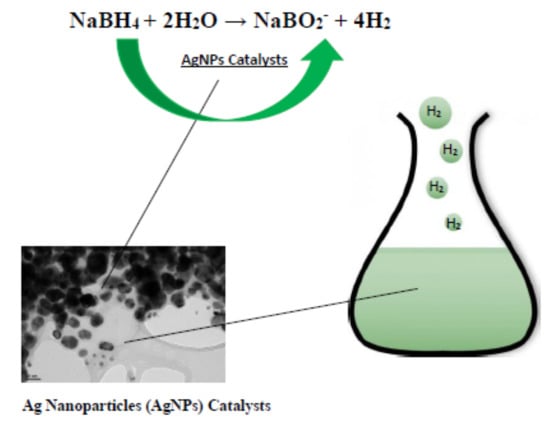
Beta-Cyclodextrin-Assisted Synthesis of Silver Nanoparticle Network and Its Application in a Hydrogen Generation Reaction
Abstract
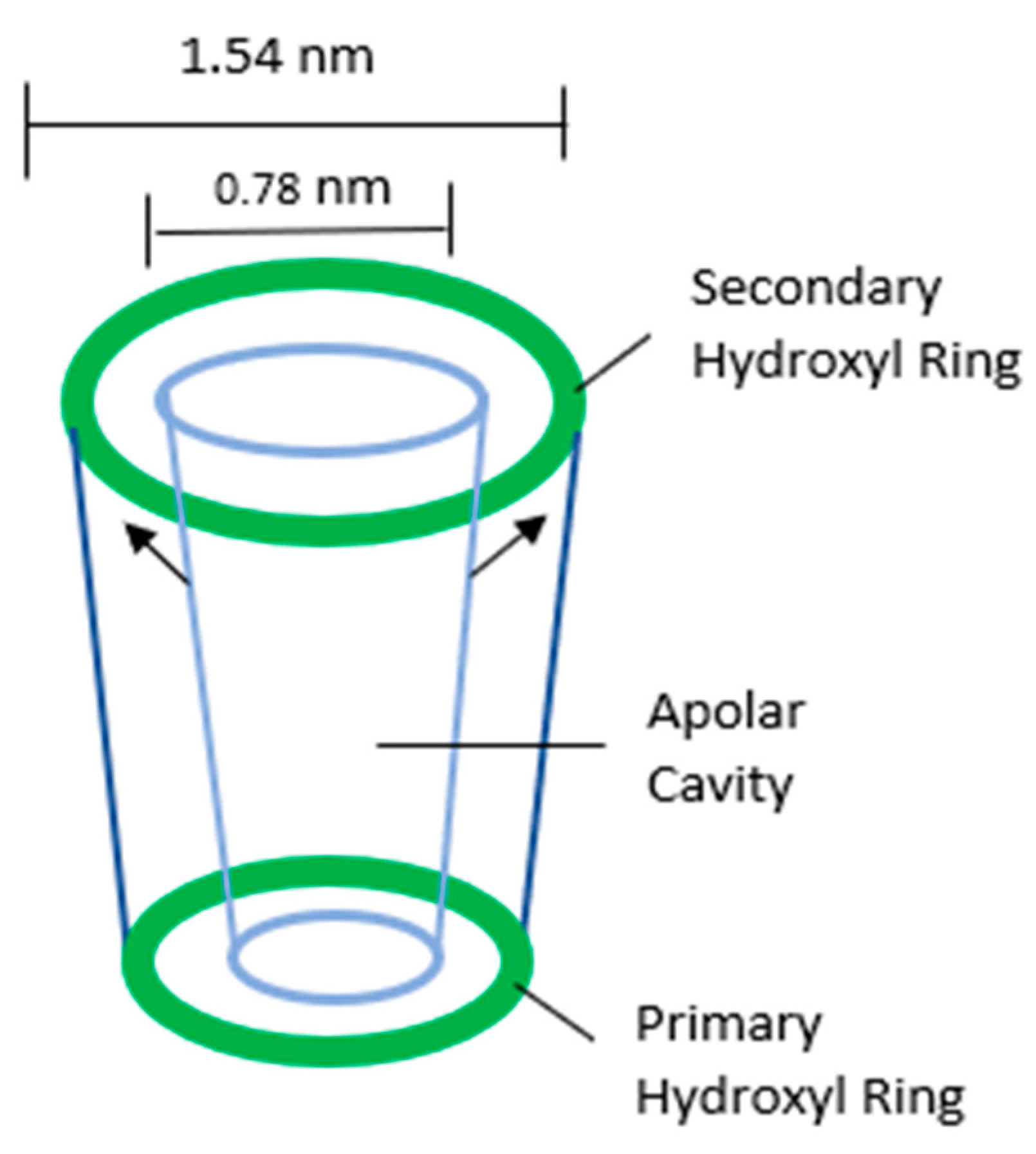
:1. Introduction
2. Results and Discussion
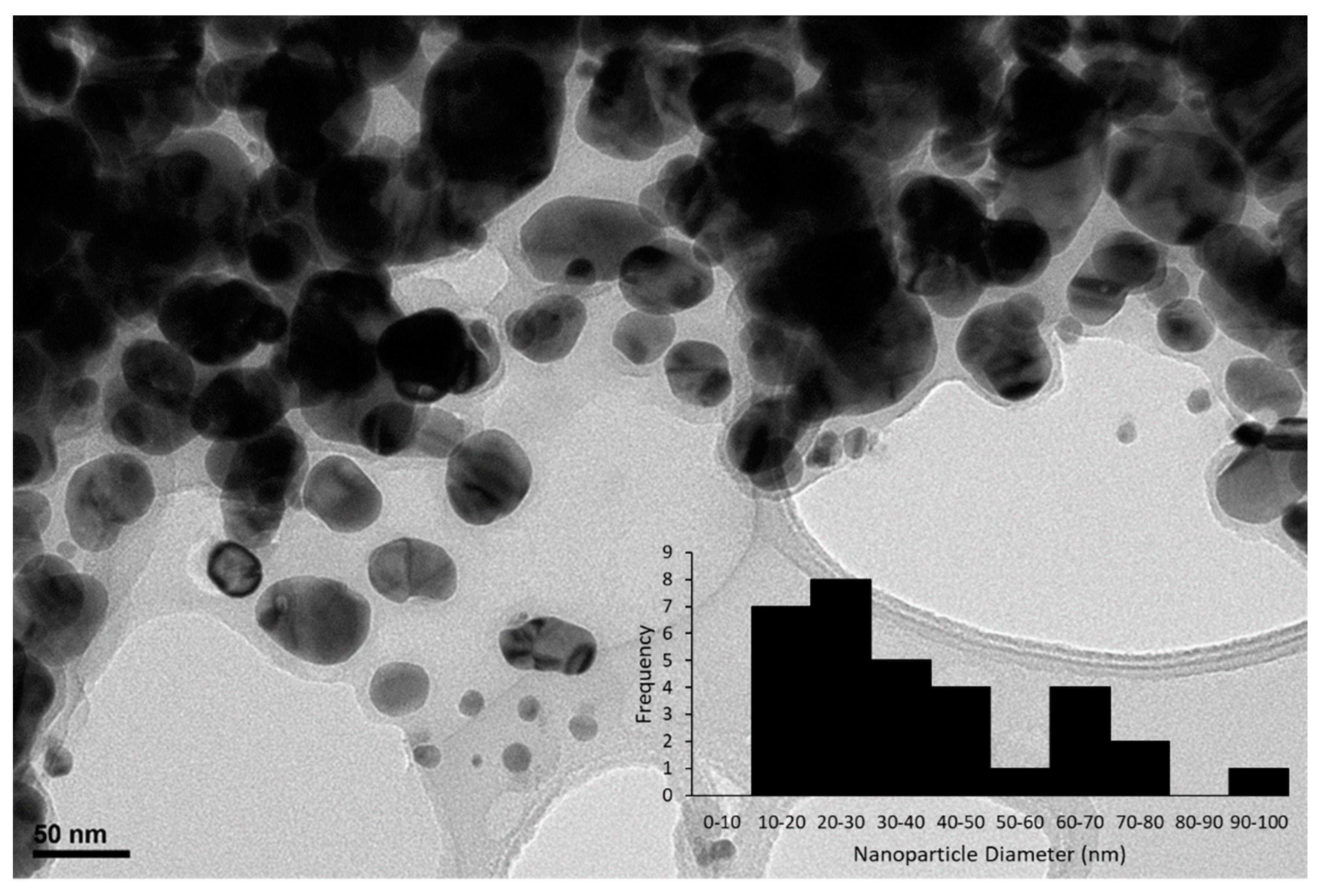
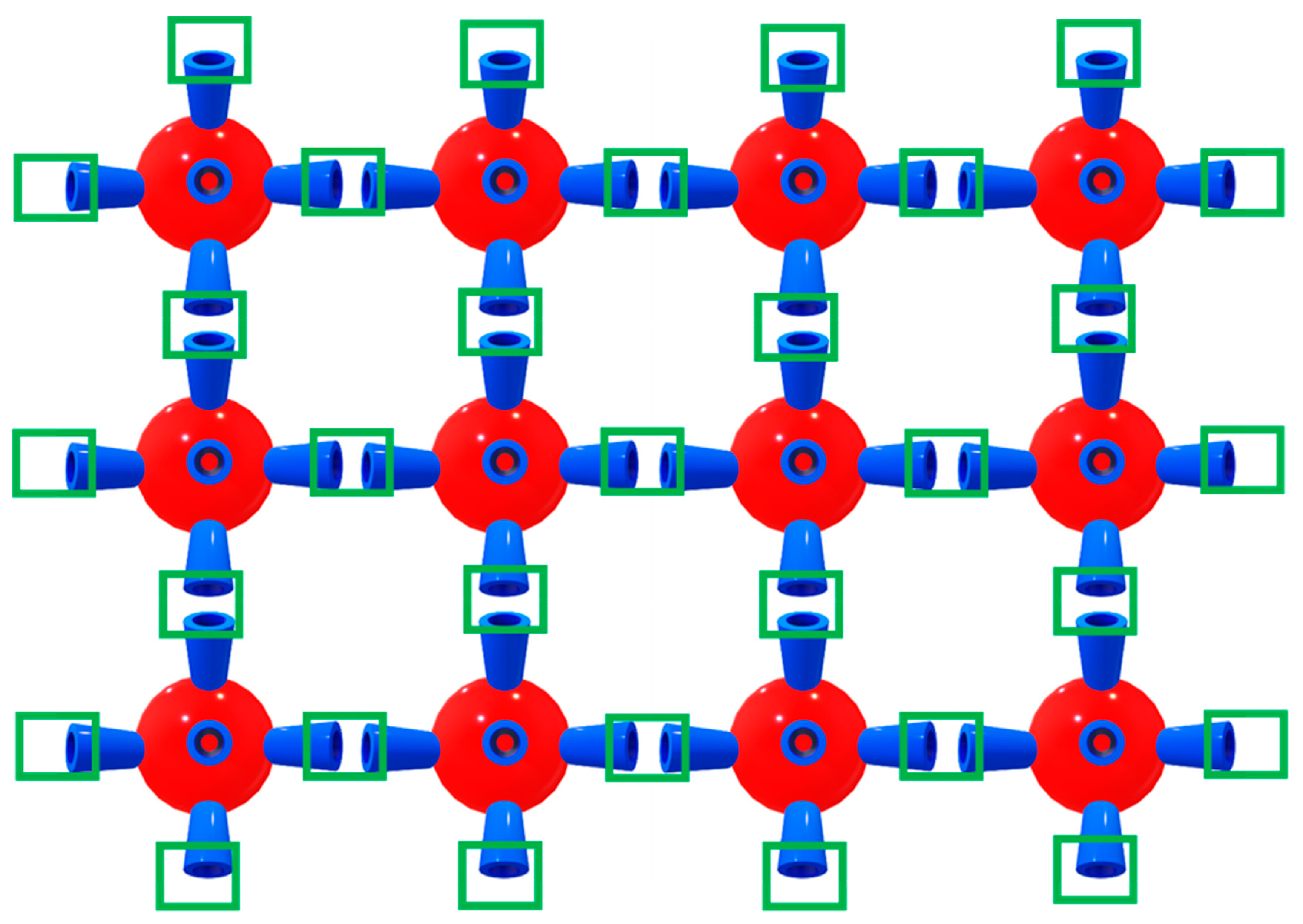
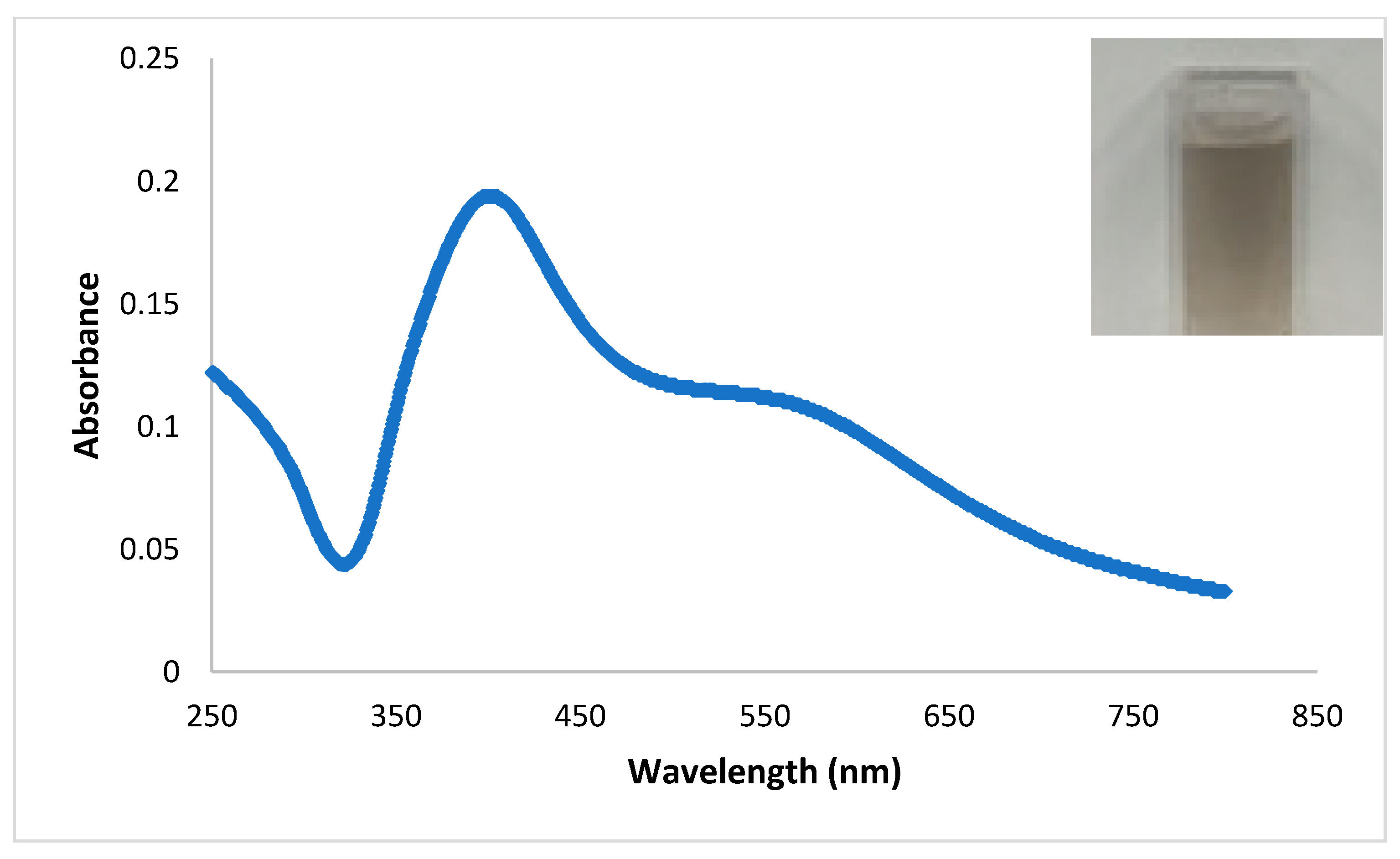
2.1. Characterization of Catalyst
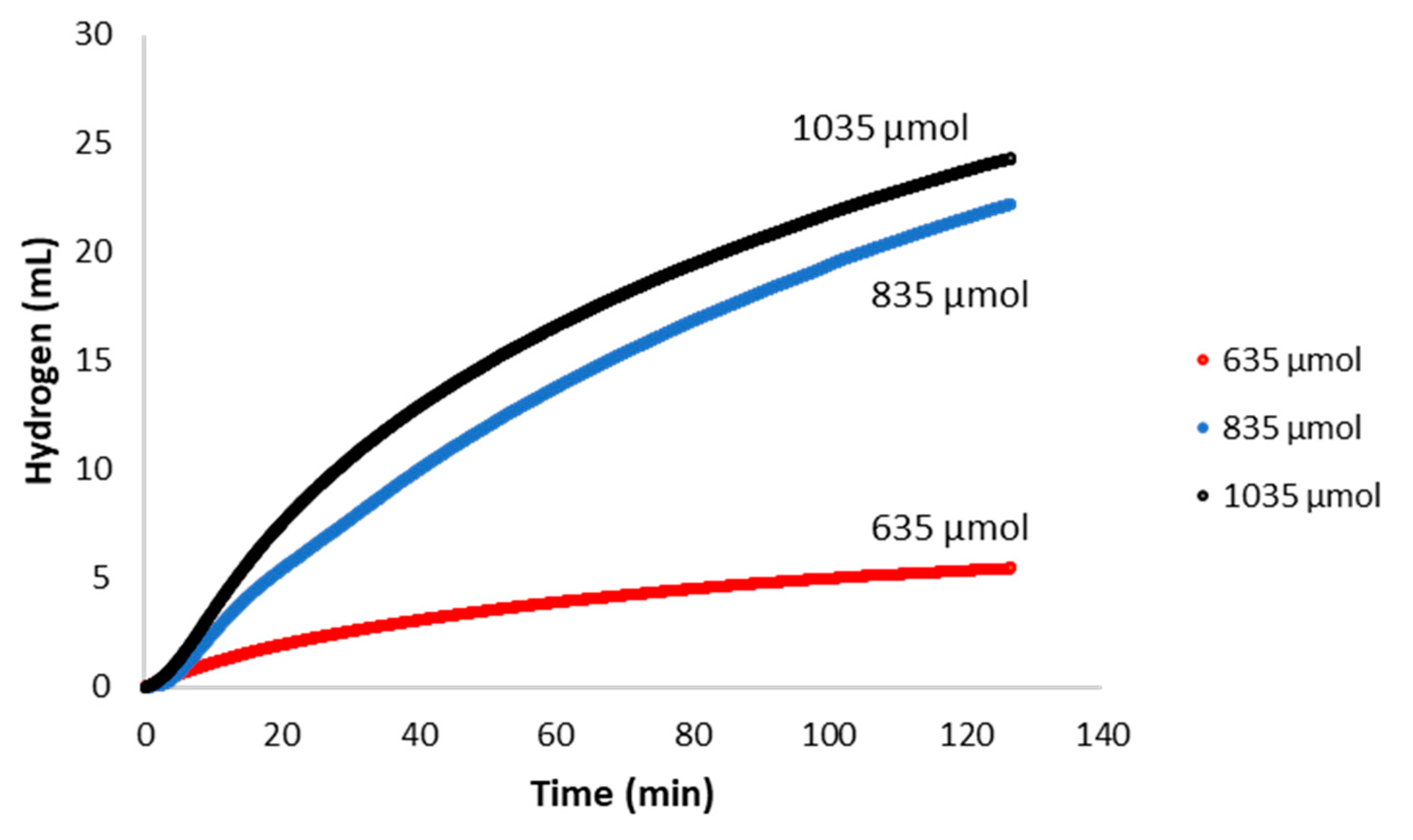
2.2. Catalytic Activity with Varied Concentrations of Reactant
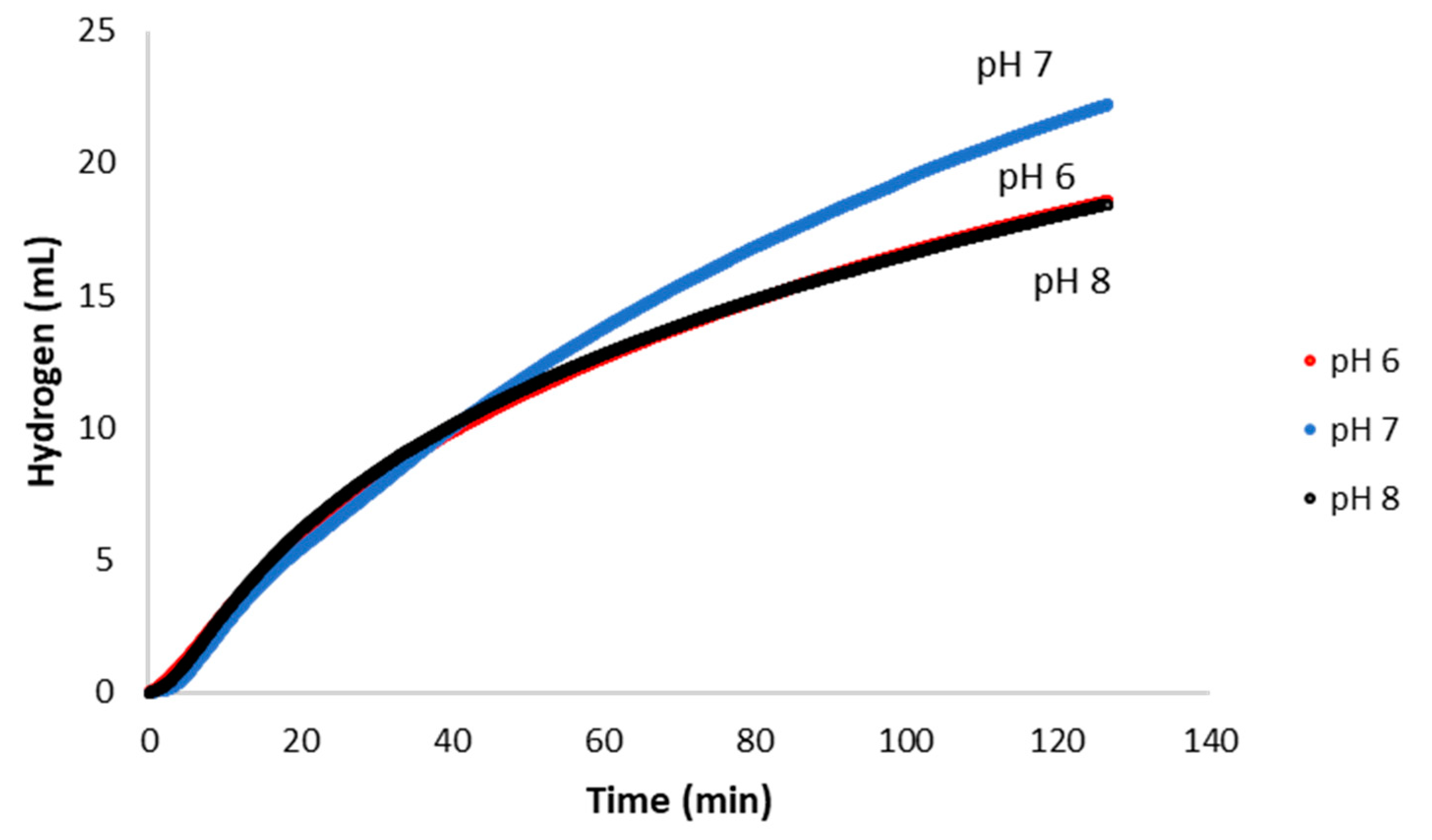
2.3. Catalytic Activity Under Varied pH Conditions
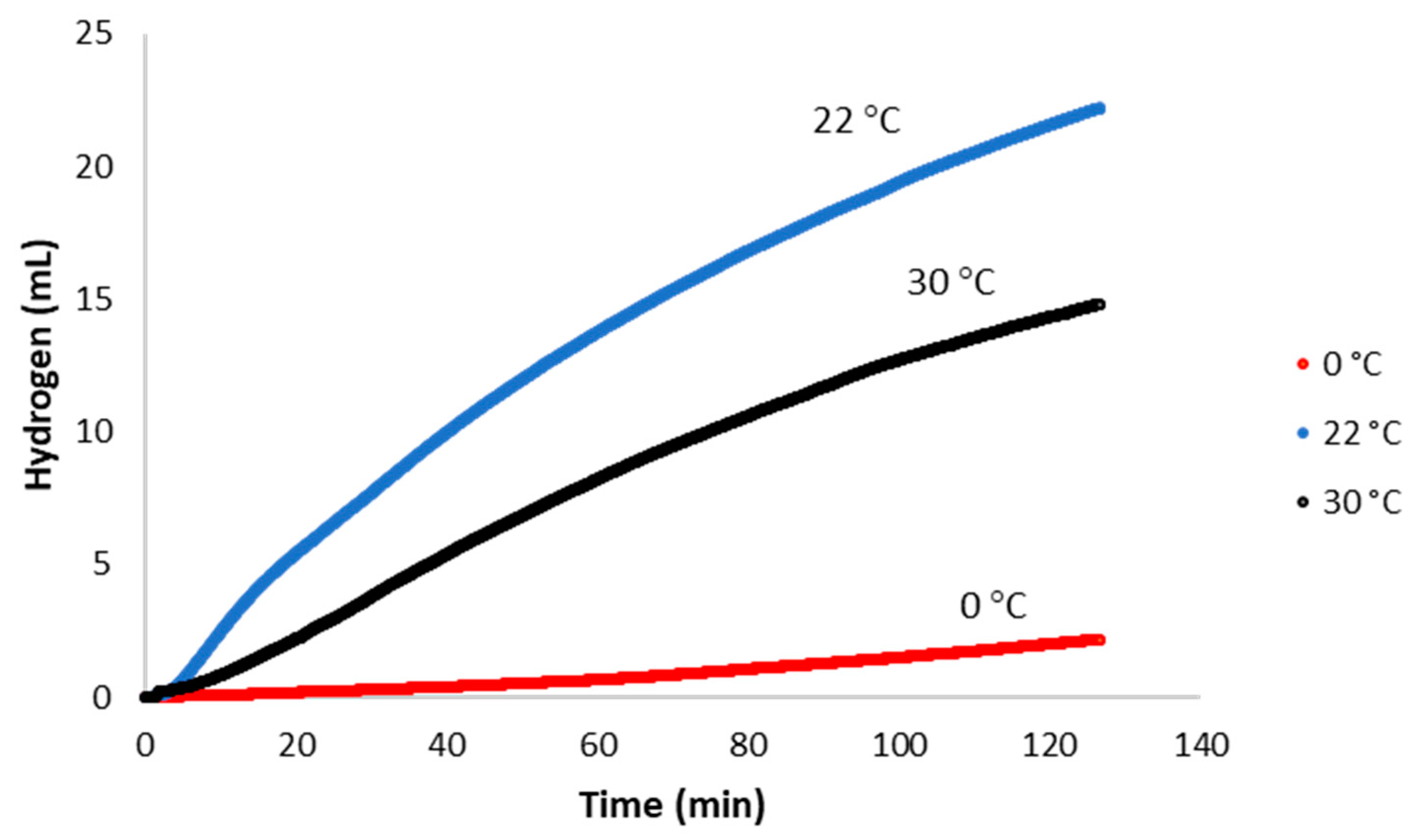
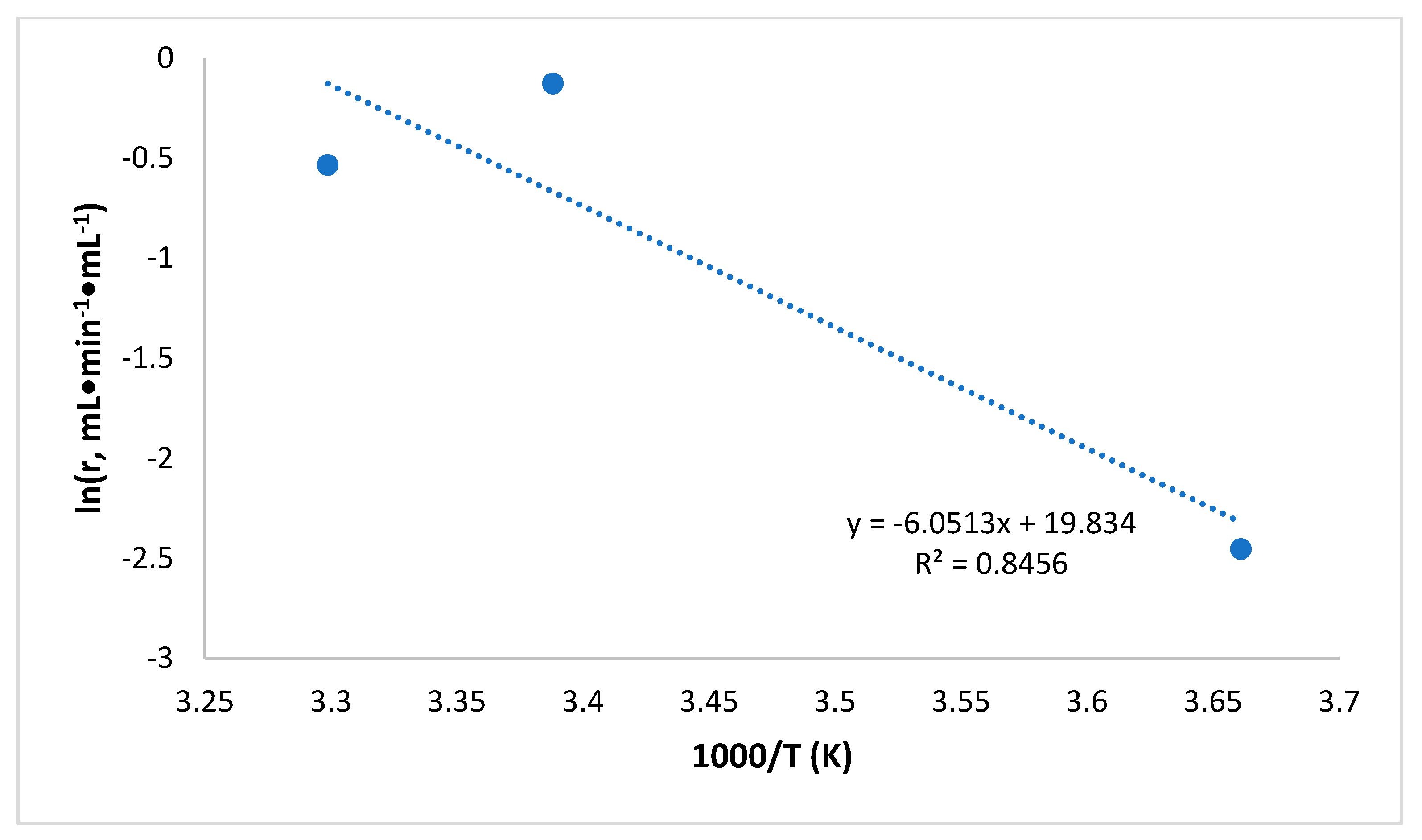
2.4. Catalytic Activity at Varied Temperatures and Activation Energies
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
3.3. Catalysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunn, S. Hydrogen futures: Toward a sustainable energy system. Int. J. Hydrog. Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Navarro, R.M.; Pena, M.A.; Fierro, J.L.G. Hydrogen Production Reactions from Carbon Feedstocks: Fossil Fuels and Biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Xu, Q. A high-performance hydrogen generation system: Transition metal-catalyzed dissociation and hydrolysis of ammonia-borane. J. Power Sources 2006, 156, 190–194. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P. A review: Hydrogen generation from borohydride hydrolysis reaction. J. Power Sources 2009, 187, 527–534. [Google Scholar] [CrossRef]
- Kojima, Y.; Suzuki, K.I.; Fukumoto, K.; Sasaki, M.; Yamamoto, T.; Kawai, Y.; Hayashi, H. Hydrogen generation using sodium borohydride solution and metal catalyst coated on metal oxide. Int. J. Hydrog. Energy 2002, 27, 1029–1034. [Google Scholar] [CrossRef]
- Özkar, S.; Zahmakıran, M. Hydrogen generation from hydrolysis of sodium borohydride using Ru (0) nanoclusters as catalysts. J. Alloys Compd. 2005, 404, 728–731. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium Borohydride, Its Hydrolysis and its Use as a Reducing Agent and in the Generation of Hydrogen. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.M.; Wixtrom, A.; Zhang, K.; Cao, W.; Baumgart, H. Highly Uniform Self-Assembled Gold Nanoparticles over High Surface Area Dense ZnO Nanorod Arrays as Novel Surface Catalysts. ECS J. Solid State Sci. Technol. 2014, 3, M61–M64. [Google Scholar] [CrossRef]
- Huff, C.; Dushatinski, T.; Barzanji, A.; Abdel-Fattah, N.; Barzanji, K.; Abdel-Fattah, T.M. Pretreatment of gold nanoparticle multi-walled carbon nanotube composites for catalytic activity toward hydrogen generation reactions. ECS J. Solid State Sci. Technol. 2017, 7, M69–M71. [Google Scholar] [CrossRef]
- Huff, C.; Dushatinski, T.; Abdel-Fattah, T.M. Gold nanoparticle/multi-walled carbon nanotube composite as novel catalyst for hydrogen evolution reactions. Int. J. Hydrog. Energy 2017, 42, 18985–18990. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.M.; Wixtrom, A. Catalytic Reduction of 4-Nitrophenol Using Gold Nanoparticles Supported on Carbon Nanotubes. ECS J. Solid State Sci. Technol. 2014, 3, M18–M20. [Google Scholar] [CrossRef]
- Zahmakiran, M.; Ozkar, S. Zeolite-confined ruthenium (0) nanoclusters catalyst: Record catalytic activity, reusability, and lifetime in hydrogen generation from hydrolysis of sodium borohydride. Langmuir 2009, 27, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Fernandes, R.; Miotello, A. Hydrogen generation by hydrolysis of NaBH4 with efficient Co-P-B catalyst: A kinetic study. J. Power Sources 2009, 188, 411–420. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Aboulatta, A.; Heyman, A.; Abdel-Fattah, T.M. Silver Nanoparticle/Multi-Walled Carbon Nanotube Composite as Catalyst for Hydrogen Production. ECS J. Solid State Sci. Technol. 2017, 6, 115–118. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.; Chen, Y.; Star, A. Corking Carbon Nanotube Cups with Gold Nanoparticles. ACS Nano 2012, 6, 6912–6921. [Google Scholar] [CrossRef]
- Kochkar, H.; Aouine, M.; Ghorbel, A.; Berhault, G. Shape-Controlled Synthesis of Silver and Palladium Nanoparticles Using β-Cyclodextrin. J. Phys. Chem. C 2011, 115, 11364–11373. [Google Scholar] [CrossRef]
- Jiang, Z.J.; Liu, C.Y.; Sun, L.W. Catalytic properties of Silver Nanoparticles Supported on Silica Spheres. J. Phys. Chem. B 2005, 109, 1730–1735. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Heyman, A.; Abdel-Fattah, T.M. Palladium Nanoparticle Multiwalled Carbon Nanotube Composite as Catalyst for Hydrogen Production by the Hydrolysis of Sodium Borohydride. ACS Appl. Energy Mater. 2018, 1, 4635–4640. [Google Scholar] [CrossRef]
