Facile Aqueous–Phase Synthesis of Pd–FePt Core–Shell Nanoparticles for Methanol Oxidation Reaction
Abstract
1. Introduction
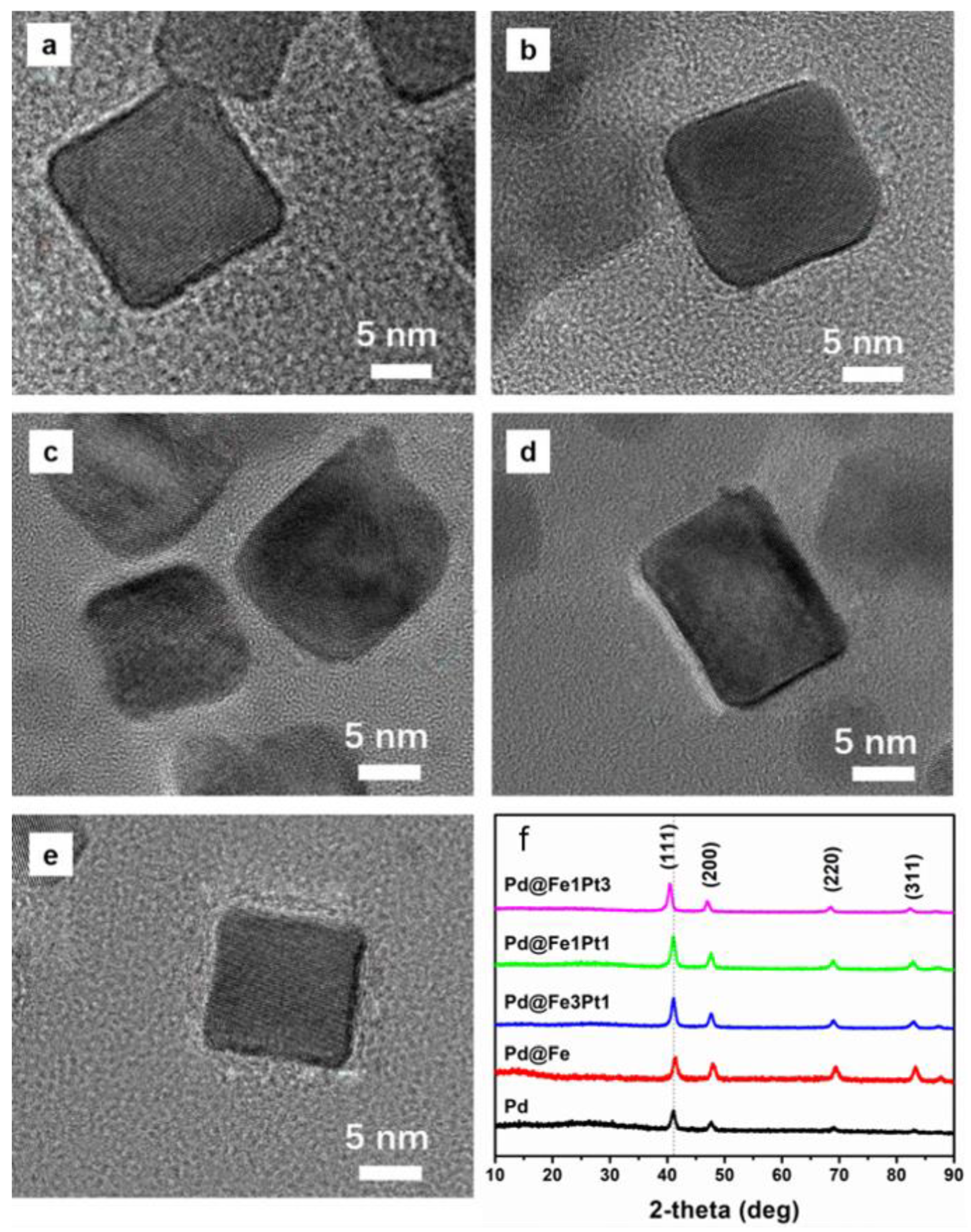
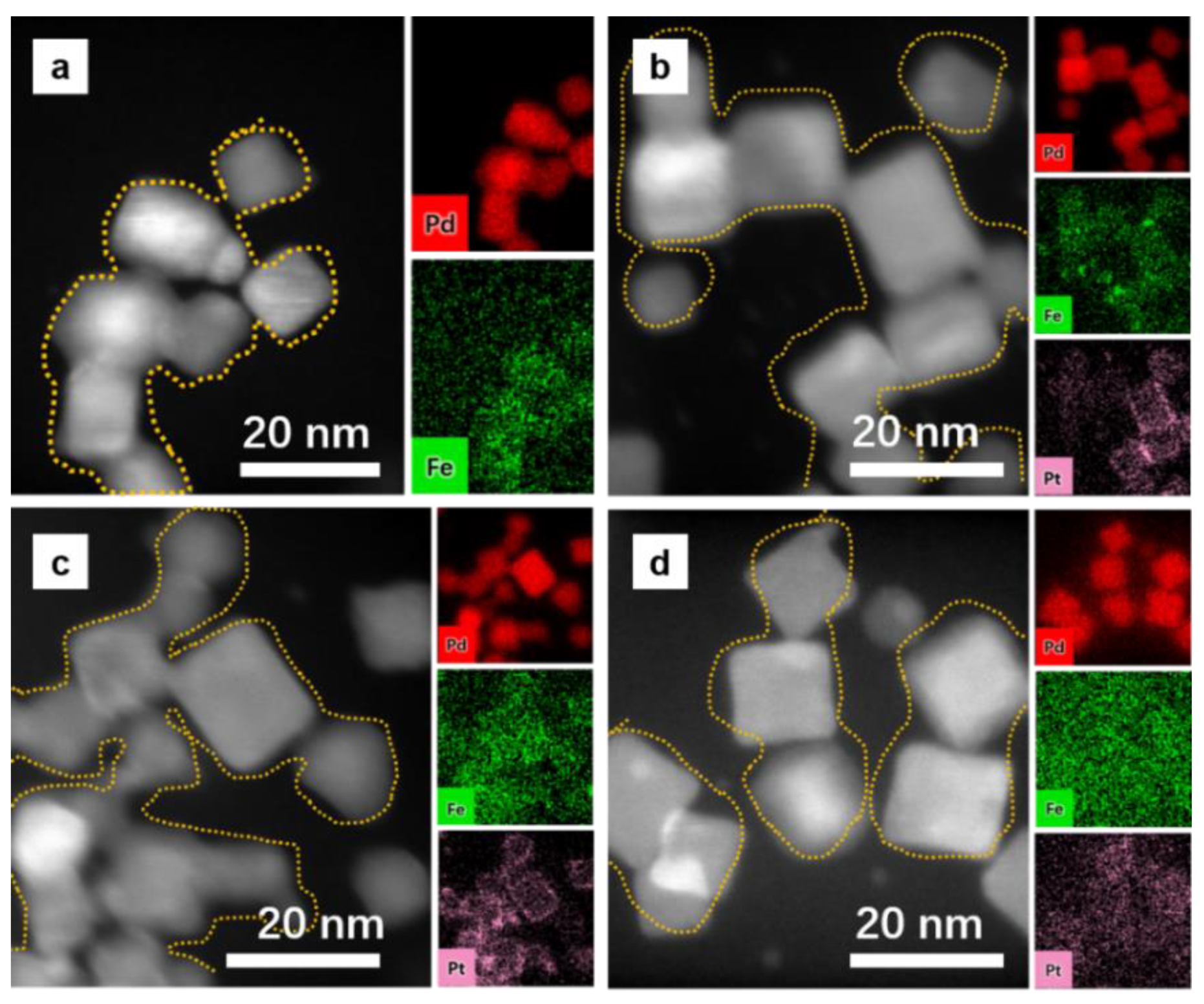
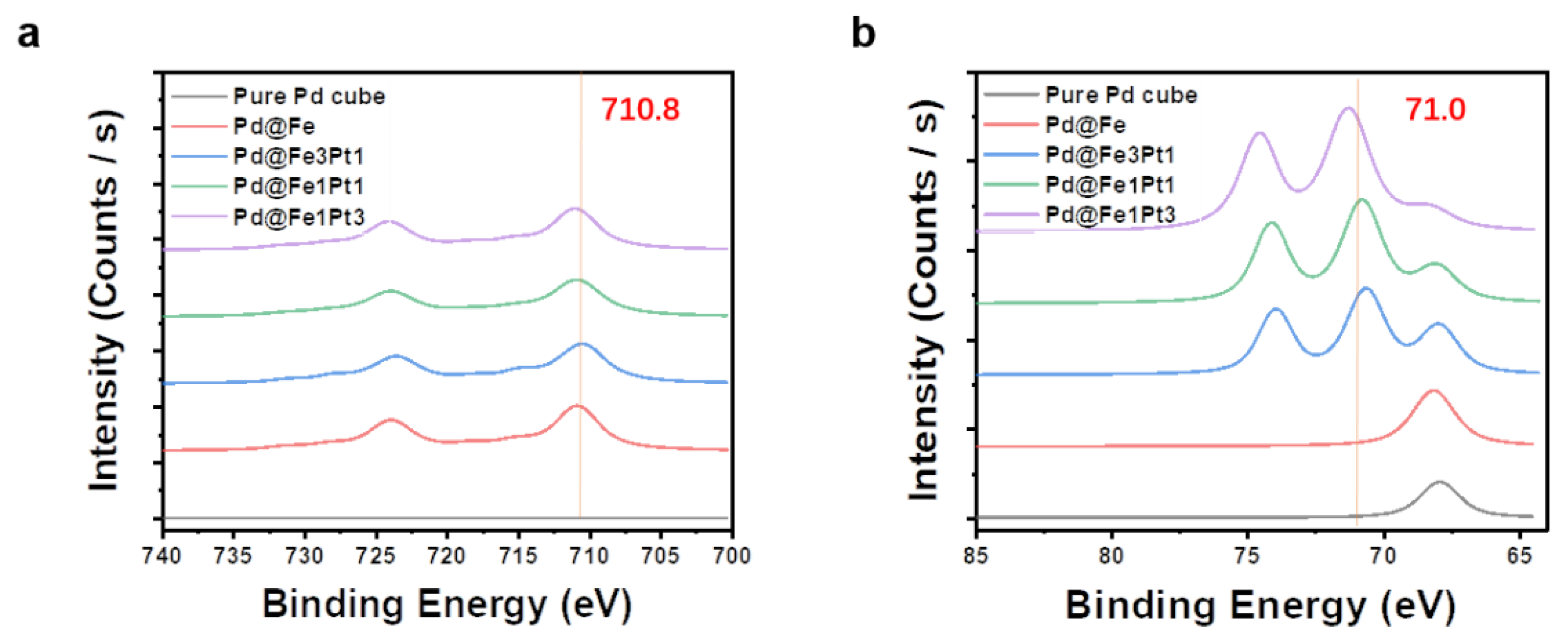
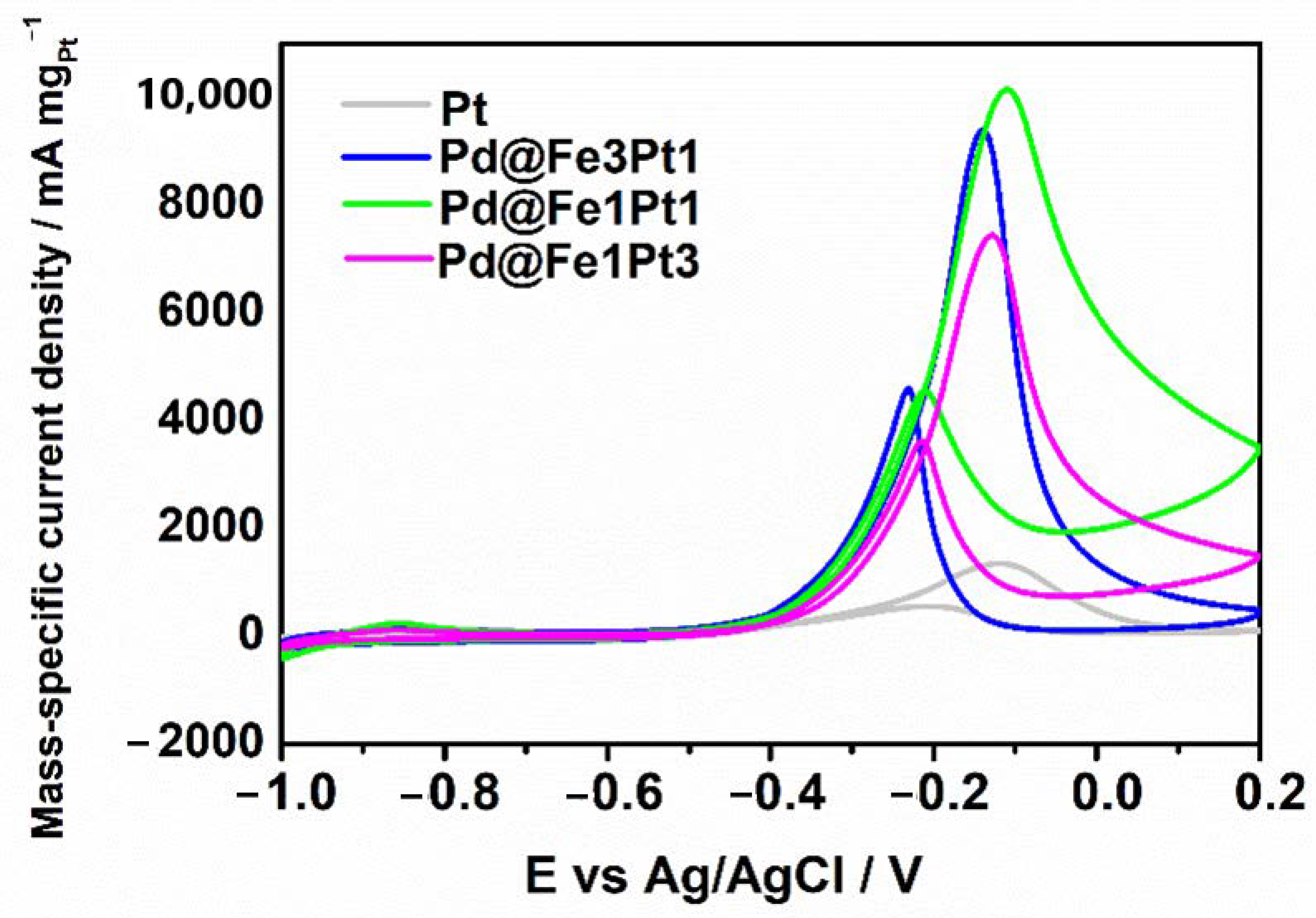
2. Results
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Pd, Pd@Fe, and Pd@FePt Core–Shell Nanoparticles
3.3. Synthesis of Carbon-Supported Pd, Pd@Fe, and Pd@FePt Nanoparticles
3.4. Characterization
3.5. Electrochemical Characterization and Catalytic Activity Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tan, L.L.; Wei, M.Y.; Shang, L.; Yang, Y.W. Cucurbiturils-Mediated noble metal nanoparticles for applications in sensing, SERS, theranostics, and catalysis. Adv. Funct. Mater. 2020, 2007277. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Du, B.J.; Huang, Y.Y.; Zheng, J. Ultrasmall noble metal nanoparticles: Breakthroughs and biomedical implications. Nano Today 2018, 21, 106–125. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.S.; da Silva, A.G.M.; Camargo, P.H.C. Nanocatalysis by noble metal nanoparticles: Controlled synthesis for the optimization and understanding of activities. J. Mater. Chem. A 2019, 7, 5857–5874. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y.N. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.Y.; Park, S.; Lee, H.; Kim, H.; Chung, Y.H.; Yoo, J.M.; Ahn, D.; Yu, S.H.; Lee, K.S.; Ahmadi, M.; et al. Activity-Stability relationship in Au@Pt nanoparticles for electrocatalysis. ACS Energy Lett. 2020, 5, 2827–2834. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Lee, W.J.; Ruqia, B.; Baik, H.; Oh, H.S.; Paek, S.M.; Lim, H.K.; Choi, C.H.; Choi, S.I. Theoretical and experimental understanding of hydrogen evolution reaction kinetics in alkaline electrolytes with Pt-based core-shell nanocrystals. J. Am. Chem. Soc. 2019, 141, 18256–18263. [Google Scholar] [CrossRef]
- Zhu, J.W.; Xie, M.H.; Chen, Z.T.; Lyu, Z.H.; Chi, M.F.; Jin, W.Q.; Xia, Y.N. Pt-Ir-Pd trimetallic nanocages as a dual catalyst for efficient oxygen reduction and evolution reactions in acidic media. Adv. Energy Mater. 2020, 10, 1904114. [Google Scholar] [CrossRef]
- Huang, X.Q.; Zhao, Z.P.; Cao, L.; Chen, Y.; Zhu, E.B.; Lin, Z.Y.; Li, M.F.; Yan, A.M.; Zettl, A.; Wang, Y.M.; et al. High-Performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef]
- Xia, B.Y.; Wu, H.B.; Wang, X.; Lou, X.W. One-Pot synthesis of cubic PtCu3 nanocages with enhanced electrocatalytic activity for the methanol oxidation reaction. J. Am. Chem. Soc. 2012, 134, 13934–13937. [Google Scholar] [CrossRef]
- Jenkinson, K.J.; Wagner, A.; Kornienko, N.; Reisner, E.; Wheatley, A.E.H. A One-pot route to faceted FePt-Fe3O4 dumbbells: Probing morphology-catalytic activity effects in O2 reduction catalysis. Adv. Funct. Mater. 2020, 30, 2002633. [Google Scholar] [CrossRef]
