The Demonstration of the Superiority of the Dual Ni-Based Catalytic System for the Adjustment of the H2/CO Ratio in Syngas for Green Fuel Technologies
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.1.1. Morphological Characterization
2.1.2. Physical Characterization
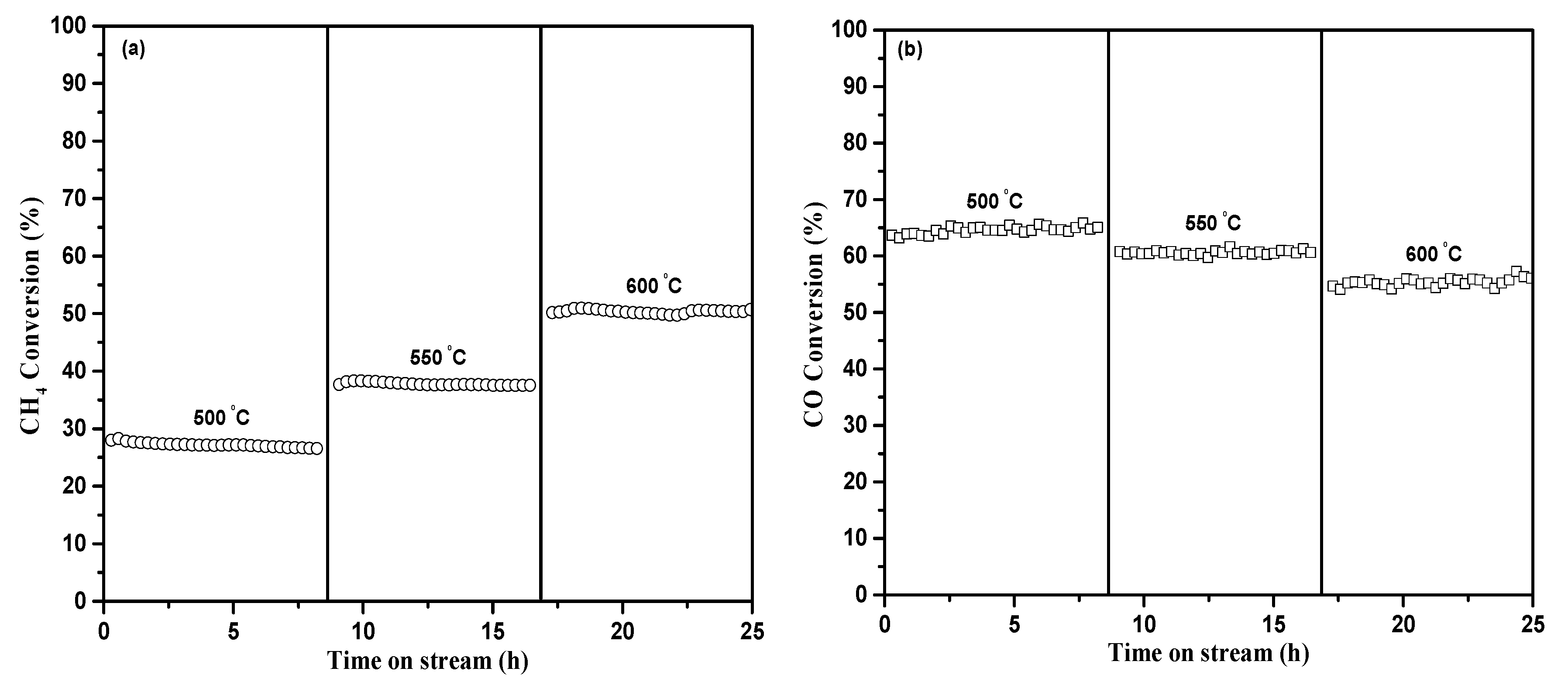
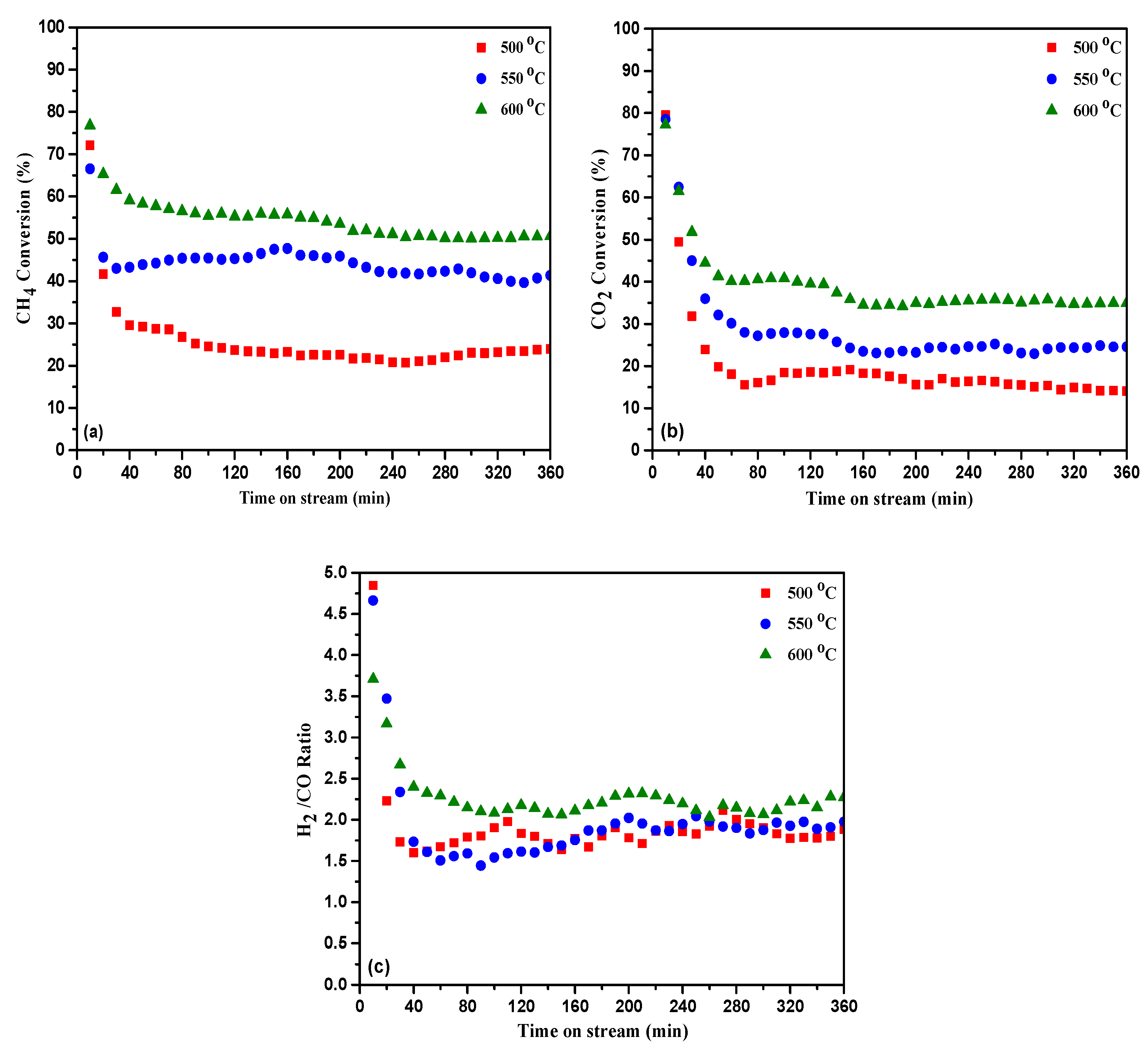
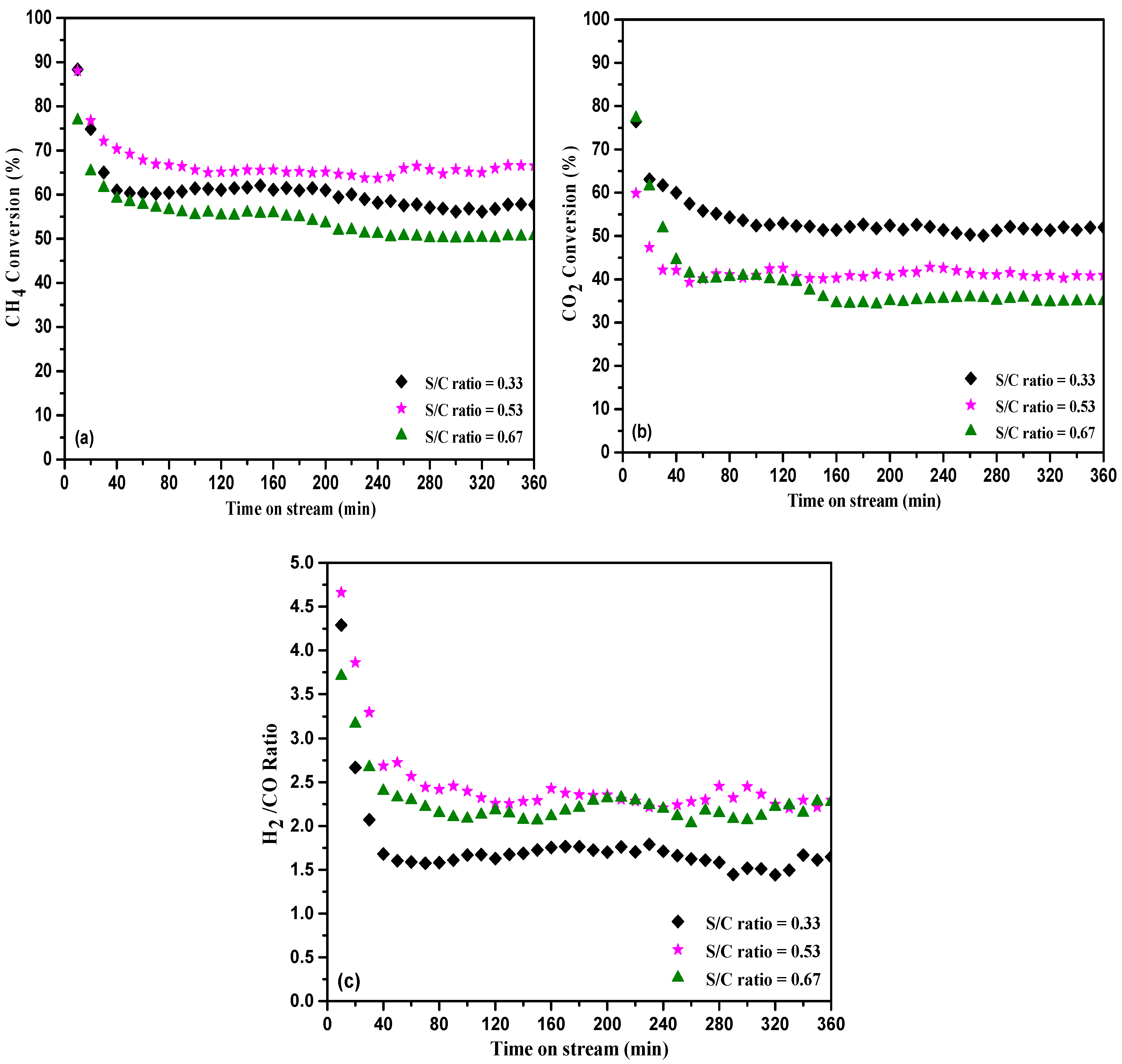
2.2. Catalytic Performance
2.2.1. Catalytic Performance for CRM and UHT-WGS Reactions
2.2.2. Catalytic Performance for DCP
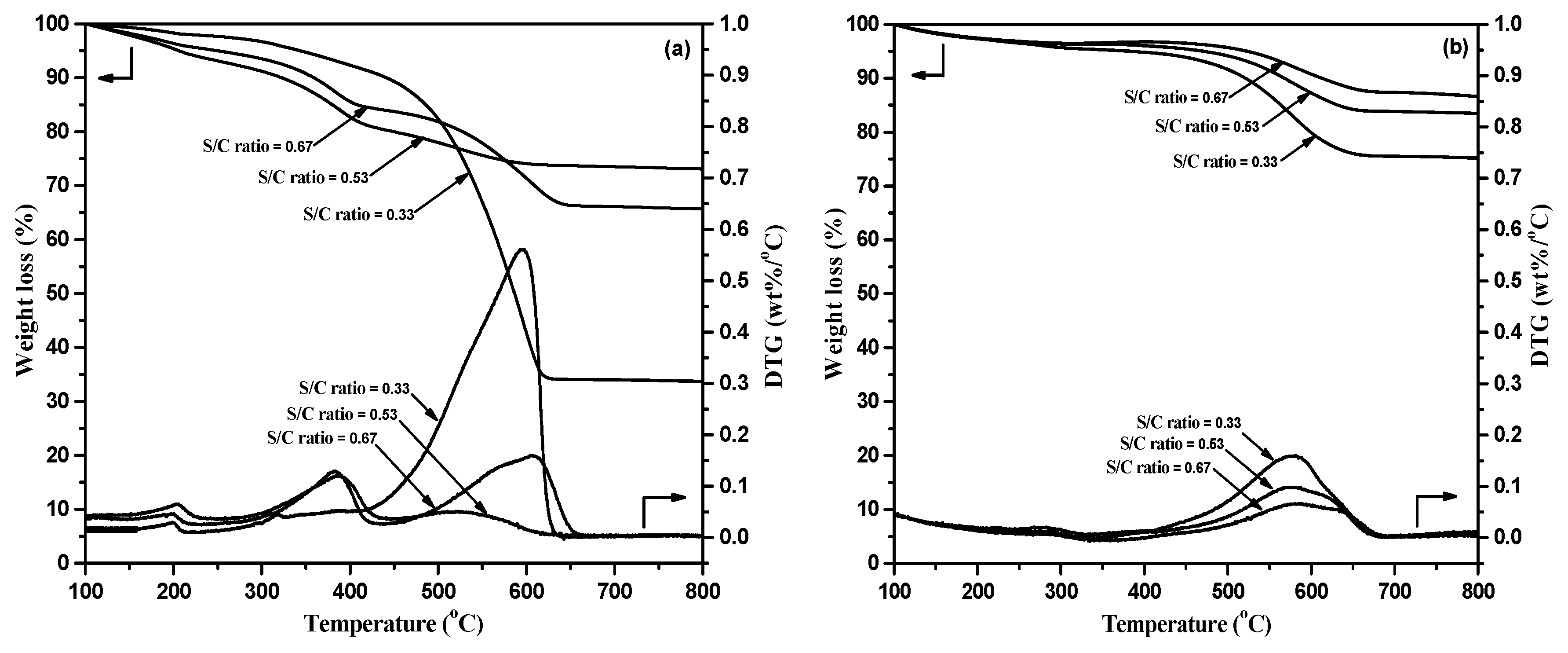
2.2.3. TGA/DTG Analysis of Spent Catalysts
3. Material and Methods
3.1. Catalyst Preparation
3.1.1. CRM Catalyst Preparation
3.1.2. UHT-WGS Catalyst Preparation
3.2. Catalyst Characterization
3.2.1. Morphological Characterization
3.2.2. Physical Characterization
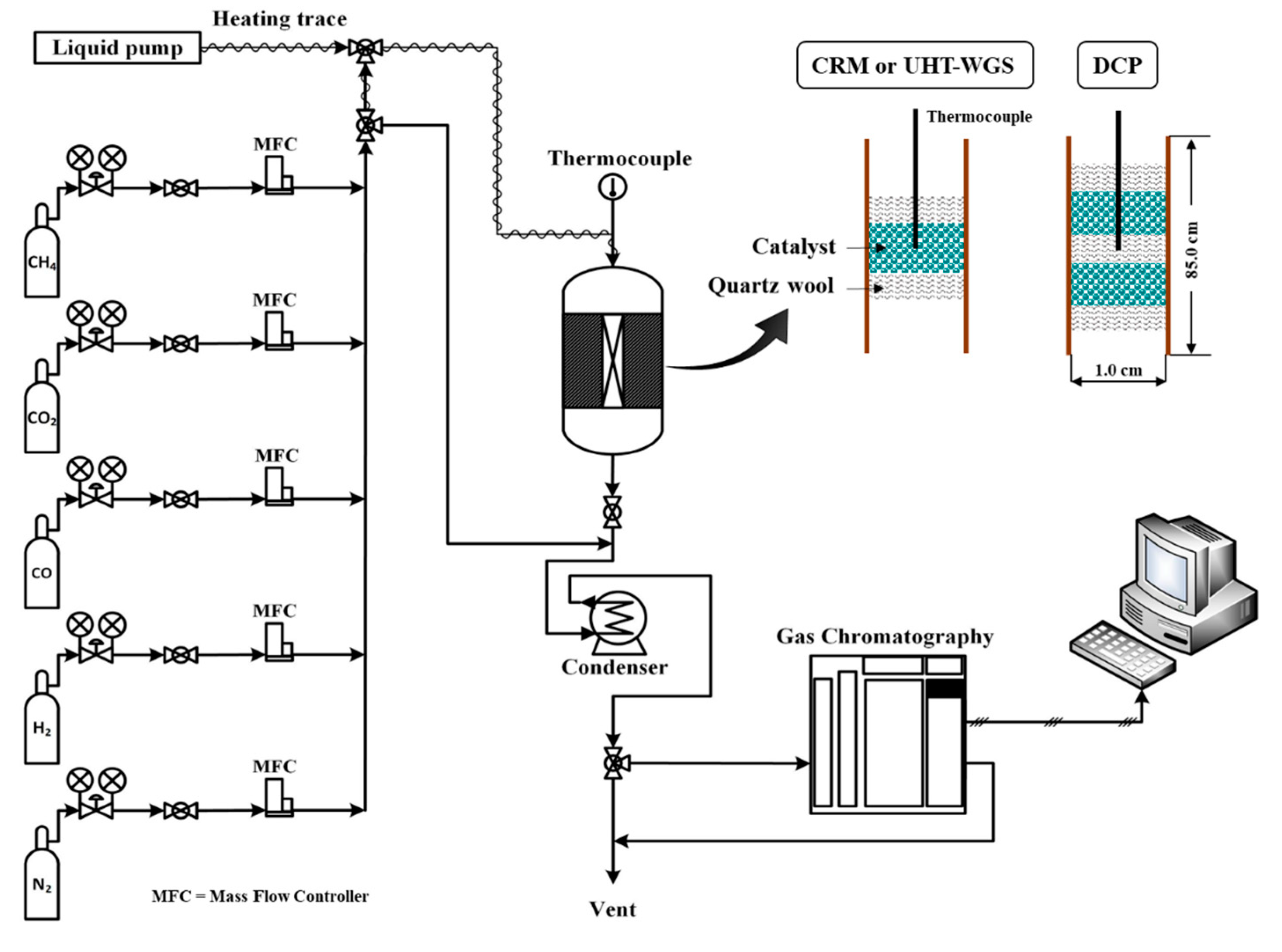
3.3. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| List of acronyms | |
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner–Halenda |
| CRM | CO2 reforming of methane |
| CSCRM | combined steam and CO2 reforming of methane |
| DCP | dual Ni-based catalytic process |
| DTG | derivative thermogravimetric analysis |
| EISA | evaporation-induced self-assembly |
| FTs | Fischer–Tropsch synthesis |
| GHSV | gas hourly space velocity |
| GTL | gas-to-liquids |
| H2-TPD | hydrogen temperature programmed desorption |
| H2-TPR | hydrogen temperature programmed reduction |
| RWGS | reverse water gas shift |
| S/C ratio | steam-to-carbon (H2O/(CH4 + CO2) ratio |
| SMSI | strong metal support interaction |
| TCD | thermal conductivity detector |
| TGA | thermogravimetric analysis |
| UHT-WGS | ultra-high-temperature water–gas shift |
| XRD | X-ray diffraction |
References
- Koo, K.Y.; Roh, H.S.; Jung, U.H.; Yoon, W.L. Combined H2O and CO2 reforming of CH4over Ce-promoted Ni/Al2O3 catalyst for gas to liquid (GTL) process: Enhancement of Ni-CeO2interaction. Catal. Today 2012, 185, 126–130. [Google Scholar] [CrossRef]
- Hadian, N.; Rezaei, M. Combination of dry reforming and partial oxidation of methane over Ni catalysts supported on nanocrystalline MgAl2O4. Fuel 2013, 113, 571–579. [Google Scholar] [CrossRef]
