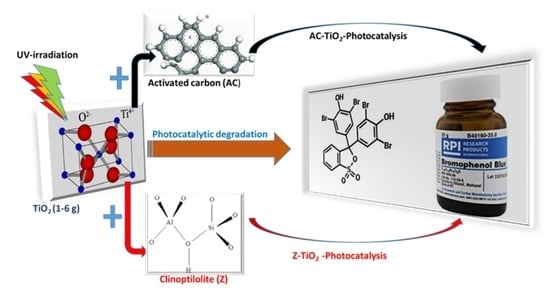
Adsorption and Photocatalytic Mineralization of Bromophenol Blue Dye with TiO2 Modified with Clinoptilolite/Activated Carbon
Abstract
1. Introduction
2. Results and Discussion
2.1. Physiochemical Properties of TiO2-Based Composite (A–TiO2/Z–TiO2)
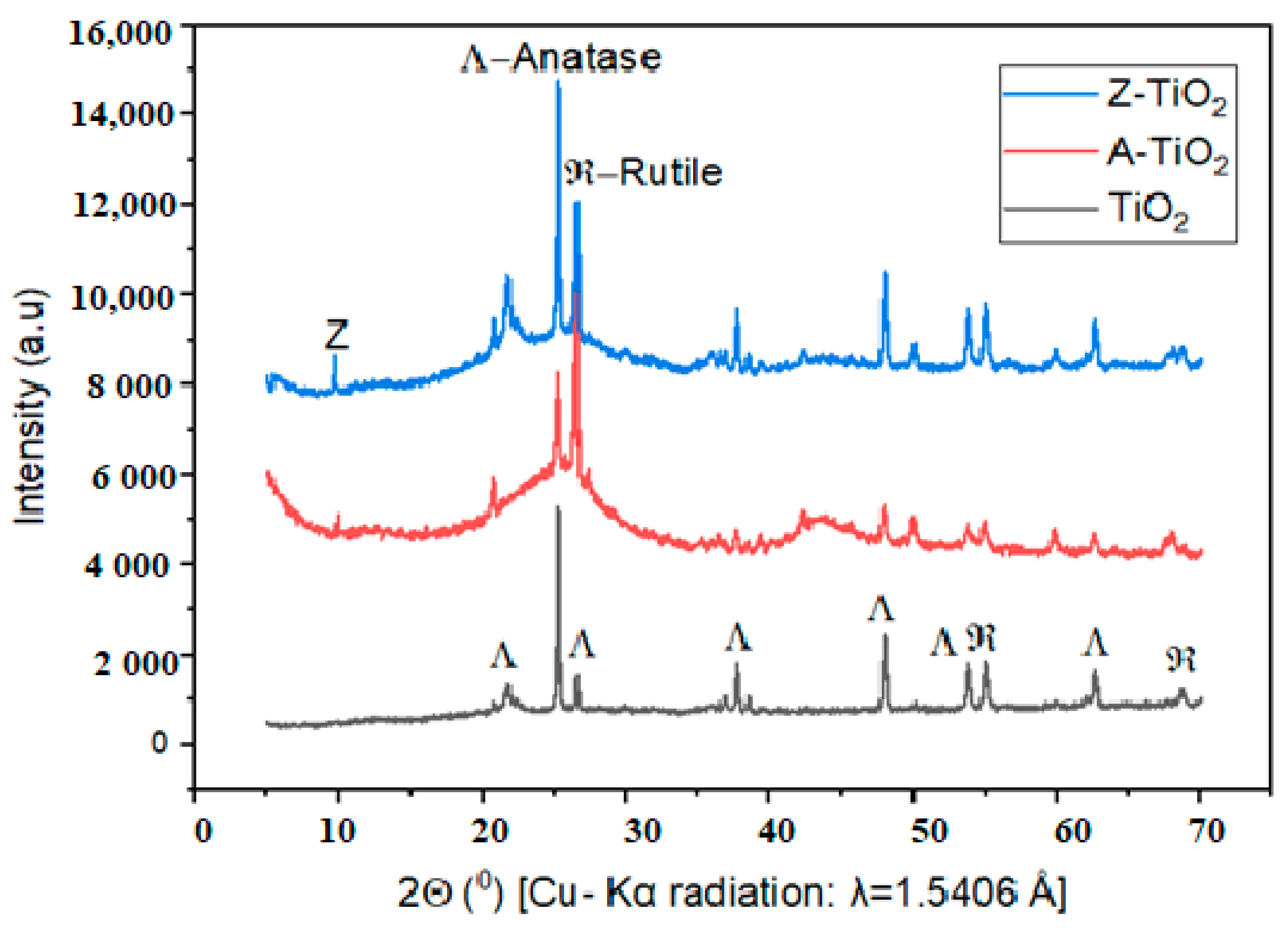
2.1.1. Crystalline Size
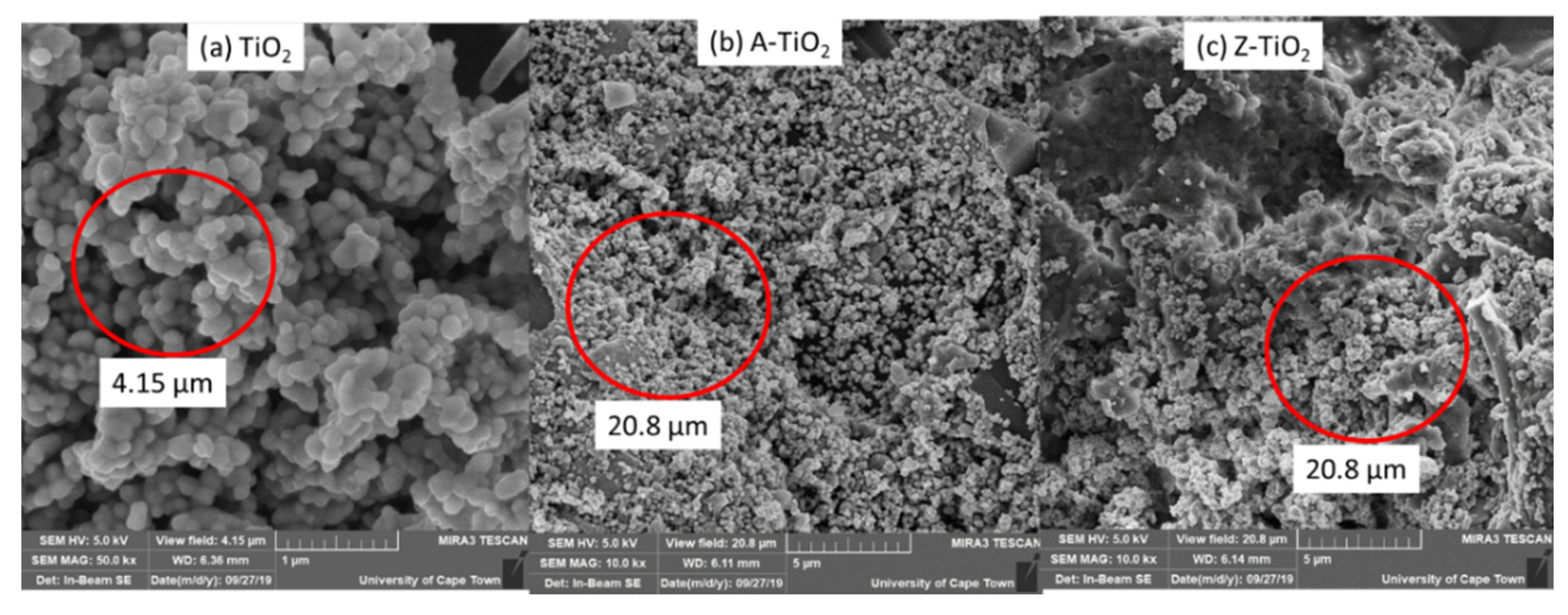
2.1.2. Morphological Analysis
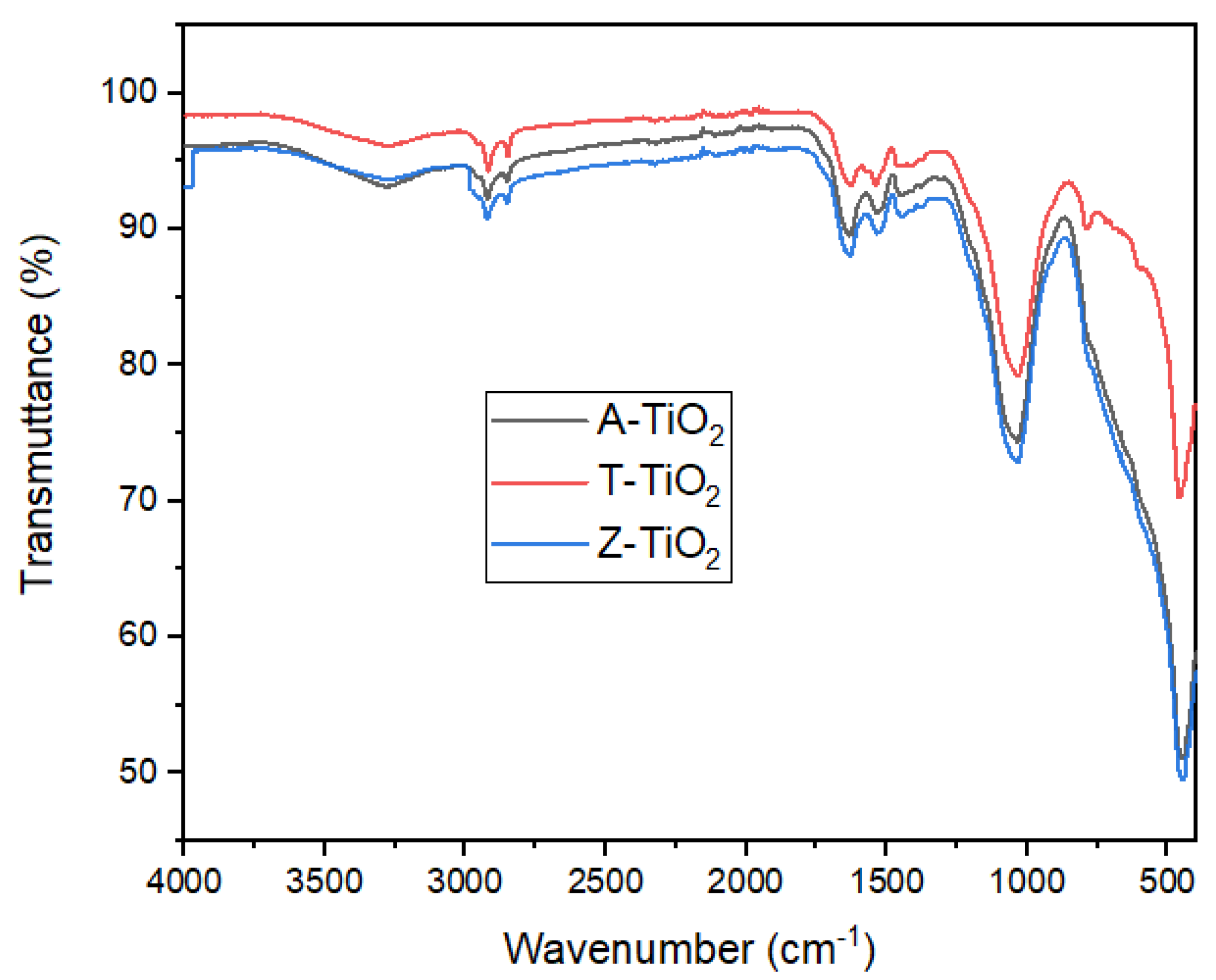
2.1.3. Molecular Structures and Functional Groups
2.2. Photocatalytic Degradation
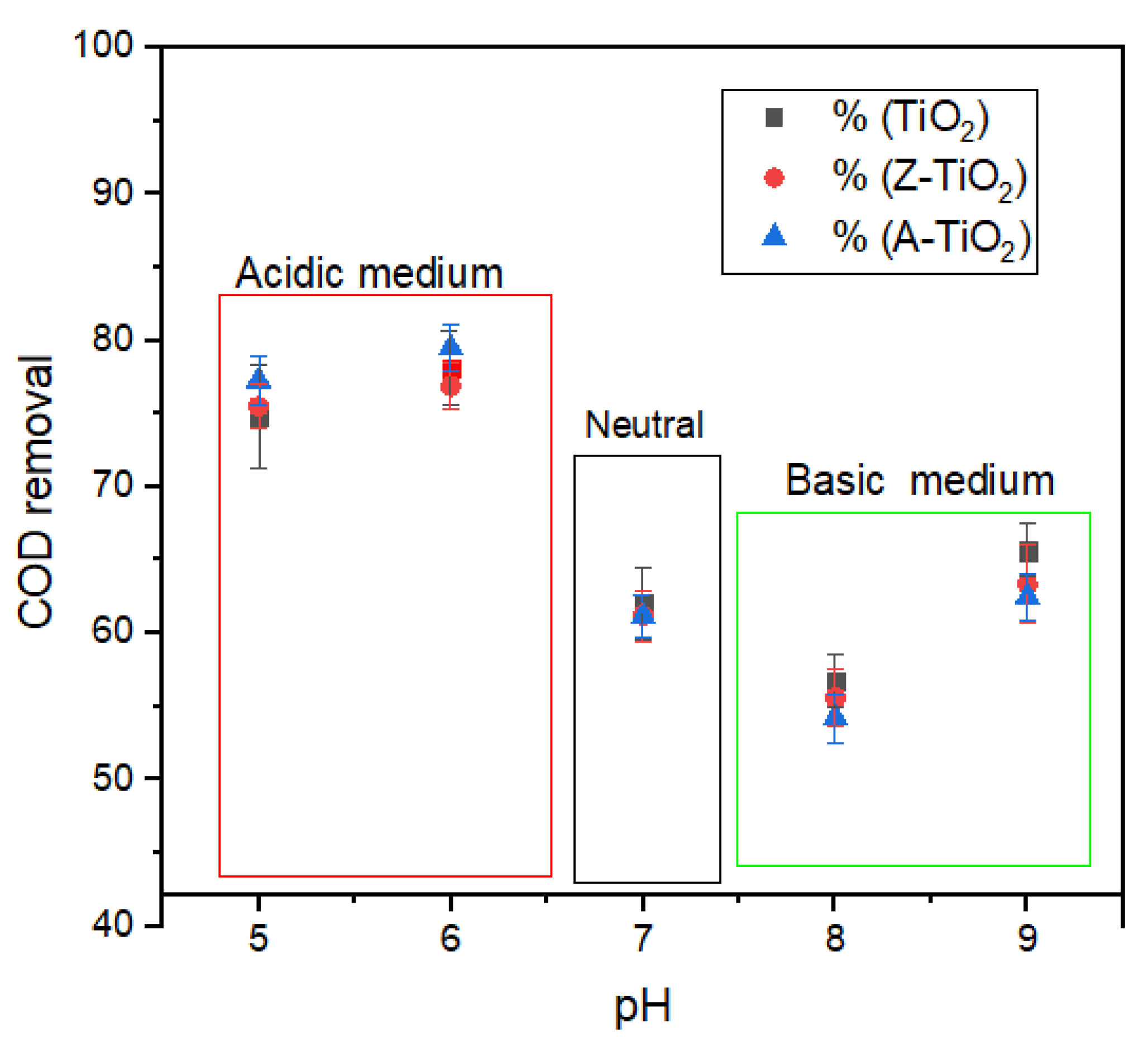
2.2.1. Effect of pH on Photocatalytic Degradation
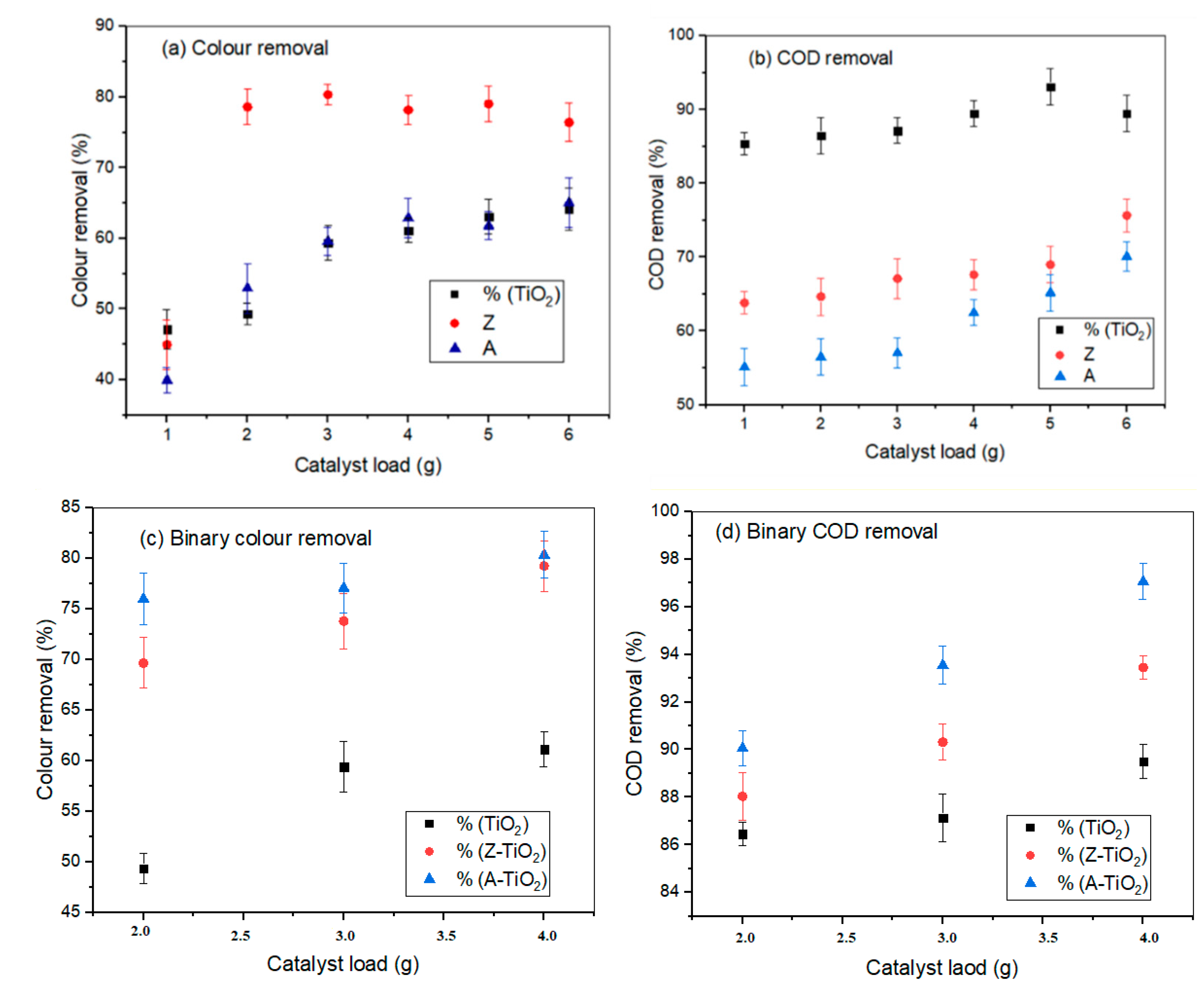
2.2.2. Effects of Photocatalyst Dosage
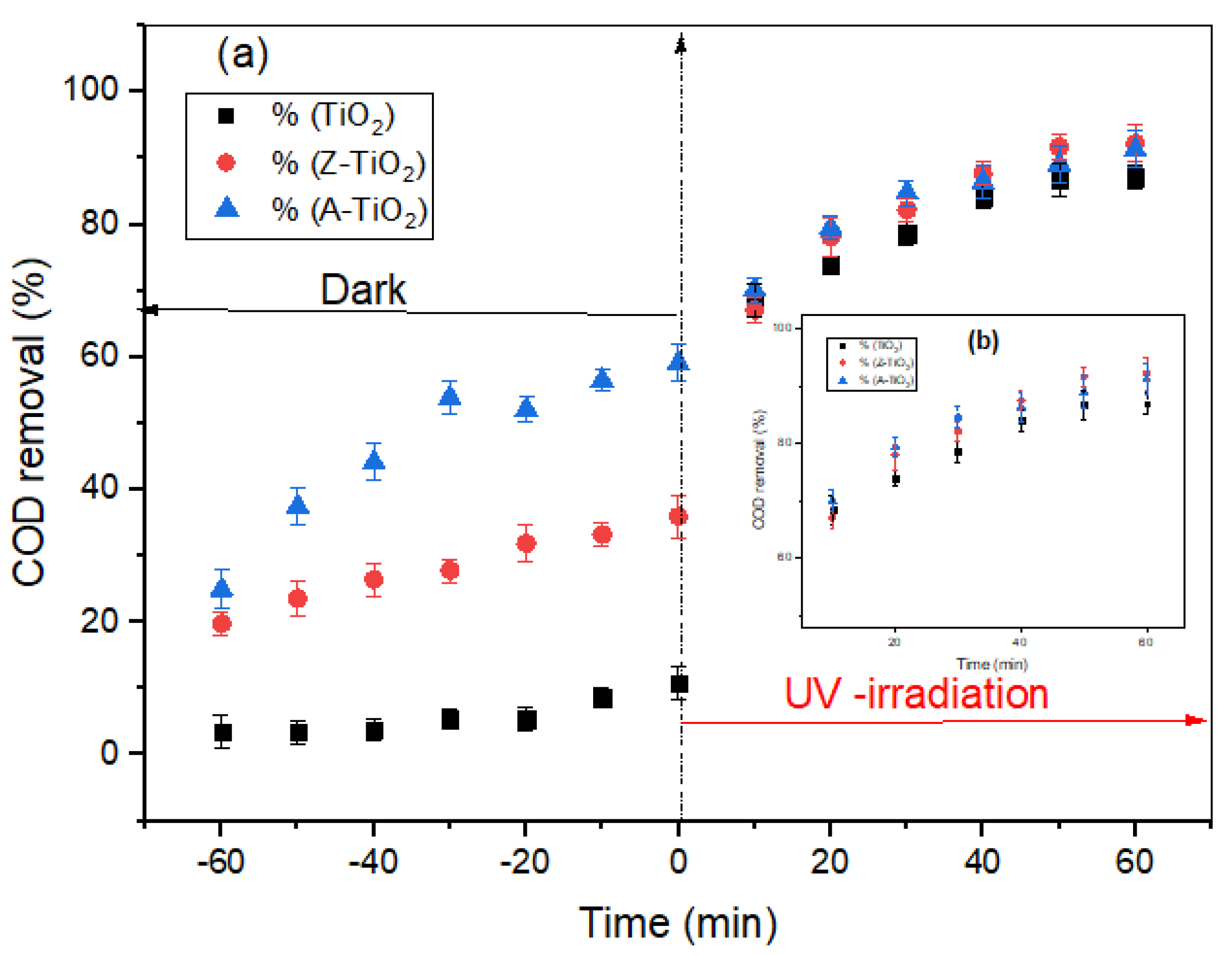
2.3. Adsorption and Photocatalysis
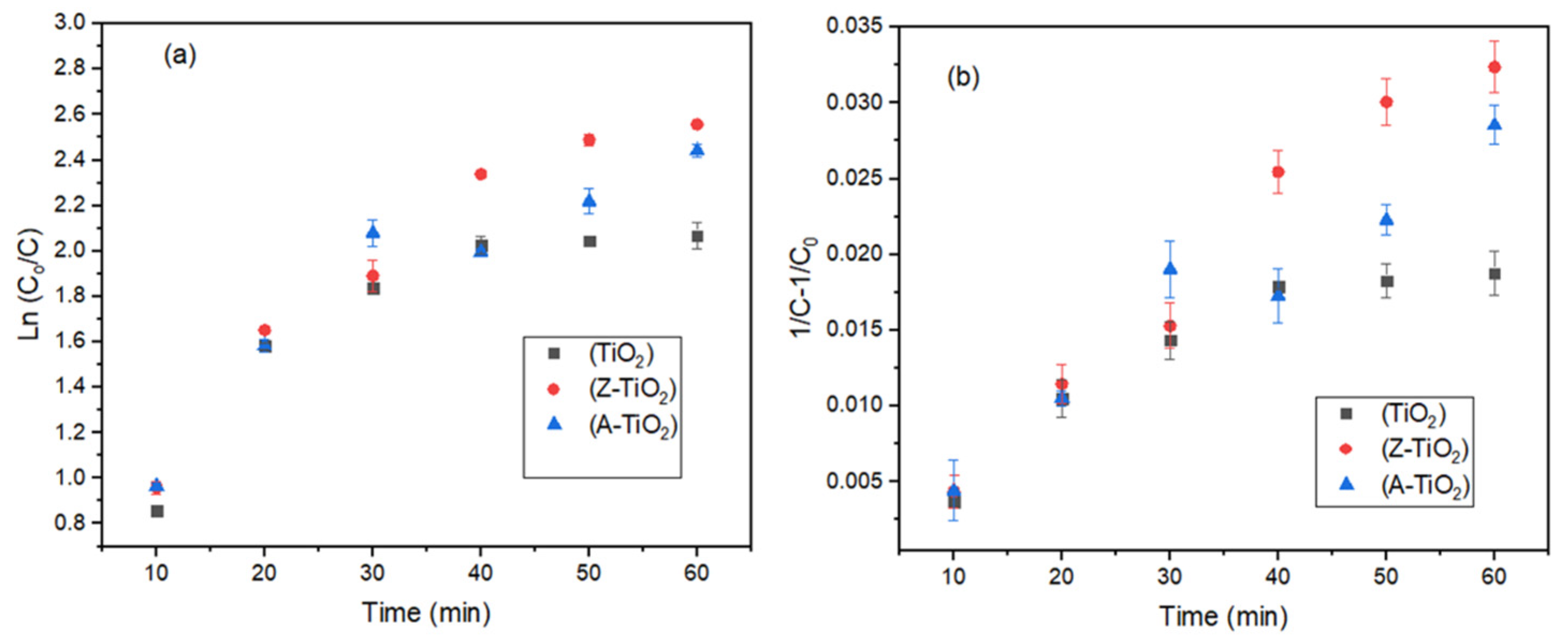
2.3.1. Kinetic Models
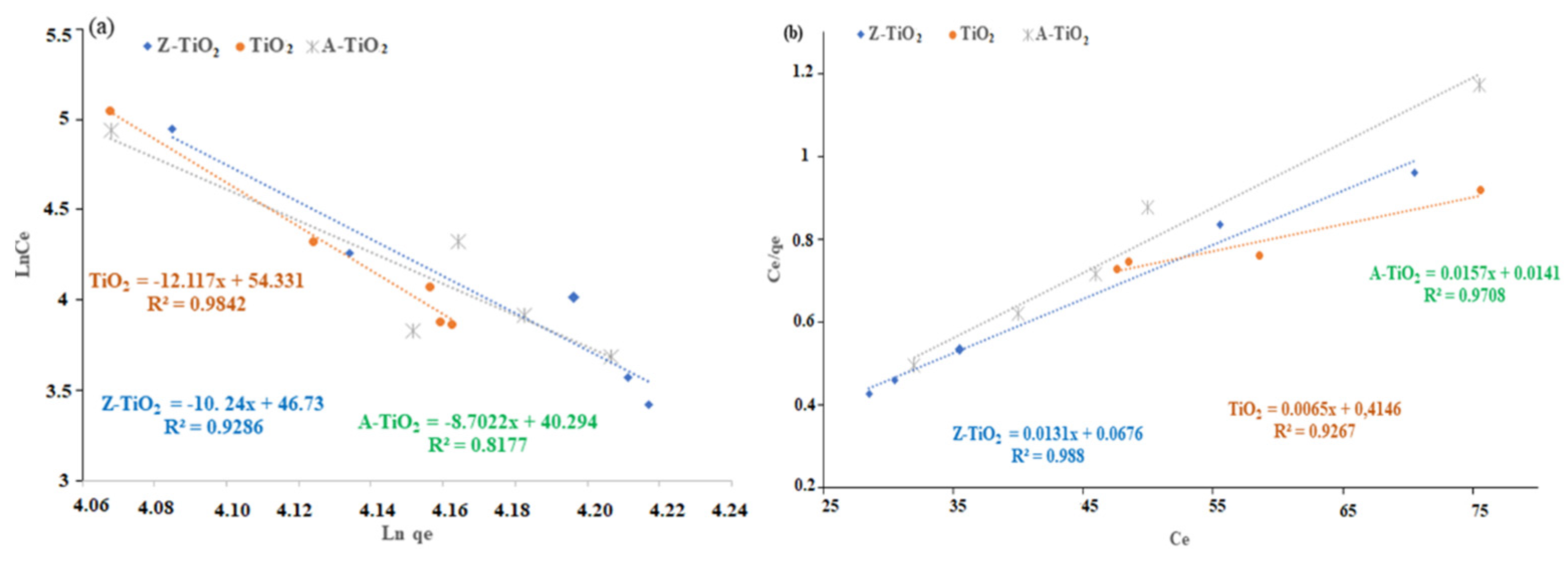
2.3.2. Kinetic Isotherms
3. Materials and Methods
3.1. Chemicals and Synthetic Effluent
Nanocomposite Synthesis (A–TiO2 and Z–TiO2)
3.2. Characterization of Composite (A–TiO2 and Z–TiO2)
3.2.1. X-Ray Diffraction (XRD) Analysis
3.2.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.2.3. Scanning Electronic Microscope (SEM) Analysis
3.3. Experimental Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gleeson, T.; Wada, Y.; Bierkens, M.F.P.; Van Beek, L.P.H. Water balance of global aquifers revealed by groundwater footprint. Nature 2012, 488, 197–200. [Google Scholar] [CrossRef]
