Metal-Free Catalysts for the Polymerization of Alkynyl-Based Monomers
Abstract
:1. Introduction
2. Organobase-Catalysts for Alkyne-Based Polymerization
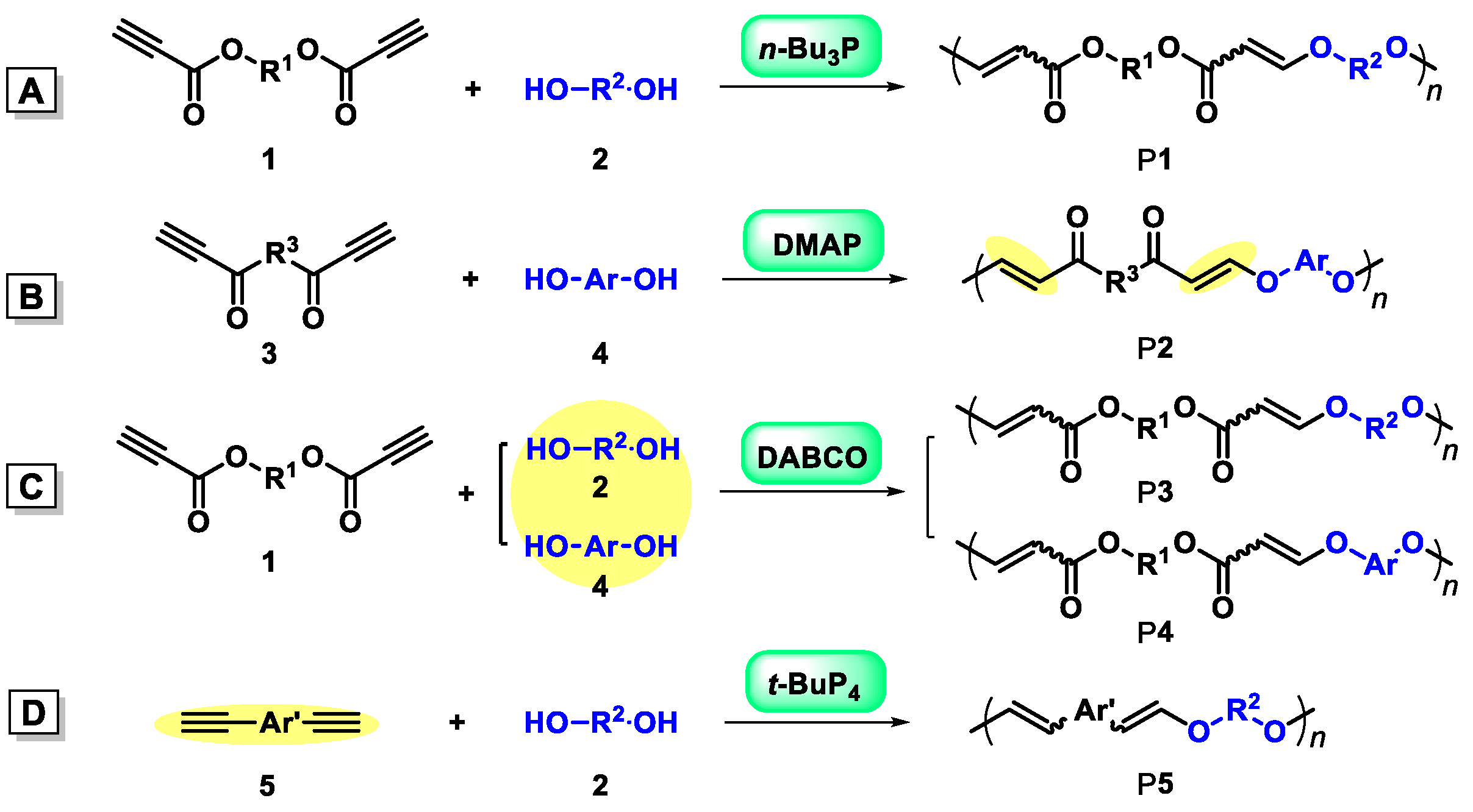
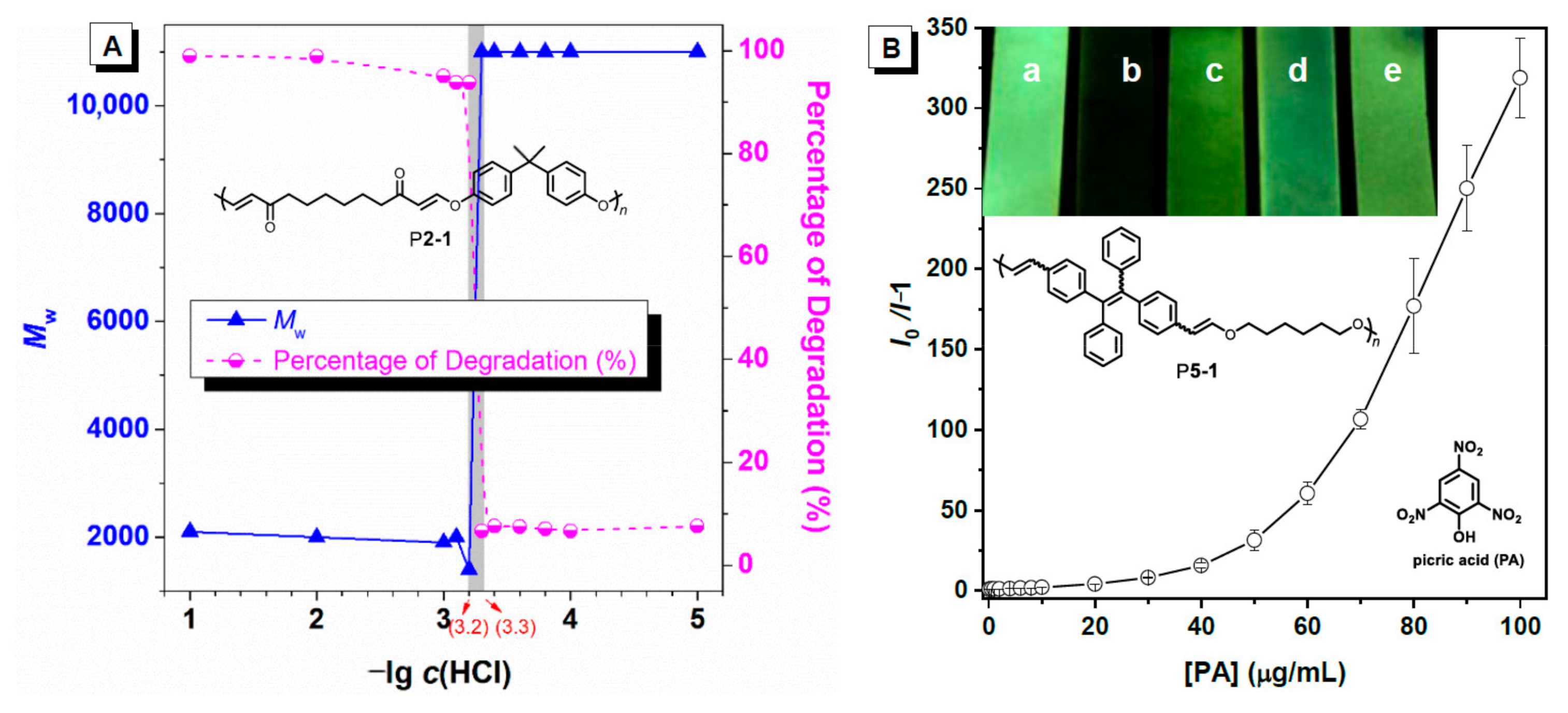
2.1. Organobase-Catalysts for Hydroxyl-yne Polymerization
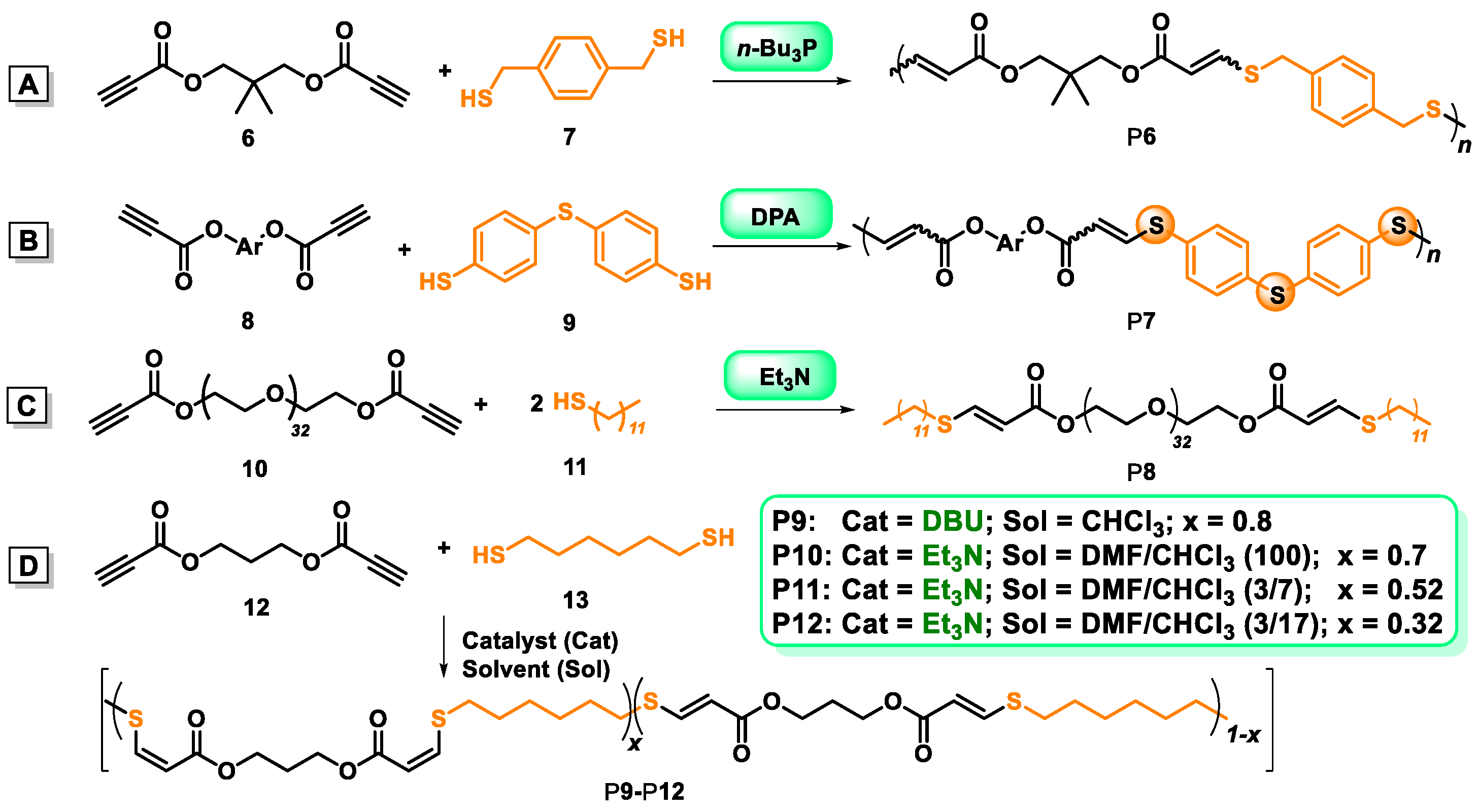
2.2. Organobase-Catalysts for Sulfydryl-yne Polymerization
2.3. Organobase-Catalysts for Alkyne-Azide Polymerization
3. Acid-Catalysts for Alkyne-Based Polymerization
4. Catalyst-Free Polymerization
4.1. Catalyst-Free Azide-Alkyne Polymerization
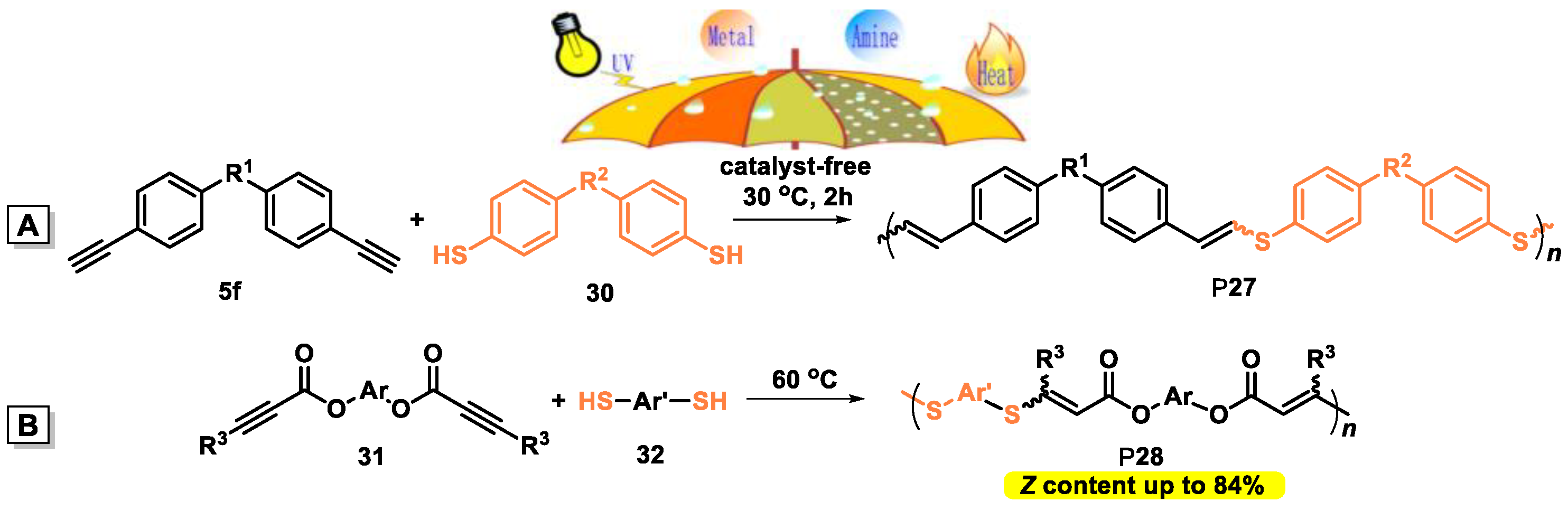
4.2. Catalyst-Free Thiol-yne Polymerization
4.3. Spontaneous Amino-yne Polymerization
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Sun, J.Z.; Tang, B.Z. Poly(disubstituted acetylene)s: Advances in polymer preparation and materials application. Prog. Polym. Sci. 2018, 79, 98–120. [Google Scholar] [CrossRef]
- Wang, K.; Amin, K.; An, Z.; Cai, Z.; Chen, H.; Chen, H.; Dong, Y.; Feng, X.; Fu, W.; Gu, J.; et al. Advanced functional polymer materials. Mater. Chem. Front. 2020, 4, 1803–1915. [Google Scholar] [CrossRef]
- Djéga-Mariadassou, G.; Boudart, M. Classical kinetics of catalytic reactions. J. Catal. 2003, 216, 89–97. [Google Scholar] [CrossRef]
- Qin, A.; Lam, J.W.Y.; Tang, B.Z. Click polymerization. Chem. Soc. Rev. 2010, 39, 2522–2544. [Google Scholar] [CrossRef] [Green Version]
- Qin, A.; Jim, C.K.W.; Lu, W.; Lam, J.W.Y.; Häussler, M.; Dong, Y.; Sung, H.H.Y.; Williams, I.D.; Wong, G.K.L.; Tang, B.Z. Click polymerization: Facile synthesis of functional poly(aroyltriazole)s by metal-free, regioselective 1,3-dipolar polycycloaddition. Macromolecules 2007, 40, 2308–2317. [Google Scholar] [CrossRef]
- Kuroda, H.; Tomita, I.; Endo, T. A novel polyaddition of diols with bifunctional acetylenes having electron-withdrawing groups. Macromolecules 1995, 28, 433–436. [Google Scholar] [CrossRef]
- Kuroda, H.; Tomita, I.; Endo, T. A novel polyaddition of bifunctional acetylenes containing electron-withdrawing groups. Iii. Synthesis of polymers having β-alkoxyenoate moieties by the reaction of internal diynes with diols. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 1597–1604. [Google Scholar] [CrossRef]
- Kuroda, H.; Tomita, I.; Endo, T. A novel polyaddition of bifunctional acetylenes containing electron-withdrawing groups: 4. Synthesis of polymers having enone moieties by the reaction of ynones with bifunctional heteronucleophiles. Polymer 1997, 38, 3655–3662. [Google Scholar] [CrossRef]
- Shi, Y.; Bai, T.; Bai, W.; Wang, Z.; Chen, M.; Yao, B.; Sun, J.Z.; Qin, A.; Ling, J.; Tang, B.Z. Phenol-yne click polymerization: An efficient technique to facilely access regio- and stereoregular poly(vinylene ether ketone)s. Chem.-Eur. J. 2017, 23, 10725–10731. [Google Scholar] [CrossRef]
