Dehydrogenation of Formic Acid to CO2 and H2 by Manganese(I)–Complex: Theoretical Insights for Green and Sustainable Route
Abstract
1. Introduction
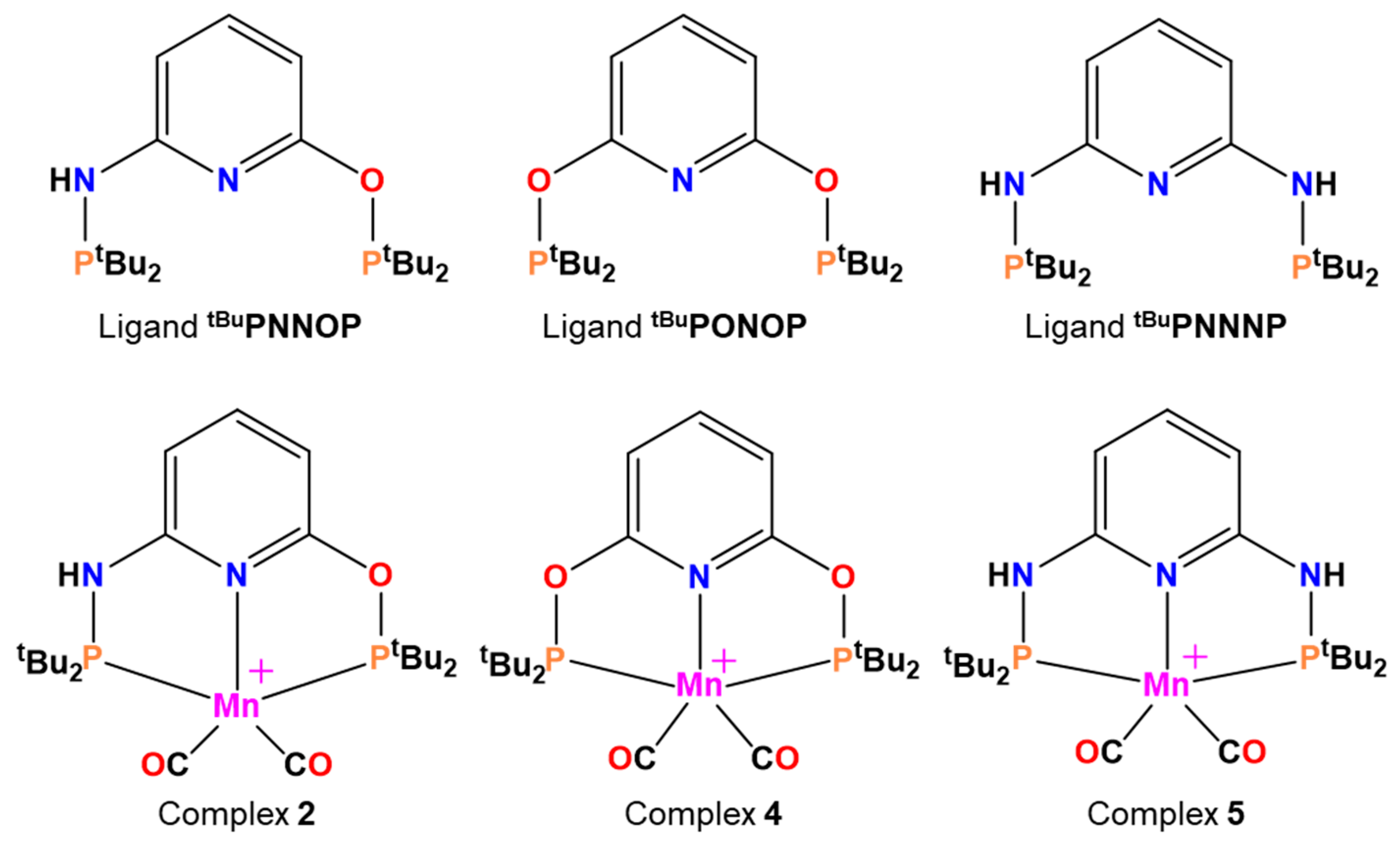
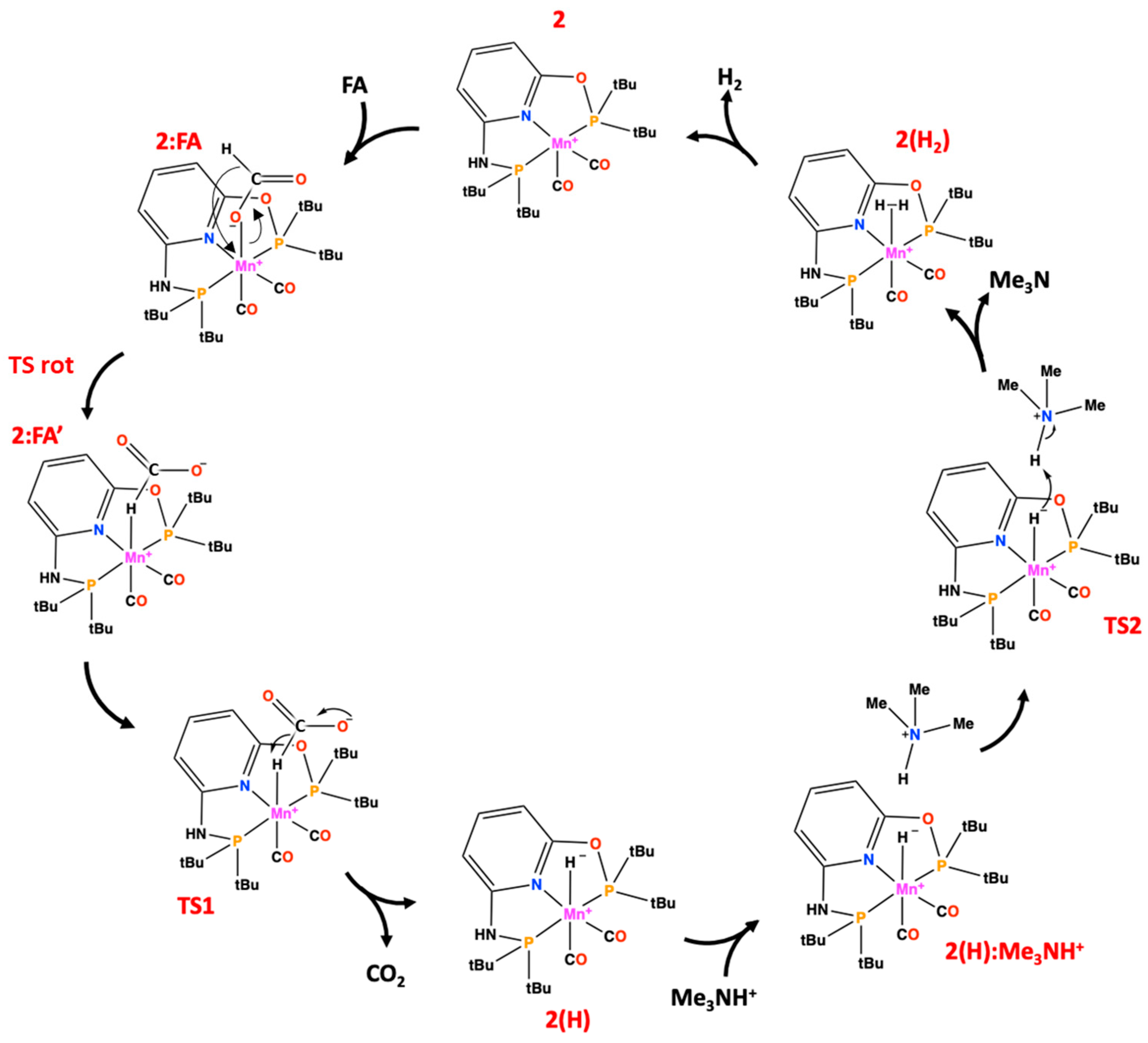
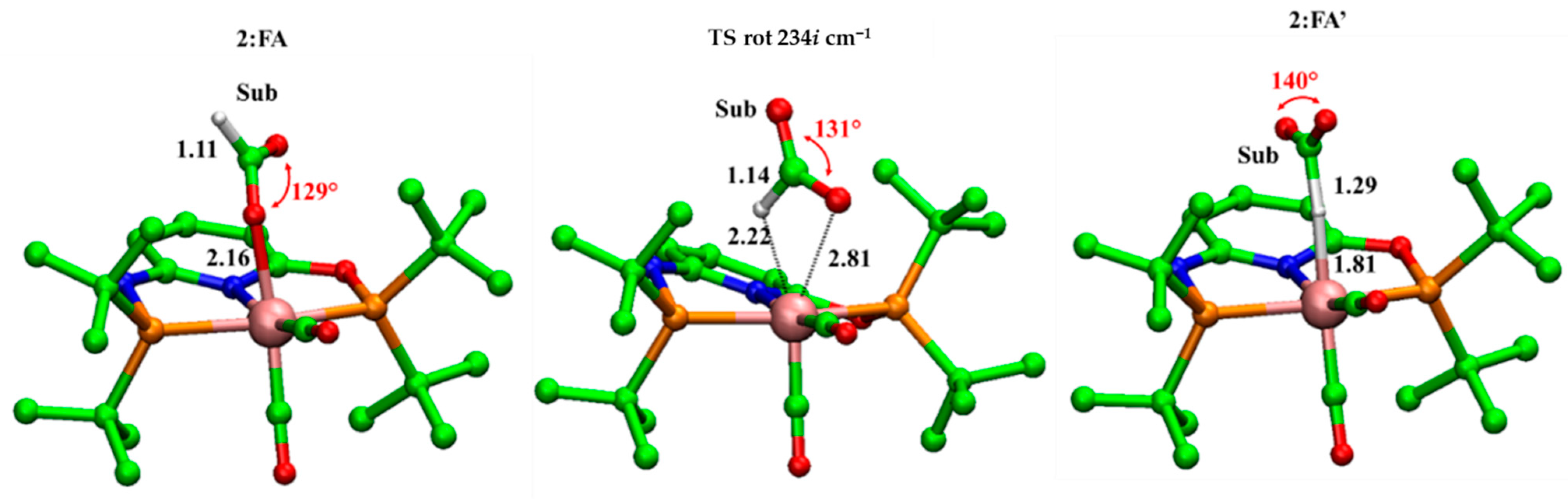
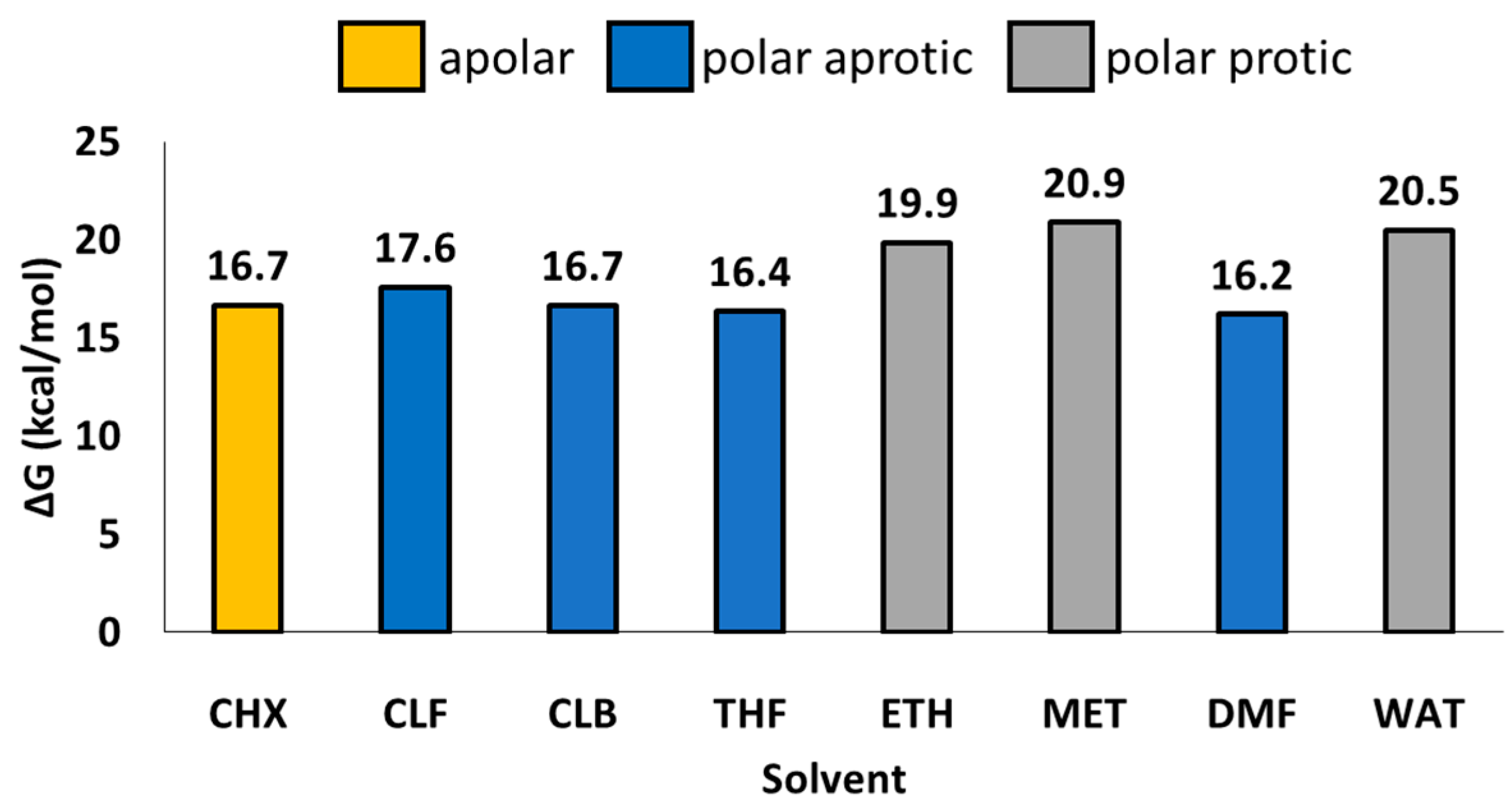
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellmann, A.; Sponholz, P.; Junge, H.; Beller, M. Formic acid as a hydrogen storage material—Development of homogeneous catalysts for selective hydrogen release. Chem. Soc. Rev. 2016, 45, 3954–3988. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, G.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Review of solutions to global warming, air pollution, and energy security. Energy Environ. Sci. 2009, 2, 148–173. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The Hydrogen Issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Princiotta, F.T. Global Climate Change and the Mitigation Challenge. In Global Climate Change—The Technology Challenge; Princiotta, F.T., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 1–50. [Google Scholar]
- Armaroli, N.; Balzani, V. The future of energy supply: Challenges and opportunities. Angew. Chem. Int. Ed. 2007, 46, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Ortenzi, F.; Pede, G.; Ramadhas, A.S. Alternative Fuels for Transportation; Ramadhas, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 243–293. [Google Scholar]
- Abuadala, A.; Dincer, I. A review on biomass-based hydrogen production and potential applications. Int. J. Energy Res. 2012, 36, 415–455. [Google Scholar] [CrossRef]
- Kırtay, E. Recent advances in production of hydrogen from biomass. Energy Convers. Manag. 2011, 52, 1778–1789. [Google Scholar] [CrossRef]