- Wada, Y.; Van Beek, L.P.H.; Van Kempen, C.M.; Reckman, J.W.T.M.; Vasak, S.; Bierkens, M.F.P. Global depletion of groundwater resources. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef]
- Richey, A.S.; Thomas, B.F.; Lo, M.H.; Famiglietti, J.S.; Swenson, S.; Rodell, M. Uncertainty in global groundwater storage estimates in a Total Groundwater Stress framework. Water Resour. Res. 2015, 51, 5198–5216. [Google Scholar] [CrossRef] [PubMed]
- Edokpayi, J.N.; Odiyo, J.O.; Durowoju, O.S. Impact of Wastewater on Surface Water Quality in Developing Countries: A Case Study of South Africa. In Water Quality; IntechOpen: London, UK, 2017. [Google Scholar]
- Barnard, J.L. Biological nutrient removal without the addition of chemicals. Water Res. 1975, 9, 485–490. [Google Scholar] [CrossRef]
- Adewumi, J.R.; Ilemobade, A.; Van Zyl, J. Treated wastewater reuse in South Africa: Overview, potential and challenges. Resour. Conserv. Recycl. 2010, 55, 221–231. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Opoku, M.A.; Armah, E.K.; Rathilal, S. Fate of COVID-19 Occurrences in Wastewater Systems: Emerging Detection and Treatment Technologies—A Review. Water 2020, 12, 2680. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, B.; Wang, Q. Biodegradation and Decolorization of Dye Wastewater: A Review. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Arora, S. Textile Dyes: It’s Impact on Environment and its Treatment. J. Bioremediat. Biodegrad. 2014, 5, 1. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, M.; Huang, K.; Liu, Z. Textile Dyeing Wastewater Treatment. In Advances in Treating Textile Effluent; IntechOpen: London, UK, 2011. [Google Scholar]
- Cheremisinoff, P.N. Handbook of Water and Wastewater Treatment Technology; Butterworth-Heinemann: Oxford, UK, 2019. [Google Scholar]
- Salgot, M.; Folch, M. Wastewater treatment and water reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 64–74. [Google Scholar] [CrossRef]
- Mohanty, K.; Purkait, M.K. Membrane Technologies and Applications; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Xu, P.; Zeng, G.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total. Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S. Application of Organic Coagulants in Water and Wastewater Treatment. Org. Polym. 2019. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S. Application of magnetized nanomaterial for textile effluent remediation using response surface methodology. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Ocon, J.D.; Chong, M.N. Electrochemical oxidation remediation of real wastewater effluents—A review. Process. Saf. Environ. Prot. 2018, 113, 48–67. [Google Scholar] [CrossRef]
- Jin, J.; El-Din, M.G.; Bolton, J.R. Assessment of the UV/Chlorine process as an advanced oxidation process. Water Res. 2011, 45, 1890–1896. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Sievers, M. Advanced Oxidation Processes. In Treatise on Water Science; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Durgalakshmi, D.; Rakkesh, R.A.; Rajendran, S.; Naushad, M. Principles and Mechanisms of Green Photocatalysis; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Sacco, O.; Vaiano, V.; Sannino, D. Main parameters influencing the design of photocatalytic reactors for wastewater treatment: A mini review. J. Chem. Technol. Biotechnol. 2020, 95, 2608–2618. [Google Scholar] [CrossRef]
- Zakaria, W.F.W.; Jalil, A.; Hassan, N.S.; Ibrahim, M.; Jalil, A.A. Visible-light driven photodegradation of phenol over niobium oxide-loaded fibrous silica titania composite catalyst. J. Chem. Technol. Biotechnol. 2020, 95, 2638–2647. [Google Scholar]
- Wafi, A.; Szabó-Bárdos, E.; Horváth, O.; Pósfai, M.; Makó, É.; Juzsakova, T.; Fónagy, O. The Photocatalytic and Antibacterial Performance of Nitrogen-Doped TiO2: Surface-Structure Dependence and Silver-Deposition Effect. Nanomaterials 2020, 10, 2261. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, E.K.; Rathilal, S.; Naidoo, D.B. Photocatalytic degradation of oily waste and phenol from a local South Africa oil refinery wastewater using response methodology. Sci. Rep. 2020, 10, 8850. [Google Scholar] [CrossRef]
- Khan, W.Z.; Najeeb, I.; Tuiyebayeva, M.; Makhtayeva, Z. Refinery wastewater degradation with titanium dioxide, zinc oxide, and hydrogen peroxide in a photocatalytic reactor. Process. Saf. Environ. Prot. 2015, 94, 479–486. [Google Scholar] [CrossRef]
- Naushad, M.; Rajendran, S.; Lichtfouse, E. Green Photocatalysts; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Raja, S.; Mattoso, L.H.C. Functionalized Polymer-BaseZd Composite Photocatalysts; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Huang, C.-W.; Wu, M.-C. Photocatalytic degradation of methylene blue by UV-assistant TiO2 and natural sericite composites. J. Chem. Technol. Biotechnol. 2020, 95, 2715–2722. [Google Scholar]
- Zanrosso, C.D.; Piazza, D.; Lansarin, M.A. Solution mixing preparation of PVDF/ZnO polymeric composite films engineered for heterogeneous photocatalysis. J. Appl. Polym. Sci. 2020, 137, 48417. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, F.; Zhang, J. Carbon-Deposited TiO2: Synthesis, Characterization, and Visible Photocatalytic Performance. J. Phys. Chem. C 2010, 114, 933–939. [Google Scholar] [CrossRef]
