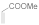 | No product | 24 | - |
| 11 |  | No product | 24 | - |
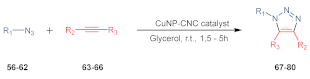
| Entry a | R1 | R2 | R3 | Product | Time, t (h) | Yield (%) b |
|---|---|---|---|---|---|---|
| 1 | Bn | Ph | H | 67 | 1.5 | 99 |
| 2 | Ph | Ph | H | 68 | 1.5 | 98 |
| 3 | 4-Br-Ph | Ph | H | 69 | 4 | 70 |
| 4 | 4-Cl-Ph | Ph | H | 70 | 4 | 69 |
| 5 | 4-MeO-Ph | Ph | H | 71 | 2 | 98 |
| 6 | 4-CN-Ph | Ph | H | 72 | 4 | 68 |
| 7 | C8H17 | Ph | H | 73 | 2 | 97 |
| 8 | 4-Br-Ph | C4H9 | H | 74 | 4.5 | 82 |
| 9 | Bn | C4H9 | H | 75 | 3.5 | 83 |
| 10 | Ph | C4H9 | H | 76 | 1.5 | 99 |
| 11 | Bn | CH2OH | H | 77 | 2.5 | 71 |
| 12 | 4-Br-Ph | CH2OH | H | 78 | 3.5 | 72 |
| 13 | Ph | CH2OH | H | 79 | 3 | 93 |
| 14 | Ph | COOMe | COOMe | 80 | 3.5 | 89 |

| Entry | Substrates 81a–81f | Products 82a–82f | Time (h) | Yield (%) a |
|---|---|---|---|---|
| 1 | 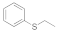 |  | 2 | 99 |