- Metin, O.; Ozkar, S. Hydrogen Generation from the Hydrolysis of Ammonia-Borane and Sodium Borohydride by Using Water-Soluble Polymer-Stabilized Cobalt (0) Nanoclusters. Catalyst. Energy Fuels 2009, 23, 3517–3526. [Google Scholar] [CrossRef]
- Zahmakıran, M.; Özkar, S. Water dispersible acetate stabilized ruthenium (0) nanoclusters as catalyst for hydrogen generation reaction of sodium borohydride. J. Mol. Catal. A Chem. 2006, 258, 95–103. [Google Scholar] [CrossRef]
- Metin, Ö.; Özkar, S. Hydrogen generation from the hydrolysis of sodium borohydride by using water dispersible, hydrogen phosphate-stabilized nickel (0) nanoclusters as catalyst. Int. J. Hydrog. Energy 2007, 32, 1707–1715. [Google Scholar] [CrossRef]
- Yılmaz, M.S.; Figen, A.K.; Pişkin, S. Production of sodium metaborate tetrahydrate (NaB(OH)4·2H2O) using ultrasonic irradiation. Powder Technol. 2012, 215, 166–173. [Google Scholar] [CrossRef]
- Pena-Alonso, R.; Sicurelli, A.; Callone, E.; Carturan, G.; Raj, R. A picoscale catalyst for hydrogen generation from NaBH4 for fuel cells. J. Power Sources 2007, 165, 315–323. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, H.; Chen, J. Improved hydrogen generation for alkaline solution using carbon-supported Co-B as catalysts. Int. J. Hydrog. Energy 2007, 32, 4711–4716. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Antibacterial and catalytic activities of green synthesized silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Vidhu, V.K.; Philip, D. Catalytic degradation of organic dyes using biosynthesized silver. Micron 2014, 56, 54–62. [Google Scholar] [CrossRef]
- Merga, G.; Wilson, R.; Lynn, G.; Milosavljevic, B.H.; Meisel, D. Redox Catalysis on “Naked” Silver Nanoparticles. J. Phys. Chem. C 2007, 111, 12220–12226. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, B.; Tripathi, B.P. Polydopamine assisted synthesis of ultrafine silver nanoparticles for heterogeneous catalysis and water remediation. Nano-Struct. Nano-Objects 2020, 23, 100489. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, K.; Qin, W.; Hu, Y. Highly efficient flow-through catalytic reduction of methylene blue using silver nanoparticles functionalized cotton. Chem. Eng. J. 2020, 388, 124252. [Google Scholar] [CrossRef]
- Khan, M.S.J.; Khan, S.B.; Kamal, T.; Asiri, A.M. Catalytic Application of Silver Nanoparticles in Chitosan Hydrogel Prepared by a Facile Method. J. Polym. Environ. 2020, 28, 962–972. [Google Scholar] [CrossRef]
- Xu, R.; Wang, D.; Zhang, J.; Li, Y. Shape-Dependent Catlytic Activity of Silver Nanoparticles for the Oxidation of Styrene. Chem. Asian J. 2006, 1, 888–893. [Google Scholar] [CrossRef]
- Andrieux, J.; Swierczynski, D.; Laversenne, L.; Garron, A.; Bennici, S.; Goutaudier, C.; Miele, P.; Auroux, A.; Bonnetot, B. Kinetics of hydrogen generation on NaBH4 powders using cobalt catalysts. Int. J. Hydrog. Energy 2008, 34, 938. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Z.; Guo, Q.; Tian, H. Progress of Nanoscience. J. Power Sources 2014, 231, 190. [Google Scholar] [CrossRef]
- Guella, G.; Patton, B.; Miotello, A. Kinetic Features of the Platinum Catalyzed Hydrolysis of Sodium Borohydride from 11B NMR Measurements. J. Phys. Chem. C 2007, 111, 18744. [Google Scholar] [CrossRef]


| Catalyst | Ea (kJ/mol) | Temperature (°C) | Reference |
|---|---|---|---|
| Co nanoparticles | 35.0 | 29–59 | [33] |
| ZrCo/C | 34.8 | 10–50 | [34] |
| Ru nanoclusters | 41.0 | 25–45 | [21] |
| Ni nanoclusters | 54.0 | 25–45 | [22] |
| Pt/C | 45.0 | 15–75 | [35] |
