Examination of Photocatalyzed Chlorophenols for Sequential Photocatalytic-Biological Treatment Optimization
Abstract
:1. Introduction
2. Results and Discussion
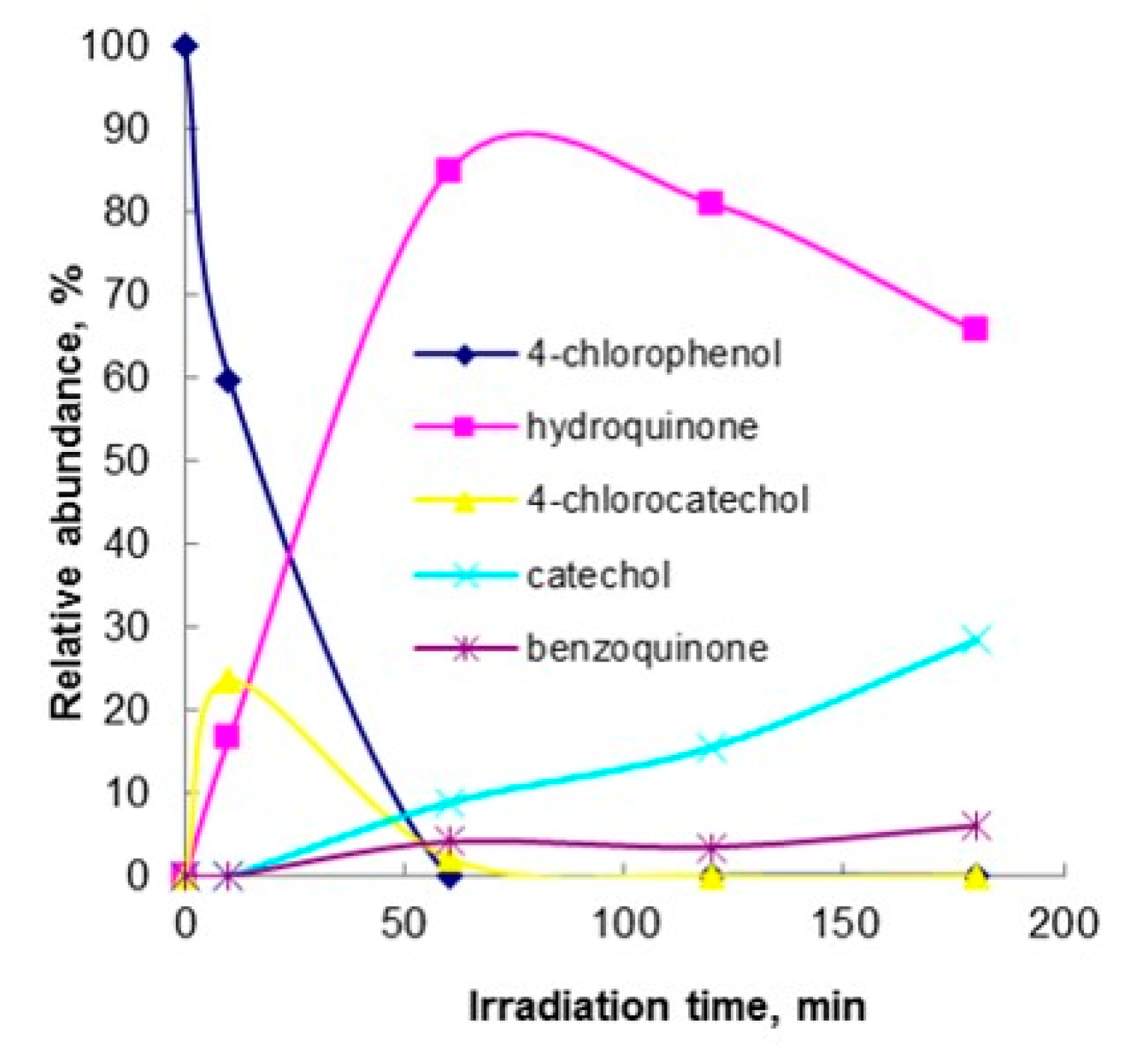
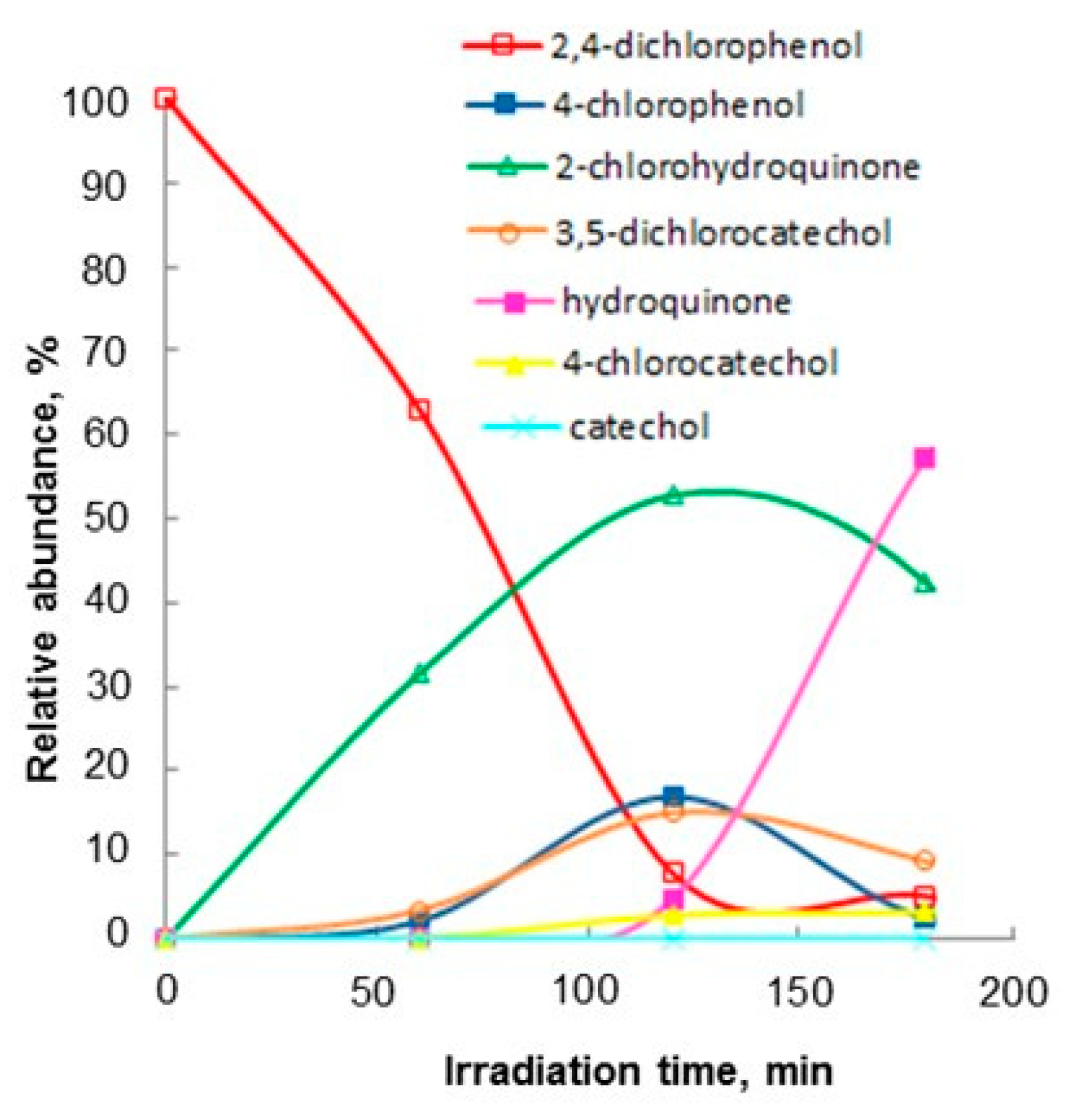
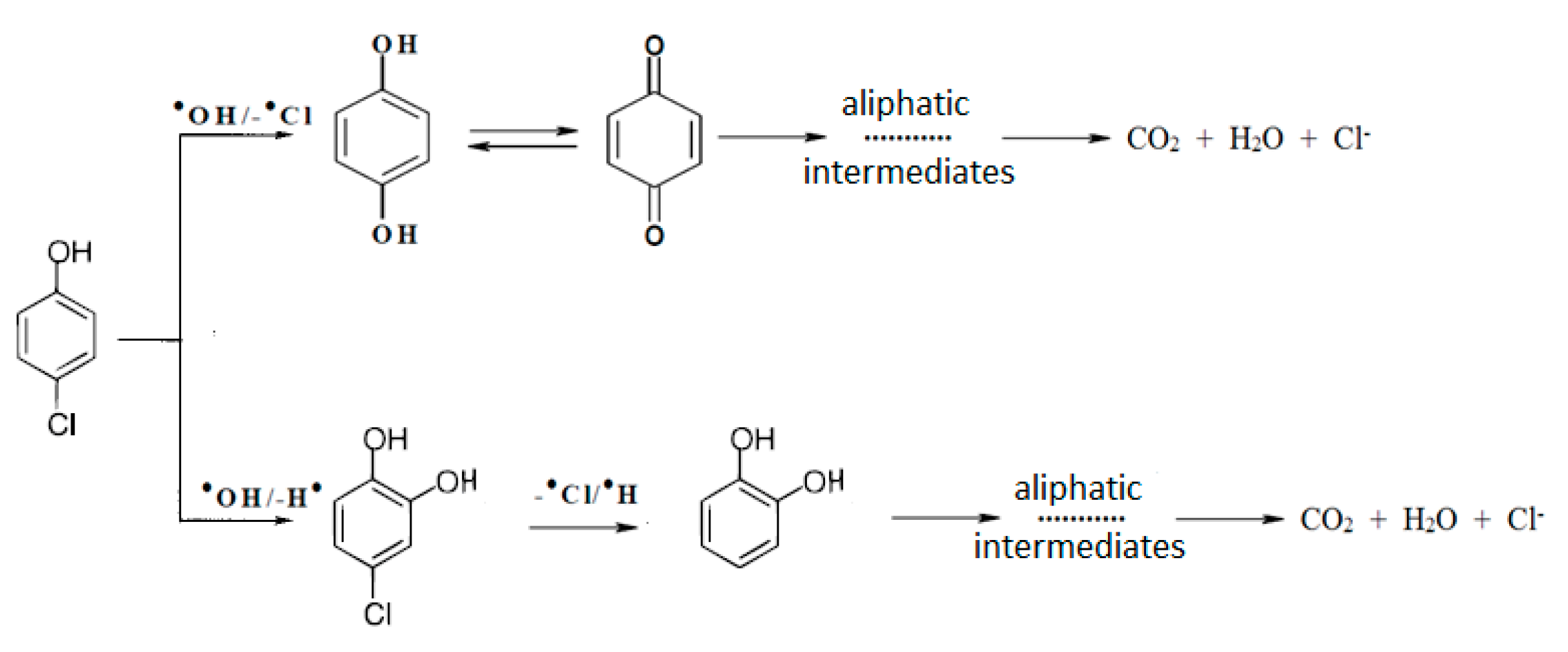
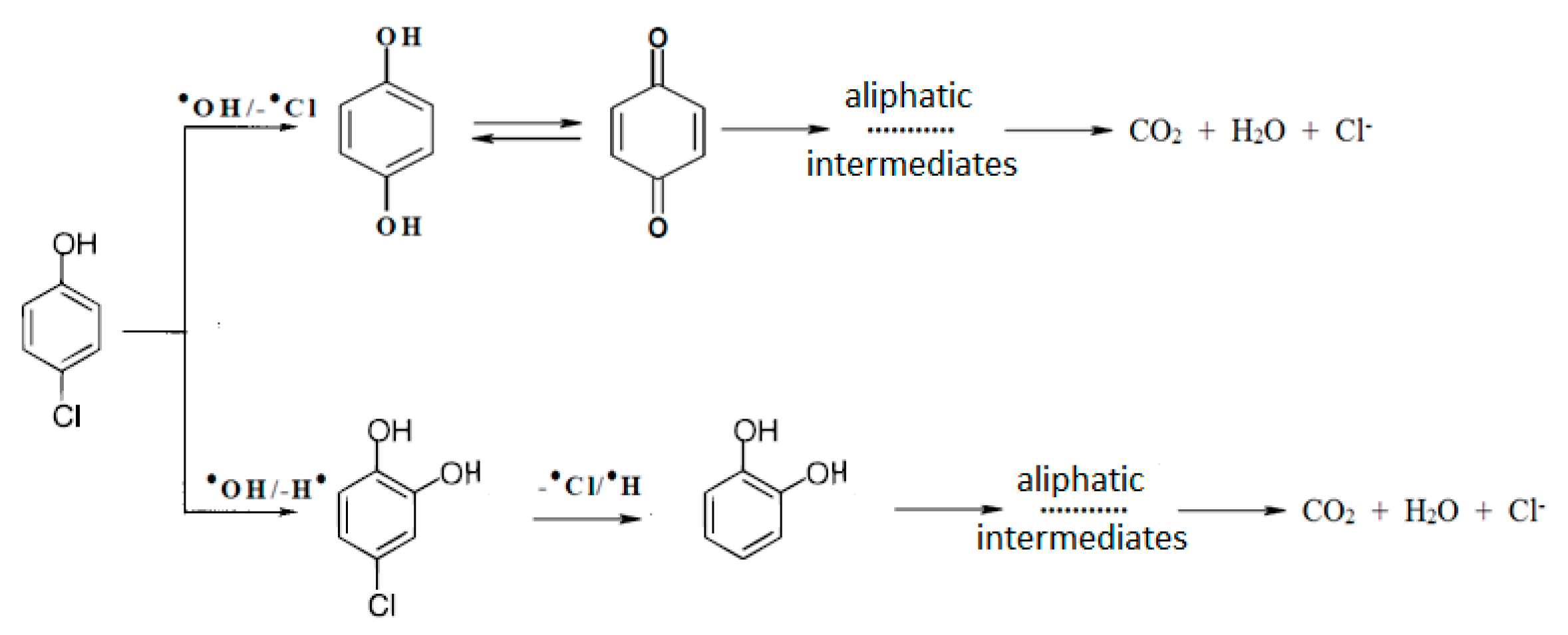
2.1. Photocatalytic Oxidation
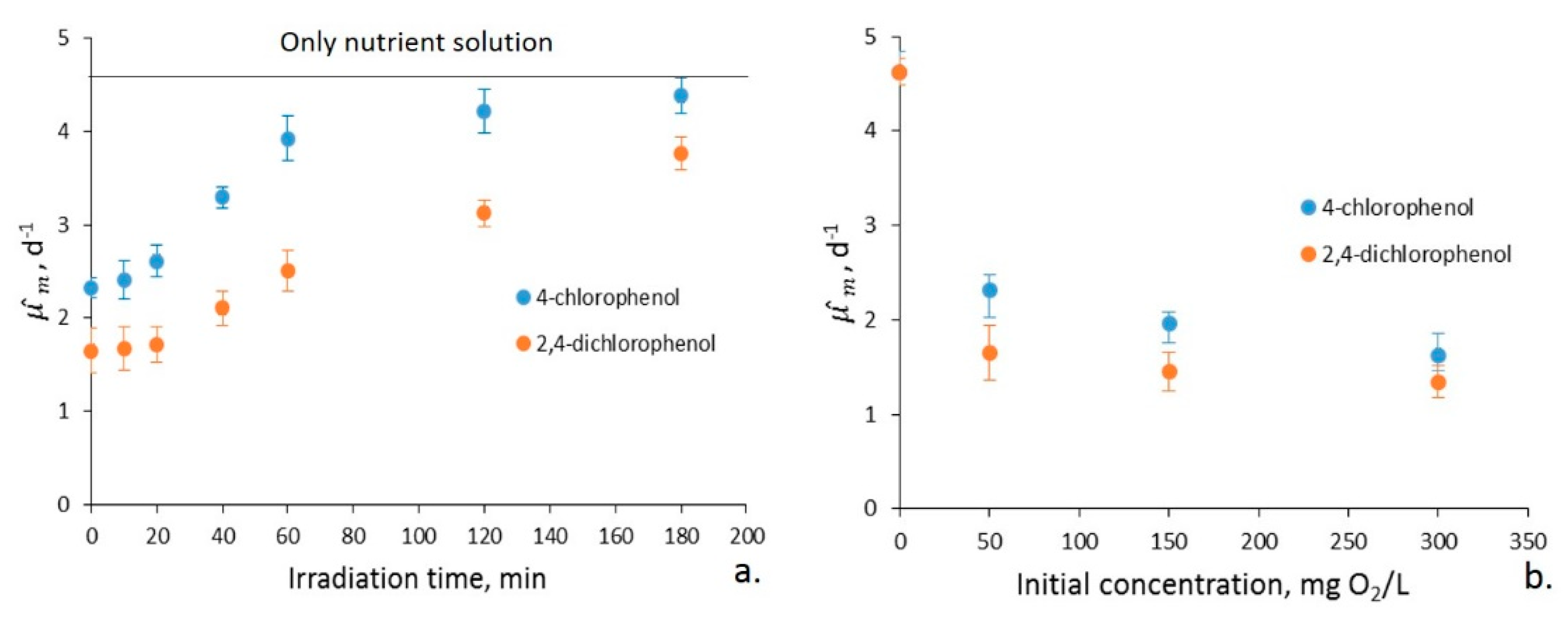
2.2. Biodegradability of Photocatalyzed Chlorophenols
3. Materials and Methods
3.1. Chemicals and Reagents
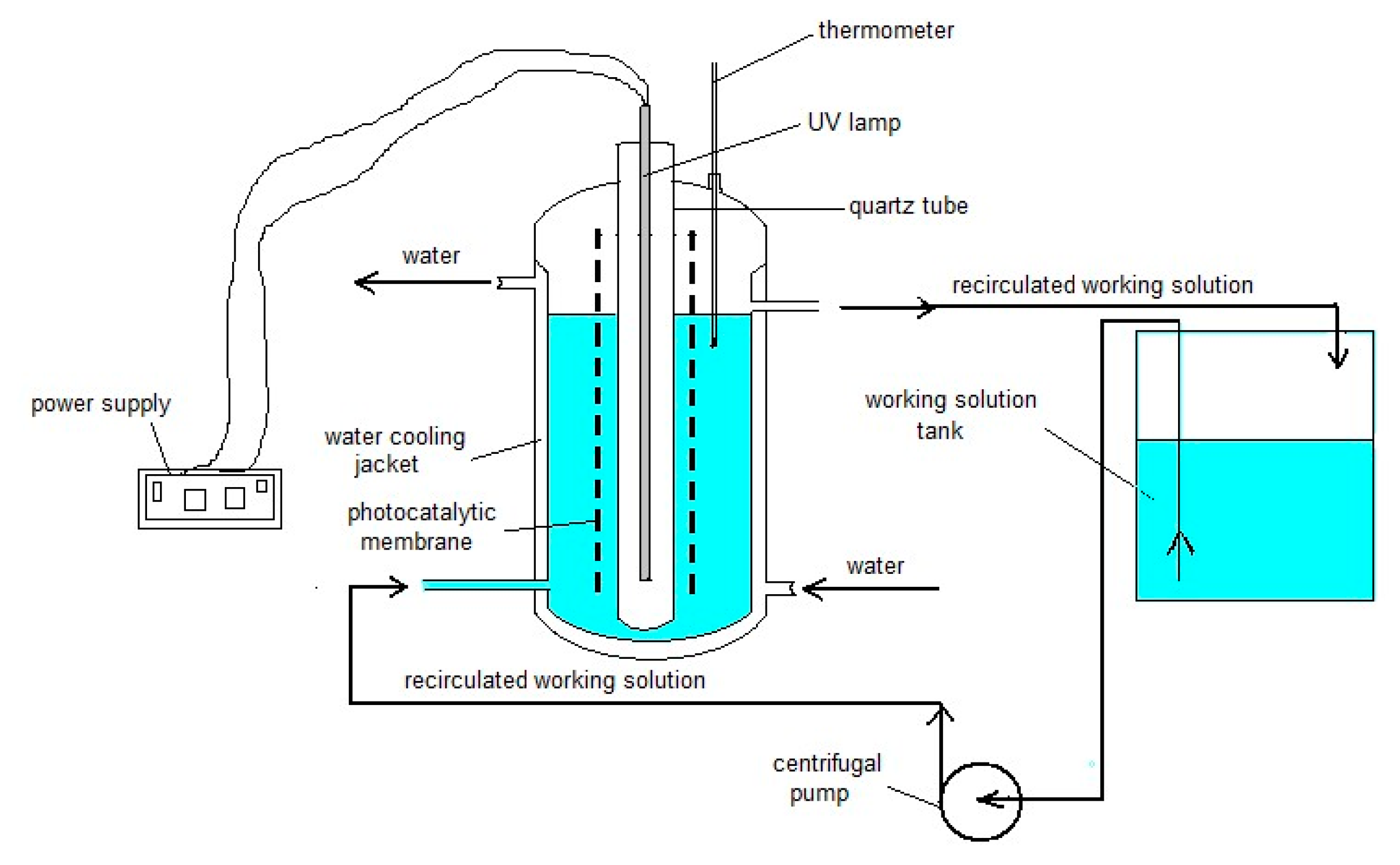
3.2. Photocatalytic Oxidation Experiments
3.3. Biodegradability Experiments
3.4. Identification of Photocatalytic Oxidation Intermediates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Słomkiewicz, P.; Szczepanik, B.; Czaplicka, M. Adsorption of phenol and chlorophenols by HDTMA modified halloysite nanotubes. Materials 2020, 13, 3309. [Google Scholar] [CrossRef] [PubMed]
- Olaniran, A.O.; Igbinosa, E.O. Chlorophenols and other related derivatives of environmental concern: Properties, distribution and microbial degradation processes. Chemosphere 2011, 83, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, X.; Wang, L.; Xu, Y.; Li, J.; Li, Y. Effects of elevated 4-chlorophenol loads on components of polysaccharides and proteins and toxicity in an activated sludge process. Chem. Eng. J. 2017, 330, 236–244. [Google Scholar] [CrossRef]