| 2 | 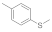 | 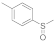 | 5 | 95 |
| 3 | 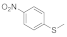 |  | 12 | 73 |
| 4 | 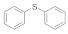 | 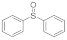 | 12 | 65 |
| 5 |  | 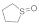 | 2 | 96 |
| 6 | 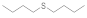 |  | 2.5 | 95 |

| Entry | Substrates 83a–83h | Products 84a–84h | Yield (%) a |
|---|---|---|---|
| 1 | 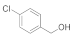 |  | 99 |
| 2 |  | 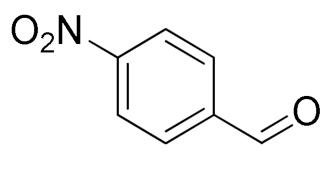 | 82 |
| 3 | 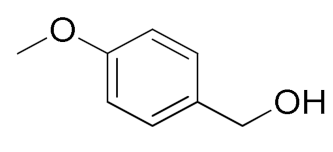 | 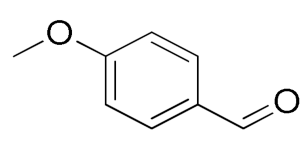 | 95 |
| 4 | 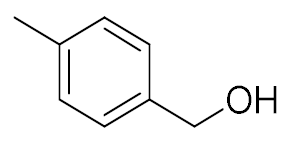 | 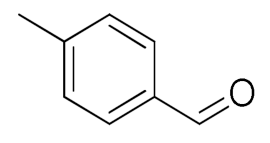 | 95 |
| 5 |  |  | 98 |
| 6 |  |  | 80 |
| 7 | 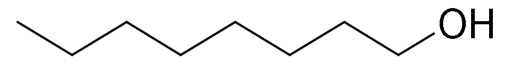 |  | 45 |
| 8 |  | 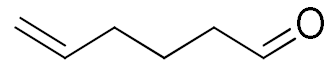 | 45 |
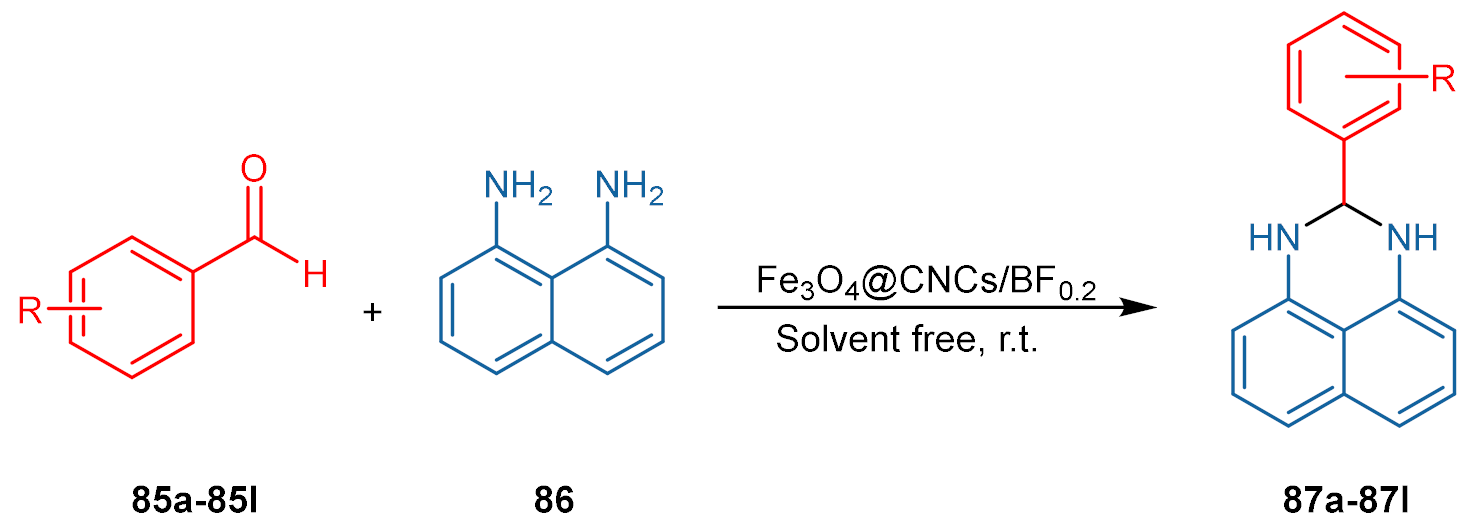
