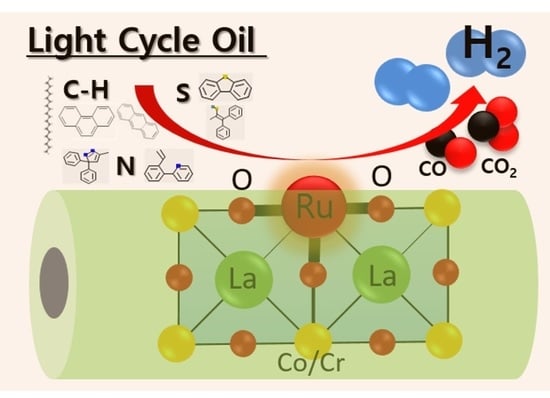
Light Cycle Oil Source for Hydrogen Production through Autothermal Reforming using Ruthenium doped Perovskite Catalysts
Abstract
1. Introduction
2. Results and Discussion
2.1. LCO Feedstock Characterization
2.2. Synthesis of Perovskite Catalysts
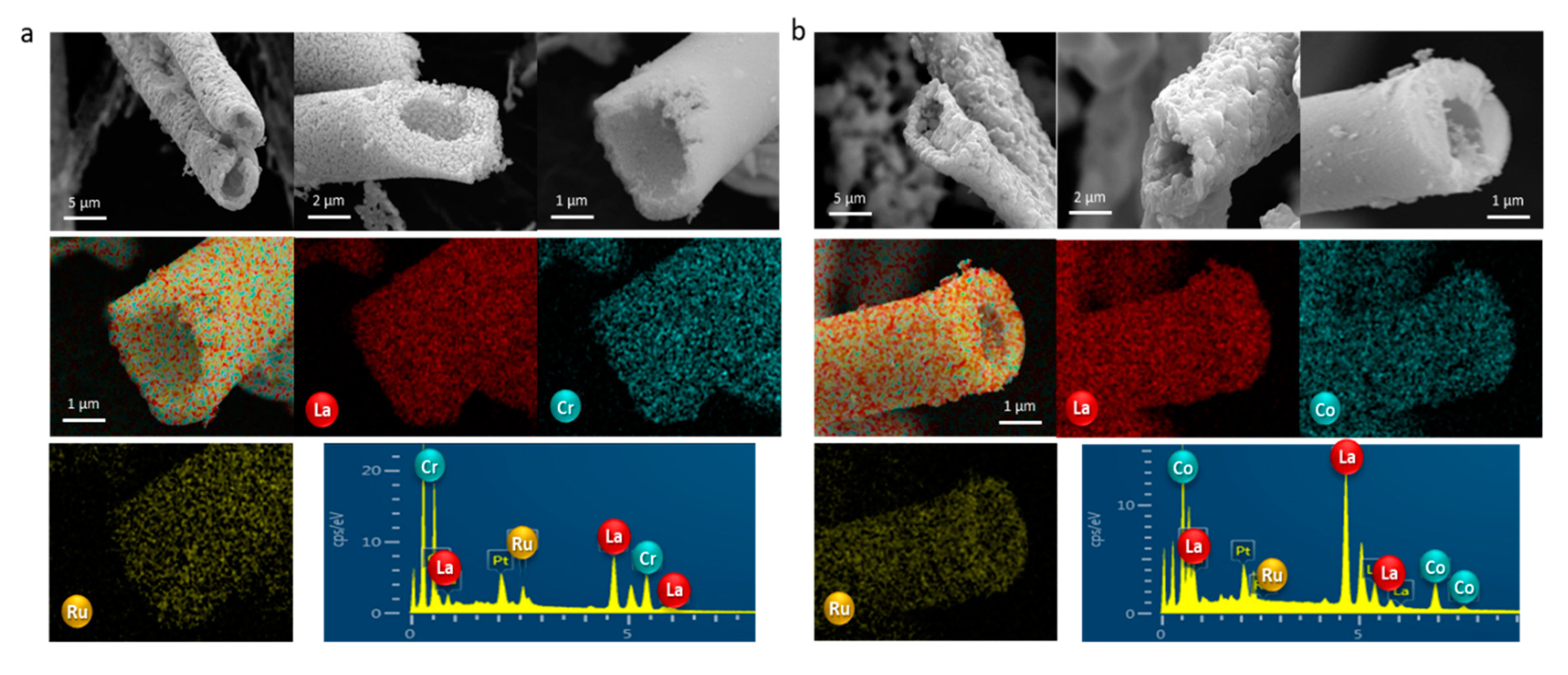
2.2.1. SEM-EDX (Scanning Electron Microscopy-Energy-Dispersive X-ray)
2.2.2. XRD (X-Ray Diffraction)
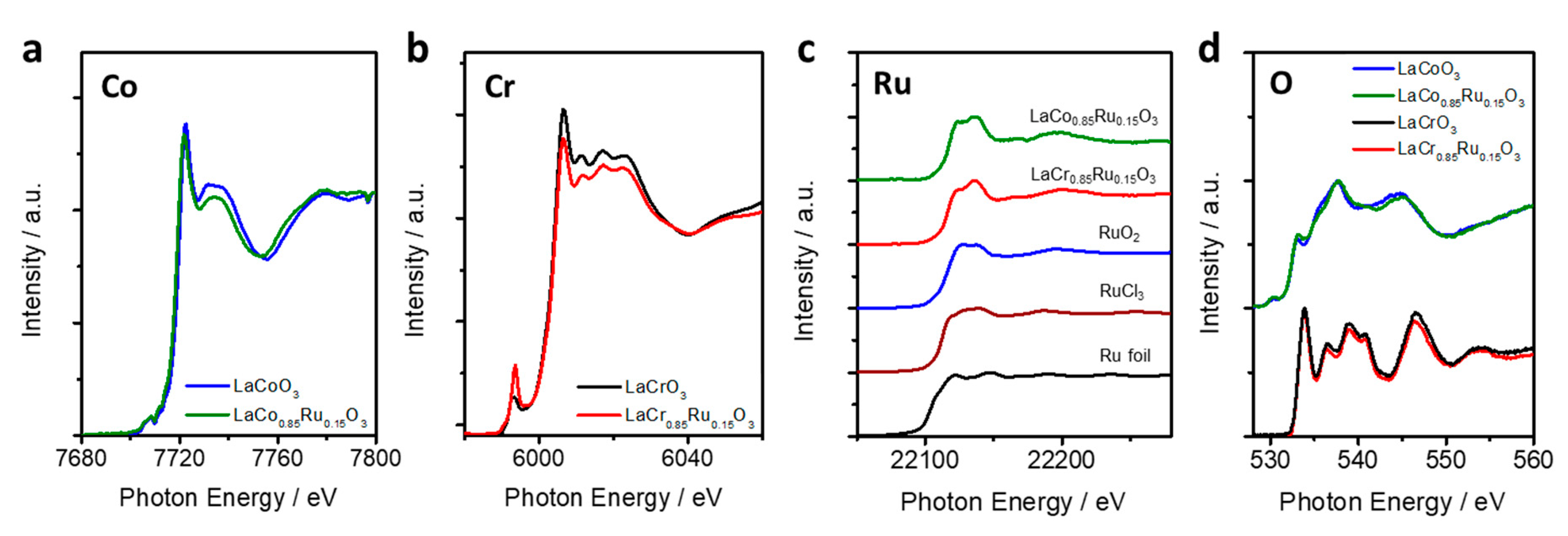
2.2.3. XANES (X-Ray Absorption Near Edge Structure)