- Garba, Z.N.; Zhou, W.; Lawan, I.; Xiao, W.; Zhang, M.; Wang, L.; Chen, L.; Yuan, Z. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: A review. J. Environ. Manag. 2019, 241, 59–75. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Descorme, C. Catalytic wastewater treatment: Oxidation and reduction processes. Recent studies on chlorophenols. Catal. Today 2017, 297, 324–334. [Google Scholar] [CrossRef]
- Melchor-Lagar, V.; Ramos-Ramírez, E.; Morales-Pérez, A.-A.; Rangel-Vázquez, I.; Angel, G.D. Photocatalytic removal of 4-chlorophenol present in water using ZrO2/LDH under UV light source. J. Photochem. Photobiol. A Chem. 2020, 389, 112251. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Fresno, F.; Lefevre, C.; Robert, D.; Keller, N. Highly robust La1-xTixFeO3 dual catalyst with combined photocatalytic and photo-CWPO activity under visible light for 4-chlorophenol removal in water. Appl. Catal. B Environ. 2020, 262, 118310. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, Q.; Zhang, X.; Zhang, G.; Chen, X.; Zhao, C. A novel tubular up-flow magnetic film photocatalytic system optimized by main factors control for efficient removal of chlorophenols wastewater. J. Hazard. Mater. 2020, 398, 122963. [Google Scholar] [CrossRef]