- Christopher, K.; Dimitrios, R. A review on exergy comparison of hydrogen production methods from renewable energy sources. Energy Environ. Sci. 2012, 5, 6640–6651. [Google Scholar] [CrossRef]
- Moriarty, P.; Honnery, D. A hydrogen standard for future energy accounting? Int. J. Hydrog. Energy 2010, 35, 12374–12380. [Google Scholar] [CrossRef]
- Hobein, B.; Krueger, R. Hydrogen and Fuel Cells—Fundamentals, Technologies and Applications; Stolten, D., Ed.; Wiley-VCH: Weinheim, Germany, 2010; pp. 377–393. [Google Scholar]
- Schüth, F. Chemical Compounds for Energy Storage. Chem. Ing. Tech. 2011, 83, 1984–1993. [Google Scholar] [CrossRef]
- Makowski, P.; Thomas, A.; Kuhn, P.; Goettmann, F. Organic materials for hydrogenstorage applications: From physisorption on organic solids to chemisorption in organic molecules. Energy Environ. Sci. 2009, 2, 480–490. [Google Scholar] [CrossRef]
- Dalebrook, A.F.; Gan, W.; Grasemann, M.; Moret, S.; Laurenczy, G. Hydrogen storage: Beyond conventional methods. Chem. Commun. 2013, 49, 8735–8751. [Google Scholar] [CrossRef]
- Li, S.-L.; Xu, Q. Metal–organic frameworks as platforms for clean energy. Energy Environ. Sci. 2013, 6, 1656–1683. [Google Scholar] [CrossRef]
- Rodrıguez-Lugo, R.E.; Trincado, M.; Vogt, M.; Tewes, F.; Santiso-Quinones, G.; Grutzmacher, H. A homogeneous transition metal complex for clean hydrogen production from methanol-water mixtures. Nat. Chem. 2013, 5, 342–347. [Google Scholar] [CrossRef]
- Nielsen, N.; Alberico, E.; Baumann, W.; Drexler, H.-J.; Junge, H.; Gladiali, S.; Beller, M. Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide. Nature 2013, 495, 85–89. [Google Scholar] [CrossRef]
