Two-Stage Catalytic Abatement of N2O Emission in Nitric Acid Plants
Abstract
1. Introduction
2. Results
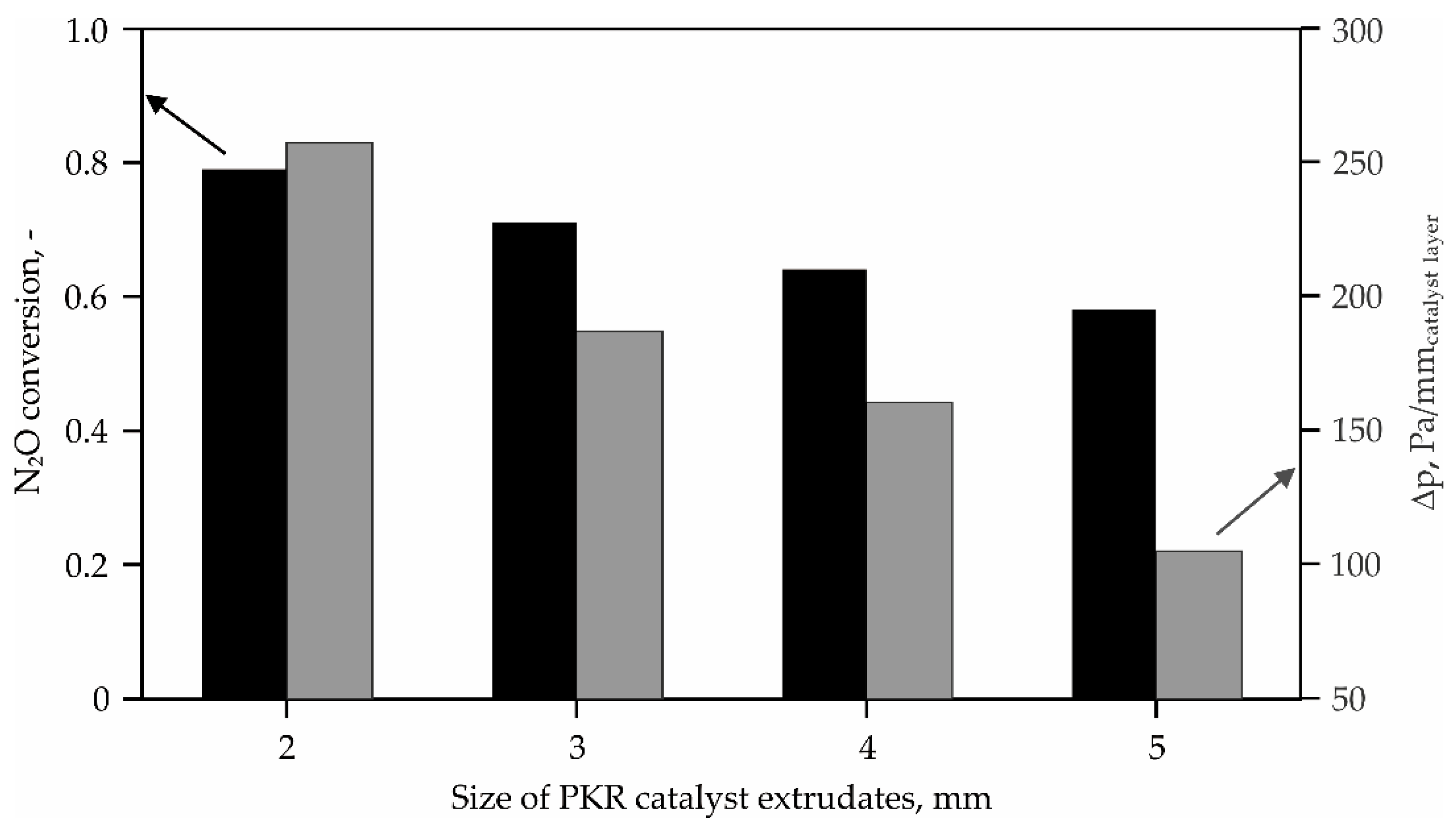
2.1. High-Temperature N2O Decomposition
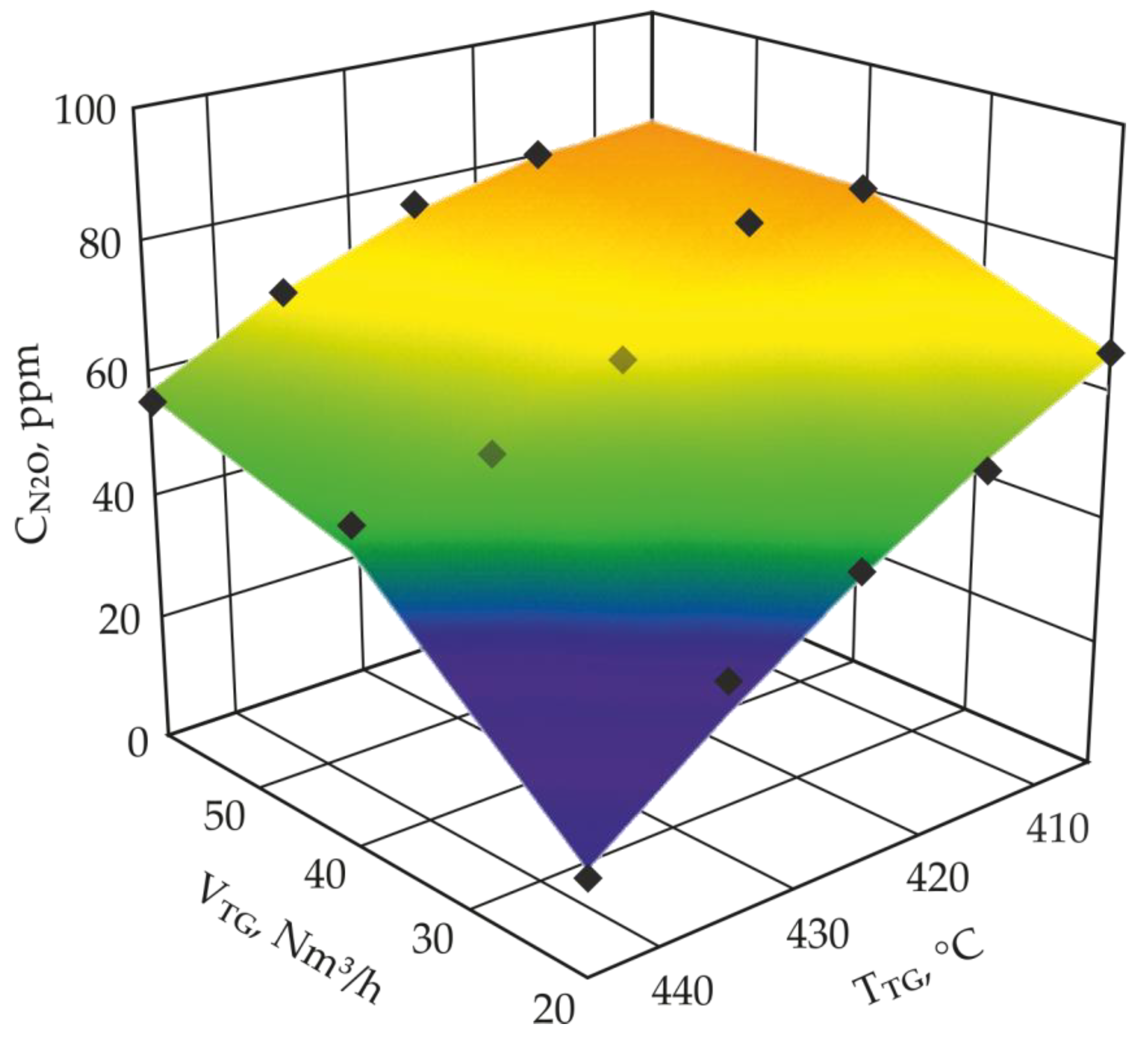
2.2. Low-Temperature N2O Decomposition
3. Discussion
4. Materials and Methods
4.1. Manufacturing of the Catalysts on a Large Scale
4.1.1. HT-deN2O Catalyst (PKR)
4.1.2. LT-deN2O Catalyst (K/Zn0.4Co2.6O4/α-Al2O3)
4.2. Activity Tests for PKR Catalyst in a Pilot Nitric Acid Plant
4.3. Activity Test of the LT-deN2O Catalyst
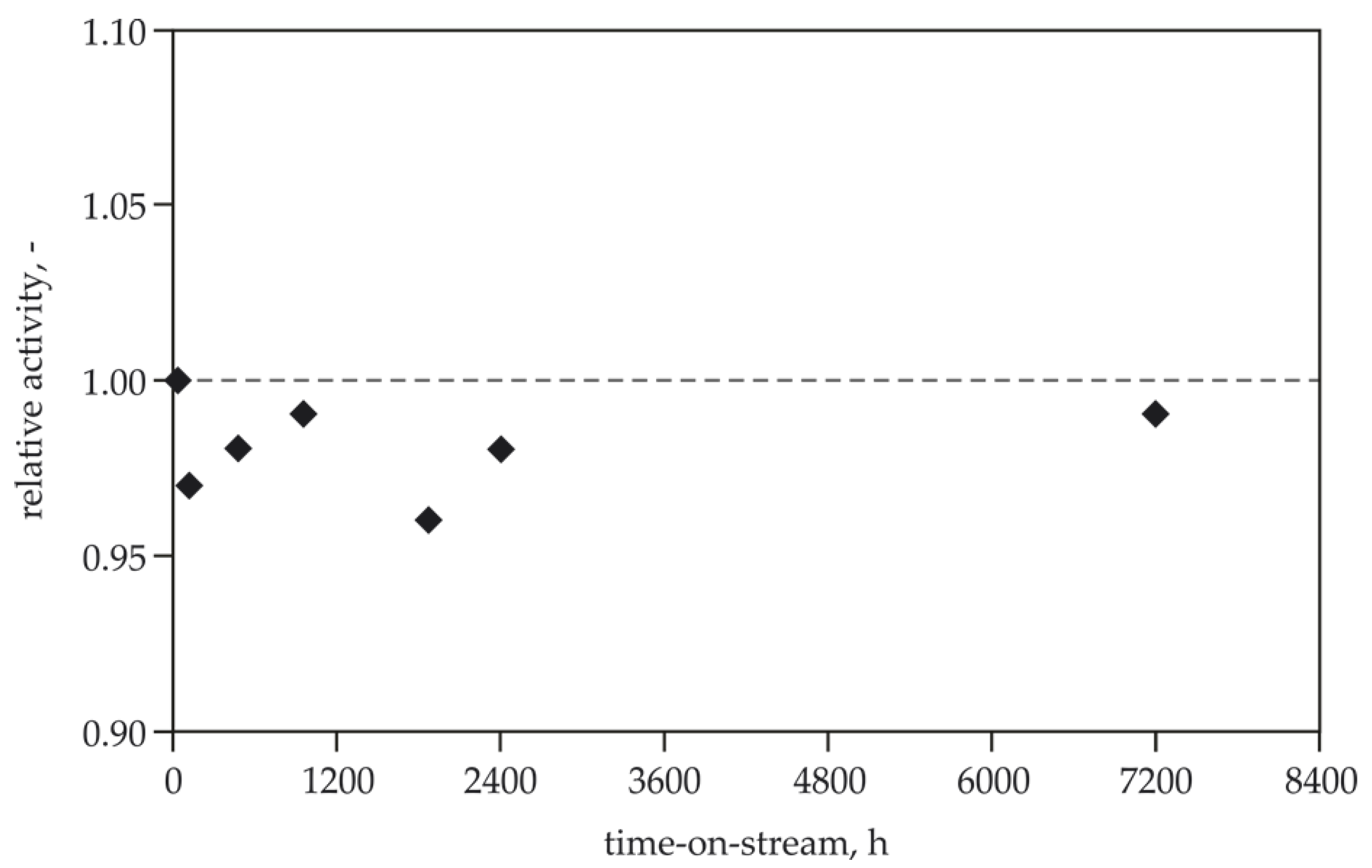
4.4. Stability Tests of the HT-deN2O and LT-deN2O Catalysts
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CN2O | concentration of N2O (mol·m−3 or ppm) |
| dext | extrudate diameter (mm) |
| E0 | activation energy (J/mol) |
| kgen | general reaction rate constant, first-order rate law (s−1) |
| k0 | Arrhenius pre-exponential factor (s−1) |
| p | operating pressure (barg) |
| R | gas constant (J·mol−1·K−1) |
| rcat | reaction rate per unit weight of catalyst (mol·kg−1·s−1) |
| XN2O | molar fraction of N2O (−) |
| T | temperature in Arrhenius equation (K) |
| TNG | nitrous gas temperature (°C) |
| TTG | tail gas temperature (°C) |
| V | catalyst bed volume (m3) |
| VNG | nitrous gases flow rate (Nm3·h−1) |
| VTG | tail gas flow rate (Nm3·h−1) |
| XN2O | N2O conversion |
| Greek symbols: | |
| ∆π | pressure drop (Pa/mmcatalyst layer) |
| τ | residence time (s) |
References