- Shen, M.; Wu, Z.; Huang, H.; Du, Y.; Zou, Z.; Yang, P. Carbon-doped anatase TiO2 obtained from TiC for photocatalysis under visible light irradiation. Mater. Lett. 2006, 60, 693–697. [Google Scholar] [CrossRef]
- Lee, Y.-F.; Chang, K.-H.; Hu, C.-C.; Lin, K.-M. Synthesis of activated carbon-surrounded and carbon-doped anatase TiO2 nanocomposites. J. Mater. Chem. 2010, 20, 5682–5688. [Google Scholar] [CrossRef]
- haq, I.U.; Ahmad, W.; Ahmad, I.; Yaseen, M. Photocatalytic oxidative degradation of hydrocarbon pollutants in refinery wastewater using TiO2 as catalyst. Water Environ. Res. 2020, 92, 2086–2094. [Google Scholar] [CrossRef]
- Derikvandi, H.; Nezamzadeh-Ejhieh, A. An effective wastewater treatment based on sunlight photodegradation by SnS2–ZnS/clinoptilolite composite. Solid State Sci. 2020, 101, 106127. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Wolf, D. Kinetics of Heterogeneous Catalytic Reactions; Springer Series in Chemical Physics; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Ross, J.R.H. The Kinetics and Mechanisms of Catalytic Reactions. In Heterogeneous Catalysis; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Vannice, M.A. Kinetics of Catalytic Reactions; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Ashfaq, A.; Khatoon, A. Waste Management of Textiles: A Solution to The Environmental Pollution. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 780–787. [Google Scholar]