- Yang, E.-h.; Noh, Y.S.; Hong, G.H.; Moon, D.J. Combined steam and CO2 reforming of methane over La1-xSrxNiO3 perovskite oxides. Catal. Today 2018, 299, 242–250. [Google Scholar] [CrossRef]
- Baek, S.-C.; Bae, J.-W.; Cheon, J.Y.; Jun, K.-W.; Lee, K.-Y. Combined Steam and Carbon Dioxide Reforming of Methane on Ni/MgAl2O4: Effect of CeO2 Promoter to Catalytic Performance. Catal. Lett. 2011, 141, 224–234. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, K.; Choudary, N.V.; Pant, K.K. Effect of support materials on the performance of Ni-based catalysts in tri-reforming of methane. Fuel Process. Technol. 2019, 186, 40–52. [Google Scholar] [CrossRef]
- Koo, K.Y.; Roh, H.S.; Jung, U.H.; Seo, D.J.; Seo, Y.S.; Yoon, W.L. Combined H2O and CO2 reforming of CH4 over nano-sized Ni/MgO-Al2O3 catalysts for synthesis gas production for gas to liquid (GTL): Effect of Mg/Al mixed ratio on coke formation. Catal. Today 2009, 146, 166–171. [Google Scholar] [CrossRef]
- Roh, H.-S.; Koo, K.Y.; Joshi, U.D.; Yoon, W.L. Combined H2O and CO2 Reforming of Methane Over Ni–Ce–ZrO2 Catalysts for Gas to Liquids (GTL). Catal. Lett. 2008, 125, 283–288. [Google Scholar] [CrossRef]
- Koh, A.C.W.; Chen, L.; Kee Leong, W.; Johnson, B.F.G.; Khimyak, T.; Lin, J. Hydrogen or synthesis gas production via the partial oxidation of methane over supported nickel–cobalt catalysts. Int. J. Hydrogen Energy 2007, 32, 725–730. [Google Scholar] [CrossRef]
- Rabenstein, G.; Hacker, V. Hydrogen for fuel cells from ethanol by steam-reforming, partial-oxidation and combined auto-thermal reforming: A thermodynamic analysis. J. Power Sources 2008, 185, 1293–1304. [Google Scholar] [CrossRef]
- Cavallaro, S.; Chiodo, V.; Vita, A.; Freni, S. Hydrogen production by auto-thermal reforming of ethanol on Rh/Al2O3 catalyst. J. Power Sources 2003, 123, 10–16. [Google Scholar] [CrossRef]
- Ma, Q.; Guo, L.; Fang, Y.; Li, H.; Zhang, J.; Zhao, T.S.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Combined methane dry reforming and methane partial oxidization for syngas production over high dispersion Ni based mesoporous catalyst. Fuel Process. Technol. 2019, 188, 98–104. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Khajenoori, M. Combined dry reforming and partial oxidation of methane to synthesis gas on noble metal catalysts. Int. J. Hydrogen Energy 2011, 36, 2969–2978. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Koo, K.-Y.; Song, I.-K. A Simulation Study on SCR (Steam Carbon Dioxide Reforming) Process Optimization for Fischer-Tropsch Synthesis. Korean Chem. Eng. Res. 2009, 47, 700–704. [Google Scholar]
- Al-Nakoua, M.A.; El-Naas, M.H. Combined steam and dry reforming of methane in narrow channel reactors. Int. J. Hydrogen Energy 2012, 37, 7538–7544. [Google Scholar] [CrossRef]
- Wilhelm, D.; Simbeck, D.; Karp, A.; Dickenson, R. Syngas production for gas-to-liquids applications: Technologies, issues and outlook. Fuel Process. Technol. 2001, 71, 139–148. [Google Scholar] [CrossRef]
- Sehested, J. Four challenges for nickel steam-reforming catalysts. Catal. Today 2006, 111, 103–110. [Google Scholar] [CrossRef]
- Kumar, N.; Shojaee, M.; Spivey, J. Catalytic bi-reforming of methane: From greenhouse gases to syngas. Curr. Opin. Chem. Eng. 2015, 9, 8–15. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Czaun, M.; Mathew, T.; May, R.B.; Prakash, G.K.S. Single Step Bi-reforming and Oxidative Bi-reforming of Methane (Natural Gas) with Steam and Carbon Dioxide to Metgas (CO-2H2) for Methanol Synthesis: Self-Sufficient Effective and Exclusive Oxygenation of Methane to Methanol with Oxygen. J. Am. Chem. Soc. 2015, 137, 8720–8729. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Czaun, M.; Prakash, G.K.S. Bi-reforming of Methane from Any Source with Steam and Carbon Dioxide Exclusively to Metgas (CO–2H2) for Methanol and Hydrocarbon Synthesis. J. Am. Chem. Soc. 2013, 135, 648–650. [Google Scholar] [CrossRef]
- Jang, W.-J.; Jeong, D.-W.; Shim, J.-O.; Kim, H.-M.; Roh, H.-S.; Son, I.H.; Lee, S.J. Combined steam and carbon dioxide reforming of methane and side reactions: Thermodynamic equilibrium analysis and experimental application. Appl. Energy 2016, 173, 80–91. [Google Scholar] [CrossRef]