- Xiao, X.; Jeong, H.; Song, J.; Ahn, J.P.; Kim, J.; Yu, T. Facile synthesis of Pd@Pt core-shell nanocubes with low Pt content via direct seed-mediated growth and their enhanced activity for formic acid oxidation. Chem. Commun. 2019, 55, 11952–11955. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Xiao, X.; Hong, J.; Lee, K.J.; Park, S.; Ahn, J.P.; Lee, K.Y.; Yu, T. Tailored palladium-platinum nanoconcave cubes as high performance catalysts for the direct synthesis of hydrogen peroxide. ACS Appl. Mater. Interfaces 2020, 12, 6328–6335. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Kim, K.Y.; Nam, H.; Kim, H.; Yoon, J.; Lee, J.H.; Kim, H.K.; Ahn, J.P.; Lee, S.Y.; Lee, K.Y.; et al. Facile direct seed-mediated growth of AuPt bimetallic shell on the surface of Pd nanocubes and application for direct H2O2 synthesis. Catalysts 2020, 10, 650. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Jung, E.; Yoo, S.; Lim, T.; Kim, J.; Yu, T. Controlling the degree of coverage of the Pt shell in Pd@Pt core-shell nanocubes for methanol oxidation reaction. Catalysts 2020, 10, 1133. [Google Scholar] [CrossRef]
- Sheng, J.L.; Kang, J.H.; Ye, H.Q.; Xie, J.Q.; Zhao, B.; Fu, X.Z.; Yu, Y.; Sun, R.; Wong, C.P. Porous octahedral PdCu nanocages as highly efficient electrocatalysts for the methanol oxidation reaction. J. Mater. Chem. A 2018, 6, 3906–3912. [Google Scholar] [CrossRef]