- Inger, M.; Dobrzyńska-Inger, A.; Rajewski, J.; Wilk, M. Optimization of ammonia oxidation using Response Surface Methodology. Catalysts 2019, 9, 249. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Kapteijn, F.; Schöffel, K.; Moulijn, J.A. Formation and control of N2O in nitric acid production: Where do we stand today? Appl. Catal. B Environ. 2003, 44, 117–151. [Google Scholar] [CrossRef]
- Isupova, L.A.; Ivanova, Y.A. Removal of nitrous oxide in nitric acid production. Kinet. Catal. 2019, 60, 744–760. [Google Scholar] [CrossRef]
- Wilk, M.; Inger, M.; Kruk, J.; Gołębiowski, A.; Jancewicz, R.; Szczepaniak, B. Obniżenie emisji podtlenku azotu z instalacji kwasu azotowego. Przem. Chem. 2009, 88, 730–733. [Google Scholar]
- Stefanova, M.; Chuturkova, R. Technical engineering for catalytic reduction of nitrous oxide emissions. J. Eng. Technol. 2015, 3, 89–94. [Google Scholar] [CrossRef]
- Konsolakis, M.I. Recent advances on nitrous oxide (N2O) decomposition over non-noble-metal oxide catalysts: Catalytic performance, mechanistic considerations, and surface chemistry aspects. ACS Catal. 2015, 5, 6397–6421. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Reduction of greenhouse gas emissions by catalytic processes. In Handbook of Climate Change Mitigation and Adaptation, 2nd ed.; Springer: Cham, Switzerland, 2016; Volume 4, pp. 2827–2880. [Google Scholar]
- Kim, M.-J.; Lee, S.J.; Ryu, I.-S.; Jeon, M.-W.; Moon, S.-H.; Roh, H.-S.; Jeon, S. Catalytic decomposition of N2O over cobalt based spinel oxides: The role of additives. Mol. Catal. 2017, 442, 202–207. [Google Scholar] [CrossRef]