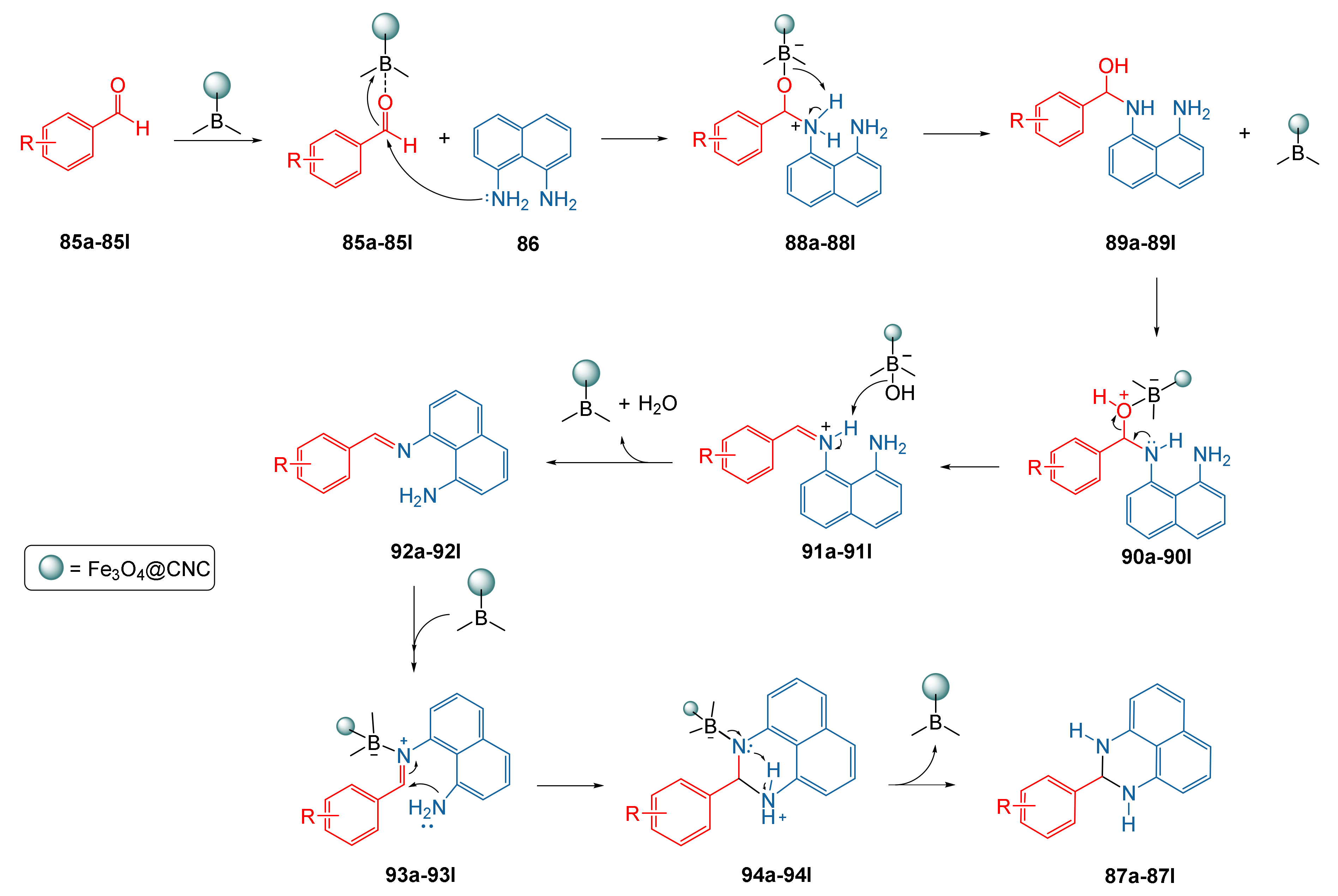
| Entry a | R | Time (min) | Product 87a–87l | Yield (%) b |
|---|---|---|---|---|
| 1 | H | 5 | a | 98 |
| 2 | 4-OMe | 15 | b | 91 |
| 3 | 4-NMe2 | 15 | c | 86 |
| 4 | 2,4-(OMe)2 | 15 | d | 75 |
| 5 | 3,4-(OMe)2 | 15 | e | 92 |
| 6 | 4-Cl | 10 | f | 96 |
| 7 | 4-COOH | 10 | g | 99 |
| 8 | 2-NO2 | 12 | h | 94 |
| 9 | 3-NO2 | 10 | i | 96 |
| 10 | 4-NO2 | 10 | j | 99 |
| 11 | 2,3-(OMe)2 | 20 | k | 85 |
| 12 | 2,3-(Cl)2 | 15 | l | 88 |
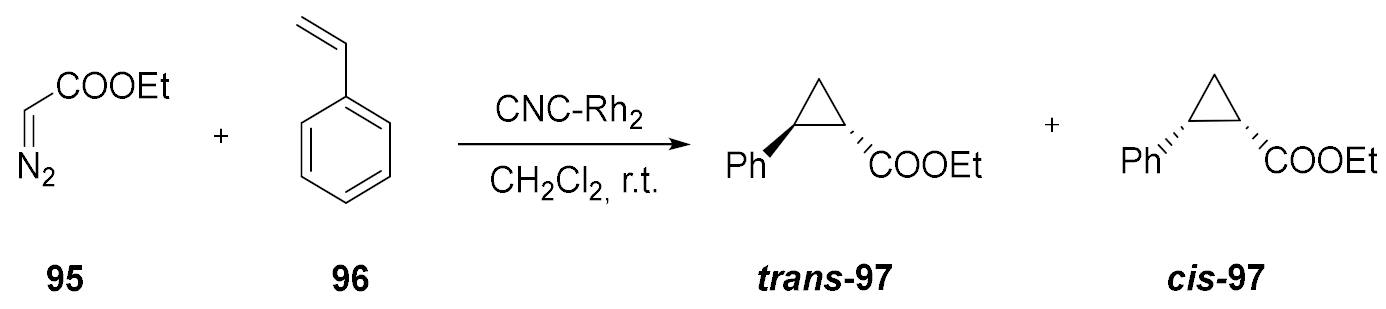
| Entry a | Reaction Time | Cis-97 Yield (%) | Trans-97 Yield (%) | Total Yield (%) |
|---|---|---|---|---|
| 1 | 15 | 20 | 22 | 42 |
| 2 | 30 | 21 | 29 | 50 |
| 3 | 45 | 28 | 34 | 62 |
| 4 | 60 | 29 | 35 | 64 |
| 5 | 120 | 33 | 39 | 72 |
| 6 | 180 | 34 | 40 | 74 |
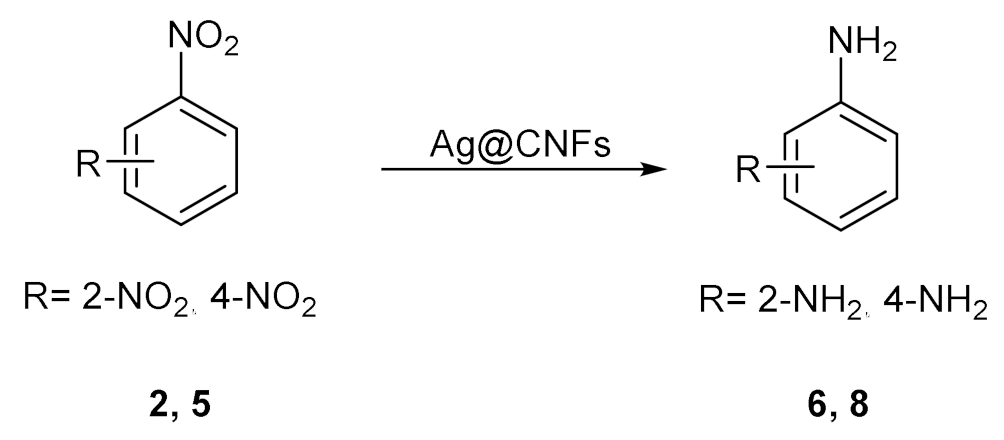
| Nitrophenol (NP) | Catalyst | Rate Constant | Reference |
|---|---|---|---|
| 2 | Ag/CNFs hydrogel | 46.6 × 10−3 s−1 | [105] |
| 2 | Ag/CNFs | 9.05 × 10−2 s−1 | [106] |