- Grasemann, M.; Laurenczy, G. Formic acid as a hydrogen source—Recent developments and future trends. Energy Environ. Sci. 2012, 5, 8171–8181. [Google Scholar] [CrossRef]
- Malacea, R.; Poli, R.; Manoury, E. Asymmetric hydrosilylation, transfer hydrogenation and hydrogenation of ketones catalyzed by iridium complexes. Coord. Chem. Rev. 2010, 254, 729–752. [Google Scholar] [CrossRef]
- Gladiali, S.; Alberico, E. Asymmetric transfer hydrogenation: Chiral ligands and applications. Chem. Soc. Rev. 2006, 35, 226–236. [Google Scholar] [CrossRef]
- King, R.B.; Bhattacharyya, N.K. Catalytic reactions of formate 4. A nitrite-promoted rhodium (III) catalyst for hydrogen generation from formic acid in aqueous solution. Inorg. Chim. Acta 1995, 237, 65–69. [Google Scholar] [CrossRef]
- Marcinkowski, M.D.; Liu, J.; Murphy, C.J.; Liriano, M.L.; Wasio, N.A.; Lucci, F.R.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Selective Formic Acid Dehydrogenation on Pt-Cu Single-Atom Alloys. ACS Catal. 2017, 7, 413–420. [Google Scholar] [CrossRef]
- Barrett, S.M.; Slattery, S.A.; Miller, A.J.M. Photochemical Formic Acid Dehydrogenation by Iridium Complexes: Understanding Mechanism and Overcoming Deactivation. ACS Catal. 2015, 5, 6320–6327. [Google Scholar] [CrossRef]
- Fink, C.; Laurenczy, G. A Precious Catalyst: Rhodium-Catalyzed Formic Acid Dehydrogenation in Water. Eur. J. Inorg. Chem. 2019, 2019, 2381–2387. [Google Scholar] [CrossRef]
- Frenklah, A.; Treigerman, Z.; Sasson, Y.; Kozuch, S. Formic Acid Dehydrogenation by Ruthenium Catalyst—Computational and Kinetic Analysis with the Energy Span Model. Eur. J. Org. Chem. 2019, 2019, 591–597. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.; Kumar, A. Hydrogen Energy Future with Formic Acid: A Renewable Chemical Hydrogen Storage System. Catal. Sci. Technol. 2016, 6, 12–40. [Google Scholar] [CrossRef]
