Nanoscale Multidimensional Pd/TiO2/g-C3N4 Catalyst for Efficient Solar-Driven Photocatalytic Hydrogen Production
Abstract
1. Introduction
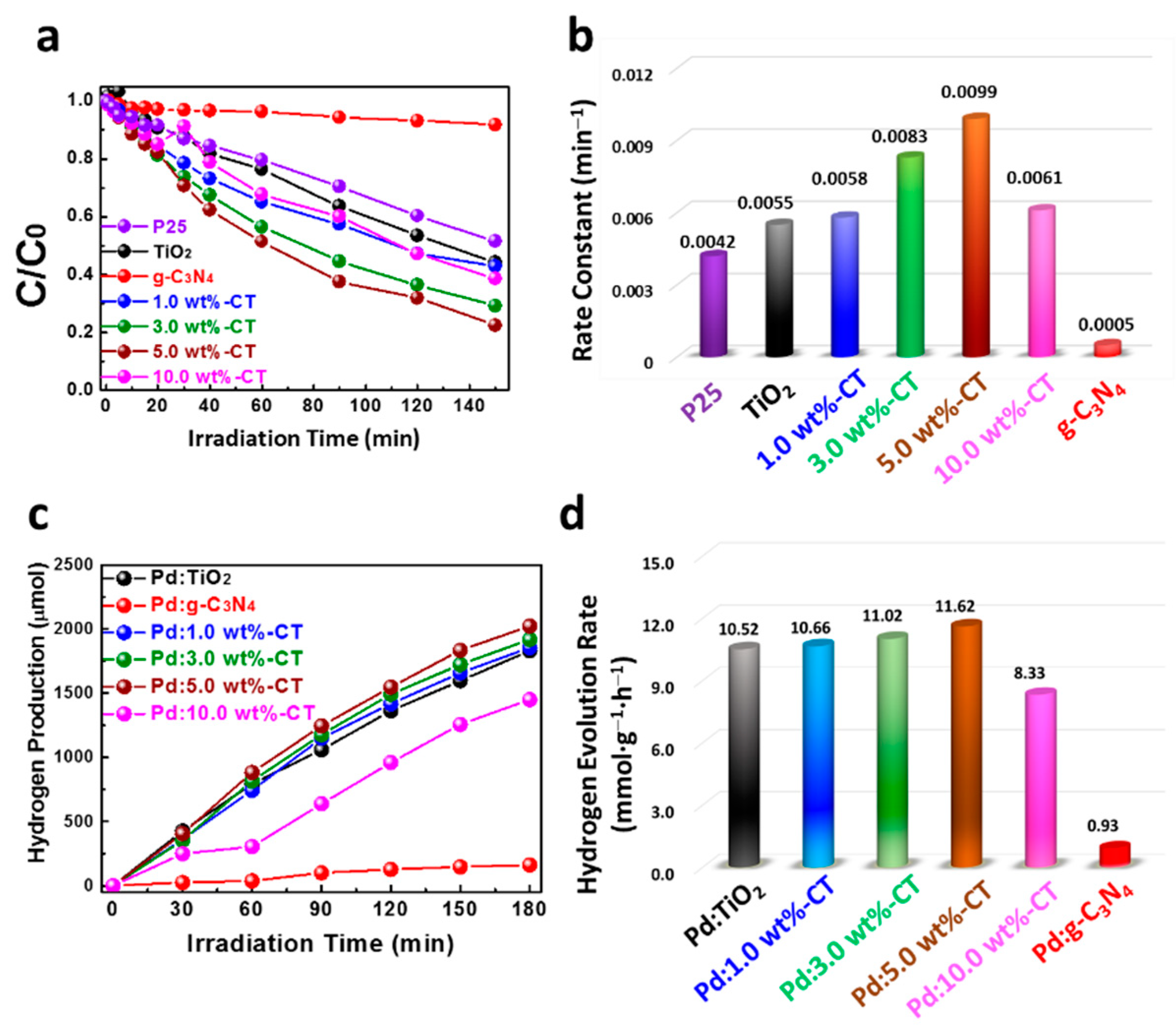
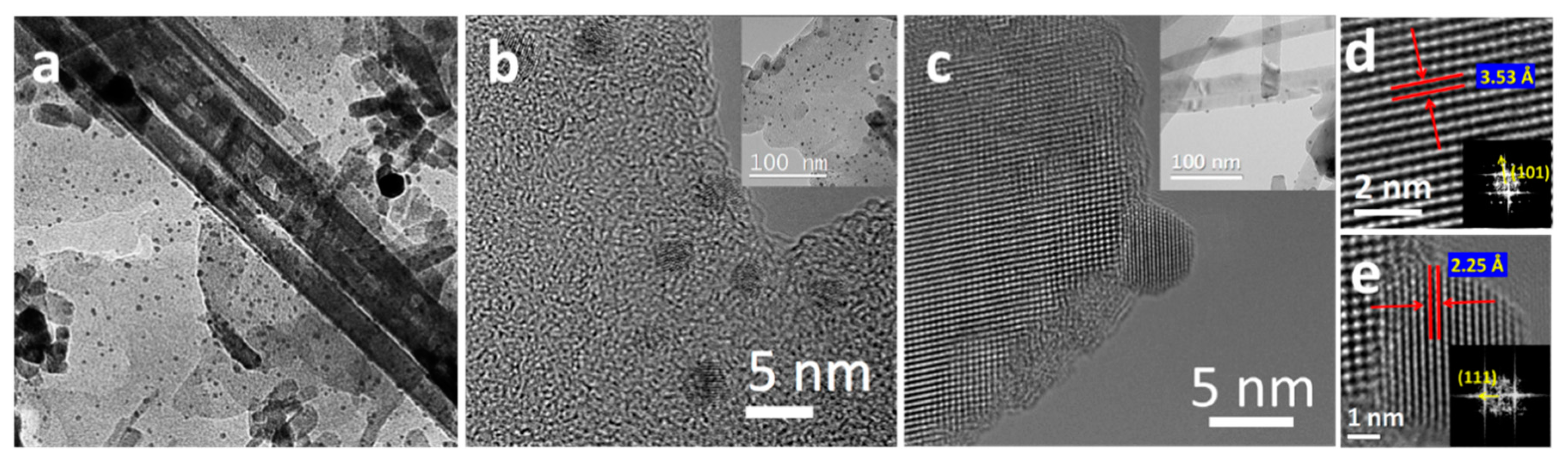
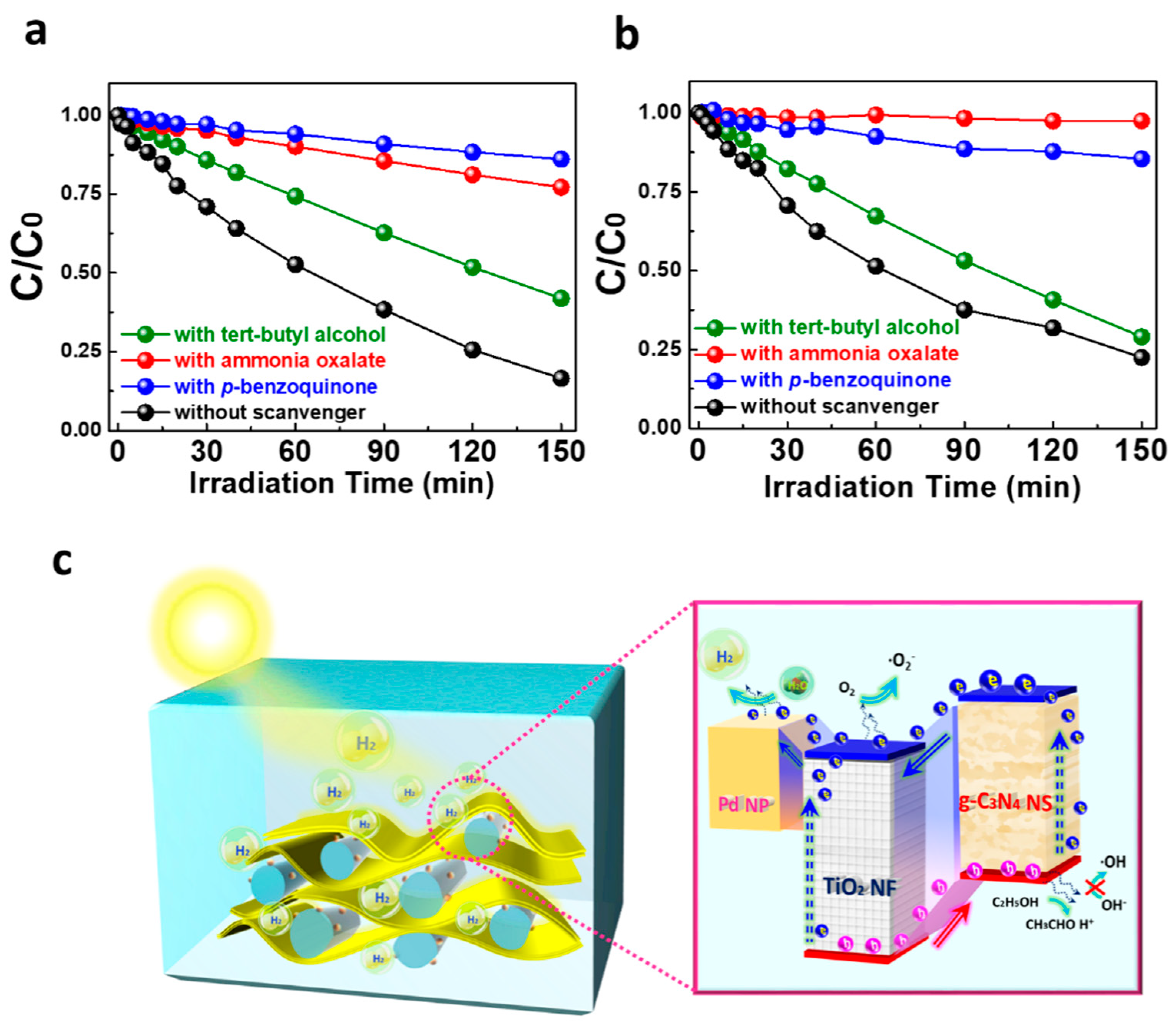
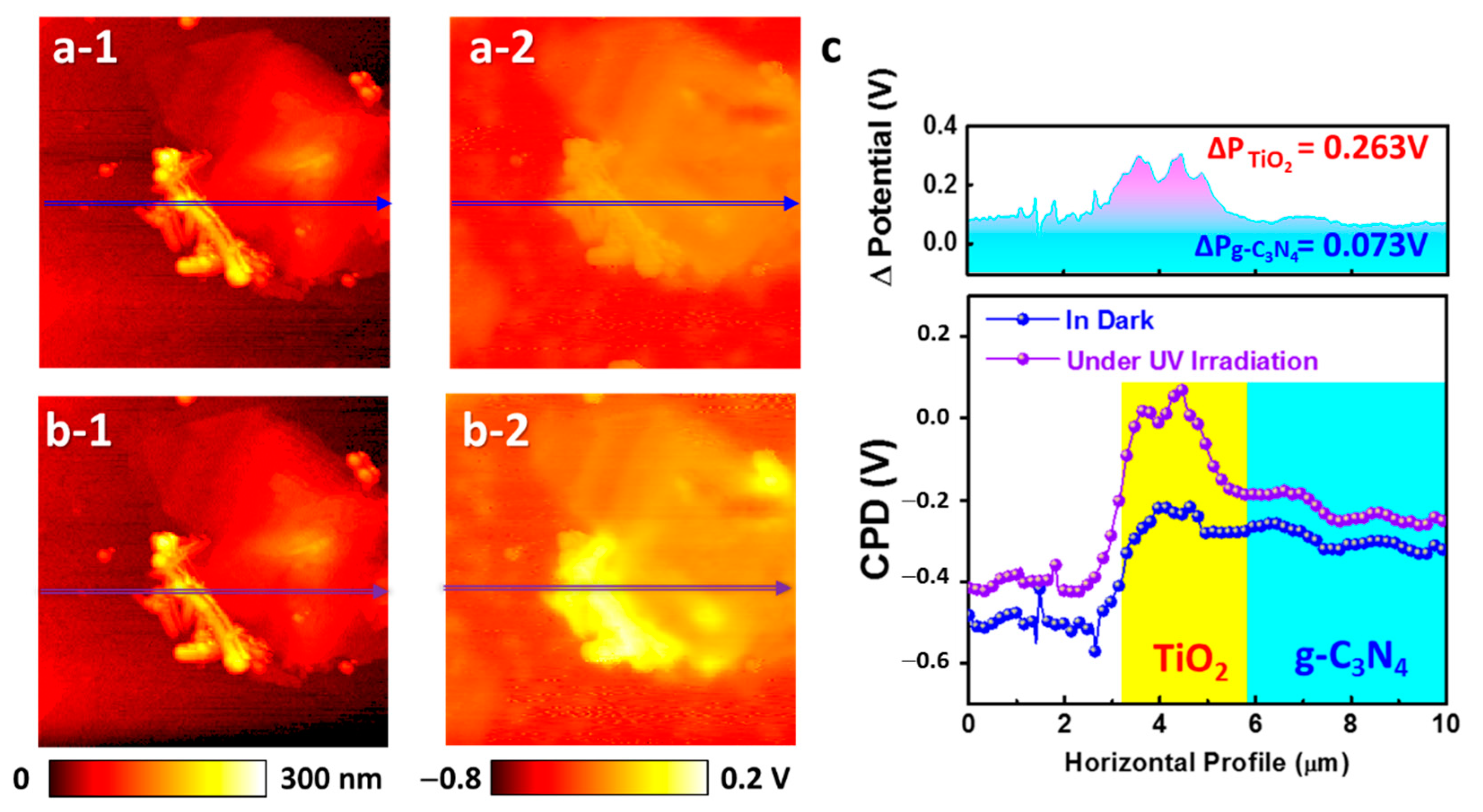
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Catalyst
3.2. Material Characterization
3.3. Photocatalytic Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.S.; Takata, T.; Domen, K. Particulate Photocatalysts for Overall Water Splitting. Nat. Rev. Mater. 2017, 2, 17. [Google Scholar] [CrossRef]
- Wang, Y.O.; Suzuki, H.; Xie, J.J.; Tomita, O.; Martin, D.J.; Higashi, M.; Kong, D.; Abe, R.; Tang, J.W. Mimicking Natural Photosynthesis: Solar to Renewable H2 Fuel Synthesis by Z-scheme Water Splitting Systems. Chem. Rev. 2018, 118, 5201–5241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, C.; Domen, K. Recent Developments in Heterogeneous Photocatalysts for Solar-driven Overall Water Splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.L.; Liu, S.H.; Dai, Z.D.; He, Y.P.; Song, X.Z.; Tan, Z.Q. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 32. [Google Scholar] [CrossRef]
- Wu, M.C.; Lin, T.H.; Hsu, K.H.; Hsu, J.F. Photo-induced Disinfection Property and Photocatalytic Activity Based on the Synergistic Catalytic Technique of Ag Doped TiO2 Nanofibers. Appl. Surf. Sci. 2019, 484, 326–334. [Google Scholar] [CrossRef]