| 5 | 11.34 × 10−2 s−1 | ||
| 2 | Ag/Au/CNFs | 22.83 × 10−2 s−1 | [107] |
| 5 | 15.59 × 10−3 s−1 |
| Substrate | Catalyst | Reference | Substrate | Catalyst | Reference |
|---|---|---|---|---|---|
| MB 1 | Ag@CNFs TiO2-NH2@CNFs/CNT Fe3O4/TiO2@CNF/Chitosan/CNT | [120] [121] | 2 25 | NiFe2O4@CNFs | [124] |
| Indigo carmine | TiO2-NH2@CNFs/CNT | [120] | Cr(VI) | Fe3O4/TiO2@CNF/Chitosan/CNT BiOBr@CNFs | [121] [122] |
| TC | Ag/CN@CNFs | [123] | As(V) | Fe3O4/TiO2@CNF/Chitosan/CNT | [121] |
| Congo red | Fe3O4/TiO2@CNF/Chitosan/CNT | [121] | Ciprofloxacin Ofloxacin | CoS2-CuS@CNFs | [125] |
| RB5 | NiFe2O4@CNFs | [124] | PMS | CNFs-CoFe2O4/PVDF | [126] |
| RhB | BiOBr@CNFs Ag/CN@CNFs | [122] [123] | |||
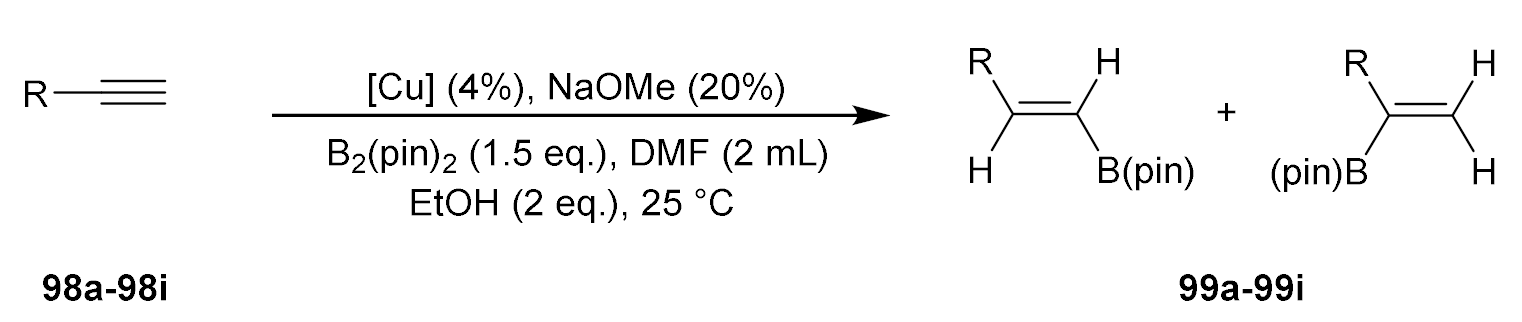
| Entry | Reactant | Product | Conv. (%) | E/Z |
|---|---|---|---|---|
| 1 | 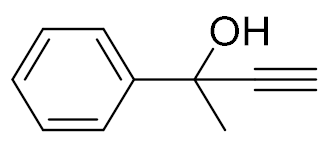 | 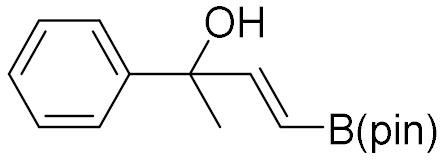 | 92 | 99:1 |
| 2 |  |  | 97 | 99:1 |