- Granger, P.; Dacquin, J.-P.; Dujardin, C. Catalytic abatement of N2O from stationary sources. In Perovskites and Related Mixed Oxides: Concepts and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 611–630. [Google Scholar]
- Schoonheydt, R.; Vanelderen, P.; Sels, B. Direct catalytic decomposition of N2O over Cu-and Fe-zeolites. In New and Future Develelopments in Catalysis: Catalysis for Remediation and Environmental Concerns; Elsevier: Amsterdam, The Netherlands, 2013; pp. 399–419. [Google Scholar]
- Ohnishi, C.; Asano, K.; Iwamoto, S.; Chikama, K.; Inoue, M. Preparation of Co3O4 catalysts for direct decomposition of nitrous oxide under industrial conditions. Stud. Surf. Sci. Catal. 2006, 162, 737–744. [Google Scholar]
- Wójcik, S.; Grzybek, G.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Bulk, surface and interface promotion of Co3O4 for the low-temperature N2O decomposition catalysis. Catalysts 2020, 10, 41. [Google Scholar] [CrossRef]
- Zhao, T.; Li, Y.; Gao, Q.; Liu, Z.; Xu, X. Potassium promoted Y2O3-Co3O4 catalysts for N2O decomposition. Catal. Commun. 2020, 137, 105948. [Google Scholar] [CrossRef]
- Richards, N.; Carter, J.H.; Nowicka, E.; Parker, L.A.; Pattisson, S.; He, Q.; Dummer, N.F.; Golunski, S.; Hutchings, G.J. Structure-sensitivity of alumina supported palladium catalysts for N2O decomposition. Appl. Catal. B Environ. 2020, 264, 118501. [Google Scholar] [CrossRef]
- Hoang Ho, P.; Jabłońska, M.; Palkovits, R.; Rodriguea-Castellon, E.; Ospitali, F.; Formasari, G.; Vaccari, A.; Benito, P. N2O catalytic decomposition on electrodeposited Rh-based open-cell metallic foams. Chem. Eng. J. 2020, 379, 122259. [Google Scholar]