- Zeng, X. Recent advances in catalytic sequential reactions involving hydroelement addition to carbon–carbon multiple bonds. Chem. Rev. 2013, 113, 6864–6900. [Google Scholar] [CrossRef]
- Oonishi, Y.; Gomez-Suarez, A.; Martin, A.R.; Nolan, S.P. Hydrophenoxylation of alkynes by cooperative gold catalysis. Angew. Chem. Int. Ed. Engl. 2013, 52, 9767–9771. [Google Scholar] [CrossRef]
- Wang, J.; Bai, T.; Chen, Y.; Ye, C.; Han, T.; Qin, A.; Ling, J.; Tang, B.Z. Palladium/Benzoic acid-catalyzed regio- and stereoselective polymerization of internal diynes and diols through C(sp3)–H activation. ACS Macro Lett. 2019, 8, 1068–1074. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.Z.; Qin, A.; Tang, B.Z. Transition-metal-free polymerization of bromoalkynes and phenols. Macromolecules 2019, 52, 2949–2955. [Google Scholar] [CrossRef]
- Si, H.; Wang, K.; Song, B.; Qin, A.; Tang, B.Z. Organobase-catalysed hydroxyl–yne click polymerization. Polym. Chem. 2020, 11, 2568–2575. [Google Scholar] [CrossRef]
- Schwesinger, R.; Hasenfratz, C.; Schlemper, H.; Walz, L.; Peters, E.-M.; Peters, K.; von Schnering, H.G. How strong and how hindered can uncharged phosphazene bases be? Angew. Chem. Int. Ed. Engl. 1993, 32, 1361–1363. [Google Scholar] [CrossRef]
- Schwesinger, R.; Schlemper, H. Peralkylated polyaminophosphazenes-extremely strong, neutral nitrogen bases. Angew. Chem. Int. Ed. Engl. 1987, 26, 1167–1169. [Google Scholar] [CrossRef]
- Kondo, Y. Superbases for Organic Synthesis: Guanidines, Amidines and Phosphazenes and Related Organocatalysts, 1st ed.; Ishikawa, T., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; Volume 5, p. 145. [Google Scholar]
- Wang, J.; Li, B.; Xin, D.; Hu, R.; Zhao, Z.; Qin, A.; Tang, B.Z. Superbase catalyzed regio-selective polyhydroalkoxylation of alkynes: A facile route towards functional poly(vinyl ether)s. Polym. Chem. 2017, 8, 2713–2722. [Google Scholar] [CrossRef]
- Takahashi, S.; Cohen, L.A.; Miller, H.K.; Peake, E.G. Calculation of the pka values of alcohols from .Sigma. Constants and from the carbonyl frequencies of their esters. J. Org. Chem 1971, 36, 1205–1209. [Google Scholar] [CrossRef]
- Kresge, A.J.; Pruszynski, P.; Stang, P.J.; Williamson, B.L. Base-catalyzed hydrogen exchange and estimates of the acid strength of benzoyl- and (trimethylsilyl)acetylene in aqueous solution. A correlation between acetylene pka estimates and hydroxide ion catalytic coefficients for hydrogen exchange. J. Org. Chem 1991, 56, 4808–4811. [Google Scholar] [CrossRef]