- Xue, S.F.; Deng, W.T.; Yang, F.; Yang, J.L.; Amiinu, I.S.; He, D.P.; Tang, H.L.; Mu, S.C. Hexapod PtRuCu nanocrystalline alloy for highly efficient and stable methanol oxidation. ACS Catal. 2018, 8, 7578–7584. [Google Scholar] [CrossRef]
- Yang, P.P.; Yuan, X.L.; Hu, H.C.; Liu, Y.L.; Zheng, H.W.; Yang, D.; Chen, L.; Cao, M.H.; Xu, Y.; Min, Y.L.; et al. Solvothermal synthesis of alloyed PtNi colloidal nanocrystal clusters (CNCs) with enhanced catalytic activity for methanol oxidation. Adv. Funct. Mater. 2018, 28, 1704774. [Google Scholar] [CrossRef]
- Christian, M.; Aguey-Zinsou, K.F. Synthesis of core-shell NaBH4@M (M = Co, Cu, Fe, Ni, Sn) nanoparticles leading to various morphologies and hydrogen storage properties. Chem. Commun. 2013, 49, 6794–6796. [Google Scholar] [CrossRef]
- Ouyang, L.; Zhu, L.H.; Jiang, J.Z.; Tang, H.Q. A surface-enhanced Raman scattering method for detection of trace glutathione on the basis of immobilized silver nanoparticles and crystal violet probe. Anal. Chim. Acta 2014, 816, 41–49. [Google Scholar] [CrossRef]
- Xia, Y.N.; Gilroy, K.D.; Peng, H.C.; Xia, X.H. Seed-Mediated growth of colloidal metal nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 60–95. [Google Scholar] [CrossRef]