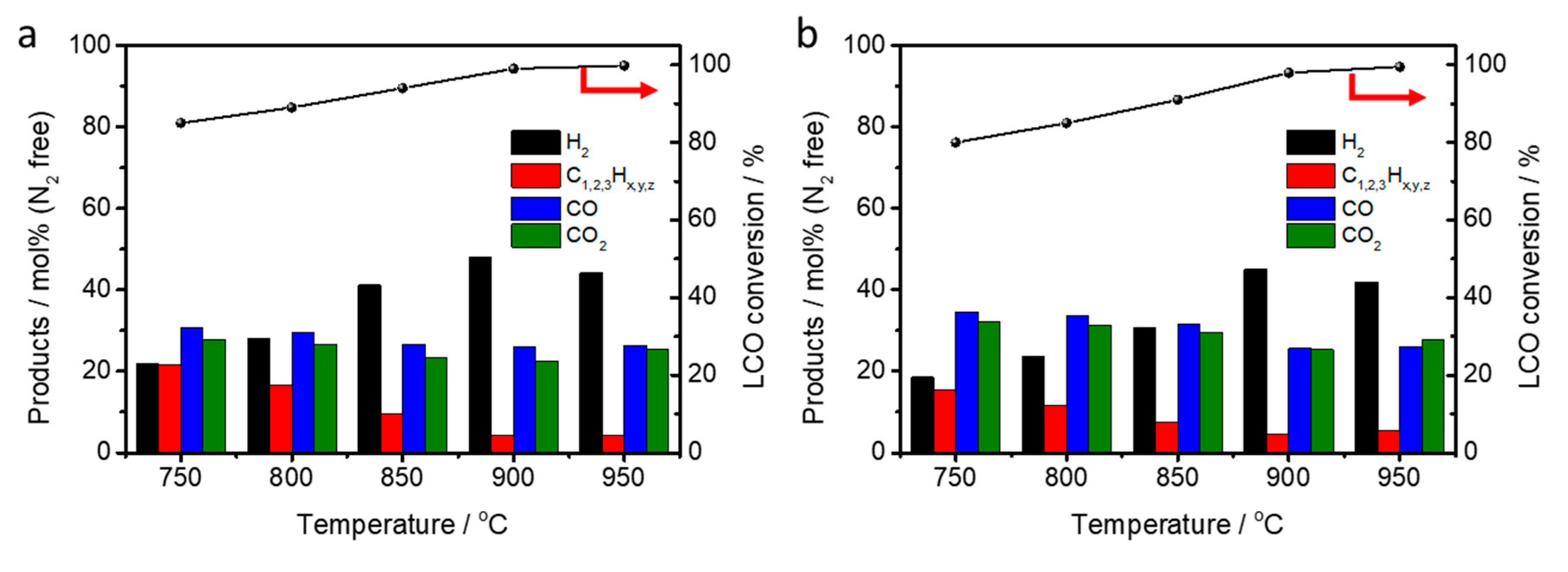
2.3. LCO-ATR by Various Reaction Temperature
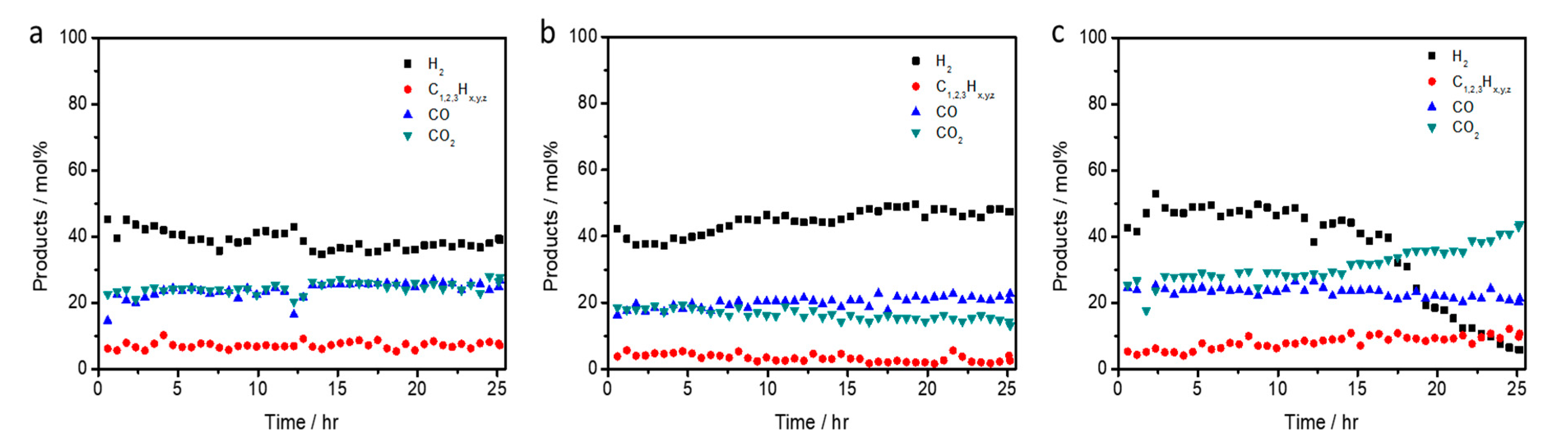
2.4. Long-Term Test of LCO-ATR Reactions
2.5. Post Characterization of the Used Perovskite Catalysts
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Damo, U.M.; Ferrari, M.L.; Turan, A.; Massardo, A.F. Solid oxide fuel cell hybrid system: A detailed review of an environmentally clean and efficient source of energy. Energy 2019, 168, 235–246. [Google Scholar] [CrossRef]
- Cai, F.; Ibrahim, J.J.; Fu, Y.; Kong, W.; Zhang, J.; Sun, Y. Low-temperature hydrogen production from methanol steam reforming on Zn-modified Pt/MoC catalysts. Appl. Catal. B Environ. 2020, 264, 118500. [Google Scholar] [CrossRef]
- United Nations Framework Convention on Climate Change (UNFCCC). Adoption of the Paris Agreement, Report No. FCCC/CP/2015/L.9/Rev.1, 2015. Available online: http://unfccc.int/resource/docs/2015/cop21/eng/l09r01.pdf (accessed on 13 August 2020).