- Wu, M.C.; Huang, W.K.; Lin, T.H.; Lu, Y.J. Photocatalytic Hydrogen Production and Photodegradation of Organic Dyes of Hydrogenated TiO2 Nanofibers Decorated Metal Nanoparticles. Appl. Surf. Sci. 2019, 469, 34–43. [Google Scholar] [CrossRef]
- Xu, F.Y.; Zhang, J.J.; Zhu, B.C.; Yu, J.G.; Xu, J.S. CuInS2 Sensitized TiO2 Hybrid Nanofibers for Improved Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2018, 230, 194–202. [Google Scholar] [CrossRef]
- Wu, M.C.; Hsiao, K.C.; Chang, Y.H.; Chan, S.H. Photocatalytic Hydrogen Evolution of Palladium Nanoparticles Decorated Black TiO2 Calcined in Argon Atmosphere. Appl. Surf. Sci. 2018, 430, 407–414. [Google Scholar] [CrossRef]
- Yin, G.H.; Huang, X.Y.; Chen, T.Y.; Zhao, W.; Bi, Q.Y.; Xu, J.; Han, Y.F.; Huang, F.Q. Hydrogenated Blue Titania for Efficient Solar to Chemical Conversions: Preparation, Characterization, and Reaction Mechanism of CO2 Reduction. ACS Catal. 2018, 8, 1009–1017. [Google Scholar] [CrossRef]
- Wu, M.C.; Hsiao, K.C.; Chang, Y.H.; Kordas, K. Core-shell Heterostructures of Rutile and Anatase TiO2 Nanofibers for Photocatalytic Solar Energy Conversion. ACS Appl. Nano Mater. 2019, 2, 1970–1979. [Google Scholar] [CrossRef]
- Ansón-Casaos, A.; Hernández-Ferrer, J.; Vallan, L.; Xie, H.; Lira-Cantú, M.; Benito, A.M.; Maser, W.K. Functionalized Carbon Dots on TiO2 for Perovskite Photovoltaics and Stable Photoanodes for Water Splitting. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Geng, R.; Yin, J.J.; Zhou, J.X.; Jiao, T.F.; Feng, Y.; Zhang, L.X.; Chen, Y.; Bai, Z.H.; Peng, Q.M. In Situ Construction of Ag/TiO2/g-C3N4 Heterojunction Nanocomposite Based on Hierarchical Co-assembly with