- Yan, M.; Jiang, Q.; Zhang, T.; Wang, J.; Yang, L.; Lu, Z.; He, H.; Fu, Y.; Wang, X.; Huang, H. Three-Dimensional low-defect carbon nanotube/nitrogen-doped graphene hybrid aerogel-supported Pt nanoparticles as efficient electrocatalysts toward the methanol oxidation reaction. J. Mater. Chem. A 2018, 6, 18165–18172. [Google Scholar] [CrossRef]
- Gao, N.; Wu, X.; Li, X.; Huang, J.; Li, D.; Yang, D.; Zhang, H. Facile synthesis of ternary PtPdCu alloy hexapods as highly efficient electrocatalysts for methanol oxidation. Nanoscale 2017, 9, 11077–11084. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.W.; Kim, M.; Han, S.W. One-Pot synthesis and electrocatalytic properties of Pd@Pt core-shell nanocrystals with tailored morphologies. Chem. Eur. J. 2014, 20, 7901–7905. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Viswanathan, B. ORR activity and direct ethanol fuel cell performance of carbon-supported Pt − M (M = Fe, Co, and Cr) alloys prepared by polyol reduction method. J. Phys. Chem. C 2009, 113, 18907–18913. [Google Scholar]
- Mazumder, V.; Chi, M.; More, K.L.; Sun, S. Core/Shell Pd/FePt Nanoparticles as an active and durable catalyst for the oxygen reduction reaction. J. Am. Chem. Soc. 2010, 132, 7848–7849. [Google Scholar] [CrossRef]
- Wang, H.; Ji, S.; Wang, W.; Linkov, V.; Pasupathi, S.; Wang, R. Pt decorated PdFe/C: Extremely high electrocatalytic activity for methanol oxidation. Int. J. Electrochem. Sci. 2012, 7, 3390–3398. [Google Scholar]