| 3 | 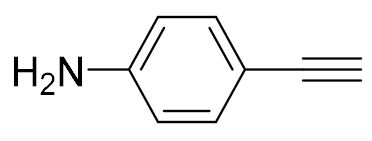 |  | 92 | 97:3 |
| 4 | 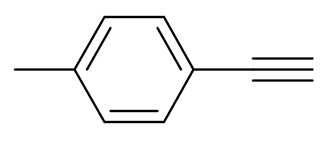 |  | 97 | 99:1 |
| 5 |  |  | 97 | 98:2 |
| 6 | 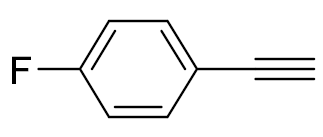 | 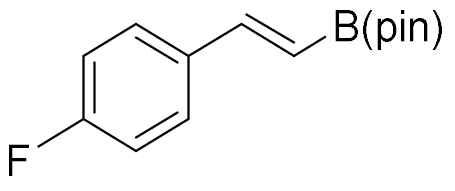 | 99 | >99:1 |
| 7 | 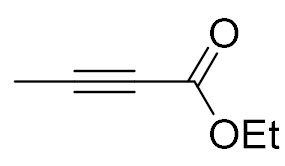 |  | 99 | 99:1 |
| 8 | 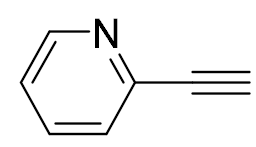 | 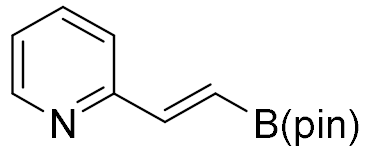 | 92 | 99:1 |
| 9 |  |  | 86 | 99:1 |
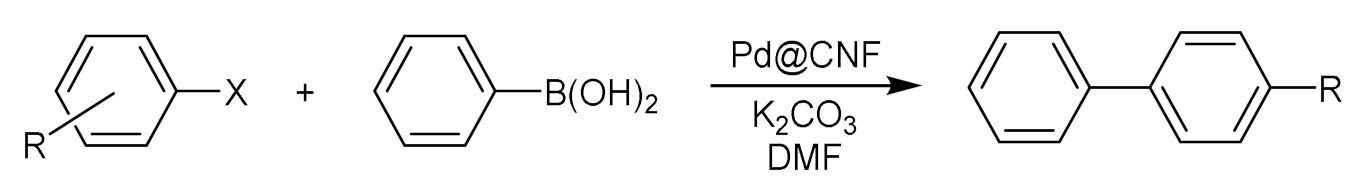
| Entry | ArX | Conv. (%) | Entry | ArX | Conv. (%) |
|---|---|---|---|---|---|
| 1 | 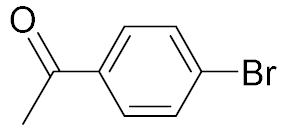 | 98 | 2 | 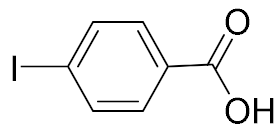 | 98 |
| 3 | 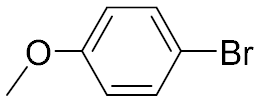 | 93 | 4 | 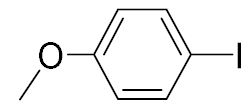 | 99 |
| 5 | 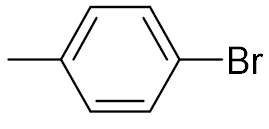 | 87 | 6 |  | 68 |