| Au/MWCNTs | 21.1 | 0–30 | [11] |
| Pd/MWCNTs | 62.66 | 0–30 | [19] |
| Ag/MWCNTs | 44.5 | 0–30 | [15] |
| AgNPNs | 50.3 | 0–30 | This Work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huff, C.; Long, J.M.; Abdel-Fattah, T.M. Beta-Cyclodextrin-Assisted Synthesis of Silver Nanoparticle Network and Its Application in a Hydrogen Generation Reaction. Catalysts 2020, 10, 1014. https://doi.org/10.3390/catal10091014
Huff C, Long JM, Abdel-Fattah TM. Beta-Cyclodextrin-Assisted Synthesis of Silver Nanoparticle Network and Its Application in a Hydrogen Generation Reaction. Catalysts. 2020; 10(9):1014. https://doi.org/10.3390/catal10091014
Chicago/Turabian StyleHuff, Clay, Julia M. Long, and Tarek M. Abdel-Fattah. 2020. "Beta-Cyclodextrin-Assisted Synthesis of Silver Nanoparticle Network and Its Application in a Hydrogen Generation Reaction" Catalysts 10, no. 9: 1014. https://doi.org/10.3390/catal10091014
APA StyleHuff, C., Long, J. M., & Abdel-Fattah, T. M. (2020). Beta-Cyclodextrin-Assisted Synthesis of Silver Nanoparticle Network and Its Application in a Hydrogen Generation Reaction. Catalysts, 10(9), 1014. https://doi.org/10.3390/catal10091014