- Kuroda, H.; Tomita, I.; Endo, T. A novel polyaddition of bifunctional acetylenes containing electron-withdrawing groups. 2. Synthesis of polymers having .Beta.-alkylmercaptoenoate moieties by the reaction with dithiols. Macromolecules 1995, 28, 6020–6025. [Google Scholar] [CrossRef]
- Jim, C.K.W.; Qin, A.; Lam, J.W.Y.; Mahtab, F.; Yu, Y.; Tang, B.Z. Metal-free alkyne polyhydrothiolation: Synthesis of functional poly(vinylenesulfide)s with high stereoregularity by regioselective thioclick polymerization. Adv. Funct. Mater. 2010, 20, 1319–1328. [Google Scholar] [CrossRef] [Green Version]
- Truong, V.X.; Dove, A.P. Organocatalytic, regioselective nucleophilic “click” addition of thiols to propiolic acid esters for polymer–polymer coupling. Angew. Chem. Int. Ed. 2013, 52, 4132–4136. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.A.; Yu, J.; Barker, I.A.; Truong, V.X.; Cao, Z.; Dobrinyin, A.V.; Becker, M.L.; Dove, A.P. Independent control of elastomer properties through stereocontrolled synthesis. Angew. Chem. Int. Ed. 2016, 55, 13076–13080. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Huang, D.; Zhang, J.; Guo, S.; Hu, R.; Zhao, Z.; Qin, A.; Tang, B.Z. Synthesis of 1,5-regioregular polytriazoles by efficient nme4oh-mediated azide–alkyne click polymerization. Polym. Chem. 2015, 6, 5545–5549. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Nie, H.; Qin, A.; Tang, B.Z. Phosphazene base-mediated azide–alkyne click polymerization toward 1,5-regioregular polytriazoles. Macromolecules 2019, 52, 4713–4720. [Google Scholar] [CrossRef]
- Hu, R.; Li, W.; Tang, B.Z. Recent advances in alkyne-based multicomponent polymerizations. Macromol. Chem. Phys. 2016, 217, 213–224. [Google Scholar] [CrossRef]
- Wei, B.; Li, W.; Zhao, Z.; Qin, A.; Hu, R.; Tang, B.Z. Metal-free multicomponent tandem polymerizations of alkynes, amines, and formaldehyde toward structure- and sequence-controlled luminescent polyheterocycles. J. Am. Chem. Soc. 2017, 139, 5075–5084. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, P.; Wei, B.; Hu, R.; Tang, B.Z. Aggregation-induced emission-active hyperbranched poly(tetrahydropyrimidine) s synthesized from multicomponent tandem polymerization. Chin. J. Polym. Sci. 2019, 37, 428–436. [Google Scholar] [CrossRef]
- Hu, Y.; Han, T.; Yan, N.; Liu, J.; Liu, X.; Wang, W.-X.; Lam, J.W.Y.; Tang, B.Z. Visualization of biogenic amines and in vivo ratiometric mapping of intestinal ph by aie-active polyheterocycles synthesized by metal-free multicomponent polymerizations. Adv. Funct. Mater. 2019, 29, 1902240. [Google Scholar] [CrossRef]
- Zhang, Y.; Tseng, N.-W.; Deng, H.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Bcl3-mediated polycoupling of alkynes and aldehydes: A facile, metal-free multicomponent polymerization route to construct stereoregular functional polymers. Polym. Chem. 2016, 7, 4667–4674. [Google Scholar] [CrossRef]