- Rogelj, J.; Den elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef]
- Jang, W.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Zhai, P.; Pörtner, H.O.; Roberts, D. (Eds.) Summary for Policymakers, in Global warming of 15 °C, An IPCC Special Report on the impacts of global warming of 15 °C above pre-industrial levels and related global greenhouse gas emission pathways. In The Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018; Volume 32. [Google Scholar]
- IEA. World Energy Outlook; IEA: Paris, France, 2018. [Google Scholar]
- Antonini, C.; Treyer, K.; Streb, A.; Spek, M.; Bauer, C.; Mazzotti, M. Hydrogen production from natural gas and biomethane with carbon capture and storage–A techno-environmental analysis. Sustain. Energy Fuels 2020, 4, 2967–2986. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Energy Technology Perspectives 2017; International Energy Agency (IEA): Paris, France, 2017. [Google Scholar]
- Lee, Y.-L.; Mnoyan, A.; Na, H.-S.; Ahn, S.-Y.; Kim, K.-J.; Shim, J.-O.; Lee, K.; Roh, H.-S. Comparison of the effects of the catalyst preparation method and CeO2 morphology on the catalytic activity of Pt/CeO2 catalysts for the water–gas shift reaction. Catal. Sci. Technol. 2020. accepted. [Google Scholar] [CrossRef]
- Du, H.; Kong, R.M.; Guo, X.; Qu, F.; Li, J. Recent progress in transition metal phosphides with enhanced electrocatalysis for hydrogen evolution. Nanoscale 2018, 10, 21617–21624. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. Harmonised life-cycle global warming impact of renewable hydrogen. J. Clean. Prod. 2017, 149, 762–772. [Google Scholar] [CrossRef]
- Parkinson, B.; Tabatabaei, M.; Upham, D.C.; Ballinger, B.; Greig, C.; Smart, S.; McFarland, E. Hydrogen production using methane: Techno-economics of decarbonizing fuels and chemicals. Int. J. Hydrogen Energy 2018, 43, 2540–2555. [Google Scholar] [CrossRef]
- Sun, P.; Young, B.; Elgowainy, A.; Lu, Z.; Wang, M.; Morelli, B.; Hawkins, T. Criteria Air Pollutants and Greenhouse Gas Emissions from Hydrogen Production in U.S. Steam Methane Reforming Facilities. Environ. Sci. Technol. 2019, 53, 7103–7113. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.M.; Álvarez-Galván, M.C.; Mota, M.; Villoria de la Mano, J.A.; Al-Zahrani, S.M.; Fierro, J.L.G. Catalysts for Hydrogen Production from Heavy Hydrocarbons. ChemCatChem 2011, 3, 440–457. [Google Scholar] [CrossRef]
- Jeon, Y.; Lee, C.; Rhee, J.; Lee, G.; Myung, J.; Park, M.; Park, J.-I.; Einaga, H.; Shul, Y.-G. Autothermal reforming of heavy-hydrocarbon fuels by morphology controlled perovskite catalysts using carbon templates. Fuel 2017, 187, 446–456. [Google Scholar] [CrossRef]