Sustainable Hydrogen Evolution. Nanomaterials 2020, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ferrer, J.; Ansón-Casaos, A.; Víctor-Román, S.; Sanahuja-Parejo, O.; Martínez, M.T.; Villacampa, B.; Benito, A.M.; Maser, W.K. Photoactivity Improvement of TiO2 Electrodes by Thin Hole Transport Layers of Reduced Graphene Oxide. Electrochim. Acta 2019, 298, 279–287. [Google Scholar] [CrossRef]
- Chen, Q.H.; Zhang, M.M.; Li, J.Y.; Zhang, G.D.; Xin, Y.J.; Chai, C. Construction of Immobilized 0D/1D Heterostructure Photocatalyst Au/CuS/CdS/TiO2 NBs with Enhanced Photocatalytic Activity towards Moxifloxacin Degradation. Chem. Eng. J. 2020, 389, 12. [Google Scholar] [CrossRef]
- Wu, M.C.; Chen, C.H.; Huang, W.K.; Hsiao, K.C.; Lin, T.H.; Chan, S.H.; Wu, P.Y.; Lu, C.F.; Chang, Y.H.; Lin, T.F.; et al. Improved Solar-driven Photocatalytic Performance of Highly Crystalline Hydrogenated TiO2 Nanofibers with Core-Shell Structure. Sci. Rep. 2017, 7, 12. [Google Scholar] [CrossRef]
- Zhou, X.J.; Shao, C.L.; Li, X.H.; Wang, X.X.; Guo, X.H.; Liu, Y.C. Three Dimensional Hierarchical Heterostructures of g-C3N4 Nanosheets/TiO2 Nanofibers: Controllable Growth via Gas-solid Reaction and Enhanced Photocatalytic Activity under Visible Light. J. Hazard. Mater. 2018, 344, 113–122. [Google Scholar] [CrossRef]
- Zhu, M.S.; Kim, S.; Mao, L.; Fujitsuka, M.; Zhang, J.Y.; Wang, X.C.; Majima, T. Metal-free Photocatalyst for H2 Evolution in Visible to Near-infrared Region: Black Phosphorus/Graphitic Carbon Nitride. J. Am. Chem. Soc. 2017, 139, 13234–13242. [Google Scholar] [CrossRef]