- Han, T.; Zhang, Y.; He, B.; Lam, J.W.Y.; Tang, B.Z. Functional poly(dihalopentadiene)s: Stereoselective synthesis, aggregation-enhanced emission and sensitive detection of explosives. Polymers 2018, 10, 821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, W.; Dong, L.; Shi, J.; Tong, B.; Cai, Z.; Zhi, J.; Dong, Y. Synthesis of polyquinolines via one-pot polymerization of alkyne, aldehyde, and aniline under metal-free catalysis and their properties. Macromolecules 2018, 51, 3254–3263. [Google Scholar] [CrossRef]
- Dong, L.; Lan, T.; Liang, Y.; Guo, S.; Zhang, H. Metal-free [2+2+1] cycloaddition polymerization of alkynes, nitriles, and oxygen atoms to functional polyoxazoles. RSC Adv. 2020, 10, 24368–24373. [Google Scholar] [CrossRef]
- Huang, D.; Liu, Y.; Qin, A.; Tang, B.Z. Recent advances in alkyne-based click polymerizations. Polym. Chem. 2018, 9, 2853–2867. [Google Scholar] [CrossRef]
- Li, B.; Huang, D.; Qin, A.; Tang, B.Z. Progress on catalytic systems used in click polymerization. Macromol. Rapid Commun. 2018, 39, 1800098. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Tang, L.; Lam, J.W.Y.; Jim, C.K.W.; Yu, Y.; Zhao, H.; Sun, J.; Tang, B.Z. Metal-free click polymerization: Synthesis and photonic properties of poly(aroyltriazole)s. Adv. Funct. Mater. 2009, 19, 1891–1900. [Google Scholar] [CrossRef]
- Wei, Q.; Deng, H.; Cai, Y.; Lam, J.W.Y.; Li, J.; Sun, J.; Gao, M.; Qin, A.; Tang, B.Z. Efficient polymerization of azide and active internal alkynes. Macromol. Rapid Commun. 2012, 33, 1356–1361. [Google Scholar] [CrossRef]
- Li, H.; Mei, J.; Wang, J.; Zhang, S.; Zhao, Q.; Wei, Q.; Qin, A.; Sun, J.; Tang, B.Z. Facile synthesis of poly(aroxycarbonyltriazole)s with aggregation-induced emission characteristics by metal-free click polymerization. Sci. China Chem. 2011, 54, 611–616. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Sun, J.Z.; Hu, R.; Qin, A.; Tang, B.Z. Metal-free click polymerization of propiolates and azides: Facile synthesis of functional poly(aroxycarbonyltriazole)s. Polym. Chem. 2012, 3, 1075–1083. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wu, H.; Zhao, E.; Li, J.; Sun, J.Z.; Qin, A.; Tang, B.Z. Hyperbranched poly(aroxycarbonyltriazole)s: Metal-free click polymerization, light refraction, aggregation-induced emission, explosive detection, and fluorescent patterning. Macromolecules 2013, 46, 3907–3914. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Wu, H.; Lam, J.W.Y.; Sun, J.Z.; Qin, A.; Tang, B.Z. Ferrocene-based poly(aroxycarbonyltriazole)s: Synthesis by metal-free click polymerization and use as precursors to magnetic ceramics. Polym. Chem. 2013, 4, 5537–5541. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, Z.; Li, J.; Zhao, E.; Sun, J.Z.; Lam, J.W.Y.; Qin, A.; Tang, B.Z. Facile preparation of light refractive poly(aroxycarbonyltriazole)s by metal-free click polymerization. Macromol. Chem. Phys. 2014, 215, 1036–1041. [Google Scholar] [CrossRef]