- Jeon, Y.; Kwon, O.; Lee, C.; Lee, G.; Myung, J.; Park, S.; Irvine, J.T.S.; Shul, Y. Positional influence of Ru on Perovskite structured catalysts for efficient H2 production process by heavy-hydrocarbon source. Appl. Catal. A Gen. 2019, 582, 117111. [Google Scholar] [CrossRef]
- Nahar, G.; Dupont, V. Recent Advances in Hydrogen Production Via Autothermal Reforming Process (ATR): A Review of Patents and Research Articles. Recent Patents Chem. Eng. 2013, 6, 8–42. [Google Scholar] [CrossRef]
- Reese, M.A.; Turn, S.Q.; Cui, H. Kinetic modeling of high pressure autothermal reforming. J. Power Sources 2010, 195, 553–558. [Google Scholar] [CrossRef]
- Jeon, Y.; Park, D.-H.; Park, J.-I.; Yoon, S.-H.; Mochida, I.; Choy, J.-H.; Shul, Y.-G. Hollow Fibers Networked with Perovskite Nanoparticles for H2 Production from Heavy Oil. Sci. Rep. 2013, 3, 2902. [Google Scholar] [CrossRef] [PubMed]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef]
- Pasadakis, N.; Karonis, D.; Mintza, A. Detailed compositional study of the Light Cycle Oil (LCO) solvent extraction products. Fuel Process. Tech. 2011, 92, 1568–1573. [Google Scholar] [CrossRef]
- Laredo, G.C.; Vega Merino, P.M.; Hernández, P.S. Light Cycle Oil Upgrading to High Quality Fuels and Petrochemicals: A Review. Ind. Eng. Chem. Res. 2018, 57, 7315–7321. [Google Scholar] [CrossRef]
- Thakkar, V.P.; Abdo, S.F.; Gembicki, V.A.; Mc Gehee, J.F. LCO Upgrading: A Novel Approach for Greater Added Value and Improved Returns; UOP Report AM-05-53; UOP LLC: Des Plaines, IL, USA, 2005. [Google Scholar]
- Kaila, R.K.; Gutierrez, A.; Krause, Q.I. Autothermal reforming of simulated and commercial diesel: The performance of zirconia-supported RhPt catalyst in the presence of sulfur. Appl. Catal. B Environ. 2008, 84, 324–331. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Navarro, R.M.; Rosa, F.; Briceno, Y.; Gordillo Alvarez, F.; Fierro, J.L.G. Performance of La,Ce-modified alumina-supported Pt and Ni catalysts for the oxidative reforming of diesel hydrocarbons. Int. J. Hydrogen Energy 2008, 33, 652–663. [Google Scholar] [CrossRef]
- Karatzas, X.; Jansson, K.; González, A.; Dawody, J.; Svensson, A.; Pettersson, L. Zone-coated Rh-based monolithic catalyst for autothermal reforming of diesel. Appl. Catal. B Environ. 2011, 101, 226–238. [Google Scholar] [CrossRef]
- Pena, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef]
- Mota, N.; Alvarez-Galván, M.C.; Navarro, R.M.; Al-Zahrani, S.M.; Goguet, A.; Daly, H.; Zhang, W.; Trunschke, A.; Schlögl, R.; Fierro, J.L.G. Insights on the role of Ru substitution in the properties of LaCoO3-based oxides as catalysts precursors for the oxidative reforming of diesel fuel. Appl. Catal. B Environ. 2012, 113, 271–282. [Google Scholar] [CrossRef]