- Hao, R.R.; Wang, G.H.; Tang, H.; Sun, L.L.; Xu, C.; Han, D.Y. Template-free Preparation of Macro/Mesoporous g-C3N4/TiO2 Heterojunction Photocatalysts with Enhanced Visible Light Photocatalytic Activity. Appl. Catal. B Environ. 2016, 187, 47–58. [Google Scholar] [CrossRef]
- Tao, R.; Li, X.H.; Li, X.W.; Shao, C.L.; Liu, Y.C. TiO2/SrTiO3/g-C3N4 Ternary Heterojunction Nanofibers: Gradient Energy Band, Cascade Charge Transfer, Enhanced Photocatalytic Hydrogen Evolution, and Nitrogen Fixation. Nanoscale 2020, 12, 8320–8329. [Google Scholar] [CrossRef]
- Wang, C.J.; Zhao, Y.L.; Xu, H.; Li, Y.F.; Wei, Y.C.; Liu, J.; Zhao, Z. Efficient Z-scheme Photocatalysts of Ultrathin g-C3N4-wrapped Au/TiO2-Nanocrystals for Enhanced Visible-Light-Driven Conversion of CO2 with H2O. Appl. Catal. B Environ. 2020, 263, 13. [Google Scholar] [CrossRef]
- Fu, J.W.; Yu, J.G.; Jiang, C.J.; Cheng, B. g-C3N4-based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 31. [Google Scholar] [CrossRef]
- Kumar, S.; Karthikeyan, S.; Lee, A.F. g-C3N4-based Nanomaterials for Visible Light-Driven Photocatalysis. Catalysts 2018, 8, 47. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We A Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Z.P.; Zhu, Y.F. Enhanced Visible-Light-Driven Photocatalytic Disinfection Performance and Organic Pollutant Degradation Activity of Porous g-C3N4 Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 27727–27735. [Google Scholar] [CrossRef]