- Zhang, Y.; MacIntosh, A.D.; Wong, J.L.; Bielinski, E.A.; Williard, P.G.; Mercado, B.Q.; Hazari, N.; Bernskoetter, W.H. Iron catalyzed CO2 hydrogenation to formate enhanced by Lewis acid co-catalysts. Chem. Sci. 2015, 6, 4291–4299. [Google Scholar] [CrossRef]
- Bielinski, E.A.; Lagaditis, P.O.; Zhang, Y.; Mercado, B.Q.; Würtele, C.; Bernskoetter, W.H.; Hazari, N.; Schneider, S. Lewis Acid-Assisted Formic Acid Dehydrogenation Using a Pincer Supported Iron Catalyst. J. Am. Chem. Soc. 2014, 136, 10234–10237. [Google Scholar] [CrossRef]
- Bernskoetter, W.H.; Hazari, N. Reversible Hydrogenation of Carbon Dioxide to Formic Acid and Methanol: Lewis Acid Enhancement of Base Metal Catalysts. Acc. Chem. Res. 2017, 50, 1049–1058. [Google Scholar] [CrossRef]
- Anderson, N.H.; Boncella, J.M.; Tondreau, A.M. Manganese-Mediated Formic Acid Dehydrogenation. Chem. Eur. J. 2019, 25, 10557–10560. [Google Scholar] [CrossRef] [PubMed]
- Andérez-Fernández, M.; Vogt, L.K.; Fischer, S.; Zhou, W.; Jiao, H.; Garbe, M.; Elangovan, S.; Junge, K.; Junge, H.; Ludwig, R.; et al. A Stable Manganese Pincer Catalyst for the Selective Dehydrogenation of Methanol. Angew. Chem. Int. Ed. 2017, 56, 559–562. [Google Scholar] [CrossRef]
- Anderson, N.H.; Boncella, J.M.; Tondreau, A.M. Reactivity of Silanes with (tBuPONOP) Ruthenium Dichloride: Facile Synthesis of Chloro-Silyl Ruthenium Compounds and Formic Acid Decomposition. Chem. Eur. J. 2017, 23, 13617–13622. [Google Scholar] [CrossRef]