- Zhao, T.; Gao, Q.; Liao, W.; Xu, X. Effect of Nd-incorporation and K-modification on catalytic performance of Co3O4 for N2O decomposition. J. Fuel Chem. Technol. 2019, 47, 1120–1128. [Google Scholar] [CrossRef]
- Basahal, S.N.; Mokhtar, M.; Ali, T.T.; Narasimharao, K. Porous Fe2O3-ZrO2 and NiO-ZrO2 nanocomposites for catalytic N2O decomposition. Catal. Today 2020, 348, 166–176. [Google Scholar] [CrossRef]
- Pietrogiacomi, D.; Campa, M.C.; Carbone, L.R.; Occhiuzzi, M. N2O decomposition and reduction on Co-MOR, Fe-MOR and Ni-MOR catalysts: In situ UV–vis DRS and operando FTIR investigation. An insight on the reaction pathways. Appl. Catal. B Environ. 2019, 240, 19–29. [Google Scholar] [CrossRef]
- Bernauer, M.; Bernauer, B.; Sádovská, G.; Sobalík, Z. High-temperature decomposition of N2O from the HNO3 production: Process feasibility using a structured catalyst. Chem. Eng. Sci. 2020, 220, 115624. [Google Scholar] [CrossRef]
- Galejová, K.; Obalová, L.; Jirátová, K.; Pacultová, K.; Kovanda, F. N2O catalytic decomposition—Effect of pelleting pressure on activity of Co-Zn-Al mixed oxide catalyst. Chem. Pap. 2009, 63, 172–179. [Google Scholar] [CrossRef]
- Obalová, L.; Jirátová, K.; Karásková, K.; Chromčáková, Ž. N2O catalytic decomposition—From laboratory experiment to industry reactor. Catal. Tod. 2012, 191, 116–120. [Google Scholar] [CrossRef]
- Inger, M.; Wilk, M.; Saramok, M.; Grzybek, G.; Grodzka, A.; Stelmachowski, P.; Makowski, W.; Kotarba, A.; Sojka, Z. Cobalt spinel catalyst for N2O abatement in the pilot plant operation—Long-term activity and stability in tail gases. Ind. Eng. Chem. Res. 2014, 53, 10335–10342. [Google Scholar] [CrossRef]