- Ong, W.J. 2D/2D Graphitic Carbon Nitride (g-C3N4) Heterojunction Nanocomposites for Photocatalysis: Why Does Face-to-Face Interface Matter? Front. Mater. 2017, 4, 10. [Google Scholar] [CrossRef]
- Mun, S.J.; Park, S.J. Graphitic Carbon Nitride Materials for Photocatalytic Hydrogen Production via Water Splitting: A Short Review. Catalysts 2019, 9, 17. [Google Scholar] [CrossRef]
- Naseri, A.; Samadi, M.; Pourjavadi, A.; Moshfegh, A.Z.; Ramakrishna, S. Graphitic carbon nitride (g-C3N4)-based Photocatalysts for Solar Hydrogen Generation: Recent Advances and Future Development Directions. J. Mater. Chem. A 2017, 5, 23406–23433. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent Advances in g-C3N4-based Heterojunction Photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Zhu, Z.D.; Murugananthan, M.; Gu, J.; Zhang, Y.R. Fabrication of a Z-scheme g-C3N4/Fe-TiO2 Photocatalytic Composite with Enhanced Photocatalytic Activity under Visible Light Irradiation. Catalysts 2018, 8, 16. [Google Scholar] [CrossRef]
- Ji, C.; Du, C.; Steinkruger, J.D.; Zhou, C.; Yang, S.Y. In-situ Hydrothermal Fabrication of CdS/g-C3N4 Nanocomposites for Enhanced Photocatalytic Water Splitting. Mater. Lett. 2019, 240, 128–131. [Google Scholar] [CrossRef]
- Liu, X.M.; Liu, Y.; Zhang, W.K.; Zhong, Q.Y.; Ma, X.Y. In Situ Self-assembly of 3d Hierarchical 2D/2D CdS/g-C3N4 Hereojunction with Excellent Photocatalytic Performance. Mater. Sci. Semicond. Process 2020, 105, 9. [Google Scholar] [CrossRef]