- Danilova, M.M.; Fedorova, Z.A.; Kuzmin, V.A.; Zaikovskii, V.I.; Porsin, A.V.; Krieger, T.A. Combined steam and carbon dioxide reforming of methane over porous nickel based catalysts. Catal. Sci. Technol. 2015, 5, 2761–2768. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Ji, S.; Lang, B.; Habimana, F.; Li, C. Effect of MgO promoter on Ni-based SBA-15 catalysts for combined steam and carbon dioxide reforming of methane. J. Nat. Gas Chem. 2008, 17, 225–231. [Google Scholar] [CrossRef]
- Jabbour, K.; Massiani, P.; Davidson, A.; Casale, S.; El Hassan, N. Ordered mesoporous “one-pot” synthesized Ni-Mg(Ca)-Al2O3 as effective and remarkably stable catalysts for combined steam and dry reforming of methane (CSDRM). Appl. Catal. B Environ. 2017, 201, 527–542. [Google Scholar] [CrossRef]
- Bae, J.W.; Kim, A.R.; Baek, S.-C.; Jun, K.-W. The role of CeO2–ZrO2 distribution on the Ni/MgAl2O4 catalyst during the combined steam and CO2 reforming of methane. React. Kinet. Mech. Catal. 2011, 104, 377–388. [Google Scholar] [CrossRef]
- Yagi, F.; Kanai, R.; Wakamatsu, S.; Kajiyama, R.; Suehiro, Y.; Shimura, M. Development of synthesis gas production catalyst and process. Catal. Today 2005, 104, 2–6. [Google Scholar] [CrossRef]
- Samavati, M.; Santarelli, M.; Martin, A.; Nemanova, V. Thermodynamic and economy analysis of solid oxide electrolyser system for syngas production. Energy 2017, 122, 37–49. [Google Scholar] [CrossRef]
- Selvatico, D.; Lanzini, A.; Santarelli, M. Low Temperature Fischer-Tropsch fuels from syngas: Kinetic modeling and process simulation of different plant configurations. Fuel 2016, 186, 544–560. [Google Scholar] [CrossRef]
- Iaquaniello, G.; Salladini, A.; Palo, E.; Centi, G. Catalytic Partial Oxidation Coupled with Membrane Purification to Improve Resource and Energy Efficiency in Syngas Production. ChemSusChem 2015, 8, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Asencios, Y.J.O.; Assaf, E.M. Combination of dry reforming and partial oxidation of methane on NiO-MgO-ZrO2 catalyst: Effect of nickel content. Fuel Process. Technol. 2013, 106, 247–252. [Google Scholar] [CrossRef]
- Li, X.; Teng, Y.; Gong, M.; Chen, Y. Combination of partial oxidation and CO2 reforming of methane over monolithic Ni/CeO2–ZrO2/γ-Al2O3 Catalyst. Chin. Chem. Lett. 2005, 16, 691–692. [Google Scholar]