- Obalová, L.; Klegova, A.; Matĕjová, L.; Pacultová, K.; Fridrichová, D. Must the best laboratory prepared catalyst also be the best in an operational application. Catalysts 2019, 9, 160. [Google Scholar]
- Melian-Cabrera, I.; Espinosa, S.; Mentruit, C.; Murray, B.; Falco, L.; Socci, J.; Kapteijn, F.; Moulijn, J.A. Overcoming the engineering constraints for scaling-up the state-of-the-art catalyst for tail-gas N2O decomposition. Ind. Eng. Chem. Res. 2018, 57, 939–945. [Google Scholar] [CrossRef]
- Axon, S.; Coupland, D.; Horner, B.; Ridland, J.; Wishart, I.; Johnson Matthey PLC. Improved Catalyst Charge Design. Patent WO 2004/096702 A2, 11 November 2004. [Google Scholar]
- Schumacher, V.; Bürger, G.; Fetzer, T.; Baier, M.; Hesse, M.; BASF. Method for the Catalytic Decomposition of N2O. Patent WO 99/55621, 4 November 1999. [Google Scholar]
- Baier, M.; Fetzer, T.; Hofstadt, O.; Hesse, M.; Buerger, G.; Harth, K.; Schumacher, V.; Wistuba, H.; Otto, B.; BASF. High-Temperature Stabile Catalysts for Decomposing N2O. Patent WO 0023176, 27 April 2000. [Google Scholar]
- Nirisen, O.; Schoeffel, K.; Waller, D.; Oevreboe, D.; Norsk Hydro, A.S. Catalyst for Decomposing Nitrous Oxide and Method for Performing Processes Comprising Formation of Nitrous Oxide. Patent WO/2002/002230, 10 January 2002. [Google Scholar]
- Neveu, B.; Hamon, C.; Grande Paroisse, S.A. Method for Reducing Nitrous Oxide in Gases and Corresponding Catalysts. Patent WO1999/64139 A1, 16 December 1999. [Google Scholar]