- Lin, B.; Li, H.; An, H.; Hao, W.B.; Wei, J.J.; Dai, Y.Z.; Ma, C.S.; Yang, G.D. Preparation of 2D/2D g-C3N4 Nanosheet@ZnIn2S4 Nanoleaf Heterojunctions with Well-designed High-speed Charge Transfer Nanochannels towards High Efficiency Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2018, 220, 542–552. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Zhang, X.Y.; Zhang, H.X.; Cheng, Q.F.; Cheng, X.W. Construction of BiOCOOH/g-C3N4 Composite Photocatalyst and Its Enhanced Visible Light Photocatalytic Degradation of Amido Black 10b. Sep. Purif. Technol. 2019, 210, 125–134. [Google Scholar] [CrossRef]
- Qin, H.; Guo, R.T.; Liu, X.Y.; Pan, W.G.; Wang, Z.Y.; Shi, X.; Tang, J.Y.; Huang, C.Y. Z-scheme MoS2/g-C3N4 Heterojunction for Efficient Visible Light Photocatalytic CO2 Reduction. Dalton Trans. 2018, 47, 15155–15163. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.W.; Fujitsuka, M.; Kim, S.; Majima, T. Faster Electron Injection and More Active Sites for Efficient Photocatalytic H2 Evolution in g-C3N4/MoS2 Hybrid. Small 2018, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, N.; Sinthiya, M.M.A.; Sarathbavan, M.; Ramamurthi, K.; Sethuraman, K.; Babu, R.R. Synergetic Effect of g-C3N4/ZnO Binary Nanocomposites Heterojunction on Improving Charge Carrier Separation through 2D/1D Nanostructures for Effective Photocatalytic Activity under The Sunlight Irradiation. Sep. Purif. Technol. 2020, 244, 116356. [Google Scholar] [CrossRef]
- Ji, H.; Du, P.; Zhao, D.; Li, S.; Sun, F.; Duin, E.C.; Liu, W. 2D/1D Graphitic Carbon Nitride/Titanate Nanotubes Heterostructure for Efficient Photocatalysis of Sulfamethazine under Solar Light: Catalytic “Hot Spots” at the Rutile–Anatase–Titanate Interfaces. Appl. Catal. B Environ. 2020, 263, 118357. [Google Scholar] [CrossRef]