- Xu, C.; Li, Q.; Liu, Y.; Wang, J.; Geng, H. Hierarchical nanoporous PtFe alloy with multimodal size distributions and its catalytic performance toward methanol electrooxidation. Langmuir 2012, 28, 1886–1892. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Jung, E.; Yu, S.; Kim, H.; Kim, H.-K.; Lee, K.-Y.; Ahn, J.-P.; Lim, T.; Kim, J.; Yu, T. Facile Aqueous–Phase Synthesis of Pd–FePt Core–Shell Nanoparticles for Methanol Oxidation Reaction. Catalysts 2021, 11, 130. https://doi.org/10.3390/catal11010130
Xiao X, Jung E, Yu S, Kim H, Kim H-K, Lee K-Y, Ahn J-P, Lim T, Kim J, Yu T. Facile Aqueous–Phase Synthesis of Pd–FePt Core–Shell Nanoparticles for Methanol Oxidation Reaction. Catalysts. 2021; 11(1):130. https://doi.org/10.3390/catal11010130
Chicago/Turabian StyleXiao, Xiangyun, Euiyoung Jung, Sehyun Yu, Hyeonjin Kim, Hong-Kyu Kim, Kwan-Young Lee, Jae-Pyoung Ahn, Taeho Lim, Jinheung Kim, and Taekyung Yu. 2021. "Facile Aqueous–Phase Synthesis of Pd–FePt Core–Shell Nanoparticles for Methanol Oxidation Reaction" Catalysts 11, no. 1: 130. https://doi.org/10.3390/catal11010130
APA StyleXiao, X., Jung, E., Yu, S., Kim, H., Kim, H.-K., Lee, K.-Y., Ahn, J.-P., Lim, T., Kim, J., & Yu, T. (2021). Facile Aqueous–Phase Synthesis of Pd–FePt Core–Shell Nanoparticles for Methanol Oxidation Reaction. Catalysts, 11(1), 130. https://doi.org/10.3390/catal11010130