- Piyapaka, K.; Tungkamani, S.; Phongaksorn, M. Effect of Strong Metal Support Interactions of Supported Ni and Ni-Co Catalyst on Metal Dispersion and Catalytic Activity toward Dry Methane Reforming Reaction. King Mongkut’s Univ. Technol. North Bangkok Int. J. Appl. Sci. Technol. 2016, 9, 255–259. [Google Scholar] [CrossRef][Green Version]
- Sangsong, S.; Ratana, T.; Tungkamani, S.; Sornchamni, T.; Phongaksorn, M. Effect of CeO2 loading of the Ce-Al mixed oxide on ultrahigh temperature water-gas shift performance over Ce-Al mixed oxide supported Ni catalysts. Fuel 2019, 252, 488–495. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Q.; Wu, R. Effect of Co–Ni ratio on the activity and stability of Co–Ni bimetallic aerogel catalyst for methane Oxy-CO2 reforming. Int. J. Hydrogen Energy 2011, 36, 2128–2136. [Google Scholar] [CrossRef]
- Akbari, E.; Alavi, S.M.; Rezaei, M. Synthesis gas production over highly active and stable nanostructured NiMgOAl2O3 catalysts in dry reforming of methane: Effects of Ni contents. Fuel 2017, 194, 171–179. [Google Scholar] [CrossRef]
- Katheria, S.; Gupta, A.; Deo, G.; Kunzru, D. Effect of calcination temperature on stability and activity of Ni/MgAl2O4 catalyst for steam reforming of methane at high pressure condition. Int. J. Hydrogen Energy 2016, 41, 14123–14132. [Google Scholar] [CrossRef]
- Profeti, L.P.R.; Ticianelli, E.A.; Assaf, E.M. Production of hydrogen via steam reforming of biofuels on Ni/CeO2–Al2O3 catalysts promoted by noble metals. Int. J. Hydrogen Energy 2009, 34, 5049–5060. [Google Scholar] [CrossRef]
- Rojas, H.; Borda, G.; Reyes, P.; Brijaldo, M.; Valencia, J. Liquid-phase hydrogenation of m-dinitrobenzene over platinum catalysts. J. Chil. Chem. Soc. 2011, 56, 793–798. [Google Scholar] [CrossRef][Green Version]
- Ferreira, A.P.; Zanchet, D.; Rinaldi, R.; Schuchardt, U.; Damyanova, S.; Bueno, J.M.C. Effect of the CeO2 content on the surface and structural properties of CeO2-Al2O3 mixed oxides prepared by sol-gel method. Appl. Catal. A Gen. 2010, 388, 45–56. [Google Scholar] [CrossRef]
- Horlyck, J.; Lawrey, C.; Lovell, E.C.; Amal, R.; Scott, J. Elucidating the impact of Ni and Co loading on the selectivity of bimetallic NiCo catalysts for dry reforming of methane. Chem. Eng. J. 2018, 352, 572–580. [Google Scholar] [CrossRef]