- Wang, X.; Hu, R.; Zhao, Z.; Qin, A.; Tang, B.Z. Self-healing hyperbranched polytriazoles prepared by metal-free click polymerization of propiolate and azide monomers. Sci. China Chem. 2016, 59, 1554–1560. [Google Scholar] [CrossRef]
- Lang, M.-N.; Chi, W.-W.; Han, T.; Zhao, Q.-Z.; Li, H.-K.; Tang, B.Z.; Li, Y.-F. Synthesis of functional hyperbranched poly(methyltriazolylcarboxylate)s by catalyst-free click polymerization of butynoates and azides. Chin. J. Polym. Sci. 2020, 38, 1171–1177. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Wei, Q.; Sun, J.Z.; Wang, J.; Zhang, X.A.; Qin, A.; Tang, B.Z. Metal-free click polymerizations of activated azide and alkynes. Polym. Chem. 2013, 4, 1396–1401. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, M.; Yao, B.; Wang, J.; Mei, J.; Sun, J.Z.; Qin, A.; Tang, B.Z. A polytriazole synthesized by 1,3-dipolar polycycloaddition showing aggregation-enhanced emission and utility in explosive detection. Macromol. Rapid Commun. 2013, 34, 796–802. [Google Scholar] [CrossRef]
- Wu, Y.; He, B.; Quan, C.; Zheng, C.; Deng, H.; Hu, R.; Zhao, Z.; Huang, F.; Qin, A.; Tang, B.Z. Metal-free poly-cycloaddition of activated azide and alkynes toward multifunctional polytriazoles: Aggregation-induced emission, explosive detection, fluorescent patterning, and light refraction. Macromol. Rapid Commun. 2017, 38, 1700070. [Google Scholar] [CrossRef]
- Wu, Y.; He, B.; Wang, J.; Hu, R.; Zhao, Z.; Huang, F.; Qin, A.; Tang, B.Z. Efficient and regioselectivity-tunable metal-free polycycloaddition of activated azide and alkynes. Macromol. Rapid Commun. 2017, 38, 1600620. [Google Scholar] [CrossRef]
- Pathigoolla, A.; Gonnade, R.G.; Sureshan, K.M. Topochemical click reaction: Spontaneous self-stitching of a monosaccharide to linear oligomers through lattice-controlled azide–alkyne cycloaddition. Angew. Chem. Int. Ed. 2012, 51, 4362–4366. [Google Scholar] [CrossRef]
- Pathigoolla, A.; Sureshan, K.M. A crystal-to-crystal synthesis of triazolyl-linked polysaccharide. Angew. Chem. Int. Ed. 2013, 52, 8671–8675. [Google Scholar] [CrossRef]