- Zouzelka, R.; Remzova, M.; Plsek, J.; Brabec, L.; Rathousky, J. Immobilized rGO/TiO2 Photocatalyst for Decontamination of Water. Catalysts 2019, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- Ismael, M.; Wark, M. Perovskite-type LaFeO3: Photoelectrochemical properties and photocatalytic degradation of organic pollutants under visible light irradiation. Catalysts 2019, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Kargi, F.; Konya, I. COD, para-chlorophenol and toxicity removal from para-chlorophenol containing synthetic wastewater in an activated sludge unit. J. Hazard. Mater. 2006, B132, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Goi, A.; Trapido, M.; Tuhkanen, T. A study of toxicity, biodegradability, and some by products of ozonised nitrophenols. Adv. Environ. Res. 2004, 8, 304–311. [Google Scholar] [CrossRef]
- Ettala, M.; Koskela, J.; Kiesilä, A. Removal of chlorophenols in a municipal sewage treatment plant using activated sludge. Water Res. 1992, 26, 797–804. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Dilek, F.B. Effects of 2,4-dichlorophenol on activated sludge. Appl. Microbiol. Biotechnol. 2002, 59, 361–367. [Google Scholar] [PubMed]
- Fang, F.; Wang, S.-N.; Li, K.-Y.; Dong, J.-Y.; Xu, R.-Z.; Zhang, L.-L.; Xie, W.-M.; Cao, J.-S. Formation of microbial products by activated sludge in the presence of a metabolic uncoupler o-chlorophenol in long-term operated sequencing batch reactors. J. Hazard. Mater. 2020, 384, 121311. [Google Scholar] [CrossRef]
- Konya, I.; Eker, S.; Kargi, F. Mathematical modelling of 4-chlorophenol inhibition on COD and 4-chlorophenol removals in an activated sludge unit. J. Hazard. Mater. 2007, 143, 233–239. [Google Scholar] [CrossRef]
- Aken, P.V.; Lambert, N.; Van den Broeck, R.; Degrève, J.; Dewil, R. Advances in ozonation and biodegradation processes to enhance chlorophenol abatement in multisubstrate wastewaters: A review. Environ. Sci. Water Res. Technol. 2019, 5, 444–481. [Google Scholar] [CrossRef]
- Goel, M.; Chovelon, J.-M.; Ferronato, C.; Bayard, R.; Sreekrishnan, T.R. The remediation of wastewater containing 4-chlorophenol using integrated photocatalytic and biological treatment. J. Photochem. Photobiol. B Biol. 2010, 98, 1–6. [Google Scholar] [CrossRef]
- Suryaman, D.; Hasegawa, K. Biological and photocatalytic treatment integrated with separation and reuse of titanium dioxide on the removal of chlorophenols in tap water. J. Hazard. Mater. 2010, 183, 490–496. [Google Scholar] [CrossRef]