- Tan, Y.G.; Shu, Z.; Zhou, J.; Li, T.T.; Wang, W.B.; Zhao, Z.L. One-step Synthesis of Nanostructured g-C3N4/TiO2 Composite for Highly Enhanced Visible-Light Photocatalytic H2 Evolution. Appl. Catal. B Environ. 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Martin, D.J.; Qiu, K.; Shevlin, S.A.; Handoko, A.D.; Chen, X.; Guo, Z.; Tang, J. Highly Efficient Photocatalytic H2 Evolution from Water Using Visible Light and Structure-Controlled Graphitic Carbon Nitride. Angew. Chem. Int. Ed. 2014, 53, 9240–9245. [Google Scholar] [CrossRef]
- Wu, P.; Wang, J.; Zhao, J.; Guo, L.; Osterloh, F.E. Structure Defects in g-C3N4 Limit Visible Light Driven Hydrogen Evolution and Photovoltage. J. Mater. Chem. A 2014, 2, 20338–20344. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.-G.; He, H.-W.; Wang, X.-X.; Zhang, J.; Zhang, Q.-Q.; Tong, Y.-F.; Liu, H.-L.; Ramakrishna, S.; Yan, S.-Y.; et al. One-step Synthesis Heterostructured g-C3N4/TiO2 Composite for Rapid Degradation of Pollutants in Utilizing Visible Light. Nanomaterials 2018, 8, 842. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-C.; Wu, P.-Y.; Lin, T.-H.; Lin, T.-F. Photocatalytic Performance of Cu-doped TiO2 Nanofibers Treated by The Hydrothermal Synthesis and Air-thermal Treatment. Appl. Surf. Sci. 2018, 430, 390–398. [Google Scholar] [CrossRef]
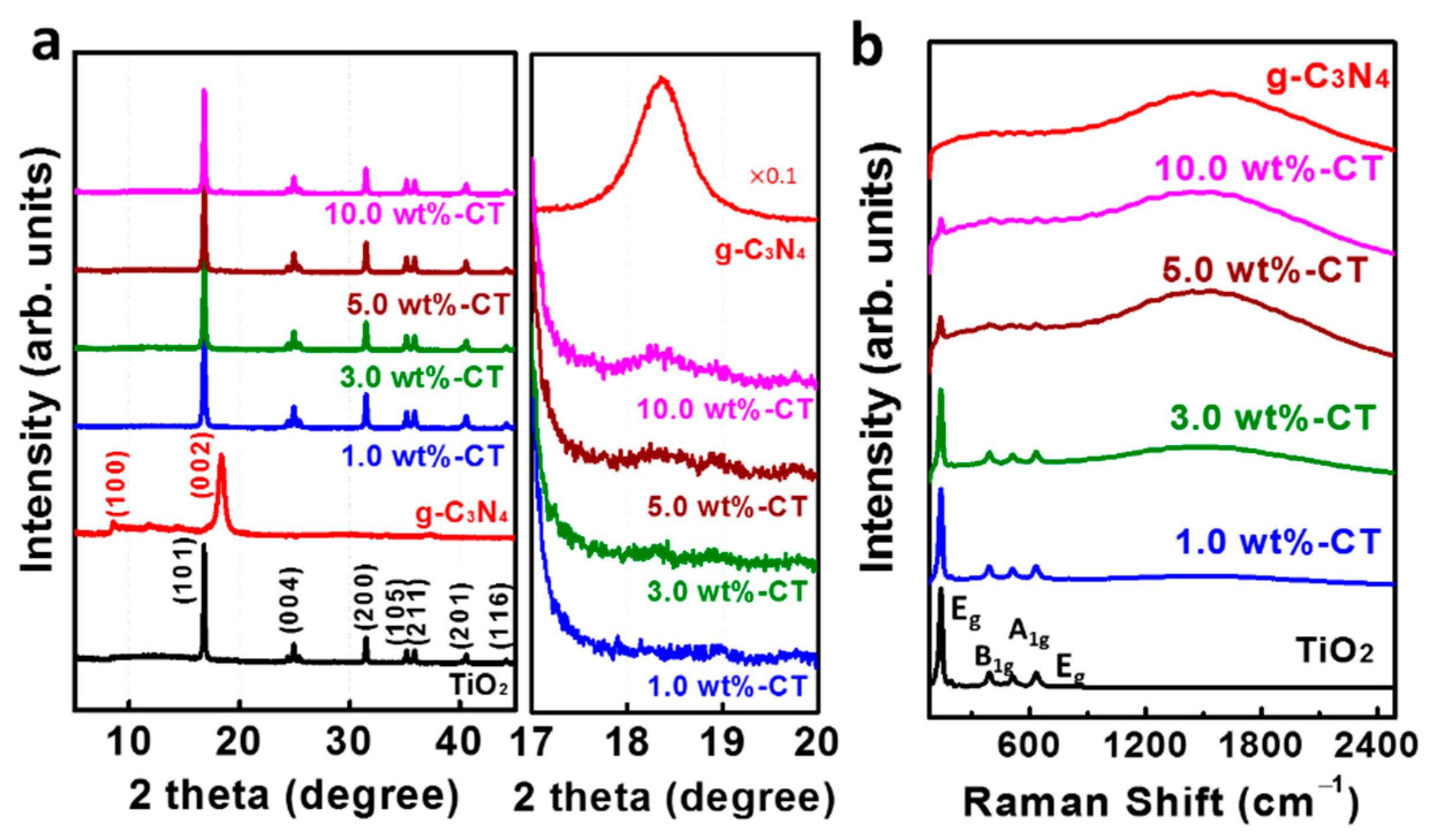
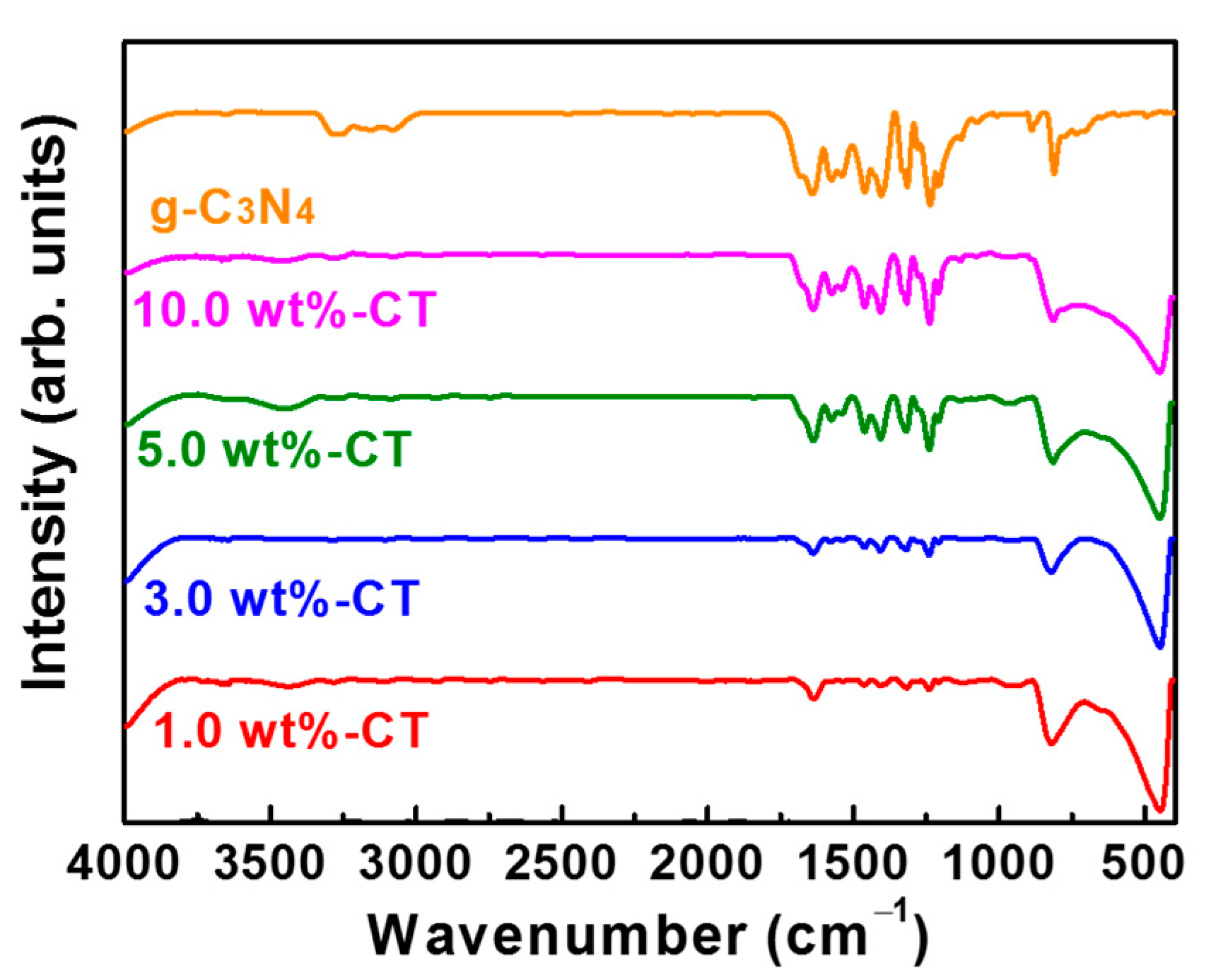
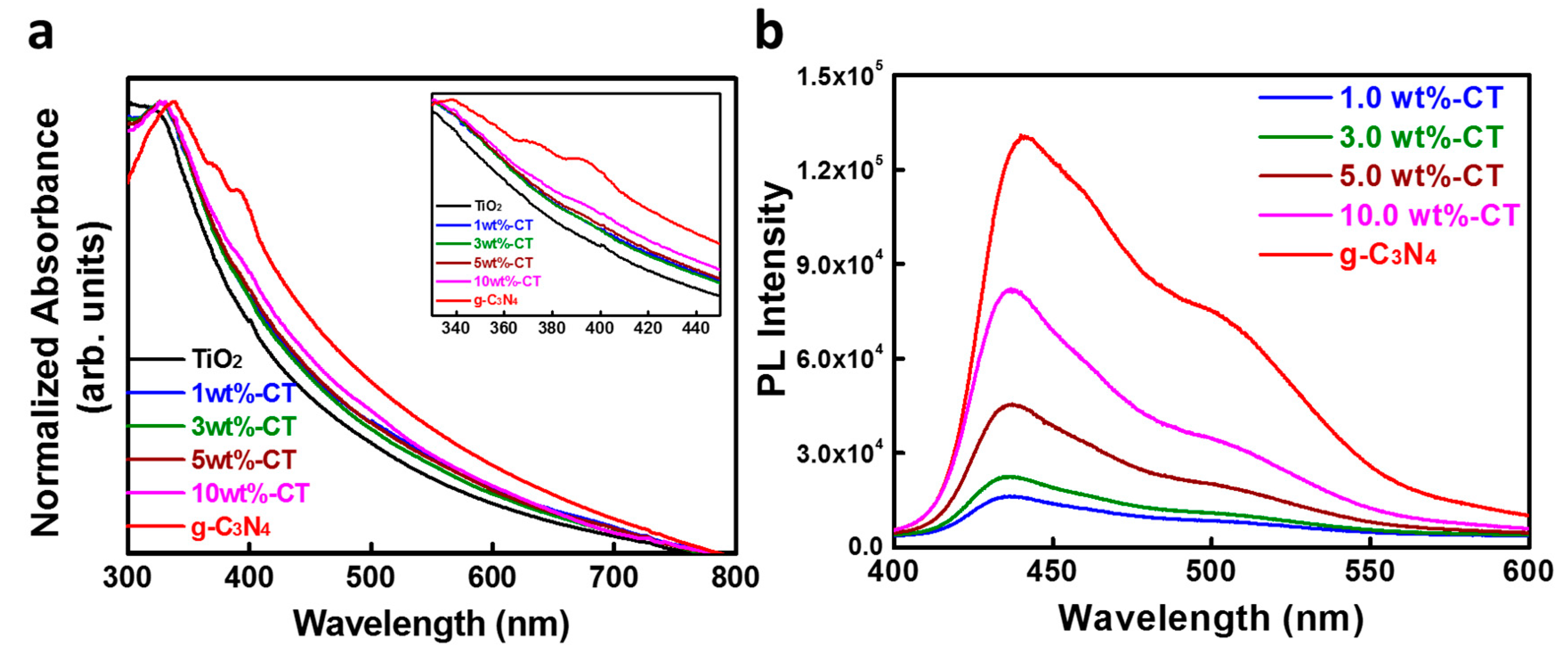
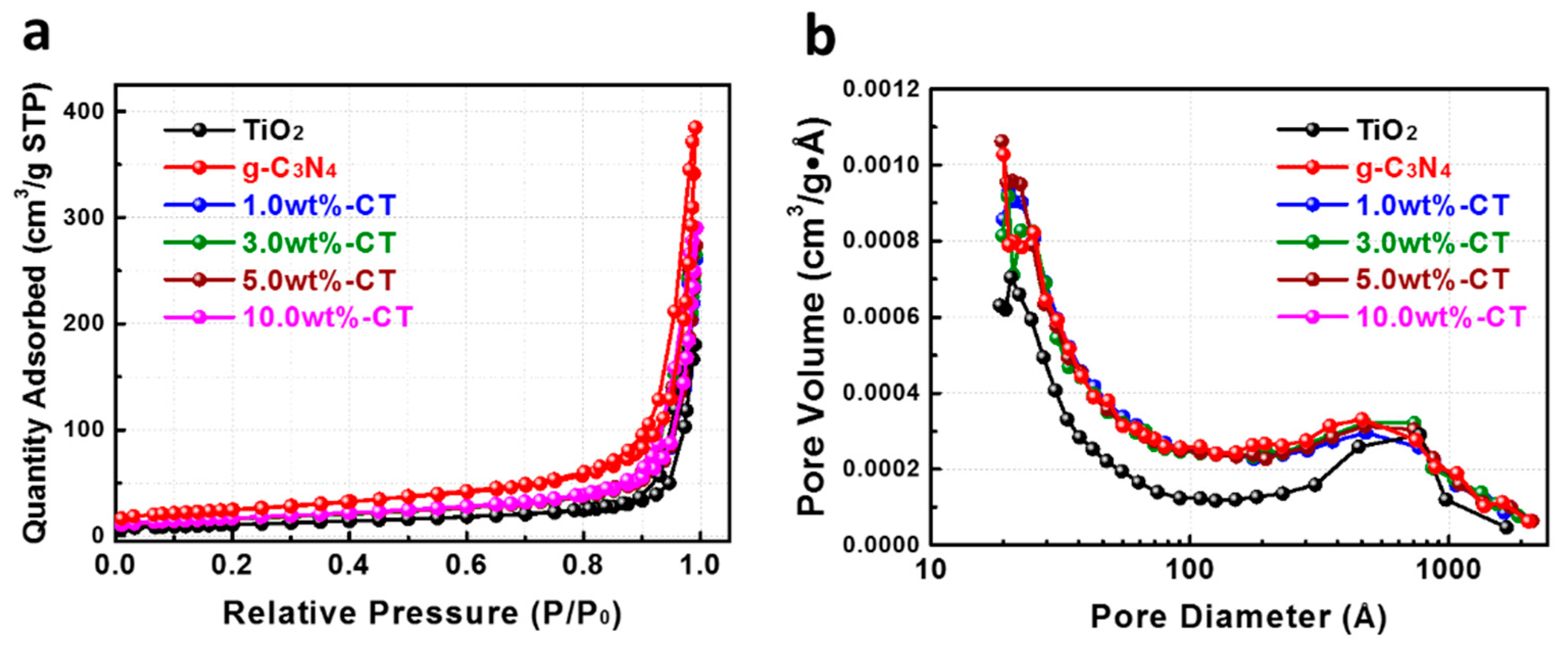