- Pathigoolla, A.; Sureshan, K.M. Synthesis of triazole-linked homonucleoside polymers through topochemical azide–alkyne cycloaddition. Angew. Chem. Int. Ed. 2014, 53, 9522–9525. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, B.P.; Rai, R.; Asokan, A.; Sureshan, K.M. Crystal-to-crystal synthesis of triazole-linked pseudo-proteins via topochemical azide–alkyne cycloaddition reaction. J. Am. Chem. Soc. 2016, 138, 14824–14827. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, B.P.; Sureshan, K.M. Topochemical azide–alkyne cycloaddition reaction in gels: Size-tunable synthesis of triazole-linked polypeptides. J. Am. Chem. Soc. 2017, 139, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Konkolewicz, D.; Gray-Weale, A.; Perrier, S. Hyperbranched polymers by thiol−yne chemistry: From small molecules to functional polymers. J. Am. Chem. Soc. 2009, 131, 18075–18077. [Google Scholar] [CrossRef] [PubMed]
- Konkolewicz, D.; Gaillard, S.; West, A.G.; Cheng, Y.Y.; Gray-Weale, A.; Schmidt, T.W.; Nolan, S.P.; Perrier, S. Luminescent hyperbranched polymers: Combining thiol-yne chemistry with gold-mediated c−h bond activation. Organometallics 2011, 30, 1315–1318. [Google Scholar] [CrossRef]
- Barbey, R.; Perrier, S. A facile route to functional hyperbranched polymers by combining reversible addition–fragmentation chain transfer polymerization, thiol–yne chemistry, and postpolymerization modification strategies. ACS Macro Lett. 2013, 2, 366–370. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Poon, C.K.; Gray-Weale, A.; Perrier, S. Hyperbranched alternating block copolymers using thiol–yne chemistry: Materials with tuneable properties. Chem. Commun. 2011, 47, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dong, C.-M. Versatile strategy for the synthesis of hyperbranched poly(ε-caprolactone)s and polypseudorotaxanes thereof. Macromolecules 2010, 43, 8447–8455. [Google Scholar] [CrossRef]
- Cai, T.; Li, M.; Zhang, B.; Neoh, K.-G.; Kang, E.-T. Hyperbranched polycaprolactone-click-poly(n-vinylcaprolactam) amphiphilic copolymers and their applications as temperature-responsive membranes. J. Mater. Chem. B 2014, 2, 814–825. [Google Scholar] [CrossRef]
- Cook, A.B.; Barbey, R.; Burns, J.A.; Perrier, S. Hyperbranched polymers with high degrees of branching and low dispersity values: Pushing the limits of thiol–yne chemistry. Macromolecules 2016, 49, 1296–1304. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Zhao, B.; Gao, Y.; Tang, A.; Gao, C. Sequential click synthesis of hyperbranched polymersvia the A2 + CB2 approach. Polym. Chem. 2011, 2, 2175–2178. [Google Scholar] [CrossRef]
- Han, J.; Zheng, Y.; Zhao, B.; Li, S.; Zhang, Y.; Gao, C. Sequentially hetero-functional, topological polymers by step-growth thiol-yne approach. Sci. Rep. 2014, 4, 4387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pötzsch, R.; Komber, H.; Stahl, B.C.; Hawker, C.J.; Voit, B.I. Radical thiol-yne chemistry on diphenylacetylene: Selective and quantitative addition enabling the synthesis of hyperbranched poly(vinyl sulfide)s. Macromol. Rapid Commun. 