- Tetteh, E.K.; Rathilal, S. Kinetics and Nanoparticle Catalytic Enhancement of Biogas Production from Wastewater Using a Magnetized Biochemical Methane Potential (MBMP) System. Catalysts 2020, 10, 1200. [Google Scholar] [CrossRef]
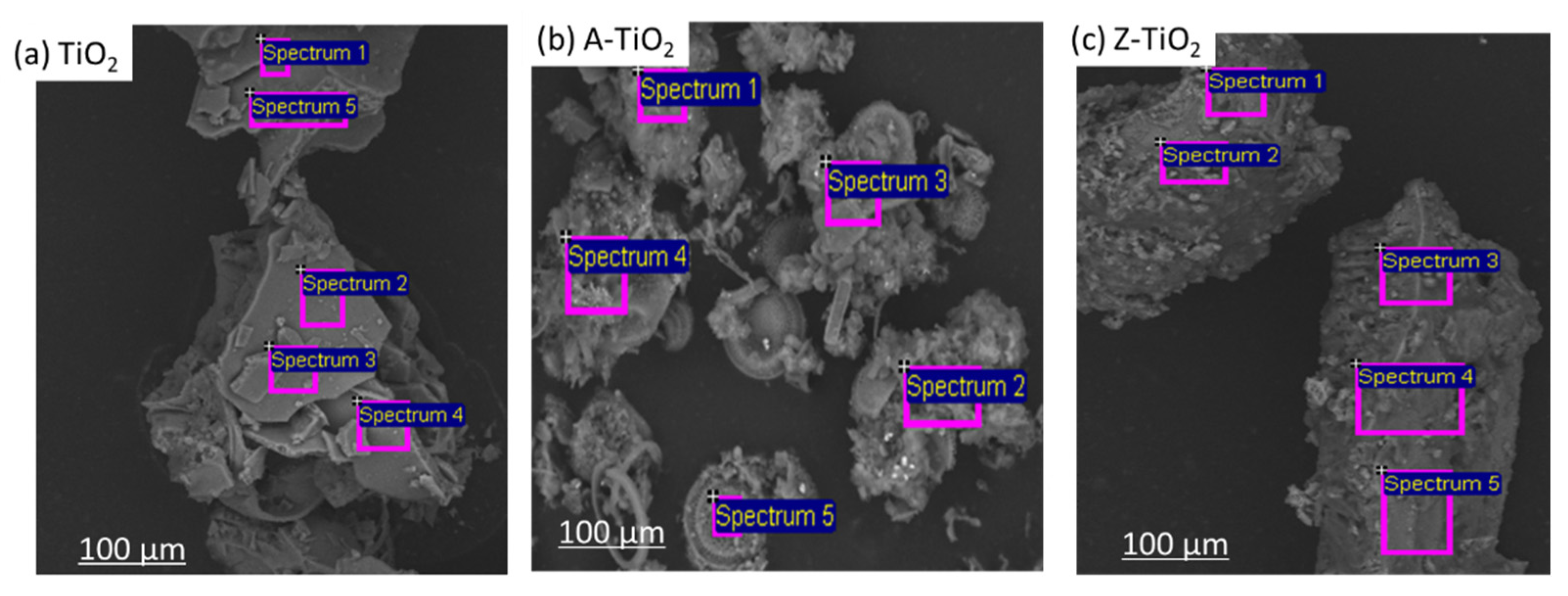
| Elemental Composition | TiO2 | A–TiO2 | Z–TiO2 | |||
|---|---|---|---|---|---|---|
| Weight (%) | Std. Dev. | Weight (%) | Std. Dev. | Weight (%) | Std. Dev. | |
| C | 13.08 | 3.69 | 17 | 1.1 | 19.72 | 6.74 |
| O | 45.64 | 2.41 | 31.4 | 11.41 | 35.64 | 6.59 |
| Na | 10.73 | 1.35 | 0.27 | 0.21 | 1.01 | 0.55 |
| Ti | 30.55 | 5.78 | 16.02 | 0.26 | 23.15 | 1.69 |
| Mg | 0.4 | 0.08 | 0.64 | 0.63 | ||
| Al | 2.66 | 0.6 | 1.18 | 1.11 | ||
| Si | 0.86 | 0.14 | 16.57 | 1.94 | ||
| K | 23.23 | 2.17 | 1.6 | 0.51 | ||
| Ca | 0.48 | 0.33 | 0.49 | 0.64 | ||
| P | 1.17 | 0.36 | ||||
| S | 0.28 | 0.07 | ||||
| Cl | 6.23 | 2.17 | ||||
| Total | 100 | 100 | 100 | |||
| Photocatalyst Type | TiO2 | Z–TiO2 | A–TiO2 |
|---|---|---|---|
| First-order Model | |||
| k1 (min−1) | 0.0218 | 0.0313 | 0.0269 |
| R2 | 0.872 | 0.955 | 0.927 |
| Adjusted R2 | 0.769 | 0.912 | 0.869 |
| Sum of squares | 0.832 | 1.714 | 1.212 |
| Second-order Model | |||
| k2 (min−1) | 0.000327 | 0.000563 | 0.000415 |
| R2 | 0.952 | 0.989 | 0.973 |
| Adjusted R2 | 0.916 | 0.978 | 0.945 |