- Jantsh, U.; Lund, J.; Kraus, M.; Gorywoda, M.; Heraeus GMBH. Catalyst for Decomposition of Nitrous Oxide under Conditions of Ostwald Process, Comprises Carrier Material, and Coating of Rhodium, Rhodium Oxide, or Palladium-Rhodium Alloy. Patent DE 102004024026 A1, 29 September 2005. [Google Scholar]
- Wilk, M.; Kruk, J.; Gołębiowski, A.; Prokop, U.; Śpiewak, Z.; Saran, H.; Instytut Nawozów Sztucznych. Sposób Wytwarzania Katalizatora do Rozkładu Podtlenku Azotu, Zwłaszcza w Gazach z Instalacji Kwasu Azotowego. Patent PL206340, 19 December 2005. [Google Scholar]
- Wilk, M.; Kruk, J.; Gołębiowski, A.; Instytut Nawozów Sztucznych. Katalizator do Rozkładu Podtlenku Azotu, Zwłaszcza w Gazach z Instalacji Kwasu Azotowego. Patent PL207666, 19 December 2005. [Google Scholar]
- Burckhardt, W.; Seifert, F.; Voigt, M.; Winterstein, G.; Hermsdorfer Institut für Technische Keramik. Ceramic Catalyst for Selectively Decomposing N2O and a Method for the Production Thereof. Patent WO2000/13789, 27 July 2000. [Google Scholar]
- Schwefer, M.; Seifert, R.; Fuchs, J.; Ruthardt, K.; Groves, M.; Thyssenkrupp Industrial Solutions AG. Method for Removing N2O and NOx From the Nitric Acid Production Process, and an Installation Suitable for Same. Patent US20170334722, 23 November 2017. [Google Scholar]
- BASF Page. Available online: https://catalysts.basf.com/literature-library/stationary-emissions-catalysts/nitrous-oxide-destruction (accessed on 30 July 2020).
- Clariant Page. Available online: https://www.clariant.com/de/Solutions/Products/2019/07/22/08/03/EnviCat-N2O (accessed on 30 July 2020).