- Naya, S.-i.; Nikawa, T.; Kimura, K.; Tada, H. Rapid and complete removal of nonylphenol by gold nanoparticle/rutile titanium(IV) oxide plasmon photocatalyst. ACS Catal. 2013, 3, 903–907. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shen, H.; Shuai, D. Simultaneous coupling of photocatalytic and biological processes: A promising synergistic alternative for enhancing decontamination of recalcitrant compounds in water. Chem. Eng. J. 2020, 126365, in press. [Google Scholar] [CrossRef]
- Rao, N.N.; Dubey, A.K.; Mohanty, S.; Khare, P.; Jain, R.; Kaul, S.N. Photocatalytic degradation of 2-chlorophenol: A study of kinetics, intermediates and biodegradability. J. Hazard. Mater. 2003, B101, 301–314. [Google Scholar] [CrossRef]
- Lindgaard-Jørgensen, P. Biodegradability of chlorophenols and mixtures of chlorophenols in seawater. Ecotoxicol. Environ. Saf. 1989, 17, 216–220. [Google Scholar] [CrossRef]
- Annachhatre, A.P.; Gheewala, S.H. Biodegradation of chlorinated phenolic compounds. Biotechnol. Adv. 1996, 14, 35–56. [Google Scholar] [CrossRef]
- Untea, I.; Orbeci, C.; Tudorache, E. Oxidative degradation of 4-chlorophenol from aqueous solution by photo-fenton advanced oxidation process. Environ. Eng. Manag. J. 2006, 5, 661–674. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Photocatalytic degradation of chlorophenols under direct solar radiation in the presence of ZnO catalyst. Res. Chem. Intermed. 