- Qia, A.; Wang, S.; Fu, G.; Ni, C.; Wu, D. La–Ce–Ni–O monolithic perovskite catalysts potential for gasoline autothermal reforming system. Appl. Catal. A Gen. 2005, 281, 233–246. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Johnson, D.W.; Remeika, J.P.; Gallagher, P.K. Perovskite oxides: Materials science in catalysis. Science 1977, 4, 827–833. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Mota, N.; Navarro, R.M.; Alvarez-Galvana, M.C.; Al-Zahrani, S.M.; Fierro, J.L.G. Hydrogen production by reforming of diesel fuel over catalysts derived from LaCo1−xRuxO3 perovskites: Effect of the partial substitution of Co by Ru (x = 0.01–0.1). J. Power Sources 2011, 196, 9087–9095. [Google Scholar] [CrossRef]
- Mota, N.; Alvarez-Galvan, M.C.; Villoria, J.A.; Rosa, F.; Fierro, J.L.G.; Navarro, R.M. Reforming of Diesel Fuel for Hydrogen Production over Catalysts Derived from LaCo1−xMxO3 (M = Ru, Fe). Top Catal. 2009, 52, 1995–2000. [Google Scholar] [CrossRef]
- Fino, D.; Russo, N.; Cauda, E.; Saracco, G.; Specchia, V. La–Li–Cr perovskite catalysts for diesel particulate combustion. Catal. Today 2006, 114, 31–39. [Google Scholar] [CrossRef]
- Sunarso, J.; Torriero, A.A.J.; Zhou, W.; Howlett, P.C.; Forsyth, M. Oxygen Reduction Reaction Activity of La-Based Perovskite Oxides in Alkaline Medium: A Thin-Film Rotating Ring-Disk Electrode Study. J. Phys. Chem. C 2012, 116, 5827–5834. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Simböck, J.; Ghiasi, M.; Schönebaum, S.; Simon, U.; de Groot, F.M.; Palkovits, R. Electronic parameters in cobalt-based perovskite-type oxides as descriptors for chemocatalytic reactions. Nat. Comm. 2020, 11, 652. [Google Scholar] [CrossRef]
- Qin, J.; Lin, L.; Wang, X. A perovskite oxide LaCoO3 cocatalyst for efficient photocatalytic reduction of CO2 with visible light. Chem. Commun. 2018, 54, 2272–2275. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, S.; Xi, S.; Ren, X.; Zhou, Y.; Zhang, G.; Yang, H.; Du, Y.; Xu, Z.J. Tailoring the Co 3d-O 2p Covalency in LaCoO3 by Fe Substitution To Promote Oxygen Evolution Reaction. Chem. Mater. 2017, 29, 10534–10541. [Google Scholar] [CrossRef]
- Song, S.; Zhou, J.; Su, X.; Wang, Y.; Li, J.; Zhang, L.; Xiao, G.; Guan, C.; Liu, R.; Chen, S.; et al. Operando X-ray spectroscopic tracking of self-reconstruction for anchored nanoparticles as high-performance electrocatalysts towards oxygen evolution. Energy Environ. Sci. 2018, 11, 2945–2953. [Google Scholar] [CrossRef]
- Getty, K.; Delgado-Jaime, M.U.; Kennepohl, P. Assignment of pre-edge features in the Ru K-edge X-ray absorption spectra of organometallic ruthenium complexes. Inorg. Chim. Acta 2008, 361, 1059–1065. [Google Scholar] [CrossRef]
- Masuda, Y.; Hosokawa, S.; Inoue, M. Combustion activities of the Ru catalysts supported on hexagonal YbFeO3. J. Ceram. Soc. Jpn. 2011, 119, 850. [Google Scholar] [CrossRef][Green Version]