- Prejanò, M.; Alberto, M.E.; Russo, N.; Marino, T. Hydration of Aromatic Nitriles Catalyzed by Mn-OH Complexes: A Rationalization from Quantum Chemical Investigations. Organometallics 2020, 39, 3352–3361. [Google Scholar] [CrossRef]
- Parise, A.; Muraca, M.C.; Russo, N.; Toscano, M.; Marino, T. The Generation of the Oxidant Agent of a Mononuclear Nonheme Fe(II) Biomimetic Complex by Oxidative Decarboxylation. A DFT Investigation. Molecules 2020, 25, 328. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Fortino, M.G.; Russo, N.; Toscano, M.; Alberto, M.E. Computational Mechanistic Insights on the NO Oxidation Reaction Catalyzed by Non-Heme Biomimetic Cr-N-Tetramethylated Cyclam Complexes. Int. J. Mol. Sci. 2019, 20, 3955. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, V. Solvent effects on the reactivities of organometallic compounds. Coord. Chem. Rev. 1976, 18, 225–255. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A., Jr.; Stratmann, R.E.; Burant, J.C.; et al. Gaussian 2009; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Phys. Chem. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Andrae, D.; Hussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Alberto, M.E. A trispyrazolylborato iron cysteinato complex efficiently mimics the cysteine dioxygenation process: Mechanistic insights. Chem. Commun. 2015, 51, 8369–8372. [Google Scholar] [CrossRef]
- Mazzone, G.; Marino, T.; Piazzetta, P.; Ponte, F.; Prejanò, M.; Sicilia, E.; Toscano, M. Quantum mechanical DFT elucidation of CO2 catalytic conversion mechanisms: Three examples. Int. J. Quantum Chem. 2018, 118, e25572. [Google Scholar] [CrossRef]