2013, 34, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Pötzsch, R.; Stahl, B.C.; Komber, H.; Hawker, C.J.; Voit, B.I. High refractive index polyvinylsulfide materials prepared by selective radical mono-addition thiol–yne chemistry. Polym. Chem. 2014, 5, 2911–2921. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Pötzsch, R.; Komber, H.; Pospiech, D.; Voit, B. High refractive index hyperbranched polymers with different naphthalene contents prepared through thiol-yne click reaction using di-substituted asymmetric bulky alkynes. Polymer 2014, 55, 5600–5607. [Google Scholar] [CrossRef]
- Wei, Q.; Zan, X.; Qiu, X.; Öktem, G.; Sahre, K.; Kiriy, A.; Voit, B. High refractive index hyperbranched polymers prepared by two naphthalene-bearing monomers via thiol-yne reaction. Macromol. Chem. Phys. 2016, 217, 1977–1984. [Google Scholar] [CrossRef]
- Gazzo, S.; Manfredi, G.; Pötzsch, R.; Wei, Q.; Alloisio, M.; Voit, B.; Comoretto, D. High refractive index hyperbranched polyvinylsulfides for planar one-dimensional all-polymer photonic crystals. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 73–80. [Google Scholar] [CrossRef]
- Yao, B.; Mei, J.; Li, J.; Wang, J.; Wu, H.; Sun, J.Z.; Qin, A.; Tang, B.Z. Catalyst-free thiol–yne click polymerization: A powerful and facile tool for preparation of functional poly(vinylene sulfide)s. Macromolecules 2014, 47, 1325–1333. [Google Scholar] [CrossRef]
- Yao, B.; Hu, T.; Zhang, H.; Li, J.; Sun, J.Z.; Qin, A.; Tang, B.Z. Multi-functional hyperbranched poly(vinylene sulfide)s constructed via spontaneous thiol–yne click polymerization. Macromolecules 2015, 48, 7782–7791. [Google Scholar] [CrossRef]
- Alameddine, B.; Baig, N.; Shetty, S.; Al-Mousawi, S.; Al-Sagheer, F. Triptycene-containing poly(vinylene sulfone) derivatives from a metal-free thiol-yne click polymerization followed by a mild oxidation reaction. Polymer 2018, 154, 233–240. [Google Scholar] [CrossRef]
- Du, J.; Huang, D.; Li, H.; Qin, A.; Tang, B.Z.; Li, Y. Catalyst-free click polymerization of thiol and activated internal alkynes: A facile strategy toward functional poly(β-thioacrylate)s. Macromolecules 2020, 53, 4932–4941. [Google Scholar] [CrossRef]
- He, B.; Su, H.; Bai, T.; Wu, Y.; Li, S.; Gao, M.; Hu, R.; Zhao, Z.; Qin, A.; Ling, J.; et al. Spontaneous amino-yne click polymerization: A powerful tool toward regio- and stereospecific poly(β-aminoacrylate)s. J. Am. Chem. Soc. 2017, 139, 5437–5443. [Google Scholar] [CrossRef]
- He, B.; Zhen, S.; Wu, Y.; Hu, R.; Zhao, Z.; Qin, A.; Tang, B.Z. Cu(I)-Catalyzed amino-yne click polymerization. Polym. Chem. 2016, 7, 7375–7382. [Google Scholar] [CrossRef]