| 7 |  | 96 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiuolo, L.; Algieri, V.; Olivito, F.; Tallarida, M.A.; Costanzo, P.; Jiritano, A.; De Nino, A. Chronicle of Nanocelluloses (NCs) for Catalytic Applications: Key Advances. Catalysts 2021, 11, 96. https://doi.org/10.3390/catal11010096
Maiuolo L, Algieri V, Olivito F, Tallarida MA, Costanzo P, Jiritano A, De Nino A. Chronicle of Nanocelluloses (NCs) for Catalytic Applications: Key Advances. Catalysts. 2021; 11(1):96. https://doi.org/10.3390/catal11010096
Chicago/Turabian StyleMaiuolo, Loredana, Vincenzo Algieri, Fabrizio Olivito, Matteo Antonio Tallarida, Paola Costanzo, Antonio Jiritano, and Antonio De Nino. 2021. "Chronicle of Nanocelluloses (NCs) for Catalytic Applications: Key Advances" Catalysts 11, no. 1: 96. https://doi.org/10.3390/catal11010096
APA StyleMaiuolo, L., Algieri, V., Olivito, F., Tallarida, M. A., Costanzo, P., Jiritano, A., & De Nino, A. (2021). Chronicle of Nanocelluloses (NCs) for Catalytic Applications: Key Advances. Catalysts, 11(1), 96. https://doi.org/10.3390/catal11010096