- Iriondo, A.; Barrio, V.L.; Cambra, J.F.; Arias, P.L.; Guemez, M.B.; Sanchez-Sanchez, M.C.; Navarro, R.M.; Fierro, J.L.G. Glycerol steam reforming over Ni catalysts supported on ceria and ceria-promoted alumina. Int. J. Hydrogen Energy 2010, 35, 11622–11633. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Lo Mastro, S.; Sodo, A. Ni/CeO2–Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Ashok, J.; Kawi, S. NiCo@NiCo phyllosilicate@CeO2 hollow core shell catalysts for steam reforming of toluene as biomass tar model compound. Energy Convers. Manag. 2019, 180, 822–830. [Google Scholar] [CrossRef]
- Sirijaruphan, A.; Horváth, A.; Goodwin Jr., J.G.; Oukaci, R. Cobalt Aluminate Formation in Alumina-Supported Cobalt Catalysts: Effects of Cobalt Reduction State and Water Vapor. Catal. Lett. 2003, 91, 89–94. [Google Scholar] [CrossRef]
- Patcas, F.; Hönicke, D. Effect of alkali doping on catalytic properties of alumina-supported nickel oxide in the selective oxidehydrogenation of cyclohexane. Catal. Commun. 2005, 6, 23–27. [Google Scholar] [CrossRef]
- Ashok, J.; Kawi, S. Steam reforming of toluene as a biomass tar model compound over CeO2 promoted Ni/CaO-Al2O3 catalytic systems. Int. J. Hydrogen Energy 2013, 38, 13938–13949. [Google Scholar] [CrossRef]
- Djinović, P.; Osojnik Črnivec, I.G.; Erjavec, B.; Pintar, A. Influence of active metal loading and oxygen mobility on coke-free dry reforming of Ni–Co bimetallic catalysts. Appl. Catal. B Environ. 2012, 125, 259–270. [Google Scholar] [CrossRef]
- Tanios, C.; Bsaibes, S.; Gennequin, C.; Labaki, M.; Cazier, F.; Billet, S.; Tidahy, H.L.; Nsouli, B.; Aboukaïs, A.; Abi-Aad, E. Syngas production by the CO2 reforming of CH4 over Ni–Co–Mg–Al catalysts obtained from hydrotalcite precursors. Int. J. Hydrogen Energy 2017, 42, 12818–12828. [Google Scholar] [CrossRef]
- Jha, A.; Jeong, D.-W.; Jang, W.-J.; Rode, C.V.; Roh, H.-S. Mesoporous NiCu–CeO2 oxide catalysts for high-temperature water–gas shift reaction. RSC Adv. 2015, 5, 1430–1437. [Google Scholar] [CrossRef]