- Harvey, J.N.; Himo, F.; Maseras, F.; Perrin, L. Scope and Challenge of Computational Methods for Studying Mechanism and Reactivity in Homogeneous Catalysis. ACS Catal. 2019, 9, 6803–6813. [Google Scholar] [CrossRef]
- Marino, T.; Ponte, F.; Mazzone, G.; Sicilia, E.; Toscano, M.; Russo, N. The ability of a zinc pyrrolidine complex to catalyze the synthesis of cyclic carbonates from carbon dioxide and epoxides: A mechanistic theoretical investigation. Dalton Trans. 2018, 46, 9030–9035. [Google Scholar] [CrossRef] [PubMed]
- Piazzetta, P.; Marino, T.; Russo, N.; Salahub, D.R. Direct hydrogenation of carbon dioxide by an artificial reductase obtained by substituting rhodium for zinc in the carbonic anhydrase catalytic center. A mechanistic study. ACS Catal. 2015, 5, 5397–5409. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chim. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, T.; Prejanò, M. Dehydrogenation of Formic Acid to CO2 and H2 by Manganese(I)–Complex: Theoretical Insights for Green and Sustainable Route. Catalysts 2021, 11, 141. https://doi.org/10.3390/catal11010141
Marino T, Prejanò M. Dehydrogenation of Formic Acid to CO2 and H2 by Manganese(I)–Complex: Theoretical Insights for Green and Sustainable Route. Catalysts. 2021; 11(1):141. https://doi.org/10.3390/catal11010141
Chicago/Turabian StyleMarino, Tiziana, and Mario Prejanò. 2021. "Dehydrogenation of Formic Acid to CO2 and H2 by Manganese(I)–Complex: Theoretical Insights for Green and Sustainable Route" Catalysts 11, no. 1: 141. https://doi.org/10.3390/catal11010141
APA StyleMarino, T., & Prejanò, M. (2021). Dehydrogenation of Formic Acid to CO2 and H2 by Manganese(I)–Complex: Theoretical Insights for Green and Sustainable Route. Catalysts, 11(1), 141. https://doi.org/10.3390/catal11010141