2013, 39, 1981–1996. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Yang, J.; Cui, S.; Qiao, J.-Q.; Lian, H.-Z. The photocatalytic dehalogenation of chlorophenols and bromophenols by cobalt doped nano TiO2. J. Mol. Catal. A Chem. 2014, 395, 42–51. [Google Scholar] [CrossRef]
- Liu, A.-L.; Li, Z.-Q.; Wu, Z.-Q.; Xia, X.-H. Study on the photocatalytic reaction kinetics in a TiO2 nanoparticles coated microreactor integrated microfluidics device. Talanta 2018, 182, 544–548. [Google Scholar] [CrossRef]
- Yang, D.-M.; Yuan, J.-M. COD and color removal from real dyeing wastewater by ozonation. Water Environ. Res. 2016, 88, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Andrio, D.; Asmura, J.; Yenie, E.; Putri, K. Enhancing BOD5/COD ratio co-substrate tofu wastewater and cow dung during ozone pretreatment. MATEC Web Conf. 2019, 276, 06027. [Google Scholar] [CrossRef] [Green Version]
- Battistoni, P.; Fava, G.; Ruello, M.L. Heavy metal shock load in activated sludge uptake and toxic effects. Water Res. 1993, 27, 821–882. [Google Scholar] [CrossRef]
- Kumar, P.; Nemati, M.; Hill, G.A. Biodegradation kinetics of 1,4-benzoquinone in batch and continuous systems. Biodegradation 2011, 22, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Ekama, G.A.; Dold, P.L.; Marais, G.V.R. Procedures for determining influent cod fractions and the maximum specific growth rate of heterotrophs in activated sludge systems. Water Sci. Technol. 1986, 18, 91–114. [Google Scholar] [CrossRef]