- Burghardt, A.; Bartelmus, G. Inżynieria Reaktorów Chemicznych; PWN: Warszawa, Poland, 2001. [Google Scholar]
- Inger, M.; Rajewski, J.; Ruszak, M.; Wilk, M. The influence of NOx presence on the catalytic N2O decomposition over the supported double-promoted cobalt spinel catalyst. Chem. Pap. 2019, 73, 1979–1986. [Google Scholar] [CrossRef]
- Giecko, G.; Borowiecki, T.; Gac, W.; Kruk, J. Fe2O3/Al2O3 catalysts for N2O decomposition in the nitric acid industry. Catal. Tod. 2008, 137, 403–409. [Google Scholar] [CrossRef]
- Kruk, J.; Stołecki, K.; Michalska, K.; Konkol, M.; Kowalik, P. The influence of modifiers on the activity of Fe2O3 catalyst for high temperature N2O decomposition (HT-deN2O). Catal. Tod. 2012, 191, 125–128. [Google Scholar] [CrossRef]
- Wilk, M.; Inger, M.; Ruszak, M.; Saramok, M.; Kowalik, P.; Antoniak-Jurak, K.; Sojka, Z.; Kotarba, A.; Grzybek, G.; Stelmachowski, P.; et al. Supported Co-Zn Spinel Catalyst for the Abatement of Nitrogen(I) Oxide Emissions Especially from Nitric Acid Plants and a Method for Its Manufacture. Patent EP 16000547, 3 March 2016. [Google Scholar]
- Grzybek, G.; Wójcik, S.; Legutko, P.; Gryboś, J.; Indyka, P.; Leszczyński, B.; Kotarba, A.; Sojka, Z. Thermal stability and repartition of potassium promoter between the support and active phase in the K-Co2.6Zn0.4O4/α-Al2O3 catalyst for N2O decomposition: Crucial role of activation temperature on catalytic performance. Appl. Catal. B Environ. 2017, 205, 597–604. [Google Scholar] [CrossRef]
- Ciura, K.; Grzybek, G.; Wójcik, S.; Indyka, P.; Kotarba, A.; Sojka, Z. Optimization of cesium and potassium promoter loading in alkali-doped Zn 0.4 Co2.6 O4/Al2 O3 catalysts for N2O abatement. React. Kinet. Mech. Catal. 2017, 121, 645–655. [Google Scholar] [CrossRef]
- Gołębiowski, A.; Kruk, J.; Wilk, M. Katalizator do wysokotemperaturowego rozkładu tlenku diazotu. Przem. Chem. 2006, 85, 709–711. [Google Scholar]



| Scenario 1 | Scenario 2 | Scenario 3 | Scenario 4 | Scenario 5 | Scenario 6 | |
|---|---|---|---|---|---|---|
| XN2O for HT-deN2O | 0.8 | 0.9 | 0.95 | 0.8 | 0.8 | 0.9 |
| XN2O for LT-deN2O | 0.8 | 0.9 | 0.9 | |||
| Vcat for HT-deN2O | V | 1.43 V | 1.875 V | V | V | 1.43 V |
| Vcat for LT-deN2O | V | 1.43 V | 1.43 V | |||
| CN2O outlet, ppm | 200 | 100 | 50 | 40 | 20 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inger, M.; Moszowski, B.; Ruszak, M.; Rajewski, J.; Wilk, M. Two-Stage Catalytic Abatement of N2O Emission in Nitric Acid Plants. Catalysts 2020, 10, 987. https://doi.org/10.3390/catal10090987
Inger M, Moszowski B, Ruszak M, Rajewski J, Wilk M. Two-Stage Catalytic Abatement of N2O Emission in Nitric Acid Plants. Catalysts. 2020; 10(9):987. https://doi.org/10.3390/catal10090987
Chicago/Turabian StyleInger, Marek, Bartosz Moszowski, Monika Ruszak, Jakub Rajewski, and Marcin Wilk. 2020. "Two-Stage Catalytic Abatement of N2O Emission in Nitric Acid Plants" Catalysts 10, no. 9: 987. https://doi.org/10.3390/catal10090987
APA StyleInger, M., Moszowski, B., Ruszak, M., Rajewski, J., & Wilk, M. (2020). Two-Stage Catalytic Abatement of N2O Emission in Nitric Acid Plants. Catalysts, 10(9), 987. https://doi.org/10.3390/catal10090987