| Sum of squares | 1.926 | 1.346 | 1.564 |
| Category | Investigating Factor | Purpose | Single Material (A, TiO2, and Z) | Binary Composite (A–TiO2 and Z–TiO2) |
| 1 | Single dosage (1–6 g) | Photocatalysis | All | N/A |
| 2 | Binary dosage (2–4 g) | Photocatalysis | TiO2 | All |
| 3 | Reaction time (±60 min) | Adsorption and photocatalysis | TiO2 | All |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kweinor Tetteh, E.; Rathilal, S. Adsorption and Photocatalytic Mineralization of Bromophenol Blue Dye with TiO2 Modified with Clinoptilolite/Activated Carbon. Catalysts 2021, 11, 7. https://doi.org/10.3390/catal11010007
Kweinor Tetteh E, Rathilal S. Adsorption and Photocatalytic Mineralization of Bromophenol Blue Dye with TiO2 Modified with Clinoptilolite/Activated Carbon. Catalysts. 2021; 11(1):7. https://doi.org/10.3390/catal11010007
Chicago/Turabian StyleKweinor Tetteh, Emmanuel, and Sudesh Rathilal. 2021. "Adsorption and Photocatalytic Mineralization of Bromophenol Blue Dye with TiO2 Modified with Clinoptilolite/Activated Carbon" Catalysts 11, no. 1: 7. https://doi.org/10.3390/catal11010007
APA StyleKweinor Tetteh, E., & Rathilal, S. (2021). Adsorption and Photocatalytic Mineralization of Bromophenol Blue Dye with TiO2 Modified with Clinoptilolite/Activated Carbon. Catalysts, 11(1), 7. https://doi.org/10.3390/catal11010007