- Ekama, G.A.; Wentzel, M.C.; Sötemann, S.W. Mass balance-based plant-wide wastewater treatment plant models—Part 2: Tracking the influent inorganic suspended solids. Water SA 2006, 32, 277–285. [Google Scholar] [CrossRef]
- Trapani, D.D.; Mannina, G.; Torregrossa, M.; Viviani, G. Quantification of kinetic parameters for heterotrophic bacteria via respirometry in a hybrid reactor. Water Sci. Technol. 2010, 61, 1757–1766. [Google Scholar] [CrossRef]
- Orbeci, C.; Modrogan, C.; Dăncilă, A.M. Degradation of pharmaceutical effluents by photo-assisted techniques. Rev. Chim. 2016, 67, 166–170. [Google Scholar]
- Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1992.
- OECD 209, Activated Sludge, Respiration Inhibition Test (Carbon and Ammonium Oxidation); OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2010.
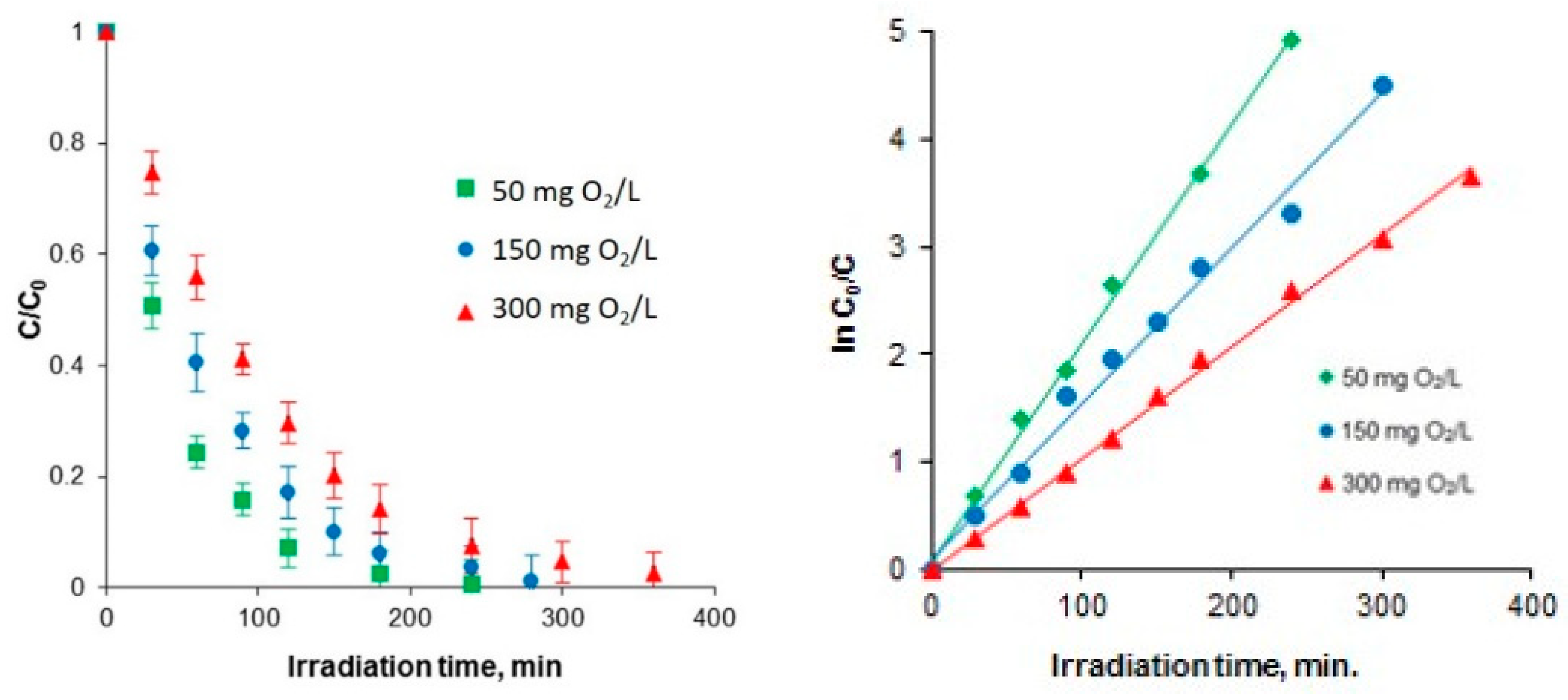
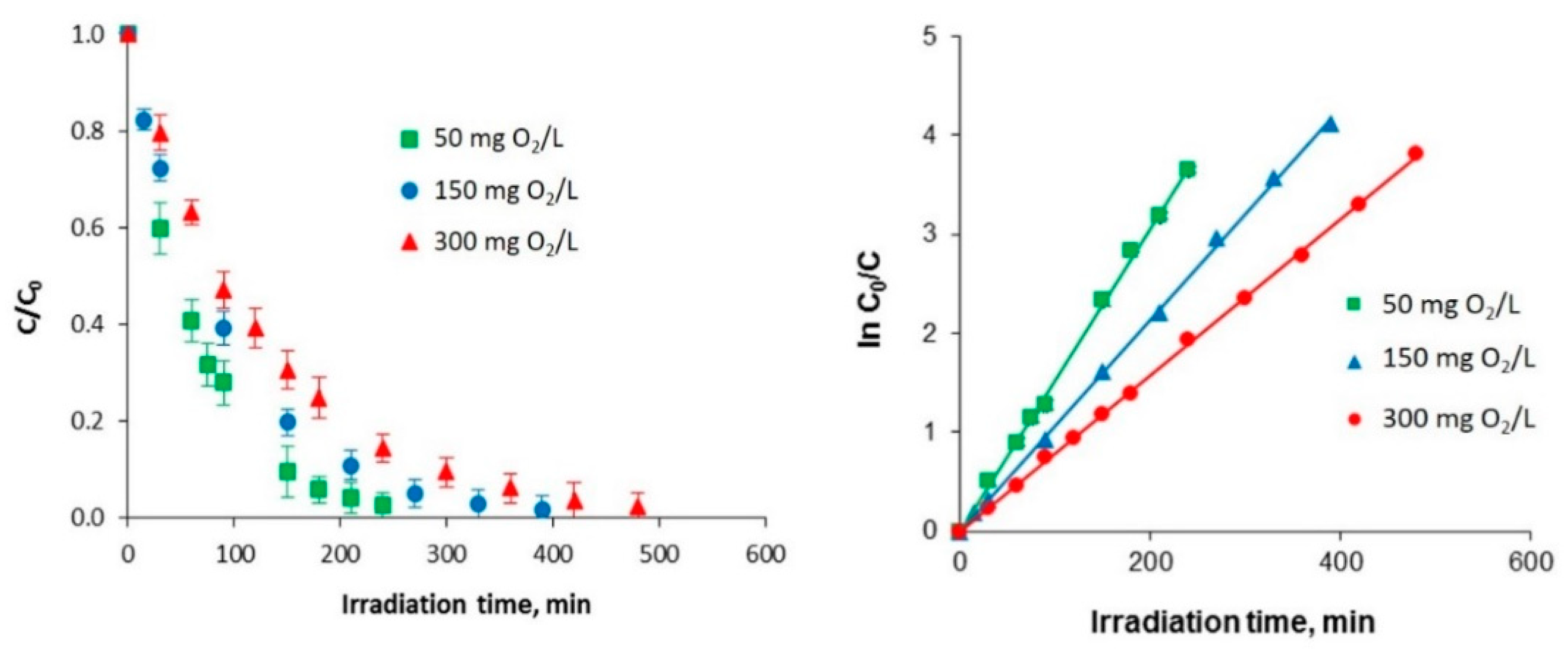
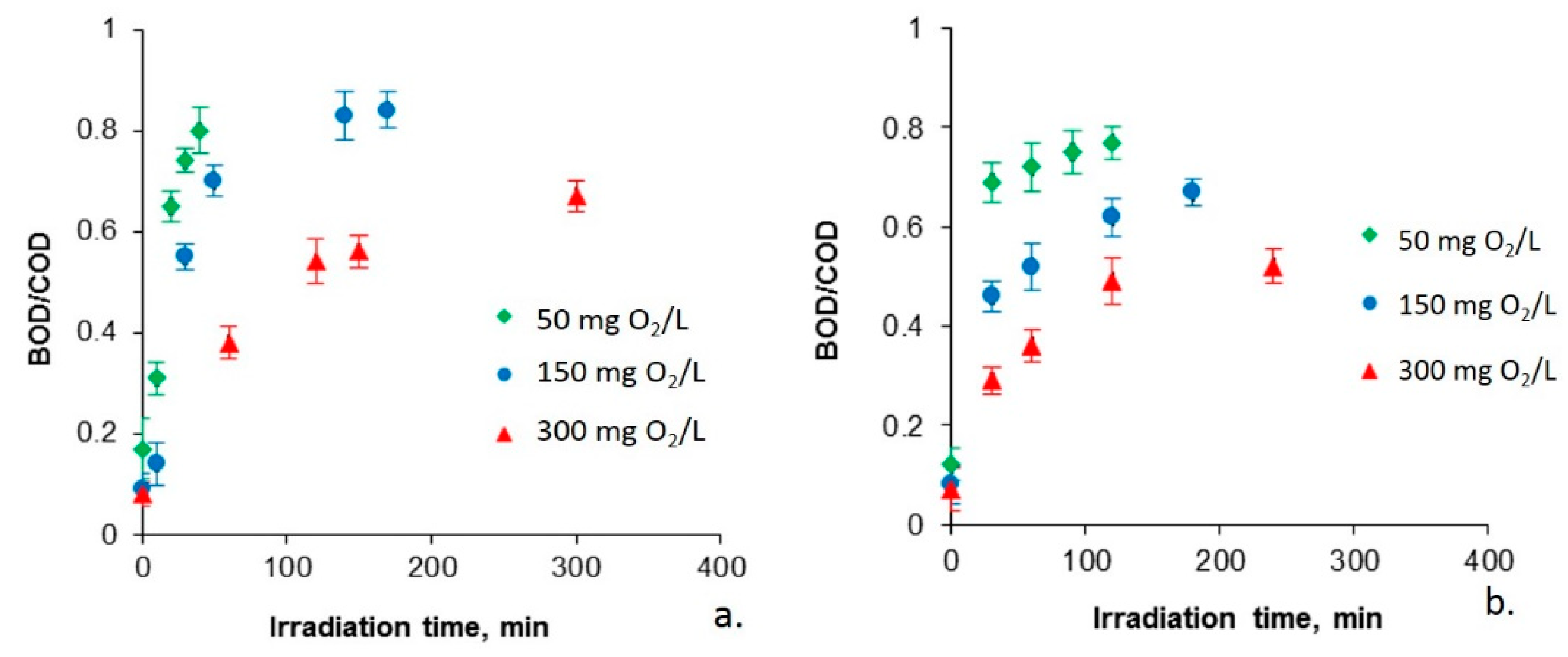
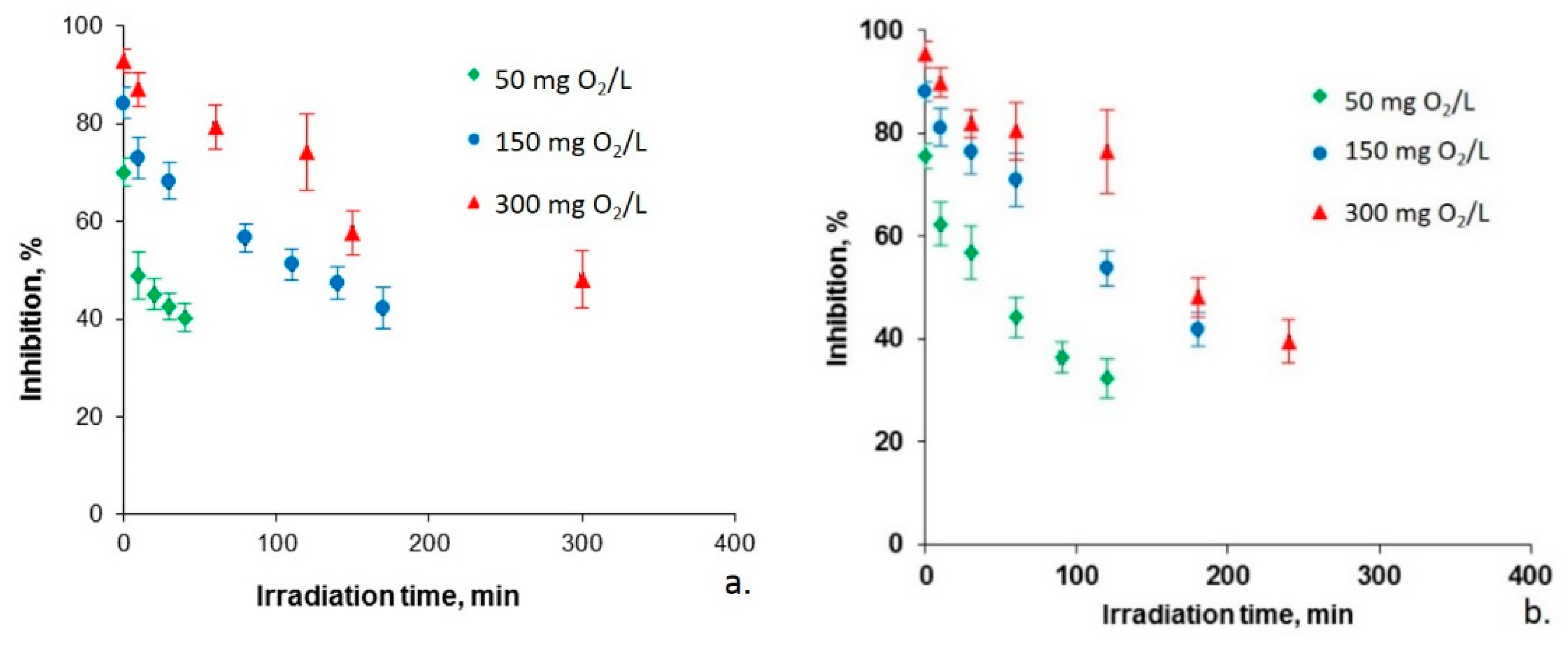
| Kinetic Parameter | Chlorophenol Initial Concentration (mg O2/L) | ||
|---|---|---|---|
| kobs, min−1 | 50 | 150 | 300 |
| 4-chlorophenol | |||