- Haryanto, A.; Fernando, S.; Adhikari, S. Ultrahigh temperature water gas shift catalysts to increase hydrogen yield from biomass gasification. Catal. Today 2007, 129, 269–274. [Google Scholar] [CrossRef]
- Oemar, U.; Bian, Z.; Hidajat, K.; Kawi, S. Sulfur resistant LaxCe1−xNi0.5Cu0.5O3 catalysts for an ultra-high temperature water gas shift reaction. Catal. Sci. Technol. 2016, 6, 6569–6580. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Jiang, X.; Song, Z.; Zhao, X.; Ma, C. Microwave-enhanced methane combined reforming by CO2 and H2O into syngas production on biomass-derived char. Fuel 2016, 185, 692–700. [Google Scholar] [CrossRef]
- Gensterblum, Y.; Busch, A.; Krooss, B.M. Molecular concept and experimental evidence of competitive adsorption of H2O, CO2 and CH4 on organic material. Fuel 2014, 115, 581–588. [Google Scholar] [CrossRef]
- Tan, K.; Zuluaga, S.; Gong, Q.; Gao, Y.; Nijem, N.; Li, J.; Thonhauser, T.; Chabal, Y.J. Competitive coadsorption of CO2 with H2O, NH3, SO2, NO, NO2, N2, O2, and CH4 in M-MOF-74 (M = Mg, Co, Ni): The role of hydrogen bonding. Chem. Mater. 2015, 27, 2203–2217. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, H.; Zhang, Z.; Chen, H.; Liu, X. Molecular simulation of CO2/CH4/H2O competitive adsorption and diffusion in brown coal. RSC Adv. 2019, 9, 3004–3011. [Google Scholar] [CrossRef]
- Singh, S.; Bahari, M.B.; Abdullah, B.; Phuong, P.T.T.; Truong, Q.D.; Vo, D.V.N.; Adesina, A.A. Bi-reforming of methane on Ni/SBA-15 catalyst for syngas production: Influence of feed composition. Int. J. Hydrogen Energy 2018, 43, 17230–17243. [Google Scholar] [CrossRef]
- Álvarez M, A.; Centeno, M.Á.; Odriozola, J.A. Ru-Ni Catalyst in the Combined Dry-Steam Reforming of Methane: The Importance in the Metal Order Addition. Top. Catal. 2016, 59, 303–313. [Google Scholar] [CrossRef]
- Lillebø, A.; Rytter, E.; Blekkan, E.A.; Holmen, A. Fischer-Tropsch Synthesis at High Conversions on Al2O3-Supported Co Catalysts with Different H2/CO Levels. Ind. Eng. Chem. Res. 2017, 56, 13281–13286. [Google Scholar] [CrossRef]
- Ashok, J.; Wai, M.H.; Kawi, S. Nickel-based Catalysts for High-temperature Water Gas Shift Reaction-Methane Suppression. ChemCatChem 2018, 10, 3927–3942. [Google Scholar] [CrossRef]