| Sample Name | SBET (m2·g−1) | Pore Size (nm) | Pore Volume (cm3·g−1) | ||
|---|---|---|---|---|---|
| BJH Adsorption | BJH Desorption | BJH Adsorption | BJH Desorption | ||
| TiO2 | 49.64 | 22.7 | 21.3 | 0.295 | 0.294 |
| 1.0 wt%-CT | 58.96 | 25.9 | 24.5 | 0.405 | 0.404 |
| 3.0 wt%-CT | 59.71 | 26.7 | 25.0 | 0.413 | 0.412 |
| 5.0 wt%-CT | 59.91 | 26.8 | 25.1 | 0.426 | 0.425 |
| 10.0 wt%-CT | 61.74 | 28.2 | 26.7 | 0.451 | 0.450 |
| g-C3N4 | 88.98 | 25.9 | 24.1 | 0.599 | 0.597 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-H.; Chang, Y.-H.; Chiang, K.-P.; Wang, J.-C.; Wu, M.-C. Nanoscale Multidimensional Pd/TiO2/g-C3N4 Catalyst for Efficient Solar-Driven Photocatalytic Hydrogen Production. Catalysts 2021, 11, 59. https://doi.org/10.3390/catal11010059
Lin T-H, Chang Y-H, Chiang K-P, Wang J-C, Wu M-C. Nanoscale Multidimensional Pd/TiO2/g-C3N4 Catalyst for Efficient Solar-Driven Photocatalytic Hydrogen Production. Catalysts. 2021; 11(1):59. https://doi.org/10.3390/catal11010059
Chicago/Turabian StyleLin, Ting-Han, Yin-Hsuan Chang, Kuo-Ping Chiang, Jer-Chyi Wang, and Ming-Chung Wu. 2021. "Nanoscale Multidimensional Pd/TiO2/g-C3N4 Catalyst for Efficient Solar-Driven Photocatalytic Hydrogen Production" Catalysts 11, no. 1: 59. https://doi.org/10.3390/catal11010059
APA StyleLin, T.-H., Chang, Y.-H., Chiang, K.-P., Wang, J.-C., & Wu, M.-C. (2021). Nanoscale Multidimensional Pd/TiO2/g-C3N4 Catalyst for Efficient Solar-Driven Photocatalytic Hydrogen Production. Catalysts, 11(1), 59. https://doi.org/10.3390/catal11010059