| 0.0203 | 0.0144 | 0.0103 | |
| 2,4-dichlorophenol | |||
| 0.0153 | 0.0107 | 0.0079 | |
| Property | 4-Cholorophenol | 2,4-Dichlorophenol |
|---|---|---|
| Formula | C6H4ClOH | C6H4Cl2O |
| Density (25 °C) | 1.306 g/cm3 | 1.38 g/cm3 |
| Molecular weight | 128.56 g/mol | 163.00 g/mol |
| Solubility in water (20 °C) | 27.1 g/L | 4.5 g/L |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobirică, L.; Bobirică, C.; Orbeci, C. Examination of Photocatalyzed Chlorophenols for Sequential Photocatalytic-Biological Treatment Optimization. Catalysts 2020, 10, 985. https://doi.org/10.3390/catal10090985
Bobirică L, Bobirică C, Orbeci C. Examination of Photocatalyzed Chlorophenols for Sequential Photocatalytic-Biological Treatment Optimization. Catalysts. 2020; 10(9):985. https://doi.org/10.3390/catal10090985
Chicago/Turabian StyleBobirică, Liliana, Constantin Bobirică, and Cristina Orbeci. 2020. "Examination of Photocatalyzed Chlorophenols for Sequential Photocatalytic-Biological Treatment Optimization" Catalysts 10, no. 9: 985. https://doi.org/10.3390/catal10090985
APA StyleBobirică, L., Bobirică, C., & Orbeci, C. (2020). Examination of Photocatalyzed Chlorophenols for Sequential Photocatalytic-Biological Treatment Optimization. Catalysts, 10(9), 985. https://doi.org/10.3390/catal10090985