- He, B.; Zhang, J.; Zhang, H.; Liu, Z.; Zou, H.; Hu, R.; Qin, A.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Catalyst-free multicomponent tandem polymerizations of alkyne and amines toward nontraditional intrinsic luminescent poly(aminomaleimide)s. Macromolecules 2020, 53, 3756–3764. [Google Scholar] [CrossRef]
- Chen, X.; Hu, R.; Qi, C.; Fu, X.; Wang, J.; He, B.; Huang, D.; Qin, A.; Tang, B.Z. Ethynylsulfone-based spontaneous amino-yne click polymerization: A facile tool toward regio- and stereoregular dynamic polymers. Macromolecules 2019, 52, 4526–4533. [Google Scholar] [CrossRef]
- Chen, X.; Bai, T.; Hu, R.; Song, B.; Lu, L.; Ling, J.; Qin, A.; Tang, B.Z. Aroylacetylene-based amino-yne click polymerization toward nitrogen-containing polymers. Macromolecules 2020, 53, 2516–2525. [Google Scholar] [CrossRef]
- Hu, R.; Chen, X.; Zhou, T.; Si, H.; He, B.; Kwok, R.T.K.; Qin, A.; Tang, B.Z. Lab-in-cell based on spontaneous amino-yne click polymerization. Sci. China Chem. 2019, 62, 1198–1203. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Zhao, Z.; Qin, A.; Hu, R.; Tang, B.Z. Catalyst-free, atom-economic, multicomponent polymerizations of aromatic diynes, elemental sulfur, and aliphatic diamines toward luminescent polythioamides. Macromolecules 2015, 48, 7747–7754. [Google Scholar] [CrossRef]

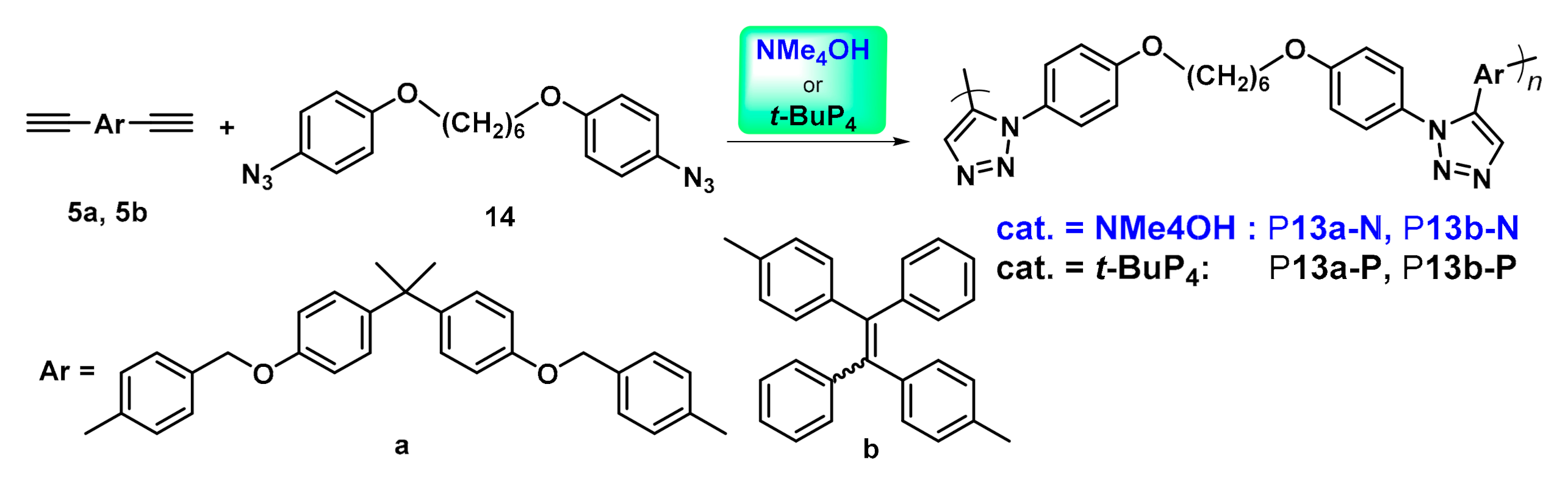
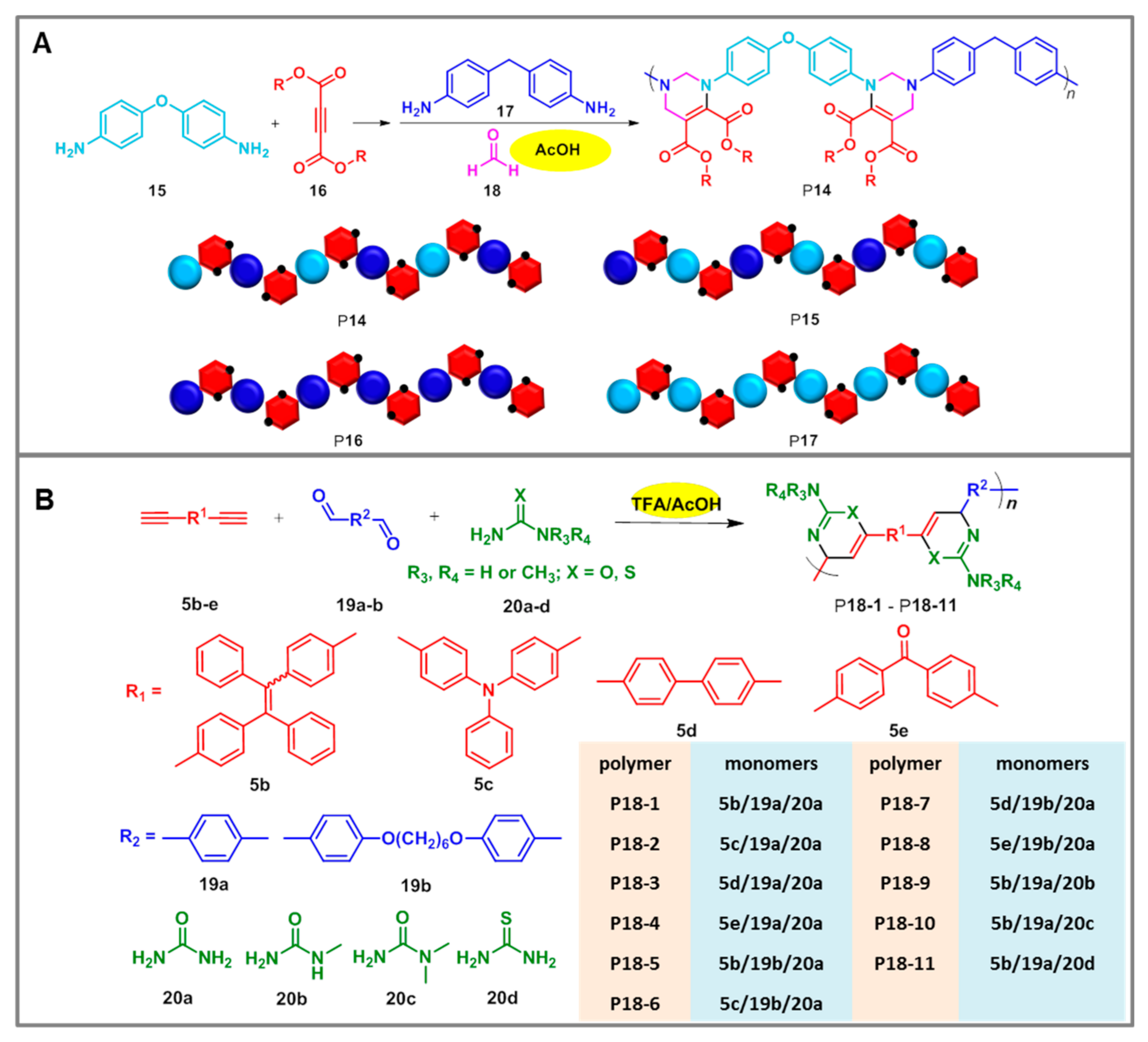
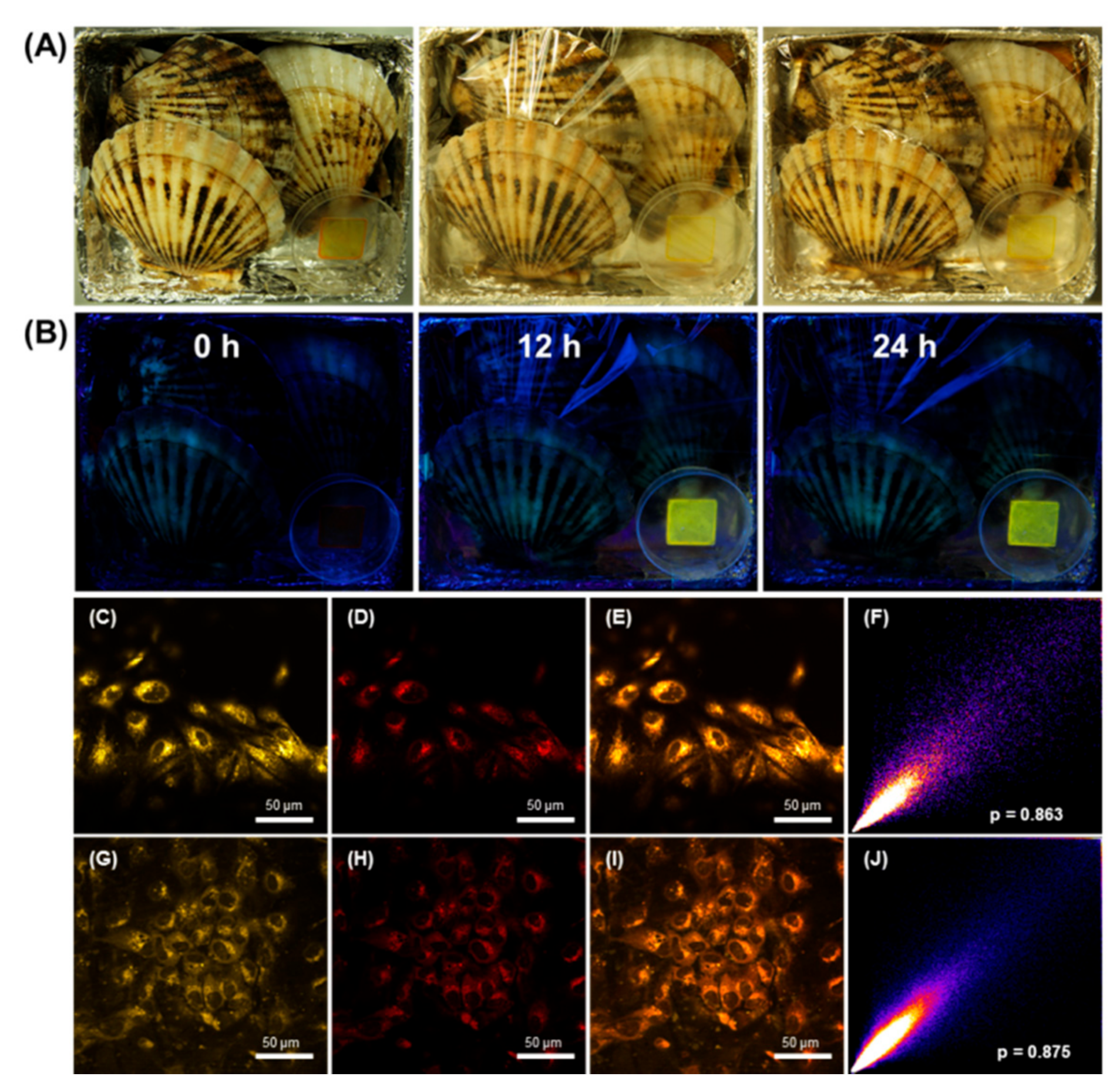

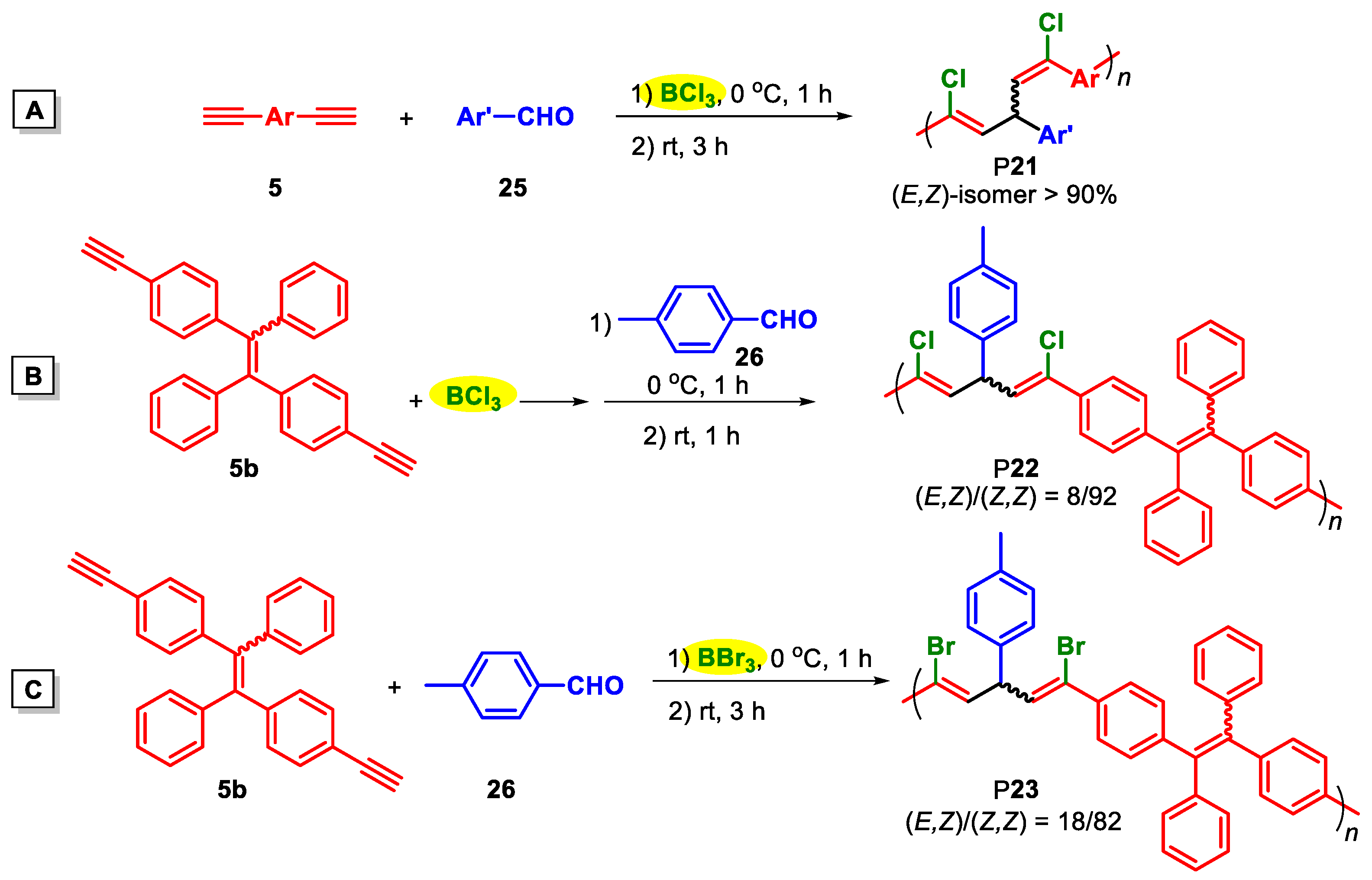
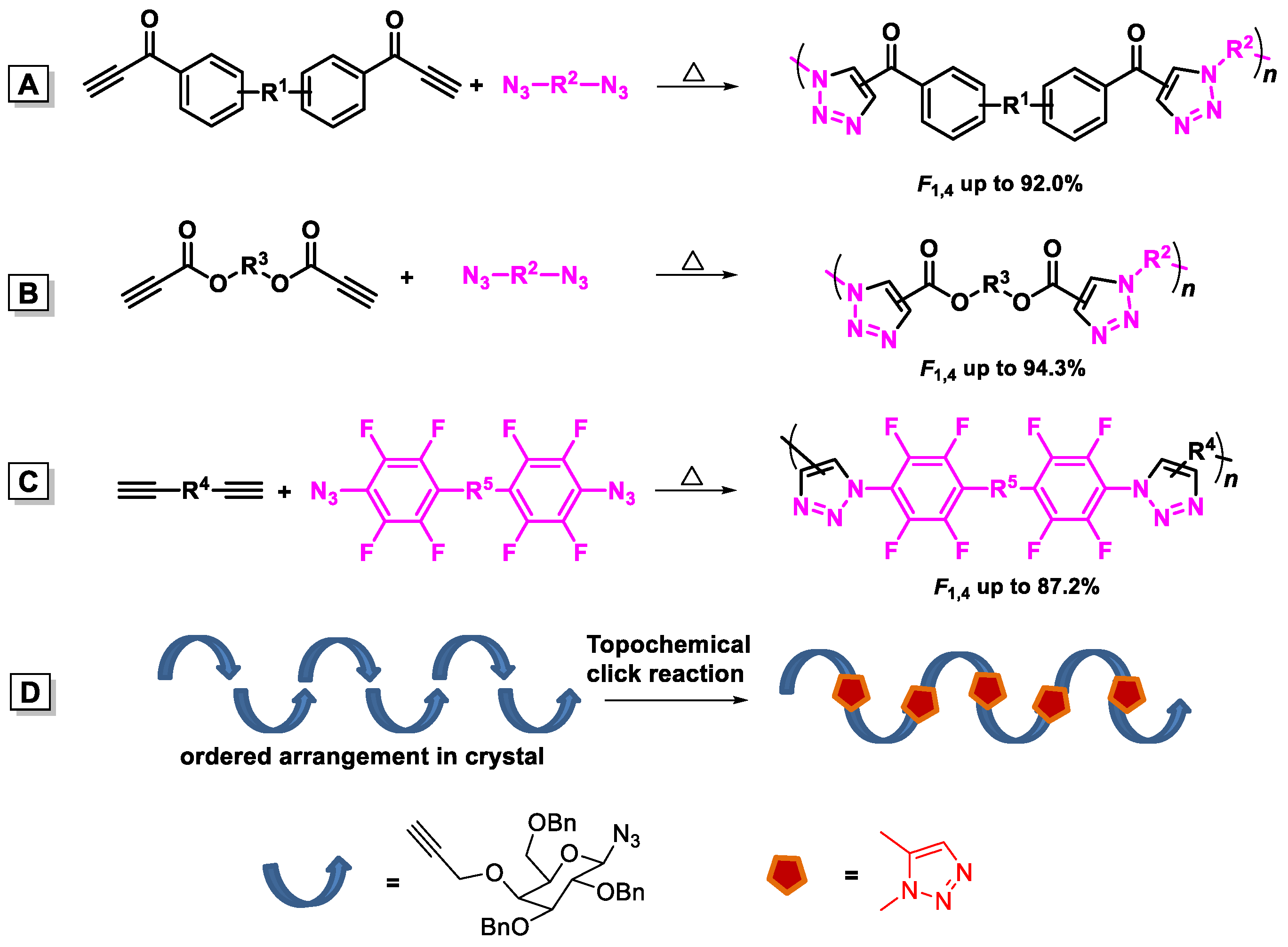



| Catalysts | Polymers | Monomers | T (°C) | [cat.]/[14] | Mw | PDI | Yield (%) | F1,5c (%) |
|---|---|---|---|---|---|---|---|---|
| NMe4OH | P10a-N | 9a + 10 | 25 | 1 | 44,000 a | 1.41 | 86 | 92 |
| t-BuP4 | P10a-P | 9a + 10 | 80 | 0.5 | 9600 b | 1.44 | 97 | 87 |
| NMe4OH | P10b-N | 9b + 10 | 25 | 1 | 40,300 a | 1.45 | 85 | 100 |
| t-BuP4 | P10a-P | 9b + 10 | 80 | 0.5 | 12,000 b | 1.43 | 99 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, Z.; Yang, F.; Zhang, H.; Sun, J.; Tang, B. Metal-Free Catalysts for the Polymerization of Alkynyl-Based Monomers. Catalysts 2021, 11, 1. https://doi.org/10.3390/catal11010001
Zhang J, Zhang Z, Yang F, Zhang H, Sun J, Tang B. Metal-Free Catalysts for the Polymerization of Alkynyl-Based Monomers. Catalysts. 2021; 11(1):1. https://doi.org/10.3390/catal11010001
Chicago/Turabian StyleZhang, Jie, Zhiming Zhang, Fulin Yang, Haoke Zhang, Jingzhi Sun, and Benzhong Tang. 2021. "Metal-Free Catalysts for the Polymerization of Alkynyl-Based Monomers" Catalysts 11, no. 1: 1. https://doi.org/10.3390/catal11010001
APA StyleZhang, J., Zhang, Z., Yang, F., Zhang, H., Sun, J., & Tang, B. (2021). Metal-Free Catalysts for the Polymerization of Alkynyl-Based Monomers. Catalysts, 11(1), 1. https://doi.org/10.3390/catal11010001