- Arcon, I.; Bencan, A.; Kodre, A.; Kosec, M. X-ray absorption spectroscopy analysis of Ru in La2RuO5. X-ray Spectrom. 2007, 36, 301–304. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Zhou, J.; Jiang, Z.; Shen, B. Chemoselectivity-induced multiple interfaces in MWCNT/Fe3O4@ZnO heterotrimers for whole X-band microwave absorption. Nanoscale 2014, 6, 12298–12302. [Google Scholar] [CrossRef]
- Hong, W.T.; Stoerzinger, K.A.; Moritz, B.; Devereaux, T.P.; Yang, W.; Shao-Horn, Y. Probing LaMO3 Metal and Oxygen Partial Density of States Using X-ray Emission, Absorption, and Photoelectron Spectroscopy. J. Phys. Chem. C 2015, 119, 2063–2072. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.; Zhang, Y.; Peng, L.; Ma, S.; Yang, T.; Guo, H.; Zhang, Z.; Su, D.S.; Zhang, J. In Situ oxidation of carbon-encapsulated cobalt nanocapsules creates highly active cobalt oxide catalysts for hydrocarbon combustion. Nat. Comm. 2015, 6, 7181. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Chen, Y.; Engelhard, M.H.; Song, C. Comparative study on the sulfur tolerance and carbon resistance of supported noble metal catalysts in steam reforming of liquid hydrocarbon fuel. ACS Catal. 2012, 2, 1127–1137. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, C.; Li, Y.; Song, C.; Bolin, T.B. Sulfur poisoning mechanism of steam reformingcatalysts: An X-ray absorption near edge structure (XANES) spectroscopic stud. Phys. Chem. Chem. Phys. 2010, 12, 5707–5711. [Google Scholar] [CrossRef] [PubMed]
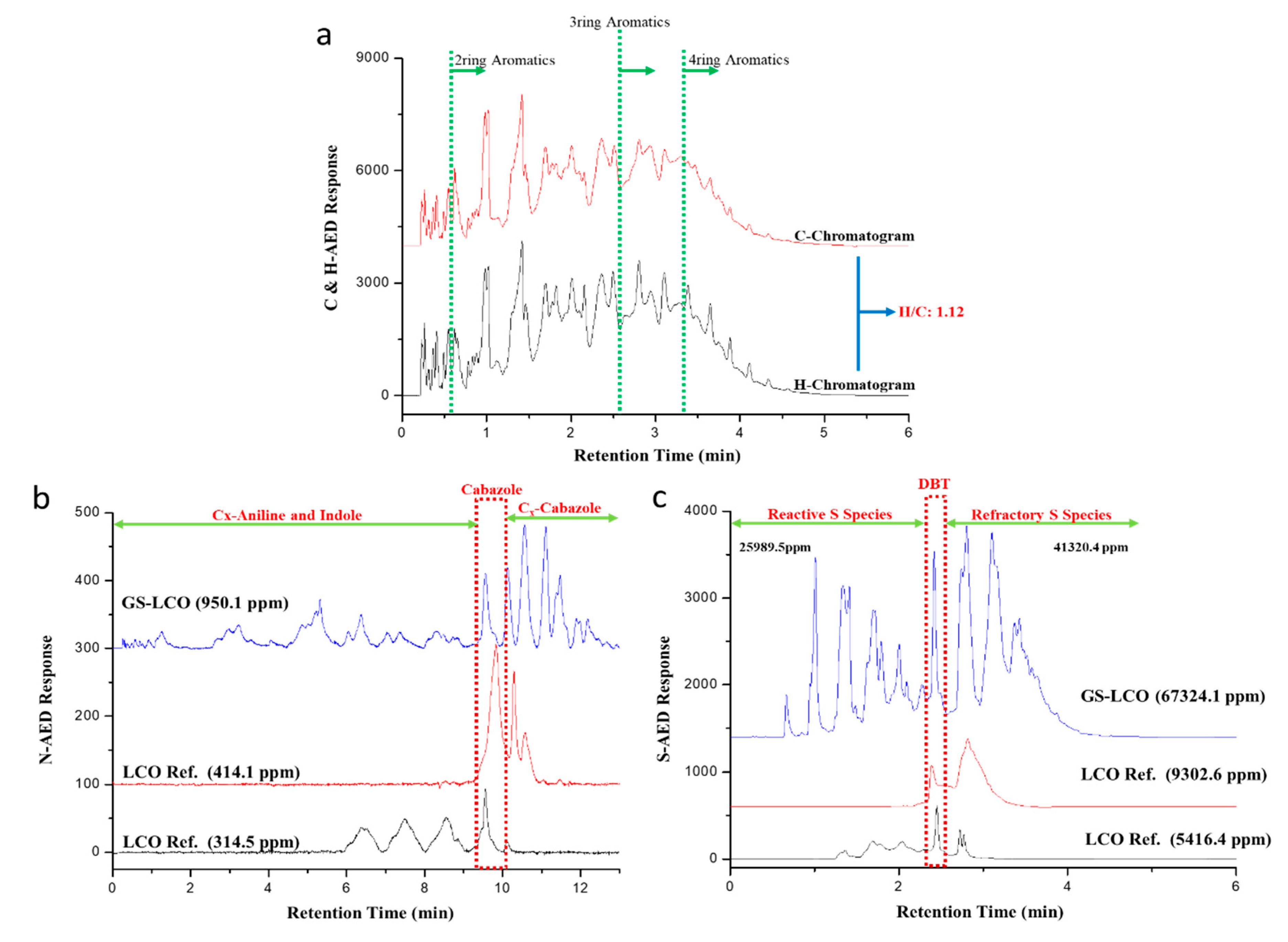


| Species | Ring | SA/USA 1 | Compositions/(%) | % |
|---|---|---|---|---|
| HC | 57.92 | |||
| 0 | - | Eicosane (2.38), Octadecane (2.50), Nonadecane (0.92), etc. | 8.94 | |
| 1 | SA | 1,7-Dimethyl-4-(1-methylethyl)cyclodecane (0.165), etc. | 0.17 | |
| 1 | USA | Benzene (1.48), Octane (0.21), Cyclopropane (0.11), etc. | 2.06 | |
| 2 | SA | Cyclopropane, 1,1’-methylenebis- (0.07), etc. | 0.01 | |
| 2 | USA | Naphthalene (5.39), 1,1’-Biphenyl (1.12), 1H-Indene (0.55), etc. | 10.65 | |
| 3 | SA | Tricyclo-octane, 8-methylene (0.01), etc. | 0.02 | |
| 3 | USA | Phenanthrene (17.57), Anthracene (6.87), Fluorene (1.47), etc. | 31.40 | |
| 4 | USA | Pyrene (2.06), Benz(a)anthracene (1.24), etc. | 4.68 | |
| S | 30.75 | |||
| 0 | - | 1-Methyl-prop-2-enyl Dithiopropanoate (0.07, 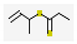 ), etc. ), etc. | 0.01 | |
| 1 | - | - | 0.00 | |
| 2 | - | 1-Propene-2-thiol (10.08, 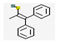 ), Benzo[b]thiophene (0.119, ), Benzo[b]thiophene (0.119,  ), etc. ), etc. | 10.78 | |
| 3 | - | Dibenzothiophene (8.94, 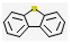 ), 2,7-Dimethyldibenzothiophene (9.63, ), 2,7-Dimethyldibenzothiophene (9.63, 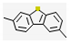 ), etc. ), etc. | 19.90 | |