- Prakash, N.; Lee, M.H.; Yoon, S.; Jung, K.D. Role of acid solvent to prepare highly active PtSn/Θ-Al2O3 catalysts in dehydrogenation of propane to propylene. Catal. Today 2017, 293–294, 33–41. [Google Scholar] [CrossRef]
- Barbier, A.; Tuel, A.; Martin, G.A.; Arcon, I.; Arcon, I.; Kodre, A.; Kodre, A. Characterization and catalytic behavior of CO/SiO2 catalysts: Influence of dispersion in the Fischer-Tropsch reaction. J. Catal. 2001, 200, 106–116. [Google Scholar] [CrossRef]
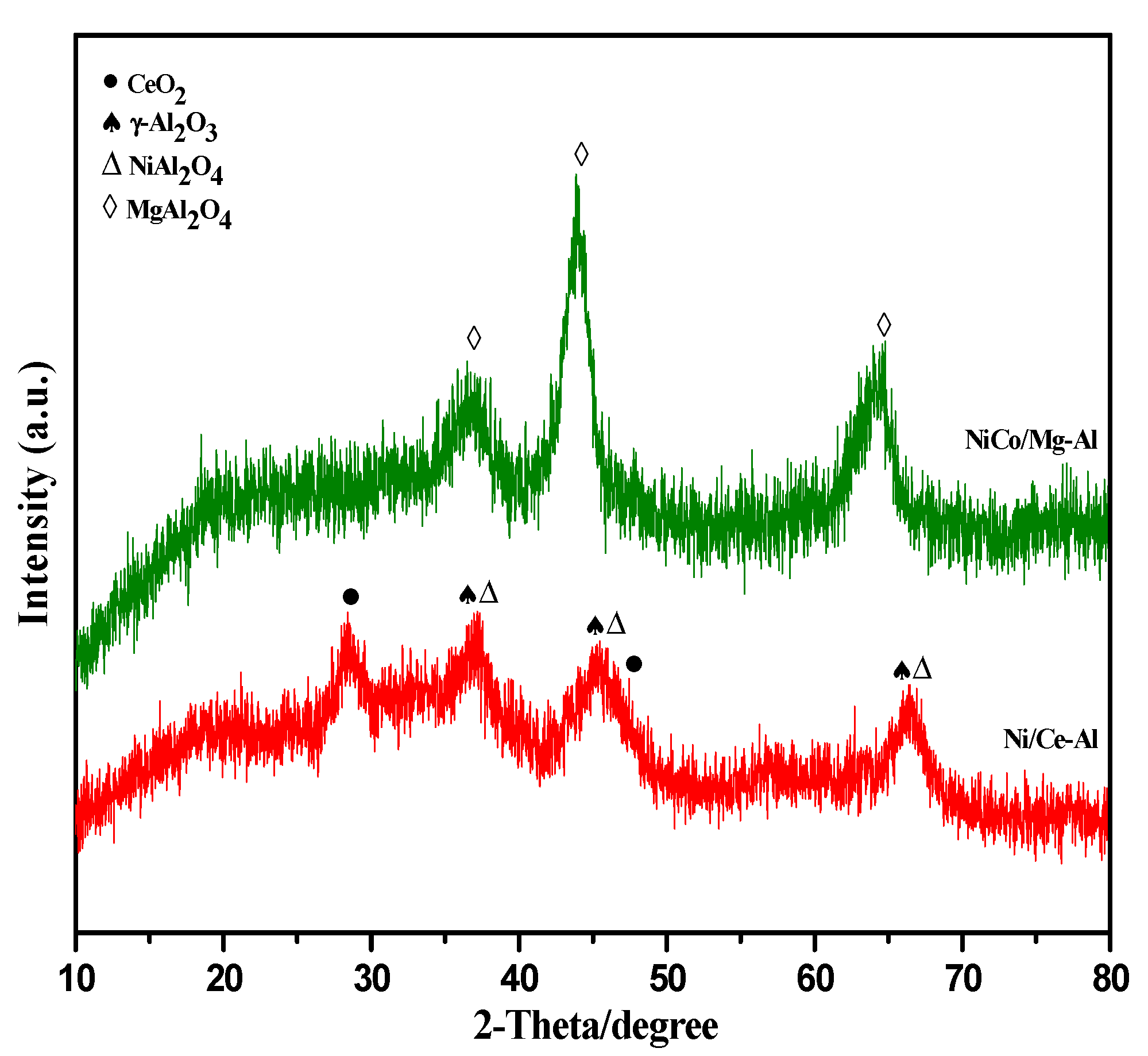
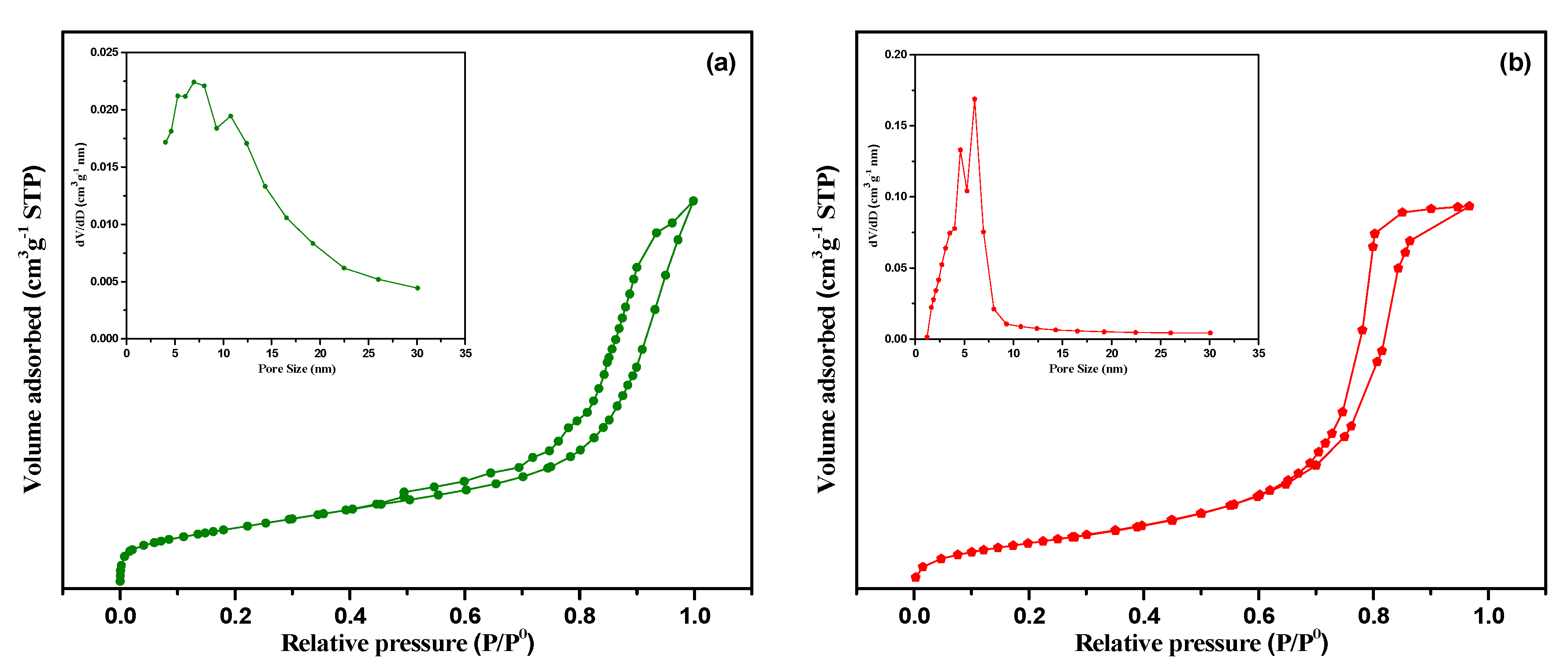
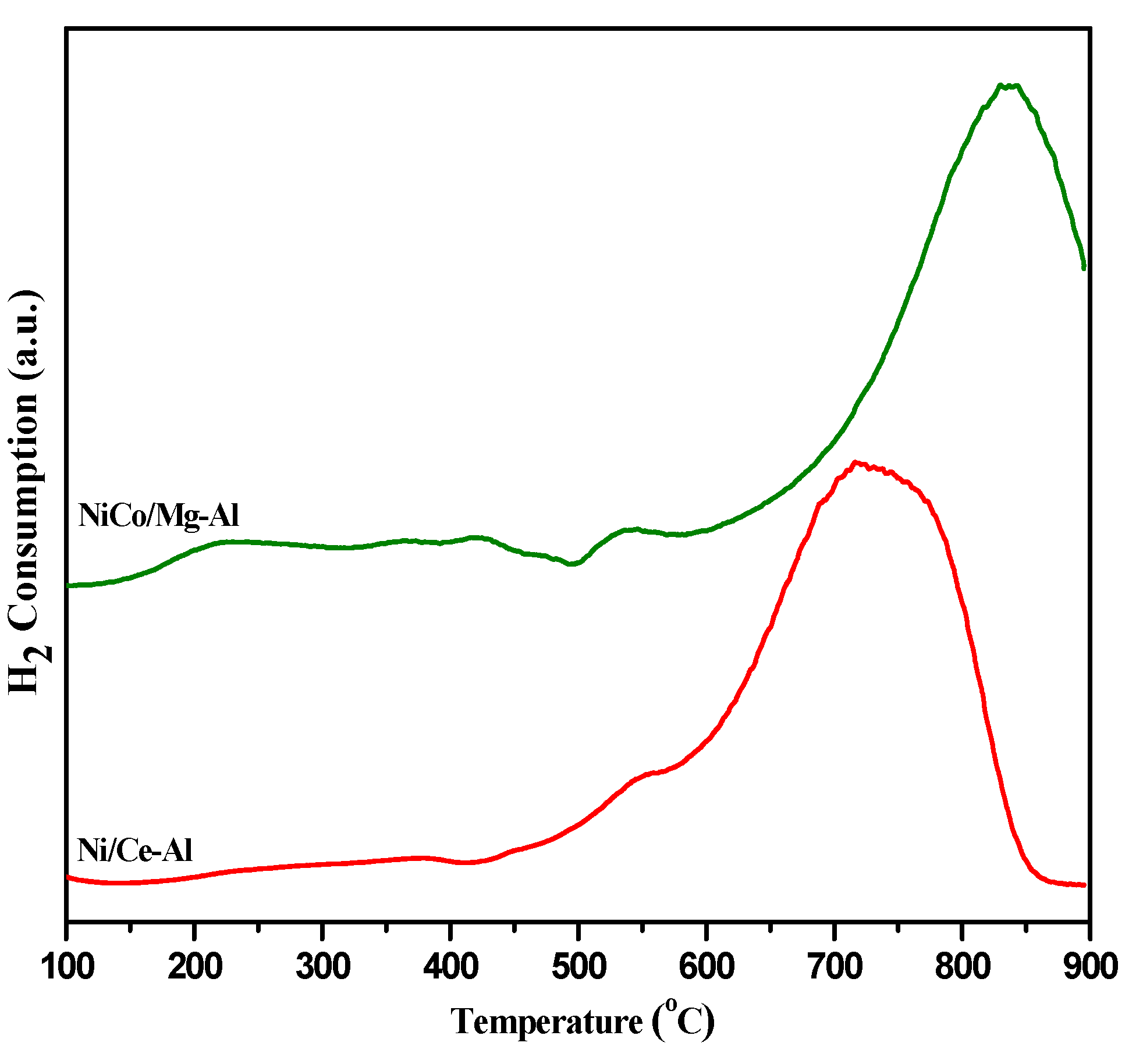
| Catalysts | SBET (m2 g−1) a | Vp (cm3 g−1) a | Average Pore Size Diameter (nm) a | % | H2-Uptakes (µmol g−1) | ||
|---|---|---|---|---|---|---|---|
| Actual c | Theoretical d | ||||||
| NiCo/Mg-Al | 130 | 0.4 | 11.2 | 32.2 | 2.0 | 1616 | 1700 |
| Ni/5Ce-Al | 183 | 0.6 | 12.5 | 23.8 | 2.7 | 1428 | 1704 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangsong, S.; Ratana, T.; Tungkamani, S.; Sornchamni, T.; Phongaksorn, M.; Croiset, E. The Demonstration of the Superiority of the Dual Ni-Based Catalytic System for the Adjustment of the H2/CO Ratio in Syngas for Green Fuel Technologies. Catalysts 2020, 10, 1056. https://doi.org/10.3390/catal10091056
Sangsong S, Ratana T, Tungkamani S, Sornchamni T, Phongaksorn M, Croiset E. The Demonstration of the Superiority of the Dual Ni-Based Catalytic System for the Adjustment of the H2/CO Ratio in Syngas for Green Fuel Technologies. Catalysts. 2020; 10(9):1056. https://doi.org/10.3390/catal10091056
Chicago/Turabian StyleSangsong, Suntorn, Tanakorn Ratana, Sabaithip Tungkamani, Thana Sornchamni, Monrudee Phongaksorn, and Eric Croiset. 2020. "The Demonstration of the Superiority of the Dual Ni-Based Catalytic System for the Adjustment of the H2/CO Ratio in Syngas for Green Fuel Technologies" Catalysts 10, no. 9: 1056. https://doi.org/10.3390/catal10091056
APA StyleSangsong, S., Ratana, T., Tungkamani, S., Sornchamni, T., Phongaksorn, M., & Croiset, E. (2020). The Demonstration of the Superiority of the Dual Ni-Based Catalytic System for the Adjustment of the H2/CO Ratio in Syngas for Green Fuel Technologies. Catalysts, 10(9), 1056. https://doi.org/10.3390/catal10091056