| 4 | - | Phenaleno [1,9-bc]thiophene (0.04, 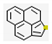 ), etc. ), etc. | 0.07 | |
| N | 1.35 | |||
| 0 | - | Butanenitrile (0.002), etc. | 0.003 | |
| 1 | - | 3-Methyl-1,2-diazirine (0.017,  ), etc. ), etc. | 0.04 | |
| 2 | - | Pyridine (0.02, 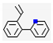 ), etc. ), etc. | 0.07 | |
| 3 | - | Carbazole (0.637,  ), 3,3-Diphenyl-5-methyl-3H-pyrazole (0.281, ), 3,3-Diphenyl-5-methyl-3H-pyrazole (0.281,  ), etc. ), etc. | 1.23 | |
| 4 | - | Benzo(a)phenazine (0.006,  ), etc. ), etc. | 0.01 | |
| Total | 90.02 |
| Catalysts | Elemental Compositions 1 | BET Analysis 2 | ||||
|---|---|---|---|---|---|---|
| La | Cr vs. Co | Ru | SBET (m2/g) | Total Pore Volume (p/p0 = 0.990) (mL/g) | Average Pore Diameter (nm) | |
| LaCo0.85Ru0.15O3 | 1.00 | 0.86 | 0.14 | 15.5 | 0.047 | 12.6 |
| LaCr0.85Ru0.15O3 | 1.00 | 0.84 | 0.16 | 14.3 | 0.051 | 15.9 |
| Peak 1 | d-Value | Cell Parameters | Particles Size 2 | |||
|---|---|---|---|---|---|---|
| Pp | d (Å) | a (Å) | b (Å) | c (Å) | Ps | |
| LaCoO3 | 33.15 | 2.783 | 5.468 | 5.453 | 13.17 | 38.2 |
| LaCo0.85Ru0.15O3 | 32.72 | 2.794 | 5.514 | 5.511 | 13.73 | 21.3 |
| LaCrO3 | 32.63 | 2.741 | 5.493 | 5.477 | 7.745 | 27.9 |
| LaCr0.85Ru0.15O3 | 31.82 | 2.761 | 5.515 | 5.512 | 7.793 | 18.3 |
| Catalysts | Weight Loss 1 (wt %) | Composition 2 (%) | |||
|---|---|---|---|---|---|
| Carbon | Hydrogen | Nitrogen | Sulfur | ||
| Pt-GDC | 31.87 | 94.87 | 0.51 | 0.43 | 4.19 |
| LaCo0.85Ru0.15O3 | 2.56 | 95.79 | 0.82 | 0.26 | 3.01 |
| LaCr0.85Ru0.15O3 | 1.12 | 96.74 | 0.82 | 0.15 | 2.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.; Jung, H.-K.; Park, C.-I.; Shul, Y.; Park, J.-i. Light Cycle Oil Source for Hydrogen Production through Autothermal Reforming using Ruthenium doped Perovskite Catalysts. Catalysts 2020, 10, 1039. https://doi.org/10.3390/catal10091039
Jeon Y, Jung H-K, Park C-I, Shul Y, Park J-i. Light Cycle Oil Source for Hydrogen Production through Autothermal Reforming using Ruthenium doped Perovskite Catalysts. Catalysts. 2020; 10(9):1039. https://doi.org/10.3390/catal10091039
Chicago/Turabian StyleJeon, Yukwon, Hoi-Kyoeng Jung, Cho-I Park, Yonggun Shul, and Joo-il Park. 2020. "Light Cycle Oil Source for Hydrogen Production through Autothermal Reforming using Ruthenium doped Perovskite Catalysts" Catalysts 10, no. 9: 1039. https://doi.org/10.3390/catal10091039
APA StyleJeon, Y., Jung, H.-K., Park, C.-I., Shul, Y., & Park, J.-i. (2020). Light Cycle Oil Source for Hydrogen Production through Autothermal Reforming using Ruthenium doped Perovskite Catalysts. Catalysts, 10(9), 1039. https://doi.org/10.3390/catal10091039